- 1Department of Radiology, Affiliated Hospital 6 of Nantong University, Yancheng Third People’s Hospital, Yancheng, China
- 2Department of Neurology, Affiliated Hospital 6 of Nantong University, Yancheng Third People’s Hospital, Yancheng, China
Background: The structural brain abnormalities associated with idiopathic dystonia (ID) remain inadequately understood. Previous voxel-based morphometry (VBM) studies examining whole-brain gray matter (GM) volume alterations in patients with ID have reported inconsistent and occasionally contradictory findings.
Methods: We performed a coordinate-based meta-analysis (CBMA) using the latest seed-based d mapping with permutation of subject images (SDM-PSI) technique to identify consistent GM alterations in patients with ID at the whole-brain level. Additionally, meta-regression analyses were conducted to explore the potential moderating effects of age, gender, and disease duration on GM volume.
Results: The CBMA incorporated 27 VBM studies, comprising 32 datasets with a total of 840 patients with ID and 834 healthy controls. Our analysis did not identify consistent or reliable GM alterations in patients with ID. The robustness of these findings was confirmed through a jackknife sensitivity analysis. Meta-regression analyses revealed that disease duration significantly influenced GM volume in the right insula.
Conclusion: Based on the best practice guidelines for CBMA, we utilized the most recent SDM-PSI algorithm to perform a new CBMA that included a larger group of individuals with ID. However, in contrast to previous CBMAs, we did not observe any consistent alterations in GM in ID. The findings suggest that using GM volume assessed by VBM as an imaging marker for ID may not be reliable. This could be attributed to ID being a functional disorder, or the inconsistency in GM alterations may be influenced by demographic and clinical variations, differences in imaging protocols and analysis methods, or small sample sizes. It is imperative to control for subject characteristics, employ standardized VBM methodologies, and enhance sample sizes in future research.
1 Introduction
Dystonia is the third most prevalent movement disorder worldwide, characterized by abnormal postures or movements in specific regions of the body resulting from persistent muscle contractions (1, 2). The global incidence of dystonia is approximately 30.85 per 100,000 individuals, significantly affecting patients’ quality of life and imposing considerable social and economic burdens (3). Idiopathic dystonia (ID), a common subtype of dystonia, occurs independently of other neurological or genetic conditions (4). While dysfunction in the basal ganglia or cerebello-thalamo-cortical circuits is widely regarded as the primary cause of ID (5, 6), its precise neuropathological and physiological mechanisms remain incompletely understood (7).
Voxel-based morphometry (VBM) facilitates automated comparisons of gray matter (GM) volume or density in T1-weighted magnetic resonance imaging (MRI) scans at the whole-brain level between different groups (8). Unlike the traditional region-of-interest (ROI) approach, VBM offers greater objectivity and efficiency (8, 9). Previous studies have investigated GM alterations in ID using VBM, but the results have been inconsistent and occasionally contradictory. For instance, Chirumamilla et al. and Bianchi et al. reported increased GM volume in the anterior cingulate cortex in patients with ID (10, 11), whereas Piccinin et al. found a decrease in GM in the same region (12). Additionally, some studies have reported no significant GM abnormalities in ID (13–17). Therefore, further research is required to identify consistent and reliable patterns of GM alterations, which could provide deeper insights into the pathophysiological mechanisms underlying ID.
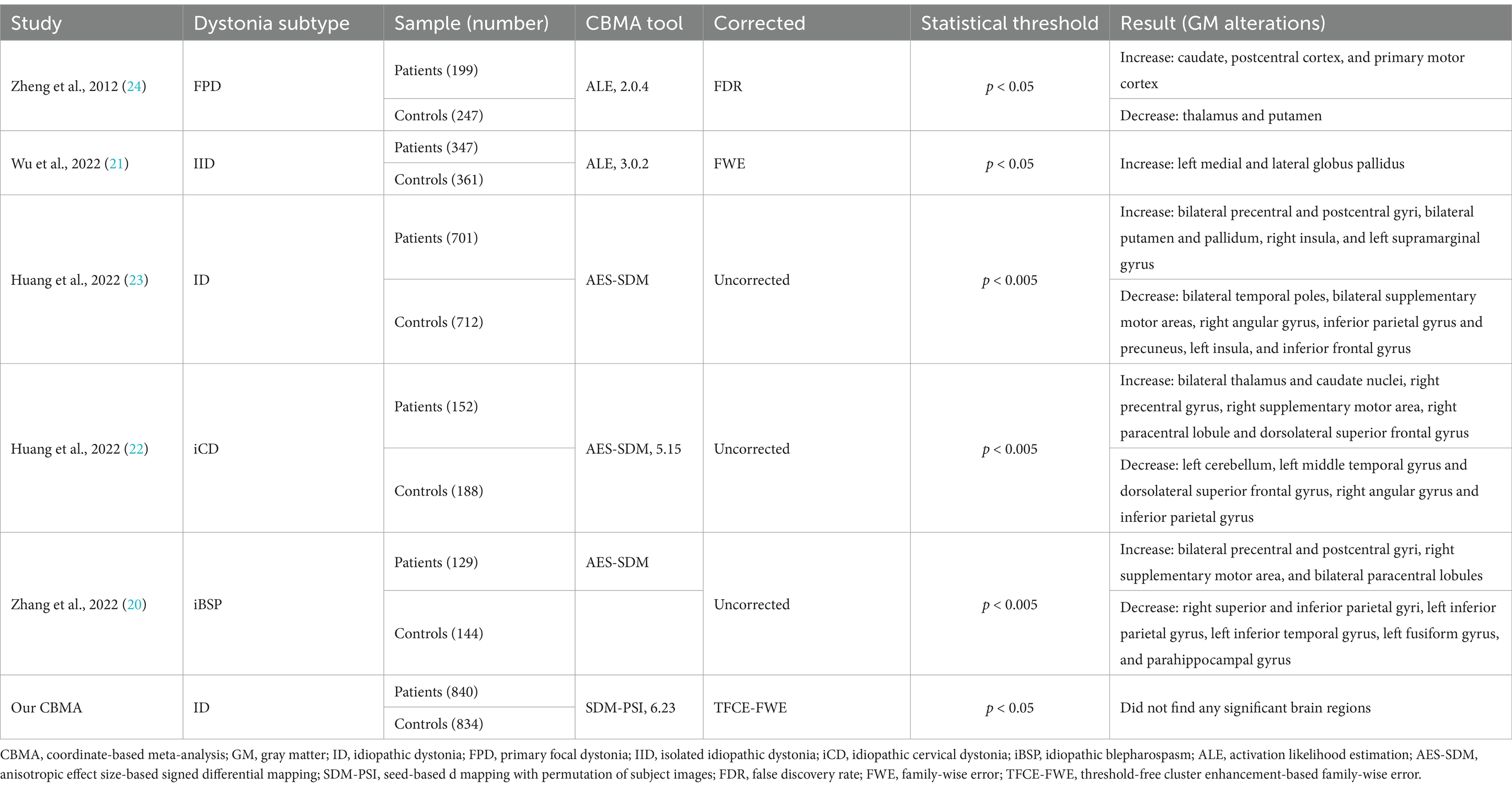
Coordinate-based meta-analysis (CBMA) enables the integration of multiple neuroimaging studies, facilitating the derivation of more generalizable and robust findings (18, 19). To date, five CBMAs have summarized whole-brain VBM studies on ID and its subtypes, yet inconsistencies in their results have been observed (20–24). This discrepancy may be attributed to the use of different CBMA algorithms. Zheng et al. and Wu et al. employed activation likelihood estimation (ALE) techniques (21, 24), whereas Huang et al. and Zhang et al. utilized anisotropic effect size-based signed differential mapping (AES-SDM) techniques (20, 22, 23). In contrast to ALE, AES-SDM can incorporate both significant and non-significant results along with their effect sizes but adopts a less stringent statistical approach (25, 26). Disparities in literature search strategies, time frames, and the focus on specific ID subtypes may have further contributed to these inconsistencies. A comprehensive summary of the information from these five CBMA studies is provided in Table 1.
In line with the latest guidelines and recommendations, several aspects of the previous five CBMAs require improvement (27–29). First, the studies by Gellea et al. and Pantano et al. should be excluded, as they employed ROI techniques rather than whole-brain approaches in their VBM analyses (30, 31). Additionally, the inclusion of studies that employed small-volume correction (SVC) should be reassessed (32–36), as this method may exaggerate results in specific brain regions and introduce bias in region selection (27). Furthermore, Zheng et al. applied an older version of ALE with false discovery rate (FDR) correction (24), which has been demonstrated to be potentially suboptimal for CBMA (37). Moreover, the inclusion of ALE analyses with a limited number of studies (9 and 14 studies, respectively) (21, 24), while a minimum of 17 studies is recommended for ALE analysis to ensure adequate statistical power (38). These factors likely compromised the robustness of the CBMA results. Notably, the number of VBM studies focusing on ID has increased in recent years. Thus, an updated CBMA of existing VBM studies on ID is crucial for identifying consistent and robust GM alterations.
The latest version of CBMA, seed-based d mapping with permutation of subject images (SDM-PSI), applies threshold-free cluster enhancement (TFCE)-based family-wise error (FWE) correction to control for multiple comparisons (39). This method is advantageous because it simultaneously addresses both FWE and cluster-level significance without requiring a predefined cluster size threshold, thus preserving statistical sensitivity while reducing the likelihood of false positive findings. It has demonstrated effectiveness in a range of neurological and psychiatric disorders, including schizophrenia and Parkinson’s disease, and has exhibited high statistical efficacy (40, 41). Therefore, we conducted an updated CBMA to identify consistent and robust GM alterations in ID, employing SDM-PSI in line with current guidelines and recommendations (27–29). Additionally, meta-regression analyses were performed to examine the potential influence of participant demographic and clinical characteristics on GM alterations.
2 Methods
2.1 Study search and selection
A systematic and comprehensive search was conducted across the PubMed, Web of Science, and Embase databases to identify relevant studies published up to September 21, 2024. The search used the keywords (“dystonia” OR “blepharospasm” OR “writer’s cramp” OR “spasmodic dysphonia” OR “Meige’s syndrome” OR “dystonic disorders”) AND (“voxel*” OR “VBM” OR “morphometry”). Additionally, potential studies were sourced through literature review and scrutiny of references in the retrieved articles. Studies that met the following criteria were included: (1) original peer-reviewed English-language articles; (2) applying VBM analysis to compare GM differences between patients with ID and healthy controls (HC) at the whole-brain level (ROI analysis or SVC analysis were not included); and (3) findings reported in stereotactic three-dimensional coordinates (x, y, z). In cases of overlapping subjects among studies, the study with the larger sample size was prioritized for inclusion. Studies were excluded if the required data could not be obtained despite efforts to contact corresponding authors.
2.2 Data extraction
Data were collected on participant characteristics (ID subtype, sample size, age, gender, handedness, and disease duration), imaging details (e.g., MRI scanner, magnetic field strength, sequence, voxel size, head coil channels, processing software, modulation, Gaussian kernel, covariates, and statistical threshold), and peak coordinates and t-values of regions showing significant GM alterations compared to HC. In cases where z- or p-values were provided, the online SDM tool was utilized to convert them to t-values. Two researchers independently reviewed and extracted data from the studies, resolving discrepancies through discussion. In instances of unresolved differences, a third author was consulted for consensus.
2.3 Quality assessment
The quality of the studies was assessed using a 10-point checklist (42), with primary evaluation criteria including sample size, demographic information, key clinical variables, methodological thoroughness, result presentation, and study limitations. Each criterion received a score of 0, 0.5, or 1, indicating non-compliance, partial compliance, or full compliance with the specified criteria. Two authors independently assessed and scored each study, resolving any discrepancies through discussion. The checklist details are available in Supplementary Table S1.
2.4 CBMA of included VBM studies
The SDM-PSI software version 6.231 was used to perform the meta-analysis. A detailed description of the SDM-PSI approach is available in previous publications (39, 43). The main steps are briefly outlined as follows: Initially, a document was created to compile the peak coordinates, effect sizes, and demographic/clinical details (e.g., sample size, age, gender, disease duration, etc.) from all original studies. Second, the upper and lower bounds of possible effect size images were calculated within a GM mask. Third, effect size analysis was conducted using the multiple imputation algorithm MetaNSUE. Fourth, Rubin’s rule was employed to voxel-wise combine meta-analysis images from various input datasets. Finally, subject images were reconstructed for permutation tests, with multiple comparisons corrected using TFCE-based FWE correction (cluster-level p < 0.05, voxel extent ≥10).
2.5 Sensitivity, heterogeneity and publication bias analyses
A jackknife sensitivity analysis was conducted on the datasets to evaluate the impact of individual studies on the overall outcomes. By systematically excluding one dataset at a time and conducting iterative analyses, brain regions that consistently showed significance were deemed highly reproducible (44). Heterogeneity across the studies was assessed using the I2 statistic, with an I2 value less than 50% indicating low heterogeneity (45). Additionally, Egger’s test was employed to examine potential publication bias.
2.6 Meta-regression analyses
Linear meta-regression analyses were conducted to investigate the impact of diverse demographic and clinical factors, with particular attention to age, gender, and disease duration. A statistical significance threshold of p < 0.05 (TFCE-based FWE correction) and a voxel extent ≥10 was applied.
3 Results
3.1 Information on included studies and participants
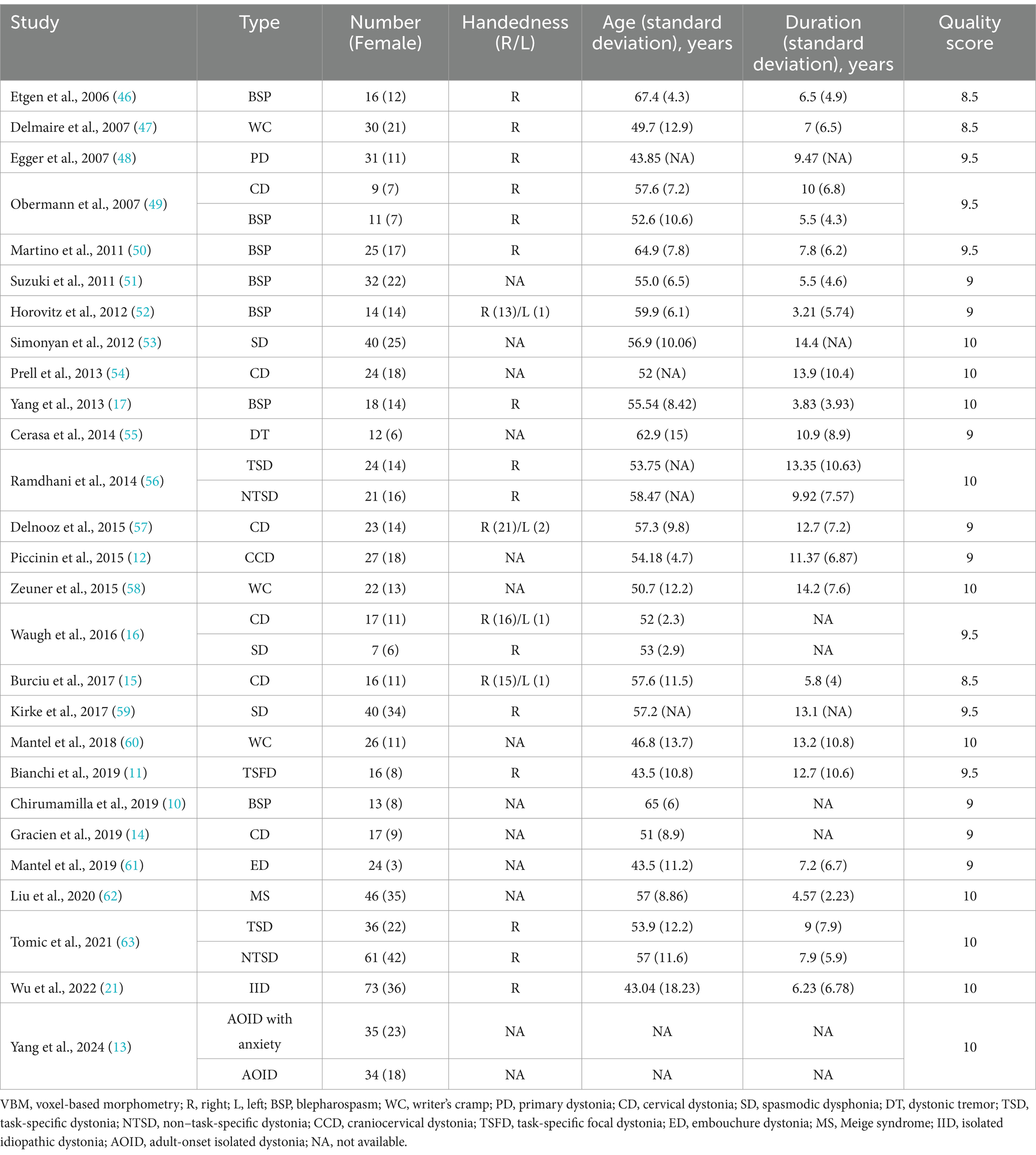
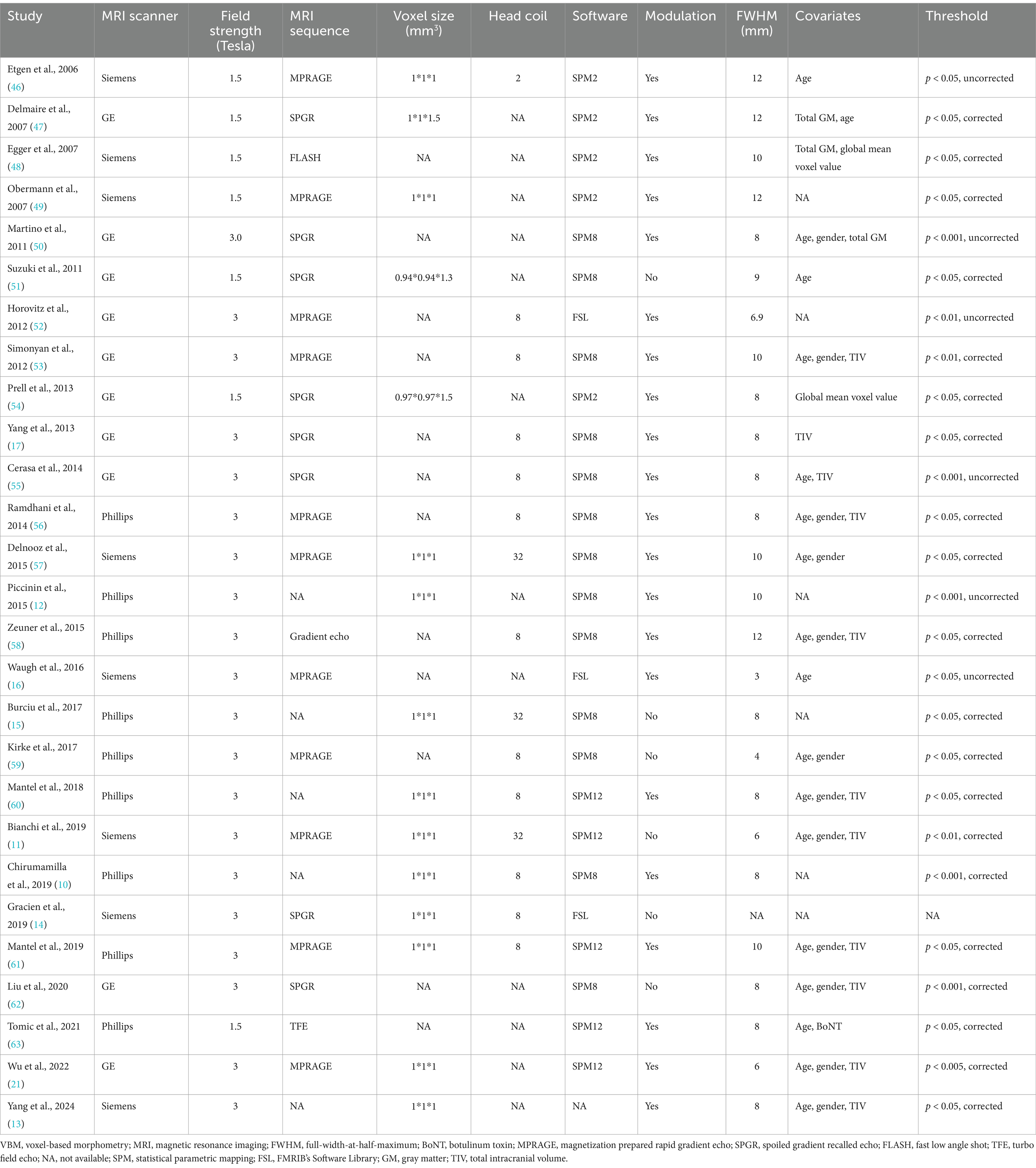
A total of 730 results were identified through the literature search, leading to the inclusion of 27 original VBM studies in this CBMA (10–17, 21, 46–63). The comprehensive literature screening process is outlined in Figure 1. Of the incorporated studies, 21 reported increases or decreases in GM volume in specific brain regions, while 6 reported no significant alterations in patients with ID. These studies comprised 32 datasets, encompassing a total of 840 patients with ID and 834 HC subjects. Sample sizes across these datasets ranged from 7 to 73 for the patient groups (mean: 26.25) and from 7 to 83 for the HC groups (mean: 26.06). Reported demographic and clinical characteristics included gender (62.6% female in the patient groups and 58.3% in the HC groups across 32 datasets), age (mean: 58.02 years for patients, 52.42 years for HC across 31 datasets), and disease duration (mean: 9.15 years across 28 datasets). The demographic and clinical data for each study are presented in Table 2. Furthermore, Table 3 outlines the imaging protocols and data processing methods. Each study underwent a quality assessment, with detailed scores provided in Supplementary Table S2.
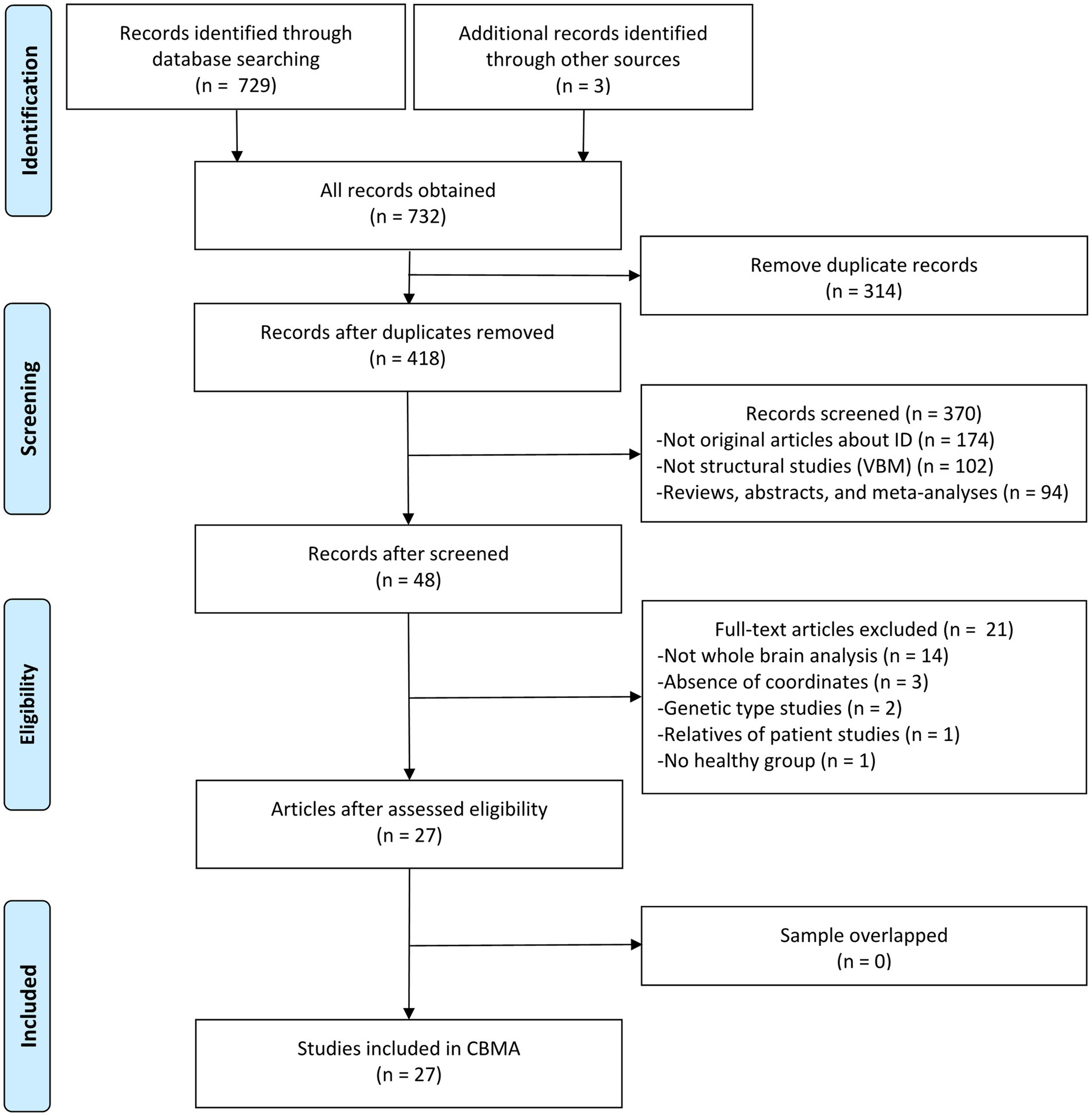
Figure 1. Literature selection flowchart in meta-analysis. ID, idiopathic dystonia; VBM, voxel-based morphometry; CBMA, coordinate-based meta-analysis.
3.2 CBMA of included VBM studies
No statistically significant and consistent differences in GM were identified between patients with ID and HC subjects across 32 datasets after applying TFCE-based FWE correction (p < 0.05, voxel extent ≥10).
3.3 Sensitivity, heterogeneity and publication bias analyses
The jackknife sensitivity analysis indicated no consistent alterations in GM between patients with ID and HC subjects across all datasets. Since no significant brain clusters were identified in the CBMA, further analyses of heterogeneity and publication bias were not conducted.
3.4 Meta-regression analyses
The meta-regression analysis demonstrated that a longer disease duration, as reported in 28 datasets, was associated with an increase in GM volume in the right insula (Montreal Neurological Institute [MNI] coordinates: x = 38, y = −14, z = 2; Brodmann area 48; SDM-Z = 3.258; voxels = 621; TFCE-based FWE correction, p < 0.01, Figure 2). Furthermore, neither age nor gender exhibited associations with GM volume (p < 0.05, TFCE-based FWE correction and voxel extent ≥10).
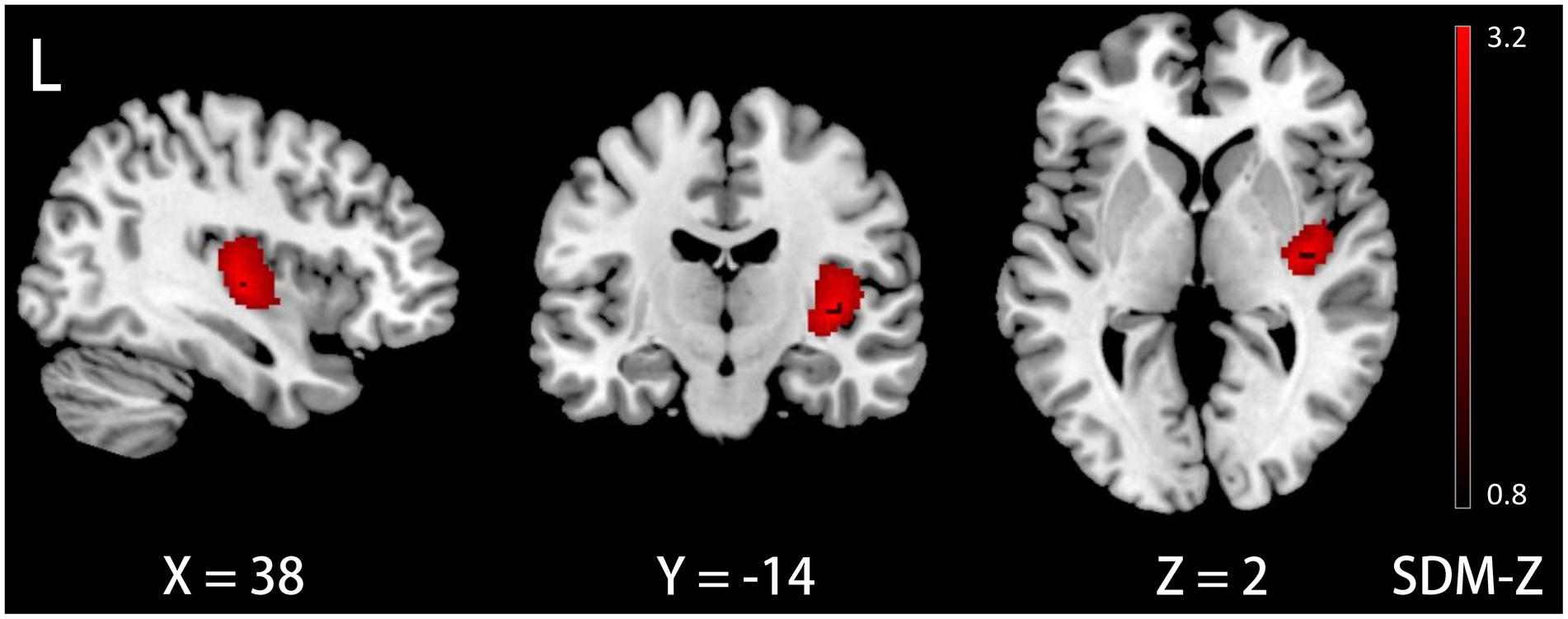
Figure 2. Meta-egression results demonstrating GM volume increases in the right insula with disease duration in ID. GM, gray matter; ID, idiopathic dystonia; L, left.
4 Discussion
This updated CBMA did not exhibit any consistent GM alterations in patients with ID compared to HC subjects, with jackknife sensitivity analysis confirming the robustness of the findings. Furthermore, meta-regression analysis revealed a significant impact of disease duration on GM in the right insula.
It is noteworthy that our findings were inconsistent with those in previous CBMAs (20–24), which may be attributed to the application of the latest SDM-PSI algorithm alongside more stringent statistical methods, and adherence to contemporary guidelines that excluded studies using ROI and SVC analyses. Additionally, our CBMA included a larger cohort comprising 32 original VBM datasets and 840 participants, thus enhancing the reliability of the results. Consequently, the absence of consistent GM alterations may suggest that GM is not a dependable neuroimaging biomarker for ID.
One potential explanation for the inconsistent GM abnormalities observed in patients with ID may be the absence of true GM alterations within this population. Previous autopsy studies and animal models have demonstrated that ID does not present with structural brain anomalies (64–66). Recent functional MRI studies have revealed significant abnormalities across several brain regions in patients with ID, including the primary motor cortex, supplementary motor area, cerebellum, thalamus, and putamen (67–71). Additionally, several studies have demonstrated a correlation between the severity of clinical symptoms and functional abnormalities in specific brain regions (68, 69, 71). Furthermore, some research suggests that symptomatic treatments, such as botulinum toxin injections or deep brain stimulation, can modulate abnormal brain functions (72–75). Collectively, these findings suggest that ID may be primarily characterized as a functional brain disorder that does not necessarily involve structural abnormalities.
The inconsistency in identifying GM alterations in ID may be attributed to the heterogeneity among participants. As illustrated in Table 2, the 32 datasets include 13 distinct subtypes of ID, highlighting significant diversity in demographic and clinical characteristics. Specific subtypes of ID are often associated with distinct patterns of GM abnormalities (76). For instance, Tomic et al. and Ramdhani et al. observed that, compared to non-task-specific dystonia (blepharospasm and cervical dystonia), task-specific dystonia (laryngeal dystonia and writer’s cramp) was associated with increased GM volume in the primary somatosensory cortex, middle frontal gyrus, temporal lobe, and occipital lobe (56, 63). Moreover, the varied motor symptoms observed in patients are frequently associated with increased GM volume in specific brain regions, providing insights into the potential mechanisms underlying GM alterations in ID (64, 77). As a result, GM alterations in ID present diverse patterns, with the disorder’s inherent heterogeneity likely contributing to the lack of consistent GM findings.
The meta-regression analysis indicated that disease duration has an impact on GM volume in the right insula, a region crucial for emotional perception and sensory processing (78, 79). Individuals with ID frequently experience non-motor symptoms such as chronic pain, anxiety, and depression (80–82). These symptoms have been linked to changes in brain structure in various psychiatric and neurological disorders, often correlating with disease duration (83–85). This suggests that the alterations in GM volume in the insula may reflect an adaptive response to these non-motor symptoms in individuals with ID. Although no significant modulatory effects of age or gender on GM volume were observed, caution should be exercised in interpreting this finding, as it is based on study-level rather than individual-level data. Age and gender are well-established factors that are known to influence brain structure (86–89). Other potential confounding variables, such as handedness (90–92), disease severity (12, 54, 57, 93), age of onset (93), medication history (12, 57, 63), and education level (94), may also contribute to discrepancies in brain structure. Unfortunately, the lack of sufficient data from the original studies limits the ability to systematically investigate these potential variables. Consequently, the demographic and clinical heterogeneity of participants in VBM studies may account for the failure to identify consistent GM alterations.
In addition to participant heterogeneity, the inconsistency in GM alterations may also arise from variations in imaging protocols. As outlined in Table 3, the 27 VBM studies employed a range of MRI scanners (GE, Philips, Siemens), magnetic field strengths (3.0 and 1.5 Tesla), head coil channels (2, 8, 32, and unreported), pulse sequences, and voxel sizes. Focke et al. and Takao et al. found that variations in MRI scanners influenced VBM analysis outcomes (95, 96), and other studies have demonstrated that magnetic field strength also influences results (97–99). Furthermore, differences in head coil channels, pulse sequences, and voxel sizes have been associated with discrepancies in GM measurements (77, 100–102). Moreover, inadequate management of head movement during image acquisition may compromise image quality and result in inaccuracies in GM quantification (103, 104). Notably, only a limited number of original studies rigorously controlled for head movement during MRI scanning or conducted comprehensive visual inspections and manual corrections of images (11–14, 17, 21, 54, 55, 57–59, 61).
The inconsistency in GM alterations may also result from variations in image processing procedures, which are a crucial aspect of VBM analysis. Initially, brain images from all subjects underwent segmentation and registration into standard space, followed by modulation to compensate for image deformation, and a smoothing process (8). Subsequently, statistical analyses were conducted to identify and interpret differences between groups. The VBM studies included in the CBMA employed various software platforms (different versions of SPM and FSL), applying diverse techniques for image segmentation, registration, modulation, and smoothing, which could potentially influence GM measurements (105–111). Correcting for multiple comparisons is crucial in neuroimaging studies to prevent inflated positive results and bias (112). However, seven out of the 32 analyzed datasets employed uncorrected thresholds (12, 16, 46, 50, 52, 55). Moreover, factors such as age, gender, and total intracranial volume serve as important covariates in VBM analysis (113, 114); however, six studies failed to incorporate any covariates in their analyses (10, 12, 14, 15, 49, 52). Therefore, the multitude of methodological decisions involved in each step of VBM analysis may contribute to inconsistencies in results.
Finally, a small sample size can constrain the ability to identify significant findings in neuroimaging studies, potentially reducing the robustness of the results (115). Fusar-Poli et al. observed that VBM studies with smaller sample sizes generally produce fewer significant findings (116). However, the current ID datasets have an average of only 26.25 participants, which reduces the statistical power necessary to detect significant GM differences. Furthermore, the non-normal distribution of the data complicates the situation further, as smaller studies are more prone to producing false positives compared to larger ones (117). This susceptibility compromises the generalizability and stability of the results. Consequently, the limited sample size likely plays a major role in the discrepancies observed in VBM findings, explaining the lack of significant GM abnormalities identified in this CBMA.
Taken together, the inconsistency in observed GM alterations may primarily arise from demographic and clinical heterogeneity, variations in imaging acquisition and analytical methods, and small sample sizes. To enhance the robustness and reproducibility of future studies, the following recommendations are proposed: (1) conduct power analyses prior to VBM studies to determine appropriate sample sizes; encourage multicenter collaborations or data sharing to increase sample sizes; (2) conduct comprehensive assessments of clinical population characteristics to minimize the potential impact of confounding factors on results; and (3) establish and implement standardized imaging acquisition and analytical methodologies, and apply rigorous statistical strategies for analysis.
It is essential to acknowledge several limitations of this study. First, CBMA relies on peak coordinates and corresponding effect sizes reported in VBM studies rather than on original datasets. Some scholars have suggested that image-based meta-analyses or hybrid meta-analyses combining images and coordinates may improve result accuracy (118, 119), although this approach depends on the availability of raw data shared by researchers. Second, the datasets included in this CBMA are incomplete, as unpublished studies, non-English publications, and studies lacking crucial information were excluded, which may have introduced selection bias. Third, the limited number of studies focusing on specific ID subtypes impeded the conduct of subgroup CBMA analyses to explore the variability within the disorder. Fourth, we did not analyze functional MRI studies in ID. Integrating structural and functional neuroimaging could help bridge the gap between the inconsistent GM changes observed and the functional abnormalities reported, which may be beneficial in revealing the neuropathological mechanisms of ID.
5 Conclusion
The CBMA of whole-brain VBM studies failed to reveal a consistent and reliable pattern of GM differences between patients with ID and HC subjects. This finding may indicate that GM is not a reliable neuroimaging marker for ID. It is possible that ID may primarily be a functional disorder. Another explanation for CBMA’s inability to detect consistent GM alterations could be the demographic and clinical heterogeneity among participants, along with variations in image acquisition, processing techniques, and small sample sizes. To improve the accuracy of future findings, upcoming VBM studies must rigorously assess potential confounding factors, adhere to standardized protocols for image acquisition and analysis, and aim to increase sample sizes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
Z-YW: Data curation, Writing – original draft. FC: Data curation, Writing – original draft. H-HS: Data curation, Writing – original draft. H-LL: Writing – original draft, Conceptualization, Methodology. J-BH: Conceptualization, Methodology, Writing – original draft. Z-YD: Conceptualization, Methodology, Supervision, Writing – review & editing. SW: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Special Funds for Science Development of the Foundation Plan for Outstanding People of The Sixth Peak of Jiangsu Province of China (2019-WSN-313), the Research Foundation of Science and Technology Bureau of Yancheng (YCBK2023041), the Clinical Teaching Hospitals of Jiangsu Vocational College of Medicine (20219112), and the Clinical Medicine Special Research Fund of Nantong University (2023JZ019).
Acknowledgments
We are grateful to all authors in the research involved in the meta-analysis for using their data and coordinates.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1510115/full#supplementary-material
Footnotes
References
1. Balint, B, Mencacci, NE, Valente, EM, Pisani, A, Rothwell, J, Jankovic, J, et al. Dystonia. Nat Rev Dis Primers. (2018) 4:25. doi: 10.1038/s41572-018-0023-6
2. Grütz, K, and Klein, C. Dystonia updates: definition, nomenclature, clinical classification, and etiology. J Neural Transm (Vienna). (2021) 128:395–404. doi: 10.1007/s00702-021-02314-2
3. Medina, A, Nilles, C, Martino, D, Pelletier, C, and Pringsheim, T. The prevalence of idiopathic or inherited isolated dystonia: a systematic review and Meta-analysis. Mov Disord Clin Pract. (2022) 9:860–8. doi: 10.1002/mdc3.13524
4. Prasad, R, Kumar, A, Pathak, A, Singh, VK, Verma, A, Chaurasia, RN, et al. Comparative study between idiopathic and non-idiopathic dystonia: a prospective observational study. Neurol Sci. (2021) 42:5029–35. doi: 10.1007/s10072-021-05176-4
5. Thomsen, M, Lange, LM, Zech, M, and Lohmann, K. Genetics and pathogenesis of dystonia. Annu Rev Pathol. (2024) 19:99–131. doi: 10.1146/annurev-pathmechdis-051122-110756
6. Lehéricy, S, Tijssen, MA, Vidailhet, M, Kaji, R, and Meunier, S. The anatomical basis of dystonia: current view using neuroimaging. Mov Disord. (2013) 28:944–57. doi: 10.1002/mds.25527
7. Madelein van der Stouwe, AM, Nieuwhof, F, and Helmich, RC. Tremor pathophysiology: lessons from neuroimaging. Curr Opin Neurol. (2020) 33:474–81. doi: 10.1097/wco.0000000000000829
8. Ashburner, J, and Friston, KJ. Voxel-based morphometry--the methods. Neuroimage. (2000) 11:805–21. doi: 10.1006/nimg.2000.0582
9. Whitwell, JL, and Josephs, KA. Voxel-based morphometry and its application to movement disorders. Parkinsonism Relat Disord. (2007) 13:S406–16. doi: 10.1016/s1353-8020(08)70039-7
10. Chirumamilla, VC, Dresel, C, Koirala, N, Gonzalez-Escamilla, G, Deuschl, G, Zeuner, KE, et al. Structural brain network fingerprints of focal dystonia. Ther Adv Neurol Disord. (2019) 12:1756286419880664. doi: 10.1177/1756286419880664
11. Bianchi, S, Fuertinger, S, Huddleston, H, Frucht, SJ, and Simonyan, K. Functional and structural neural bases of task specificity in isolated focal dystonia. Mov Disord. (2019) 34:555–63. doi: 10.1002/mds.27649
12. Piccinin, CC, Pioyesana, LG, Santos, MCA, Guimaraes, RP, De Campos, BM, Rezende, TJR, et al. Diffuse decreased gray matter in patients with idiopathic craniocervical dystonia: a voxel-based morphometry study. Front Neurol. (2015) 5:283. doi: 10.3389/fneur.2014.00283
13. Yang, Z, Liu, H, Zhang, J, Luo, Y, Weng, A, Zhang, Y, et al. Neural correlates of anxiety in adult-onset isolated dystonia. Neuroscience. (2024) 558:50–7. doi: 10.1016/j.neuroscience.2024.08.018
14. Gracien, RM, Petrov, F, Hok, P, van Wijnen, A, Maiworm, M, Seiler, A, et al. Multimodal quantitative mri reveals no evidence for tissue pathology in idiopathic cervical dystonia. Front Neurol. (2019) 10:941. doi: 10.3389/fneur.2019.00914
15. Burciu, RG, Hess, CW, Coombes, SA, Ofori, E, Shukla, P, Chung, JW, et al. Functional activity of the sensorimotor cortex and cerebellum relates to cervical dystonia symptoms. Hum Brain Mapp. (2017) 38:4563–73. doi: 10.1002/hbm.23684
16. Waugh, JL, Kuster, JK, Levenstein, JM, Makris, N, Multhaupt-Buell, TJ, Sudarsky, LR, et al. Thalamic volume is reduced in cervical and laryngeal Dystonias. PLoS One. (2016) 11:e0155302. doi: 10.1371/journal.pone.0155302
17. Yang, J, Luo, C, Song, W, Chen, Q, Chen, K, Chen, X, et al. Altered regional spontaneous neuronal activity in blepharospasm: a resting state fMRI study. J Neurol. (2013) 260:2754–60. doi: 10.1007/s00415-013-7042-8
18. Caspers, J, Zilles, K, Beierle, C, Rottschy, C, and Eickhoff, SB. A novel meta-analytic approach: mining frequent co-activation patterns in neuroimaging databases. Neuroimage. (2014) 90:390–402. doi: 10.1016/j.neuroimage.2013.12.024
19. Tench, CR, Tanasescu, R, Constantinescu, CS, Auer, DP, and Cottam, WJ. Easy to interpret coordinate based meta-analysis of neuroimaging studies: analysis of brain coordinates (ABC). J Neurosci Methods. (2022) 372:109556. doi: 10.1016/j.jneumeth.2022.109556
20. Zhang, M, Huang, X, Li, B, Shang, H, and Yang, J. Gray matter structural and functional alterations in idiopathic Blepharospasm: a multimodal Meta-analysis of VBM and functional neuroimaging studies. Front Neurol. (2022) 13:889714. doi: 10.3389/fneur.2022.889714
21. Wu, Y, Zhang, C, Li, Y, Feng, J, Zhang, M, Li, H, et al. Imaging insights of isolated idiopathic dystonia: voxel-based morphometry and activation likelihood estimation studies. Front Neurol. (2022) 13:823882. doi: 10.3389/fneur.2022.823882
22. Huang, X, Zhang, M, Li, B, Shang, H, and Yang, J. Structural and functional brain abnormalities in idiopathic cervical dystonia: a multimodal meta-analysis. Parkinsonism Relat Disord. (2022) 103:153–65. doi: 10.1016/j.parkreldis.2022.08.029
23. Huang, X, Lin, J, Shang, H, and Yang, J. Voxel-based meta-analysis of gray matter abnormalities in idiopathic dystonia. J Neurol. (2022) 269:2862–73. doi: 10.1007/s00415-022-10961-y
24. Zheng, Z, Pan, P, Wang, W, and Shang, H. Neural network of primary focal dystonia by an anatomic likelihood estimation meta-analysis of gray matter abnormalities. J Neurol Sci. (2012) 316:51–5. doi: 10.1016/j.jns.2012.01.032
25. Radua, J, Mataix-Cols, D, Phillips, ML, El-Hage, W, Kronhaus, DM, Cardoner, N, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. (2012) 27:605–11. doi: 10.1016/j.eurpsy.2011.04.001
26. Liu, J-L, Sun, J-T, Hu, H-L, Wang, H-Y, Kang, Y-X, Chen, T-Q, et al. Structural and functional neural alterations in internet addiction: a study protocol for systematic review and Meta-analysis. Psychiatry Investig. (2023) 20:69–74. doi: 10.30773/pi.2021.0383
27. Tahmasian, M, Sepehry, AA, Samea, F, Khodadadifar, T, Soltaninejad, Z, Javaheripour, N, et al. Practical recommendations to conduct a neuroimaging meta-analysis for neuropsychiatric disorders. Hum Brain Mapp. (2019) 40:5142–54. doi: 10.1002/hbm.24746
28. Müller, VI, Cieslik, EC, Laird, AR, Fox, PT, Radua, J, Mataix-Cols, D, et al. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. (2018) 84:151–61. doi: 10.1016/j.neubiorev.2017.11.012
29. Manuello, J, Costa, T, Cauda, F, and Liloia, D. Six actions to improve detection of critical features for neuroimaging coordinate-based meta-analysis preparation. Neurosci Biobehav Rev. (2022) 137:104659. doi: 10.1016/j.neubiorev.2022.104659
30. Gallea, C, Herath, P, Voon, V, Lerner, A, Ostuni, J, Saad, Z, et al. Loss of inhibition in sensorimotor networks in focal hand dystonia. Neuroimage Clin. (2018) 17:90–7. doi: 10.1016/j.nicl.2017.10.011
31. Pantano, P, Totaro, P, Fabbrini, G, Raz, E, Contessa, GM, Tona, F, et al. A transverse and longitudinal MR imaging voxel-based morphometry study in patients with primary cervical dystonia. AJNR Am J Neuroradiol. (2011) 32:81–4. doi: 10.3174/ajnr.A2242
32. Draganski, B, Thun-Hohenstein, C, Bogdahn, U, Winkler, J, and May, A. "motor circuit" gray matter changes in idiopathic cervical dystonia. Neurology. (2003) 61:1228–31. doi: 10.1212/01.wnl.0000094240.93745.83
33. Garraux, G, Bauer, A, Hanakawa, T, Wu, T, Kansaku, K, and Hallett, M. Changes in brain anatomy in focal hand dystonia. Ann Neurol. (2004) 55:736–9. doi: 10.1002/ana.20113
34. Filip, P, Gallea, C, Lehericy, S, Bertasi, E, Popa, T, Marecek, R, et al. Disruption in cerebellar and basal ganglia networks during a visuospatial task in cervical dystonia. Mov Disord. (2017) 32:757–68. doi: 10.1002/mds.26930
35. Draganski, B, Schneider, SA, Fiorio, M, Klöppel, S, Gambarin, M, Tinazzi, M, et al. Genotype-phenotype interactions in primary dystonias revealed by differential changes in brain structure. Neuroimage. (2009) 47:1141–7. doi: 10.1016/j.neuroimage.2009.03.057
36. Granert, O, Peller, M, Jabusch, H-C, Altenmueller, E, and Siebner, HR. Sensorimotor skills and focal dystonia are linked to putaminal grey-matter volume in pianists. J Neurol Neurosurg Psychiatry. (2011) 82:1225–31. doi: 10.1136/jnnp.2011.245811
37. Chumbley, JR, and Friston, KJ. False discovery rate revisited: FDR and topological inference using Gaussian random fields. Neuroimage. (2009) 44:62–70. doi: 10.1016/j.neuroimage.2008.05.021
38. Eickhoff, SB, Nichols, TE, Laird, AR, Hoffstaedter, F, Amunts, K, Fox, PT, et al. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage. (2016) 137:70–85. doi: 10.1016/j.neuroimage.2016.04.072
39. Albajes-Eizagirre, A, Solanes, A, Vieta, E, and Radua, J. Voxel-based meta-analysis via permutation of subject images (PSI): theory and implementation for SDM. Neuroimage. (2019) 186:174–84. doi: 10.1016/j.neuroimage.2018.10.077
40. Wang, L, Liu, R, Liao, J, Xiong, X, Xia, L, Wang, W, et al. Meta-analysis of structural and functional brain abnormalities in early-onset schizophrenia. Front Psych. (2024) 15:1465758. doi: 10.3389/fpsyt.2024.1465758
41. Xie, H, Yang, Y, Sun, Q, Li, ZY, Ni, MH, Chen, ZH, et al. Abnormalities of cerebral blood flow and the regional brain function in Parkinson's disease: a systematic review and multimodal neuroimaging meta-analysis. Front Neurol. (2023) 14:1289934. doi: 10.3389/fneur.2023.1289934
42. Cai, M, Wang, R, Liu, M, Du, X, Xue, K, Ji, Y, et al. Disrupted local functional connectivity in schizophrenia: an updated and extended meta-analysis. Schizophrenia. (2022) 8:93. doi: 10.1038/s41537-022-00311-2
43. Albajes-Eizagirre, A, Solanes, A, Fullana, MA, Ioannidis, JPA, Fusar-Poli, P, Torrent, C, et al. Meta-analysis of voxel-based neuroimaging studies using seed-based d mapping with permutation of subject images (SDM-PSI). J Vis Exp. (2019) 153:e59841. doi: 10.3791/59841
44. Vera, JF. Distance stability analysis in multidimensional scaling using the jackknife method. Br J Math Stat Psychol. (2017) 70:25–41. doi: 10.1111/bmsp.12079
45. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
46. Etgen, T, Muehlau, M, Gaser, C, and Sander, D. Bilateral grey-matter increase in the putamen in primary blepharospasm. J Neurol Neurosurg Psychiatry. (2006) 77:1017–20. doi: 10.1136/jnnp.2005.087148
47. Delmaire, C, Vidailhet, M, Elbaz, A, Bourdain, F, Bleton, JP, Sangla, S, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer's cramp. Neurology. (2007) 69:376–80. doi: 10.1212/01.wnl.0000266591.49624.1a
48. Egger, K, Mueller, J, Schocke, M, Brenneis, C, Rinnerthaler, M, Seppi, K, et al. Voxel based morphometry reveals specific gray matter changes in primary dystonia. Mov Disord. (2007) 22:1538–42. doi: 10.1002/mds.21619
49. Obermann, M, Yaldizli, O, De Greiff, A, Lenard Lachenmayer, M, Buhl, AR, Tumczak, F, et al. Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord. (2007) 22:1117–23. doi: 10.1002/mds.21495
50. Martino, D, Di Giorgio, A, D'Ambrosio, E, Popolizio, T, Macerollo, A, Livrea, P, et al. Cortical gray matter changes in primary blepharospasm: a voxel-based morphometry study. Mov Disord. (2011) 26:1907–12. doi: 10.1002/mds.23724
51. Suzuki, Y, Kiyosawa, M, Wakakura, M, Mochizuki, M, and Ishii, K. Gray matter density increase in the primary sensorimotor cortex in long-term essential blepharospasm. Neuroimage. (2011) 56:1–7. doi: 10.1016/j.neuroimage.2011.01.081
52. Horovitz, SG, Ford, A, Najee-Ullah, MA, Ostuni, JL, and Hallett, M. Anatomical correlates of blepharospasm. Transl Neurodegener. (2012) 1:12. doi: 10.1186/2047-9158-1-12
53. Simonyan, K, and Ludlow, CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex. (2012) 22:417–25. doi: 10.1093/cercor/bhr120
54. Prell, T, Peschel, T, Koehler, B, Bokemeyer, MH, Dengler, R, Guenther, A, et al. Structural brain abnormalities in cervical dystonia. BMC Neurosci. (2013) 14:123. doi: 10.1186/1471-2202-14-123
55. Cerasa, A, Nistico, R, Salsone, M, Bono, F, Salvino, D, Morelli, M, et al. Neuroanatomical correlates of dystonic tremor: a cross-sectional study. Parkinsonism Relat Disord. (2014) 20:314–7. doi: 10.1016/j.parkreldis.2013.12.007
56. Ramdhani, RA, Kumar, V, Velickovic, M, Frucht, SJ, Tagliati, M, and Simonyan, K. What's special about task in dystonia? A voxel-based morphometry and diffusion weighted imaging study. Mov Disord. (2014) 29:1141–50. doi: 10.1002/mds.25934
57. Delnooz, CCS, Pasman, JW, and van de Warrenburg, BPC. Dynamic cortical gray matter volume changes after botulinum toxin in cervical dystonia. Neurobiol Dis. (2015) 73:327–33. doi: 10.1016/j.nbd.2014.10.013
58. Zeuner, KE, Knutzen, A, Granert, O, Goetz, J, Wolff, S, Jansen, O, et al. Increased volume and impaired function: the role of the basal ganglia in writer's cramp. Brain Behav. (2015) 5:e00301. doi: 10.1002/brb3.301
59. Kirke, DN, Battistella, G, Kumar, V, Rubien-Thomas, E, Choy, M, Rumbach, A, et al. Neural correlates of dystonic tremor: a multimodal study of voice tremor in spasmodic dysphonia. Brain Imaging Behav. (2017) 11:166–75. doi: 10.1007/s11682-016-9513-x
60. Mantel, T, Meindl, T, Li, Y, Jochim, A, Gora-Stahlberg, G, Kraeenbring, J, et al. Network-specific resting-state connectivity changes in the premotor-parietal axis in writer's cramp. Neuroimage Clin. (2018) 17:137–44. doi: 10.1016/j.nicl.2017.10.001
61. Mantel, T, Altenmueller, E, Li, Y, Meindl, T, Jochim, A, Lee, A, et al. Abnormalities in grey matter structure in embouchure dystonia. Parkinsonism Relat Disord. (2019) 65:111–6. doi: 10.1016/j.parkreldis.2019.05.008
62. Liu, J, Li, L, Chen, L, Liu, R, Jiang, Y, Fang, J, et al. Grey matter changes in Meige syndrome: a voxel-based morphology analysis. Sci Rep. (2020) 10:14533. doi: 10.1038/s41598-020-71479-9
63. Tomic, A, Agosta, F, Sarasso, E, Svetel, M, Kresojevic, N, Fontana, A, et al. Brain structural changes in focal dystonia-what about task specificity? A multimodal MRI study. Mov Disord. (2021) 36:196–205. doi: 10.1002/mds.28304
64. Albanese, A, Bhatia, K, Bressman, SB, Delong, MR, Fahn, S, Fung, VSC, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. (2013) 28:863–73. doi: 10.1002/mds.25475
65. Gibb, WRG, Lees, AJ, and Marsden, CD. Pathological report of four patients presenting with cranial dystonias. Mov Disord. (1998) 3:211–21. doi: 10.1002/mds.870030305
66. Tanabe, LM, Kim, CE, Alagem, N, and Dauer, WT. Primary dystonia: molecules and mechanisms. Nat Rev Neurol. (2009) 5:598–609. doi: 10.1038/nrneurol.2009.160
67. Luo, Y, Guo, Y, Zhong, L, Liu, Y, Dang, C, Wang, Y, et al. Abnormal dynamic brain activity and functional connectivity of primary motor cortex in blepharospasm. Eur J Neurol. (2022) 29:1035–43. doi: 10.1111/ene.15233
68. Nieuwhof, F, Toni, I, Dirkx, MF, Gallea, C, Vidailhet, M, Buijink, AWG, et al. Cerebello-thalamic activity drives an abnormal motor network into dystonic tremor. Neuroimage Clin. (2022) 33:102919. doi: 10.1016/j.nicl.2021.102919
69. Sarasso, E, Emedoli, D, Gardoni, A, Zenere, L, Canu, E, Basaia, S, et al. Cervical motion alterations and brain functional connectivity in cervical dystonia. Parkinsonism Relat Disord. (2024) 120:106015. doi: 10.1016/j.parkreldis.2024.106015
70. Giannì, C, Pasqua, G, Ferrazzano, G, Tommasin, S, De Bartolo, MI, Petsas, N, et al. Focal dystonia: functional connectivity changes in cerebellar-basal ganglia-cortical circuit and preserved global functional architecture. Neurology. (2022) 98:e1499–509. doi: 10.1212/wnl.0000000000200022
71. Liu, Y, Yang, L, Yan, H, Feng, C, Jiang, W, Li, W, et al. Increased functional connectivity coupling with supplementary motor area in blepharospasm at rest. Brain Res. (2023) 1817:148469. doi: 10.1016/j.brainres.2023.148469
72. Feng, L, Yin, D, Wang, X, Xu, Y, Xiang, Y, Teng, F, et al. Brain connectivity abnormalities and treatment-induced restorations in patients with cervical dystonia. Eur J Neurol. (2021) 28:1537–47. doi: 10.1111/ene.14695
73. Hok, P, Hvizdošová, L, Otruba, P, Kaiserová, M, Trnečková, M, Tüdös, Z, et al. Botulinum toxin injection changes resting state cerebellar connectivity in cervical dystonia. Sci Rep. (2021) 11:8322. doi: 10.1038/s41598-021-87088-z
74. Huang, XF, Hao, XQ, Yin, XX, Ren, L, Wang, D, Jin, F, et al. Functional connectivity alterations in the frontoparietal network and sensorimotor network are associated with behavioral heterogeneity in blepharospasm. Front Neurol. (2023) 14:1273935. doi: 10.3389/fneur.2023.1273935
75. Hart, MG, Polyhronopoulos, N, Sandhu, MK, and Honey, CR. Deep brain stimulation improves symptoms of spasmodic dysphonia through targeting of thalamic sensorimotor connectivity. Neurosurgery. (2024) 94:1291–300. doi: 10.1227/neu.0000000000002836
76. Mac Iver, CL, Tax, CMW, Jones, DK, and Peall, KJ. Structural magnetic resonance imaging in dystonia: a systematic review of methodological approaches and findings. Eur J Neurol. (2022) 29:3418–48. doi: 10.1111/ene.15483
77. Ramdhani, RA, and Simonyan, K. Primary dystonia: conceptualizing the disorder through a structural brain imaging lens. Tremor Other Hyperkinet Mov (N Y). (2013) 3:tre-03-152-3638-4. doi: 10.7916/d8h70dj7
78. Uddin, LQ, Nomi, JS, Hébert-Seropian, B, Ghaziri, J, and Boucher, O. Structure and function of the human insula. J Clin Neurophysiol. (2017) 34:300–6. doi: 10.1097/wnp.0000000000000377
79. Summerfield, C, Horing, B, and Büchel, C. The human insula processes both modality-independent and pain-selective learning signals. PLoS Biol. (2022) 20:e3001540. doi: 10.1371/journal.pbio.3001540
80. Medina Escobar, A, Pringsheim, T, Goodarzi, Z, and Martino, D. The prevalence of depression in adult onset idiopathic dystonia: systematic review and metaanalysis. Neurosci Biobehav Rev. (2021) 125:221–30. doi: 10.1016/j.neubiorev.2021.02.036
81. Medina Escobar, A, Martino, D, and Goodarzi, Z. The prevalence of anxiety in adult-onset isolated dystonia: a systematic review and meta-analysis. Eur J Neurol. (2021) 28:4238–50. doi: 10.1111/ene.15050
82. Listik, C, Listik, E, de Paiva Santos Rolim, F, Meneses Cury Portela, DM, Perez Lloret, S, de Alves Araújo, NR, et al. Development and validation of the dystonia-pain classification system: a multicenter study. Mov Disord. (2023) 38:1163–74. doi: 10.1002/mds.29423
83. Moayedi, M, Weissman-Fogel, I, Crawley, AP, Goldberg, MB, Freeman, BV, Tenenbaum, HC, et al. Contribution of chronic pain and neuroticism to abnormal forebrain gray matter in patients with temporomandibular disorder. Neuroimage. (2011) 55:277–86. doi: 10.1016/j.neuroimage.2010.12.013
84. Chen, Y, Cui, Q, Fan, Y-S, Guo, X, Tang, Q, Sheng, W, et al. Progressive brain structural alterations assessed via causal analysis in patients with generalized anxiety disorder. Neuropsychopharmacology. (2020) 45:1689–97. doi: 10.1038/s41386-020-0704-1
85. Andreescu, C, Butters, MA, Begley, A, Rajji, T, Wu, M, Meltzer, CC, et al. Gray matter changes in late life depression—a structural MRI analysis. Neuropsychopharmacology. (2007) 33:2566–72. doi: 10.1038/sj.npp.1301655
86. Sun, J, Tu, Z, Meng, D, Gong, Y, Zhang, M, and Xu, J. Interpretation for individual brain age prediction based on gray matter volume. Brain Sci. (2022) 12:1517. doi: 10.3390/brainsci12111517
87. Lotze, M, Domin, M, Gerlach, FH, Gaser, C, Lueders, E, Schmidt, CO, et al. Novel findings from 2, 838 adult brains on sex differences in gray matter brain volume. Sci Rep. (2019) 9:1671. doi: 10.1038/s41598-018-38239-2
88. Farokhian, F, Yang, C, Beheshti, I, Matsuda, H, and Wu, S. Age-related gray and white matter changes in Normal adult brains. Aging Dis. (2017) 8:899–909. doi: 10.14336/ad.2017.0502
89. Ruigrok, AN, Salimi-Khorshidi, G, Lai, MC, Baron-Cohen, S, Lombardo, MV, Tait, RJ, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. (2014) 39:34–50. doi: 10.1016/j.neubiorev.2013.12.004
90. Hervé, P-Y, Crivello, F, Perchey, G, Mazoyer, B, and Tzourio-Mazoyer, N. Handedness and cerebral anatomical asymmetries in young adult males. Neuroimage. (2006) 29:1066–79. doi: 10.1016/j.neuroimage.2005.08.031
91. Kavaklioglu, T, Guadalupe, T, Zwiers, M, Marquand, AF, Onnink, M, Shumskaya, E, et al. Structural asymmetries of the human cerebellum in relation to cerebral cortical asymmetries and handedness. Brain Struct Funct. (2016) 222:1611–23. doi: 10.1007/s00429-016-1295-9
92. Kang, SJ, Kang, KA, Jang, H, Lee, JY, Lee, KI, Kwoen, MS, et al. Brain morphology according to age, sex, and handedness. Ann Clin Neurophysiol. (2017) 19:93–100. doi: 10.14253/acn.2017.19.2.93
93. Wu, Y, Wang, T, Ding, Q, Li, H, Wu, Y, Li, D, et al. Cortical and subcortical structural abnormalities in patients with idiopathic cervical and generalized dystonia. Front Neuroimaging. (2022) 1:807850. doi: 10.3389/fnimg.2022.807850
94. Cercignani, M, Rzezak, P, Squarzoni, P, Duran, FL, de Toledo Ferraz Alves, T, Tamashiro-Duran, J, et al. Relationship between brain age-related reduction in gray matter and educational attainment. PLoS One. (2015) 10:e0140945. doi: 10.1371/journal.pone.0140945
95. Takao, H, Hayashi, N, and Ohtomo, K. Effects of the use of multiple scanners and of scanner upgrade in longitudinal voxel-based morphometry studies. J Magn Reson Imaging. (2013) 38:1283–91. doi: 10.1002/jmri.24038
96. Focke, NK, Helms, G, Kaspar, S, Diederich, C, Tóth, V, Dechent, P, et al. Multi-site voxel-based morphometry--not quite there yet. Neuroimage. (2011) 56:1164–70. doi: 10.1016/j.neuroimage.2011.02.029
97. Marchewka, A, Kherif, F, Krueger, G, Grabowska, A, Frackowiak, R, and Draganski, B. Influence of magnetic field strength and image registration strategy on voxel-based morphometry in a study of Alzheimer's disease. Hum Brain Mapp. (2014) 35:1865–74. doi: 10.1002/hbm.22297
98. Tardif, CL, Collins, DL, and Pike, GB. Regional impact of field strength on voxel-based morphometry results. Hum Brain Mapp. (2009) 31:943–57. doi: 10.1002/hbm.20908
99. Seiger, R, Hahn, A, Hummer, A, Kranz, GS, Ganger, S, Küblböck, M, et al. Voxel-based morphometry at ultra-high fields. A comparison of 7T and 3T MRI data. Neuroimage. (2015) 113:207–16. doi: 10.1016/j.neuroimage.2015.03.019
100. Panman, JL, To, YY, van der Ende, EL, Poos, JM, Jiskoot, LC, Meeter, LHH, et al. Bias introduced by multiple head coils in MRI research: an 8 channel and 32 channel coil comparison. Front Neurosci. (2019) 13:729. doi: 10.3389/fnins.2019.00729
101. Streitbürger, DP, Pampel, A, Krueger, G, Lepsien, J, Schroeter, ML, Mueller, K, et al. Impact of image acquisition on voxel-based-morphometry investigations of age-related structural brain changes. Neuroimage. (2014) 87:170–82. doi: 10.1016/j.neuroimage.2013.10.051
102. Yan, S, Qian, T, Maréchal, B, Kober, T, Zhang, X, Zhu, J, et al. Test-retest variability of brain morphometry analysis: an investigation of sequence and coil effects. Ann Transl Med. (2020) 8:12. doi: 10.21037/atm.2019.11.149
103. Zacà, D, Hasson, U, Minati, L, and Jovicich, J. Method for retrospective estimation of natural head movement during structural MRI. J Magn Reson Imaging. (2018) 48:927–37. doi: 10.1002/jmri.25959
104. Reuter, M, Tisdall, MD, Qureshi, A, Buckner, RL, van der Kouwe, AJW, and Fischl, B. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage. (2015) 107:107–15. doi: 10.1016/j.neuroimage.2014.12.006
105. Li, C, Liu, W, Guo, F, Wang, X, Kang, X, Xu, Y, et al. Voxel-based morphometry results in first-episode schizophrenia: a comparison of publicly available software packages. Brain Imaging Behav. (2020) 14:2224–31. doi: 10.1007/s11682-019-00172-x
106. De Bondt, T, Pullens, P, Van Hecke, W, Jacquemyn, Y, and Parizel, PM. Reproducibility of hormone-driven regional grey matter volume changes in women using SPM8 and SPM12. Brain Struct Funct. (2016) 221:4631–41. doi: 10.1007/s00429-016-1193-1
107. Popescu, V, Schoonheim, MM, Versteeg, A, Chaturvedi, N, Jonker, M, Xavier de Menezes, R, et al. Grey matter atrophy in multiple sclerosis: clinical interpretation depends on choice of analysis method. PLoS One. (2016) 11:e0143942. doi: 10.1371/journal.pone.0143942
108. Farokhian, F, Beheshti, I, Sone, D, and Matsuda, H. Comparing CAT12 and VBM8 for detecting brain morphological abnormalities in temporal lobe epilepsy. Front Neurol. (2017) 8:428. doi: 10.3389/fneur.2017.00428
109. Rajagopalan, V, and Pioro, EP. Disparate voxel based morphometry (VBM) results between SPM and FSL softwares in ALS patients with frontotemporal dementia: which VBM results to consider? BMC Neurol. (2015) 15:32. doi: 10.1186/s12883-015-0274-8
110. Eckert, MA, Tenforde, A, Galaburda, AM, Bellugi, U, Korenberg, JR, Mills, D, et al. To modulate or not to modulate: differing results in uniquely shaped Williams syndrome brains. Neuroimage. (2006) 32:1001–7. doi: 10.1016/j.neuroimage.2006.05.014
111. Radua, J, Canales-Rodríguez, EJ, Pomarol-Clotet, E, and Salvador, R. Validity of modulation and optimal settings for advanced voxel-based morphometry. Neuroimage. (2014) 86:81–90. doi: 10.1016/j.neuroimage.2013.07.084
112. Celle, S, Delon-Martin, C, Roche, F, Barthelemy, JC, Pepin, JL, and Dojat, M. Desperately seeking grey matter volume changes in sleep apnea: a methodological review of magnetic resonance brain voxel-based morphometry studies. Sleep Med Rev. (2016) 25:112–20. doi: 10.1016/j.smrv.2015.03.001
113. Barnes, J, Ridgway, GR, Bartlett, J, Henley, SM, Lehmann, M, Hobbs, N, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. (2010) 53:1244–55. doi: 10.1016/j.neuroimage.2010.06.025
114. Pell, GS, Briellmann, RS, Chan, CH, Pardoe, H, Abbott, DF, and Jackson, GD. Selection of the control group for VBM analysis: influence of covariates, matching and sample size. Neuroimage. (2008) 41:1324–35. doi: 10.1016/j.neuroimage.2008.02.050
115. Button, KS, Ioannidis, JP, Mokrysz, C, Nosek, BA, Flint, J, Robinson, ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. (2013) 14:365–76. doi: 10.1038/nrn3475
116. Fusar-Poli, P, Radua, J, Frascarelli, M, Mechelli, A, Borgwardt, S, Di Fabio, F, et al. Evidence of reporting biases in voxel-based morphometry (VBM) studies of psychiatric and neurological disorders. Hum Brain Mapp. (2014) 35:3052–65. doi: 10.1002/hbm.22384
117. Viviani, R, Beschoner, P, Ehrhard, K, Schmitz, B, and Thone, J. Non-normality and transformations of random fields, with an application to voxel-based morphometry. Neuroimage. (2007) 35:121–30. doi: 10.1016/j.neuroimage.2006.11.037
118. Radua, J, and Mataix-Cols, D. Meta-analytic methods for neuroimaging data explained. Biol Mood Anxiety Disord. (2012) 2:6. doi: 10.1186/2045-5380-2-6
Keywords: idiopathic dystonia, voxel-based morphometry, gray matter, coordinate-based meta-analysis, seed-based d mapping
Citation: Wang Z-Y, Chen F, Sun H-H, Li H-L, Hu J-B, Dai Z-Y and Wang S (2025) No reliable gray matter alterations in idiopathic dystonia. Front. Neurol. 16:1510115. doi: 10.3389/fneur.2025.1510115
Edited by:
Qing Ye, Nanjing Drum Tower Hospital, ChinaReviewed by:
Jie Lu, Capital Medical University, ChinaLiang Gong, Chengdu Second People’s Hospital, China
Copyright © 2025 Wang, Chen, Sun, Li, Hu, Dai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Wang, eWNzeV93c0AxNjMuY29t; Zhen-Yu Dai, eWNzeWR6eUAxNjMuY29t
†These authors have contributed equally to this work
 Zhen-Yu Wang
Zhen-Yu Wang Fei Chen
Fei Chen Hai-Hua Sun2†
Hai-Hua Sun2† Zhen-Yu Dai
Zhen-Yu Dai Shu Wang
Shu Wang