1 Introduction
All activities of entities occur within specific temporal and spatial scales. These scales are mutually restrictive. The spatiotemporal scales of neural activity are subject to the structural and functional organization of the brain. There is a strong connection between the size of the brain and the frequency of neural activity (1), while each brain region or neural circuit has its own spectral characteristics (2) (see Figure 1A). Consequently, cognitive-specific spectral fingerprints should be present if each cognitive function is realized by a distinct neural circuit. This phenomenon is elucidated by the spectral fingerprint hypothesis of cognition (3) (see Figure 1B). Recent studies have not only uncovered the frequency characteristics of specific brain regions or neural circuits but also shown that each cognitive process fluctuates at a preferential frequency (4).
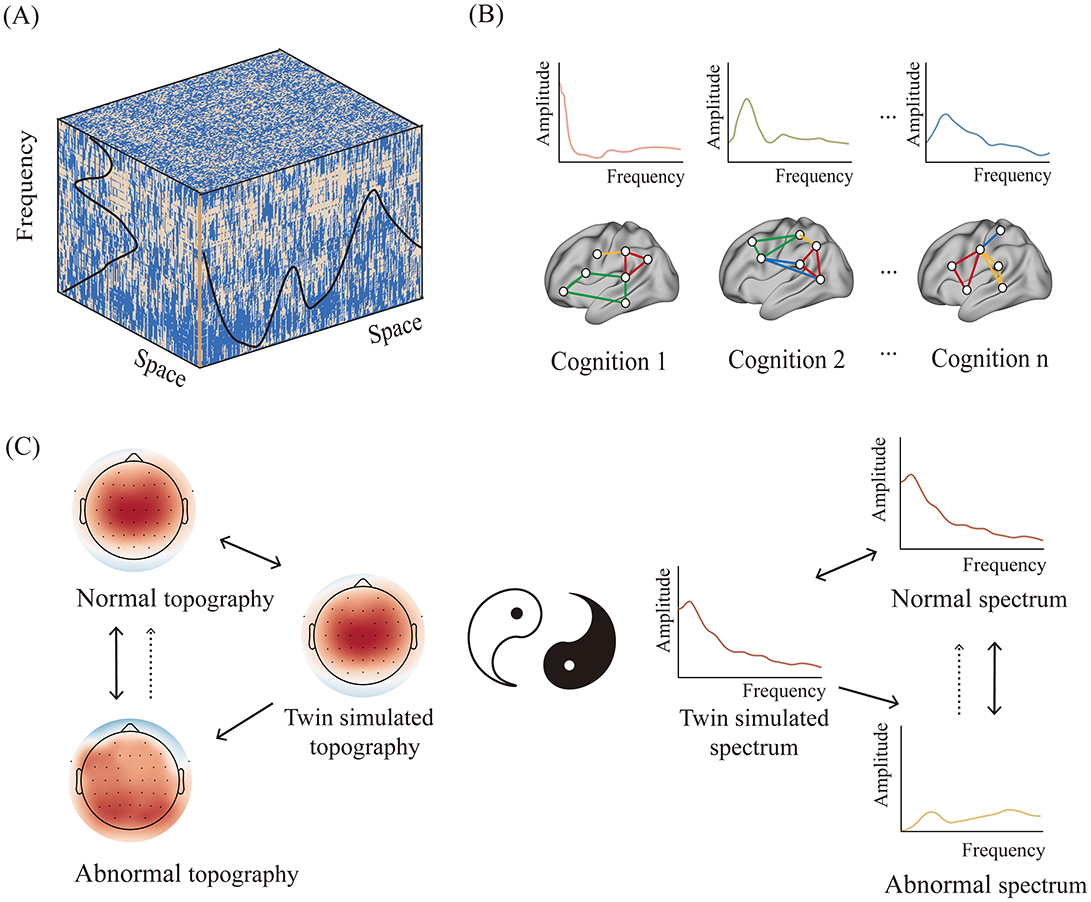
Figure 1. The intrinsic spatiotemporal structure of cognition and its implication for the intervention of brain functions. (A) The cognitive-specific spatiotemporal brain structure. (B) Cognitive fingerprints defined by specific neural circuits and power spectra. (C) Intervening in abnormal brain functions through the simulated twin spatiotemporal structure of normal brain functions.
In specificity, if a comprehensive cognitive function is divided into distinct cognitive processing stages, each associated with different neural circuits, then this cognitive function can be characterized by the spatiotemporal structure of nested neural circuits and corresponding spectral profiles. Assessing the level achievable by each cognitive function depends on the resemblance between the spatiotemporal structure of its neural circuit and the optimal spatiotemporal structure. Therefore, the intrinsic spatiotemporal structure of cognition is crucial for understanding the neural mechanisms that govern normal and pathological cognitive processes, as well as for cognitive rehabilitation and interventions in brain function.
2 Brain stimulation based on the intrinsic spatiotemporal structure of cognitions
By engaging in long-term cognitive training, individuals can optimize their cognitive abilities and gradually stabilize the neural circuits associated with those cognitive abilities, resulting in the emergence of specific spatiotemporal structures (5). The spatiotemporal structure of the neural circuit associated with optimal cognitive functioning can be defined as the intrinsic spatiotemporal structure of that cognitive function. Young adults, prodigies, or individuals with professional training often provide models that closely approximate the intrinsic spatiotemporal structure (6). Although many cognitive functions may appear similar at first glance, they are difficult to transfer from one to another due to their exclusive spatiotemporal structure. Different cognitive functions may share many brain regions, networks, and neural oscillations. However, this overlap is insufficient to produce transfer effects across cognitive functions after cognitive training. A specific cognitive function is defined by the complete spatiotemporal structure formed by both shared and non-shared components. Due to differences in intrinsic spatiotemporal structures, each cognitive function is unique, which may be a key reason for the lack of far-transfer effects in cognitive training. We refer to this phenomenon as cognitive isolation. Similar to productive isolation, cognitive isolation impedes the transition between two distinct cognitive functions. This may be a key reason for the absence of far-transfer effects in cognitive training (7).
The intrinsic spatiotemporal structure of cognitive functions may provide advantageous targets for brain stimulation. Current interventions for brain function are limited in their efficacy as they are restricted to non-specific neural circuits or frequencies, making it difficult to achieve an ideal brain state (6). On the other hand, interventions that target specific spatial or frequency elements of brain function have yielded remarkable outcomes (8). Therefore, it is exciting to anticipate the potential outcomes of brain stimulation techniques that are based on intrinsic spatiotemporal structures. In other words, achieving the optimal frequency of activity in each neural circuit, along with their interactions, may lead to the attainment of optimal cognitive function.
With the advancement of digital twin technology, it may become possible to mirror the twin spatiotemporal structure of every cognitive function in the future. According to the neural entrainment theory, this simulated twin spatiotemporal structure may cause the neural system to resonate in specific patterns (9), thereby optimizing the corresponding cognitive functions (see Figure 1C). It is encouraging that efforts have been made to combine high-definition transcranial electrical stimulation with broadband or band-removed spectral profiles, yielding favorable outcomes (10). This has promising implications for brain function rehabilitation and cognitive enhancement.
3 Discussion
The implementation of precision interventions is pivotal in improving the efficacy of non-invasive brain stimulation techniques. As each cognitive function is tied to specific neural circuits, stimulating a neural circuit at its intrinsic frequency inevitably yields more precise regulation of that cognitive function. However, when multiple cognitive processes or neural circuits are impaired, as commonly observed in mental disorders, the regulation of a single circuit or frequency may not necessarily achieve the desired outcome. Similarly, to enhance holistic cognitive functions, rather than focusing solely on improving a single stage of cognitive processing, it is essential to coordinate multiple circuits at various frequencies. In this case, the intrinsic spatiotemporal structure of cognitive functions can provide a more optimal solution.
Author contributions
YW: Conceptualization, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. CZ: Software, Visualization, Writing – original draft. QL: Supervision, Writing – review & editing. XJ: Validation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Social Science Foundation of China (BBA200030).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Buzsáki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. (2013) 80:751–64. doi: 10.1016/j.neuron.2013.10.002
2. Keitel A, Gross J. Individual human brain areas can be identified from their characteristic spectral activation fingerprints. PLoS Biol. (2016) 14:e1002498. doi: 10.1371/journal.pbio.1002498
3. Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nature Reviews Neuroscience. (2012) 13:121–34. doi: 10.1038/nrn3137
4. Palva S, Palva JM. Roles of brain criticality and multiscale oscillations in temporal predictions for sensorimotor processing. Trends Neurosci. (2018) 41:729–43. doi: 10.1016/j.tins.2018.08.008
5. Yang GR, Joglekar MR, Song HF, Newsome WT, Wang X-J. Task representations in neural networks trained to perform many cognitive tasks. Nat Neurosci. (2019) 22:297–306. doi: 10.1038/s41593-018-0310-2
6. Qiao J, Wang Y, Wang S. Natural frequencies of neural activities and cognitions may serve as precise targets of rhythmic interventions to the aging brain. Front Aging Neurosci. (2022) 14:988193. doi: 10.3389/fnagi.2022.988193
7. Sala G, Gobet F. Cognitive training does not enhance general cognition. Trends Cogn Sci. (2019) 23:9–20. doi: 10.1016/j.tics.2018.10.004
8. Cash RFH, Cocchi L, Lv J, Fitzgerald PB, Zalesky A. Functional magnetic resonance imaging–guided personalization of transcranial magnetic stimulation treatment for depression. JAMA psychiatry. (2021) 78:337–9. doi: 10.1001/jamapsychiatry.2020.3794
9. Zhang S, Qin Y, Wang J, Yu Y, Wu L, Zhang T. Noninvasive electrical stimulation neuromodulation and digital brain technology: a review. Biomedicines. (2023) 11:1513. doi: 10.3390/biomedicines11061513
Keywords: intrinsic spatiotemporal structure, precise intervention, spectral fingerprint, digital twin, brain stimulation
Citation: Wang Y, Zhang C, Liu Q and Jing X (2025) The intrinsic spatiotemporal structure of cognitive functions inspires the intervention of brain functions. Front. Neurol. 16:1494673. doi: 10.3389/fneur.2025.1494673
Received: 13 September 2024; Accepted: 24 January 2025;
Published: 13 February 2025.
Edited by:
Mariella Pazzaglia, Sapienza University of Rome, ItalyReviewed by:
Jiansong Zhou, Central South University, ChinaCopyright © 2025 Wang, Zhang, Liu and Jing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yifeng Wang, d3lmQHNpY251LmVkdS5jbg==; Xiujuan Jing, anhqc2ljbnVAMTYzLmNvbQ==
 Yifeng Wang
Yifeng Wang Chi Zhang
Chi Zhang Qiang Liu
Qiang Liu Xiujuan Jing
Xiujuan Jing