
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 31 January 2025
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1501500
Background: Despite existing treatments of generalized myasthenia gravis (gMG), there remains a need for more effective therapies with fewer side effects. Telitacicept, targeting B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL), emerges as a potential novel therapy for gMG.
Case presentation: In our study, four patients with gMG to standard treatments underwent an 8-week course of telitacicept monotherapy. Post-treatment, all patients exhibited satisfactory improvements. The Myasthenia Gravis Foundation of America Quantitative Myasthenia Gravis (MGFA-QMG) scores, 15-item Myasthenia Gravis Quality of Life (MGQOL-15) scores, and MG-associated Activities of Daily Living (MG-ADL) scores showed a marked reduction, indicating decreased disease severity and enhanced quality of life. Additionally, immunological assessments revealed a decrease in CD19+B lymphocyte counts and acetylcholine receptor (AChR) antibodies. Only one patient reported a mild, transient injection reaction.
Conclusion: Favorable clinical improvement and mild adverse events for gMG in treated with telitacicept were observed. However, larger-scale and longer-term studies are necessary to confirm these results and fully establish the role of telitacicept in the treatment of gMG.
Myasthenia gravis (MG), an severe autoimmune neuromuscular disorder, predominantly manifests through skeletal muscle weakness and fatigability, impacting approximately 12.4 individuals per 100,000 population worldwide (1, 2). This condition is characterized by the production of pathogenic antibodies, most commonly targeting the acetylcholine receptors (AchR) or muscle-specific kinase (MuSK) at the neuromuscular junction, leading to impaired neuromuscular transmission (3). Initial therapeutic strategies for MG typically involve anticholinesterase inhibitors, such as pyridostigmine, which provide symptomatic relief but do not alter the underlying disease course (4). For rapid, albeit short-term improvement in clinical status, intravenous immunoglobulin (IVIG) and plasma exchange (PE) are frequently employed (5, 6). The long-term management of MG often necessitates the use of corticosteroids and other immunosuppressive agents like azathioprine, mycophenolate mofetil, and methotrexate (4). However, these traditional treatments, while effective in many cases, can lead to significant side effects and may not suffice for all patients (7, 8). Particularly challenging is the management of refractory generalized myasthenia gravis (gMG), which affects an estimated 10–15% of MG patients (9–12). These clinical challenges are compounded by increased healthcare utilization and a diminished quality of life for patients (13).
Telitacicept, a novel biologic agent, heralds a new era in the management of autoimmune diseases. This recombinant fusion protein, comprising the transmembrane activator and CAML interactor (TACI) receptor fused with the fragment crystallizable (Fc) region of human immunoglobulin G (IgG), operates by neutralizing the activities of B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL) (14). This dual blockade results in a significant reduction of circulating B cells, pro-inflammatory cytokines, and IgG levels, thereby modulating the immune response. In the realm of rheumatic diseases, telitacicept has demonstrated efficacy and safety in conditions such as systemic lupus erythematosus (SLE), neuromyelitis optica spectrum disorders, Sjogren’s syndrome, and autoimmune nephropathy (15–18). Its mechanism of action, targeting key pathways in the pathogenesis of autoimmune disorders, offers a unique therapeutic approach by concurrently inhibiting the proliferation and maturation of B and T lymphocytes (19). Despite these advances, the precise therapeutic efficacy of telitacicept in gMG remains an area of ongoing research and clinical interest, underscoring the need for further studies to elucidate its role in the treatment paradigm of this complex autoimmune disorder.
Therefore, in this context, we aim to share our experience of telitacicept in treating gMG in four cases. This report may provide a novel approach for patient management, contributing valuable insights to the evolving landscape of gMG treatment strategies.
Patient 1, a 67-year-old female, presented with a complex, progressive course of myasthenia gravis, initially diagnosed 5 years ago. Her initial symptomatology included right eyelid ptosis, which manifested acutely and displayed diurnal fluctuations, notably more pronounced in the evening and ameliorating with rest. This ocular manifestation led to a diagnosis of ocular MG, for which she commenced treatment with pyridostigmine, dosed at 60 mg thrice daily. Approximately 3 years after the initial diagnosis, the patient’s condition evolved, marked by the emergence of limb weakness and left eyelid ptosis, substantially impairing her vision. Despite therapeutic adjustments to the pyridostigmine regimen, these symptoms persisted. This refractory response necessitated the addition of oral prednisone (10 mg daily) and tacrolimus (3 mg daily), resulting in a gradual improvement of both limb weakness and eyelid ptosis. However, 4 years post-diagnosis, the patient experienced an exacerbation of her symptoms. This relapse was characterized by an intensified limb weakness, notably impacting her ability to perform tasks such as climbing stairs and lifting heavy objects. Concurrently, she developed new symptoms including hoarseness and dysphagia, predominantly with solid foods. These clinical manifestations prompted her admission to our hospital, where she was re-evaluated and diagnosed with gMG.
Upon admission, assessments revealed a Myasthenia Gravis Foundation of America Quantitative Myasthenia Gravis (MGFA-QMG) score of 19, indicative of moderate disease severity. Complementary to this, her 15-item Myasthenia Gravis Quality of Life (MGQOL-15) and MG-associated Activities of Daily Living (MG-ADL) scores were 20 and 15, respectively, reflecting a considerable impact on her quality of life and daily functioning. Diagnostic confirmation was obtained through an Enzyme-Linked Immunosorbent Assay (ELISA), which identified the presence of AChR antibodies in her blood, corroborating the diagnosis of MG. In light of her clinical presentation and laboratory findings, a decision was made to initiate treatment with telitacicept. This therapeutic transition involved discontinuing her existing regimen of tacrolimus and prednisone. The response to telitacicept was promising. Merely 2 weeks post-initiation, marked improvement in clinical symptoms was noted, particularly in swallowing difficulties and hoarseness. Objective measures supported this clinical observation; her MGFA-QMG, MGQOL-15, and MG-ADL scores decreased by 6, 4, and 3 points, respectively, The patient’s improvement continued positively; by the eighth injection, which was administered 7 weeks after the initial dose, further amelioration of symptoms was evident. The scores, as compared to the baseline, were marked reduced (MGFA-QMG by 17 points, MGQOL-15 by 20 points, and MG-ADL by 15 points). In response to these favorable outcomes, it was decided to discontinue the medication, transitioning the patient to a monitoring phase. During the one-month clinical follow-up, the patient’s condition remained stable, affirming the efficacy of the telitacicept treatment in managing her gMG symptoms.
Concurrently, three other patients, each with a diagnosis of gMG, were treated with telitacicept monotherapy. The clinical characteristics and medical history of these four individuals are comprehensively summarized in Table 1 and Figure 1. Echoing the experience of patient 1, patients 2, 3, and 4 exhibited notable improvements in their gMG clinical scores within the initial 2–4 weeks of starting telitacicept treatment. This rapid clinical response was characterized by a marked reduction in MGFA-QMG, MGQOL-15, and MG-ADL scores, reaching a peak around the fifth week of therapy. Notably, this enhanced clinical state was sustained, as demonstrated by the stable scores observed in these patients (Figure 2). Upon the conclusion of the 8-week telitacicept course, treatment was discontinued for all patients. Interestingly, during the one-month follow-up period post-telitacicept withdrawal, their gMG clinical scores remained unchanged, suggesting a sustained therapeutic effect of the drug (Figure 2). Patient 3 experienced a transient injection reaction, which manifested as a mild flu-like reaction with symptoms of fever, sore throat, and headache. These symptoms were self-limiting and resolved spontaneously without necessitating discontinuation of telitacicept. However, no other adverse event was observed, underscoring the tolerability of this therapeutic agent in the management of gMG.
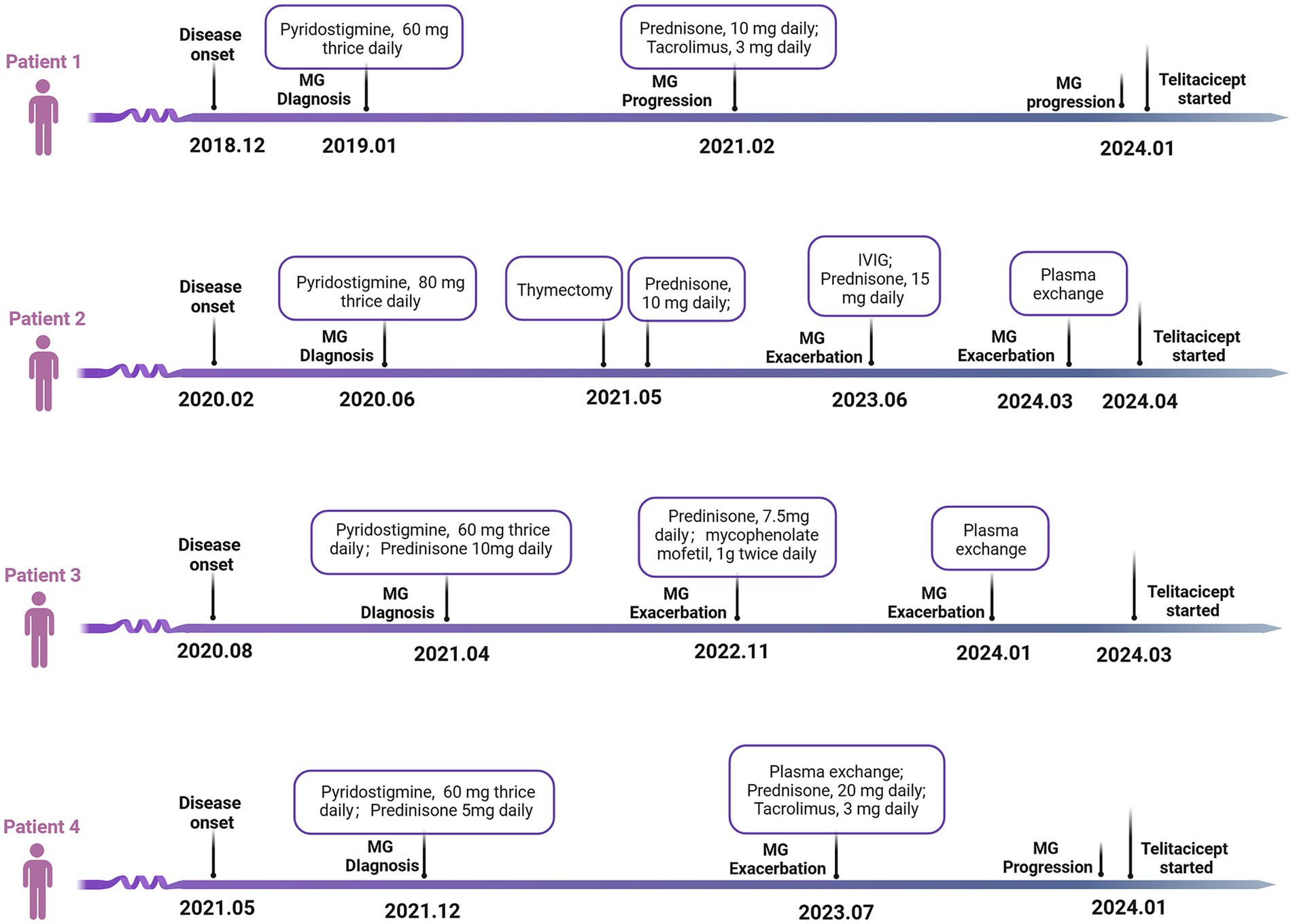
Figure 1. Medical history of the four refractory gMG patients. gMG, generalized myasthenia gravis; IVIG, intravenous immunoglobulin.

Figure 2. Clinical scale changes following telitacicept treatment from basline to week 12. (A) Change from baseline to week 12 in MGFA-QMG. (B) Change from baseline to week 12 in MGQOL-15. (C) Change from baseline to week 12 in MG-ADL. MGFA-QMG, Myasthenia Gravis Foundation of America Quantitative Myasthenia Gravis Score; MGQOL-15, 15-item Myasthenia Gravis Quality of Life Scale; MG-ADL, MG-associated Activities of Daily Living score.
In the course of routine clinical management, we conducted dynamic monitoring of key immunological parameters in these patients, including CD19+B lymphocyte counts, and levels of IgA, IgM, IgG, and AChR antibodies. These assessments, integral to our therapeutic protocol, were performed following the initiation of telitacicept treatment. Using flow cytometry, we observed a marked decrease in CD19+B lymphocytes as early as one-week post-initial telitacicept administration. This trend of declining CD19+B cell counts was consistent throughout the treatment duration, up to week 8 (Figure 3A). Parallel to the changes in CD19+B cells, we noted a weekly decrement in the concentrations of IgA, IgM, and IgG antibodies in the peripheral blood of all four patients. This pattern is indicative of the immunomodulatory effect of telitacicept (Figures 3B–D). Additionally, the levels of AChR antibodies, a pivotal marker in the management of GMG, were evaluated at four-week intervals. Consistent with the therapeutic goal, there was an overall downward trajectory in AChR antibody levels throughout the treatment period, further supporting the clinical effectiveness of telitacicept in these patients (Figure 3E).
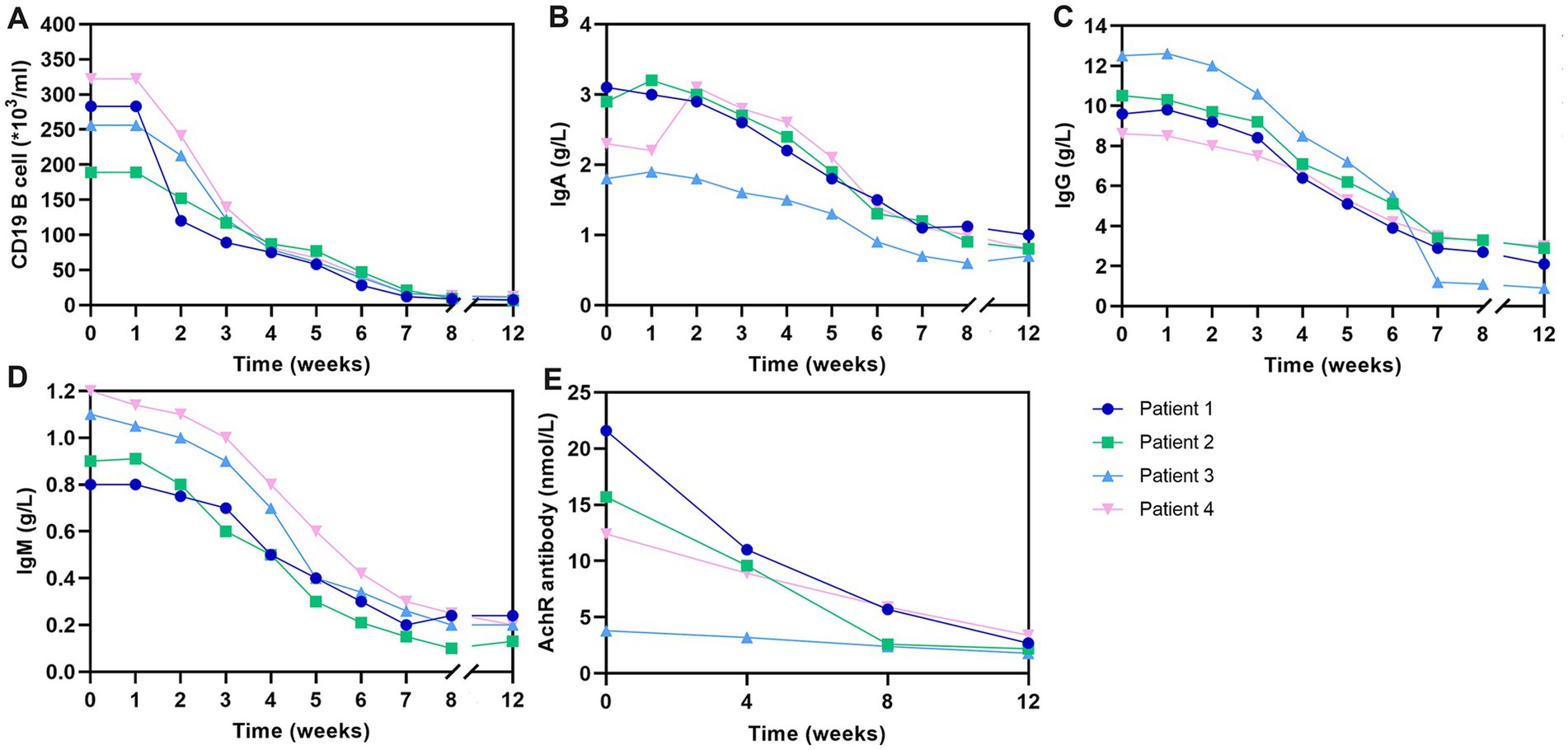
Figure 3. Change in CD19+ B cells and antibody levels over the 12-week study period. (A) Changes in CD19+ B-cell counts over the study period. (B) Changes in IgA levels over the study period. (C) Changes in IgG levels over the study period. (D) Changes in IgG levels over the study period. (E) Changes in AChR levels over the study period. AChR, acetylcholine receptor; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M.
In this case series, we explored the clinical efficacy of telitacicept in the treatment of gMG, a novel approach in the therapeutic landscape of this autoimmune disorder. Our study involved four patients, each with distinct manifestations of gMG, all of whom showed significant improvements following an 8-week course of telitacicept monotherapy. This was reflected in marked reductions in gMG clinical scores, including the MGFA-QMG score, and improvements in patients’ quality of lives, as measured by the MGQOL-15 and MG-ADL scores. These findings suggest that telitacicept not only reduces disease severity but also improves functional capacity, making it a promising alternative to traditional corticosteroid therapy, which is often associated with adverse side effects (7, 8).
Our findings align with three published case reports describing treatment with telitacicept. For instance, a 75-year-old female with gMG showed significant improvement in both clinical symptoms and MG scores following treatment with telitacicept (20). Similarly, two other patients—one with concurrent gMG and rheumatoid arthritis, and another with an eight-year history of gMG—also experienced substantial symptom reductions after receiving the same therapy (21). These observations are further supported by a recent phase II randomized controlled trial, which confirmed that telitacicept effectively and rapidly alleviates gMG symptoms over a six-month period, while maintaining a favorable safety profile (22).
The safety profile of telitacicept observed in our study was favorable, with only one patient experiencing a mild, transient injection site reaction. Immunologically, telitacicept led to a decrease in CD19 ± B lymphocyte counts and reduced levels of IgA, IgM, IgG, and AChR antibodies. These changes align with the drug’s mechanism of action, which involves neutralizing BLyS and APRIL to suppress autoimmune humoral immunity and lower autoimmune antibody levels. However, the reduction in IgG levels observed in our study raises concerns about potential infection risks. To mitigate this risk, we recommend regular immune function monitoring and vaccination before starting treatment, particularly for influenza and pneumonia.
Recent advances in the treatment of gMG have provided more targeted therapeutic options. Agents like complement inhibitors and neonatal Fc receptor blockers offer promising approaches due to their specificity in targeting disease pathways (5, 23, 24). B-cell depletion therapies, such as rituximab, have demonstrated efficacy in refractory gMG (25–27), though its effect on reducing steroid dependence in mild-to-moderate cases remains limited (28). Targeting key cytokines like B-cell activating factor (BAFF or BLyS), which is crucial for B-cell survival, is another strategy gaining attention. While belimumab, a BAFF inhibitor, has shown efficacy in systemic lupus erythematosus (29), its benefit in gMG is uncertain, as demonstrated by a phase II trial (30). These developments underscore the complexity of gMG treatment and the need for continued exploration of more targeted therapies. Our findings suggest that telitacicept could be particularly valuable for gMG who have not responded to conventional therapies or are unable to tolerate their adverse effects. However, given the limitations of our study, including the small sample size and short follow-up, further research is essential to better define its role in clinical practice.
In conclusion, telitacicept demonstrates promising efficacy in treating gMG, improving both clinical scores and quality of life while maintaining a favorable safety profile in the short term. For clinicians, these findings provide valuable guidance when considering telitacicept as a treatment option, particularly for gMG patients who are unresponsive to or intolerant of traditional therapies. However, further research with larger cohorts and longer follow-up is essential to establish the long-term safety and efficacy of telitacicept in gMG patients.
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of Peking University People’s Hospital (Approval number: 2023PHB402-006). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) and/or their next of kin for the publication of any potentially identifiable images or data included in this article.
XS: Validation, Writing – original draft. YH: Data curation, Visualization, Writing – original draft. HJ: Writing – original draft. YY: Writing – original draft. YS: Writing – original draft, Writing – review & editing. ZZ: Conceptualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Nos. 72304012 and 81303013).
We would like to thank all the participants for their support and contribution.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gilhus, NE, Tzartos, S, Evoli, A, Palace, J, Burns, TM, and Verschuuren, J. Myasthenia gravis. Nat Rev Dis Prim. (2019) 5:30. doi: 10.1038/s41572-019-0079-y
2. Salari, N, Fatahi, B, Bartina, Y, Kazeminia, M, Fatahian, R, Mohammadi, P, et al. Global prevalence of myasthenia gravis and the effectiveness of common drugs in its treatment: a systematic review and meta-analysis. J Transl Med. (2021) 19:516. doi: 10.1186/s12967-021-03185-7
3. Gilhus, NE, and Verschuuren, JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. (2015) 14:1023–36. doi: 10.1016/S1474-4422(15)00145-3
5. Howard, JF, Utsugisawa, K, Benatar, M, Murai, H, Barohn, RJ, Illa, I, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. (2017) 16:976–86. doi: 10.1016/S1474-4422(17)30369-1
6. Silvestri, NJ, and Wolfe, GI. Treatment-refractory myasthenia gravis. J Clin Neuromuscul Dis. (2014) 15:167–78. doi: 10.1097/CND.0000000000000034
7. Basta, IZ, Pekmezovic, TD, Peric, SZ, Kisic-Tepavcevic, DB, Rakocevic-Stojanovic, VM, Stevic, ZD, et al. Assessment of health-related quality of life in patients with myasthenia gravis in Belgrade (Serbia). Neurol Sci. (2012) 33:1375–81. doi: 10.1007/s10072-012-1170-2
8. Sanders, DB, Wolfe, GI, Benatar, M, Evoli, A, Gilhus, NE, Illa, I, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. (2016) 87:419–25. doi: 10.1212/WNL.0000000000002790
9. Suh, J, Goldstein, JM, and Nowak, RJ. Clinical characteristics of refractory myasthenia gravis patients. Yale J Biol Med. (2013) 86:255–60.
10. Anderson, D, Phan, C, Johnston, WS, and Siddiqi, ZA. Rituximab in refractory myasthenia gravis: a prospective, open-label study with long-term follow-up. Ann Clin Transl Neurol. (2016) 3:552–5. doi: 10.1002/acn3.314
11. Silvestri, NJ, and Wolfe, GI. Rituximab in treatment-refractory myasthenia gravis. JAMA Neurol. (2017) 74:21–3. doi: 10.1001/jamaneurol.2016.4367
12. Tran, C, Biswas, A, Mendoza, M, Katzberg, H, Bril, V, and Barnett, C. Performance of different criteria for refractory myasthenia gravis. Eur J Neurol. (2021) 28:1375–84. doi: 10.1111/ene.14675
13. Engel-Nitz, NM, Boscoe, A, Wolbeck, R, Johnson, J, and Silvestri, NJ. Burden of illness in patients with treatment refractory myasthenia gravis. Muscle Nerve. (2018) 58:99–105. doi: 10.1002/mus.26114
14. Fan, Y, Gao, D, and Zhang, Z. Telitacicept, a novel humanized, recombinant TACI-fc fusion protein, for the treatment of systemic lupus erythematosus. Drugs Today. (2022) 58:23–32. doi: 10.1358/dot.2022.58.1.3352743
15. Chen, R, Fu, R, Lin, Z, Huang, C, and Huang, W. The efficacy and safety of telitacicept for the treatment of systemic lupus erythematosus: a real life observational study. Lupus. (2023) 32:94–100. doi: 10.1177/09612033221141253
16. Ding, J, Jiang, X, Cai, Y, Pan, S, Deng, Y, Gao, M, et al. Telitacicept following plasma exchange in the treatment of subjects with recurrent neuromyelitis optica spectrum disorders: a single-center, single-arm, open-label study. CNS Neurosci Ther. (2022) 28:1613–23. doi: 10.1111/cns.13904
17. Xu, D, Fang, J, Zhang, S, Huang, C, Huang, C, Qin, L, et al. Efficacy and safety of telitacicept in primary Sjögren's syndrome: a randomized, double-blind, placebo-controlled, phase 2 trial. Rheumatology. (2023) 63:698–705. doi: 10.1093/rheumatology/kead265
18. Cai, J, Gao, D, Liu, D, and Liu, Z. Telitacicept for autoimmune nephropathy. Front Immunol. (2023) 14:1169084. doi: 10.3389/fimmu.2023.1169084
19. Dhillon, S. Telitacicept: first approval. Drugs. (2021) 81:1671–5. doi: 10.1007/s40265-021-01591-1
20. Guo, Q, Huang, Y, Wang, F, and Fang, L. Case report: telitacicept in severe myasthenia gravis: a case study with multiple autoantibodies. Front Immunol. (2023) 14:1270011. doi: 10.3389/fimmu.2023.1270011
21. Zhang, Z, Wang, Z, Du, X, Huang, X, and Zhang, Y. Refractory generalized myasthenia gravis treated successfully with telitacicept: two cases report. J Neurol. (2024) 271:584–8. doi: 10.1007/s00415-023-12036-y
22. Yin, J, Zhao, M, Xu, X, Zhang, M, Xu, Z, Li, Z, et al. A multicenter, randomized, open-label, phase 2 clinical study of telitacicept in adult patients with generalized myasthenia gravis. Eur J Neurol. (2024) 31:e16322. doi: 10.1111/ene.16322
23. Bril, V, Drużdż, A, Grosskreutz, J, Habib, AA, Mantegazza, R, Sacconi, S, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. (2023) 22:383–94. doi: 10.1016/S1474-4422(23)00077-7
24. Howard, JF, Bril, V, Vu, T, Karam, C, Peric, S, Margania, T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. (2021) 20:526–36. doi: 10.1016/S1474-4422(21)00159-9
25. Piehl, F, Eriksson-Dufva, A, Budzianowska, A, Feresiadou, A, Hansson, W, Hietala, MA, et al. Efficacy and safety of rituximab for new-onset generalized myasthenia gravis: the RINOMAX randomized clinical trial. JAMA Neurol. (2022) 79:1105–12. doi: 10.1001/jamaneurol.2022.2887
26. Castiglione, JI, Rivero, AD, Barroso, F, Brand, P, Lautre, A, and Kohler, AA. Long-term remission with low-dose rituximab in myasthenia gravis: a retrospective study. J Clin Neuromuscul Dis. (2022) 24:18–25. doi: 10.1097/CND.0000000000000420
27. Keung, B, Robeson, KR, DiCapua, DB, Rosen, JB, O'Connor, KC, Goldstein, JM, et al. Long-term benefit of rituximab in MuSK autoantibody myasthenia gravis patients. J Neurol Neurosurg Psychiatry. (2013) 84:1407–9. doi: 10.1136/jnnp-2012-303664
28. Nowak, RJ, Coffey, CS, Goldstein, JM, Dimachkie, MM, Benatar, M, Kissel, JT, et al. Phase 2 trial of rituximab in acetylcholine receptor antibody-positive generalized myasthenia gravis: the BeatMG study. Neurology. (2022) 98:e376–89. doi: 10.1212/wnl.0000000000013121
29. Singh, JA, Shah, NP, and Mudano, AS. Belimumab for systemic lupus erythematosus. Cochrane Database Syst Rev. (2021) 2021. doi: 10.1002/14651858.CD010668.pub2
Keywords: generalized myasthenia gravis, telitacicept, B lymphocyte stimulator, a proliferation-inducing ligand, treatment
Citation: Song X, He Y, Jiang H, Yu Y, Sun Y and Zhang Z (2025) Successful treatment of generalized myasthenia gravis with telitacicept: a Chinese case series and literature review. Front. Neurol. 16:1501500. doi: 10.3389/fneur.2025.1501500
Received: 25 September 2024; Accepted: 20 January 2025;
Published: 31 January 2025.
Edited by:
Jian-Quan Shi, Nanjing Medical University, ChinaReviewed by:
Katherine Buzzard, Monash University, AustraliaCopyright © 2025 Song, He, Jiang, Yu, Sun and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoxu Zhang, emhhbmd6aGFveHUzM0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.