- 1Molecular Development of the Immune System Section, Laboratory of Immune System Biology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, United States
- 2Clinical Genomics Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, United States
- 3Immunology Graduate Group, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, United States
- 4Translational Neuroradiology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States
- 5Academic Unit of Neuropathology, Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, Oxford, United Kingdom
- 6Emory University MD/PhD Program, Emory University School of Medicine, Atlanta, GA, United States
- 7Department of Medical Genetics, University of British Columbia, Vancouver, BC, Canada
- 8Department of Medicine, Division of Neurology, University of British Columbia, Vancouver, BC, Canada
Background: Previous genetic and epidemiological studies have examined subpopulations from the Canadian Collaborative Project on Genetic Susceptibility to Multiple Sclerosis (CCPGSMS) patient cohort, but an encompassing analysis of the study population has not yet been carried out.
Objective: This retrospective study examines patterns of multiple sclerosis (MS) prevalence in 13,663 cohort members, including 4,821 persons with MS or suspected MS and 8,842 family members.
Methods: We grouped participants into epidemiologic subgroups based on age of MS onset, clinical stage at diagnosis, symptom type at disease onset, sex, proband status, disability as measured by the EDSS, and ancestry based on reported ethnicity.
Results: We observed a 2.7:1 MS prevalence ratio of women to men, though disease severity was greater for male patients. Variation in the age of disease onset between patients was only slightly associated with sex and strongly associated with disease type. Specific types of clinical symptoms at disease onset were associated with the prognosis. Regional residence did not correlate with disease onset, type, or severity.
Conclusion: Population trends, as presented here, are not explained by environmental factors alone, highlighting the need for a comprehensive genetic analysis to understand disease variance across families.
1 Introduction
Multiple sclerosis (MS) is the leading cause of non-traumatic brain injury in young adults and is characterized by immune-mediated damage to the myelin nerve sheath and central nervous system (CNS) degeneration (1). MS has varying forms and stages of the disease, including relapsing–remitting MS (RRMS, ~85% of cases), secondary-progressive MS (SPMS, ~60–70% of RRMS cases develop into this form), and primary-progressive MS (PPMS, ~10–15% of cases) (2). Many patients first present with an acute “clinically isolated syndrome” (CIS). If the patient’s initial magnetic resonance imaging (MRI) demonstrates CNS lesions, CIS will likely progress to relapsing disease (3). MS pathogenesis is believed to involve environmental and genetic factors (4, 5). While the increasing prevalence of MS (6) may be due to changes in diagnostic criteria and improvements in disease awareness in health systems around the world, alterations in environmental factors may also play a role. Distance from the equator, vitamin D deficiency, and infection with Epstein–Barr virus (EBV) are significant MS susceptibility factors (7–9). In the United States, the highest MS prevalence is in northeastern and midwestern states, generally at higher latitudes (10). Prevalence in Manitoba, Canada, is among the highest worldwide (11). The correlation between higher latitudes and increased risk of MS may involve decreased exposure to solar ultraviolet (UV) radiation (8). Reduced exposure to UV radiation was first proposed as an environmental susceptibility factor for MS to explain how vitamin D protects from MS (8). Vitamin D controls the promoter region of the human leukocyte antigen (HLA) allele HLA-DRB1, the main susceptibility locus for MS (12). The specific haplotype is HLA-DRB1*1501, dominant in Northern European populations (12). Like MS, EBV prevalence is also increased at higher latitudes and a risk factor for MS (13, 14). However, the specific pathogenic mechanisms of environmental factors are unknown, leading to interest in defining genetic factors.
The most strongly associated genetic risk factors identified with MS are certain HLA II genes, such as DQA1, DQB1, and DRB1 (15, 16). Genome-wide association studies (GWASs) have identified a host of genes, mainly immunological, that may contribute to MS (17). How these genes contribute to MS pathogenesis remains unknown. Given the persisting questions regarding MS etiology and pathogenesis, it is valuable to examine historical cohorts using modern epidemiological techniques and genomic analyses. One classic study of MS is the Canadian Collaborative Project on Genetic Susceptibility to Multiple Sclerosis (CCPGSMS), for which information is still publicly available (4, 18). A total of 13,663 patients and family members were included before enrollment ended. Exome sequencing analyses have been performed for a subset of CCPGSMS families, revealing candidate risk variants associated with MS (19, 20). Here, we provide information on population structure and disease etiology of the complete CCPGSMS cohort.
2 Methods
This study is a retrospective analysis of data assembled by the Canadian Collaborative Study Group. The investigation uncovered MS patients with pediatric onset of disease, families with multiplex MS disease, and MS co-occurring with other autoimmune diseases. Participants provided written informed consent under an IRB protocol approved by the Research Ethics Review Board (REB # H08-01669) at the University of British Columbia. Data were collected over 18 years, providing a large patient and family member cohort with thorough background information (18). Enrollment ended approximately 10 years ago. Genetic screens, geography questionnaires, and co-morbidity questionnaires were administered to all patients.
When the study first began in 1993, patients were diagnosed using the Poser criteria. These now obsolete criteria required evidence of two or more relapses lasting over 24 h, occurring a minimum of 1 month apart, in addition to evidence of CNS lesions (21). Where possible, patients and family members were later re-evaluated using the McDonald criteria, which requires evidence of CNS damage over time (22, 23). Patients and their families were categorized as “affected,” “unaffected, blood-related,” and “unaffected, married-in” based on disease status and relationship to the proband.
The investigators of the present study did not collect the original specimens or clinical information, which were de-identified for use in the present study. NIH reviewed the study and determined an IRB-approved protocol was not necessary because it did not meet the basic requirements to be considered human subject research. Data collected during the original CCPGSMS study were further divided by age of MS onset (i.e., age at date of diagnosis for clinical symptoms), clinical stage at diagnosis, symptom type at disease onset, sex, proband status, disability as measured by the EDSS, and ancestry based on reported ethnicity.
The mean ages of onset in different groups of patients were compared using the Kruskal-Wallis test. The differences in the types of symptoms at disease onset in different patient groups that were defined based on disability scores and clinical stages were tested using the chi-squared test for trend. All statistical analyses were performed with R (v4.2.1) in RStudio (v2023.06.1). The package ms.sev (v1.0.4) was used to perform global age-related MS severity (ARMSS) score calculations (24). We used ggplot2 (v3.3.6) and plotrix (v3.8.2) to generate plots and display the results of statistical tests. The distribution of age at MS onset was analyzed in 10-year increments (Table 1, Figure 1). Using cancensus (25) (v0.5.5) and tidycensus (v1.4.4), we mapped the probands across Canadian provinces. The package cancensus allowed us to access Canadian census data available via Statistics Canada (26).
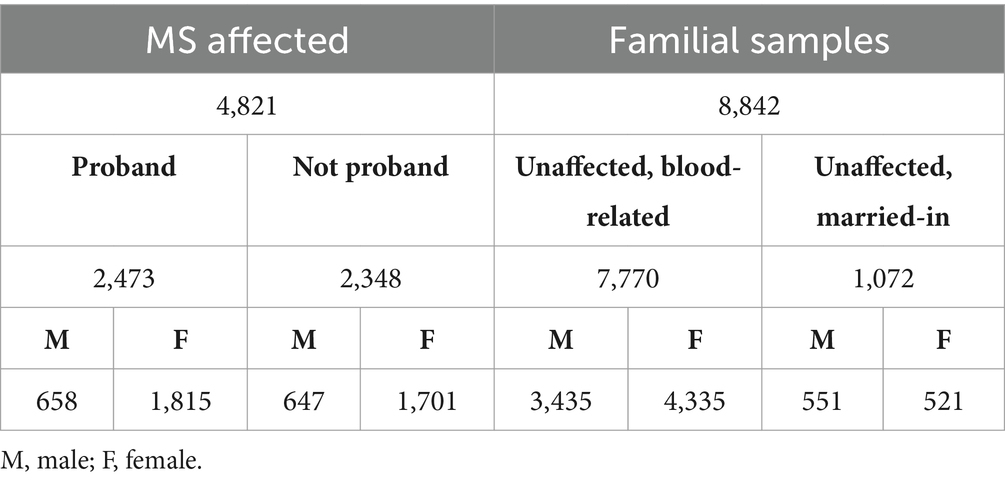
Table 1. Summary of population data included in the study, subdivided based on disease status, blood-relation or marriage status, proband status, and sex (n = 13,663).
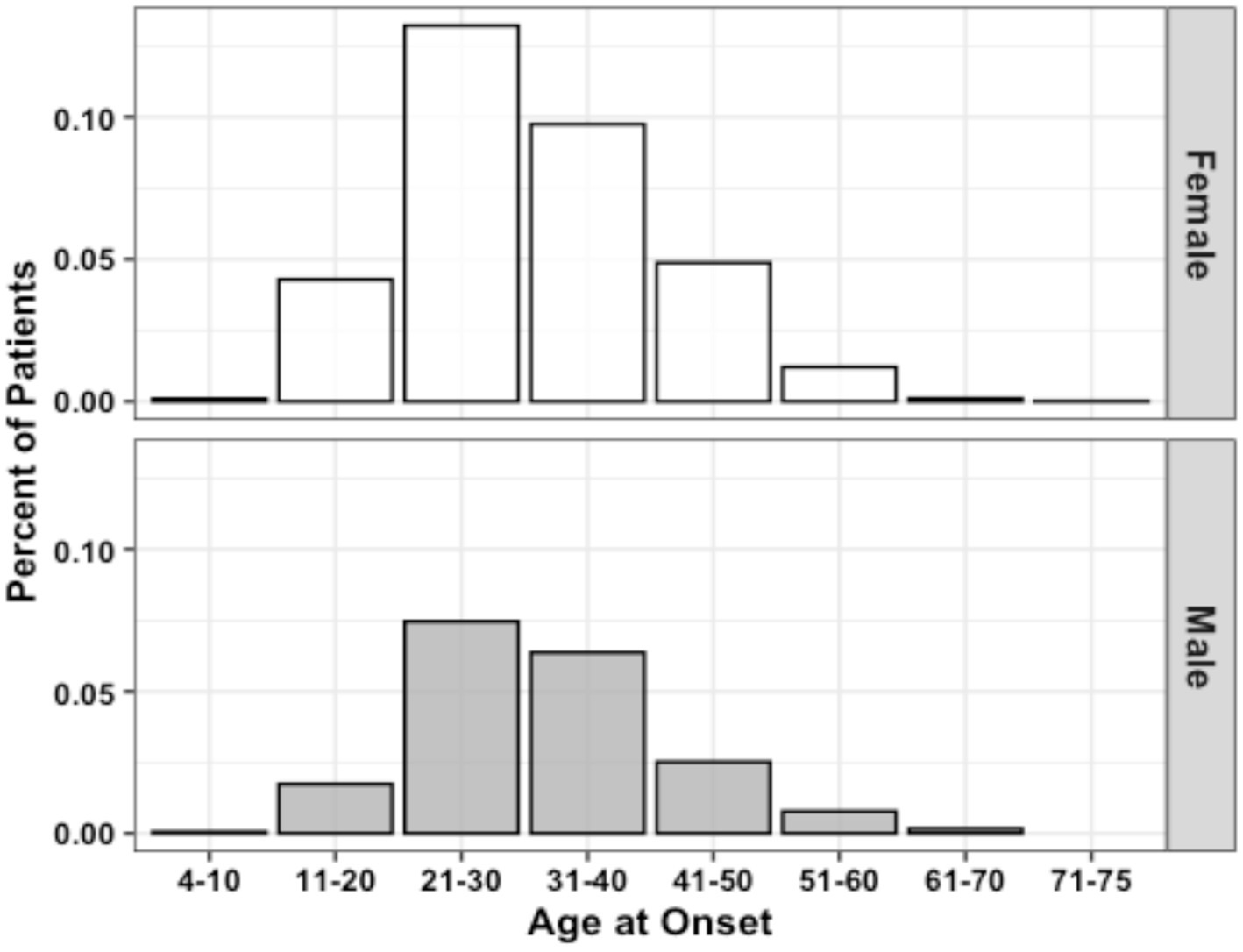
Figure 1. Age of MS onset distribution by sex (n = 3,787). Data represent all patients for whom age at initial diagnosis was provided.
EDSS scores were converted to ARMSS scores (24). This disability measure normalizes EDSS scores relative to disease duration to detect disability differences between different age groups. We used an ARMSS-based disability metric instead of the MS Severity Score (based on disease duration), as well as the EDSS (snapshot disability indicator), to represent disease severity status better since disease duration information can be incorrect due to the possibility of subclinical MS stages with varying periods. ARMSS scores were only calculated for patients with known age at EDSS collection available (n = 1,746). ARMSS scores were used to generate global ARMSS (gARMSS) scores for each patient using the age of data collection and EDSS data. Because sample sizes were not equal between the male and female proband groups, a Monte Carlo simulation was used to randomly sample 475 patients from the female proband group over a series of 10,000 iterations with the male population and calculate an average gARMSS within a sample size equal to that of the male proband group (n = 475).
3 Results
The population data gathered by the CCPGSMS represented a total of 13,663 individuals: 4,821 persons with MS (approximately 35%), subdivided into probands (51%) and affected relatives (49%), and 8,842 unaffected family members (Table 1). Patients included male = 27% and female = 73%. Unaffected family members were grouped by blood relation (88%) or marital status (12%) and again divided by sex (male = 45%, female = 55%) (Table 1). We found that although the sex ratio of unaffected individuals was comparable (females/males = 1.2), the affected individuals were skewed toward women (females/males = 2.7). We next examined patterns of MS incidence (Table 2, Figure 1), which was highest among individuals 21 to 30 years old, independent of sex (Figure 1, Supplementary Figure 1), followed by those aged 31 to 40 years. MS was extremely rare under the age of 10 (n = 7) and over 60 (n = 19), probably due to lower awareness of early- and late-onset MS at the time of the sample collection period. Detailed clinical and demographic characteristics of the study group are given in Supplementary Table S1.
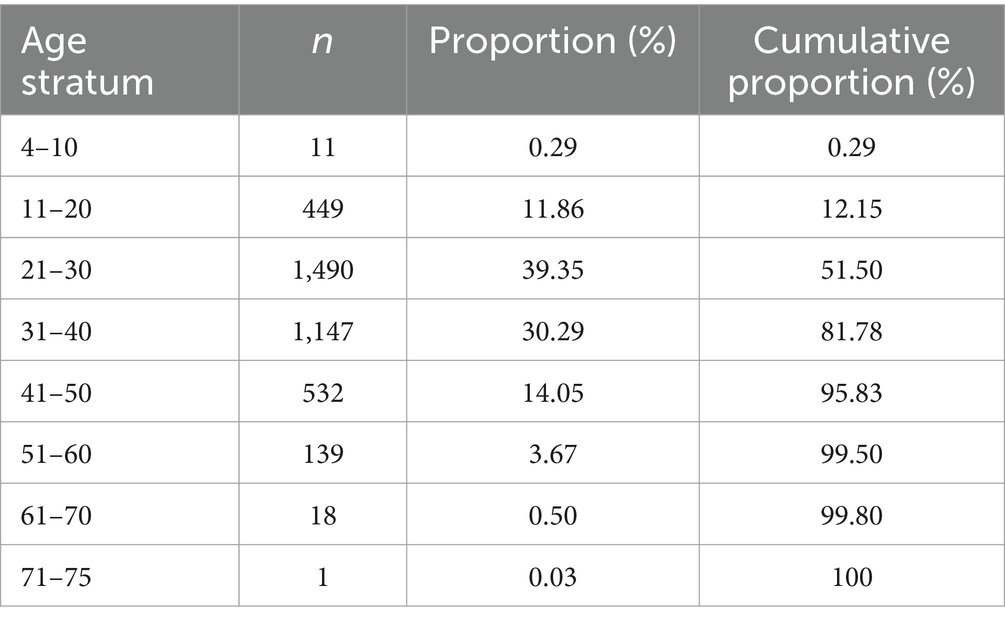
Table 2. Proportion of the affected population grouped by age at the time of MS diagnosis (n = 3,787).
RRMS was the most common clinical form (34.8%), followed by chronic-progressive MS (CPMS) (10.4%) and relapsing-progressive MS (RPMS) (7.5%). Benign diagnoses describing a less severe version of RRMS with few mild relapses were grouped with the larger RRMS group. SPMS (4.1%) and PPMS (1.7%) were the least frequently reported clinical forms. Though “chronic progressive” is no longer commonly used, we believe it is important to reflect the cohort demographics of the original study. We also did not have enough clinical information to associate CPMS data with more specific categories such as SPMS or PPMS. Nevertheless, we examined the similarities and differences among the three subgroups in terms of sex, age of onset, and disability scores. The female-to-male ratios in the CPMS, PPMS, and SPMS groups were 1.9, 1.4, and 2.75, respectively. The ratio of female patients was significantly higher in the SPMS group (73.4%) than in the CPMS (65.6%, p = 0.04) and PPMS (58.2%, p = 0.01) groups. The age of onset was significantly higher in the PPMS (39.2 ± 9.6) group than in the CPMS (34 ± 10.9, p < 0.0001) and SPMS (30.9 ± 8.8, p < 0.0001) groups. While there were no significant differences in EDSS scores at the last follow-up among the three progressive MS groups, the mean gARMSS score was significantly lower in the PPMS group (7.8 ± 2.2) compared with the CPMS (8.7 ± 1.6, p = 0.009) and SPMS (9.1 ± 1.1, p = 0.001) groups, probably due to the shorter follow-up time of the PPMS group (13.3 years vs. 19.8 in CPMS and 19.5 in SPMS). Patients with benign phenotypes were defined as having an EDSS score equal to or lower than 3.0 after 15 or more years from disease onset and having never received MS therapies (19, 27). Affected patient diagnoses that were unreported at the time of data collection were considered “unknown” (41.5%) (Figure 2). Disease type contributed strongly to the variation in age of onset between patients. Mean age of onset of patients with a progressive disease was significantly higher than that of patients with benign and relapsing forms of MS (both p < 0.0001).
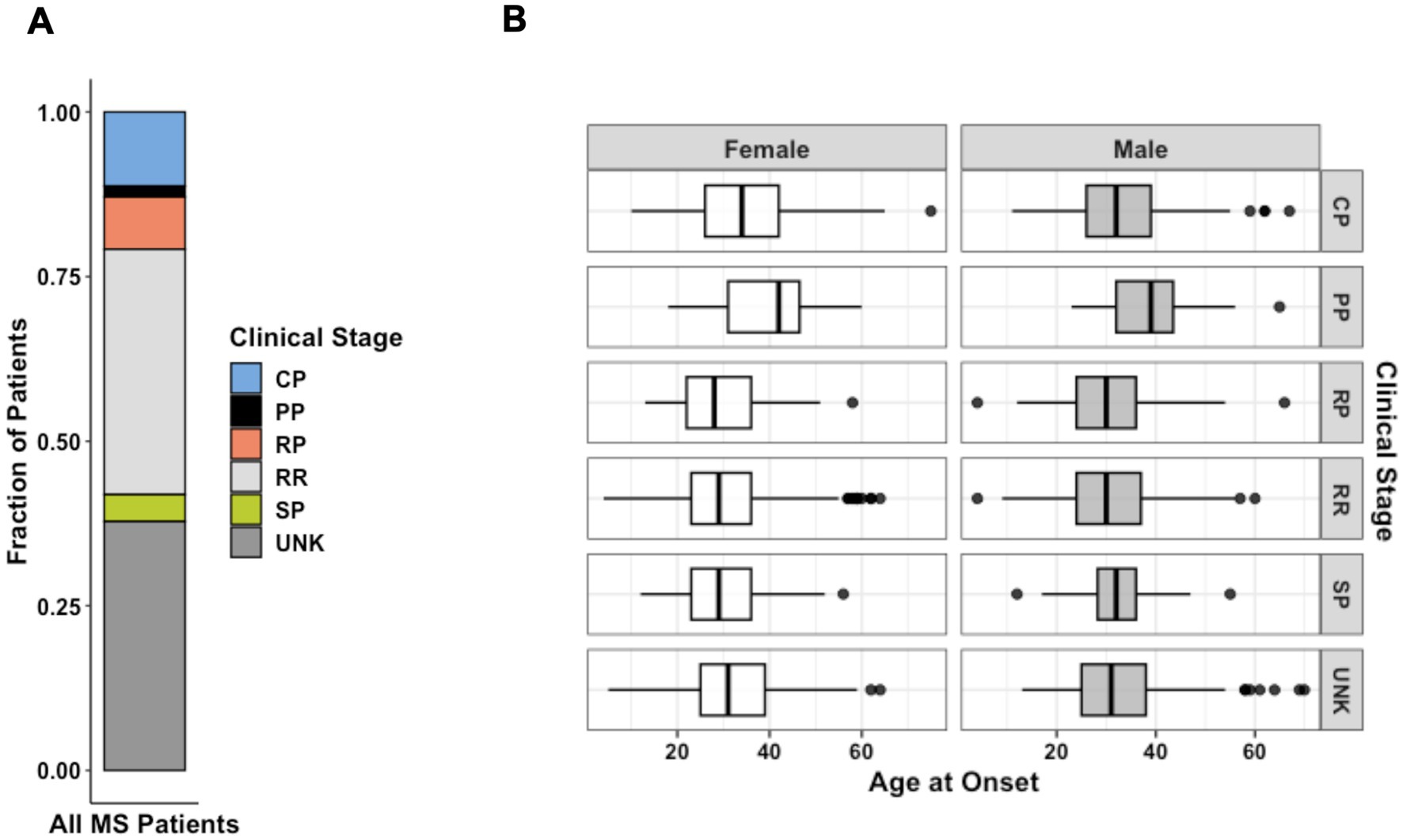
Figure 2. (A) Distribution of MS forms for all patients diagnosed as affected from the patient cohort (n = 4,821). (B) Faceted boxplots characterizing age of onset by sex and MS clinical stage at the time of data collection (n = 3,787). Data represent all patients for whom age at initial diagnosis was provided. CP, chronic-progressive; PP, primary- progressive; RP, relapsing-progressive; RR, relapsing–remitting; SP, secondary- progressive; UNK, Unknown.
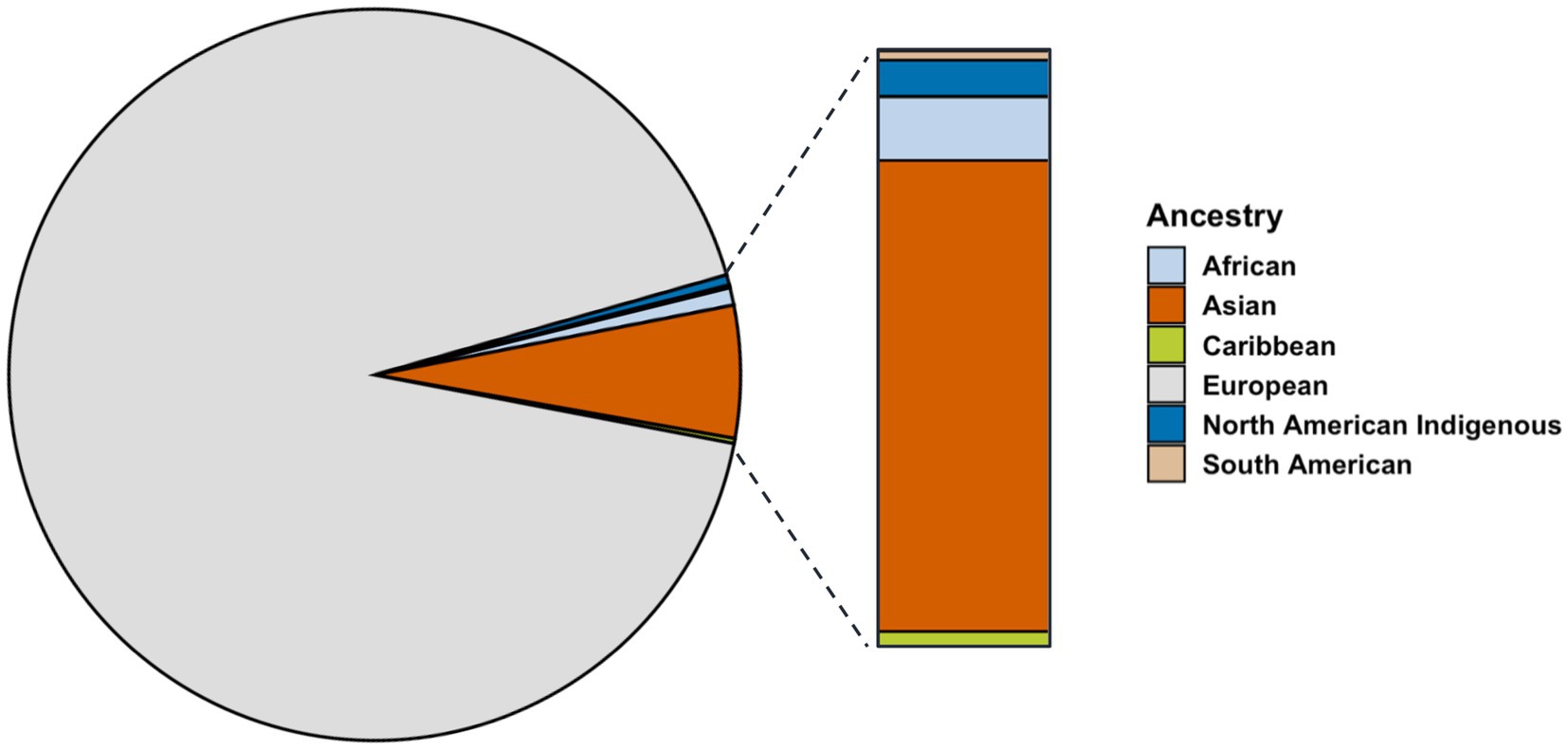
The ancestry breakdown was examined for proband patients for whom data were available (n = 905). Most participants reported ancestry from Europe (92.5%), far exceeding those reporting ancestry from Asia (5.7%), Africa (1%), and Indigenous North America (0.05%) (Figure 3). Reported ancestry did not significantly contributed to variation in age of onset (p = 0.06).
Residency at the time of data collection was also examined and plotted by province to show the distribution of proband patients across Canada as a dot density map (Figure 4A). The total population per province, as estimated by the 1996 Census, was indicated on the same dot density map to highlight the influence of geographic distribution on overall cohort demographics. Patients were mainly from Ontario (33.2%) and Quebec (17.7%), the most populous provinces, followed by British Columbia (15.2%), Nova Scotia (10.8%), and Saskatchewan (10.6%) (Figure 4A). There appeared to be an ascertainment bias toward patients recruited in British Columbia and Ontario, as the study was spearheaded in parallel by MS clinics in Vancouver and London, though we observed enrichments in probands (individuals with familial MS) in Nova Scotia and Saskatchewan (Figure 4B). Provincial residency did not appear to contribute significantly to variation in onset age.
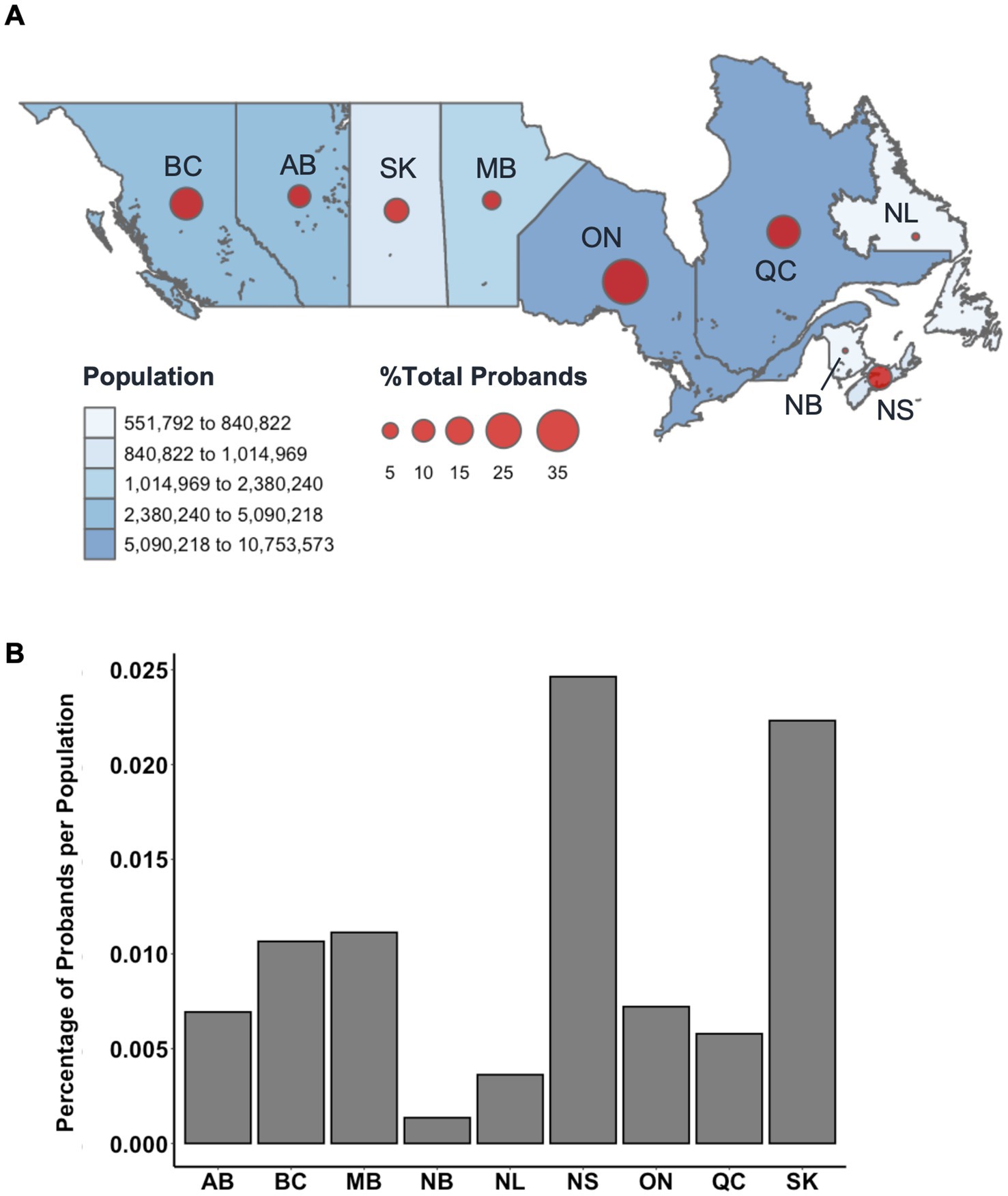
Figure 4. (A) Relative percentage distribution of proband patients by Canadian province (n = 2,372). Datapoints are proportionally sized to the relative percentage of probands and overlayed with total population per province as estimated by the 1996 Census (accessed via Statistics Canada). Probands from unknown provinces (n = 101) were excluded from this distribution. (B) Relative percentage of MS proband patients per province compared to the relative percentage of the total Canadian population per province. BC, British Columbia; AB, Alberta; SK, Saskatchewan; MB, Manitoba; ON, Ontario; QC, Quebec; NL, Newfoundland; NB, New Brunswick; NS, Nova Scotia.
To interpret differences between subsets of persons with MS, disability status was examined using ARMSS scores. Affected males had significantly higher gARMSS scores compared to affected females (p = 0.00095) (Figure 5A). To account for the discrepancy in sample size between male and female proband subsets, this sex-biased difference in disease severity was further corroborated by a probabilistic model with a Monte Carlo simulation generating 10,000 simulated data sets built from female proband EDSS data equaling the sample size of the male proband EDSS data (n = 475). The mean ARMSS of each simulated data set was compared to the mean gARMSS score of the male proband group (p < 2.2×10−16). Across all iterations of the simulation, the mean difference in gARMSS scores between groups remained small but constant, with male proband scores maintaining an average 0.53 increase over female proband scores (Figure 5B).
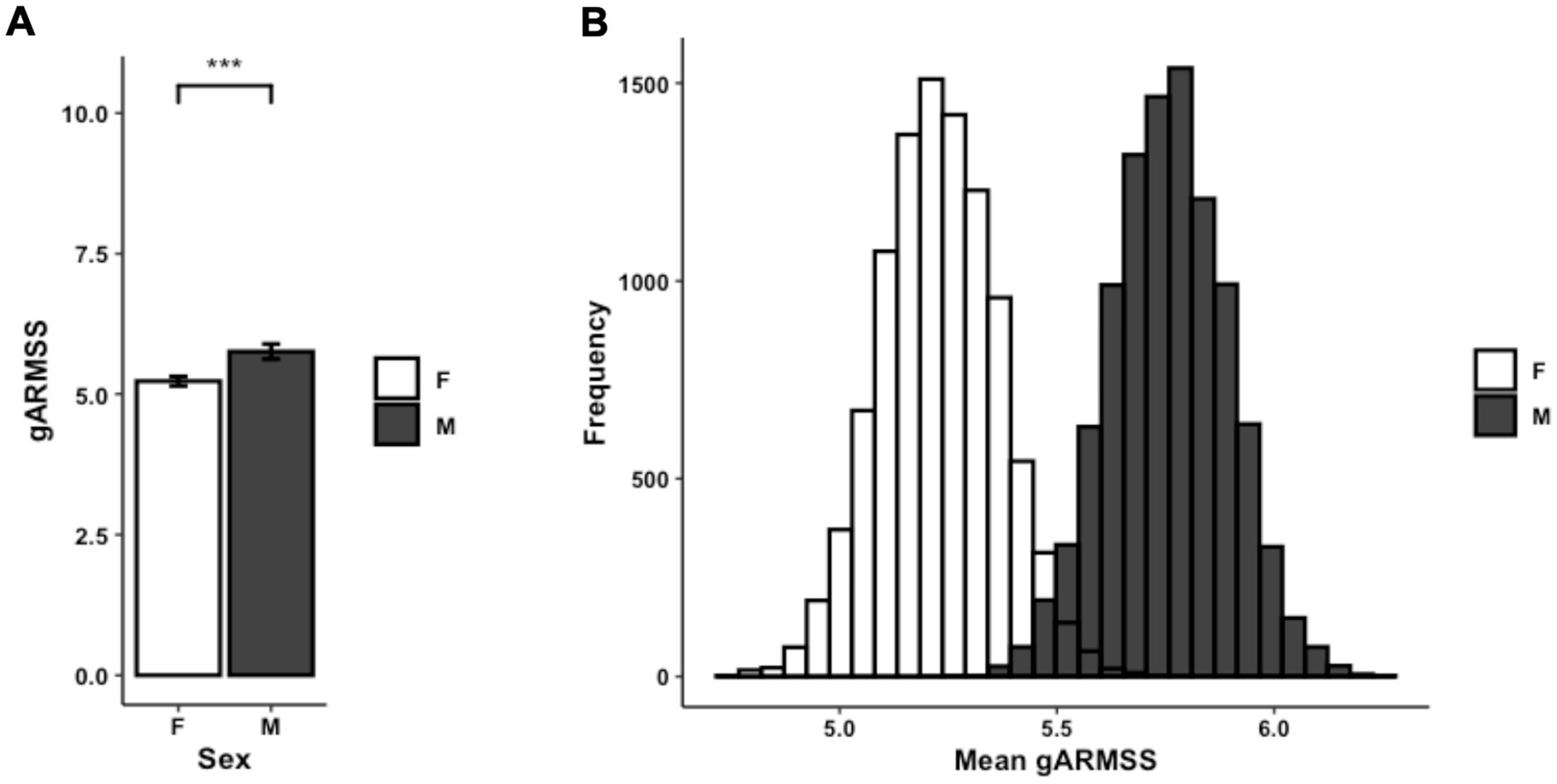
Figure 5. (A) Average gARMSS of proband patients by sex with error bars indicating standard of the mean (n = 1,746; p = 0.00095). Data represent all proband patients for whom EDSS at initial diagnosis was provided. Two-tailed paired Student t-test p-value indicate statistical significance (***, p < 0.001). (B) Histogram of gARMSS means of proband patients by sex, randomly sampled by Monte Carlo simulation with 10,000 iterations to equalize sample size. EDSS, Expanded Disability Status Scale; gARMSS, global age-related MS severity.
To assess associations between symptoms at disease onset and prognosis, patients with available gARMSS scores were stratified into two groups: those with a gARMSS score ≥ 5 (indicative of an unfavorable disease course, n = 1,264) and those with a gARMSS score < 5 (indicative of a favorable disease course, n = 495). We used this cutoff based on a previous study indicating that the cutoff well-distinguished the patients based on their disability statuses (28). Indeed, the mean gARMSS score in the current patient cohort was significantly higher in the unfavorable disease course group (p < 0.0001), showing that the disability scores of each group were not closely aggregated below and above the cut-off value of 5. It is important to note that the higher number of patients with a gARMSS score ≥ 5 than those with a gARMSS score < 5 is likely due to the long follow-up period (>18 years) of the cohort, given that the development of a progressive course is likely an age-related phenomenon (29). The symptoms at disease onset were categorized as follows: fatigue, diplopia, retrobulbar neuritis, balance problems, gait disturbances, upper limb ataxia, sensory-face, sensory-spinal cord, pain, motor-acute, motor-slow, transverse myelitis, bladder dysfunction, Lhermitte’s sign, and vertigo. Among individuals with available data on symptoms at disease onset, the correlation between the type of symptom and disease course was examined using the chi-squared test for trend, comparing the unfavorable and favorable course groups. A significantly higher proportion of patients in the unfavorable course group presented with gait problems and slow motor dysfunction at disease onset compared with those in the favorable course group (p = 0.0027 and p = 0.005, respectively), while sensory spinal cord symptoms were more prevalent at disease onset in the favorable course group (p = 0.017) (Figure 6A). Consistently, within the subgroup of patients with a well-defined disease course history (benign/relapsing–remitting with complete remission vs. definitive progressive course), a greater percentage of individuals with progressive MS exhibited balance problems (p = 0.022), gait disturbances (p = 0.012), and slow motor dysfunction (p = 0.026) at disease onset. Conversely, patients with a benign course more frequently manifested sensory spinal cord symptoms as their initial disease presentation (p = 0.04) (Figure 6B). Given the higher disease severity observed in male patients, we tested whether there was a difference between prognosis-associated symptoms at disease onset in males and females. This revealed that sensory spinal cord symptoms were significantly more frequent in females at disease onset (p < 0.0001). Other comparisons did not show a difference in severity that could shed light on MS pathogenesis. For example, disease severity in different provinces appeared to relate mainly to the population density of different provinces (Supplementary Figure 2).
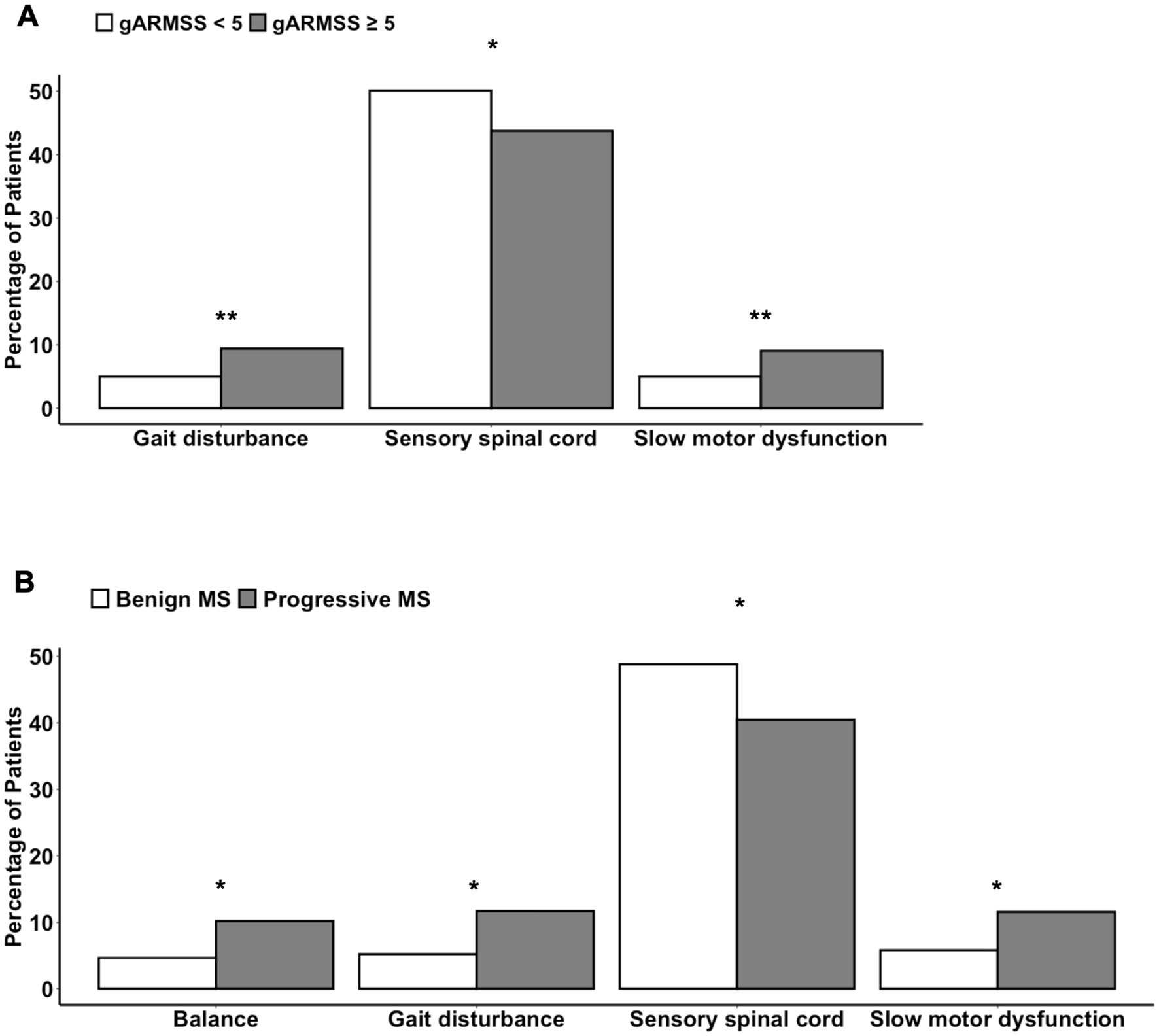
Figure 6. Differences in the symptoms at disease onset (A) between patients with a gARMSS score lower than 5 (favorable disease course) and those with a gARMSS score equal to higher than 5 (unfavorable disease course) and (B) between persons with a benign or progressive MS type. Chi-squared test for trend (*, p < 0.05; **, p < 0.01). gARMSS, global age-related MS severity.
4 Discussion
Our study population of 13,663 participants comprises one of the largest familial specimen sets assembled for an autoimmune disease. Patterns of MS incidence found in this cohort corroborate those examined in previous studies. Among CCPGSMS patients, peak incidence was observed between ages 21 and 40. In both males and females, persons with CPMS and PPMS were diagnosed at slightly older ages than those with other types of MS (Figure 3B), aligning with the notion that older age predisposes patients to more progressive phenotypes or that progression reflects the natural history of the disease as patients age. We also observed that RRMS was the most common form of MS; 82% of patients in this study were younger than 41 years at the time of diagnosis, which is consistent with existing reports (14, 30).
Over a third of proband patients reported residency in Ontario (32.7%). The remaining proband population was largely from Quebec (17.4%), British Columbia (16.7%), Nova Scotia (9.4%), and Saskatchewan (9.3%). Though the 1996 Census reported that less than 6% of the total population of Canada resided in Nova Scotia and Saskatchewan at the time, more proband patients of the CCPGSMS cohort were found to be residents of these provinces than residents of Alberta (7.9%) or Manitoba (5.2%). Studies of MS prevalence in Canada have reported the Canadian prairies (SK, AB, MB) as having the highest incidence rates of all regions (11). Proband incidences in this study were proportionally comparable to the relative populations of most Canadian provinces, apart from Alberta and Nova Scotia. Notably, the relative proportion of probands in Nova Scotia was three times greater than the relative proportion of Canadians residing in the province in 1996. It is also important to note that many rural communities have reduced access to medical care, which sometimes forces affected patients to move to urban centers for better access. Conversely, some patients with increased severity may be limited in their ability to move to major centers.
Increased disease severity in males was observed in the present cohort, validating previous observations that although women with RRMS relapse more frequently than men with RRMS, the latter group exhibits more rapid disability accumulation following each relapse (31) and thus a worse overall outcome. The ratio of affected females to affected males in our study is comparable to the ratio measured globally (32). This demographic imbalance in sex is relatively constant across age strata in this study and has been observed in numerous other cohorts. However, different hypotheses have been proposed to explain this distribution (33, 34). Regarding the differences in clinical presentation at onset, there are a limited number of studies investigating differences in attack locations between female and male persons with MS, yielding inconsistent results (35, 36). A comprehensive study involving 14,969 persons with MS examined the relationship between 49,279 phenotypically characterized MS attacks and associated demographic and clinical parameters (36), showing that sensory attacks are more commonly observed in female patients and associated with a non-progressive course. This is in line with our current observations that sensory spinal cord symptoms were more frequent in female patients and in those with a lower disability score and benign course, and male patients had higher disability scores. The reasons underlying the differences in clinical presentation and progression between sexes are currently unclear; however, cellular and animal studies have indicated possible molecular differences between sexes, involving differences in oligodendrocyte differentiation, remyelination capacity, or axonal vulnerability (37).
In addition to sex, ancestry is a risk factor for MS. Here, we report a skewed number of affected patients with ambiguous European ancestry, confounding risk attributable to proximity to the equator. This limitation is due to the biased geographical ascertainment in this study and the small sample size of proband patients for which complete demographic information was provided (n = 905). In our analysis, the sample size was strictly limited to proband patients for which all criteria (i.e., sex, age, clinical stage, ancestry, and residence) were available. Though most enrolled participants reported European ancestry, it is unclear whether this distribution is indicative of trends unique to the etiology of MS, as ~70% of Canadians are ethnically European (26). This ascertainment bias is a weakness of this dataset. Regional distance from the equator was previously shown to be a risk factor in MS, but alone, it does not determine disease status. Reduced vitamin D due to more limited sunlight exposure, rather than proximity to the equator, has been discussed as the relevant correlated risk factor. In rural areas where more populations engage in outdoor work such as farming, vitamin D exposure may not always accurately correlate with latitude (38). Confounding studies of MS prevalence in Canada have reported a higher incidence among patients from the Canadian Prairies, which are north of other Canadian regions but also experience the most average annual sunlight hours (39). Further, many countries across the world have seen an increase in the prevalence of MS, regardless of geographical location. Interestingly, more significant increases in female-to-male ratios are associated with a latitudinal gradient, with the ratio being more drastically skewed toward female persons with RRMS in northern latitudes (40). Findings such as these have led many researchers to reassess the idea of a latitudinal gradient as a causative factor, further suggesting that this gradient effect has been attenuated.
A major limitation of the current study is that MS diagnostic criteria have changed since the data were initially collected, resulting in non-identical, multilateral patient diagnoses and severity assessments. Another limitation is that the effect of disease-modifying treatments was not accounted for in the analysis of MS severity. Lastly, there is a possible selection bias in different sub-comparisons since we could not include the whole cohort in some analyses due to missing data—most notably, 41.5% of the patients had an unknown MS subtype. The next steps for this CCPGSMS cohort will be combining the population data with genomic DNA sequencing. As multiplex MS families are common in this cohort, we plan to focus on identifying high-risk genetic variants in MS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Research Ethics Review Board (REB # H08-01669) at the University of British Columbia. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
AP: Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. EE: Formal analysis, Writing – original draft, Writing – review & editing. SR: Formal analysis, Visualization, Writing – original draft. DR: Conceptualization, Writing – review & editing. AW: Writing – review & editing. DS: Data curation, Resources, Writing – review & editing. CV-G: Data curation, Resources, Writing – review & editing. ML: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Division of Intramural Research of NIAID and NINDS, NIH.
Acknowledgments
We thank Justin Gabrielski for critical reading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1509371/full#supplementary-material
References
1. Reich, DS, Lucchinetti, CF, and Calabresi, PA. Multiple Sclerosis. N Engl J Med. (2018) 378:169–80. doi: 10.1056/NEJMra1401483
2. Dutta, R, and Trapp, BD. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Curr Opin Neurol. (2014) 27:271–8. doi: 10.1097/WCO.0000000000000094
3. Doshi, A, and Chataway, J. Multiple sclerosis, a treatable disease. Clin Med. (2016) 16:s53–9. doi: 10.7861/clinmedicine.16-6-s53
4. Sadovnick, AD. Genetic background of multiple sclerosis. Autoimmun Rev. (2012) 11:163–6. doi: 10.1016/j.autrev.2011.05.007
5. Sadovnick, AD, Yee, IM, Criscuoli, M, and DeLuca, GC. Genes and environment in multiple sclerosis: impact of temporal changes in the sex ratio on recurrence risks. Mult Scler J. (2022) 28:359–68. doi: 10.1177/13524585211020221
6. Walton, C, King, R, Rechtman, L, Kaye, W, Leray, E, Marrie, RA, et al. Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS, third edition. Mult Scler. (2020) 26:1816–21. doi: 10.1177/1352458520970841
7. Sabel, CE, Pearson, JF, Mason, DF, Willoughby, E, Abernethy, DA, and Taylor, BV. The latitude gradient for multiple sclerosis prevalence is established in the early life course. Brain. (2021) 144:2038–46. doi: 10.1093/brain/awab104
8. Hayes, CE, Cantorna, MT, and DeLuca, HF. Vitamin D and multiple sclerosis. Exp Biol Med. (1997) 216:21–7. doi: 10.3181/00379727-216-44153A
9. Hedström, AK. Risk factors for multiple sclerosis in the context of Epstein-Barr virus infection. Front Immunol. (2023) 14:1212676. doi: 10.3389/fimmu.2023.1212676
10. Dilokthornsakul, P, Valuck, RJ, Nair, KV, Corboy, JR, Allen, RR, and Campbell, JD. Multiple sclerosis prevalence in the United States commercially insured population. Neurology. (2016) 86:1014–21. doi: 10.1212/WNL.0000000000002469
11. Marrie, RA, Yu, N, Blanchard, J, Leung, S, and Elliott, L. The rising prevalence and changing age distribution of multiple sclerosis in Manitoba. Neurology. (2010) 74:465–71. doi: 10.1212/WNL.0b013e3181cf6ec0
12. Handunnetthi, L, Ramagopalan, SV, and Ebers, GV. Multiple sclerosis, vitamin D, and HLA-DRB1*15. Neurology. (2010) 74:1905–10. doi: 10.1212/WNL.0b013e3181e24124
13. Disanto, G, Pakpoor, J, Morahan, JM, Hall, C, Meier, UC, Giovannoni, G, et al. Epstein-Barr virus, latitude and multiple sclerosis. Mult Scler. (2013) 19:362–5. doi: 10.1177/1352458512451942
14. Gbaguidi, B, Guillemin, F, Soudant, M, Debouverie, M, Mathey, G, and Epstein, J. Age-period-cohort analysis of the incidence of multiple sclerosis over twenty years in Lorraine, France. Sci Rep. (2022) 12:1001. doi: 10.1038/s41598-022-04836-5
15. Fogdell, A, Hillert, J, Sachs, C, and Olerup, O. The multiple sclerosis- and narcolepsy-associated HLA class II haplotype includes DRB5*0101 allele. Tissue Antigens. (1995) 46:333–6. doi: 10.1111/j.1399-0039.1995.tb02503.x
16. Alcina, A, Abad-Grau, MD, Fedetz, M, Izquierdo, G, Lucas, M, Fernandez, O, et al. Multiple sclerosis risk variant HLA-DRB1*1501 associates with high expression of DRB1 gene in different human populations. PLoS One. (2012) 7:e29819. doi: 10.1371/journal.pone.0029819
17. International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. (2019) 365:eaav7188. doi: 10.1126/science.aav7188
18. Sadovnick, AD, Risch, NJ, and Ebers, GC. Canadian collaborative project on genetic susceptibility to MS, phase 2: rationale and method. Can J Neurol Sci. (1998) 25:216–21. doi: 10.1017/S0317167100034041
19. Wang, Z, Sadovnick, AD, Traboulsee, AL, Ross, JP, Bernales, CQ, Encarnacion, M, et al. Nuclear Receptor NR1H3 in Familial Multiple Sclerosis. Neuron. (2016) 90:948–54. doi: 10.1016/j.neuron.2016.04.039
20. Sadovnick, AD, Traboulsee, AL, Zhao, Y, Bernales, CQ, Encarnacion, M, Ross, JP, et al. Genetic modifiers of multiple sclerosis progression, severity and onset. Clin Immunol. (2017) 180:100–5. doi: 10.1016/j.clim.2017.05.009
21. Poser, CM, Paty, DW, Scheinberg, L, McDonald, WI, Davis, FA, Ebers, GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. (1983) 13:227–31. doi: 10.1002/ana.410130302
22. McDonald, WI, Compston, A, Edan, G, Goodkin, D, Hartung, HP, Lublin, FD, et al. Recommended diagnostic criteria for MS: guidelines from the international panel on the diagnosis of MS. Ann Neurol. (2001) 50:121–7. doi: 10.1002/ana.1032
23. Thompson, AJ, Banwell, BL, Barkhof, F, Carroll, WM, Coetzee, T, Comi, G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2017) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2
24. Manouchehrinia, A, Westerlind, H, Kingwell, E, Zhu, F, Carruthers, R, Ramanujam, R, et al. Age related multiple sclerosis severity score: disability ranked by age. Mult Scler. (2017) 23:1938–46. doi: 10.1177/1352458517690618
25. Von Bergmann, J, Jacobs, A, and Shkolnik, D. (2022). Cancensus: R package to access, retrieve, and work with Canadian Census data and geography. Available at: https://mountainmath.github.io/cancensus/ (Accessed January 21, 2023).
26. Statistics Canada. (2022). Broad age groups, gender and census year: Canada, provinces and territories. Available at: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=9810003601 (Accessed August 21, 2023).
27. Amato, MP, Zipoli, V, Goretti, B, Portaccio, E, de Caro, MF, Ricchiuti, L, et al. Benign multiple sclerosis. J Neurol. (2006) 253:1054–9. doi: 10.1007/s00415-006-0161-8
28. Everest, E, Uygunoglu, U, Tutuncu, M, Bulbul, A, Onat, UI, Unal, M, et al. Prospective outcome analysis of multiple sclerosis cases reveals candidate prognostic cerebrospinal fluid markers. PLoS One. (2023) 18:e0287463. doi: 10.1371/journal.pone.0287463
29. Tutuncu, M, Tang, J, Zeid, NA, Kale, N, Crusan, DJ, Atkinson, EJ, et al. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler J. (2013) 19:188–98. doi: 10.1177/1352458512451510
30. Westerlind, H, Ramanujam, R, Uvehag, D, Kuja-Halkola, R, Boman, M, Bottai, M, et al. Modest familial risks for multiple sclerosis: a registry-based study of the population of Sweden. Brain. (2014) 137:770–8. doi: 10.1093/brain/awt356
31. Ribbons, KA, McElduff, P, Boz, C, Trojano, M, Izquierdo, G, Duquette, P, et al. Male sex is independently associated with faster disability accumulation in relapse-onset MS but not in primary progressive MS. PLoS One. (2015) 10:e0122686. doi: 10.1371/journal.pone.0122686
32. O’Connell, K, Tubridy, N, Hutchinson, M, and McGuigan, C. Incidence of multiple sclerosis in the Republic of Ireland: a prospective population-based study. Mult Scler Relat Disord. (2017) 13:75–80. doi: 10.1016/j.msard.2017.02.010
33. Dunn, SE, Lee, H, Pavri, FR, and Zhang, MA. Sex-based differences in multiple sclerosis (part I): biology of disease incidence. Curr Top Behav Neurosci. (2015) 26:29–56. doi: 10.1007/7854_2015_371
34. Teuscher, C, Noubade, R, Spach, K, McElvany, B, Bunn, JY, Fillmore, PD, et al. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc Natl Acad Sci USA. (2006) 103:8024–9. doi: 10.1073/pnas.0600536103
35. Tsantes, E, Leone, MA, Curti, E, Cantello, R, Vecchio, D, and Granella, F. Location of first attack predicts the site of subsequent relapses in multiple sclerosis. J Clin Neurosci. (2020) 74:175–9. doi: 10.1016/j.jocn.2020.02.017
36. Kalincik, T, Buzzard, K, Jokubaitis, V, Trojano, M, Duquette, P, Izquierdo, G, et al. Risk of relapse phenotype recurrence in multiple sclerosis. Mult Scler J. (2014) 20:1511–22. doi: 10.1177/1352458514528762
37. Marin-Husstege, M, Muggironi, M, Raban, D, Skoff, RP, and Casaccia-Bonnefil, P. Oligodendrocyte progenitor proliferation and maturation is differentially regulated by male and female sex steroid hormones. Dev Neurosci. (2004) 26:245–54. doi: 10.1159/000082141
38. Kearns, PK, Paton, M, O’Neill, M, Waters, C, Colville, S, McDonald, J, et al. Regional variation in the incidence rate and sex ratio of multiple sclerosis in Scotland 2010-2017: findings from the Scottish multiple sclerosis register. J Neurol. (2019) 266:2376–86. doi: 10.1007/s00415-019-09413-x
39. Beck, CA, Metz, LM, Svenson, LW, and Patten, SB. Regional variation of multiple sclerosis in Canada. Mult Scler. (2005) 11:516–9. doi: 10.1191/1352458505ms1192oa
Keywords: epidemiology, demographics, multiple sclerosis, population trends, prognosis, risk factors
Citation: Pagalilauan AM, Everest E, Rachimi S, Reich DS, Waldman AD, Sadovnick AD, Vilarino-Guell C and Lenardo MJ (2025) The Canadian collaborative project on genetic susceptibility to multiple sclerosis cohort population structure and disease etiology. Front. Neurol. 16:1509371. doi: 10.3389/fneur.2025.1509371
Edited by:
Achim Berthele, University Hospital Rechts der Isar, Technical University of Munich, GermanyReviewed by:
Joachim Havla, Ludwig Maximilian University of Munich, GermanyAksel Siva, Istanbul University Cerrahpasa, Türkiye
Copyright © 2025 Pagalilauan, Everest, Rachimi, Reich, Waldman, Sadovnick, Vilarino-Guell and Lenardo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael J. Lenardo, bWxlbmFyZG9AbmlhaWQubmloLmdvdg==
 Alison M. Pagalilauan1,2
Alison M. Pagalilauan1,2 Elif Everest
Elif Everest Daniel S. Reich
Daniel S. Reich A. Dessa Sadovnick
A. Dessa Sadovnick Michael J. Lenardo
Michael J. Lenardo