- 1Cognitive Neuroscience Laboratory, Brain Injury Research, Shirley Ryan AbilityLab, Chicago, IL, United States
- 2Department of Physical Medicine and Rehabilitation, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
- 3Institute of Cognitive Sciences Marc Jeannerod, CNRS/UMR 5229, Bron, France
- 4University Claude Bernard Lyon 1, Villeurbanne, France
- 5Department of Psychology, National University, San Diego, CA, United States
- 6Department of Neurology, Boston University, Boston, MA, United States
- 7School of Systems Biology, George Mason University, Fairfax, VA, United States
- 8Department of Psychology, University of Mannheim, Mannheim, Germany
- 9Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 10Department of Cognitive Science, Johns Hopkins University, Baltimore, MD, United States
- 11Departments of Neurology, Northwestern University, Chicago, IL, United States
- 12Departments of Psychiatry, Northwestern University, Chicago, IL, United States
- 13Cognitive Neurology & Alzheimer's Disease, Feinberg School of Medicine, Northwestern University, Chicago, IL, United States
- 14Department of Psychology, Northwestern University, Chicago, IL, United States
Attachment style shapes one's connections with important figures in their life. One such unique relationship is the connection to God (CTG), which may be shaped by attachment style. Stronger CTG has been associated with secure attachment, yet the neural mechanisms underlying this relationship remain unclear. While previous research has implicated the prefrontal cortex (PFC) in CTG, findings have been mixed and may depend on attachment style—an idea that has yet to be directly tested. This study aimed to (1) examine whether individuals with a secure attachment style report higher levels of CTG compared to those with a non-secure attachment style, and (2) identify the brain regions associated with CTG in individuals with secure vs. non-secure attachment. We assessed attachment style and CTG in a sample of male combat veterans (N = 150), the majority of whom had focal traumatic brain injuries (pTBI; N = 119). Brain imaging (CT scans) was also obtained. Behaviorally, after controlling for age, years of education, and brain volume loss, individuals with a secure attachment style reported stronger CTG. Voxel-based lesion-symptom mapping revealed that damage to the right orbitofrontal cortex was associated with stronger CTG in individuals with secure—but not insecure—attachment. These findings suggest that attachment style shapes CTG at both behavioral and neural levels. Moreover, they highlight the potential role of attachment style in TBI recovery, offering insights that could inform spiritually integrated therapeutic interventions and support strategies.
1 Introduction
The desire to form meaningful relationships with others is a fundamental human need. Attachment theory (1) is a psychological framework that suggests that early relationships with caregivers shape an individual's emotional bonds and how they relate to others throughout adult life (2, 3). Adult attachment is classified into four styles: a secure style and three insecure subtypes—fearful, preoccupied, and dismissive. A large body of research suggests that attachment style can impact one's self-esteem, romantic relationships and friendships (4–6).
Just as attachment patterns influence interpersonal relationships, they can also extend to one's connection with God, influencing the perception of God as a secure or insecure attachment figure (7–9). Specifically, people with secure attachment styles were shown to be more likely to develop a secure, positive relationship with God, viewing God as a supportive and loving figure (10, 11).
Traumatic brain injury (TBI) is a major global health challenge with profound impacts on survivors and their families (12). Despite its widespread effects, there is limited knowledge on how attachment style influences the recovery journey. Specifically, the impact of attachment style on an individual's connection to God after suffering a TBI, and the neuronal mechanisms underlying such impact, remain unexplored.
1.1 Neural basis of connection to God
Religious belief and connection to God are uniquely human experiences, yet their neural basis was unexamined for years. However, over the past two decades, a growing number of studies have aimed to uncover the neuronal underpinnings of these experiences (13, 14). For instance, regions within the social cognition network—such as the temporopolar region, medial prefrontal cortex, temporoparietal junction, and precuneus—were shown to be active during personal prayers to God (15). This aligns with the idea that religious individuals often perceive God as a relational partner in their religious practices.
In particular, the prefrontal cortex (PFC) has been suggested as a key region associated with religious belief and connection to God. This is consistent with the PFC's role in social relationships, affective processing, and its connections to the reward network. However, while some studies found that the PFC plays a role in religiosity and the experience of connection to God (16, 17), others have reported no significant involvement of this region (18, 19).
In summary, the current literature presents mixed findings regarding the PFC's role in one's connection to God (20, 21). While some studies suggest its involvement, it remains unclear whether this relationship is consistent or influenced by individual differences. One possibility is that the PFC's role on connection to God depends on attachment style, but this hypothesis has yet to be directly tested. However, although attachment theory has generated a rich body of research, the neural bases of its potential impact remain unknown. Investigating this intersection could provide valuable insights into the role of attachment in the spiritual aspects of recovery, potentially informing targeted therapeutic interventions.
1.2 Current study
The primary objective of this study is to investigate how attachment style can impact one's connection to God on the behavioral and neuronal level. The sample included 150 combat veterans who participated in the Vietnam Head Injury Study (VHIS) (22, 23). The majority of this sample had localized penetrating traumatic brain injuries (N = 119).
To examine impact of attachment style on connection to God on the behavioral level, we collected data from two surveys assessing attachment style [i.e., the relationship questionnaire (RQ) (24), and the Relationship Scales Questionnaire (RSQ) (25)], as well as three surveys assessing connection to God [i.e., selected items from the God Image Inventory (26); the religious experience scale (27); and the religious emphasis scale (28), see detailed description below].
To examine the underlying neuronal involvement, we conducted a Voxel-Based Lesion Symptom Mapping (VLSM) on individuals with secure and insecure attachment style. This technique analyzes voxel-wise brain damage across individuals to identify brain regions associated with a behavioral outcome.
This an exploratory study with two aims. The first aim of this study was to examine whether individuals with a secure attachment style demonstrate elevated levels of connection to God compared to individuals with a non-secure attachment style, regardless of brain damage. The second aim was to identify, using VLSM, the brain areas associated with connection to God in individuals with secure attachment, relative to individuals with non-secure attachment.
2 Materials and methods
2.1 Participants
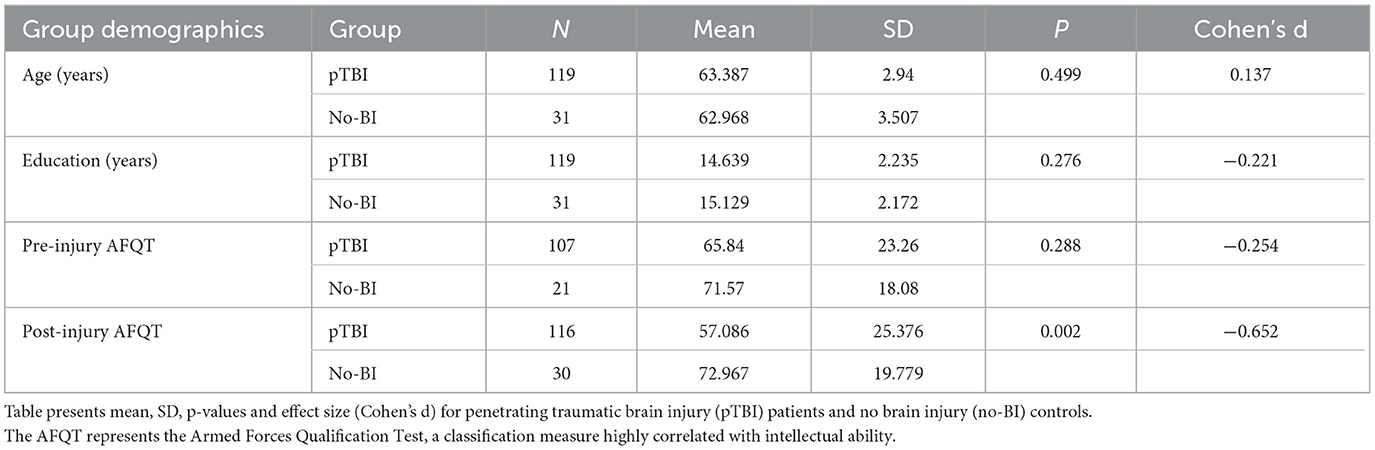
Participants were drawn from phase IV of the W.F. Caveness Vietnam Head Injury Study (VHIS) registry, which is a prospective, long-term follow-up observational study of male veterans with focal penetrating traumatic brain injury (pTBI) and with no brain injury (no-BI). During phase four of the study (2008–2012; ~40–45 years post-injury) we assessed 150 individuals, 119 of them with pTBI and 31 with no brain injury. The age range of participants was 59–81 years, with 98% (146/150) being 69 years or younger. The pTBI and no-BI groups were matched with respect to age, level of education, and pre-injury general intelligence, measured using the Armed Forces Qualification Test (AFQT; Table 1).
As one would expect, the pTBI group scored lower than controls on a measure of post-injury cognitive abilities (AFQT) (29). However, both groups scored within the normal range of the test scores (above the 50th percentile). The lesion overlay density map for all participants in the pTBI group can be found in Figure 1.
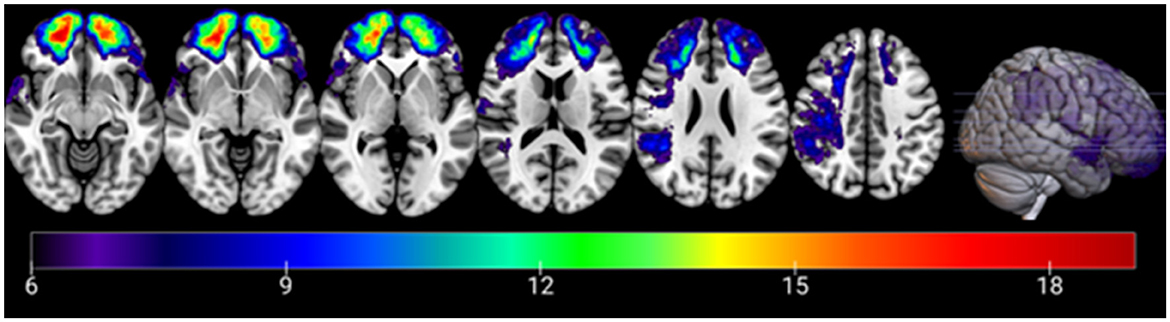
Figure 1. Overlay density map of the lesions in the penetrating traumatic brain injury group. Numbers on the scale represent the number of patients with lesion in a specific voxel (6 patients threshold). The color legend indicates the number of patients with damage to a particular voxel. Z values from left to right: −10, −5, 0, 15, 25, 40. Images are in radiological space (i.e., right is left).
The study was approved by an Institutional Review Board at the National Institute of Neurological Disorders and Stroke at the National Institute of Health, Bethesda, MD, USA, and the current analysis was approved by the Northwestern University Institutional Review Board.
2.2 CT acquisition and analysis
Axial CT scans without contrast were obtained using a GE Medical Systems Light Speed Plus CT scanner at the Bethesda Naval Hospital, Bethesda, MD. These scans were conducted during Phase 3 of the Vietnam Head Injury Study (2003–2006). Although more recent CT scans were conducted during Phase 4 (2008–2012) for clinical purposes, an NIH staff radiologist reviewed them and reported no new lesions or significant pathological changes compared to Phase 3. CT scans were utilized because most of the pTBI participants had retained metal in their brain.
Structural neuroimaging data were reconstructed with an in-plane voxel size of 0.4 x 0.4 mm, an overlapping slice thickness of 2.5 mm, and a 1-mm slice interval. Lesion location and volume from CT images were determined using the interactive ABLe software (30), implemented in MEDx v3.44 (Medical Numerics) with enhancements to support the Automated Anatomical Labeling (AAL) atlas (31).
Each scan was normalized to a CT template brain image in Montreal Neurological Institute (MNI) space. A trained neuropsychiatrist performed manual tracing for each lesion, which was later reviewed by a blinded observer (J.G.) to reach a consensus on lesion extent.
2.3 Voxel-based lesion-symptom mapping
A VLSM analysis (32) was applied to test the association between the traced brain lesions for each participant and their responses on the surveys measuring CTG. In this VLSM analysis, the CTG scores of patients with a lesion in each voxel are compared to CTG score of patients without a lesion in this voxel using a t-test (see detailed description of CTG measures below). To have sufficient statistical power and to be able to test regions all over the brain, voxels that did not have at least 6 patients with damage were excluded from the analysis. Note that the overlay map of lesion locations for 119 patients (Figure 1) map shows brain regions with lesions present for at least six participants in each voxel consistent with the constraints of the VLSM as described above. The map shows a sufficient degree of overlap to draw conclusions for all the target brain regions. Participants' lesion size was used as a covariate in this analysis.
The analysis was carried out using the VLSM package version 2.60 (https://aphasialab.org/vlsm/) on MATLAB R2022a (Mathworks, Natick, MA) software. Anatomic labeling was performed with Automated Anatomical Labeling (AAL) atlas, and Natbrainlab atlas of white matter pathways (33). VLSM results were visualized in MRICronGL (https://www.nitrc.org/projects/mricrogl) overlaid on an MNI standard brain. All t-tests were two-tailed and a P < 0.05 was considered statistically significant.
2.4 Neuropsychological testing
Participants were assessed from 2009 to 2012 at the National Institutes of Health in Bethesda, MD, over a 5- to 7-day period with tests that measured a wide variety of cognitive, social and personality measures. For this study, we focused on the assessment of connection to God and attachment style.
2.4.1 Connection to God
Participants completed the following 3 scales:
1. God Image Inventory (26). This inventory reflects an internal model of the sort of person that the individual imagines God to be. The original God Image Inventory consists of 156 items. Since our participants were assessed in a variety of tasks and questionnaires over a week, we decided to select 14 items from the Presence and Salience subscales of the God Image Inventory. The Cronbach's alpha for the God Image Inventory is 0.958. Examples of the items are: “I can talk to God on an intimate basis,” “God tells me what he wants from me.” A higher score indicates a stronger sense of attachment to God and the presence of God. See Supplementary Text S1 for the complete list of God Image Inventory items we used.
2. Religious experience scale (27). The scale reflects the perceived influence of God in one's life, including feelings of being forgiven for sins and referring to God when making decisions. This scale consists of 12 items. The Cronbach's alpha for the Religious Experience Scale is 0.96. Examples of items are “I experience an awareness of God's love” and “I pray privately in places other than church.” A higher score indicates a stronger influence of God in the individual's life. See Supplementary Text S1 for the complete list of the religious experience scale items.
3. Religious emphasis scale (28). This scale reflects the extent to which parents emphasized religious behaviors during development. The scale consists of 10 items. The Cronbach's alpha for the God Emphasis Scale is 0.92. Examples of the items involved different religious behavior such as “taking part in religious youth groups,” “praying before a meal,” or “Going to church: attending religious services.” A higher score indicates a stronger emphasis parents gave to religious behaviors. See Supplementary Test S1 for the complete list of the religious emphasis scale.
An exploratory factor analysis (EFA) was conducted on the items of the God Image Inventory, Religious Experience Scale, and Religious Emphasis Scale. We then extracted the individualized factor scores for further use in subsequent analyses. The number of items per scale (10–14) and the sample size (N = 150) aligns with recent guidelines for exploratory factor analysis (34).
Specifically, we used the factor score regression method (35), standardizing the computed factor scores to a mean of zero and a standard deviation of one. This approach was chosen to enable standardized comparisons across scales.
2.4.2 Attachment style measures
Assessment styles were measured using two different methods. The first method provided a categorical measure of attachment, based on participants' selection of the one attachment style that best described them [the Relationship Questionnaire (RQ); (24)]. The second method provided continuous scores for each attachment style by asking participants to rate themselves on four different attachment dimensions [the Relationship Scales Questionnaire (RSQ); (25)]. Both measures are widely used in the adult attachment literature (36).
1. The RQ (24) consists of a single-item measure, where participants were asked to identify their attachment style by reading four short paragraphs, each describing close relationships in adulthood, and selecting the one that best described them. In the second part, RQ2, participants are asked to rate their agreement with each prototype on a 7-point scale. The highest of the four attachment prototype ratings is then used to classify participants into an attachment category. The RQ was shown to be a valid tool in assessing attachment styles, in both healthy and clinical (37) samples.
2. The RSQ (25) is a 30-item self-report measure assessing four attachment styles: secure, fearful, preoccupied, and dismissive. Items are rated on a five-point scale, with mean scores calculated for each style. The measure has adequate reliability and convergent validity (25).
2.5 Statistical analyses
Prior to analyses, missing values (Religious emphasis scale, n = 5; God Image Inventory n = 10; Religious experience scale n = 9) were replaced by mean of the scale. Behavioral data analysis was carried out using JASP 0.19.3 (38) with the alpha level set to P < 0.05 (two-tailed).
First, given that we define CTG as a single construct measured repeatedly using three different tools, and that the assumption of homogeneity of variance was met (Levene's test, all P > 0.16), we used a repeated measures analysis of variance to evaluate the effect of attachment style on CTG measures. Effect size (η2) was calculated. Following recent recommendations for multiple statistical analyses to assess robustness (39), we complemented the frequentist approach with an equivalent Bayesian analysis. We report Bayes Factor (BF10) as an odds ratio, indicating the likelihood of the data under one hypothesis compared to another (40). A correlation analysis was used to evaluate the association between the level of endorsement of each attachment style (as measured by RSQ), with the three measures of connection to God. The assumption of data normality was not met (Shapiro-Wilk, P < 0.01), hence Spearman's Rho correlations were used.
3 Results
3.1 Associations between attachment style and measures of connection to God
3.1.1 Secure attachment vs. non-secure attachment styles
The pTBI sample was split into a secure group (i.e., 57 individuals who endorsed a secure attachment style) and a non-secure group (i.e., 93 individuals who endorsed dismissing, fearful, or preoccupied style), based on responses to the categorical question from the RQ.
First, we conducted a repeated measures analysis of variance with the scores in the three measures of connection to God as repeated measures, and the attachment style (secure vs. non-secure) as a between-participant factor. We included age, years of education, and total brain volume loss (measured in cc) as covariates in this analysis.
We found a significant main effect for attachment style, such that individuals with secure attachment scored higher on the measures of connection to God compared to individuals with a non-secure attachment style (F1,145= 4.272, P = 0.041, η2 = 0.020; BF10= 0.618, Figure 2). There was no main effect of CTG measure nor was there interaction. A post-hoc analysis was subsequently conducted using the Bonferroni correction. No significant differences were found between secure and non-secure attachment styles on any individual CTG measure (all Pbonf > 0.5).
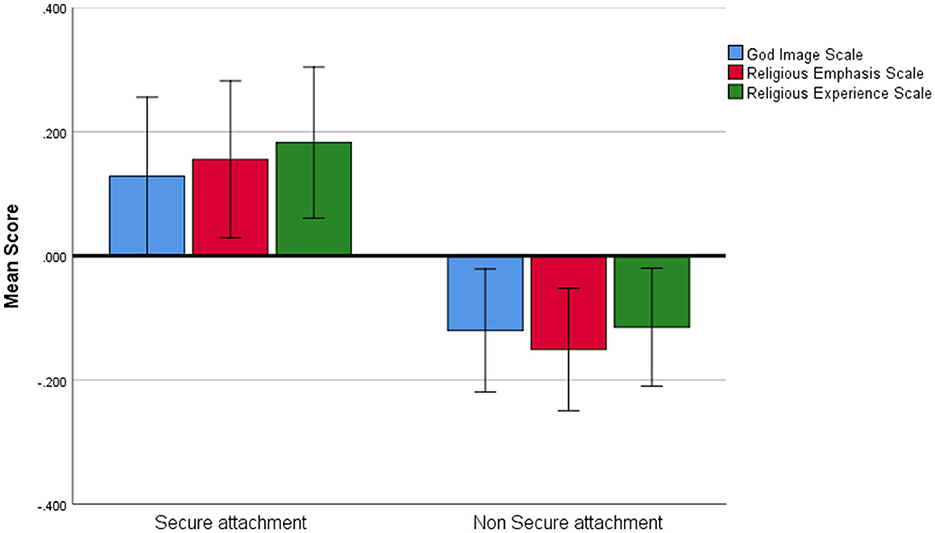
Figure 2. Measures of connection to God. Average scores on the three measures of connection to God in individuals with secure and non-secure attachment styles. Error bars represent one standard error.
3.1.2 Correlations between attachment styles and connection to God
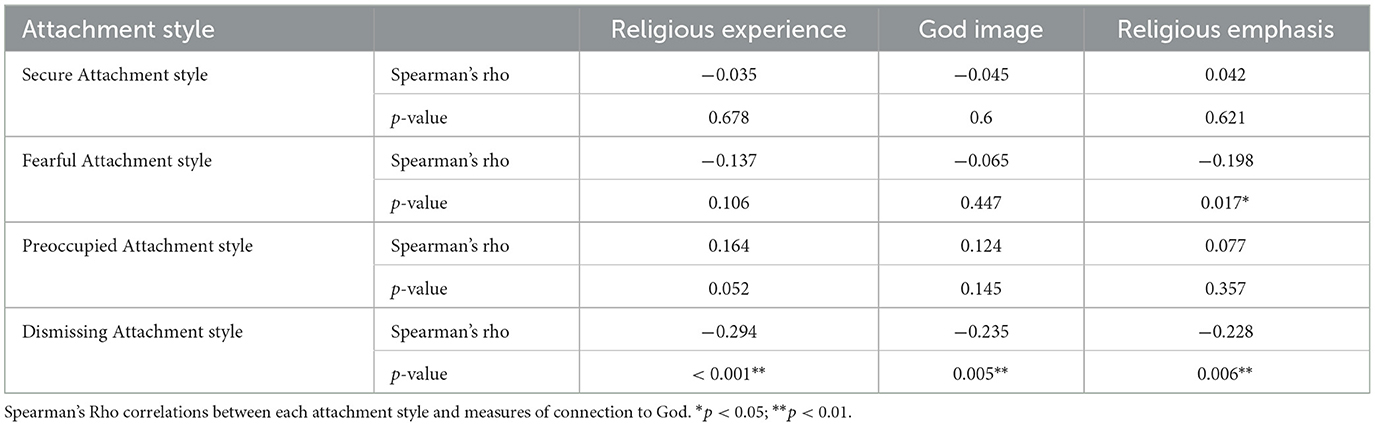
Endorsements of each specific attachment style (as measured by RSQ) were correlated with the three measures of connection to God. A negative correlation was found between dismissing attachment style and all three measures of connection to God (see Table 2). This finding indicates that participants with a more dismissing orientation (avoiding intimacy, self-reliant) score lower on measures of CTG (report weaker connection to God and religious practice).
3.2 VLSM analysis
A whole-brain VLSM analysis was performed for pTBI participants with secure and non-secure attachment separately (grouping was made based on responses to the RQ). For each group, three analyses were conducted with the three connection to God measures as the outcome, and total brain volume loss as a covariate.
3.2.1 VLSM results: secure attachment
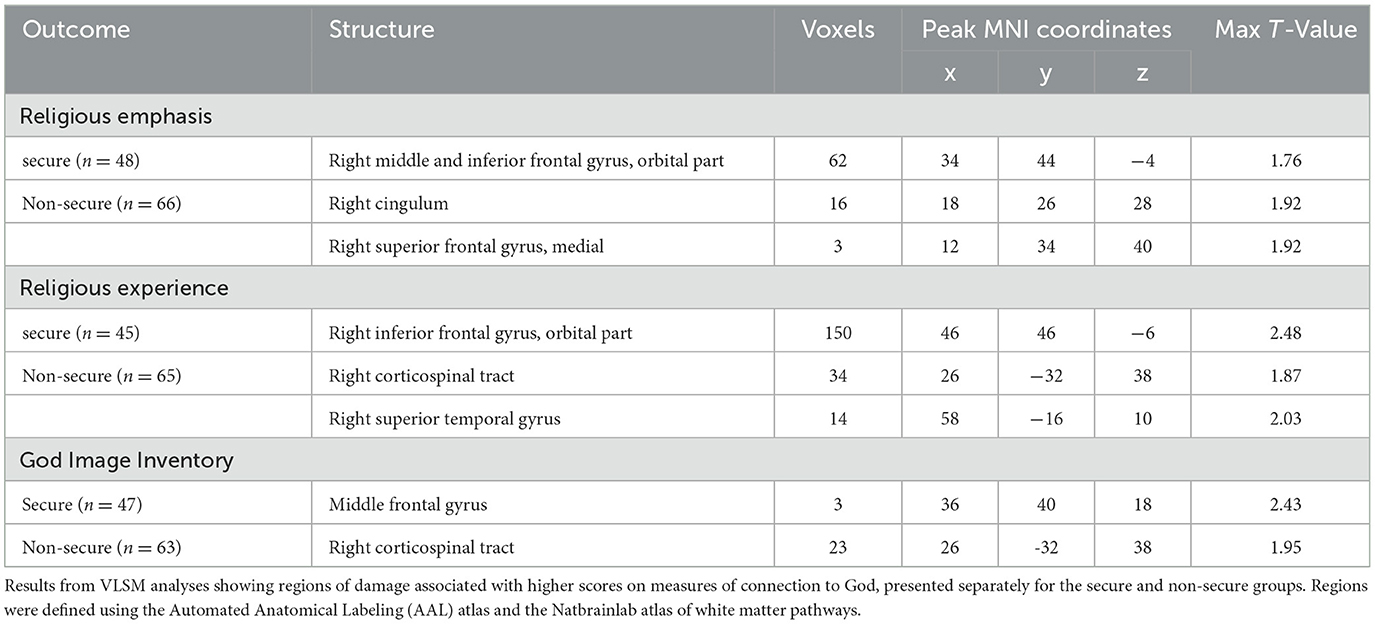
Whole-brain VLSM analysis on participants with secure attachment (n = 48) revealed two significant overlapping clusters. The first, for the religious emphasis scale (volume = 62 voxels, Max t = 1.76) was located predominantly in the orbital part of the middle and inferior frontal gyrus in the right hemisphere. The peak MNI coordinates were (34 44 −4). The second, for the religious experience scale (volume = 150 voxels, Max t = 2.48), cluster was located primarily within the orbital part of the inferior frontal gyrus in the right hemisphere. The peak MNI coordinates were (46 46−6). A smaller (volume = 3 voxels, Max t = 2.43) cluster was associated with the God image inventory, in the Middle frontal gyrus The peak MNI coordinates were (36 40 18; see Table 3 and Figure 3A).
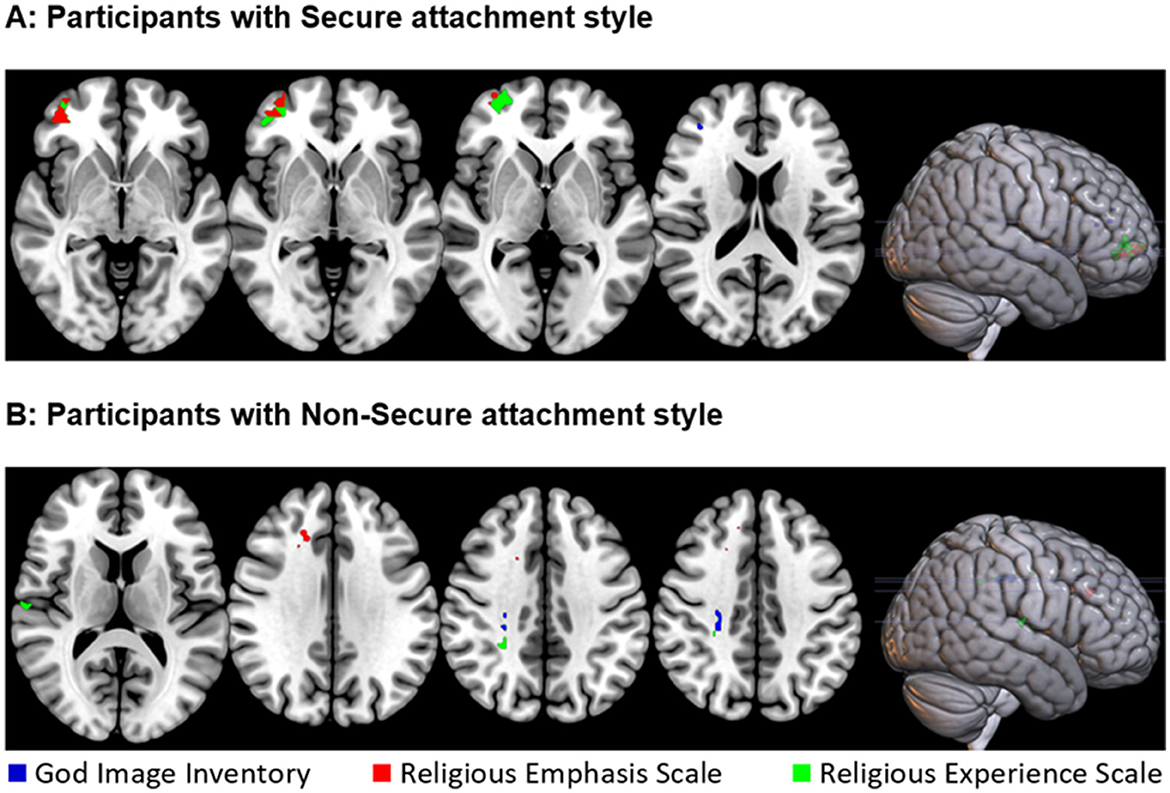
Figure 3. Location of brain lesions which significantly associates with stronger connection to God, as measured by the God image inventory (in blue), the religious emphasis scale (in red), and the religious experience (in green). All images are presented in radiological space (i.e., the right is shown on the left). (A) Results for participants with secure attachment. Z values (from left to right): −4, −2, 0, 18. (B) Results for participants with non-secure attachment. Z values (from left to right): 12, 32, 38, 40.
3.2.2 VLSM results: non-secure attachment
Whole-brain VLSM analysis on participants with an insecure attachment style (n = 66) revealed a few small clusters of < 50 voxels, mostly in white matter areas, including overlapping clusters for the religious experience and God Image scores at the right corticospinal tract. Other clusters were identified at the right cingulum, the right medial superior frontal gyrus, and the right superior temporal gyrus (see Table 3 and Figure 3B).
4 Discussion
This is the first study to investigate the neural correlates of connection to God in individuals with different attachment styles. Our findings suggest that attachment style can shape one's connection to God on the behavioral and the neuronal level, showing that (1) individuals with a secure attachment style report a stronger connection to God, and (2) the role of the right orbitofrontal cortex (OFC) in underlying CTG is different for individuals with secure and insecure attachment style. We will discuss the results and their implications, as well as the challenges in this area of research and future potential directions next.
4.1 Attachment style and connection to God: behavioral findings
Findings from this study suggest that individuals with secure attachment style form a stronger connection to God compared to individuals with an insecure attachment style, regardless of brain lesion location. These results are in line with previous studies reporting an association between secure attachment style and a closer connection to God or religious practice in the general population (41).
This finding supports the correspondence hypothesis, a concept in the psychology of religion that suggests that the nature of an individual's attachment style will correspond to their relationship with God. Specifically, if someone had a secure attachment with their caregivers, they are likely to perceive God as loving and caring, while those with insecure attachments might view God as distant or punitive (8, 42). However, it is important to note that the correspondence hypothesis is referring to attachment to God, while in this study we measured Connection to God which is a broader term.
We also found that dismissive attachment style is specifically associated with a weaker connection to God, regardless of brain injury. People with dismissing attachment style withdraw from intimate relationships and tend to be self-reliant. Our data is therefore in line with the expectation that people with dismissing attachment style will be more likely to face problems on their own and not reach out to God for support. Moreover, these results support previous findings reporting an association between dismissing attachment style and lack of intimacy in a relationship with God, in a sample of undergraduate college students (10). It is also in line with a study that showed that people with dismissing attachment style were less likely to plead to God compared to people with a preoccupied and secure attachment style (43).
4.2 Attachment style and connection to God: neuronal findings
Our findings indicate that the brain regions involved in an individual's CTG are influenced by their attachment style. For individuals with secure attachment, lesions to the right orbital part of inferior and middle frontal gyrus are associated with stronger connection to God, while for individuals with insecure attachment, those regions were not associated with a connection to God.
The observed link between the right-hemisphere connection to God aligns with previous research suggesting that the right hemisphere plays a dominant role in emotional processing and affective experiences (44–46), including those related to spirituality and religiosity (17, 47, 48).
4.2.1 The orbitofrontal cortex and connection to God
The OFC is functionally related to the ventromedial prefrontal cortex (49), which is often associated with religious beliefs and a relationship with God (17, 21, 50). Moreover, the medial OFC uniquely is associated with subjectively rewarding and pleasant stimuli (51–53), which may include positive religious beliefs and experiences (54).
There are several potential explanations to the finding that individuals with a secure attachment style show a stronger connection to God after a lesion to the right OFC (rOFC). First, it is important to note that findings from this study do not necessarily imply that the rOFC directly impacts connection to God, even when it is intact. Instead, one possible interpretation is related to the rOFC role in modulating balanced emotional processing (55). It is possible that when the rOFC is damaged, individuals may experience reduced emotional stability. For those with a secure attachment style, a strong connection to God may serve as a compensatory source of emotional comfort, whereas individuals with an insecure attachment style may not find the same solace in their religious beliefs, and therefor do not show elevated connection to God following damage in this area.
Another possible interpretation is that the rOFC inhibits other intact brain areas that enable the strong relationship with God observed in the securely attached patients. This interpretation is challenged by the fact that regions that are known to share an antagonistic relationship with the OFC and the vmPFC [e.g., frontoparietal and lateral parietal regions (56, 57)] have shown to be involved in doubting religious belief and spiritual concepts (58). For instance, excitatory TMS to the right inferior parietal cortex reduced biases toward religious and spiritual concepts (47), and disbelief in the efficacy of prayer is associated with deactivations in the temporopolar/orbitofrontal regions (15). Yet, a different imaging study showed an association between activation in the right OFC to increased religious experience (48). Future research on the brain basis of connection to God is needed to identify areas directly regulated by the OFC, that when released from its inhibition allows for a stronger relationship with God, at least in securely attached patients.
Lastly, it is possible that given the role of the rOFC in psychological flexibility (59, 60) a damage in this area might result in stronger beliefs in religious fundamentalism and authoritarianism (61, 62).
4.3 Study limitations and future research direction
This study has a unique methodological strength which is achieved by combining behavioral data (attachment and connection to God) and neuroanatomical data (brain lesion mapping) in a large sample. Nevertheless, as with any study targeting a complex social concept, this study is not free of limitations which we acknowledge here.
First, we assessed attachment using brief, self-report measures. The assessment of attachment therefore reflects individuals' subjective perceptions of their close relationships, which may be vulnerable to reporting bias. Future research may benefit from adding interview methodologies over self-report assessments of adult attachment (63). Yet, it is important to note that the tools that were used in this study are among the most reliable of their kind, and are frequently used in research on adult attachment, providing consistent results (36).
Second, the sample in our study was composed of older male participants. Men and women may exhibit different attachment styles (64). Moreover, aging is associated with more important role for religion in one's life (65), and can influence cognitive and emotional changes. Therefore, the results may not be generalizable to younger or female samples and should be interpreted with these considerations in mind. However, it is important to note that age and gender cannot account for the observed differences between secure and insecure attachment groups reported in this study. Future studies are required to evaluate the generalizability of the results reported here on diverse samples of men and women, in different age groups and from different cultural backgrounds.
Lastly, it is important to note that the reported VLSM analysis did not include a correction for multiple comparisons, which fits the exploratory nature of the study. Correcting for multiple comparisons in this case could potentially obscure meaningful associations that may guide future research directions. Importantly, we were able to replicate our findings by conducting three separate VLSM analyses—one for each outcome measure—and obtaining similar results. Furthermore, the observed difference between secure and insecure attachment styles cannot be attributed to the lack of correction for multiple comparisons, as it remained consistent across all analyses. Nonetheless, future studies can focus on the rOFC a-priori as a region of interest and incorporate permutation analysis as correction (66, 67).
Although our study leaves some questions unresolved, it nevertheless offers new data that ties attachment style to connection to God using both behavioral and lesion mapping methods.
5 Conclusions
In conclusion, the present study provides evidence that attachment style impacts one's connection to God, with secure attachment being associated with stronger connection to God regardless of prior brain injury. It also suggests that attachment style impacts the brain regions associated with connection to God, with a lesion in the rOFC being associated with stronger connection to God in individuals with secure- and not insecure- attachment style. These findings deepen our understanding of how attachment style influences spiritual experiences, which can have implications for personalized approaches to spiritual care. Furthermore, this study enhances our understanding of the brain mechanisms underlying spiritual experiences, offering valuable insights into the neural substrates of faith and religiosity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board at the National Institute of Neurological Disorders and Stroke at the National Institute of Health, Bethesda, MD, USA, and the current analysis was approved by the Northwestern University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SCZ: Conceptualization, Formal analysis, Methodology, Writing – original draft. IC: Conceptualization, Writing – original draft. PM: Funding acquisition, Writing – review & editing. FK: Data curation, Project administration, Writing – review & editing. BG: Writing – review & editing. JG: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The VHIS study was funded by the Department of Defense and the National Institute of Neurological Disorders and Stroke. The current analysis was supported by a grant from the John F. Templeton Foundation (JG and PM) and the Therapeutic Cognitive Neuroscience Fund (BG). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors would like to thank all the Vietnam veterans who participated in this study. We would also like to thank The National Naval Medical Center and the National Institute of Neurological Disorders and Stroke for providing their facilities and supporting this research. We thank V. Raymont, S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, K. Reding, G. Tasick, S. Bonifant, M. Tierney, L. Glass, L. Yozawitz, C. Noury, V. Tsen, and A. Leopold for testing participants and organizing the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1488890/full#supplementary-material
References
1. Ainsworth MS, Bowlby J. An ethological approach to personality development. Am Psychol. (1991) 46:333. doi: 10.1037//0003-066X.46.4.333
2. Mikulincer M, Shaver PR. Attachment in Adulthood: Structure, Dynamics, and Change. New York: Guilford Publications (2010).
3. Hazan C, Shaver P. Romantic love conceptualized as an attachment process. J Pers Soc Psychol. (1987) 52:511–24. doi: 10.1037//0022-3514.52.3.511
4. Bylsma WH, Cozzarelli C, Sumer N. Relation between adult attachment styles and global self-esteem. Basic Appl Soc Psych. (1997) 19:1–16. doi: 10.1207/15324839751037101
5. Shi L. The Association between adult attachment styles and conflict resolution in romantic relationships. Am J Fam Ther. (2003) 31:143–57. doi: 10.1080/01926180301120
6. Grabill CM, Kerns KA. Attachment style and intimacy in friendship. Pers Relatsh. (2000) 7:363–78. doi: 10.1111/j.1475-6811.2000.tb00022.x
7. Kirkpatrick LA, Shaver PR. Attachment theory and religion: childhood attachments, religious beliefs, and conversion. J Sci Stud Relig. (1990) 28:315–34. doi: 10.2307/1386461
8. Kirkpatrick LA. God as a substitute attachment figure: a longitudinal study of adult attachment style and religious change in college students. Pers Soc Psychol Bull. (1998) 24:961–73. doi: 10.1177/0146167298249004
9. Kirkpatrick LA, Shaver PR. An attachment-theoretical approach to romantic love and religious belief. Pers Soc Psychol Bull. (1992) 18:266–75. doi: 10.1177/0146167292183002
10. McDonald A, Beck R, Allison S, Norswortby L. Attachment to god and parents: testing the correspondence vs. compensation hypotheses. J Psychol Christ. (2005) 24. Available online at: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=0e3b08c4ba5718bdbee58102e33bda77bf778938
11. Granqvist P, Kirkpatrick LA. Attachment and religious representations and behavior. In:Cassidy J, Shaver PR, , editors. Handbook of Attachment: Theory, Research, and Clinical Applications. 2nd ed. New York, NY: The Guilford Press (2008). p. 906–33.
12. Johnson WD, Griswold DP. Traumatic brain injury: a global challenge. Lancet Neurol. (2017) 16:949–50. doi: 10.1016/S1474-4422(17)30362-9
13. McNamara P, Newsome W, Linkenhoker B, Grafman J. Neuroscientists must not be afraid to study religion. Nature. (2024) 631:25–7. doi: 10.1038/d41586-024-02153-7
14. Grafman J, Cristofori I, Zhong W, Bulbulia J. The neural basis of religious cognition. Curr Dir Psychol Sci. (2020) 29:126–33. doi: 10.1177/0963721419898183
15. Schjoedt U, Stødkilde-Jørgensen H, Geertz AW, Lund TE, Roepstorff A. The power of charisma—perceived charisma inhibits the frontal executive network of believers in intercessory prayer. Soc Cogn Affect Neurosci. (2011) 6:119–27. doi: 10.1093/scan/nsq023
16. Pelletier-Baldelli A, Dean DJ, Lunsford-Avery JR, Smith Watts AK, Orr JM, Gupta T, et al. Orbitofrontal cortex volume and intrinsic religiosity in non-clinical psychosis. Psych Res Neuro. (2014) 222:124–30. doi: 10.1016/j.pscychresns.2014.03.010
17. Cohen-Zimerman S, Cristofori I, Zhong W, Bulbulia J, Krueger F, Gordon B, et al. Neural underpinning of a personal relationship with God and sense of control: a lesion-mapping study. Cogn Affect Behav Neurosci. (2020) 20:575–87. doi: 10.3758/s13415-020-00787-4
18. Schjødt U, Stødkilde-Jørgensen H, Geertz AW, Roepstorff A. Rewarding prayers. Neurosci Lett. (2008) 443:165–8. doi: 10.1016/j.neulet.2008.07.068
19. Kapogiannis D, Barbey AK, Su M, Zamboni G, Krueger F, Grafman J. Cognitive and neural foundations of religious belief. Proc Nat Acad Sci. (2009) 106:4876–81. doi: 10.1073/pnas.0811717106
20. Rim JI, Ojeda JC, Svob C, Kayser J, Drews E, Kim Y, et al. Current understanding of religion, spirituality, and their neurobiological correlates. Harv Rev Psychiatry. (2019) 27:303. doi: 10.1097/HRP.0000000000000232
21. Cristofori I, Zhong W, Cohen-Zimerman S, Bulbulia J, Gordon B, Krueger F, et al. Brain networks involved in the influence of religion on empathy in male Vietnam War veterans. Sci Rep. (2021) 11:11047. doi: 10.1038/s41598-021-90481-3
22. Raymont V, Salazar AM, Krueger F, Grafman J. “Studying injured minds” - the Vietnam head injury study and 40 years of brain injury research. Front Neurol. (2011) 28:2:15. doi: 10.3389/fneur.2011.00015
23. Cristofori I, Cohen-Zimerman S, Krueger F, Jabbarinejad R, Delikishkina E, Gordon B, et al. Studying the social mind: an updated summary of findings from the Vietnam head injury study. Cortex. (2024) 174:164–88. doi: 10.1016/j.cortex.2024.03.002
24. Bartholomew K, Horowitz LM. Attachment styles among young adults: a test of a four-category model. J Pers Soc Psychol. (1991) 61:226–44. doi: 10.1037//0022-3514.61.2.226
25. Griffin DW, Bartholomew K. Models of the self and other: fundamental dimensions underlying measures of adult attachment. J Pers Soc Psychol. (1994) 67:430. doi: 10.1037//0022-3514.67.3.430
26. Lawrence RT. Measuring the image of god: the god image inventory and the god image scales. J Psychol Theol. (1997) 25:214–26. doi: 10.1177/009164719702500206
27. Edwards KJ. Sex-role behavior and religious experience. Research in mental health and religious behavior: an introduction to research in the integration of Christianity and the behavioral sciences. (1976) 224–38.
28. Altemeyer B. Enemies of Freedom: Understanding Right-wing Authoritarianism. Hoboken, NJ: Jossey-Bass (1988).
29. Plag JA, Goffman JM. The armed forces qualification test: its validity in predicting military effectiveness for naval enlistees. Pers Psychol. (1967) 20:323–40. doi: 10.1111/j.1744-6570.1967.tb01527.x
30. Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions (ABLe). Comput Methods Programs Biomed. (2007) 86:245–54. doi: 10.1016/j.cmpb.2007.02.006
31. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. (2002) 15:273–89. doi: 10.1006/nimg.2001.0978
32. Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion–symptom mapping. Nat Neurosci. (2003) 6:448–50. doi: 10.1038/nn1050
33. Thiebaut de. Schotten M, Bizzi A, Dell'Acqua F, Allin M, Walshe M, Murray R, et al. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage. (2011) 54:49–59. doi: 10.1016/j.neuroimage.2010.07.055
34. de Winter JCF, Dodou D, Wieringa PA. Exploratory factor analysis with small sample sizes. Multivariate Behav Res. (2009) 44:147–81. doi: 10.1080/00273170902794206
35. DiStefano C, Zhu M, Mindrila D. Understanding and using factor scores: considerations for the applied researcher. Pract Assess Res Eval. (2009) 14.
36. Both LE Best LA A A comparison of two attachment measures in relation to personality factors and facets. Pers Individ Dif. (2017) 112:1–5. doi: 10.1016/j.paid.2017.02.040
37. Wongpakaran N, DeMaranville J, Wongpakaran T. Validation of the relationships questionnaire (rq) against the experience of close relationship-revised questionnaire in a clinical psychiatric sample. Healthcare. (2021) 9:1174. doi: 10.3390/healthcare9091174
39. Wagenmakers EJ, Sarafoglou A, Aczel B. One statistical analysis must not rule them all. Nature. (2022) 605:423–5. doi: 10.1038/d41586-022-01332-8
40. Jarosz AF, Wiley J. What are the odds? A practical guide to computing and reporting Bayes factors. J Prob Solv. (2014) 7:2. doi: 10.7771/1932-6246.1167
41. Eurelings-Bontekoe EHM, Hekman-Van Steeg J, Verschuur MJ. The association between personality, attachment, psychological distress, church denomination and the god concept among a non-clinical sample. Ment Health Relig Cult. (2005) 8:141–54. doi: 10.1080/13674670412331304320
42. Granqvist P. Religiousness and perceived childhood attachment: on the question of compensation or correspondence. J Sci Study Reli. (1998) 350–67. doi: 10.2307/1387533
43. Cooper LB, Bruce AJ, Harman MJ, Boccaccini MT. Differentiated styles of attachment to god and varying religious coping efforts. J Psychol Theol. (2009) 37:134–41. doi: 10.1177/009164710903700205
44. Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the patient activation measure (pam): conceptualizing and measuring activation in patients and consumers. Health Serv Res. (2004) 39:1005–26. doi: 10.1111/j.1475-6773.2004.00269.x
45. Hartikainen KM. Emotion-attention interaction in the right hemisphere. Brain Sci. (2021) 11:1006. doi: 10.3390/brainsci11081006
46. Schore AN. Attachment and the regulation of the right brain. Attach Hum Dev. (2000) 2:23–47. doi: 10.1080/146167300361309
47. Crescentini C, Di Bucchianico M, Fabbro F, Urgesi C. Excitatory stimulation of the right inferior parietal cortex lessens implicit religiousness/spirituality. Neuropsychologia. (2015) 70:71–9. doi: 10.1016/j.neuropsychologia.2015.02.016
48. Beauregard M, Paquette V. Neural correlates of a mystical experience in Carmelite nuns. Neurosci Lett. (2006) 405:186–90. doi: 10.1016/j.neulet.2006.06.060
49. Delgado MR, Beer JS, Fellows LK, Huettel SA, Platt ML, Quirk GJ, et al. Viewpoints: dialogues on the functional role of the ventromedial prefrontal cortex. Nat Neurosci. (2016) 19:1545–52. doi: 10.1038/nn.4438
50. Ferguson MA, Nielsen JA, King JB, Dai L, Giangrasso DM, Holman R, et al. Reward, salience, and attentional networks are activated by religious experience in devout Mormons. Soc Neurosci. (2018) 13:104–16. doi: 10.1080/17470919.2016.1257437
51. Rolls ET, Cheng W, Feng J. The orbitofrontal cortex: reward, emotion and depression. Brain Commun. (2020) 2:fcaa196. doi: 10.1093/braincomms/fcaa196
52. Xie C, Jia T, Rolls ET, Robbins TW, Sahakian BJ, Zhang J, et al. Reward versus nonreward sensitivity of the medial versus lateral orbitofrontal cortex relates to the severity of depressive symptoms. Biol. Psychiatry Cogn Neurosci Neuro. (2021) 6:259–69. doi: 10.1016/j.bpsc.2020.08.017
53. Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Rev Neurosci. (2005) 6:691–702. doi: 10.1038/nrn1747
54. McNamara P, Butler PM. The Neuropsychology of Religious Experience. In:Paloutzian RF, Park CL, , editors, Handbook of the psychology of religion and spirituality (2nd Edn). New York, NY : The Guilford Press. (2013) p. 215–233.
55. Schore AN. Modern attachment theory. In:Gold SN, , editor. APA Handbook of Trauma Psychology: Foundations in Knowledge. Washington, DC: American Psychological Association (2017). p. 389–406. doi: 10.1037/0000019-020
56. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Nat Acad Sci. (2005) 102:9673–8. doi: 10.1073/pnas.0504136102
57. Uddin LQ, Kelly AM, Biswal BB, Xavier Castellanos F, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality human brain mapping. (2009) 30:625–37. doi: 10.1002/hbm.20531
58. Jack AI, Friedman JP, Boyatzis RE, Taylor SN. Why do you believe in god? relationships between religious belief, analytic thinking, mentalizing and moral concern. PLoS ONE. (2016) 11:e0149989. doi: 10.1371/journal.pone.0149989
59. Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. (2000) 123:2189–202. doi: 10.1093/brain/123.11.2189
60. Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. (2012) 16:147–56. doi: 10.1016/j.tics.2012.01.005
61. Asp E, Ramchandran K, Tranel D. Authoritarianism, religious fundamentalism, and the human prefrontal cortex. Neuropsychology. (2012) 26:414. doi: 10.1037/a0028526
62. Zhong W, Cristofori I, Bulbulia J, Krueger F, Grafman J. Biological and cognitive underpinnings of religious fundamentalism. Neuropsychologia. (2017) 100:18–25. doi: 10.1016/j.neuropsychologia.2017.04.009
63. Jacobvitz D, Curran M, Moller N. Measurement of adult attachment: the place of self-report and interview methodologies. Attach Hum Dev. (2002) 4:207–15. doi: 10.1080/14616730210154225
64. Mickelson KD, Kessler RC, Shaver PR. Adult attachment in a nationally representative sample. J Pers Soc Psychol. (1997) 73:1092. doi: 10.1037//0022-3514.73.5.1092
66. Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. (2007) 19:1081–8. doi: 10.1162/jocn.2007.19.7.1081
Keywords: attachment style, connection to God, voxel-based lesion-symptom mapping (VLSM), traumatic brain injury (TBI), orbitofrontal cortex (OFC)
Citation: Cohen-Zimerman S, Cristofori I, McNamara P, Krueger F, Gordon B and Grafman J (2025) Attachment style and its impact on connection to God in individuals with brain injury: behavioral and lesion-based findings. Front. Neurol. 16:1488890. doi: 10.3389/fneur.2025.1488890
Received: 30 August 2024; Accepted: 31 March 2025;
Published: 24 April 2025.
Edited by:
Marianna Delussi, University of Bari Aldo Moro, ItalyReviewed by:
Xinyang Liu, East China Normal University, ChinaEva Pettemeridou, University of Limassol, Cyprus
Brick Johnstone, Shepherd Center, United States
Copyright © 2025 Cohen-Zimerman, Cristofori, McNamara, Krueger, Gordon and Grafman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shira Cohen-Zimerman, c2NvaGVuemltZUBzcmFsYWIub3Jn; Jordan Grafman, amdyYWZtYW5Abm9ydGh3ZXN0ZXJuLmVkdQ==
 Shira Cohen-Zimerman
Shira Cohen-Zimerman Irene Cristofori
Irene Cristofori Patrick McNamara
Patrick McNamara Frank Krueger
Frank Krueger Barry Gordon
Barry Gordon Jordan Grafman
Jordan Grafman