- 1Department of Basic Sciences, College of Science and Health Professions, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
- 2King Abdullah International Medical Research Center, Jeddah, Saudi Arabia
- 3College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Riyadh, Saudi Arabia
- 4Department of Pharmacy Practice, Faculty of Pharmacy, University of Tabuk, Tabuk, Saudi Arabia
- 5Department of Pharmacy Practice, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia
Background: Stroke recurrence is a serious and prevalent complication of ischemic stroke that warrants additional investigation.
Methods: A hospital-based retrospective observational study included acute-subacute ischemic stroke adult patients. The primary aim was to determine the risk factors associated with recurrent stroke within 365 days. Additionally, a combined outcome consisting of any stroke recurrence or all-cause mortality within 365 days was considered secondary outcome. Univariate and multivariable Cox proportional-hazards models were used to examine the association of risk factors with stroke recurrence and composite death/stroke recurrence.
Results: Of 1,244 patients, 112 (9%) experienced stroke recurrence. The multivariable analysis identified risk factors for stroke recurrence including history of previous stroke (HR = 3.65, 95% CI:2.28–5.99, p = 0.0001), tissue plasminogen activator (tPA) treatment (HR = 2.84, 95% CI:1.57–4.86, p = 0.0003), seizure (HR = 1.96, 95% CI:1.14–3.22, p = 0.0105), and depression (HR = 2.26, 95% CI:1.33–3.69, p = 0.0016). Only previous stroke history (HR = 2.37, 95% CI:1.74–3.26, p = 0.0001) remained significantly associated with the combined outcome of stroke recurrence/death. Additional risk factors for the composite outcome included older age of patients (HR = 1.02, 95% CI:1.01–1.03, p = 0.0009), admission to the intensive care unit (ICU) (HR = 3.70, 95% CI:2.63–5.22, p = 0.0105), pneumonia (HR = 1.47, 95% CI:1.05–2.05, p = 0.0249), and brain edema (HR = 2.36, 95% CI:1.58–3.46, p = 0.0001).
Conclusion: Key findings include a stroke recurrence rate of 9.96% and a combined death/stroke recurrence rate of 21.83% within 365 days. Multivariable analysis confirmed that history of stroke, receiving tPA, experiencing seizures, and depression were significantly associated with stroke recurrence. Implementing additional preventive measures for individuals in these high-risk categories is essential. Further studies are needed to validate our findings.
1 Introduction
Stroke stands as the global second-leading cause of death and the third-leading cause of combined mortality and disability (1). It represents a significant health burden, with an increased global incidence rate of 70% over the last three decades (1). Ischemic stroke is the most prevalent type, accounting for 87% of all stroke cases. Recurrent stroke presents an ominous complication for patients who have experienced an initial ischemic stroke (2). Compared to initial stroke, recurrence stroke is more difficult to treat and carries higher mortality rates. Despite advancements in clinical care, the risk of recurrence remains alarmingly high, with nearly 40% of patients experiencing a recurrent stroke within 5 years (3). Therefore, prevention is of considerable importance to both individual and public health. Early identification of recurrent stroke and optimal management are essential to improve patient care and quality of life.
A thorough understanding of the risk factors for stroke recurrence is vital for enhancing secondary prevention strategies. On a global scale, hypertension emerges as the most dominant risk factor, followed by hyperlipidemia, ischemic heart disease, atrial fibrillation (AF), and diabetes mellitus (DM) (3, 4). In addition, other studies have identified hypercholesterolemia and obesity as significant risk factors for recurrent stroke (5, 6). Locally, one prospective study held in one region of Saudi Arabia identified hypertension and DM as substantial risk factors for recurrent stroke, with both conditions coexisting in a high proportion of recurrent stroke patients. Ischemic heart disease was featured prominently in almost half of the enrolled subjects, as well as smoking and AF (7).
Despite the existing evidence related to some of the risk factors associated with the incidence of recurrent stroke, many potential contributors have not been extensively investigated. Thus, this study aimed to determine the incidence and contributing risk factors of stroke recurrence among patients with acute and subacute ischemic stroke in two tertiary hospitals.
2 Patients and methods
2.1 Study design
A hospital-based retrospective observational study was carried out on individuals with acute and subacute ischemic stroke treated at two tertiary hospitals in different cities between June 2016 and September 2022. Electronic medical records (BESTCare® 2.0) were used to screen eligible patients based on the inclusion and exclusion criteria, Figure 1. Data was extracted using a secured data collection sheet in Excel.
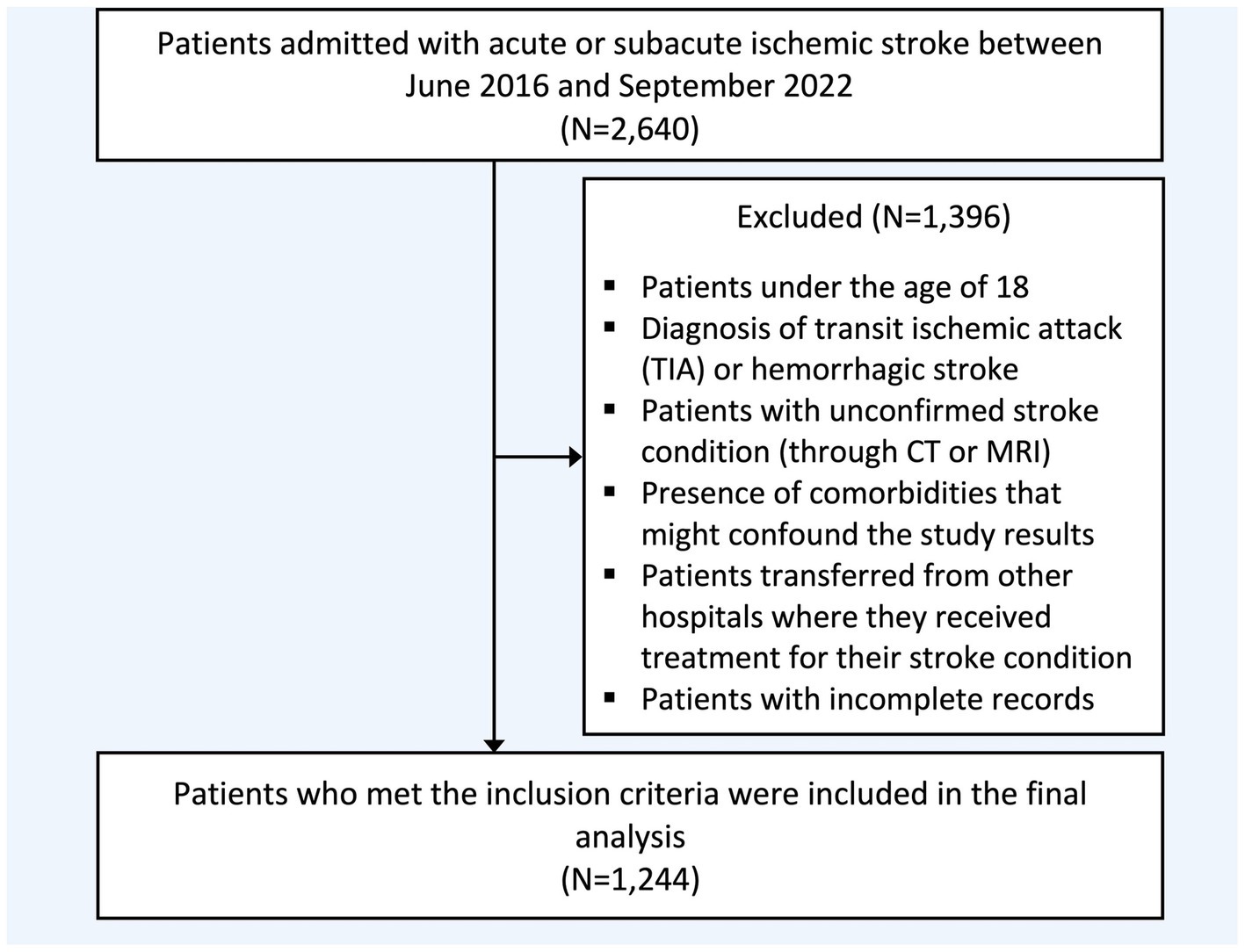
Figure 1. Flowchart of study population selection. CT, Computed tomography; MRI, Magnetic resonance imaging.
2.2 Study participants
Inclusion criteria comprised patients who are 18 years or older and arrived at the emergency room within 7 days of stroke symptoms or were admitted at the incident time with a confirmed ischemic stroke diagnosis using either a magnetic resonance imaging (MRI) or a computed tomography (CT) scan. Exclusion criteria comprised patients with a diagnosis of transient ischemic attack (TIA) or hemorrhagic stroke, the presence or history of significant comorbidities that could confound the study results (intracranial pathology, tumors, or traumatic brain injury), those transferred from other hospitals, and individuals with missing or incomplete medical or laboratory records, Figure 1.
2.3 Outcomes
Patients were followed up retrospectively for up to 1 year. The primary outcome was the incidence of stroke recurrence within 365 days in patients with ischemic stroke. The definition of stroke recurrence is ischemic or hemorrhagic stroke qualifying for the following conditions: A new neurological deficit or deterioration of the previous deficit not considered to be due to toxic effects of drug therapy, hemorrhagic transformation, or concomitant acute brain injury occurring within 7 days of the index stroke (8, 9). Furthermore, we also considered a combined secondary outcome comprising death or stroke recurrence within 365 days following the initial stroke onset. Death was defined as mortality from any cause (10).
2.4 Data collection and potential confounders
The collected data included demographic variables such as age and gender, date of arrival, and date of stroke. Potential modifiable risk factors included the National Institutes of Health Stroke Scale (NIHSS) score, previous history of stroke, intensive care unit (ICU) admission, and comorbidities, including hypertension, DM, dyslipidemia, AF, dementia, and depression (11). In addition, data regarding treatment intervention, including mechanical thrombectomy and tissue-type plasminogen activator (tPA), were also collected. Other potential confounders included post-stroke complications such as seizure, pneumonia, cerebral edema, deep vein thrombosis-pulmonary embolism (DVT-PE), and impaired consciousness. The stroke subtype was determined according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification. The TOAST classification denotes five subtypes of ischemic stroke: (1) large-artery atherosclerosis, (2) cardioembolism, (3) small-vessel occlusion, (4) stroke of other determined etiology, and (5) stroke of undetermined etiology (9).
2.5 Statistical analysis
Normally distributed continuous variables were presented as mean ± standard deviation, while non-normally distributed variables were expressed as median and interquartile range (IQR). Gray’s Test for Equality of Cumulative Incidence Functions was employed to assess the equality of cumulative risks. Cox proportional-hazards models were used to analyze univariate associations of risk factors with stroke recurrence and death/stroke recurrence. Furthermore, multivariable Cox proportional-hazards models with the backward elimination method were used to examine the association of risk factors with stroke recurrence and death/stroke recurrence. Patients were monitored until the earliest occurrence of stroke recurrence, death, or the censoring date, defined as either 1 year from the index date (stroke onset) or September 1, 2023, whichever came first. Censoring for stroke recurrence occurred if the cause of death was unspecified or attributed to a cause other than stroke. All statistical analyses were performed using SAS software version 9.4.
3 Results
3.1 Baseline and clinical characteristics of patients included in the study
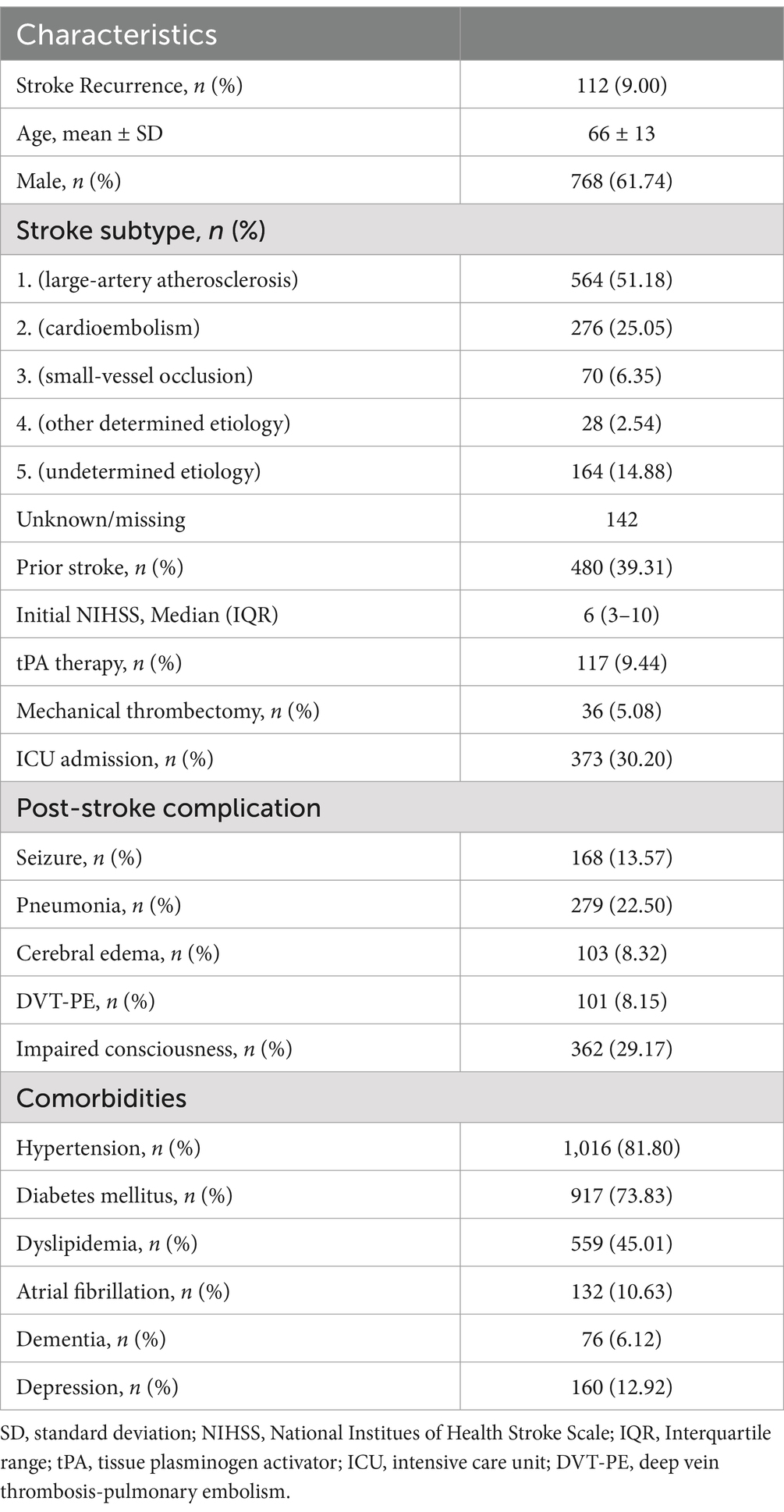
A total of 2,640 patients underwent screening, with 1,244 included in the final analysis, Table 1. The mean age was 66 years, and 38.26% were female. Patients with a history of more than one stroke at baseline accounted for 39.31% of the sample. The median NIHSS score, reflecting initial clinical severity, was 6 (IQR: 3–10). The rate of individuals receiving tPA and mechanical thrombectomy was 9.44 and 5.08%, respectively. ICU admission during hospitalization was documented in 30.20% of the sample. The reported comorbidities included hypertension (n = 1,016, 81.80%), DM (n = 917, 73.83%), dyslipidemia (n = 559, 45.01%), AF (n = 132, 10.63%), dementia (n = 76, 6.12%), and depression (n = 160, 12.92%). Reported post-stroke complications were seizure (n = 168, 13.57%), pneumonia (n = 279, 22.50%), cerebral edema (n = 103, 8.32%), DVT-PE (n = 101, 8.15%), and impaired consciousness (n = 362, 29.17%).
3.2 Cumulative risk of developing stroke recurrence or death/stroke recurrence
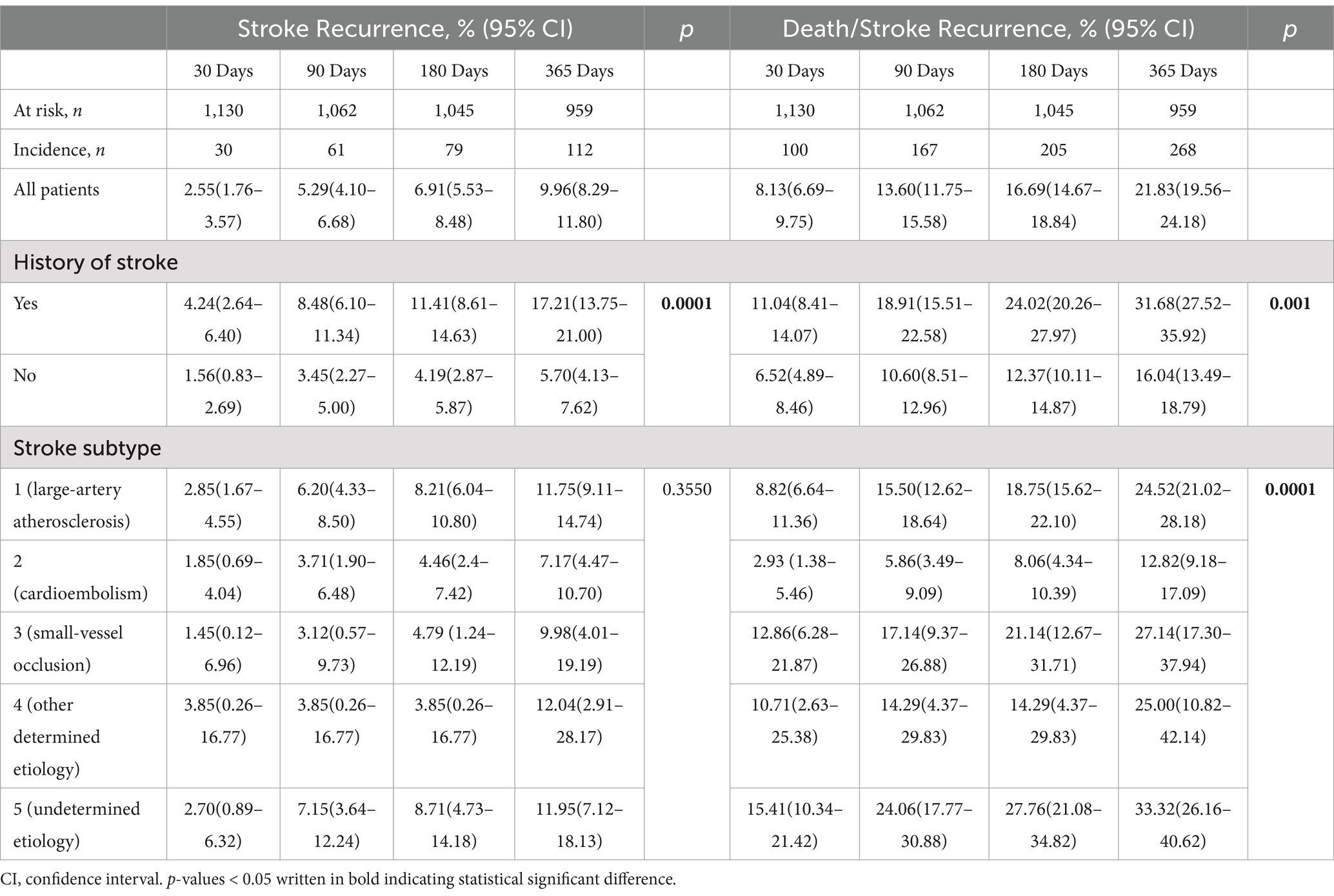
The incidence rate of stroke recurrence in the first 365 days after stroke was 9.96% (95% CI: 8.29–11.80). Incidence rates at specific intervals were as follows: 2.55% (95% CI: 1.76–3.57) at 30 days, 5.29% (95% CI: 4.10–6.68) at 90 days, and 6.91% (95% CI: 5.53–8.48) at 180 days. Previous history of stroke was significantly associated with an increased risk of recurrence (p = 0.0001). Recurrence rates for patients with a prior stroke history were notably higher compared to those without, at 30 days (4.24% vs. 1.56%), 90 days (8.48% vs. 3.45%), 180 days (11.41% vs. 4.19%), and 365 days (17.21% vs. 5.70%), Table 2.
The incidence rate of death or stroke recurrence within the first 365 days after stroke was 21.83% (95% CI: 19.56–24.18). Cumulative incidence rates at specific time intervals were as follows: 8.13% (95% CI: 6.69–9.75) at 30 days, 13.60% (95% CI: 11.75–15.58) at 90 days, and 16.69% (95% CI: 14.67–18.84) at 180 days. Patients with a history of stroke experienced significantly higher rates of death or stroke recurrence compared to those without a prior history. Incidence rates were markedly higher across all periods: 11.04% vs. 6.52% at 30 days, 18.91% vs. 10.60% at 90 days, 24.02% vs. 12.37% at 180 days, and 31.68% vs. 16.04% at 365 days. Furthermore, variations in stroke subtypes significantly influenced the cumulative incidence of death or stroke recurrence over time.
3.3 Risk factors associated with stroke recurrence
Univariate analysis (Table 3) identified several risk factors significantly associated with stroke recurrence. These included age (HR = 1.03, 95% CI: 1.01–1.03, p < 0.0001), history of stroke (HR = 3.17, 95% CI: 2.16–4.73, p < 0.0001), stroke subtype two vs. one as a reference (HR = 0.59, 95% CI: 0.35–0.98, p = 0.0477), tPA treatment (HR = 2.07, 95% CI: 1.23–3.31, p = 0.0038), ICU admission (HR = 1.89, 95% CI: 1.28–2.77, p = 0.0012), seizures (HR = 2.52, 95% CI: 1.63–3.78, p < 0.0001), pneumonia (HR = 1.58, 95% CI:1.03–2.37, p = 0.0305), brain edema (HR = 1.93, 95% CI:1.01–3.38, p = 0.0311), DVT-PE (HR = 1.86, 95% CI:1.01–3.14, p = 0.0306), hypertension (HR = 1.95, 95% CI:1.12–3.73, p = 0.0294), dyslipidemia (HR = 1.70, 95% CI:1.17–2.50, p = 0.0057), and depression (HR = 2.56, 95% CI:1.66–3.85, p < 0.0001). Upon multivariable analysis (Table 4), risk of stroke recurrence remained significantly associated with a prior history of stroke (HR = 3.65, 95% CI:2.28–5.99, p = 0.0001), tPA (HR = 2.84, 95% CI:1.57–4.86, p = 0.0003), seizure (HR = 1.96, 95% CI:1.14–3.22, p = 0.0105), and depression (HR = 2.26, 95% CI:1.33–3.69, p = 0.0016).
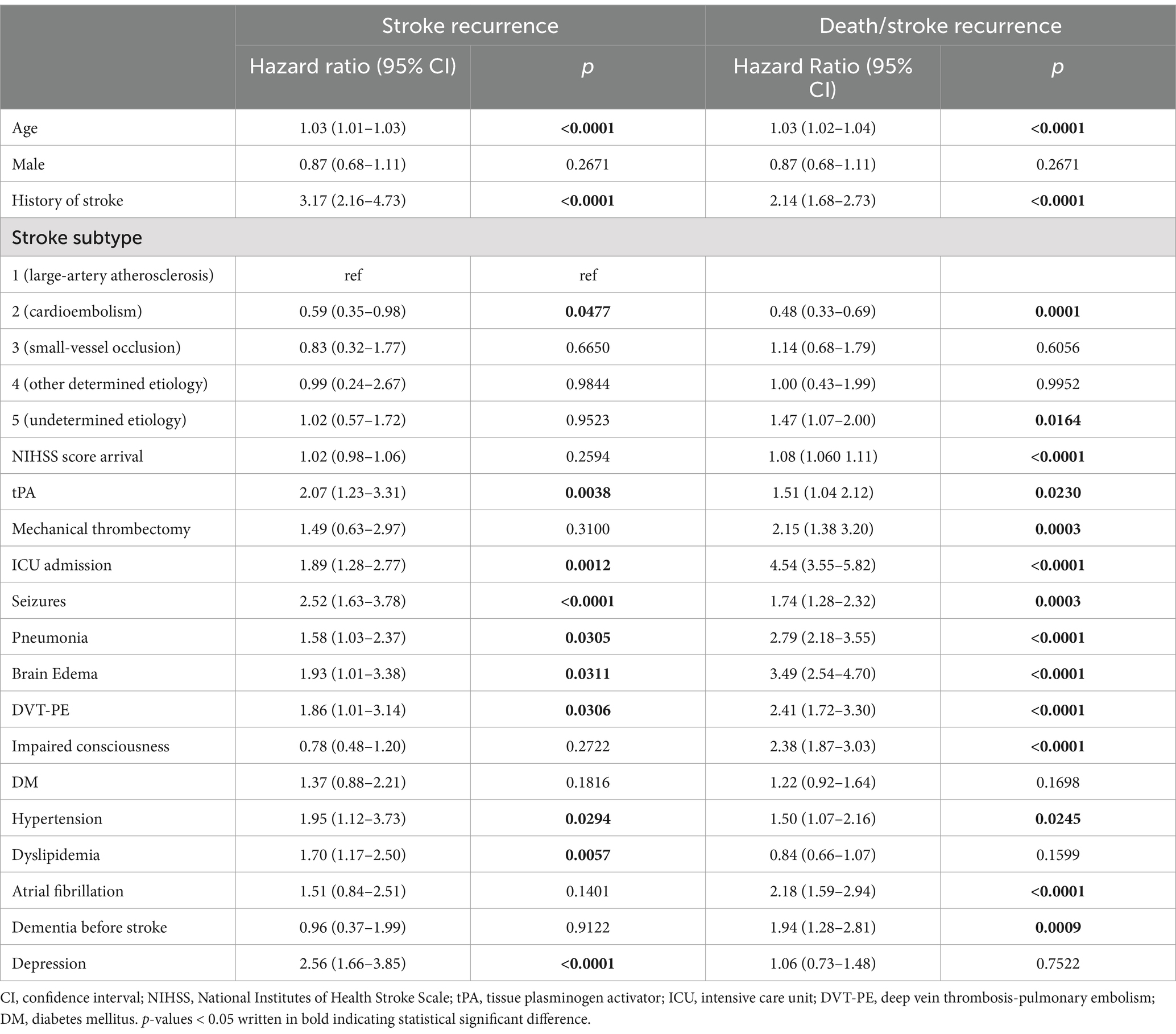
Table 3. Univariate associations of risk factors with first stroke recurrence and stroke recurrence or death.
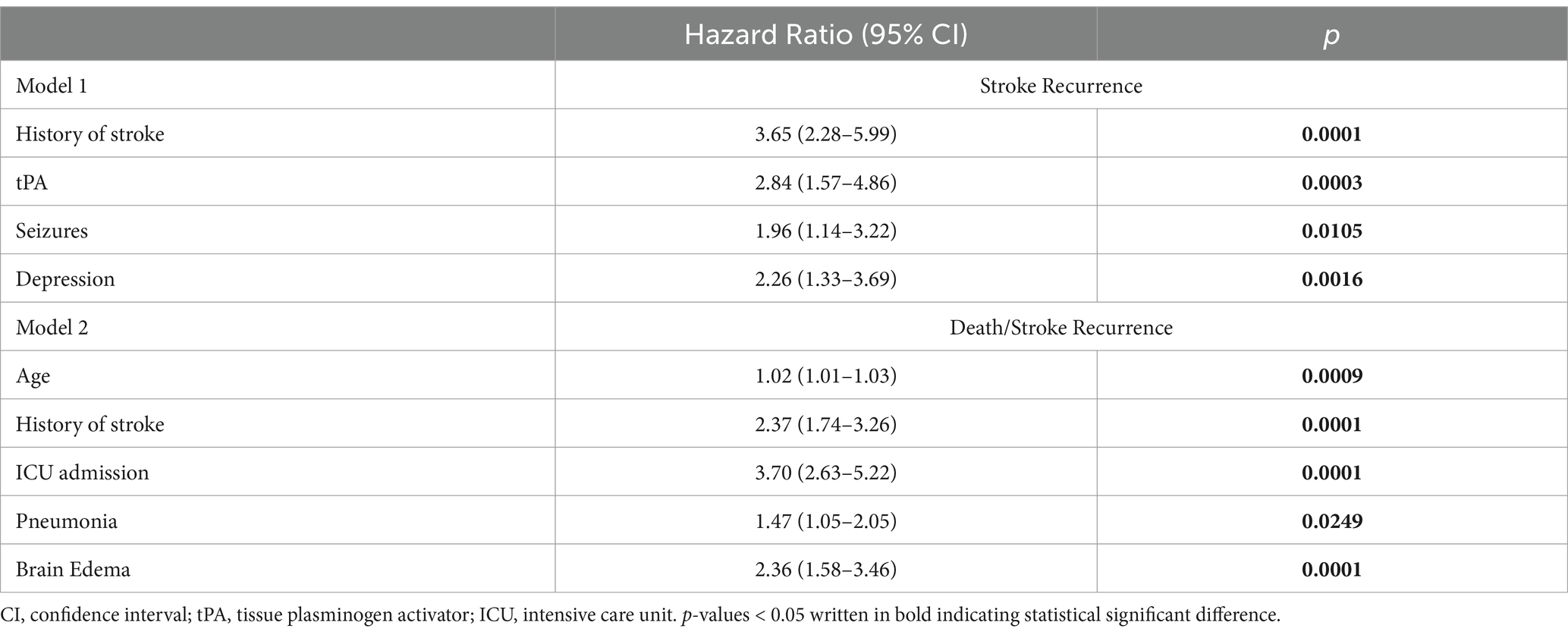
Table 4. Multivariable associations of risk factors with first stroke recurrence and stroke recurrence or death.
3.4 Risk factors associated with stroke recurrence/death
Univariate analysis identified several risk factors significantly associated with stroke recurrence. These included age (HR = 1.03, 95% CI: 1.02–1.04, p < 0.0001), history of stroke (HR = 2.14, 95% CI: 1.68–2.73, p < 0.0001), stroke subtype two vs. one as a reference (HR = 0.48, 95% CI: 0.33–0.69, p = 0.0001), stroke subtype five vs. one (HR = 1.47, 95% CI: 1.07–2.00, p = 0.0164), initial NIHSS score (HR = 1.08, 95% CI: 1.06–1.11, p < 0.0001), tPA treatment (HR = 1.51, 95% CI: 1.04–2.12, p = 0.0230), mechanical thrombectomy (HR = 2.15, 95% CI: 1.38–3.20, p = 0.0003), ICU admission (HR = 4.54, 95% CI: 3.55–5.82, p < 0.0001), seizures (HR = 1.74, 95% CI: 1.28–2.32, p = 0.0003), pneumonia (HR = 2.79, 95% CI: 2.18–3.55, p < 0.0001), brain edema (HR = 3.49, 95% CI: 2.54–4.70, p < 0.0001), DVT-PE (HR = 2.41, 95% CI:1.72–3.30, p < 0.0001), impaired consciousness (HR = 2.38, 95% CI:1.87–3.03, p < 0.0001) hypertension (HR = 1.50, 95% CI:1.07–2.16, p = 0.0245), AF (HR = 2.18, 95% CI: 1.59–2.94, p < 0.0001), and dementia (HR = 1.94, 95% CI:1.28–2.81, p = 0.0009). Upon multivariable analysis (Table 4), the risk of stroke recurrence/death remained significantly associated with age (HR = 1.02, 95% CI:1.01–1.03, p = 0.0009), history of stroke (HR = 2.37, 95% CI:1.74–3.26, p = 0.0001), ICU admission (HR = 3.70, 95% CI:2.63–5.22, p = 0.0001), pneumonia (HR = 1.47, 95% CI:1.05–2.05, p = 0.0249), and brain edema (HR = 2.36, 95% CI:1.58–3.46, p = 0.0001).
4 Discussion
This study addresses a critical gap in understanding the incidence and risk factors associated with recurrent stroke in Saudi Arabia. The findings revealed that the cumulative risk of recurrent stroke was 2.55% within 30 days, increasing to 5.29% at 90 days, 6.91% at 180 days, and reaching ~10% within 365 days following the index stroke. Our findings align with a prospective cohort study by Modrego et al., reporting a cumulative risk of recurrence of 2.1% at 30 days and 9.5% at 365 days (12). In comparison, a systematic review and meta-analysis reported a pooled cumulative risk of stroke recurrence of 3.1% (95% CI, 1.7–4.4) at 30 days and 11.1% (95% CI, 9.0–13.3) at 365 days (13). Similarly, a meta-analysis by Lin et al. reported a cumulative recurrence risk of 7.7% at 90 days, 9.5% at 180 days, and 10.4% at 365 days. These rates are slightly higher than those observed in our study at 90 and 180 days; however, the 365-day recurrence rate aligns with our findings (14). Variations in study design, inclusion criteria (e.g., different stroke subtypes), patient demographics such as age, and the prevalence of comorbidities may explain the observed discrepancies in recurrence rates. Given the differences in demographics and comorbidities, future research should focus on intracranial atherosclerotic disease (ICAD), as it may represent a significant factor in the etiology that was not explored in this study (15).
Moreover, Rucker et al. and Flach et al. reported a 90-day ischemic stroke recurrence rate of 3.1% (95% CI: 2.5–3.7) and 2.2% (95% CI: 1.8–2.6), respectively, lower than our findings (16, 17). This discrepancy may stem from the inclusion of all stroke types in their studies rather than focusing solely on ischemic stroke. Additionally, differences in ischemic stroke subtype distribution could explain the variation; for instance, subtype one accounted for 50% of strokes in our study, compared to only 8.4% in Rucker et al. and 16% in Flach et al. Furthermore, their studies’ lack of reported comorbidities may contribute to these differences (16, 17). Lastly, Arsava et al., in a multi-center study across the USA, South Korea, and Brazil, reported a 90-day recurrence rate of 4.2% (95% CI: 3.2–5.2), which aligns more closely with our results. Their study also noted that 34% of recurrent strokes were subtype one (18). These findings highlight the significance of considering stroke subtype distribution and comorbidities in interpreting recurrence rate variations across studies.
In the current study, the cumulative incidence rates of death or stroke recurrence were as follows: 8.13% at 30 days, 13.60% at 90 days, 16.69% at 180 days, and 21.83% at 365 days after stroke. Patients with a prior stroke history showed significantly higher incidence rates. These findings align with literature emphasizing elevated risks in individuals with previous stroke history (19). Variations among stroke subtypes also influenced outcomes, consistent with a report linking different subtypes to varying risks of recurrence and mortality. For instance, a previous study indicated that specific ischemic stroke subtypes, such as large artery atherosclerosis and cardioembolic strokes, are associated with higher risks of recurrence compared to other subtypes (20).
Univariate analysis identified multiple predictors of stroke recurrence, including advanced age, history of stroke, tPA treatment, ICU admission, seizures, pneumonia, brain edema, DVT-PE, hypertension, dyslipidemia, and depression. Multivariable analysis confirmed the independent association of stroke recurrence with a history of stroke, tPA treatment, seizures, and depression. Previous history of stroke was identified as an independent predictor of stroke recurrence, consistent with findings by Xu et al., who reported a 2- to 3-fold increased risk of stroke recurrence in patients with prior stroke history (20). This underscores the critical need for tailored secondary prevention in this high-risk population.
The observed association between tPA, an essential treatment for improving acute stroke outcomes, and an increased risk of recurrence highlights the need for further investigation. Potential mechanisms underlying this association may include the influence of underlying stroke etiologies, predisposing factors, or complications related to reperfusion therapies. Future research should focus on elucidating these mechanisms and assessing the long-term impact of tPA on recurrent stroke risk, considering both patient-specific factors and treatment-related variables.
Moreover, post-stroke seizures and depression were independent stroke recurrence predictors, aligning with studies such as Doria et al. and Zeng et al. (21, 22). Seizures likely reflect more significant cortical damage or vascular instability (23), while depression may impact treatment adherence and recovery (24). It is crucial to educate stroke patients with seizures and depression about their risk of recurrence. Similarly, finding ways to control seizures and depression in these patients is vital in clinical settings and future studies.
Similarly, Univariate analysis revealed numerous predictors for stroke recurrence/death, including age, history of stroke, stroke subtypes, initial NIHSS, ICU admission, mechanical thrombectomy, pneumonia, brain edema, and comorbidities like AF and dementia. Multivariable analysis confirmed significant associations with age, history of stroke, ICU admission, pneumonia, and brain edema. Studies have shown that older adults are at increased risk due to age-related vascular changes, comorbidities, and reduced physiological reserve. For example, a meta-analysis by Mohan et al. reported a similar association between advancing age and stroke recurrence rates, underscoring the need for age-tailored secondary prevention strategies (13). Additionally, pneumonia and brain edema, as markers of post-stroke complications, have also been implicated in poorer outcomes. For instance, a study by Finlayson et al. found that post-stroke pneumonia is associated with increased mortality and more extended hospital stays. The study emphasizes the importance of early identification and management of pneumonia in stroke patients to improve outcomes (25). Similarly, a previously published study found that brain edema is an independent predictor of poor outcomes after ischemic stroke (26). Additionally, a review article by Zhang et al. emphasized that cerebral edema is a common complication of acute ischemic stroke, leading to poorer functional outcomes and substantially increasing mortality rates. The review highlights the importance of early identification of patients at high risk for severe edema to implement intensive monitoring and evidence-based interventions (27).
Our study possesses several notable strengths and limitations. A key strength is that, to the best of our knowledge, this is the first study to report the long-term incidence of recurrent stroke and mortality in Saudi Arabia, utilizing a larger sample size. This unique contribution provides a foundation for understanding stroke recurrence and mortality patterns for ischemic stroke patients in this region. Additionally, the study identifies a comprehensive range of risk factors, such as history of stroke, administration of tPA, seizures, and depression, many of which have been underexplored in the existing literature. Another significant strength lies in the study’s inclusion of data from two hospitals located in different regions of Saudi Arabia. This approach enhances the external validity of our findings by capturing a more diverse patient population and reducing the likelihood of regional biases.
However, the study is not without limitations. Its retrospective design inherently relies on the accuracy and completeness of medical record documentation, which may affect the reliability of the results. While we captured recurrence incidence at various time points, data limitations prevented the evaluation of recurrence beyond 1 year, which could have provided a more comprehensive understanding of long-term risks. Moreover, our study did not include data regarding carotid stenosis, surgical interventions (e.g., carotid endarterectomy), or interventional procedures (e.g., stenting). Furthermore, this study did not assess common risk factors, such as smoking and chronic kidney disease, leaving potential gaps in the analysis. Future research should focus on evaluating secondary prevention interventions, patient adherence, and their impact on stroke recurrence to provide a more comprehensive understanding of these critical factors. Further, while our study focused on interventions related to ischemic stroke, we acknowledge that we did not explore the influence of other medication use on stroke recurrence. Future research should consider the comparative effects of treatment versus non-treatment, as well as the potential impact of additional medications, to provide a more comprehensive understanding of factors influencing stroke recurrence. Finally, while our study focused on dyslipidemia and the TOAST criteria of stroke etiology, the lack of data on intracranial atherosclerotic disease (ICAD) is an important gap. We recommend that future studies specifically investigate the role of ICAD in the pathophysiology of stroke among Saudi patients, as it could provide valuable insights. Addressing these omissions will help present a more holistic view of the risk factors contributing to stroke recurrence. Despite these limitations, the study offers valuable insights and establishes a foundation for further investigations in this essential area.
5 Conclusion
This study highlights the significant burden of stroke recurrence and mortality among patients within the first year following a stroke, emphasizing the importance of identifying and managing risk factors. Key findings include a stroke recurrence rate of 9.96% and a combined death/stroke recurrence rate of 21.83% within 365 days.
A variety of risk factors were identified, with multivariable analysis revealing that a history of stroke, administration of tPA, seizure, and depression were significantly associated with stroke recurrence. However, only a prior stroke history remained significantly associated with the combined outcome of stroke recurrence and death. Additional risk factors for the composite outcome included older age, ICU admission, pneumonia, and brain edema.
These findings highlight the importance of early, focused interventions to reduce preventable complications and better manage comorbidities. Special attention should be given to patients with a history of stroke or severe presentations, as they are most vulnerable.
Further research is needed to validate these findings and deepen our understanding of the factors contributing to stroke recurrence and related outcomes.
Data availability statement
The data analyzed in this study is available upon request. Requests to access these datasets should be directed to Faisal Alamri, YWxhbXJpZkBrc2F1LWhzLmVkdS5zYQ==.
Ethics statement
The studies involving human participants were reviewed and approved by the King Abdullah International Medical Research Center Institutional Review Board, Riyadh, Saudi Arabia. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
EA: Formal analysis, Writing – original draft, Writing – review & editing. HA: Data curation, Investigation, Writing – original draft, Writing – review & editing. YA: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. AAlj: Investigation, Writing – original draft, Writing – review & editing. AAlg: Investigation, Writing – original draft, Writing – review & editing. FAlt: Investigation, Writing – original draft, Writing – review & editing. FAla: Conceptualization, Data curation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Diseases, GBD, and Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
2. Alamri, FF, Almarghalani, DA, Alraddadi, EA, Alharbi, A, Algarni, HS, Mulla, OM, et al. The utility of serum glucose potassium ratio as a predictive factor for haemorrhagic transformation, stroke recurrence, and mortality among ischemic stroke patients. Saudi Pharm J. (2024) 32:102082. doi: 10.1016/j.jsps.2024.102082
3. Leoo, T, Lindgren, A, Petersson, J, and von Arbin, M. Risk factors and treatment at recurrent stroke onset: results from the recurrent stroke quality and epidemiology (RESQUE) study. Cerebrovasc. Dis. (2008) 25:254–60. doi: 10.1159/000113864
4. Kariasa, IM, Nurachmah, E, and Koestoer, RA. Analysis of participants’ characteristics and risk factors for stroke recurrence. Enfermeria Clin. (2019) 29:286–90. doi: 10.1016/j.enfcli.2019.04.035
5. Laloux, P, Lemonnier, F, and Jamart, J. Risk factors and treatment of stroke at the time of recurrence. Acta Neurol. Belg. (2010) 110:299–302.
6. Rahayu, LP, Sudrajat, DA, Nurdina, G, Agustina, EN, and Kusuma Putri, TAR. The risk factor of recurrence stroke among stroke and transient ischemic attack patients in Indonesia. KnE Life Sci. (2019) 2019:87–97. doi: 10.18502/kls.v4i13.5229
7. el-gohary, T, Alshenqiti, AM, Ibrahim, SR, Khaled, O, Ali, A, and Ahmed, M. Risk factors and types of recurrent stroke: a Saudi hospital based study. J. Phys. Ther. Sci. (2019) 31:743–6. doi: 10.1589/jpts.31.743
8. Lovett, J, Coull, A, and Rothwell, P. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. (2004) 62:569–73. doi: 10.1212/01.WNL.0000110311.09970.83
9. Alraddadi, EA, Alatawi, Y, Kumar, RS, Bukhari, JI, Alghamdi, AE, Lughbi, S, et al. Insights into risk factors and outcomes of post-stroke seizures in Saudi Arabia: a multicenter analysis. Metab. Brain Dis. (2024) 40:72. doi: 10.1007/s11011-024-01508-3
10. Hillen, T, Coshall, C, Tilling, K, Rudd, AG, McGovern, R, and Wolfe, CDA. Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London stroke register. Stroke. (2003) 34:1457–63. doi: 10.1161/01.STR.0000072985.24967.7F
11. Alharbi, AR, Alali, AS, Samman, Y, Alghamdi, NA, Albaradie, O, Almaghrabi, M, et al. Vitamin D serum level predicts stroke clinical severity, functional independence, and disability-a retrospective cohort study. Front. Neurosci. (2022) 16:951283. doi: 10.3389/fnins.2022.951283
12. Modrego, PJ, Mainar, R, and Turull, L. Recurrence and survival after first-ever stroke in the area of Bajo Aragon, Spain. A prospective cohort study. J. Neurol. Sci. (2004) 224:49–55. doi: 10.1016/j.jns.2004.06.002
13. Mohan, KM, Wolfe, CD, Rudd, AG, Heuschmann, PU, Kolominsky-Rabas, PL, and Grieve, AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. (2011) 42:1489–94. doi: 10.1161/STROKEAHA.110.602615
14. Lin, B, Zhang, Z, Mei, Y, Wang, C, Xu, H, Liu, L, et al. Cumulative risk of stroke recurrence over the last 10 years: a systematic review and meta-analysis. Neurol. Sci. (2021) 42:61–71. doi: 10.1007/s10072-020-04797-5
15. Moonis, M. Intracranial atherosclerosis disease: a preventable epidemic. Ann. Indian Acad. Neurol. (2020) 23:246–7. doi: 10.4103/aian.AIAN_82_20. Epub 2020 Jun 10.
16. Rucker, V, Heuschmann, PU, O'Flaherty, M, Weingartner, M, Hess, M, Sedlak, C, et al. Twenty-year time trends in long-term case-fatality and recurrence rates after ischemic stroke stratified by etiology. Stroke. (2020) 51:2778–85. doi: 10.1161/STROKEAHA.120.029972
17. Flach, C, Muruet, W, Wolfe, CDA, Bhalla, A, and Douiri, A. Risk and secondary prevention of stroke recurrence: a Population-Base cohort study. Stroke. (2020) 51:2435–44. doi: 10.1161/STROKEAHA.120.028992
18. Arsava, EM, Kim, GM, Oliveira-Filho, J, Gungor, L, Noh, HJ, Lordelo Mde, J, et al. Prediction of early recurrence after acute ischemic stroke. JAMA Neurol. (2016) 73:396–401. doi: 10.1001/jamaneurol.2015.4949
19. Xu, J, Zhang, X, Jin, A, Pan, Y, Li, Z, Meng, X, et al. Trends and risk factors associated with stroke recurrence in China, 2007-2018. JAMA Netw. Open. (2022) 5:e2216341. doi: 10.1001/jamanetworkopen.2022.16341
20. Langanay, L, Gonzalez Sanchez, R, Hamroun, A, Dauchet, L, Amouyel, P, Dallongeville, J, et al. Ischemic stroke subtypes: risk factors, treatments, and 1-month prognosis - the Lille, France stroke registry. J. Stroke Cerebrovasc. Dis. (2024) 33:107761. doi: 10.1016/j.jstrokecerebrovasdis.2024.107761
21. Doria, JW, and Forgacs, PB. Incidence, implications, and Management of Seizures Following Ischemic and Hemorrhagic Stroke. Curr Neurol Neurosci Rep. (2019) 19:37. doi: 10.1007/s11910-019-0957-4
22. Zeng, YY, Wu, MX, Zhou, SN, Geng, DD, Cheng, L, Fan, KL, et al. Corrigendum: early-onset depression in stroke patients: effects on unfavorable outcome 5 years post-stroke. Front. Psychol. (2021) 12:732437. doi: 10.3389/fpsyt.2021.732437
23. Smigelskyte, A, Rimkuviene, G, Zukaite, D, Repeckaite, G, and Jurkeviciene, G. The Association of Epileptic Seizures after acute ischemic stroke with cerebral cortical involvement and electroencephalographic changes. Medicina (Kaunas). (2024) 60:768. doi: 10.3390/medicina60050768
24. Wang, J, Kuo, WY, Chen, MC, and Chen, CY. Impact of rehabilitation adherence and depressive symptoms on post-stroke self-care ability and quality of life: a longitudinal study. Top. Stroke Rehabil. (2024) 31:361–71. doi: 10.1080/10749357.2023.2259652
25. Hadley, G, Beard, DJ, Couch, Y, Neuhaus, AA, Adriaanse, BA, DeLuca, GC, et al. Rapamycin in ischemic stroke: old drug, new tricks? J. Cereb. Blood Flow Metab. (2019) 39:20–35. doi: 10.1177/0271678X18807309
26. Battey, TW, Karki, M, Singhal, AB, Wu, O, Sadaghiani, S, Campbell, BC, et al. Brain edema predicts outcome after nonlacunar ischemic stroke. Stroke. (2014) 45:3643–8. doi: 10.1161/STROKEAHA.114.006884
Keywords: stroke recurrence, mortality, ischemic stroke, risk factors, stroke complication
Citation: Alraddadi EA, Alotaibi HF, Alatawi Y, Aljabri A, Alghamdi AA, Alturki F and Alamri FF (2025) A multicenter analysis to identify the risk factors for stroke recurrence and mortality within 1 year. Front. Neurol. 16:1478175. doi: 10.3389/fneur.2025.1478175
Edited by:
Majaz Moonis, UMass Memorial Medical Center, United StatesReviewed by:
Hipólito Nzwalo, University of Algarve, PortugalChristian Urbanek, Klinikum Ludwigshafen, Germany
Copyright © 2025 Alraddadi, Alotaibi, Alatawi, Aljabri, Alghamdi, Alturki and Alamri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faisal F. Alamri, YWxhbXJpZkBrc2F1LWhzLmVkdS5zYQ==
†These authors have contributed equally to this work
 Eman A. Alraddadi
Eman A. Alraddadi Hashim F. Alotaibi
Hashim F. Alotaibi Yasser Alatawi
Yasser Alatawi Ahmed Aljabri
Ahmed Aljabri Ahmed A. Alghamdi
Ahmed A. Alghamdi Fahad Alturki
Fahad Alturki Faisal F. Alamri
Faisal F. Alamri