
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 02 April 2025
Sec. Dementia and Neurodegenerative Diseases
Volume 16 - 2025 | https://doi.org/10.3389/fneur.2025.1496382
Objectives: This meta-analysis aims to systematically evaluate the effects of virtual reality (VR)-based interventions on cognitive function, emotional state, and quality of life in patients with mild cognitive impairment (MCI).
Methods: A comprehensive literature search was conducted using five databases from their inception to June 2024. The inclusion criteria focused on randomized controlled trials (RCTs) that examined VR-based interventions in adults aged 60 or older diagnosed with MCI. The primary outcome was cognitive function, while secondary outcomes included emotional state, quality of life, and dynamic balance. To investigate potential sources of heterogeneity, subgroup analyses and meta-regression were conducted. Subgroup analyses were stratified by VR parameters (immersion level, duration, session, and frequency) and demographic factors (geographic region, education level, and male proportion). Publication bias was assessed using funnel plots and Egger’s regression test. A “trim and fill” method was employed to adjust for any detected publication bias. The certainty of the evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework with the GRADEpro GDT software.
Results: A total of 30 RCTs involving 1,365 participants from 9 countries across 4 continents were included. The meta-analysis revealed that VR-based interventions significantly improved global cognition, as assessed by the Montreal Cognitive Assessment (MoCA; SMD = 0.82, 95% CI: 0.27 to 1.38, p = 0.003, GRADE: moderate) and the Mini-Mental State Examination (MMSE; SMD = 0.83, 95% CI: 0.40 to 1.26, p = 0.0001, GRADE: low). Additionally, VR interventions enhanced attention, as measured by the Digit Span Backward (DSB; SMD = 0.61, 95% CI: 0.21 to 1.02, p = 0.003, GRADE: low) and Digit Span Forward (DSF; SMD = 0.89, 95% CI: 0.34 to 1.45, p = 0.002, GRADE: low). Improvements were also observed in quality of life, as indicated by scores on the Instrumental Activities of Daily Living (IADL; SMD = 0.22, 95% CI: 0.00 to 0.45, p = 0.049, GRADE: moderate). However, no significant effects were found for executive function, memory, verbal fluency, visual abilities, emotional status, or dynamic balance (p > 0.05). Subgroup analysis revealed that VR interventions were more effective when using semi-immersive VR, with session durations of ≤60 min and a frequency of more than twice per week. Participants from Asia and Europe demonstrated better outcomes, and a lower proportion of male participants (≤ 40%) was also associated with improvements in targeted cognitive domains.
Conclusion: The findings indicate that VR interventions can significantly improve global cognition, attention, and quality of life in individuals with MCI. Subgroup analyses further revealed that optimal cognitive outcomes were associated with semi-immersive VR, session durations of ≤60 min, intervention frequencies exceeding twice per week, studies conducted in Asia and Europe, and participant groups with a male proportion of ≤40%. Moreover, the study provides valuable insights into secondary outcomes, suggesting that VR interventions may positively impact emotional state and dynamic balance when appropriately tailored to factors such as immersion level, duration, frequency, and other relevant parameters.
Mild cognitive impairment (MCI) is a clinical condition that represents a transitional stage between the cognitive decline associated with normal aging and the more severe impairment seen in dementia, particularly Alzheimer’s disease (AD) (1, 2). Individuals diagnosed with MCI are at a significantly higher risk of progressing to dementia, with a mean annual conversion rate of approximately 10%, compared to the annual incidence of 1–2% in the general population (3, 4). This critical period offers an opportunity for intervention, making the identification of effective therapeutic strategies to delay or prevent the progression to dementia of utmost importance.
The increasing prevalence of MCI, driven by the aging global population, has intensified the need for innovative interventions that not only address cognitive decline but also improve emotional well-being and overall quality of life. Traditional cognitive rehabilitation methods, including memory training (5), cognitive exercises (6), and pharmacotherapy (7), have shown some efficacy but often suffer from limitations such as low patient engagement and adherence. These limitations have spurred interest in alternative and more engaging therapeutic approaches.
Virtual reality (VR) technology has emerged as a promising tool in the field of cognitive rehabilitation due to its unique ability to create immersive and interactive environments (8). VR allows for the simulation of real-world scenarios in a controlled and customizable manner, making it an ideal platform for cognitive training (9). Unlike traditional cognitive exercises, VR can engage multiple senses simultaneously, potentially leading to more robust cognitive benefits (8). Moreover, the interactive nature of VR can enhance patient motivation and adherence to therapy (9, 10), which are critical factors in the success of any long-term intervention.
In recent years, a growing body of research has explored the application of VR-based interventions for cognitive rehabilitation in various populations, including stroke, Parkinson, MCI, and dementia, and et. For instance, a recent meta-analysis demonstrated that VR has significant beneficial effects on cognitive function in individuals who have sustained a stroke (11). Similarly, Lei et al. reported that VR not only achieves similar effects to conventional rehabilitation training but also improves gait and balance performance in patients with Parkinson’s disease (12). Another review also found that computerized cognitive training or VR technology could improve cognition, executive functions, and attention of MCI or AD patients to some extent (13).
However, much of the existing literature tends to combine MCI and dementia in analyses, which may obscure important differences in intervention effectiveness between these groups (1, 14–17). Given that MCI and dementia represent distinct stages of cognitive decline, it is critical to separately analyze the effects of VR-based interventions on individuals with MCI. Such an approach allows for a more precise understanding of how VR can be utilized to target the specific needs of this population and potentially prevent the progression to dementia.
This meta-analysis aims to address this gap by systematically evaluating the effects of VR-based interventions on cognitive function, emotional state, and quality of life in patients with MCI. By focusing specifically on MCI, this study seeks to provide a clearer picture of the therapeutic potential of VR for this population and offer evidence-based recommendations for its application in clinical practice.
The protocol was prospectively registered on the PROSPERO International Prospective Register for Systematic Reviews website (Registration #: CRD42023489464) in December 2023. Design and reporting of this review have followed “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) statement (Supplementary Table 1) (18).
English language articles were retrieved by title and abstract from the earliest record up to June 2024 from PubMed, Embase, Elsevier, Web of Science, and SciELO by two independent authors (X.L. and Y.Z.). The search strategy (based on Medical Subject Headings) combined the following terms: “Mild Cognitive Impairment”; “MCI”; “Virtual Reality”; “VR”; “Cognitive Function” (the full search strategy is reported in Supplementary Table 2). All literature was imported into Endnote X9 (Thomson Reuters, Carlsbad, CA, USA), which also removed duplications. Two reviewers (L.T. and L.Y.) screened all titles and abstracts. Once abstracts suggested that studies were potentially suitable, the full-text versions were screened and then included in the review if they fulfilled the selection criteria. A third reviewer (M.T.) was consulted in cases of disagreement.
The inclusion criteria were defined with the PICOS approach:
(a) P (population): all populations were aged more than 60 years, with a diagnosis of MCI or cognitive impairment. Diagnostic criteria for MCI patients included Mini-Mental State Examination (MMSE) (11–26 score), Montreal Cognitive Assessment (MoCA) (< 26 score), Monongahela-Youghiogheny Healthy Aging Team assessment, subjective cognitive decline and diagnosis by doctors.
(b) I (intervention): The experimental group (EG) received VR-based rehabilitation training. The VR intervention should be the use of interactive simulations created with computer hardware and software to present users with a virtual figure to engage in environments that appear and feel similar to real world objects and events (1).
(c) C (comparison): The control group (CG) received no intervention, or received conventional training, or received an alternative intervention such as health education.
(d) O (outcomes): The primary outcome of this study was cognitive function: (I) Global Cognition: MoCA, MMSE, Symbol Digit Substitution Test (SDST), and Cognitive Failure Questionnaire (CFQ); (II) Execution Cognition: Trail Making Test–Part A (TMT-A) and Trail Making Test–Part B (TMT-B); (III) Attention: Digit Span Backward (DSB) and Digit Span Forward (DSF); (IV) Memory: Rey Auditory Verbal Learning Test-Immediate Recall (RAVLT-IR), Rey Auditory Verbal Learning Test-Delayed Recall (RAVLT-DR), Chinese Version Verbal Learning Test-Immediate Recall (CVVLT-IR), and Chinese Version Verbal Learning Test-Delayed Recall (CVVLT-DR); (V) Verbal Fluency: Animal Word and “ㅅ” Word; (VI) Visual Ability: Wechsler Adult Intelligence Scale-Block Design Test (WAIS-BDT) and Clock Drawing Test (CDT).
Secondary outcomes were (I) Emotional State: Geriatric Depression Scale-15 (GDS-15) and Geriatric Depression Scale-30 (GDS-30); (II) Quality of Life: Instrumental Activity of Daily Living (IADL) and Quality of Life-Alzheimer Disease (QoL-AD); and (III) Dynamic Balance: Timed Up-and-Go Test (TUG) and Berg Balance Scale (BBS).
(e) S (study design): randomized controlled trials (RCTs).
a. Patients with a history of other neurological diseases (e.g., Parkinson’s disease or stroke,) or psychiatric disorders (e.g., anxiety disorders or depressive).
b. During the follow-up period, medications for MCI (cholinesterase inhibitors or memantine) were prescribed.
c. Studies not published in English.
d. Case reports, cross-sectional, retrospective, systematic reviews, editorial letters, or conference abstracts without the full text available.
Two authors (X.L. and Y.Z.) extracted data independently, with any discrepancies discussed until a consensus was reached. Data on study characteristics (author, published year, and country), sample characteristics (male/female size, age, education years, MMSE, MoCA, and IADL score), EG characteristics (immersive level, intervention component, session, frequency, and duration), CG characteristics (intervention component), and outcome characteristics was extracted. If a study reported results for different periods, each of them was treated as a separate trial (19).
The quality assessment was conducted using the risk of bias tool from RevMan 5.4.1 (The Cochrane Collaboration in Oxford, England) (20). This tool evaluates seven aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Each aspect was rated by the researchers as high risk (−), low risk (+), or uncertain risk (?). In cases of disagreement on the ratings, a consultation process was implemented to reach a consensus.
The Cochrane systematic review software RevMan 5.4.1 (The Cochrane Collaboration in Oxford, England) was used to create forest plots with 95% confidence intervals (CI). In order to avoid the impression of differences between studies, the standardized mean difference (SMD) was used in the data description. The heterogeneity was quantitatively determined by I2, where I2 values of <25, 26–74, and > 75% represented small, moderate, and large levels of heterogeneity, respectively. Fixed-effects models were applied when heterogeneity was graded as small, whereas random-effects models were utilized for moderate or large heterogeneity. For pooled effects with moderate or large heterogeneity, subgroup analyses were conducted based on VR parameters (immersion level, duration, session, and frequency) and demographic factors (geographic region, education level, and male proportion) to identify potential sources and influencing factors. Immersion levels were categorized as non-immersive, semi-immersive, and full immersive. Interactions using a PC monitor, keyboard, and mouse were classified as non-immersive, while more advanced graphics with larger surface displays were categorized as semi-immersive. Full immersive VR, representing the highest level of immersion, was defined as utilizing 3D displays (21). The subgroup classification criteria are presented in Table 1.
If sufficient studies were available (≥ 10 study groups), meta-regression was performed to further analyze the impact of specific covariates. Moreover, publication bias was determined through funnel plot and Egger’s regression test when a sufficiently large sample of studies (≥ 10 study groups) was available for the EG vs. CG comparison (22, 23). In case of publication bias, the “trim and fill” method was used to further evaluate the influence of publication bias on pooled results (24). Sensitivity analyses were performed using the “leave-one-out” method, whereby individual studies were sequentially excluded to examine their influence on the overall estimates (25).
We applied the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) framework to evaluate the certainty of the evidence (26). The assessment was conducted using GRADEPro GDT software (version 2022). This systematic approach evaluates the overall certainty of evidence for each outcome based on five key domains: risk of bias, imprecision, inconsistency, indirectness, and publication bias. Evidence certainty was categorized into four levels: high, moderate, low, or very low, reflecting the confidence that the reported effect is close to the true effect.
Figure 1 shows the flow diagram of study selection. The initial search generated 4,947 articles of which 1,260 were duplicates. 3,386 articles were excluded by screening titles and abstracts. Out of the remaining 301 articles screened by full text. Finally, 30 original research articles were selected for further analysis. Results of the literature quality and publication bias risk assessment are presented in Figure 2.
Table 2 summarizes the characteristics of the included studies. All 30 studies were RCTs, and all the included studies were related to elderly people with cognitive disorders or MCI. The 30 studies had a total of 1,365 participants in 9 countries across 4 continents, including 489 male and 876 female. In terms of immersive level, nine studies reported full-immersive VR, seven reported semi-immersive VR, and the remaining fourteen reported non-immersive VR. Training frequency varied from 2 to 5 times per week and the duration per session varied from 18 to 100 min. The duration of interventions ranged from brief 4 weeks programs to 24 weeks of training.
MoCA, MMSE, SDST, and CFQ were used to evaluate global cognition in the studies. Eleven, fourteen, two, and three studies reported MoCA, MMSE, SDST, and CFQ results for 466, 661, 134, and 160 participants, respectively. The meta-analysis revealed that the MoCA (SMD = 0.82, 95% CI: 0.27 to 1.38, I2 = 86%, p = 0.003, GRADE: moderate) and MMSE (SMD = 0.83, 95% CI: 0.40 to 1.26, I2 = 84%, p = 0.0001, GRADE: low) in the EG than in the CG. There was no difference in SDST (SMD = 1.14, 95% CI: −0.50 to 2.78, I2 = 95%, p = 0.17, GRADE: very low) and CFQ (SMD = −0.09, 95% CI: −0.40 to 0.22, I2 = 7%, p = 0.59, GRADE: low) between groups (Table 3; Supplementary Figure 1).
The subgroup analysis of MoCA scores showed that the following factors were significantly associated with better outcomes: semi-immersive level (p < 0.0001), duration ≤16 weeks (p < 0.05), session ≤60 min (p < 0.05), frequency > 2 times/week (p = 0.005), participants from Asia and Europe (p < 0.05), and male proportion ≤ 40% (p < 0.0001) (Table 4; Supplementary Figure 2). Meta-regression analysis further validated these results, indicating that immersive level (p = 0.08), duration (p = 0.011), session (p = 0.012), frequency (p = 0.011), geographic region (p = 0.010), and education level (p = 0.007) were significant factors affecting the improvement in MoCA scores (Table 5).
The subgroup analysis of MMSE scores showed that the following factors were significantly associated with better outcomes: full or semi or non-immersive level (p < 0.05), session ≤60 min (p < 0.05), frequency > 2 times/week (p = 0.0003), participants from Europe (p < 0.0001), and male proportion ≤ 40% (p = 0.0005) (Table 4; Supplementary Figure 3). Meta-regression analysis indicating that frequency (p = 0.019) and male proportion (p = 0.019) were significant factors affecting the improvement in MMSE scores (Table 5).
Patients’ execution cognition was evaluated based on TMT-A and TMT-B. Ten studies reported TMT-A results and fifteen reported TMT-B results. No significant difference was observed between the EG and CG in TMT-A (SMD = −0.26, 95% CI: −0.55 to −0.03, I2 = 52%, p = 0.08, GRADE: moderate) and TMT-B (SMD = −0.29, 95% CI: −0.64 to 0.07, I2 = 80%, p = 0.11, GRADE: moderate) (Table 3; Supplementary Figure 4).
The subgroup analysis of TMT-A showed that the following factors were significantly associated with better outcomes: full or semi-immersive level (p < 0.05), duration ≤8 weeks (p = 0.04), session ≤30 min or > 60 min (p < 0.05), education level ≤ 9 years (p = 0.0009), and male proportion ≤ 40% (p = 0.03) (Table 4, Supplementary Figure 5). Meta-regression analysis indicating that session (p = 0.048) and frequency (p = 0.040) were significant factors affecting the improvement in TMT-A (Table 5).
The subgroup analysis of TMT-B demonstrated that semi-immersive level (p = 0.02) and duration more than 16 weeks (p < 0.0001) had significant positive effects on performance (Table 4; Supplementary Figure 6). However, meta-regression analysis showed that immersive level, duration, session, frequency, geographic region, education level, and male proportion were not significant factors influencing improvements in TMT-B outcomes (Table 5).
DSB and DSF was used to evaluate patients’ attention. Seven and five studies reported DSB and DSF results for 383 and 251 participants, respectively. DSB (SMD = 0.61, 95% CI: 0.21 to 1.02, I2 = 72%, p = 0.003, GRADE: low) and DSF (SMD = 0.89, 95% CI: 0.34 to 1.45, I2 = 75%, p = 0.002, GRADE: low) was significantly higher in the EG than in the CG (Table 3; Supplementary Figure 7).
The subgroup analysis of DSB showed that the following factors were significantly associated with better outcomes: semi-immersive level (p = 0.0002), 8 < duration ≤16 weeks (p = 0.007), 30 < session ≤60 min (p < 0.0001), frequency > 2 times/week (p = 0.006), participants from Asia and Europe (p < 0.05), and male proportion ≤ 40% (p = 0.0001) (Table 4; Supplementary Figure 8).
The subgroup analysis of DSF showed that the following factors were significantly associated with better outcomes: semi-immersive level (p < 0.0001), duration ≤8 weeks or > 16 weeks (p < 0.05), session ≤30 min or > 60 min (p < 0.05), participants from Asia and Europe (p < 0.05), frequency > 2 times/week and male proportion > 40% (p < 0.0001) (Table 4; Supplementary Figure 9).
Patients’ memory was evaluated based on RAVLT-IR, RAVLT-DR, CVVLT-IR, and CVVLT-DR. There was no difference in RAVLT-IR (SMD = −0.01, 95% CI: −0.38 to 0.36, I2 = 57%, p = 0.95, GRADE: low), RAVLT-DR (SMD = 0.13, 95% CI: −0.35 to 0.62, I2 = 66%, p = 0.59, GRADE: low), CVVLT-IR (SMD = 0.13, 95% CI: −0.24 to 0.50, I2 = 0%, p = 0.49, GRADE: low), and CVVLT-DR (SMD = 0.41, 95% CI: −0.58 to 1.39, I2 = 85%, p = 0.42, GRADE: very low) between groups (Table 3; Supplementary Figure 10).
Subgroup analysis of RAVLT-IR showed that semi-immersive level (p = 0.006) and session >60 min (p = 0.006) had a positive effect (Table 4; Supplementary Figure 11).
A subgroup analysis based on immersive level, session, and education level revealed that participants in the EG who engaged in non-immersive level or 30 < session ≤60 min, as well as those with an education level > 9 years, demonstrated significant positive effects on RAVLT-DR (p = 0.0003, Table 4; Supplementary Figure 12).
Subgroup analysis of CVVLT-DR showed that education level ≤ 9 years had a positive effect (p < 0.0001) (Table 4; Supplementary Figure 13).
Animal Word and “ㅅ” Word was used to evaluate patients’ verbal fluency. The meta-analysis revealed that the animal Word (SMD = 0.20, 95% CI: −0.06 to 0.47, I2 = 0%, p = 0.14, GRADE: moderate) and “ㅅ” Word (SMD = 0.00, 95% CI: −0.48 to 0.47, I2 = 0%, p = 1.00, GRADE: low) was no difference between groups (Table 3; Supplementary Figure 14).
Two and five studies reported WAIS-BDT and CDT results for 134 and 167 participants to evaluate visual ability, respectively. However, there was no difference in WAIS-BDT (SMD = 0.44, 95% CI: −0.37 to 1.24, I2 = 81%, p = 0.29, GRADE: very low) and CDT (SMD = 0.21, 95% CI: −0.30 to 0.72, I2 = 63%, p = 0.41, GRADE: very low) between groups (Table 3; Supplementary Figure 15).
A subgroup analysis based on immersive level, duration, session, and male proportion revealed that participants who engaged in full-immersive level, duration >16 weeks, or session ≤30 min, as well male proportion ≤ 40%, demonstrated significant positive effects on CDT (p = 0.002, Table 4; Supplementary Figure 16).
GDS-15 and GDS-30 was used to evaluate patients’ emotional state. There was no difference in GDS-15 (SMD = −0.40, 95% CI: −1.17 to −0.37, I2 = 85%, p = 0.31, GRADE: low) and GDS-30 (SMD = −1.38, 95% CI: −4.51 to 1.76, I2 = 98%, p = 0.39, GRADE: very low) between EG and CG (Table 3; Supplementary Figure 17).
A subgroup analysis by frequency and geographic region showed that EG of >2 times/week and participants from Europe (p < 0.0001) had a positive effect on GDS-15 (Table 4; Supplementary Figure 18).
IADL and QoL-AD was used to evaluate patients’ quality of life. IADL (SMD = 0.22, 95% CI: 0.00 to 0.45, I2 = 0%, p = 0.049, GRADE: moderate) was significantly higher in the EG than in the CG. There was no difference in QoL-AD (SMD = −0.06, 95% CI: −0.39 to 0.26, I2 = 0%, p = 0.71, GRADE: low) between groups (Table 3; Supplementary Figure 19).
Five and three studies reported TUG and BBS results for 280 and 208 participants to evaluate dynamic balance, respectively. There was no difference in TUG (SMD = 0.05, 95% CI: −0.45 to 0.56, I2 = 76%, p = 0.83, GRADE: low) and BBS (SMD = 0.45, 95% CI: −0.51 to 1.41, I2 = 91%, p = 0.35, GRADE: low) between groups (Table 3; Supplementary Figure 20).
Subgroup analysis of TUG showed that full-immersive level (p = 0.002) and duration ≤8 weeks or > 16 weeks (p < 0.05) had a positive effect (Table 4; Supplementary Figure 21).
A subgroup analysis by immersive level and frequency showed that EG of semi-immersive level and frequency ≤ 2 times/weeks (p < 0.0001) had a positive effect on BBS (Table 4; Supplementary Figure 22).
In a word, the summary of optimal VR parameter and demographic factors effects on cognitive domains and dynamic balance are shown in Table 6.
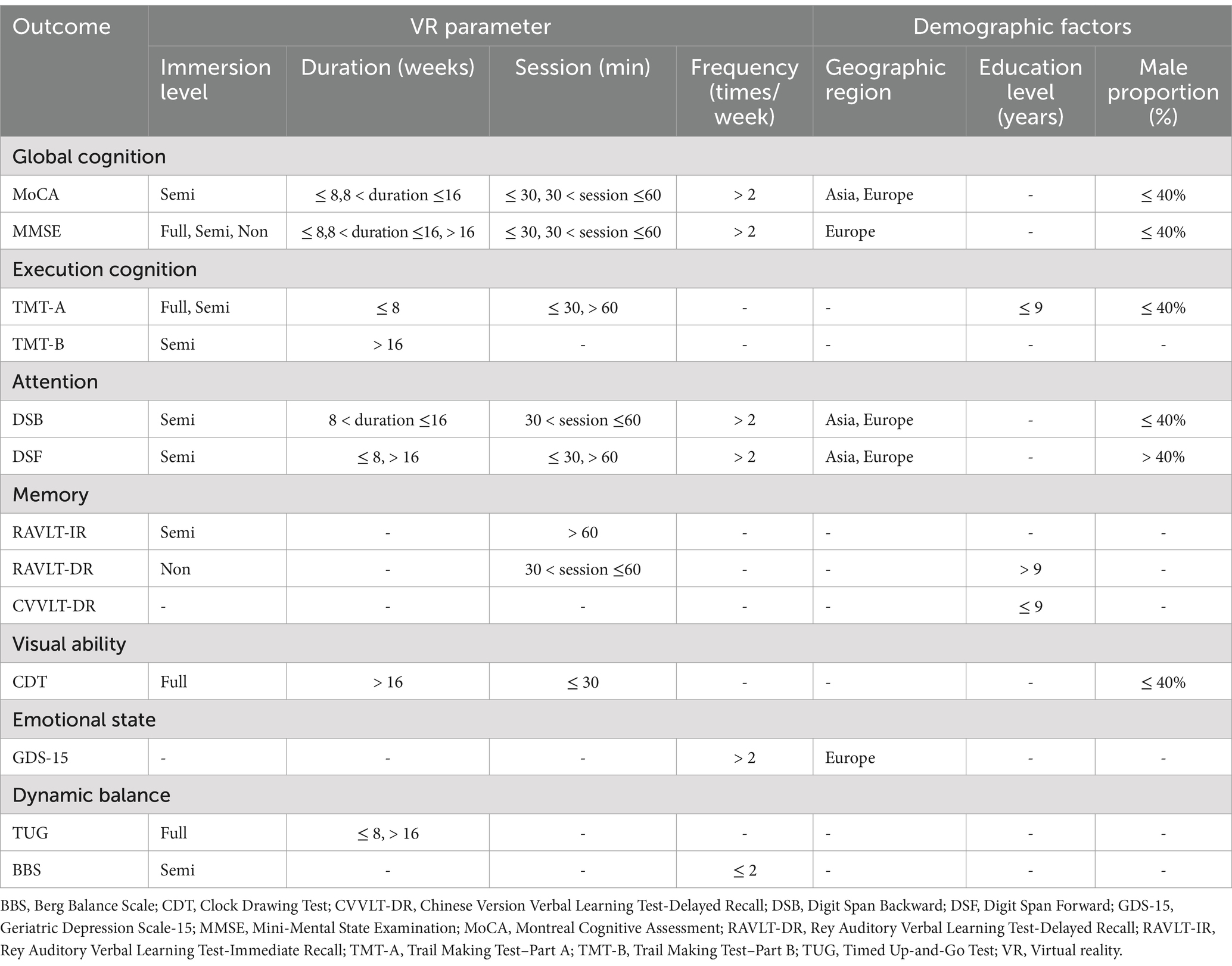
Table 6. Summary of optimal VR parameter and demographic factors effects on cognitive domains and dynamic balance.
Figure 3 showed the funnel plot of MoCA, MMSE, TMT-A, and TMT-B. The results of Egger’s test showed that MoCA (t = 2.03, p = 0.073), TMT-A (t = 0.93, p = 0.381), and TMT-B (t = 0.16, p = 0.872) was no significant publication bias. However, Egger’s test showed that there was publication bias in the MMSE (t = 2.55, p = 0.026). Pooled results using the “trim and fill” method showed no obvious changes (SMD = 0.872, p < 0.0001), indicating this result was robust. Additionally, sensitivity analysis showed that all pooled estimates were not materially altered after removal of a single study.
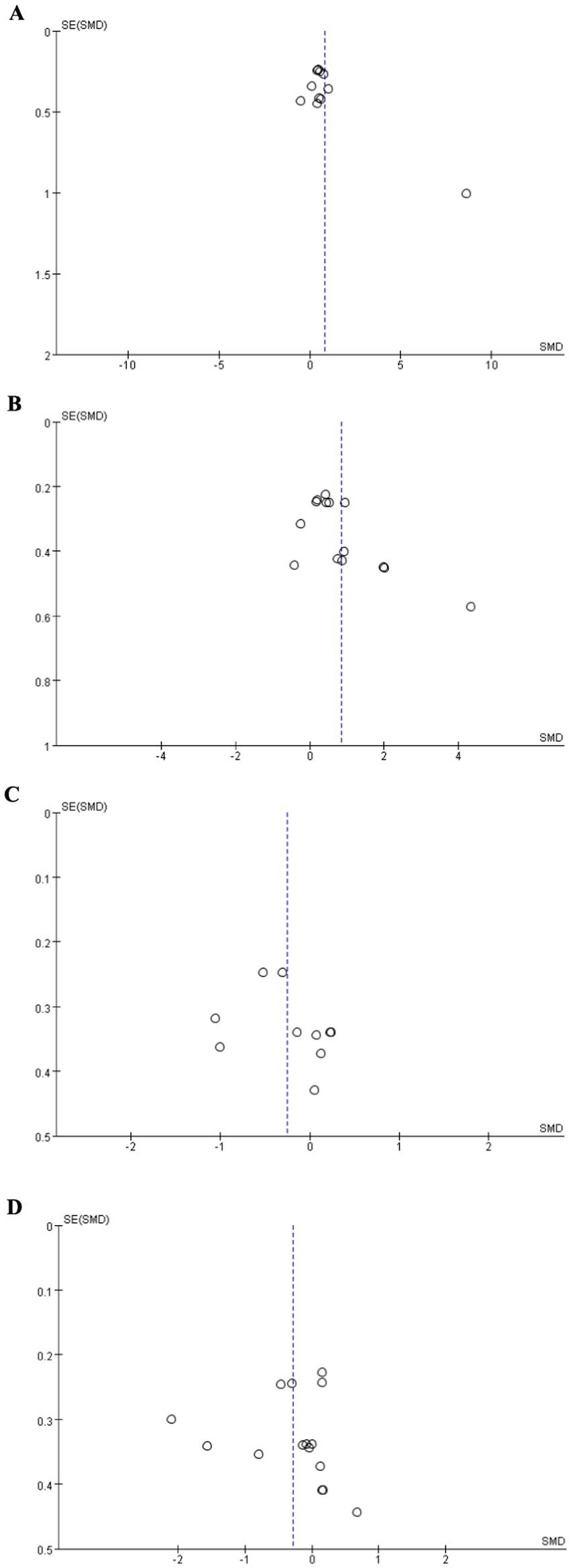
Figure 3. Funnel plot. (A) MoCA, (B) MMSE, (C) TMT-A, (D) TMT-B. MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; TMT-A, Trail Making Test–Part A; TMT-B, Trail Making Test–Part B.
This meta-analysis evaluated the effectiveness of VR-based interventions on cognitive function, emotional state, and quality of life in individuals with MCI. The findings indicate that VR interventions significantly improve global cognition, attention, and quality of life. However, their effects on executive function, memory, visuospatial abilities, emotional state, and dynamic balance appear to be influenced by specific VR parameters, including immersion level, duration, session, and frequency, as well as demographic factors such as geographic region, education level, and male proportion. These results align with previous research (1, 14, 27) supporting VR as a promising tool for cognitive rehabilitation in older adults with cognitive impairments.
MCI is characterized by cognitive decline and represents a crucial window for AD prevention and treatment (2, 8). This study confirms that VR-based interventions significantly enhance global cognition (MoCA and MMSE scores) and attention (DSB and DSF scores) in individuals with MCI, highlighting VR as a promising non-pharmacological therapy for this population. However, no significant improvements were observed in other cognitive domains, such as executive function, memory, and visuospatial abilities. This finding is consistent with some reviews suggesting that VR interventions do not significantly enhance these cognitive functions (8, 28). In contrast, other studies have indicated that VR, when combined with additional interventions, can improve these domains (17, 29). This discrepancy may stem from differences in study populations—while our analysis focused exclusively on individuals with MCI, previous studies included both MCI and dementia patients, potentially influencing outcomes. Additionally, differences in study design may contribute to these inconsistencies. Our meta-analysis included only RCTs, ensuring higher methodological rigor, whereas other reviews incorporated non-RCTs, increasing the risk of bias. Further research is needed to determine whether VR interventions can effectively enhance executive function, memory, and other cognitive domains in MCI populations.
Subgroup analysis revealed that VR intervention efficacy is influenced by VR parameters such as immersion level, duration, session, and frequency. Among different immersion levels, semi-immersive VR demonstrated the most significant benefits across multiple cognitive outcomes (MoCA, MMSE, TMT-A, TMT-B, DSB, DSF, and RAVLT-IR), consistent with previous findings (14, 21, 28). From a human-computer interaction perspective, its superior effectiveness can be attributed to an optimal balance between engagement and cognitive load (30). Prior research emphasizes that user acceptance and cognitive workload are crucial factors in determining VR intervention efficacy (30). Non-immersive VR may lack sufficient sensory stimulation for meaningful cognitive engagement, whereas fully immersive VR, while providing an enriched environment, may induce discomfort, fatigue, and cybersickness, leading to cognitive overload (31, 32). Semi-immersive VR strikes a balance, maximizing engagement while minimizing adverse effects, making it a promising approach for cognitive rehabilitation in individuals with MCI.
Regarding intervention duration, interventions lasting ≤16 weeks demonstrated the most significant improvements in several cognitive outcomes (e.g., MoCA, TMT-A, DSB). However, longer interventions (> 16 weeks) were more effective for tasks requiring higher-order cognitive functions, such as executive function and visuospatial abilities (e.g., TMT-B, CDT). This may be due to the different cognitive processes targeted by short- and long-term interventions. Shorter programs may enhance general cognitive function through repeated stimulation, while longer interventions allow for the gradual reinforcement of complex cognitive skills (33). However, prolonged interventions may also lead to participant fatigue or reduced adherence, potentially affecting outcomes. Future research should explore the optimal intervention duration to balance effectiveness and user engagement.
When categorized by session and frequency, the most effective interventions involved sessions lasting ≤60 min and occurring more than twice per week, yielding significant improvements across multiple cognitive outcomes (e.g., MoCA, MMSE, DSB). From a cognitive psychology perspective, shorter, more frequent sessions optimize learning by preventing cognitive fatigue and sustaining attention, which enhances memory consolidation and cognitive engagement (34). Frequent training reinforces neural plasticity, strengthens synaptic connections, and aligns with the principles of spaced learning, improving long-term retention and skill transfer (35). This structured approach may be particularly beneficial for individuals with MCI, as consistent cognitive stimulation helps build cognitive resilience while minimizing the risk of mental fatigue, a common concern in aging populations. Prolonged or infrequent sessions, by contrast, may lead to disengagement and suboptimal cognitive gains. These findings suggest that VR interventions should prioritize an optimal balance between session and frequency to maximize cognitive benefits while mitigating the risk of overexertion.
Session and frequency also influenced intervention effectiveness, with the most beneficial interventions involving session lengths of ≤60 min and a frequency of more than twice per week. From a cognitive psychology perspective, shorter, more frequent sessions optimize learning by preventing cognitive fatigue and maintaining attention, thereby improving memory consolidation and engagement (34). Frequent training reinforces neural plasticity and strengthens synaptic connections, aligning with the principles of spaced learning to enhance long-term retention and skill transfer (35). This approach may be particularly advantageous for individuals with MCI, as consistent cognitive stimulation fosters resilience while minimizing mental fatigue. In contrast, prolonged or infrequent sessions may lead to disengagement and suboptimal cognitive gains. These findings suggest that VR interventions should prioritize an optimal balance between session duration and frequency to maximize cognitive benefits while mitigating fatigue.
Subgroup analysis further demonstrated that VR intervention effectiveness varies by demographic factors. Notably, VR-based interventions showed greater efficacy in studies conducted in Asia and Europe and in populations with ≤40% male participants. Regional differences may be influenced by socio-cultural factors such as digital literacy, attitudes toward technology, and access to digital health interventions. Both Asia and Europe have implemented policies and investments that actively support VR development, fostering advancements in hardware and software applications (36). The integration of VR into education and healthcare in these regions has led to greater familiarity and acceptance, potentially enhancing user engagement and intervention effectiveness (36). Additionally, the well-established VR industry in these areas has improved accessibility to high-quality VR systems, further supporting their application in cognitive rehabilitation. Gender differences in VR effectiveness may stem from variations in psychophysiological engagement and behavioral tendencies. Women tend to exhibit greater emotional responsiveness and immersion in virtual environments, which may enhance the impact of VR-based interventions, particularly those utilizing emotional stimuli to reinforce learning. This heightened engagement may lead to stronger cognitive gains. Additionally, behavioral tendencies may play a role—men often prioritize efficiency and task completion speed, whereas women are more likely to immerse themselves in the experience. This aligns well with the design principles of VR-based cognitive training, which emphasize emotional stimulation and deep cognitive processing rather than rapid execution.
The secondary outcomes revealed varied effects of VR interventions on emotional state, quality of life, and dynamic balance in MCI patients. Significant improvements in emotional state were observed in the EG when using higher frequency (>2 times/week) and in European studies, as reflected by GDS-15 scores. This suggests that regional factors and intervention frequency may influence emotional outcomes. However, no differences were found in GDS-30 scores, suggesting that longer assessments may not fully capture the emotional benefits of VR. In terms of quality of life, significant improvements in IADL scores indicate that VR interventions may enhance daily living activities. These findings align with those of Son et al., who reported similar improvements in MCI and AD patients following VR-based cognitive training (37). However, no significant changes were detected in QoL-AD scores, suggesting that while VR interventions may promote functional independence, their impact on overall quality-of-life perceptions may be limited. Regarding dynamic balance, no significant overall differences were found in TUG or BBS scores. However, subgroup analyses revealed that full-immersive VR interventions and durations of ≤8 weeks or > 16 weeks positively influenced TUG performance. Similarly, semi-immersive VR interventions with lower frequency (≤ 2 times per week) improved BBS scores. These findings suggest that both immersion level and intervention duration play critical roles in enhancing dynamic balance. Future studies should further investigate these factors to optimize intervention strategies.
Even though this study has clinical implications, there were several limitations. First, it only included English-language publications, potentially overlooking relevant non-English studies, particularly from East Asia, a major contributor to VR research. Future reviews should incorporate multilingual studies for a more comprehensive perspective. Second, many outcomes relied on subjective self-reported measures (e.g., MoCA, MMSE), which are prone to bias and may not accurately reflect objective cognitive or functional improvements. Future studies should integrate standardized objective assessments, such as biomarkers or physiological metrics, to enhance reliability. Third, most studies lacked long-term follow-up, making it unclear whether VR’s benefits are sustained. Lastly, confounding factors—such as baseline cognitive function, comorbidities, and technology familiarity—were often unaddressed, potentially influencing results. Standardized participant selection and rigorous statistical controls are needed to mitigate these effects. Additionally, publication bias may have influenced our results, as positive findings are more likely to be published, potentially inflating effect sizes. Although we assessed publication bias using funnel plots and Egger’s test, the limited number of studies may have affected the reliability of these analyses. Future research should focus on developing standardized VR intervention protocols to enhance replicability and comparability across studies.
The findings indicate that VR interventions can significantly improve global cognition, attention, and quality of life in individuals with MCI. Subgroup analyses further revealed that optimal cognitive outcomes were associated with semi-immersive VR, session durations of ≤60 min, intervention frequencies exceeding twice per week, studies conducted in Asia and Europe, and participant groups with a male proportion of ≤40%. Moreover, the study provides valuable insights into secondary outcomes, suggesting that VR interventions may positively impact emotional state and dynamic balance when appropriately tailored to factors such as immersion level, duration, frequency, and other relevant parameters.
XL: Conceptualization, Writing – original draft, Writing – review & editing. YZ: Writing – original draft. LT: Writing – original draft. LY: Methodology, Writing – review & editing. MT: Investigation, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Key research project of Ningbo Rehabilitation Hospital (Project Number: 2024KY01), the 2023 Annual Pathophysiology Technology Research Key Laboratory Open Fund of Zhejiang Province (Project Number: 202307), Medicine and Health Care in Zhejiang Province Science and Technology Plan Projects of Traditional Chinese Medicine (Project Number: 2024ZL954), and Ningbo Key Research and Development Plan Project (Project Number: 2023Z173).
We thank all the colleagues for participating in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1496382/full#supplementary-material
1. Zhu, S, Sui, Y, Shen, Y, Zhu, Y, Ali, N, Guo, C, et al. Effects of virtual reality intervention on cognition and motor function in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Front Aging Neurosci. (2021) 13:586999. doi: 10.3389/fnagi.2021.586999
2. Yan, M, Zhao, Y, Meng, Q, Wang, S, Ding, Y, Liu, Q, et al. Effects of virtual reality combined cognitive and physical interventions on cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis. Ageing Res Rev. (2022) 81:101708. doi: 10.1016/j.arr.2022.101708
3. Petersen, R, Lopez, O, Armstrong, MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of neurology. Neurology. (2018) 90:126–35. doi: 10.1212/WNL.0000000000004826
4. Meng, Q, Yin, H, Wang, S, Shang, B, Meng, X, Yan, M, et al. The effect of combined cognitive intervention and physical exercise on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Aging Clin Exp Res. (2022) 34:261–76. doi: 10.1007/s40520-021-01877-0
5. Olchik, MR, Farina, J, Steibel, N, Teixeira, AR, and Yassuda, MS. Memory training (MT) in mild cognitive impairment (MCI) generates change in cognitive performance. Arch Gerontol Geriatr. (2013) 56:442–7. doi: 10.1016/j.archger.2012.11.007
6. Train the Brain Consortium. Randomized trial on the effects of a combined physical/cognitive training in aged MCI subjects: the train the brain study. Sci Rep. (2017) 7:39471. doi: 10.1038/srep39471
7. Gauthier, S. Pharmacotherapy of mild cognitive impairment. Dialogues Clin Neurosci. (2004) 6:391–5. doi: 10.31887/DCNS.2004.6.4/sgauthier
8. Wu, J, Ma, Y, and Ren, Z. Rehabilitative effects of virtual reality technology for mild cognitive impairment: a systematic review with meta-analysis. Front Psychol. (2020) 11:1811. doi: 10.3389/fpsyg.2020.01811
9. Kim, A, Darakjian, N, and Finley, JM. Walking in fully immersive virtual environments: an evaluation of potential adverse effects in older adults and individuals with Parkinson’s disease. J Neuroeng Rehabil. (2017) 14:1–12. doi: 10.1186/s12984-017-0225-2
10. Penke, L, Maniega, SM, Murray, C, Gow, AJ, Hernández, MCV, Clayden, JD, et al. A general factor of brain white matter integrity predicts information processing speed in healthy older people. J Neurosci. (2010) 30:7569–74. doi: 10.1523/JNEUROSCI.1553-10.2010
11. Aminov, A, Rogers, JM, Middleton, S, Caeyenberghs, K, and Wilson, PH. What do randomized controlled trials say about virtual rehabilitation in stroke? A systematic literature review and meta-analysis of upper-limb and cognitive outcomes. J Neuroeng Rehabil. (2018) 15:1–24. doi: 10.1186/s12984-018-0370-2
12. Lei, C, Sunzi, K, Dai, F, Liu, X, Wang, Y, Zhang, B, et al. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson's disease: a systematic review. PLoS One. (2019) 14:e0224819. doi: 10.1371/journal.pone.0224819
13. Coyle, H, Traynor, V, and Solowij, N. Computerized and virtual reality cognitive training for individuals at high risk of cognitive decline: systematic review of the literature. Am J Geriatr Psychiatry. (2015) 23:335–59. doi: 10.1016/j.jagp.2014.04.009
14. Ren, Y, Wang, Q, Liu, H, Wang, G, and Lu, A. Effects of immersive and non-immersive virtual reality-based rehabilitation training on cognition, motor function, and daily functioning in patients with mild cognitive impairment or dementia: a systematic review and meta-analysis. Clin Rehabil. (2024) 38:305–21. doi: 10.1177/02692155231213476
15. Hill, NT, Mowszowski, L, Naismith, SL, Chadwick, VL, Valenzuela, M, and Lampit, A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am J Psychiatry. (2017) 174:329–40. doi: 10.1176/appi.ajp.2016.16030360
16. Sayma, M, Tuijt, R, Cooper, C, and Walters, K. Are we there yet? Immersive virtual reality to improve cognitive function in dementia and mild cognitive impairment. The Gerontologist. (2020) 60:e502–12. doi: 10.1093/geront/gnz132
17. Papaioannou, T, Voinescu, A, Petrini, K, and Stanton, FD. Efficacy and moderators of virtual reality for cognitive training in people with dementia and mild cognitive impairment: a systematic review and meta-analysis. J Alzheimers Dis. (2022) 88:1341–70. doi: 10.3233/JAD-210672
18. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
19. Borenstein, M, Hedges, LV, Higgins, JP, and Rothstein, HR. Introduction to meta-analysis. Hoboken, NJ: John Wiley & Sons (2021).
20. Higgins, JP, Altman, DG, Gøtzsche, PC, Jüni, P, Moher, D, Oxman, AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
21. Kim, O, Pang, Y, and Kim, J-H. The effectiveness of virtual reality for people with mild cognitive impairment or dementia: a meta-analysis. BMC Psychiatry. (2019) 19:1–10. doi: 10.1186/s12888-019-2180-x
22. Page, MJ, Higgins, JP, and Sterne, JA. Assessing risk of bias due to missing results in a synthesis In: JPT Higgins, J Thomas, J Chandler, M Cumpston, T Li, and MJ Page, editors. Cochrane handbook for systematic reviews of interventions. London: Cochrane (2019). 349–74.
23. Egger, M, Smith, GD, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
24. Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
25. Zhou, Z, Zhou, R, Wei, W, Luan, R, and Li, K. Effects of music-based movement therapy on motor function, balance, gait, mental health, and quality of life for patients with Parkinson’s disease: a systematic review and meta-analysis. Clin Rehabil. (2021) 35:937–51. doi: 10.1177/0269215521990526
26. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
27. Yu, J, Wu, J, Liu, B, Zheng, K, and Ren, Z. Efficacy of virtual reality technology interventions for cognitive and mental outcomes in older people with cognitive disorders: an umbrella review comprising meta-analyses of randomized controlled trials. Ageing Res Rev. (2024) 94:102179. doi: 10.1016/j.arr.2023.102179
28. Kim, H, Jung, J, and Lee, S. Therapeutic application of virtual reality in the rehabilitation of mild cognitive impairment: a systematic review and meta-analysis. Vision. (2022) 6:68. doi: 10.3390/vision6040068
29. Hwang, J-H, and Park, M-S. Effect of a dual-task virtual reality program for seniors with mild cognitive impairment. Korean J Clin Lab Sci. (2018) 50:492–500. doi: 10.15324/kjcls.2018.50.4.492
30. Wu, P-F, Yen, S-W, Fan, K-Y, Wang, W-F, and Wu, F-C. Investigating the mental workload of experiencing virtual reality on people with mild cognitive impairment In: International conference on human-computer interaction. New York: Springer (2023). 642–54.
31. Hassandra, M, Galanis, E, Hatzigeorgiadis, A, Goudas, M, Mouzakidis, C, Karathanasi, EM, et al. Α virtual reality app for physical and cognitive training of older people with mild cognitive impairment: mixed methods feasibility study. JMIR serious games. (2021) 9:e24170. doi: 10.2196/24170
32. Sánchez, A, Millán-Calenti, JC, Lorenzo-López, L, and Maseda, A. Multisensory stimulation for people with dementia: a review of the literature. Am J Alzheimers Dis Other Dement. (2013) 28:7–14. doi: 10.1177/1533317512466693
33. Zhong, D, Chen, L, Feng, Y, Song, R, Huang, L, Liu, J, et al. Effects of virtual reality cognitive training in individuals with mild cognitive impairment: a systematic review and meta-analysis. Int J Geriatr Psychiatry. (2021) 36:1829–47. doi: 10.1002/gps.5603
34. Hess, TM. Selective engagement of cognitive resources: motivational influences on older adults’ cognitive functioning. Perspect Psychol Sci. (2014) 9:388–407. doi: 10.1177/1745691614527465
35. Hopstaken, JF, Van Der Linden, D, Bakker, AB, and Kompier, MA. A multifaceted investigation of the link between mental fatigue and task disengagement. Psychophysiology. (2015) 52:305–15. doi: 10.1111/psyp.12339
36. Yu, Z, and Xu, W. A meta-analysis and systematic review of the effect of virtual reality technology on users' learning outcomes. Comput Appl Eng Educ. (2022) 30:1470–84. doi: 10.1002/cae.22532
37. Son, C, and Park, J-H. Ecological effects of VR-based cognitive training on ADL and IADL in MCI and AD patients: a systematic review and meta-analysis. Int J Environ Res Public Health. (2022) 19:15875. doi: 10.3390/ijerph192315875
38. Liao, Y-Y, Chen, I-H, Lin, Y-J, Chen, Y, and Hsu, W-C. Effects of virtual reality-based physical and cognitive training on executive function and dual-task gait performance in older adults with mild cognitive impairment: a randomized control trial. Front Aging Neurosci. (2019) 11:162. doi: 10.3389/fnagi.2019.00162
39. Liao, Y-Y, Tseng, H-Y, Lin, Y-J, Wang, C-J, and Hsu, W-C. Using virtual reality-based training to improve cognitive function, instrumental activities of daily living and neural efficiency in older adults with mild cognitive impairment. Eur J Phys Rehabil Med. (2019) 56:47–57. doi: 10.23736/S1973-9087.19.05899-4
40. Park, J-H, and Park, J-H. Does cognition-specific computer training have better clinical outcomes than non-specific computer training? A single-blind, randomized controlled trial. Clin Rehabil. (2018) 32:213–22. doi: 10.1177/0269215517719951
41. Park, J-H, Liao, Y, Kim, D-R, Song, S, Lim, JH, Park, H, et al. Feasibility and tolerability of a culture-based virtual reality (VR) training program in patients with mild cognitive impairment: a randomized controlled pilot study. Int J Environ Res Public Health. (2020) 17:3030. doi: 10.3390/ijerph17093030
42. Park, J-S, Jung, Y-J, and Lee, G. Virtual reality-based cognitive–motor rehabilitation in older adults with mild cognitive impairment: a randomized controlled study on motivation and cognitive function. Healthcare. (2020) 8:335. doi: 10.3390/healthcare8030335
43. Park, J-H. Does the virtual shopping training improve executive function and instrumental activities of daily living of patients with mild cognitive impairment? Asian J Psychiatr. (2022) 69:102977. doi: 10.1016/j.ajp.2021.102977
44. Park, J-H. Effects of virtual reality-based spatial cognitive training on hippocampal function of older adults with mild cognitive impairment. Int Psychogeriatr. (2022) 34:157–63. doi: 10.1017/S1041610220001131
45. Kang, JM, Kim, N, Lee, SY, Woo, SK, Park, G, Yeon, BK, et al. Effect of cognitive training in fully immersive virtual reality on visuospatial function and frontal-occipital functional connectivity in predementia: randomized controlled trial. J Med Internet Res. (2021) 23:e24526. doi: 10.2196/24526
46. Schwenk, M, Sabbagh, M, Lin, I, Morgan, P, Grewal, GS, Mohler, J, et al. Sensor-based balance training with motion feedback in people with mild cognitive impairment. J Rehabil Res Dev. (2016) 53:945–58. doi: 10.1682/JRRD.2015.05.0089
47. Delbroek, T, Vermeylen, W, and Spildooren, J. The effect of cognitive-motor dual task training with the biorescue force platform on cognition, balance and dual task performance in institutionalized older adults: a randomized controlled trial. J Phys Ther Sci. (2017) 29:1137–43. doi: 10.1589/jpts.29.1137
48. Tarnanas, I, Tsolakis, A, and Tsolaki, M. Assessing virtual reality environments as cognitive stimulation method for patients with MCI. Technol Inclusive Well-Being. (2014):39–74. doi: 10.1007/978-3-642-45432-5_4
49. Yang, J-G, Thapa, N, Park, H-J, Bae, S, Park, KW, Park, J-H, et al. Virtual reality and exercise training enhance brain, cognitive, and physical health in older adults with mild cognitive impairment. Int J Environ Res Public Health. (2022) 19:13300. doi: 10.3390/ijerph192013300
50. Thapa, N, Park, HJ, Yang, J-G, Son, H, Jang, M, Lee, J, et al. The effect of a virtual reality-based intervention program on cognition in older adults with mild cognitive impairment: a randomized control trial. J Clin Med. (2020) 9:1283. doi: 10.3390/jcm9051283
51. Choi, W, and Lee, S. The effects of virtual kayak paddling exercise on postural balance, muscle performance, and cognitive function in older adults with mild cognitive impairment: a randomized controlled trial. J Aging Phys Act. (2019) 27:861–70. doi: 10.1123/japa.2018-0020
52. Hagovská, M, and Olekszyová, Z. Impact of the combination of cognitive and balance training on gait, fear and risk of falling and quality of life in seniors with mild cognitive impairment. Geriatr Gerontol Int. (2016) 16:1043–50. doi: 10.1111/ggi.12593
53. Hsieh, C-C, Lin, P-S, Hsu, W-C, Wang, J-S, Huang, Y-C, Lim, A-Y, et al. The effectiveness of a virtual reality-based tai chi exercise on cognitive and physical function in older adults with cognitive impairment. Dement Geriatr Cogn Disord. (2019) 46:358–70. doi: 10.1159/000494659
54. Amjad, I, Toor, H, Niazi, IK, Pervaiz, S, Jochumsen, M, Shafique, M, et al. Xbox 360 kinect cognitive games improve slowness, complexity of EEG, and cognitive functions in subjects with mild cognitive impairment: a randomized control trial. Games Health J. (2019) 8:144–52. doi: 10.1089/g4h.2018.0029
55. Law, LL, Mok, VC, and Yau, MM. Effects of functional tasks exercise on cognitive functions of older adults with mild cognitive impairment: a randomized controlled pilot trial. Alzheimers Res Ther. (2019) 11:98–100. doi: 10.1186/s13195-019-0548-2
56. Law, LL, Mok, VC, Yau, MK, and Fong, KN. Effects of functional task exercise on everyday problem-solving ability and functional status in older adults with mild cognitive impairment—a randomised controlled trial. Age Ageing. (2022) 51. doi: 10.1093/ageing/afab210
57. Lin, F, Heffner, KL, Ren, P, Tivarus, ME, Brasch, J, Chen, DG, et al. Cognitive and neural effects of vision-based speed-of-processing training in older adults with amnestic mild cognitive impairment: a pilot study. J Am Geriatr Soc. (2016) 64:1293–8. doi: 10.1111/jgs.14132
58. Optale, G, Urgesi, C, Busato, V, Marin, S, Piron, L, Priftis, K, et al. Controlling memory impairment in elderly adults using virtual reality memory training: a randomized controlled pilot study. Neurorehabil Neural Repair. (2010) 24:348–57. doi: 10.1177/1545968309353328
59. Yang, H-L, Chu, H, Kao, C-C, Chiu, H-L, Tseng, I-J, Tseng, P, et al. Development and effectiveness of virtual interactive working memory training for older people with mild cognitive impairment: a single-blind randomised controlled trial. JA Ageing. (2019) 48:519–25. doi: 10.1093/ageing/afz029
60. Lim, EH, Kim, D-S, Won, Y-H, Park, S-H, Seo, J-H, Ko, M-H, et al. Effects of home based serious game training (brain talk™) in the elderly with mild cognitive impairment: randomized, a single-blind, controlled trial. Brain Neurorehabil. (2023) 16. doi: 10.12786/bn.2023.16.e4
61. Man, DW, Chung, JC, and Lee, GY. Evaluation of a virtual reality-based memory training programme for Hong Kong Chinese older adults with questionable dementia: a pilot study. Int J Geriatr Psychiatry. (2012) 27:513–20. doi: 10.1002/gps.2746
62. Wu, J, He, Y, Liang, S, Liu, Z, Huang, J, Tao, J, et al. Computerized cognitive training enhances episodic memory by down-modulating posterior cingulate-precuneus connectivity in older persons with mild cognitive impairment: a randomized controlled trial. Am J Geriatr Psychiatry. (2023) 31:820–32. doi: 10.1016/j.jagp.2023.04.008
63. Nousia, A, Martzoukou, M, Siokas, V, Aretouli, E, Aloizou, A-M, Folia, V, et al. Beneficial effect of computer-based multidomain cognitive training in patients with mild cognitive impairment. J Appl Neuropsychol Adult. (2021) 28:717–26. doi: 10.1080/23279095.2019.1692842
64. Manenti, R, Gobbi, E, Baglio, F, Macis, A, Ferrari, C, Pagnoni, I, et al. Effectiveness of an innovative cognitive treatment and telerehabilitation on subjects with mild cognitive impairment: a multicenter, randomized, active-controlled study. Front Aging Neurosc. (2020) 12:585988. doi: 10.3389/fnagi.2020.585988
65. Poptsi, E, Lazarou, I, Markou, N, Vassiloglou, M, Nikolaidou, E, Diamantidou, A, et al. A comparative single-blind randomized controlled trial with language training in people with mild cognitive impairment. Am J Alzheimers Dis Other Dement. (2019) 34:176–87. doi: 10.1177/1533317518813554
66. Hyer, L, Scott, C, Atkinson, MM, Mullen, CM, Lee, A, Johnson, A, et al. Cognitive training program to improve working memory in older adults with MCI. Clin Gerontol. (2016) 39:410–27. doi: 10.1080/07317115.2015.1120257
67. Finn, M, and McDonald, S. Neuropsychology, cognition. Repetition-lag training to improve recollection memory in older people with amnestic mild cognitive impairment. A randomized controlled trial. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. (2015) 22:244–58. doi: 10.1080/13825585.2014.915918
AD - Alzheimer’s disease
BBS - Berg Balance Scale
CDT - Clock Drawing Test
CG - Control group
CFQ - Cognitive Failure Questionnaire
CI - Confidence intervals
CVVLT-DR - Chinese Version Verbal Learning Test-Delayed Recall
CVVLT-IR - Chinese Version Verbal Learning Test-Immediate Recall
DSB - Digit Span Backward
DSF - Digit Span Forward
EG - Experimental group
GDS-15 - Geriatric Depression Scale-15
GDS-30 - Geriatric Depression Scale-30
GRADE - Grading of Recommendations, Assessment, Development, and Evaluation
IADL - Instrumental activities of daily living
MCI - Mild cognitive impairment
M/F - Male/Female
MMSE - Mini-Mental State Examination
MoCA - Montreal Cognitive Assessment
NC - Unrecorded
PRISMA - Preferred Reporting Items for Systematic Reviews and Meta-Analyses
QoL-AD - Quality of life for Alzheimer’s disease
RAVLT-DR - Rey Auditory Verbal Learning Test-Delayed Recall
RAVLT-IR - Rey Auditory Verbal Learning Test-Immediate Recall
RCTs - Randomized controlled trials
SDST - Symbol Digit Substitution Test
SMD - Standardized mean difference
TMT-A - Trail Making Test Part A
TMT-B - Trail Making Test Part B
TUG - Timed Up-and-Go Test
VR - Virtual reality
WAIS-BDT - Wechsler Adult Intelligence Scale-Block Design Test
Keywords: virtual reality, mild cognitive impairment, cognitive function, emotional state, quality of life
Citation: Li X, Zhang Y, Tang L, Ye L and Tang M (2025) Effects of virtual reality-based interventions on cognitive function, emotional state, and quality of life in patients with mild cognitive impairment: a meta-analysis. Front. Neurol. 16:1496382. doi: 10.3389/fneur.2025.1496382
Received: 14 September 2024; Accepted: 10 March 2025;
Published: 02 April 2025.
Edited by:
Panagiotis Kourtesis, American College of Greece, GreeceReviewed by:
Tsegaye Alemu, Hawassa University, EthiopiaCopyright © 2025 Li, Zhang, Tang, Ye and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Tang, dGFuZ21pbjg4NzI1OTJAc2luYS5jb20=
†These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.