- Department of Pediatric Neurology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
Introduction: Glucocorticoids and intravenous immunoglobulin (IVIG) have been established as the primary therapeutic agents for treating autoimmune encephalitis (AE). Methylprednisolone is the most frequently utilized glucocorticoid; however, the potential advantages of dexamethasone (DEX) in the management of encephalitis have yet to be fully elucidated. This study aimed to assess the efficacy of DEX in combination with IVIG in the treatment of pediatric AE.
Methods: This retrospective study included 41 pediatric patients who were diagnosed with AE and were categorized into two groups on the basis of their treatment history. Group A (n = 29) comprised children who initially received immunotherapy at other healthcare institutions but were referred to our hospital for DEX+IVIG treatment because of inadequate response to prior therapies. Group B (n = 12) consisted of children who were administered DEX+IVIG treatment early in the acute phase of AE at our hospital. The therapeutic outcomes of DEX+IVIG treatment in children with nonacute AE (Group A) and acute AE (Group B) were evaluated. The modified Rankin scale (mRS) was used to assess the clinical status of all participants.
Results: Ninety percent of the patients were severely ill prior to DEX+IVIG treatment (mRS = 3.8 ± 1.0). Following treatment, the clinical symptoms of children in both the nonacute stage (Group A) and the acute stage (Group B) significantly improved. At the final follow-up, 90.2% of patients (mRS = 0–2) exhibited a favorable prognosis, with a complete response rate (mRS = 0) of 43.9% and a relapse rate of 2.4%. Children who experienced relapse were treated with DEX+IVIG, leading to a positive outcome. No severe adverse events were observed during treatment. The results of this study indicated that DEX+IVIG is an effective treatment for children with acute, nonacute, and relapsing AE.
Discussion: DEX+IVIG was shown to be beneficial at the acute, nonacute, sequelae, and recurrence stages of AE.
1 Introduction
Pediatric autoimmune encephalitis (AE) is a rare but severe neuroimmune disorder that occurs in approximately one in 100,000 patients. Children with AE may develop significant neurological symptoms within a short period, including cognitive dysfunction, movement disorders, seizures, and disturbances of consciousness (1–3). The pathogenesis of AE had yet to be fully elucidated and is typically caused by various factors that trigger the abnormal production of autoantibodies (4). The most common antibodies are anti-N-methyl-D-aspartate receptor (NMDAR) antibodies, while others, such as contactin-associated protein-2 (CASPR2) antibodies, leucine-rich glioma-inactivated-1 (LGI1) antibodies, and gamma-aminobutyric acid B receptor (GABABR) antibodies, are also frequently detected in patients with AE (5, 6). In recent years, there have been an increasing number of cases of AE in which patients present with typical clinical symptoms but test negative for known antibodies. These cases may easily be misdiagnosed, leading to delays in treatment.
The current standard immunotherapies for AE include glucocorticoids, intravenous immunoglobulin (IVIG), and plasma exchange (7, 8). Early initiation of immunotherapy is linked to improved outcomes in children with AE (9). However, the optimal glucocorticoid type and dosage remain unclear, and there are no established guidelines for specific immunoglobulin regimens. Intravenous methylprednisolone is often the glucocorticoid of choice, but the potential benefits of dexamethasone (DEX) in AE treatment have received limited attention. From an anti-inflammatory perspective, DEX has an anti-inflammatory potency ratio of 25 (compared with 1 for hydrocortisone), whereas methylprednisolone has a ratio of 5, indicating that DEX is five times more potent. DEX is a long-acting glucocorticoid with a duration of 36–54 h, whereas methylprednisolone is a medium-acting agent (12–36 h). Studies have shown that in the treatment of childhood acute lymphoblastic leukemia, DEX reduces central nervous system recurrence by 50% compared with other glucocorticoids, and replacing prednisolone with dexamethasone has been shown to decrease the incidence of meningeal leukemia (10, 11). Additionally, DEX has superior central nervous system permeability and a longer half-life in cerebrospinal fluid than prednisolone (12). We hypothesize that DEX may offer distinct advantages over other glucocorticoids for treating immune-inflammatory diseases of the central nervous system. This study aimed to investigate the efficacy and potential mechanisms of DEX combined with IVIG in treating children with AE.
2 Methods
2.1 Participants and samples
This retrospective cohort study included 41 children with autoimmune encephalitis (AE) who were treated at our hospital between March 2013 and March 2023. The participants were diagnosed with either antibody-positive or antibody-negative AE, both of which were diagnosed in accordance with the criteria established by Graus et al. (13). These criteria included changes in memory, altered consciousness, or psychiatric symptoms lasting less than 3 months, combined with at least one of the following criteria: (1) emerging focal neurological signs; (2) unexplained seizures unrelated to prior epileptic disorders; (3) cerebrospinal fluid abnormalities (elevated protein or white blood cell count >5/mm3); or (4) magnetic resonance imaging (MRI) findings indicating encephalitis-related changes. Children who met the diagnostic criteria for either antibody-positive autoimmune encephalitis or the consensus criteria for antibody-negative autoimmune encephalitis were included. Children with a history of motor or speech delays, epilepsy, or psychiatric disorders, including depression, anxiety, or other primary mood disorders, were excluded from the study.
2.2 Study design and treatment strategy
The patients were divided into two groups. Group A included 29 patients who initially received intravenous methylprednisolone (IVMP) and IVIG at other hospitals but experienced limited effectiveness during the acute stage. These patients were subsequently transferred to our hospital for treatment with DEX+IVIG during the nonacute phrase of AE. Group B comprised 12 patients who were treated with DEX+IVIG immunotherapy at our hospital during the acute phase of AE. The following medical data were collected from the patients’ records: age, sex, clinical symptoms, diagnosis, laboratory results, brain MRI findings, electroencephalography (EEG) findings, immunotherapy regimens, and adverse reactions. The modified Rankin Scale (mRS) was used to assess the recovery of neurological function, with a focus on patients’ ability to live independently. The mRS evaluates physical function, activity levels, and participation in daily life, with five distinct levels. The mRS was used to assess the clinical status and effects of DEX+IVIG treatment in both nonacute (Group A) and acute (Group B) AE patients.
2.2.1 DEX+IVIG treatment regimen
1. Dexamethasone was administered intravenously at a dosage of 0.3–0.5 mg/kg/day over 0.5–1 h for 5 consecutive days, followed by gradual tapering, with a typical course lasting 7–10 days. This treatment was combined with IVIG at a dose of 2 g/kg, which was administered over 3–5 days.
2. Children with severe or relapsing conditions may have required multiple rounds of this immunotherapy regimen, depending on their clinical response.
2.3 Definitions
Antibody-positive AE cases are characterized by the presence of antibodies in the serum, cerebrospinal fluid, or both. Antibody-negative AE cases are characterized by the absence of antibodies in the serum and cerebrospinal fluid during initial or follow-up assessments. A relapse of AE is defined as any acute worsening of neuropsychiatric symptoms, epilepsy, or other neurological symptoms after at least 1 month of clinical stability following acute immunotherapy. The “acute phase” refers to the period within 2 months of disease onset; the “subacute phase” refers to the period between 2 and 3 months after disease onset; and the “nonacute phase” refers to the period at least 3 months after disease onset in the absence of complete remission. The severity of AE was assessed using the mRS, which ranges from 0 to 5 (scores of 0 indicate complete recovery, scores of 0–2 indicate mild conditions or a favorable prognosis, and scores ≥3 indicate severe conditions or a poor prognosis).
2.4 Statistical analysis
Data analyses were performed using SPSS 25 software. Normally distributed measurement data are presented as the mean ± standard deviation (x ± s). Paired t tests were used to compare mRS scores and lymphocyte cytokine levels before and after treatment. One-way ANOVA was used to assess differences in continuous variables between multiple groups. Post hoc pairwise comparisons were conducted via the least significant difference (LSD) method. A p value <0.05 was considered to indicate statistical significance.
3 Results
3.1 Demographic data
3.1.1 Clinical features
A total of 41 cases fully met the study inclusion criteria. The ages of the patients ranged from 1 to 16 years, with an average age of 6.8 years. The age distribution was as follows: ≤3 years old: 3 children; 4–6 years old: 15 children; and ≥ 7 years old: 22 children. The majority were children over 4 years of age. There were 27 male and 14 female patients, resulting in a male-to-female ratio of 1.93:1. The median follow-up period was 24 months.
3.1.2 Antibody distribution characteristics
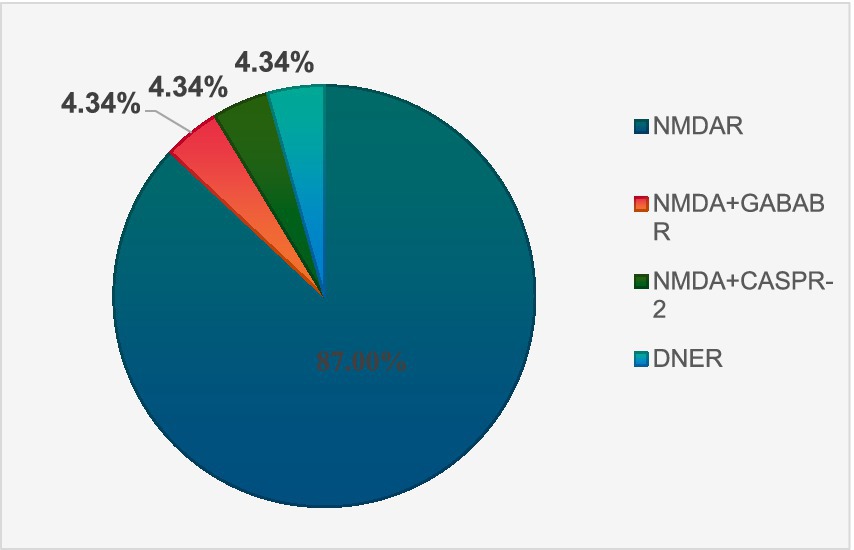
There were 23 antibody-positive cases and 18 antibody-negative cases. There were 20 cases of NMDA encephalitis and 1 case of anti-DNER antibody AE. NMDA and GABAB antibodies were detected in 1 patient, and NMDA and CASPR-2 antibodies were detected in 1 patient. The antibody distribution of 23 AE antibody-positive patients is shown in Figure 1.
3.1.3 Clinical symptoms during the acute phase
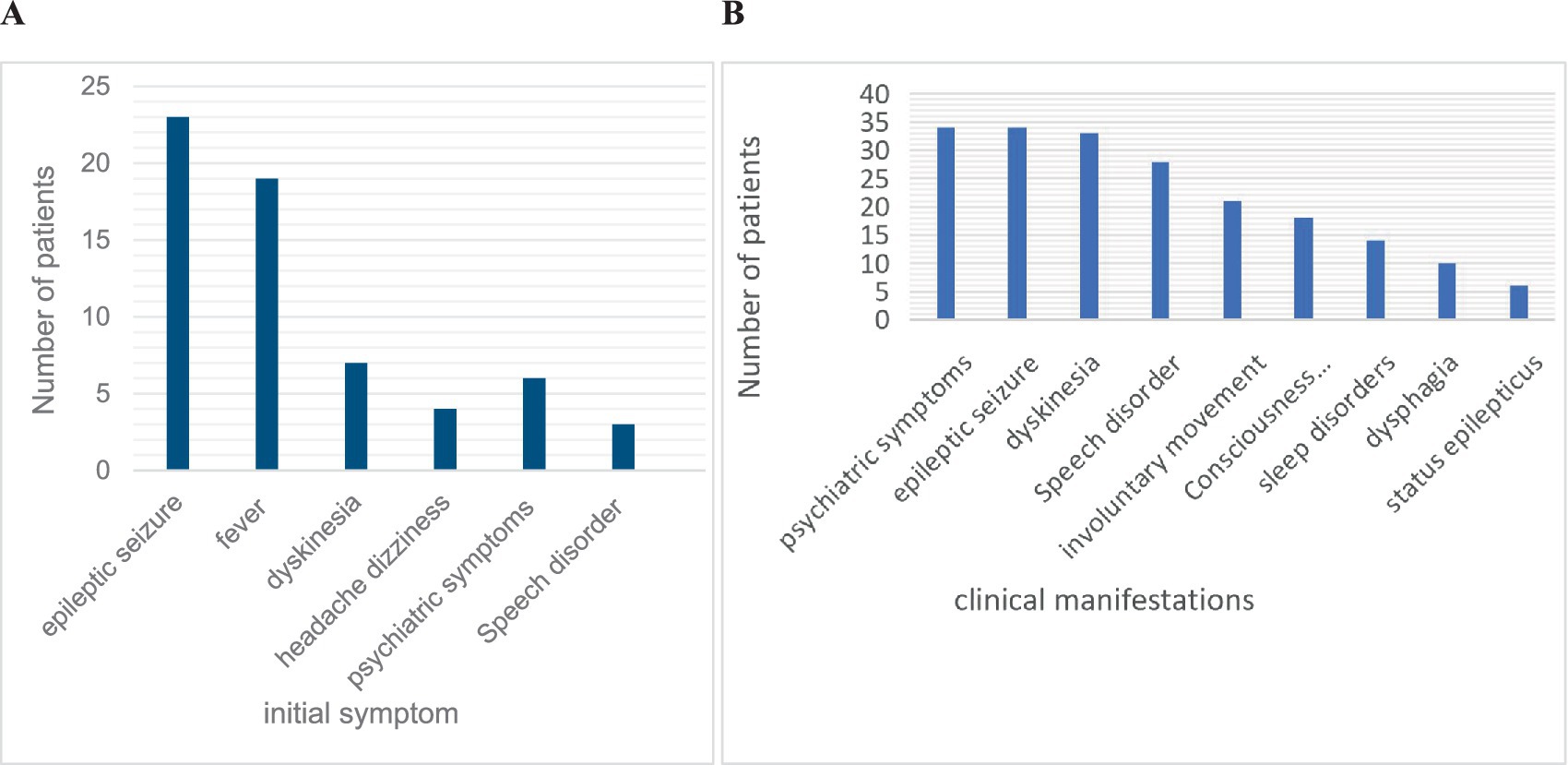
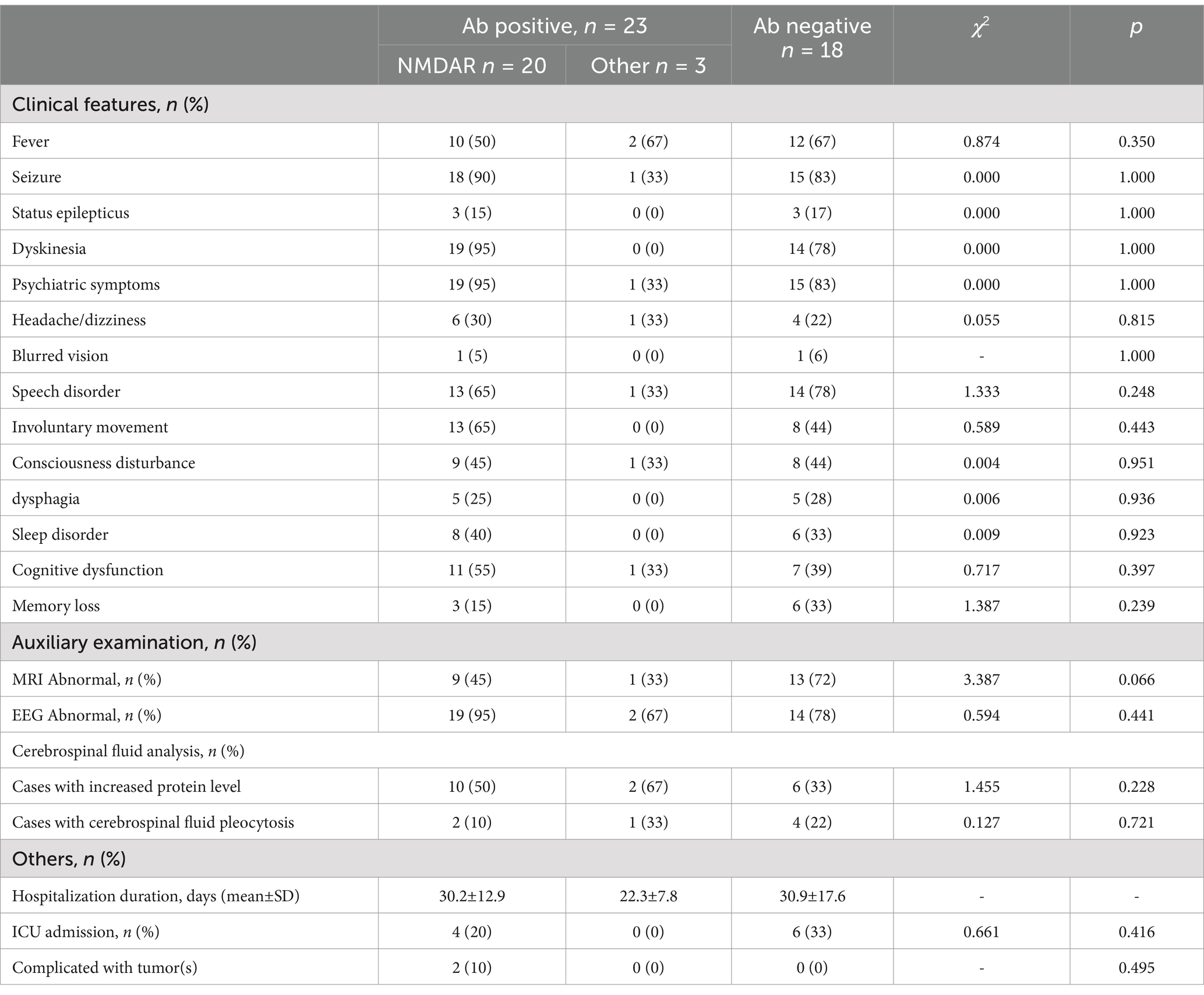
The most common initial symptoms included seizures (56.1%, n = 23), fever (46.3%, n = 19), and dyskinesia (17.1%, n = 7). The most common symptoms throughout the disease were psychiatric symptoms (82.9%, n = 34), seizures (82.9%, n = 34), dyskinesia (80.5%, n = 33), and speech disorders (62.3%, n = 28). Abnormal brain MRI findings (34.8%) were most common in the frontal lobe, followed by the basal ganglia and thalamus. EEG abnormalities (85.4%, n = 35) were mostly characterized by diffuse or focal slow waves and epileptic waves. Abnormal cerebrospinal fluid (61.0%) was detected in 25 children, mainly manifesting as slightly elevated white blood cells and proteins in the cerebrospinal fluid. The clinical symptoms and auxiliary examinations of all the children are shown in Figures 2A,B and Table 1.
3.2 Comparison of the efficacy of DEX+IVIG between group A and group B
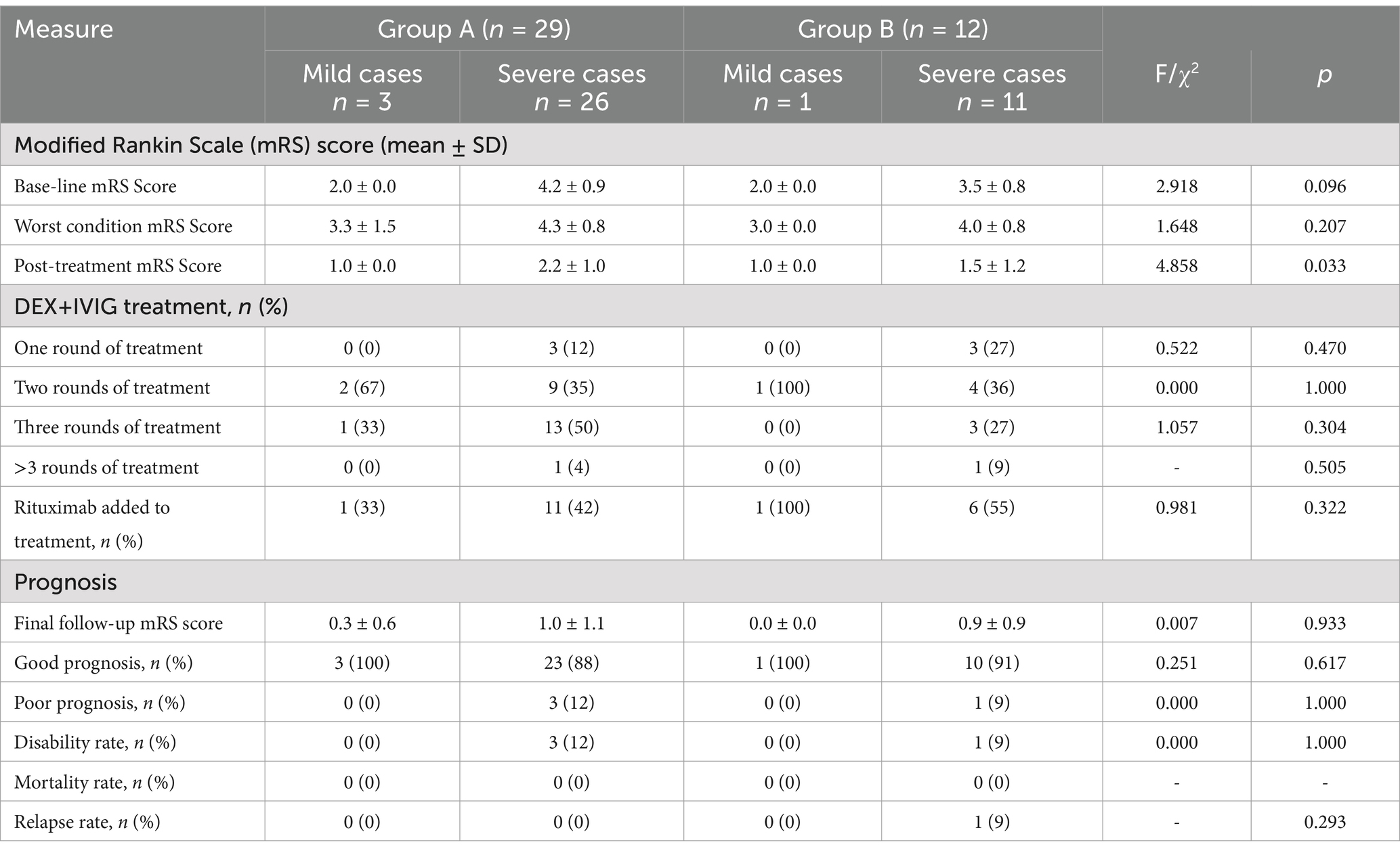
In Group A and Group B, 89.7% and 91.7% of the children, respectively, had severe conditions. After receiving 1–4 rounds of DEX+IVIG treatment, we observed significant improvements in clinical symptoms in both groups, regardless of the severity of the condition. The posttreatment mRS scores were significantly lower than the baseline scores (p < 0.05). Children in Group A had previously received IVMP+IVIG treatment at other hospitals with unsatisfactory results. By the time these children arrived at our hospital, they were already in the subacute or nonacute phase. We adjusted their treatment plan to DEX+IVIG, which yielded favorable outcomes. Children in Group B, who received DEX+IVIG treatment during the early stage of their illness at our hospital, also achieved good results. However, we found that most of these children, particularly those with more severe conditions, required 2–3 rounds of DEX+IVIG treatment. After DEX+IVIG treatment, some children in both groups continued receiving rituximab therapy, with a significantly higher rate of rituximab use in Group B than in Group A. A comparison of the clinical efficacy between Group A and Group B is shown in Table 2.
3.3 Efficacy of DEX+IVIG treatment in children with nonacute phase AE (group A)
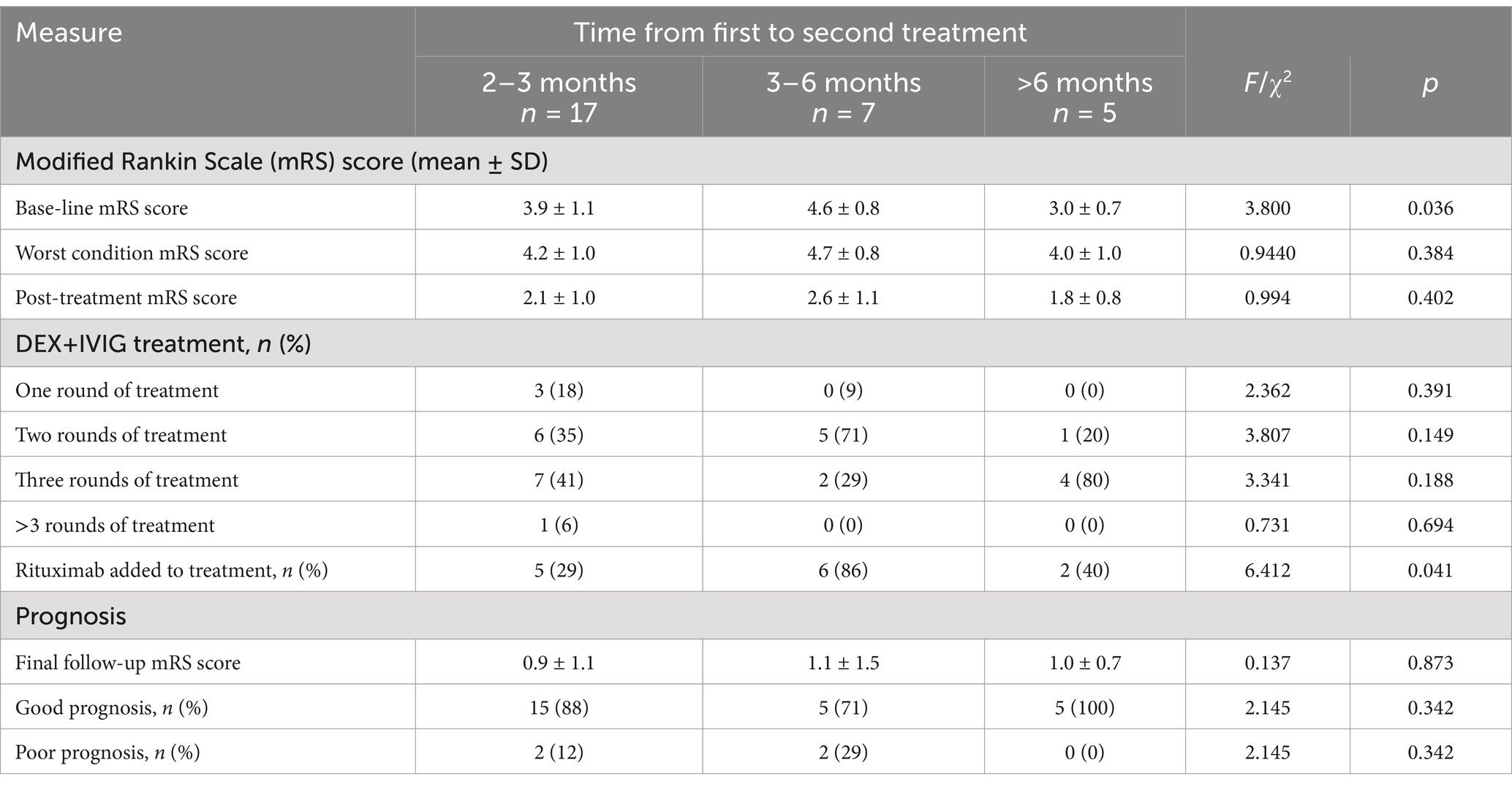
Patients in Group A initially received treatment with IVMP+IVIG at other hospitals during the acute phase of the disease; however, this approach did not yield satisfactory results. Upon transitioning to the nonacute phase and receiving DEX+IVIG therapy, the patients exhibited significant improvement. To determine whether these improvements were attributable to DEX+IVIG, we conducted a data analysis. Our findings revealed that 58.6% of the children in Group A were in the subacute phase and had not received glucocorticoids or immunoglobulin for 2–3 months. Seven children had not received immunotherapy for 3–6 months, and five children had not received any immunotherapy for more than 6 months. As shown in Table 3, the symptoms of these children had not fully resolved, with 90.0% of them presenting an mRS score of ≥3, which is indicative of severe conditions or poor prognosis.
Following 1–4 cycles of DEX+IVIG treatment, the clinical symptoms of these patients improved significantly, and their mRS scores were markedly reduced. At the last follow-up, the rate of good prognosis was 89.2%. Furthermore, the majority of children required 2–3 cycles of DEX+IVIG, and those who had been ill for 3–6 months were more likely to receive rituximab therapy. Notably, one child who underwent four cycles of DEX+IVIG but declined rituximab treatment experienced significant sequelae. On the basis of these findings, we suggest that if symptoms are not fully resolved after two cycles of DEX+IVIG treatment, second-line immunotherapy, such as rituximab, should be considered. Table 3 summarizes the outcomes of DEX+IVIG treatment in children in Group A.
3.4 Efficacy of DEX+IVIG at varying intensities in the treatment of pediatric AE
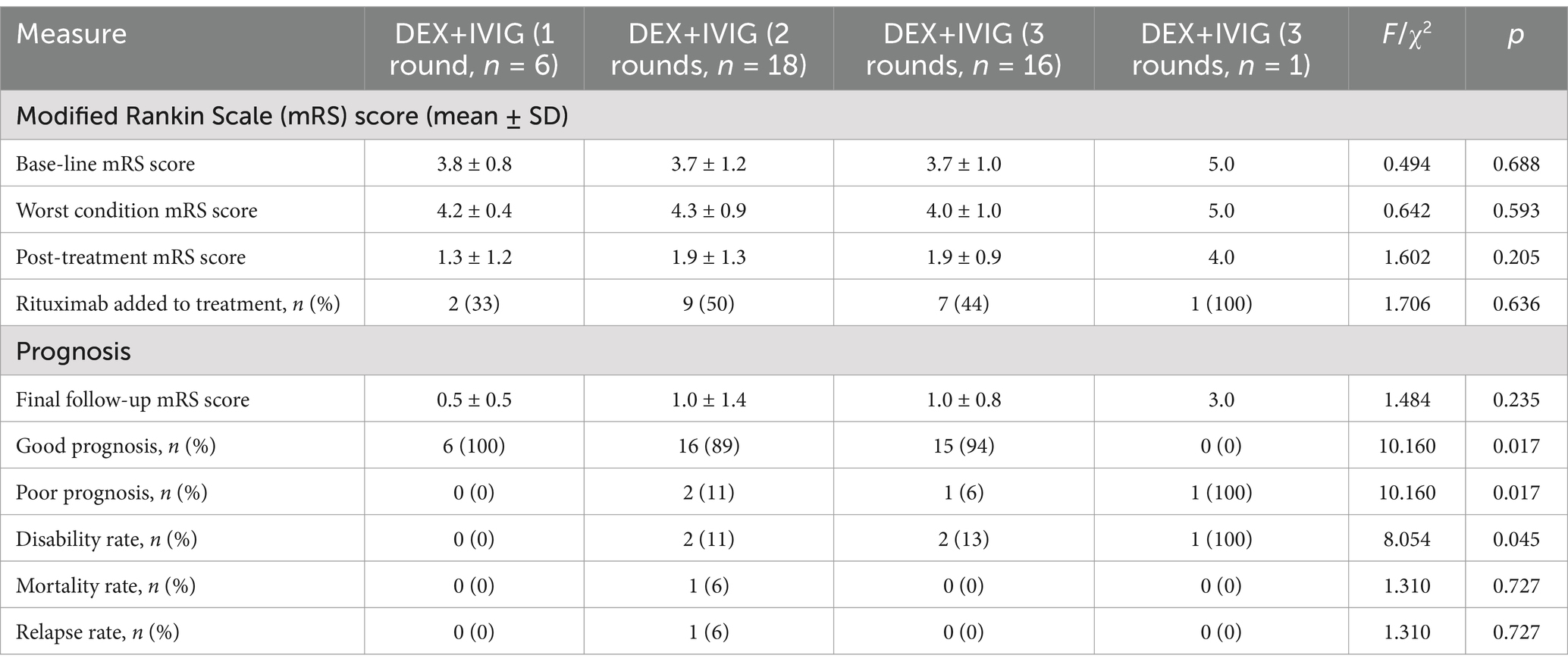
We aimed to evaluate the impact of different intensities of DEX+IVIG treatment on the clinical outcomes of children with AE, by considering the clinical status of 41 children at admission as the baseline (Table 4). The results indicated that 100% of the children who received a single round of DEX+IVIG achieved favorable outcomes. Among the children who received two rounds of DEX+IVIG, 89% had favorable outcomes, with one patient experiencing relapse and another resulting in death due to severe COVID-19 pneumonia. Among those who underwent three rounds of DEX+IVIG, 94% demonstrated favorable outcomes. One patient, who did not receive second-line immunotherapy and was treated with four rounds of DEX+IVIG, had a poor treatment response and significant sequelae. The mRS scores of children treated with one to three rounds of DEX+IVIG were significantly lower than those at baseline (Table 4). Additionally, 35% of the NMDA antibody-positive patients and 45% of the antibody-negative patients required three rounds of DEX+IVIG treatment. These findings suggest that children with NMDA antibody positivity may exhibit greater sensitivity to immunotherapy. Furthermore, brain MRI abnormalities appeared to have a minimal correlation with the number of immunotherapy rounds needed.
3.5 Impact of DEX+IVIG therapy on peripheral blood lymphocyte subsets in children with AE
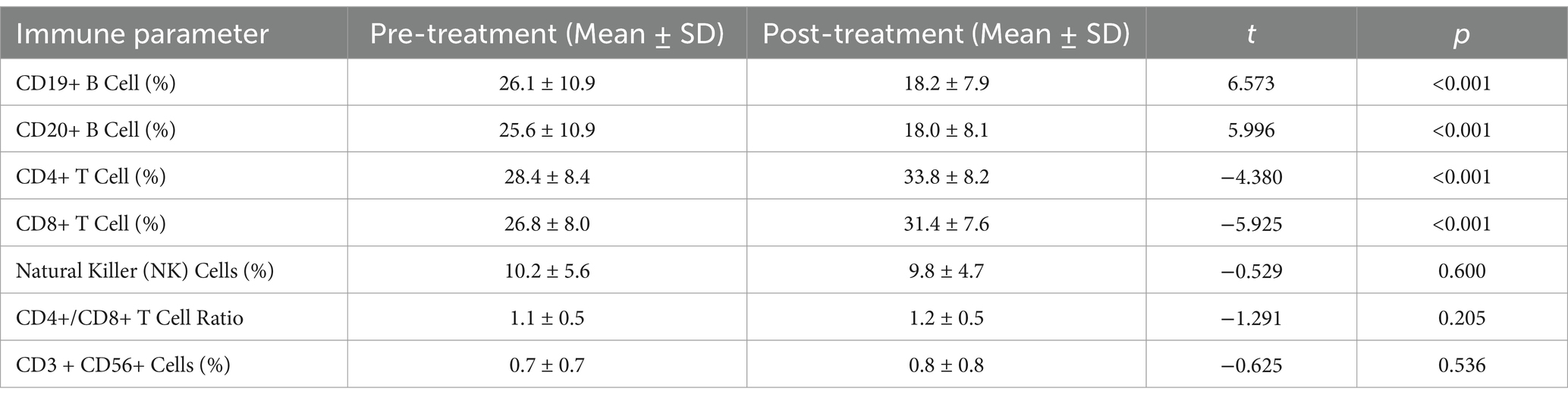
Four patients were not assessed for cellular immunology following treatment for personal reasons. Ultimately, peripheral blood lymphocyte subsets were collected before and after the initial round of DEX+IVIG immunotherapy in 37 children, and the normal reference range of peripheral blood lymphocyte subsets was based on data from Chinese children (14). The results indicated that the proportions of CD19 + B and CD20 + B cells in children with AE prior to treatment were significantly greater than those in healthy controls. After treatment, the proportions of CD19+ B cells (18.2 ± 7.9 vs. 26.1 ± 10.9) and CD20+ B cells (18.0 ± 8.1 vs. 25.6 ± 10.9) were significantly reduced. However, no significant increases were observed in the proportions of CD4+ T, CD8+ T, or NK cells or in the CD4+ T/CD8+ T ratio in the peripheral blood of children with AE prior to treatment. This lack of significant change may be attributed to the fact that some children in our cohort were not in the acute phase of the disease (Table 5).
3.6 Medium- and long-term efficacy and safety analysis
In this study, 90% of the children were classified as having severe disease or a poor prognosis (mRS score ≥ 3). The median follow-up duration was 24 months. At the final follow-up, 90.2% of patients (mRS = 0–2) had a favorable prognosis, with a complete response rate (mRS = 0) of 43.9% and a relapse rate of 2.4%. Following treatment, cerebrospinal fluid or serum antibody titers decreased in 65.2% of the children, whereas they remained unchanged in 34.8%. Most children who showed improvement had negative or reduced antibody titers. One antibody-negative child in Group A experienced relapse and was promptly and effectively treated with DEX+IVIG immunotherapy. However, owing to incomplete remission after DEX+IVIG treatment, rituximab was ultimately administered. The mRS score of the child who experienced relapse at the final follow-up was 1. In Group B, one child died from severe COVID-19 pneumonia. Mild rash and low fever were occasionally observed during IVIG infusion. During dexamethasone treatment, 12.2% (n = 5) of the children experienced mild excitement and irritability, and 9.8% (n = 4) experienced nausea, which was manageable with gastric protection therapy. Given that dexamethasone was used for a short duration (7–10 days), no significant adverse events, such as endocrine or electrolyte disturbances or concurrent infections, were observed.
4 Discussion
Pediatric AE is an autoimmune disorder that is primarily characterized by brain inflammation. With appropriate immunotherapy, the prognosis is generally favorable. While first-line treatment options are well established (15, 16), the choice of glucocorticoid remains debated (17), with most research focusing on intravenous methylprednisolone. As noted in the introduction, the potential benefits of DEX in treating encephalitis may have been underappreciated. Our observations indicate that the combination of DEX and IVIG yields significant results in treating both acute and nonacute AE in children.
In this study, 90.2% of the patients presented with severe conditions or poor prognoses. Patients in Group A were in the nonacute phase prior to receiving DEX+IVIG treatment, and most of these patients had not received immunotherapy for 2 to 6 months. The treatment outcomes were unexpectedly positive, with significant improvement in their clinical symptoms (Tables 2, 3). Each DEX+IVIG treatment session resulted in symptom improvement in Group A, which may explain why some patients did not transition quickly to second-line immunotherapy. Similarly, Group B patients, who received DEX+IVIG during the acute phase, also had favorable outcomes (Table 2). Furthermore, DEX+IVIG was effective among patients who experienced relapse. Our findings suggest that DEX+IVIG is beneficial during the acute, nonacute, and relapse phases of AE. Importantly, neither Group A nor Group B patients received second-line immunotherapy during this phase. Some children were later treated with rituximab due to incomplete remission. Analysis of 24-month follow-up data revealed a favorable prognosis rate of 90.2%, a complete remission rate of 43.9%, and an overall relapse rate of 2.4%. At the final follow-up, patients in the acute phase (Group B) had a higher favorable prognosis rate than did those in the nonacute phase (Group A). Previous studies have reported that approximately 80% of patients with anti-NMDAR encephalitis achieve functional recovery, with relapse rates ranging from 12.0 to 31.4% and mortality rates for severe cases ranging from 2.3 to 9.5% (18, 19). In contrast, our cohort, despite a high proportion of severe cases, demonstrated higher rates of favorable prognosis and complete remission, along with a lower relapse rate. These findings suggest that the DEX+IVIG regimen is an effective treatment for patients with severe AE.
Numerous previous studies support our findings. DEX has been shown to penetrate the blood–brain barrier more effectively and has a longer half-life in cerebrospinal fluid than other glucocorticoids (10), thus enabling it to exert stronger and more sustained anti-inflammatory effects in the central nervous system. DEX also offers distinct advantages in reducing capillary permeability and protecting the blood–brain barrier, thereby alleviating inflammation and brain edema and enhancing nerve conduction in children (10, 20). Several studies have reported rapid clinical improvements and favorable follow-up outcomes in patients with GABAAR antibody, anti-GAD65, and anti-Hu-associated encephalitis who were treated with DEX combined with immunoglobulin (21–23). A systematic review and meta-analysis of bacterial meningitis suggests that dexamethasone can be used as a first-line adjuvant therapy (24). Furthermore, as the preferred treatment for viral encephalitis, dexamethasone provides superior anti-inflammatory effects compared with other glucocorticoids without requiring liver metabolism or causing significant short-term inhibition of adrenal function, and its efficacy and safety are well established (25). Consistent with these findings, our data confirm that DEX+IVIG regimens are both effective and safe for the medium- and long-term treatment of AE in children.
How can the number of rounds of DEX+IVIG required for the treatment of children with AE be determined? Previous studies have suggested the use of corticosteroids in combination with IVIG for the treatment of severe AE, with the consideration of intensive (repetitive) first-line immunotherapy, including multiple rounds of IVIG (7, 26, 27). However, no standardized criteria have been established. Our findings indicate that the mRS score at onset may not be correlated with the intensity of immunotherapy needed and should instead be evaluated on the basis of the clinical efficacy following first-line treatment. In this study, two of the four children with mild AE received two rounds of DEX+IVIG, one child received one round, and two patients eventually required second-line immunotherapy. Notably, some severe cases achieved favorable outcomes with just one round of DEX+IVIG treatment (Table 2). We also observed that three rounds of first-line immunotherapy during the acute phase of AE did not yield the desired clinical results, suggesting that continued first-line therapy would be ineffective and that second-line immunotherapy should be initiated promptly. These findings are consistent with international guidelines, which recommend a second round of DEX+IVIG immunotherapy if the clinical response after the first round is unsatisfactory. Disease status should be reassessed 2 weeks after the second round of treatment, and if symptoms persist, second-line immunotherapy (such as rituximab) should be considered. However, in cases where second-line immunotherapy is not feasible due to patient or family preferences or the unavailability of second-line agents, a third round of DEX+IVIG may be attempted.
Our study demonstrated that DEX+IVIG is effective in treating all stages of AE in children; however, the underlying mechanism remains unclear. Several studies have suggested that lymphocyte subsets may contribute significantly to the pathogenesis of AE. For example, the presence of CD20+ B cells and CD3+ T cells has been reported in the lesion areas of patients with anti-NMDA and anti-GABA encephalitis (28, 29). Additionally, increased proportions of B cells and CD4+ T/CD8+ T cells have been observed in the peripheral blood of patients with autoimmune borderline encephalitis, as well as in the cerebrospinal fluid of mice with AE (30, 31). Our findings indicated that the proportions of CD19+ B cells and CD20+ B cells in the peripheral blood increased at the onset of AE and significantly decreased after treatment. These findings suggested that CD19+ and CD20+ cells may play key roles in both the pathogenesis of AE and its response to immunotherapy, which requires further investigation. However, no changes were observed in other lymphocyte markers in children with AE, possibly because some participants were not in the acute phase of the disease.
This study has several limitations, primarily due to its retrospective design. Some subjects may not have been fully tested for antibodies before 2018; therefore, children diagnosed with antibody-negative AE could actually be antibody-positive. Additionally, given the heterogeneity of autoimmune encephalitis, the appropriateness of studying both antibody-positive and antibody-negative children remains unclear and warrants further exploration. Nevertheless, our data suggest that these children respond well to immunotherapy, regardless of their antibody status. The median follow-up period was 24 months, with a minimum of 12 months. This follow-up duration may have led to the underreporting of potential relapses, and some patients with slow recovery could have been prematurely classified as having a poor prognosis. Since all the children were treated with both DEX and IVIG, it was difficult to determine the individual effectiveness of each drug. Furthermore, the lack of a control group limits comparisons with other immune treatments, such as IVMP and rituximab. To address these limitations, future prospective studies with control or comparative groups are needed.
5 Conclusion
This study analyzed the clinical features of 41 children with AE and evaluated the efficacy and safety of a DEX+IVIG immunotherapy regimen. The results indicated that DEX+IVIG may be beneficial at different stages of AE, including the acute, nonacute, sequelae, and recurrence phases. However, owing to the small sample size, the possibility of selection bias remains. Larger prospective controlled studies are needed to confirm and strengthen these findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Review Committee of Sun Yat-sen Memorial Hospital at Sun Yat-sen University approved the study protocol. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. XL: Conceptualization, Data curation, Methodology, Writing – review & editing. ZH: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing. DT: Data curation, Investigation, Project administration, Software, Writing – review & editing. YL: Data curation, Investigation, Software, Writing – review & editing. PL: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank all enrolled patients and their parents or guardians.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1512908/full#supplementary-material
References
1. Cellucci, T, Van Mater, H, Graus, F, Muscal, E, Gallentine, W, Klein-Gitelman, MS, et al. Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e73. doi: 10.1212/NXI.0000000000000663
2. Abboud, H, Probasco, JC, Irani, S, Ances, B, Benavides, DR, Bradshaw, M, et al. Autoimmune encephalitis: proposed best practice recommendations for diagnosis and acute management. J Neurol Neurosurg Psychiatry. (2021) 92:757–68. doi: 10.1136/jnnp-2020-325300
3. Mu, Y, and Yajun, L. Clinical features and early recognition of 242 cases of autoimmune encephalitis. Front Neurol. (2022) 12:803752. doi: 10.3389/fneur.2021.803752
4. Hermetter, C, Fazekas, F, and Hochmeister, S. Systematic review: syndromes, early diagnosis, and treatment in autoimmune encephalitis. Front Neurol. (2018) 9:706. doi: 10.3389/fneur.2018.00706
5. Dutra, LA, Abrantes, F, Toso, FF, Pedroso, JL, Barsottini, OGP, Hoftberger, R, et al. Autoimmune encephalitis: a review of diagnosis and treatment. Arq Neuropsiquiatr. (2018) 76:41–9. doi: 10.1590/0004-282X20170176
6. Shan, W, Yang, H, and Wang, Q. Neuronal surface antibody-mediated autoimmune encephalitis (limbic encephalitis) in China: a multicenter, retrospective study. Front Immunol. (2021) 12:621599. doi: 10.3389/fimmu.2021.621599
7. Nosadini, M, Eyre, M, Molteni, E, Thomas, T, Irani, SR, Dalmau, J, et al. Use and safety of immunotherapeutic management of N-methyl-D-aspartate receptor antibody encephalitis: a meta-analysis. JAMA Neurol. (2021) 78:1333–44. doi: 10.1001/jamaneurol.2021.3188
8. Wang, H. Efficacies of treatments for anti-NMDA receptor encephalitis. Front Biosci. (2016) 21:651–63. doi: 10.2741/4412
9. Nosadini, M, Thomas, T, Eyre, M, Anlar, B, Armangue, T, Benseler, SM, et al. International consensus recommendations for the treatment of pediatric NMDAR antibody encephalitis. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1052. doi: 10.1212/NXI.0000000000001052
10. Mitchell, CD, Thomas, T, Eyre, M, Anlar, B, Armangue, T, Benseler, SM, et al. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukemia: results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol. (2005) 129:734–45. doi: 10.1111/j.1365-2141.2005.05509.x
11. Jones, B, Freeman, AI, Shuster, JJ, Jacquillat, C, Weil, M, Pochedly, C, et al. Lower incidence of meningeal leukemia when prednisolone is replaced by dexamethasone in the treatment of acute lymphoblastic leukemia. Med Pediatr Oncol. (1991) 19:269–75. doi: 10.1002/mpo.2950190411
12. Balis, FM, Lester, CM, Chrousos, GP, Heideman, RL, and Poplack, DG. Differences in cerebrospinal fluid penetration of corticosteroids: possible relationship to the prevention of meningeal leukemia. J Clin Oncol. (1987) 5:202–7. doi: 10.1200/JCO.1987.5.2.202
13. Graus, F, Titulaer, MJ, Balu, R, Benseler, S, Bien, CG, Cellucci, T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
14. Yuan, D. A multicenter study and clinical application of establishing normal reference values for peripheral blood lymphocyte classification in children. Chongqing: Chongqing Medical University (2018).
15. Dale, RC, Gorman, MP, and Lim, M. Autoimmune encephalitis in children: clinical phenomenology, therapeutics, and emerging challenges. Curr Opin Neurol. (2017) 30:334–44. doi: 10.1097/WCO.0000000000000443
16. Wagner, JN, Kalev, O, Sonnberger, M, Krehan, I, and von Oertzen, TJ. Evaluation of clinical and paraclinical findings for the differential diagnosis of autoimmune and infectious encephalitis. Front Neurol. (2018) 9:434. doi: 10.3389/fneur.2018.00434
17. Gong, X, Luo, R, Liu, J, Guo, K, Li, A, Liu, X, et al. Efficacy and tolerability of intravenous immunoglobulin versus intravenous methylprednisolone treatment in anti-N-methyl-D-aspartate receptor encephalitis. Eur J Neurol. (2022) 29:1117–27. doi: 10.1111/ene.15214
18. Jaka, S, Singh, S, Vashist, S, Pokhrel, S, Saldana, E, Sejdiu, A, et al. Pediatric anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis: exploring psychosis, related risk factors, and hospital outcomes in a nationwide inpatient sample: a cross-sectional study. PLoS One. (2024) 19:e0296870. doi: 10.1371/journal.pone.0296870
19. Titulaer, MJ, Höftberger, R, Iizuka, T, Leypoldt, F, McCracken, L, Cellucci, T, et al. Overlapping demyelinating syndromes and anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. (2014) 75:411–28. doi: 10.1002/ana.24117
20. Handa, H, Suzuki, K, Chou, T, and Matsushima, T. Phase 1 study of the investigational proteasome inhibitor ixazomib alone or in combination with lenalidomide and dexamethasone (Rd) in Japanese patients with relapsed and/or refractory multiple myeloma (RRMM). Blood. (2014) 124:5752–2. doi: 10.1182/blood.V124.21.5752.5752
21. Huenerfauth, EI, Bien, CG, Bien, C, Volk, HA, and Meyerhoff, N. Case report: anti-GABA a receptor encephalitis in a dog. Front Vet Sci. (2022) 9:886711. doi: 10.3389/fvets.2022.886711
22. Salazar, K, Massey, C, Ankar, A, Yarimi, J, Sudheendra, M, Cokley, J, et al. Treatment of anti-GAD65 autoimmune encephalitis presenting as NORSE with intrathecal dexamethasone. Auto Neurol. 102:208. doi: 10.1212/WNL.0000000000208208
23. Marion, P, Chalus, AD, Giorgi, L, Bellesme, C, Crétien, P, Maurey, H, et al. Early and aggressive treatment may modify anti-Hu-associated encephalitis prognosis. Neuropediatrics. (2022) 54:64–7. doi: 10.1055/a-1896-6687
24. Hasbun, R. Progress and challenges in bacterial meningitis: a review. JAMA. (2022) 328:2147–2154. doi: 10.1001/jama.2022.20521
25. Urak, R, Munoz, A, Hsieh, HJ, Taus, E, Forman, SJ, and Wang, X. Dexamethasone enhanced CAR T-cell persistence and function through upregulation of interleukin-7 receptor. Blood. (2021) 138:1715–5. doi: 10.1182/blood-2021-153507
26. Xu, X, Lu, Q, Huang, Y, Fan, S, Zhou, L, Yuan, J, et al. Anti-NMDAR encephalitis: a single-center, longitudinal study in China. Neurol Neuroimmunol Neuroinflamm. (2019) 7:e633. doi: 10.1212/NXI.0000000000000633
27. Dubey, D, Britton, J, McKeon, A, Gadoth, A, Zekeridou, A, Lopez Chiriboga, SA, et al. Randomized placebo-controlled trial of intravenous immunoglobulin in autoimmune LGI1/CASPR2 epilepsy. Ann Neurol. (2020) 87:313–23. doi: 10.1002/ana.25655
28. Golombeck, KS, Bönte, K, Mönig, C, van Loo, KM, Hartwig, M, Schwindt, W, et al. Evidence of a pathogenic role for CD8+ T cells in anti-GABA(B) receptor limbic encephalitis. Neurol Neuroimmunol Neuroinflamm. (2016) 3:e232. doi: 10.1212/NXI.0000000000000232
29. Martinez-Hernandez, E, Horvath, J, Shiloh-Malawsky, Y, Sangha, N, Martinez-Lage, M, and Dalmau, J. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. (2011) 77:589–93. doi: 10.1212/WNL.0b013e318228c136
30. Hansen, N, Schwing, K, Önder, D, Widman, G, Leelaarporn, P, Prusseit, I, et al. Low CSF CD4/CD8+ T-cell proportions are associated with blood-CSF barrier dysfunction in limbic encephalitis. Epilepsy Behav. (2020) 102:106682. doi: 10.1016/j.yebeh.2019.106682
Keywords: autoimmune encephalitis, children, methylprednisolone, dexamethasone, immunoglobulin
Citation: Zhou X, Luo X, He Z, Tang D, Li Y and Li P (2025) Efficacy of dexamethasone combined with intravenous immunoglobulin for the treatment of pediatric autoimmune encephalitis. Front. Neurol. 16:1512908. doi: 10.3389/fneur.2025.1512908
Edited by:
Jorge Correale, Fundación Para la Lucha Contra las Enfermedades Neurológicas de la Infancia (FLENI), ArgentinaReviewed by:
Paolo Ragonese, University of Palermo, ItalyMats Johnson, University of Gothenburg, Sweden
Copyright © 2025 Zhou, Luo, He, Tang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pinggan Li, bGlwaW5nZ0BtYWlsLnN5c3UuZWR1LmNu
 Xiaolin Zhou
Xiaolin Zhou Xiangyang Luo
Xiangyang Luo Zhanwen He
Zhanwen He Pinggan Li
Pinggan Li