
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 14 April 2025
Sec. Stroke
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1512355
This article is part of the Research TopicFrom bench to bedside: Inflammation in Neurovascular Disorders and StrokeView all 9 articles
Introduction: The purpose of this study was to evaluate the association between four easy-to-measure inflammatory markers and the 90-day outcomes with acute ischemic stroke (AIS) patients who received intravenous thrombolytic therapy with recombinant tissue plasminogen activator (rt-PA). These included the neutrophil percentage-to-albumin ratio (NPAR), the neutrophil-to-lymphocyte ratio (NLR), the platelet-to-lymphocyte ratio (PLR), and the combined NPAR+NLR index.
Methods: This study enrolled 151 AIS patients who were treated with rt-PA (0.9 mg/kg). Clinical data were collected and NPAR, NLR, PLR were calculated from the admission blood work. The patients were followed up for 90 days after stroke onset and subsequently categorized into two groups based on the modified Rankin Scale (mRS): a favorable outcome group (111 patients, mRS ≤ 2) and a poor outcome group (40 patients, mRS > 2).
Results: In this study, we foud elevated level of albumin and lymphocyte counts are protective factors for short-term prognosis. Age, neutrophil percentage, NPAR, NLR, PLR, NIHSS score, and fasting blood glucose (FBG) are associated with poor short-term prognosis. Among these, age, NPAR, NLR, and NIHSS score are independent risk factors for poor short-term prognosis.
Discussion: NPAR, NLR, PLR, and the combined NPAR+NLR index may have predictive value for poor short-term outcomes in AIS patients following thrombolysis. NPAR demonstrates the highest predictive capability, in the following order: NPAR > NPAR+NLR > NLR > PLR.
AIS is a prevalent neurological disorder among the elderly, with high rates of incidence, mortality, and disability. For AIS patients presenting within 4.5 h of symptom onset, intravenous thrombolytic therapy (IVT) using rt-PA is considered the optimal treatment. This intervention is effective in salvaging the ischemic penumbra and restoring cerebral blood flow, thereby improving patient outcomes (1). However, despite stringent adherence to IVT indications and contraindications, some patients still experience adverse outcomes.
Inflammation and oxidative stress are critical factors influencing reperfusion prognosis in AIS patients (2). During the acute phase of AIS, there is a substantial release of pro-inflammatory cytokines and damage-associated molecular patterns (DAMPs), leading to the recruitment and activation of neutrophils. Neutrophils, which are among the first inflammatory cells to accumulate in the cerebral microvasculature, release reactive oxygen species (ROS), which are major contributors to reperfusion injury following IVT (3–5). Neutrophils serve as markers of both acute and chronic inflammation, promoting inflammation (6, 7) and thrombosis (7). Additionally, neutrophils are implicated in the pathogenesis of atherosclerosis (8) and may contribute to recurrent arterial occlusive events.
Serum albumin plays a crucial role in scavenging ROS (9) and may exert anti-inflammatory effects by inhibiting neutrophil dispersion (10), thus offering neuroprotection and potentially reducing the risk of recurrent ischemic stroke (RIS), thereby improving patient prognosis. Conversely, low serum albumin levels indicate poor nutritional status and inflammation (11). The NPAR represents a novel inflammatory biomarker. Compared to single factors, NPAR provides a more comprehensive reflection of systemic immune and inflammatory responses. However, the relationship between NPAR and outcomes in AIS patients undergoing thrombolysis remains incompletely understood.
Lymphocytes play a vital role in tissue repair and inflammation control due to their involvement in various inflammatory pathways (12). A low NLR at admission may serve as a valuable marker for predicting neurological recovery 90 days after a stroke (13). Moreover, admission NLR could be a significant biomarker for predicting outcomes in patients with large vessel occlusive stroke (14).
Platelets are crucial for both thrombosis and inflammation (15). During AIS, platelets are rapidly activated, adhering to damaged endothelial surfaces and releasing inflammatory factors that recruit additional inflammatory cells to the injury site and amplify the inflammatory response (16). In contrast, acute stress typically reduces lymphocyte counts, which are essential for coordinating healing and controlling the spread of inflammation (12). Recent evidence suggests that a higher PLR is associated with increased rates of recurrent stroke, mortality, and poor functional outcomes following stroke. PLR has emerged as a potential novel biomarker in IVT for AIS, showing promise in predicting functional outcomes (17, 18).
This study aims to evaluate the predictive value of NPAR, NLR, PLR, and the combined NPAR+NLR index for the 90-day prognosis of AIS patients receiving rt-PA IVT, to help clinicians more effectively identify high-risk patients with poor outcomes.
This study was approved by the Ethics Committee of Xinhua Hospital, affiliated with Dalian University (No. 2023–151-01). A single-center, retrospective study was conducted, involving 168 AIS patients treated at the Neurology Department of Xinhua Hospital between May 2017 and November 2023. The inclusion criteria were as follows: (1) a new AIS diagnosis based on clinical symptoms and neuroimaging, with intracranial hemorrhage excluded by CT; (2) presentation within 4.5 h of symptom onset and treatment with IVT using rt-PA (0.9 mg/kg), with a maximum dose of 90 mg; (3) age ≥ 18 years. The exclusion criteria included: (1) intracranial hemorrhage or a history of intracranial hemorrhage; (2) intra-arterial bridging therapy, mechanical thrombectomy, or thrombus aspiration following thrombolysis; (3) a history of stroke with a pre-stroke mRS score > 1; (4) significant trauma, surgery, or burns within 90 days before symptom onset; (5) acute or chronic infection, or use of anti-infective drugs, steroids, or immunosuppressants; (6) systemic diseases such as severe liver or renal dysfunction, malignancy, acute myocardial infarction, or hematological disorders; (7) other severe conditions within 90 days post-thrombolysis that could affect the mRS score.
Neurological function was assessed using the mRS 90 days after thrombolysis. A score of ≤2 was considered indicative of a good prognosis, while a score > 2 indicated a poor prognosis. Trained neurologists conducted face-to-face or telephone interviews with patients and their families 90 days after discharge to determine the mRS score. Thrombolysis was administered according to American guidelines for early management of AIS and the Chinese Stroke Guidelines (19, 20). The rt-PA dose was 0.9 mg/kg (maximum 90 mg), prepared as a 1 mg/mL solution. Ten percent of the total dose was administered intravenously as a bolus over 1 min, followed by the remaining 90% as a continuous infusion over 1 h.
Blood samples for routine laboratory tests were collected before any treatment (pre-thrombolysis, within 4.5 h of symptom onset). Complete blood counts were analyzed using the Sysmex XN-B3 automated hematology analyzer (Japan), and liver function tests were conducted using the Hitachi 7,600–020 automated biochemical analyzer (Japan).
Statistical analyses were conducted using Excel and SPSS 27.0 software. The normality of continuous variables was assessed with the Shapiro–Wilk test. Normally distributed data were expressed as mean ± standard deviation (SD) and compared between groups using the independent samples t-test. Non-normally distributed data were presented as median and interquartile range (IQR) and compared using the Mann–Whitney U test. Categorical data were described with frequency and percentage (n, %) and compared using the χ2 test. Baseline characteristics were compared between the good and poor prognosis groups. Variables with significant differences were included in univariate logistic regression analysis. Significant variables from univariate analysis were then incorporated into multivariate logistic regression analysis to identify independent risk factors for poor prognosis post-thrombolysis. The diagnostic value of each predictive factor was evaluated using ROC curves, with accuracy assessed by the AUC, sensitivity, and specificity. Statistical significance was set at p < 0.05.
A total of 168 AIS patients who underwent IVT were initially included in this study. After applying the inclusion and exclusion criteria, 17 patients were excluded due to missing or incomplete follow-up data, resulting in a final cohort of 151 AIS patients. Of these, 102 were male (67.5%) and 49 were female (32.5%; Figure 1). The favorable prognosis group, defined as having an mRS score ≤ 2, consisted of 111 patients (77 male, 34 female) with a mean age of 66.08 ± 10.66 years. The poor prognosis group, defined as having an mRS score > 2, comprised 40 patients (25 male, 15 female) with a mean age of 74.28 ± 8.84 years. A comparison of baseline characteristics between the two groups revealed no significant differences in gender, history of stroke, hypertension, type 2 diabetes, coronary heart disease, smoking, alcohol consumption, platelet count, or the duration from symptom onset (p > 0.05). However, the poor outcome group exhibited significantly higher values in age, neutrophil percentage, absolute neutrophil count, NIHSS score, FBG levels, NPAR, NLR, PLR (Figure 2) compared to the favorable outcome group (p < 0.05). Additionally, albumin and lymphocyte counts were significantly lower in the poor outcome group (p < 0.05). Refer to Table 1 for a detailed comparison.
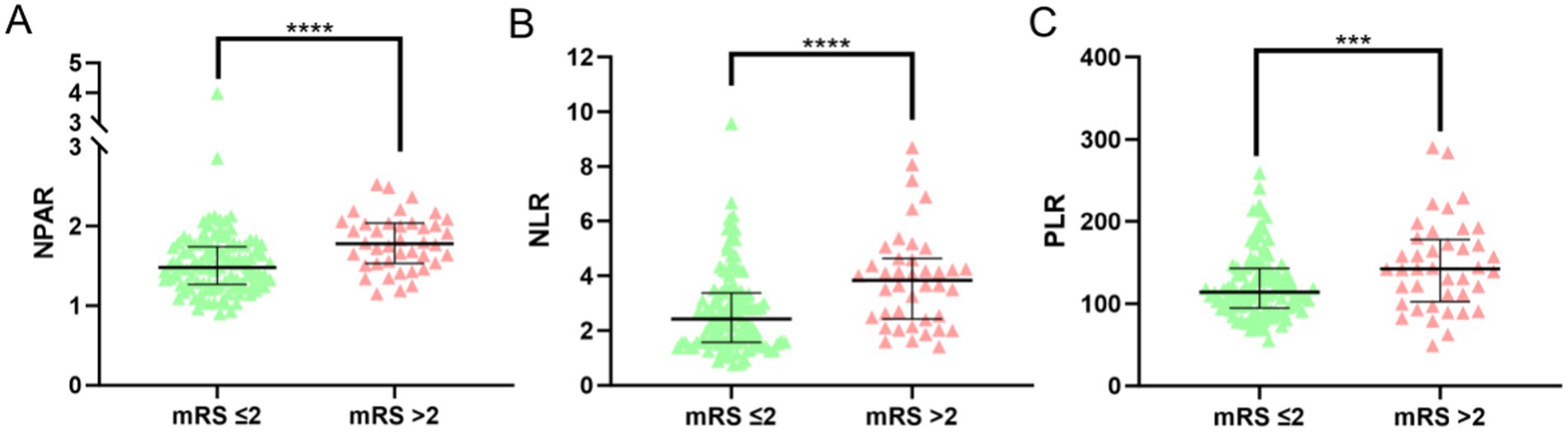
Figure 2. NPAR, NLR, PLR levels and distributions in favorable outcome and poor outcome groups. (Statistical significance is denoted by asterisks: ***p < 0.05, ****p < 0.001).
Univariate logistic regression analysis revealed that gender, history of stroke, hypertension, type 2 diabetes, coronary artery disease, smoking, alcohol consumption, neutrophil count, platelet count, and the duration from symptom onset were not significantly associated with 90-day outcomes (p > 0.05). In contrast, age, neutrophil percentage, NPAR, NLR, PLR, NIHSS score, and FBG levels were identified as significant risk factors for poor outcomes in AIS patients receiving intravenous thrombolysis (p < 0.05). Conversely, elevated albumin levels and high lymphocyte counts were protective factors for short-term prognosis (p < 0.05; refer to Table 2).
Multivariate logistic regression analysis, adjusting for confounding factors such as neutrophil percentage, albumin, lymphocyte count, PLR, and FBG levels, revealed that age (OR = 1.116, 95% CI: 1.047–1.190, p < 0.05), NPAR (OR = 3.898, 95% CI: 1.079–14.087, p < 0.05), NLR (OR = 1.672, 95% CI: 1.056–2.647, p < 0.05), and NIHSS score (OR = 1.499, 95% CI: 1.280–1.756, p < 0.05) were independent risk factors for poor short-term prognosis (p < 0.05; refer to Table 3).
The predictive value of NPAR, NLR, PLR, and their combination (NPAR + NLR) for 90-day post-thrombolysis outcomes in AIS patients was assessed using ROC curves (Figure 3). The ROC analysis revealed that NPAR exhibited the highest predictive accuracy for adverse outcomes, with an AUC of 0.723 (95% CI: 0.633–0.814, p < 0.05). The NLR, with an AUC of 0.711 (95% CI: 0.622–0.800, p < 0.05), had an optimal threshold of 3.495, with a sensitivity of 62.5% and a specificity of 79.3%. For the combined predictor (NPAR + NLR), the ROC curve yielded an AUC of 0.719 (95% CI: 0.629–0.809, p < 0.05), with a sensitivity of 67.5% and a specificity of 71.2%. Detailed data are provided in Table 4.
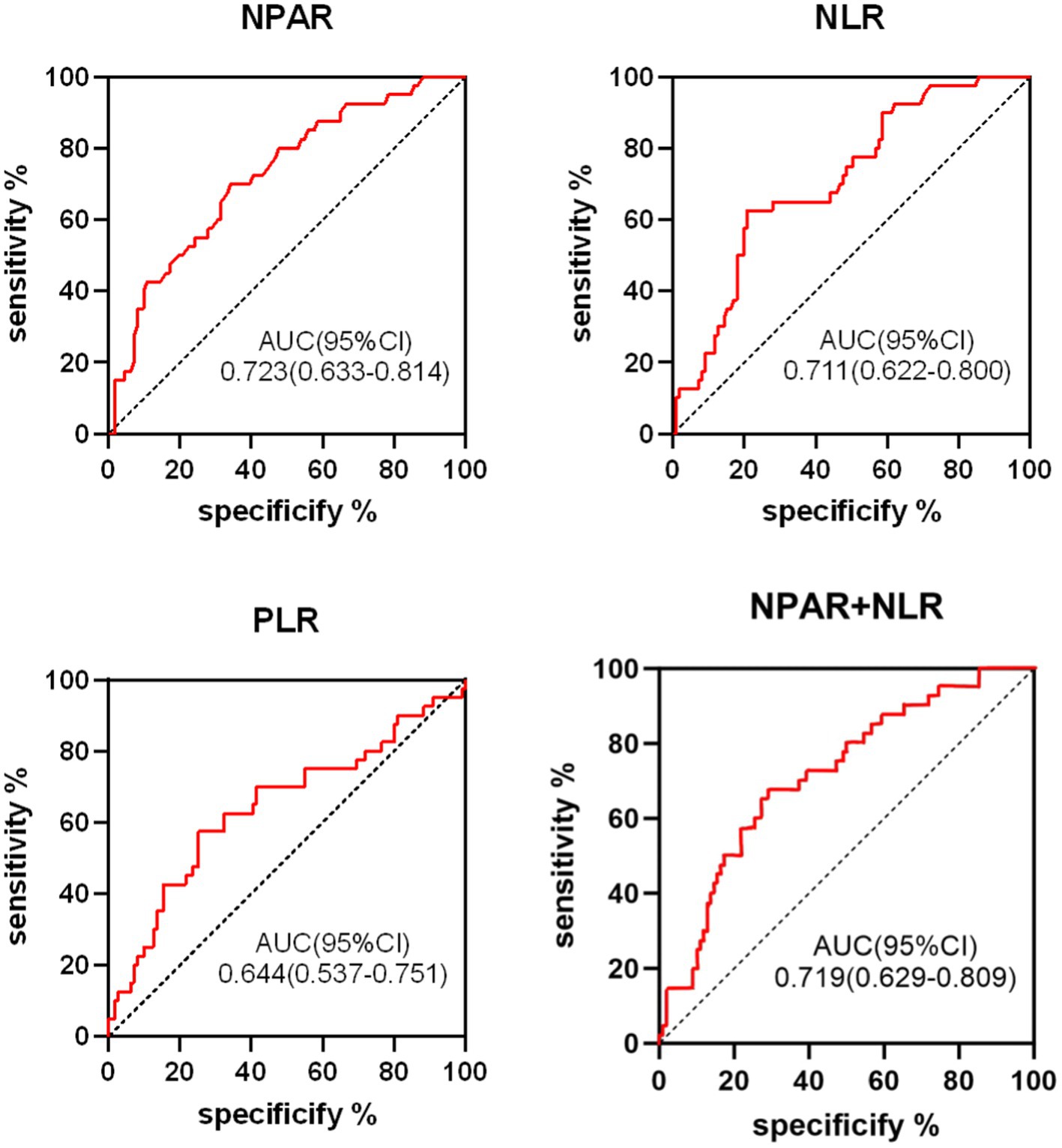
Figure 3. ROC curve analysis of NPAR, NLR, PLR, NPAR+NLR in predicting poor short-term outcomes in AIS patients following thrombolysis.
The ROC curve analysis indicates that NPAR offers superior predictive sensitivity, whereas NLR provides greater specificity. AUC values ranging from 0.5 to 0.7 are considered to have low diagnostic value, whereas values between 0.7 and 0.9 are indicative of moderate diagnostic value. Accordingly, levels of NPAR, NLR, PLR, and the combined NPAR + NLR exhibit notable prognostic values for short-term adverse outcomes. Specifically, NPAR exhibits the highest predictive efficacy, followed by the combined NPAR + NLR, NLR, and PLR in descending order of predictive value.
This study included 151 patients who underwent intravenous thrombolysis for AIS, among whom 40 experienced adverse outcomes, corresponding to an incidence rate of 26.5%. Enhancing the efficacy of intravenous thrombolysis for a greater number of AIS patients, reducing disability and mortality rates, and improving the prediction of treatment outcomes are critical public health concerns. This study aimed to evaluate the prognostic value of routine blood and liver function biomarkers in predicting short-term outcomes after intravenous thrombolysis in AIS patients. This research seeks to provide clinicians with more objective, convenient, and efficient indicators for assessing prognosis.
Neutrophils are among the first white blood cells to infiltrate ischemic regions, breaching the blood–brain barrier within 6 h of AIS onset and peaking in cerebral infiltration between 24 and 48 h (21). These cells secrete various factors, including reactive oxygen species (ROS), proteases, cytokines, and chemokines (22), which contribute to neuronal cell death and exacerbate reperfusion injury. Post-stroke neutrophil-driven inflammation is associated with increased infarct volume, hemorrhagic transformation, stroke severity, and worse functional outcomes (23, 24). Clinically, stroke patients are more susceptible to infections, which may result in poorer functional outcomes. Regardless of the severity of infection, leukocyte levels typically rise post-stroke. To control for potential confounding factors, patients showing signs of infection were excluded from this study. Our findings are consistent with prior research, which shows higher neutrophil levels in the adverse outcome group compared to the favorable outcome group.
Albumin reflects both the body’s inflammatory and nutritional status. Low albumin levels are associated with cytokine activation and chronic inflammation (25). Serum albumin not only acts as a specific inhibitor of endothelial cell apoptosis (26), but also has significant antioxidant properties (27). Additionally, albumin may reduce post-AIS inflammatory responses by inhibiting neutrophil-endothelial adhesion (28). Malnutrition is a well-known risk factor for poor outcomes following stroke (29). Hypoalbuminemia in stroke patients may arise from malnutrition and/or underlying disease processes, such as renal or hepatic failure or malignancies. To ensure patient homogeneity, individuals with severe liver disease, renal failure, or malignancies were excluded. Our study found that serum albumin levels were lower in the adverse outcome group compared to the favorable outcome group. Univariate logistic regression analysis indicated that albumin (OR: 0.884, p = 0.006) is a protective factor for short-term prognosis following intravenous thrombolysis in AIS patients, which is consistent with previous literature.
NPAR, a novel composite biomarker for inflammation and oxidative stress, is considered an accurate reflection of inflammatory status and disease prognosis. A recent retrospective study involving 829 AIS patients demonstrated that NPAR is independently associated with the risk of stroke recurrence within 3 months, suggesting it may be a more effective biomarker for predicting stroke recurrence (30). Moreover, elevated NPAR can predict stroke-associated infections (SAI), and compared to albumin, neutrophil percentage, and NLR, NPAR may serve as a more effective biomarker for predicting SAI (31). While existing studies have confirmed that neutrophils and albumin are associated with functional outcomes in AIS patients undergoing reperfusion therapy (32, 33), there is insufficient evidence to suggest that NPAR predicts outcomes in AIS patients receiving intravenous thrombolysis. Our study reveals a positive correlation between NPAR and adverse functional outcomes. Multivariate logistic regression analysis, adjusting for confounding factors, identified NPAR (OR: 3.898, p = 0.038) as an independent risk factor for short-term adverse outcomes following thrombolysis. ROC curve analysis indicates that NPAR (AUC = 0.723, 95% CI: 0.633–0.814, p < 0.05) demonstrates higher predictive value for adverse outcomes compared to NPAR + NLR, NLR, and PLR, reflecting greater sensitivity and reliability in predicting poor outcomes in AIS patients. The combined NPAR + NLR indices demonstrated lower predictive value compared to NPAR alone, possibly due to overlapping information from the neutrophil count and sample size limitations. Further research with larger sample sizes is necessary to validate these findings.
Post-ischemic stroke lymphocyte count reduction indicates ongoing brain injury, heightened stress responses, and increased infection risk (34), correlating with poorer outcomes (35). Lymphocytes play a crucial role in anti-inflammatory and endothelial protective mechanisms, and their apoptosis is exacerbated by worsening atherosclerosis (36). To control for the impact of lymphocyte count influenced by systemic inflammation, patients with active infections, malignancies, and chronic diseases were excluded. Our study found a correlation between reduced lymphocyte counts and adverse outcomes. Univariate logistic regression analysis yielded an OR of 0.355, reinforcing the notion that lymphocyte count is a significant protective factor for prognosis in AIS patients undergoing intravenous thrombolysis, consistent with existing literature.
NLR is a reliable indicator of systemic inflammation and disease prognosis, and is widely used in various disease studies. In AIS patients, NLR correlates with stroke severity, short-term functional outcomes, and infarct recurrence (37). Additionally, NLR serves as an independent predictor of 3-month mortality in AIS patients (38). A prospective observational study of 341 ischemic stroke patients revealed that, after adjusting for initial NIHSS scores, both diabetes and NLR were independently associated with stroke progression, with NLR predicting adverse functional outcomes (39). Other research highlights that elevated NLR, measured 24–36 h post-thrombolysis, significantly predicts stroke-associated pneumonia and can forecast short- and long-term adverse outcomes, hemorrhagic transformation, and 1-year mortality (40). In our study, univariate logistic regression analysis indicated that NLR levels (OR: 1.512, p < 0.001) were significantly higher in the adverse outcome group compared to the favorable outcome group. Multivariate logistic regression identified NLR (OR = 1.672, p = 0.025) as an independent predictor of short-term adverse outcomes following thrombolysis. ROC curve analysis yielded an AUC of 0.711 (95% CI: 0.622–0.800, p < 0.05), demonstrating moderate predictive value with higher specificity.
In the context of AIS, thrombosis and vascular occlusion are primarily driven by the abnormal activation and excessive aggregation of platelets (41). Circulating platelets are critical effectors in the onset, progression, and resolution of stroke, exerting direct effects on endothelial cells, and their interactions with leukocytes can exacerbate inflammatory and thrombotic conditions. While rt-PA facilitates the conversion of plasminogen to plasmin, it also increases platelet activation and aggregation (42). Following thrombolysis, an increase in platelet count may trigger delayed thrombotic events, potentially leading to re-occlusion and recurrent thrombosis (43). Previous studies have suggested that elevated platelet counts increase the risk of ischemic stroke, but are not significantly associated with adverse outcomes (44). In our study, platelet counts in the adverse outcome group were not significantly higher than those in the favorable outcome group.
PLR serves as a marker of atherosclerotic inflammation, offering insights into the mechanisms underlying AIS, including hemostasis, thrombosis, and inflammatory pathways. Elevated PLR levels are potentially linked to the severity of coronary atherosclerosis and symptomatic carotid artery stenosis (45, 46). Additionally, high PLR can serve as an indirect marker for estimating infarct size, post-thrombectomy recanalization rates, and adverse outcomes (47). PLR has been shown to predict the likelihood of hemorrhagic transformation in younger AIS patients, highlighting its clinical significance in preventing such transformations (48). In patients undergoing mechanical thrombectomy, admission PLR may predict 3-month prognosis and mortality, with lower PLR levels associated with better clinical outcomes at 3 months (49). An observational study of patients receiving rt-PA treatment found that admission PLR before thrombolysis was associated with early neurological improvement and deterioration (22). Our study’s univariate logistic regression analysis indicated that the PLR level in the adverse outcome group (OR: 1.011, p = 0.006) was higher compared to the favorable outcome group, and PLR demonstrated predictive value for prognosis in AIS patients undergoing intravenous thrombolysis (AUC = 0.644, 95% CI: 0.537–0.751, p < 0.05).
Additionally, our study found that the poor outcome group had higher values for age (OR: 1.085, p < 0.001), NIHSS score (OR: 1.367, p < 0.001), and FBG level (OR: 1.152, p = 0.013) compared to the favorable outcome group. Age (OR = 1.116, 95% CI: 1.047–1.190, p < 0.05) and NIHSS score (OR = 1.499, 95% CI: 1.280–1.756, p < 0.05) were also identified as independent risk factors for short-term adverse outcomes, consistent with traditional predictors found in previous research.
As indicators that can be applied even in underdeveloped medical settings, NPAR, NLR, and PLR are simple, cost-effective, and readily available. Composite indices are more stable than single markers, which may be influenced by various physiological and pathological conditions. Importantly, these composite indicators enhance the prognostic value of individual markers by integrating various mechanisms to predict outcomes, as demonstrated by ROC curve analysis. A notable advantage of our study is the use of baseline data from pre-thrombolysis blood samples, offering an ideal context for evaluating the impact of these indicators on outcomes. However, our study has limitations: it is a small-sample, single-center retrospective observational study, which may introduce selection bias and limit generalizability. Additionally, dynamic monitoring of NPAR, NLR, and PLR levels was not conducted, and their changes over time and correlation with patient outcomes require further investigation. Moreover, due to data limitations, we were unable to explore the association between these indicators and early neurological deterioration. The causal relationship between NPAR, NLR, PLR, and functional outcomes in AIS thrombolysis patients remains unclear, emphasizing the need for larger prospective studies to confirm causality and generalize findings to broader populations.
In summary, NPAR, NLR, PLR, and combined NPAR + NLR levels may predict short-term adverse outcomes in AIS patients receiving intravenous thrombolysis. Among these, NPAR demonstrates superior predictive performance. Using NPAR, NLR, and PLR to predict outcomes in AIS patients undergoing thrombolysis is cost-effective, readily accessible, and holds significant potential for broader application.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Committee of Xinhua Hospital affiliated to Dalian University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
CD: Data curation, Investigation, Software, Validation, Visualization, Writing – review & editing, Writing – original draft. BL: Formal analysis, Methodology, Software, Writing – original draft. MW: Data curation, Supervision, Validation, Writing – review & editing. CZ: Formal analysis, Writing – review & editing. YX: Conceptualization, Writing – review & editing. JL: Data curation, Writing – review & editing. YB: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
We sincerely thank all participants of the study. Including staff and students from the Dalian university and Xinhua Hospital affiliated with Dalian University who assisted with conducting exercise sessions, sample, and data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1512355/full#supplementary-material
1. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
2. Carbone, F, Bonaventura, A, and Montecucco, F. Neutrophil-related oxidants drive heart and brain remodeling after ischemia/reperfusion injury. Front Physiol. (2019) 10:1587. doi: 10.3389/fphys.2019.01587
3. Tang, C, Wang, C, Zhang, Y, Xue, L, Li, Y, Ju, C, et al. Recognition, intervention, and monitoring of neutrophils in acute ischemic stroke. Nano Lett. (2019) 19:4470–7. doi: 10.1021/acs.nanolett.9b01282
4. Lakhan, SE, Kirchgessner, A, and Hofer, M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. (2009) 7:97. doi: 10.1186/1479-5876-7-97
5. Dhanesha, N, Patel, RB, Doddapattar, P, Ghatge, M, Flora, GD, Jain, M, et al. PKM2 promotes neutrophil activation and cerebral thromboinflammation: therapeutic implications for ischemic stroke. Blood. (2022) 139:1234–45. doi: 10.1182/blood.2021012322
6. Ruhnau, J. Thrombosis, Neuroinflammation, and Poststroke infection: the multifaceted role of neutrophils in stroke. J Immunol Res. (2017) 2017:5140679. doi: 10.1155/2017/5140679
7. Zhu, B. Elevated neutrophil and presence of intracranial artery stenosis increase the risk of recurrent stroke. Stroke. (2018) 49:2294–300. doi: 10.1161/STROKEAHA.118.022126
8. Taleb, S. Inflammation in atherosclerosis. Arch Cardiovasc Dis. (2016) 109:708–15. doi: 10.1016/j.acvd.2016.04.002
9. Bourdon, E, and Blache, D. The importance of proteins in defense against oxidation. Antioxid Redox Signal. (2001) 3:293–311. doi: 10.1089/152308601300185241
10. Forster, C, Clark, HB, Ross, ME, and Iadecola, C. Inducible nitric oxide synthase expression in human cerebral infarcts. Acta Neuropathol. (1999) 97:215–20. doi: 10.1007/s004010050977
11. Bologa, RM, Levine, DM, Parker, TS, Cheigh, JS, Serur, D, Stenzel, KH, et al. Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis. (1998) 32:107–14. doi: 10.1053/ajkd.1998.v32.pm9669431
12. Miró-Mur, F, Urra, X, Gallizioli, M, Chamorro, A, and Planas, AM. Antigen Presentation After Stroke. Neurotherapeutics. (2016) 13:719–28. doi: 10.1007/s13311-016-0469-8
13. Li, LH, Chen, CT, Chang, YC, Chen, YJ, Lee, IH, and How, CK. Prognostic role of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune inflammation index in acute ischemic stroke: a STROBE-compliant retrospective study. Medicine (Baltimore). (2021) 100:e26354. doi: 10.1097/MD.0000000000026354
14. Yilmaz, G, Arumugam, TV, Stokes, KY, and Granger, DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. (2006) 113:2105–12. doi: 10.1161/CIRCULATIONAHA.105.593046
15. Jennings, LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. (2009) 102:248–57. doi: 10.1160/TH09-03-0192
16. Rawish, E, Nording, H, Münte, T, and Langer, HF. Platelets as mediators of Neuroinflammation and thrombosis. Front Immunol. (2020) 11:548631. doi: 10.3389/fimmu.2020.548631
17. Gong, P, Liu, Y, Gong, Y, Chen, G, Zhang, X, Wang, S, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. (2021) 18:51. doi: 10.1186/s12974-021-02090-6
18. Xu, JH. Higher platelet-to-lymphocyte ratio is associated with worse outcomes after intravenous thrombolysis in acute Ischaemic stroke. Front Neurol. (2019) 10:1192. doi: 10.3389/fneur.2019.01192
19. Kleindorfer, D, Kissela, B, Schneider, A, Woo, D, Khoury, J, Miller, R, et al. Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population-based study. Stroke. (2004) 35:e27–9. doi: 10.1161/01.STR.0000109767.11426.17
20. Liesz, A, Zhou, W, Na, SY, Hämmerling, GJ, Garbi, N, Karcher, S, et al. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J Neurosci. (2013) 33:17350–62. doi: 10.1523/JNEUROSCI.4901-12.2013
21. Zhang, RL, Chopp, M, Chen, H, and Garcia, JH. Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the rat. J Neurol Sci. (1994) 125:3–10. doi: 10.1016/0022-510X(94)90234-8
22. Kolaczkowska, E, and Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. (2013) 13:159–75. doi: 10.1038/nri3399
23. Buck, BH, Liebeskind, DS, Saver, JL, Bang, OY, Yun, SW, Starkman, S, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. (2008) 39:355–60. doi: 10.1161/STROKEAHA.107.490128
24. Kumar, AD, Boehme, AK, Siegler, JE, Gillette, M, Albright, KC, and Martin-Schild, S. Leukocytosis in patients with neurologic deterioration after acute ischemic stroke is associated with poor outcomes. J Stroke Cerebrovasc Dis. (2013) 22:e111–7. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.008
25. Ritchie, RF, Palomaki, GE, Neveux, LM, Navolotskaia, O, Ledue, TB, and Craig, WY. Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. (1999) 13:273–9. doi: 10.1002/(SICI)1098-2825(1999)13:6<273::AID-JCLA4>3.0.CO;2-X
26. Zoellner, H, Höfler, M, Beckmann, R, Hufnagl, P, Vanyek, E, Bielek, E, et al. Serum albumin is a specific inhibitor of apoptosis in human endothelial cells. J Cell Sci. (1996) 109:2571–80. doi: 10.1242/jcs.109.10.2571
27. Halliwell, B. Albumin—An important extracellular antioxidant? Biochem Pharmacol. (1988) 37:569–71. doi: 10.1016/0006-2952(88)90126-8
28. Belayev, L, Pinard, E, Nallet, H, Seylaz, J, Liu, Y, Riyamongkol, P, et al. Albumin therapy of transient focal cerebral ischemia: in vivo analysis of dynamic microvascular responses. Stroke. (2002) 33:1077–84. doi: 10.1161/hs0402.105555
29. Tang, H, Gong, F, Guo, H, Dai, Z, Wang, J, Liu, B, et al. Malnutrition and risk of mortality in ischemic stroke patients treated with intravenous thrombolysis. Front Aging Neurosci. (2022) 14:834973. doi: 10.3389/fnagi.2022.834973
30. Yang, D, Niu, C, Li, P, du, X, Zhao, M, and Jing, W. Study of the neutrophil percentage-to-albumin ratio as a biomarker for predicting recurrence of first-episode ischemic stroke. J Stroke Cerebrovasc Dis. (2024) 33:107485. doi: 10.1016/j.jstrokecerebrovasdis.2023.107485
31. Zhang, H, Wu, T, Tian, X, Lyu, P, Wang, J, and Cao, Y. High neutrophil percentage-to-albumin ratio can predict occurrence of stroke-associated infection. Front Neurol. (2021) 12:705790. doi: 10.3389/fneur.2021.705790
32. Parkkinen, J, Ojala, P, Niiranen, J, and Jolkkonen, J. Molecular mechanisms underlying neuroprotective effects of albumin after ischemic stroke. Stroke. (2007) 38:255. doi: 10.1161/01.STR.0000254506.06583.2d
33. Gao, J, Zhao, Y, du, M, Guo, H, Wan, T, Wu, M, et al. Serum albumin levels and clinical outcomes among ischemic stroke patients treated with endovascular Thrombectomy. Neuropsychiatr Dis Treat. (2021) 17:401–11. doi: 10.2147/NDT.S293771
34. Urra, X, Cervera, Á, Villamor, N, Planas, AM, and Chamorro, Á. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience. (2009) 158:1174–83. doi: 10.1016/j.neuroscience.2008.06.014
35. Vogelgesang, A, Becker, KJ, and Dressel, A. Immunological consequences of ischemic stroke. Acta Neurol Scand. (2014) 129:1–12. doi: 10.1111/ane.12165
36. Núñez, J, Sanchis, J, Bodí, V, Núñez, E, Mainar, L, Heatta, AM, et al. Relationship between low lymphocyte count and major cardiac events in patients with acute chest pain, a non-diagnostic electrocardiogram and normal troponin levels. Atherosclerosis. (2009) 206:251–7. doi: 10.1016/j.atherosclerosis.2009.01.029
37. Xue, J, Huang, W, Chen, X, Li, Q, Cai, Z, Yu, T, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:650–7. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010
38. Kocaturk, O, Besli, F, Gungoren, F, Kocaturk, M, and Tanriverdi, Z. The relationship among neutrophil to lymphocyte ratio, stroke territory, and 3-month mortality in patients with acute ischemic stroke. Neurol Sci. (2019) 40:139–46. doi: 10.1007/s10072-018-3604-y
39. Xu, C, Cai, L, Yi, T, Yi, X, and Hu, Y. Neutrophil-to-lymphocyte ratio is associated with stroke progression and functional outcome in patients with ischemic stroke. Brain Behav. (2023) 13:e3261. doi: 10.1002/brb3.3261
40. Chen, LZ, Luan, XQ, Wu, SZ, Xia, HW, Lin, YS, Zhan, LQ, et al. Optimal time point for neutrophil-to-lymphocyte ratio to predict stroke-associated pneumonia. Neurol Sci. (2023) 44:2431–42. doi: 10.1007/s10072-023-06654-7
41. Franks, ZG, Campbell, RA, Weyrich, AS, and Rondina, MT. Platelet-leukocyte interactions link inflammatory and thromboembolic events in ischemic stroke. Ann N Y Acad Sci. (2010) 1207:11–7. doi: 10.1111/j.1749-6632.2010.05733.x
42. Rubenstein, MH, Finn, AV, Leinbach, RC, Hollenbach, S, Aretz, HT, Virmani, R, et al. Short-term intravenous eptifibatide infusion combined with reduced dose recombinant tissue plasminogen activator inhibits platelet recruitment at sites of coronary artery injury. J Am Coll Cardiol. (2004) 43:287–94. doi: 10.1016/j.jacc.2003.08.039
43. Marta-Enguita, J, Navarro-Oviedo, M, Muñoz, R, Olier-Arenas, J, Zalba, G, Lecumberri, R, et al. Inside the Thrombus: Association of Hemostatic Parameters with Outcomes in large vessel stroke patients. Front Neurol. (2021) 12:599498. doi: 10.3389/fneur.2021.599498
44. Du, J. Association of mean platelet volume and platelet count with the development and prognosis of ischemic and hemorrhagic stroke. Int J Lab Hematol. (2016) 38:233–9. doi: 10.1111/ijlh.12474
45. Akboga, MK, Canpolat, U, Yayla, C, Ozcan, F, Ozeke, O, Topaloglu, S, et al. Association of Platelet to lymphocyte ratio with inflammation and severity of coronary atherosclerosis in patients with stable coronary artery disease. Angiology. (2016) 67:89–95. doi: 10.1177/0003319715583186
46. Massiot, N, Lareyre, F, Voury-Pons, A, Pelletier, Y, Chikande, J, Carboni, J, et al. High neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with symptomatic internal carotid artery stenosis. J Stroke Cerebrovasc Dis. (2019) 28:76–83. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.001
47. Altintas, O, Altintas, MO, Tasal, A, Kucukdagli, OT, and Asil, T. The relationship of platelet-to-lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone endovascular therapy. Neurol Res. (2016) 38:759–65. doi: 10.1080/01616412.2016.1215030
48. Wen, H, Wang, N, Lv, M, Yang, Y, and Liu, H. The early predictive value of platelet-to-lymphocyte ratio to hemorrhagic transformation of young acute ischemic stroke. Asian Biomed (Res Rev News). (2023) 17:267–72. doi: 10.2478/abm-2023-0069
Keywords: acute ischemic stroke, outcome, neutrophil percentage-to-albumin ratio, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio
Citation: Deng C, Liu B, Wang M, Zhu C, Xu Y, Li J and Bai Y (2025) Analysis of the correlation between neutrophil percentage-to-albumin ratio, neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with short-term prognosis in acute ischemic stroke patients undergoing intravenous thrombolysis. Front. Neurol. 16:1512355. doi: 10.3389/fneur.2025.1512355
Received: 03 November 2024; Accepted: 25 March 2025;
Published: 14 April 2025.
Edited by:
Mohd Farooq Shaikh, Charles Sturt University, AustraliaReviewed by:
Miao Chen, Hainan Medical University, ChinaCopyright © 2025 Deng, Liu, Wang, Zhu, Xu, Li and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Bai, MTAyOTk4MTgyNUBxcS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.