- 1Department of Child and Adolescent Health, School of Public Health, Guangdong Pharmaceutical University, Guangzhou, China
- 2Department of Nutrition and Food Health, School of Public Health, Guangdong Pharmaceutical University, Guangzhou, China
- 3State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangdong Provincial Key Laboratory of Ophthalmology and Visual Science, Guangdong Provincial Clinical Research Center for Ocular Diseases, Guangzhou, China
- 4Division of Birth Cohort Study, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
Objective: To examine the association of ADHD and LD with visual impairment, uncorrected refractive error, and refractive error (myopia, hyperopia, and astigmatism) among US children and adolescents.
Method: A population-based cross-sectional study included 3,385 participants aged 12–15 years from the large, representative sample of US NHANES. The diagnoses of ADHD and LD in children and adolescents, as reported by parents or adolescents themselves, were analyzed. All participants’ right eyes were used to calculate the spherical equivalent refractive errors (SER) during the investigation. Myopia, hyperopia, and astigmatism were classified by SER. Visual acuity was categorized into normal, uncorrected refractive error (URE), and visual impairment (VI) according to objectively assessed for each eye. Logistic regression analysis was used to estimate the associations between ADHD and LD and vision abnormalities.
Results: Among a total of 3,385 children and adolescents aged 12–15 years, 279 were reported to have a diagnosis of ADHD, and 427 were reported to have a diagnosis of LD. Compared with those without ADHD, children and adolescents with ADHD had an increased risk of hyperopia, with odds ratios (ORs) of 1.66 (95% CI, 1.03–2.67). LD was associated with higher risks of hyperopia (OR = 1.85, 95% CI, 1.17–2.90) and astigmatism (OR = 1.63, 95% CI: 1.18–2.26). After controlling for confounding variables, the results remained stable. LD also increased the risk of vision impairment (OR = 3.05, 95% CI: 1.05–8.90) after controlling for confounders. Stratified analyses showed that ADHD was a risk factor for hyperopia in boys compared with girls (OR = 1.62, 95%CI = 1.03–2.72), in 12–13-year-old individuals compared with 14–15-year-olds (OR = 1.69, 95%CI = 1.05–3.42). LD was a risk factor for hyperopia and astigmatism in girls compared with boys (OR = 2.81, 95%CI = 1.53–5.14; OR = 2.18, 95%CI = 1.22–3.90), and in 12–13-year-old individuals compared with 14–15-year-olds (OR = 1.99, 95%CI = 1.16–3.42; OR = 1.65, 95%CI = 1.07–2.56).
Conclusion: Children and adolescents diagnosed with ADHD and LD may be at a greater risk of developing hyperopia and astigmatism. To accurately diagnose and treat children affected by ADHD and LD, healthcare practitioners from various medical specialties should take this association into account.
1 Introduction
Vision has wide-ranging and significant effects on the economy, sustainable development, health, and many other aspects of life. Vision abnormalities, including refractive errors (myopia, hyperopia, astigmatism), strabismus, uncorrected refractive error, and vision impairment, can cause reading difficulties, blindness, anxiety, anti-social behavior, and problems with quality of life (1, 2). An estimated 596 million individuals worldwide suffered from distance vision impairment in 2020, 43 million of whom were blind. A further 510 million people lacked reading glasses, which resulted in uncorrected near vision impairment (3). It was anticipated that the number of people with myopia and high myopia would have significantly increased worldwide. Not only this, but hyperopia and other vision problems should also be of concern. Refractive errors are common in children, which suggests that they are a particularly vulnerable population (4). The prevalence of uncorrected refractive errors and strabismus in children ranged from 7.7 to 10.3% (5, 6), and uncorrected refractive problems cause vision impairment in about two-thirds of children worldwide (7).
Vision is the main sensory modality through which human beings acquire information, and Treichler stated that 80% of an individual’s information comes from vision (8). After light strikes the retina in the back of our eyes, the visual information is transmitted to the primary visual cortex (V1) and dorsal lateral geniculate nuclei (LGN), which together form the image-forming visual circuit. Among these, dorsal and ventral pathways are involved in visual perception and visuo-motor coordination (9, 10). Vision is critical in the development of movement and cognition and influences all skills learned in early life. Through the brain’s processing of visual information, we are able to identify and perceive the world around us, including colors, shapes, contours, and motion. Also, vision guides and controls our behavior. Our behavior is based on our perception and understanding of our environment. In infants, it promotes the development of early motor functions such as head control, facial expressions, imitation, and grasping objects (11, 12).
Children’s behavior and development, especially their attentional skills, learning, and reading processes, are influenced by their visual perception (13). Refractive errors can lead to deficits in these abilities and their development. Refractive error studies have shown that children with hyperopia perform worse on visual cognition and visuomotor tasks compared to children without refractive error. Children with hyperopia performed worse on reading and writing examinations as well as on standardized assessment tests in science, math, and English (14).
Attention deficit/hyperactivity disorder (ADHD) and Learning disabilities (LD) are the most frequently encountered neurodevelopmental disorders in childhood. Attention-deficit hyperactivity disorder (ADHD) is characterized by a recurrent pattern of inattention, hyperactivity, and impulsivity (15), affecting 5% of children and adolescents and 3% of adults (16, 17). Learning disabilities can affect neurocognitive processes and may limit the capacity to read, listen, spell, speak, write, concentrate, or organize information (18). The most prevalent form of learning disability is dyslexia, or a reading disability (19). ADHD and LD are neurodevelopmental disorders that manifest as structural abnormalities in the brain (20), and the same embryological tissue from which the brain develops also gives rise to the structures of the eye (21).
Some studies have elucidated the link between visual problems and ADHD/LD. There is recent meta-analytic evidence of disorders of the eye, such as astigmatism, hyperopia, strabismus, and altered measures of visual function, in people with ADHD compared to those without ADHD (22). The complex relationship between vision and ADHD/LD was further demonstrated by early abnormalities in visual sensory integration utilizing event-related potentials assessed in the visual cortex of children with ADHD (23), and the people with ADHD processed visual stimuli more slowly. Moreover, a literature review of the visual search performance of children with ADHD reported that these children were significantly slower on a sequential search task compared to their normally developing peers (24). People with nonverbal learning disabilities were impaired in visuospatial processing speed, visual perceptual ability, visual construction ability, and visuospatial working memory (25). In addition, although it is widely accepted that dyslexia is due to deficits in phonological and verbal information processing (26, 27), a growing body of data suggests that visual attention deficits contribute to reading difficulties in children with dyslexia. Therefore, it is reasonable to hypothesize that visual cognition abnormalities are more likely to be comorbid with ADHD or LD, potentially stemming from shared neurobiological factors.
After reviewing the evidence regarding visual anomalies and LD or ADHD, we identified the following limitations. Only a few studies have investigated the relationship between visual anomalies and LD or ADHD, and the findings have been inconsistent. For example, while some studies have indicated an increase in the prevalence of vision issues, including amblyopia, strabismus, hyperopia, astigmatism, heterotopia (28, 29), altered color vision, and contrast sensitivity in individuals with ADHD, another study found no association (30). Currently, there is insufficient scientific evidence to support the view that children with ADHD and LD have more visual problems. Therefore, in this study, based on the nationally representative sample in US, we aimed to investigate the prevalence of visual problems in children and adolescents with and without ADHD or LD, and to examine the association between ADHD and LD and visual abnormalities in adolescents.
2 Methods
2.1 Study population
The data used in this study were derived from the merger of three cycles (1999–2004) of the National Health and Nutrition Examination Survey (NHANES). NHANES is a nationally representative, cross-sectional sample obtained through a stratified, multistage probability design. It was routinely conducted by the Centers for Disease Control and Prevention of the non-institutionalized civilian populations in the United States (31).
Initially, the present analysis involved a total of 3,393 participants with and without ADHD and LD aged 12–15 years with valid information regarding vision examination data. Participants with a history of previous refractive surgery (4), history of cataract surgery (1) and corneal disease (3), as indicated by keratometry readings of greater than 50 D (32, 33), were excluded. Consequently, 3,385 adolescents with and without ADHD and LD were ultimately included for the primary analysis.
2.2 Measurements and variable
2.2.1 Refractive errors
Objective refraction data was obtained by the auto-refractor/keratometer (model ARK-760A) (34). In order to facilitate the analysis, spherical equivalent refractive errors, which are the average of the refractions in the two principal meridians, were calculated from the data of the right eyes of all participants (35). Myopia was defined as a SER equal to or less than-0.75 D, this more cautious definition of myopia was selected to avoid misclassification of myopia because cycloplegic medications were not used to detect refractive abnormalities. Hyperopia as a SER equal to or greater than +0.5 D, and astigmatism as a cylinder equal to or greater than 1.0 DC.
2.2.2 Visual acuity
Presenting visual acuity was assessed, and participants were asked to wear any regular distance vision correction. The 20/50 line was introduced first. The 20/200 line was shown if the participant was unable to read the 20/50 line. Participants’ visual acuity was rated as being worse than 20/200 if they were unable to read the 20/200 line. Participants were permitted to continue to the next line of smaller characters as long as they could properly read 4 or 5 items for the 20/50 line. This continued until the contestant missed two consecutive lines of two or more characters each or read the 20/20 line correctly. The last line for which four or more characters were read correctly was recorded as representing present visual acuity. It was not evaluated for visual acuity.
Corrected lenses were taken out after measuring the presenter’s visual acuity, and the autorefractor was used to determine each eye’s refraction. The measured refractive error correction was used to calculate the corrected visual acuity for eyes with a presenting visual acuity of less than 20/25. The better-seeing eye’s visual acuity was employed to describe visual impairment status.
Individuals with a presenting visual acuity of 20/40 or better were considered to have normal vision. URE was defined as those whose postrefraction visual acuity was 20/40 or greater but whose presenting visual acuity was less than 20/40. Individuals classified as VI were those whose visual acuity remained below 20/40 even after autorefraction.
2.2.3 Measurement of diagnosed LD and ADHD
Our primary outcome variables, LD and ADHD, were based on self-report/guardian responses to two NHANES interview questions: “Has a representative from a school or a health professional ever told (the child) that (he/she) had a learning disability?” and “Has a doctor or health professional ever told (the child) that (he/she) had attention deficit disorder?” The identical ADHD questions were asked of kids who were 16 years old or older, but the terms in parenthesis were changed to (you) and (you), respectively.
2.2.4 Assessment of covariates
Questionnaires were used to gather data on participant gender, age, race/ethnicity, household income, and BMI. Non-Hispanic white, non-Hispanic black, Mexican-American, and other races/ethnicities were the categories used to classify race and ethnicity. The 2,000 Centers for Disease Control Growth Charts were used as the basis for a computerized algorithm that estimated BMI in kilograms per square meter. The result was a conversion to BMI percentile values that were particular to age and sex (36). Each individual was placed in one of three BMI strata: obesity (95th percentile), overweight (85th to 94th percentile), or normal (85th percentile).
2.3 Data analyses
The NHANES analytical guidelines for 1999–2004 are available at www.cdc.gov/nchs/data/series/sr_02/sr02_161.pd. The survey modules of SAS software, version 9.4, were used to compute descriptive analysis and concurrent and prospective associations. For continuous variables, ANOVA was used to evaluate the means and proportions of the baseline characteristics; for categorical variables, chi-square testing was used. We used binary logistic regression to estimate the association between refractive error (myopia, hyperopia, astigmatism), visual acuity, and ADHD or LD. Model 1 adjusted for adolescent sex and age. Model 2 adjusted for age, sex, race/ethnicity, household income level, and BMI. In addition, we ran a stratified analysis to examine whether this association differed by sex, age, and ethnicity. Statistical significance was evaluated two-sided p-value at the 5% level.
3 Results
3.1 Characteristics of participants with ADHD and LD
The characteristics of participants with ADHD and LD are described in Table 1. The study population of 3,385 adolescents aged 12–15 years comprised 279 adolescents with ADHD (8.24%) and 3,106 adolescents without ADHD (91.76%), and 427 adolescents with LD (12.61%) and 2,958 adolescents without LD (87.19%), with a weighted mean (SE) age of 13.46 (0.02) years; 1,660 boys (weighted, 51.38%) and 1725 girls (weighted, 48.62%). Within the ADHD group, 15.37% (N = 66) children showed hyperopia, while 10.97% (N = 623) of the control group had hyperopia (χ2 = 4.16; p < 0.05). But, we found no differences observed for myopia, hyperopia, astigmatism, visual acuity, and ADHD. Within the LD group, 18.70% (N = 66) children showed hyperopia, while 11.08% (N = 305) of the control group had hyperopia (χ2 = 5.17; p = 0.023). Furthermore, significant group differences appeared between the LD group and the control group regarding astigmatism. Over 21% (N = 86) of the LD and 14.18% (N = 465) of the control group suffered from astigmatism (χ2 = 7.98; p = 0.005). But, we found no significant differences were observed for myopia, visual acuity and LD.
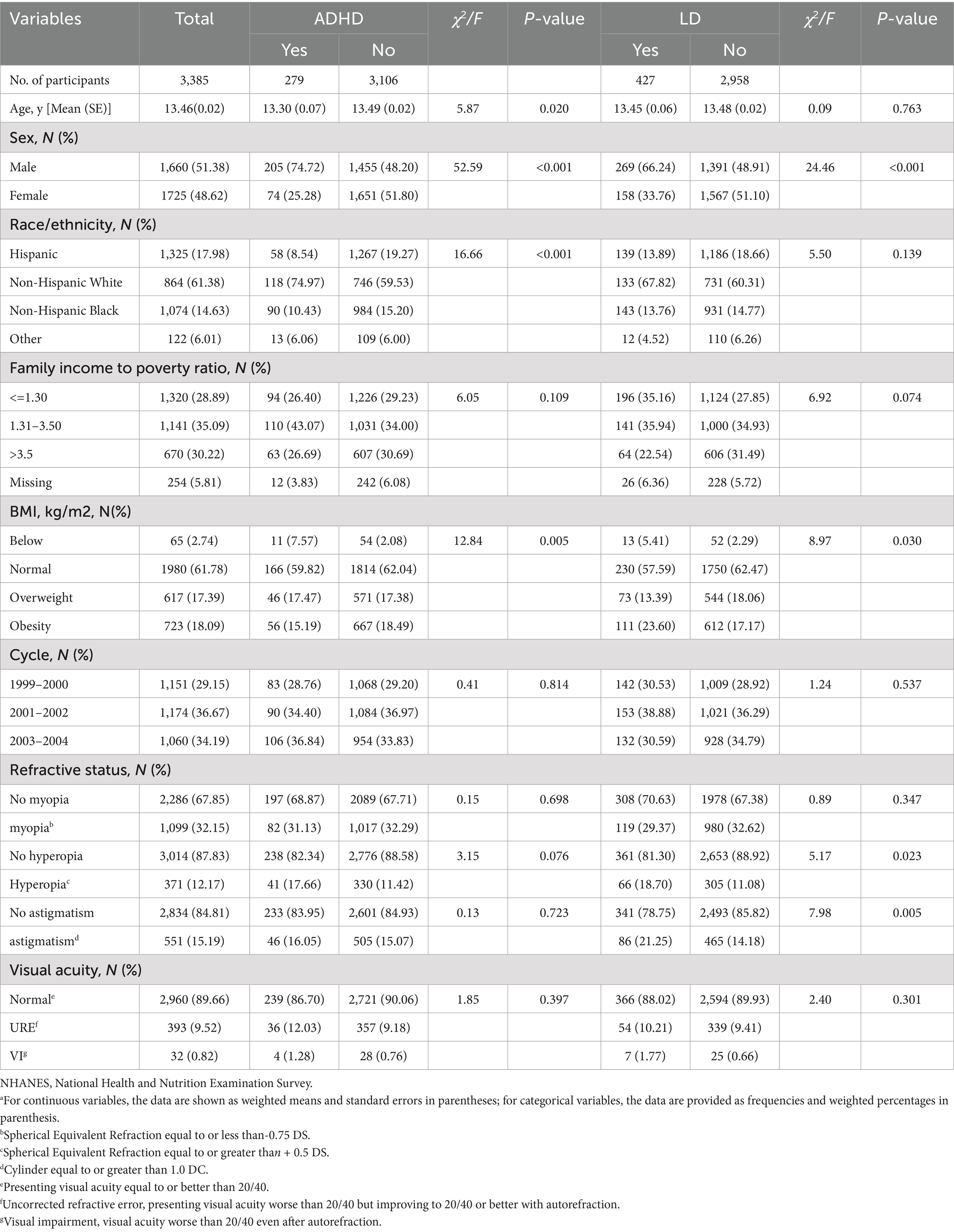
Table 1. Characteristics of overall participants aged 12–15 years according to ADHD and LD (N = 3,385) in NHANES, 1999-2004.a
3.2 Association of vision abnormalities with ADHD and LD
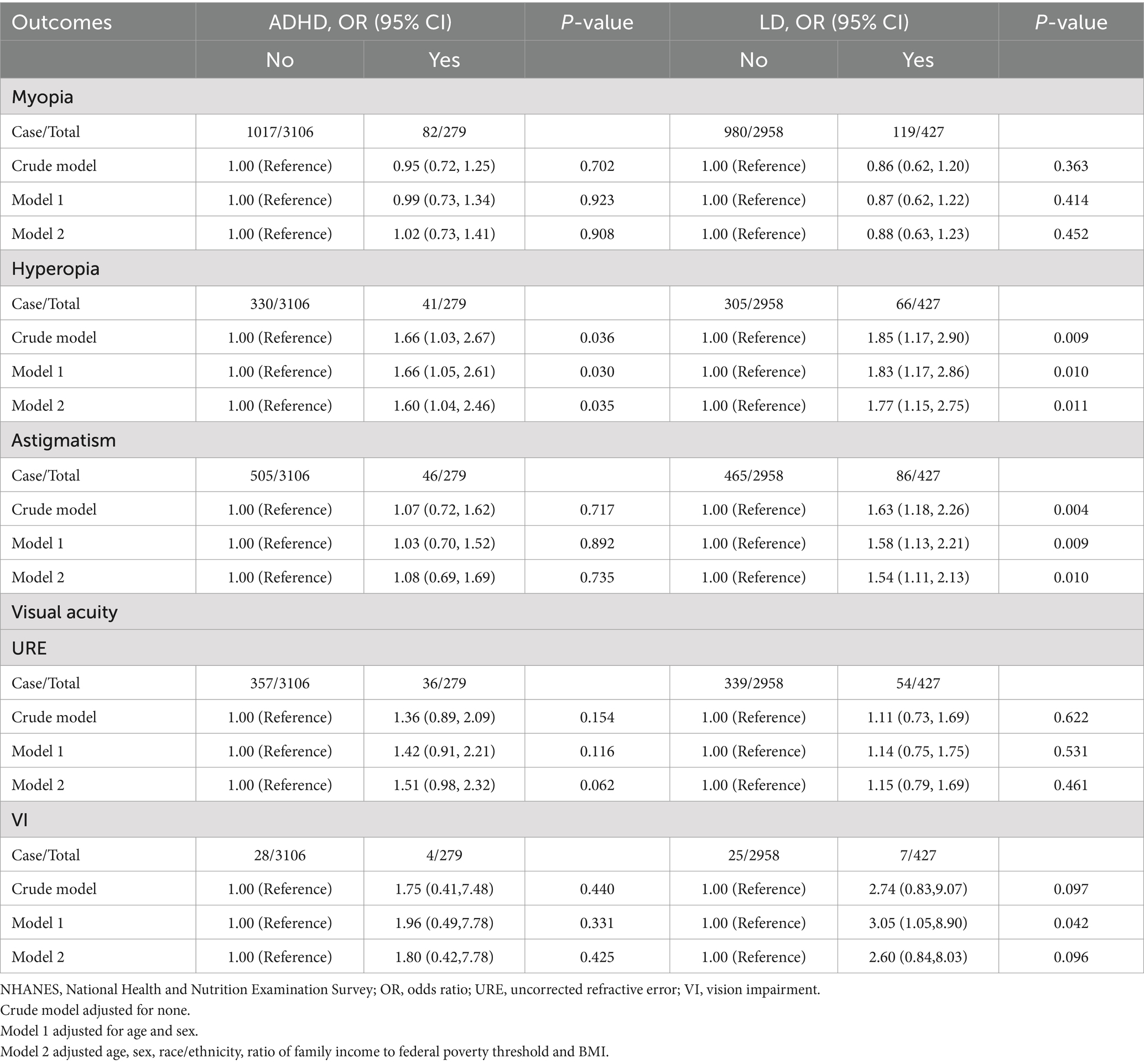
The odds ratios (ORs) of myopia, hyperopia, and astigmatism with ADHD and LD in adolescents are listed in Table 2. Within the ADHD group, in the crude model, children and adolescents with ADHD had a risk for the prevalence of hyperopia with odds ratio (ORs) of 1.66 (95% CI, 1.03–2.67) compared with those without ADHD. After adjustment for age and sex, the odds ratio of ADHD associated with hyperopia was 1.66 (95% CI, 1.05–2.61). In Model 2, which was additionally adjusted for age, sex, race/ethnicity, poverty-income ratio, and body mass index (BMI), the ORs and 95% CIs remained stable.
Within the LD group, in the crude model, children and adolescents with LD had a risk for the prevalence of hyperopia with odds ratios (ORs) of 1.85 (95% CI, 1.17–2.90) and astigmatism with odds ratios (ORs) of 1.63 (95% CI, 1.18–2.26) compared with those without LD. After adjustment for age and sex, results indicated a higher risk for prevalence of hyperopia (OR = 1.83; 95% CI, 1.17–2.86) and astigmatism (OR = 1.58, 95% CI, 1.13–2.21) in children with LD. In Model 2, which was additionally adjusted for potential confounding variables (age, sex, race/ethnicity, poverty-income ratio, and BMI), the ORs and 95% CIs remained stable. Moreover, there was a significantly association between LD and vision impairmen (OR = 3.05, 95% CI:1.05–8.90) after controlling for confounding variables. However, no significant associations were observed between children and adolescents with ADHD and myopia, uncorrected refractive error and vision impairment, adjusted ORs remained nonsignificant after controlling for other confounding variables. Similarly, no significant associations were observed between LD and myopia and uncorrected refractive error.
3.3 Stratified analysis
Stratified analyses showed that ADHD was a risk factor for hyperopia in boys compared girls (OR = 1.62, 95%CI = 1.03–2.72), in 12–13-year-old individuals compared with 14–15-year-olds (OR = 1.69, 95%CI = 1.05–3.42), and in Non-Hispanic White/Others compared with Hispanic and Non-Hispanic Black (OR = 1.74, 95%CI = 1.05–2.91). Having LD was a risk factor for prevalence of hyperopia and astigmatism in girls compared with boys (OR = 2.81, 95%CI = 1.53–5.14; OR = 2.18, 95%CI = 1.22–3.90), and in 12–13-year-old individuals compared with 14–15-year-olds (OR = 1.99, 95%CI = 1.16–3.42; OR = 1.65, 95%CI = 1.07–2.56) (Table 3).
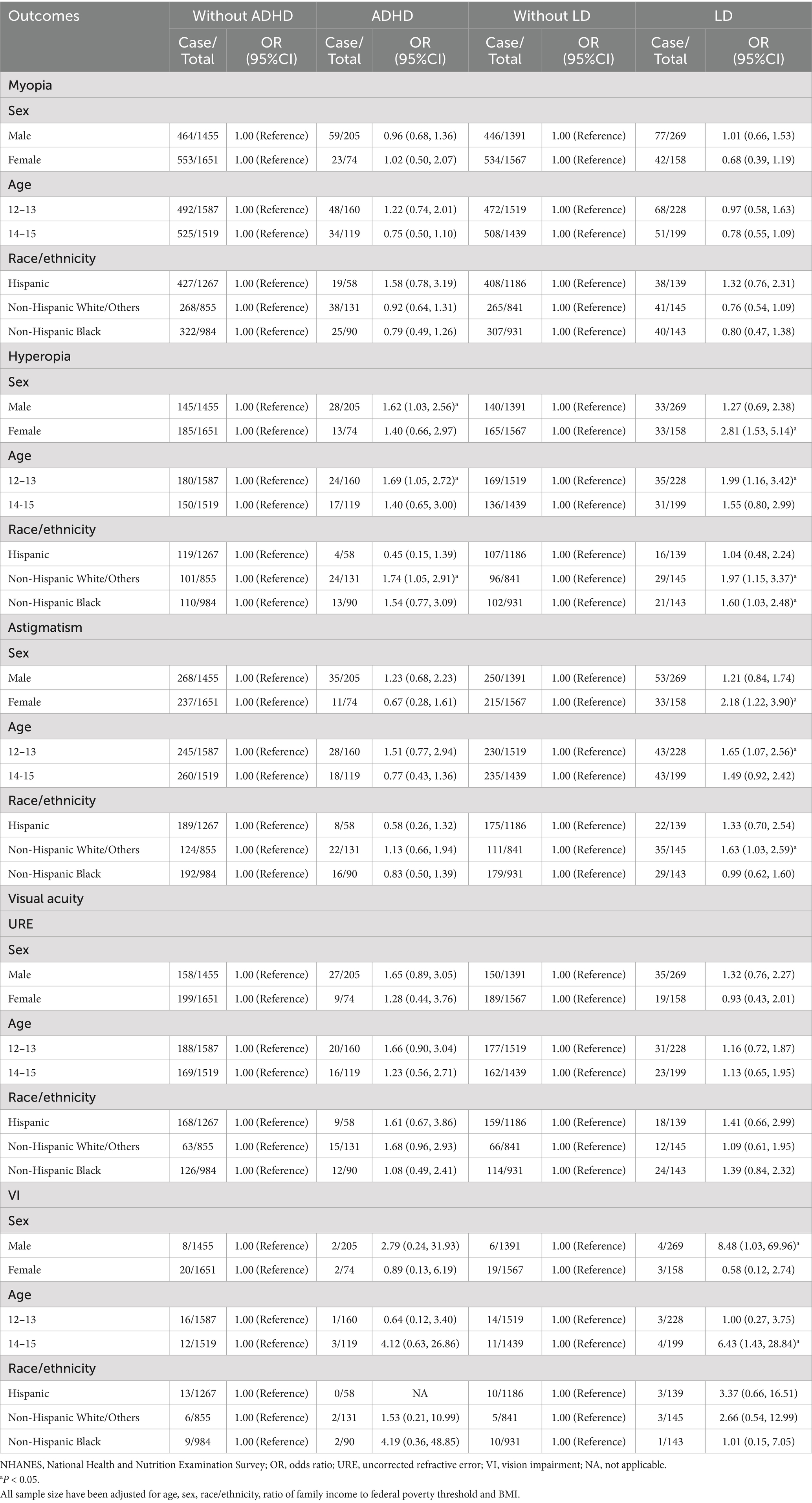
Table 3. Stratified analyses of the association of ADHD and LD with vision abnormalities in US adolescents aged 12–15 years in NHANES, 1999–2004.
4 Discussion
The present study examined the association of myopia, hyperopia, astigmatism, and visual acuity with ADHD and LD, based on a large, representative sample in the US. There is a positive association of hyperopia with ADHD and LD, astigmatism with LD among the children and adolescents in the US. Even after full adjustment, the association remained significant. No significant association was observed between myopia, uncorrected refractive error, visual impairment, and ADHD or LD.
Our results were generally in line with other studies, which also demonstrated an association between hyperopia and ADHD (22). The ability to see is a critical sensory function that is required for information acquisition. Refractive errors, such as astigmatism and hyperopia, can impair a person’s vision and focus, resulting in signs of hyperactivity and inattention. According to studies, a child who has hyperopia may experience headaches, eyestrain, intermittent blur, trouble concentrating on close objects, and poor reading and academic performance due to the need for extra accommodative effort (37, 38).
ADHD and ocular abnormalities are likely interlinked through multiple mechanisms. First, neurodevelopmental dysfuntions during crucial phases of brain growth can affect neural circuits associated with both attention and visual processing. For example, changes in the dopaminergic and noradrenergic systems, which play a vital role in regulating attention and visual perception, have been identified in ADHD. Imbalances in these neurotransmitters may affect the visual cortex and associated visual pathways, potentially leading to visual impairments (39, 40). Some studies have attempted to link alterations in dopaminergic neurotransmitter systems to neuropsychological deficits associated with ADHD. For instance, working memory and attention problems in ADHD may be explained by decreased dopaminergic inputs to the prefrontal cortex (41). Additionally, links have been found between variations of a dopamine receptor gene and sustained attention in ADHD (42). It is commonly recognized that components of human vision as well as higher cognitive abilities depend on an intact dopaminergic neurotransmitter system (43). Secondly, brain regions such as the prefrontal cortex, parietal cortex, and basal ganglia, involved in attentional control and visual information processing, frequently exhibit structural and functional abnormalities in Reduced connectivity between the prefrontal cortex and visual in ADHD. As reported in individuals those with ADHD, reduced connectivity between the prefrontal cortex and visual areas can impede the integration and interpretation of visual stimuli, thereby leading to visual abnormalities. Furthermore, genetic factors and environmental risk factors associated with ADHD may also influence the development of the visual system. The same genetic factors indicated a common biological foundation for the co-existence of these conditions. There was established evidence regarding the role of environmental risk factors in the etiology of ADHD and LD (44), and biological factors in the environment, such as preterm birth (21, 45), systemic infections (46, 47), and prematurity (48, 49) are known to influence vision problems. It is possible that affected neurodevelopment, influenced by these factors, could simultaneously trigger the onset of vision disorders and the symptoms of LD and ADHD. These multiple mechanisms together contribute to the complex relationship between ADHD and visual abnormalities.
Despite finding an association between hyperopia and ADHD or LD, we did not detect a relationship between myopia or visual acuity, which is inconsistent with other findings (50, 51). This is probably because astigmatism and hyperopia affect both far and near vision. By contrast, myopia preserves sharp close vision while only impairing far vision. Due to the increased need for focusing on close objects, astigmatism and hyperopia may become more noticeable in the home or school setting. Consequently, it’s possible that astigmatism and hyperopia have a greater impact on an individual’s ability to focus than myopia (29).
A strength of this research is that the NHANES is a large national sample that was designed to be representative of non-institutionalized children in the US, and thus the results can be generalized. However, our study also has several limitations that should be recognized. First, this is a cross-sectional observational study. As such, we are unable to infer the causal relationship between ADHD and LD and visual impairment in adolescents. Second, the diagnoses of ADHD and LD were based on self-reported information, which might be prone to recall bias. Moreover, our study did not differentiate between specific subtypes of ADHD, such as inattentive (ADHD-I), hyperactive and impulsive (ADHD-H), and combined (ADHD-C) types. Moreover, it lacked qualitative information regarding the specific forms and severity of refractive error and ADHD/LD. Furthermore, we use an older dataset, changes may have occurred in various aspects that could potentially affect the relationship between ADHD and LD and refractive errors. For example, lifestyle changes, such as increased screen time, changes in educational methods, and enhancements in eye-care awareness, may have affected the prevalence and characteristics of ADHD, LD and refractive errors. Furthermore, medication treatment may confound the relationship between visual measurements and ADHD. ADHD drugs that contain stimulants or non-stimulants may have an affect the autonomic nervous system’s regulation of ocular structures (43, 52, 53). Some studies have reported ocular side - effects like accommodation dysfunction, cataracts, mydriasis, and increased intraocular pressure (54–56), while others found no relationship between intraocular pressure and medication treatment (57, 58).
Therefore, more convincing evidence will be required from the prospective cohort studies. Future research should conduct accurate medical examinations to confirm the diagnoses of ADHD and LD, Additionally, it should explore the influence of a broader range of clinical factors on the relationship between ADHD/LD and ocular abnormalities.
5 Conclusion
In summary, our research suggests a potential association that children and adolescents diagnosed with ADHD and LD may be at a greater risk of developing hyperopia and astigmatism, while myopia and uncorrected refractive error appear to show no significant association. To accurately diagnose and treat children affected by ADHD and LD, healthcare practitioners from various medical specialties should take this association into account.
Data availability statement
The datasets used in this study can be found at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Author contributions
JAL: Conceptualization, Data curation, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. KZ: Data curation, Software, Writing – original draft, Writing – review & editing. YL: Conceptualization, Supervision, Writing – review & editing. QL: Conceptualization, Supervision, Writing – review & editing. JZ: Conceptualization, Supervision, Writing – review & editing. XZ: Conceptualization, Supervision, Writing – review & editing. TS: Conceptualization, Supervision, Writing – review & editing. CS: Conceptualization, Supervision, Writing – review & editing. XY: Conceptualization, Supervision, Writing – review & editing. JNL: Conceptualization, Supervision, Writing – review & editing. WY: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was written with the support of grants from the National Natural Science Foundation of China (Grant No. 81973063).
Acknowledgments
The authors would like to acknowledge the efforts of all the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Łazarczyk, JB, Urban, B, Konarzewska, B, Szulc, A, Bakunowicz-Łazarczyk, A, Żmudzka, E, et al. The differences in level of trait anxiety among girls and boys aged 13–17 years with myopia and emmetropia. BMC Ophthalmol. (2016) 16:201. doi: 10.1186/s12886-016-0382-2
2. Naidoo, KS, Leasher, J, Bourne, RR, Flaxman, SR, Jonas, JB, Keeffe, J, et al. Global vision impairment and blindness due to uncorrected refractive error, 1990-2010. Optom Vis Sci. (2016) 93:227–34. doi: 10.1097/OPX.0000000000000796
3. Burton, MJ, Ramke, J, Marques, AP, Bourne, RRA, Congdon, N, Jones, I, et al. The lancet Global Health Commission on global eye health: vision beyond 2020. Lancet Glob Health. (2021) 9:e489–551. doi: 10.1016/S2214-109X(20)30488-5
4. Xiao O, Morgan IG, Ellwein LB, He M, Refractive Error Study in Children Study Group. Prevalence of amblyopia in school-aged children and variations by age, gender, and ethnicity in a multi-country refractive error study. Ophthalmology. (2015) 122:1924–31. doi: 10.1016/j.ophtha.2015.05.034
5. Lança, C, Serra, H, and Prista, J. Strabismus, visual acuity, and uncorrected refractive error in portuguese children aged 6 to 11 years. Strabismus. (2014) 22:115–9. doi: 10.3109/09273972.2014.932395
6. Kvarnström, G, Jakobsson, P, Lennerstrand, G, and Dahlgaard, J. Preventable vision loss in children: a public health concern? Am Orthopt J. (2006) 56:3–6. doi: 10.3368/aoj.56.1.3
7. Pirindhavellie, GP, Yong, AC, Mashige, KP, Naidoo, KS, and Chan, VF. The impact of spectacle correction on the well-being of children with vision impairment due to uncorrected refractive error: a systematic review. BMC Public Health. (2023) 23:1575. doi: 10.1186/s12889-023-16484-z
9. Diao, Y, Chen, Y, Zhang, P, Cui, L, and Zhang, J. Molecular guidance cues in the development of visual pathway. Protein Cell. (2018) 9:909–29. doi: 10.1007/s13238-017-0490-7
10. Stein, J. Dyslexia: the role of vision and visual attention. Curr Dev Disord Rep. (2014) 1:267–80. doi: 10.1007/s40474-014-0030-6
11. Chokron, S, and Dutton, GN. From vision to cognition: potential contributions of cerebral visual impairment to neurodevelopmental disorders. J Neural Transm (Vienna). (2023) 130:409–24. doi: 10.1007/s00702-022-02572-8
12. Ionta, S. Visual neuropsychology in development: Anatomo-functional brain mechanisms of action/perception binding in health and disease. Front Hum Neurosci. (2021) 15:689912. doi: 10.3389/fnhum.2021.689912
13. Buttross, S. Attention deficit-hyperactivity disorder and its deceivers. Curr Probl Pediatr. (2000) 30:41–50. doi: 10.1016/S0045-9380(00)80044-4
14. Williams, W, Latif, A, Hannington, L, and Watkins, D. Hyperopia and educational attainment in a primary school cohort. Arch Dis Child. (2005) 90:150–3. doi: 10.1136/adc.2003.046755
15. Polanczyk, GV, Willcutt, EG, Salum, GA, Kieling, C, and Rohde, LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. (2014) 43:434–42. doi: 10.1093/ije/dyt261
16. Faraone, SV, Asherson, P, Banaschewski, T, Biederman, J, Buitelaar, JK, Ramos-Quiroga, JA, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. (2015) 1:1–23. doi: 10.1038/nrdp.2015.20
17. The World Federation of ADHD International Consensus Statement. 208 evidence-based conclusions about the disorder. Neurosci Biobehav Rev. (2021) 128:789–818. doi: 10.1016/j.neubiorev.2021.01.022
18. Hammill, DD, Leigh, JE, McNutt, G, and Larsen, SC. A new definition of learning disabilities. J Learn Disabil. (1987) 20:109–13. doi: 10.1177/002221948702000207
19. Handler, SM, and Fierson, WM. The section on ophthalmology and council on children with disabilities AA of O American Association for Pediatric Ophthalmology and Strabismus, and American Association of Certified Orthoptists. Learning disabilities, dyslexia, and vision. Pediatrics. (2011) 127:e818–56. doi: 10.1542/peds.2010-3670
20. London, A, Benhar, I, and Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. (2013) 9:44–53. doi: 10.1038/nrneurol.2012.227
21. Leung, MP, Thompson, B, Black, J, Dai, S, and Alsweiler, JM. The effects of preterm birth on visual development. Clin Exp Optom. (2018) 101:4–12. doi: 10.1111/cxo.12578
22. Bellato, A, Perna, J, Ganapathy, PS, Solmi, M, Zampieri, A, Cortese, S, et al. Association between ADHD and vision problems. A systematic review and meta-analysis. Mol Psychiatry. (2023) 28:410–22. doi: 10.1038/s41380-022-01699-0
23. Nazari, MA, Berquin, P, Missonnier, P, Aarabi, A, Debatisse, D, De Broca, A, et al. Visual sensory processing deficit in the occipital region in children with attention-deficit/hyperactivity disorder as revealed by event-related potentials during cued continuous performance test. Neurophysiol Clin. (2010) 40:137–49. doi: 10.1016/j.neucli.2010.03.001
24. Mullane, JC, and Klein, RM. Literature review: visual search by children with and without ADHD. J Atten Disord. (2008) 12:44–53. doi: 10.1177/1087054707305116
25. Cardillo, R, Vio, C, and Mammarella, IC. A comparison of local-global visuospatial processing in autism spectrum disorder, nonverbal learning disability, ADHD and typical development. Res Dev Disabil. (2020) 103:103682. doi: 10.1016/j.ridd.2020.103682
26. Peterson, RL, and Pennington, BF. Developmental dyslexia. Annu Rev Clin Psychol. (2015) 11:283–307. doi: 10.1146/annurev-clinpsy-032814-112842
27. Nittrouer, S, and Pennington, B. New approaches to the study of childhood language disorders. Curr Dir Psychol Sci. (2010) 19:308–13. doi: 10.1177/0963721410383976
28. DeCarlo, DK, Swanson, M, McGwin, G, Visscher, K, and Owsley, C. ADHD and vision problems in the National Survey of Children’s health. Optom Vis Sci. (2016) 93:459–65. doi: 10.1097/OPX.0000000000000823
29. Reimelt, C, Wolff, N, Hölling, H, Mogwitz, S, Ehrlich, S, and Roessner, V. The underestimated role of refractive error (hyperopia, myopia, and astigmatism) and strabismus in children with ADHD. J Atten Disord. (2021) 25:235–44. doi: 10.1177/1087054718808599
30. Fabian, ID, Kinori, M, Ancri, O, Spierer, A, Tsinman, A, and Ben Simon, GJ. The possible association of attention deficit hyperactivity disorder with undiagnosed refractive errors. J AAPOS. (2013) 17:507–11. doi: 10.1016/j.jaapos.2013.06.005
31. NHANES Tutorials. (2023). Available at: https://wwwn.cdc.gov/nchs/NHANES/tutorials/default.aspx (Accessed August 8, 2023).
32. Friling, R, Weinberger, D, Kremer, I, Avisar, R, Sirota, L, and Snir, M. Keratometry measurements in preterm and full term newborn infants. Br J Ophthalmol. (2004) 88:8–10. doi: 10.1136/bjo.88.1.8
33. Mutti, DO, Mitchell, GL, Jones, LA, Friedman, NE, Frane, SL, Lin, WK, et al. Axial growth and changes in lenticular and corneal power during emmetropization in infants. Invest Ophthalmol Vis Sci. (2005) 46:3074–80. doi: 10.1167/iovs.04-1040
34. NHANES - National Health and Nutrition Examination Survey Homepage. (2023). Available at: https://www.cdc.gov/nchs/nhanes/index.htm (Accessed August 8, 2023).
35. Harb, EN, and Wildsoet, CF. Nutritional factors and myopia: an analysis of National Health and nutrition examination survey data. Optometry Vision Sci. (2021) 98:458–68. doi: 10.1097/OPX.0000000000001694
36. Barlow, SE, and Dietz, WH. Obesity evaluation and treatment: expert committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics. (1998) 102:e29. doi: 10.1542/peds.102.3.e29
37. Grisham, JD, and Simons, HD. Refractive error and the reading process: a literature analysis. J Am Optom Assoc. (1986) 57:44–55.
38. VIP-HIP Study GroupKulp, MT, Ciner, E, Maguire, M, Moore, B, Pentimonti, J, et al. Uncorrected Hyperopia and Preschool Early Literacy: Results of the Vision in Preschoolers-Hyperopia in Preschoolers (VIP-HIP) Study. Ophthalmology. (2016) 123:681–9. doi: 10.1016/j.ophtha.2015.11.023
39. Ho, JD, Sheu, JJ, Kao, YW, Shia, BC, and Lin, HC. Associations between attention-deficit/hyperactivity disorder and ocular abnormalities in children: a population-based study. Ophthalmic Epidemiol. (2020) 27:194–9. doi: 10.1080/09286586.2019.1704795
40. Rubia, K, Overmeyer, S, Taylor, E, Brammer, M, Williams, SC, Simmons, A, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. (1999) 156:891–6. doi: 10.1176/ajp.156.6.891
41. Arnsten, AFT, and Li, BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. (2005) 57:1377–84. doi: 10.1016/j.biopsych.2004.08.019
42. Bellgrove, MA, Hawi, Z, Lowe, N, Kirley, A, Robertson, IH, and Gill, M. DRD4 gene variants and sustained attention in attention deficit hyperactivity disorder (ADHD): effects of associated alleles at the VNTR and −521 SNP. Am J Med Genet B Neuropsychiatr Genet. (2005) 136B:81–6. doi: 10.1002/ajmg.b.30193
43. Müller, CP, and Huston, JP. Dopamine activity in the occipital and temporal cortices of rats: dissociating effects of sensory but not pharmacological stimulation. Synapse. (2007) 61:254–8. doi: 10.1002/syn.20366
45. Frequently Asked Questions - International Dyslexia Association. (2023). Available at: https://dyslexiaida.org/frequently-asked-questions-2/ (Accessed August 4, 2023).
46. Kalogeropoulos, D, Sakkas, H, Mohammed, B, Vartholomatos, G, Malamos, K, Sreekantam, S, et al. Ocular toxoplasmosis: a review of the current diagnostic and therapeutic approaches. Int Ophthalmol. (2022) 42:295–321. doi: 10.1007/s10792-021-01994-9
47. Nayeri, T, Sarvi, S, Moosazadeh, M, Hosseininejad, Z, Amouei, A, and Daryani, A. Toxoplasma gondii infection and risk of attention-deficit hyperactivity disorder: a systematic review and meta-analysis. Pathog Glob Health. (2020) 114:126–35. doi: 10.1080/20477724.2020.1738153
48. Gustafsson, P, and Källén, K. Perinatal, maternal, and fetal characteristics of children diagnosed with attention-deficit-hyperactivity disorder: results from a population-based study utilizing the Swedish Medical Birth Register. Dev Med Child Neurol. (2011) 53:263–8. doi: 10.1111/j.1469-8749.2010.03820.x
49. Familial-Environmental Risk Factors in South African Children With Attention-Deficit Hyperactivity Disorder (ADHD): A Case-Control Study. J Child Neurol. (2015) 30:1327–32. doi: 10.1177/0883073814560630
50. Grönlund, MA, Aring, E, Landgren, M, and Hellström, A. Visual function and ocular features in children and adolescents with attention deficit hyperactivity disorder, with and without treatment with stimulants. Eye. (2007) 21:494–502. doi: 10.1038/sj.eye.6702240
51. Mezer, E, and Wygnanski-Jaffe, T. Do children and adolescents with attention deficit hyperactivity disorder have ocular abnormalities? Eur J Ophthalmol. (2012) 22:931–5. doi: 10.5301/ejo.5000145
52. Lachkar, Y, and Bouassida, W. Drug-induced acute angle closure glaucoma. Curr. Opin. Ophthalmol. (2007) 18:129. doi: 10.1097/ICU.0b013e32808738d5
53. Martin, L, Aring, E, Landgren, M, Hellström, A, and Grönlund, MA. Visual fields in children with attention-deficit / hyperactivity disorder before and after treatment with stimulants. Acta Ophthalmol. (2008) 86:259–64. doi: 10.1111/j.1755-3768.2008.01189.x
54. Bahali, K, Ipek, H, Yalcin, O, and Orum, O. Atomoxetine-induced mydriasis in a child patient. Eur Child Adolesc Psychiatry. (2014) 23:1231–2. doi: 10.1007/s00787-013-0491-x
55. Izci, F, and Gode, OE. Methylphenidate induced intraocular pressure increase. Dusunen Adam. (2016) 23:387–8. doi: 10.5350/DAJPN2016290413
56. Soyer, J, Jean-Louis, J, Ospina, LH, Bélanger, SA, Bussières, JF, and Kleiber, N. Visual disorders with psychostimulants: A paediatric case report. Paediatr Child Health. (2019) 24:153–5. doi: 10.1093/pch/pxz012
57. Larrañaga-Fragoso, P, Noval, S, Rivero, JC, and Boto-de-los-Bueis, A. The effects of methylphenidate on refraction and anterior segment parameters in children with attention deficit hyperactivity disorder. J AAPOS. (2015) 19:322–6. doi: 10.1016/j.jaapos.2015.04.005
Keywords: refractive error, attention-deficit/hyperactivity disorder, ADHD, learning disabilities, vision
Citation: Lu J, Zhou K, Liang Y, Li Q, Zhong J, Zeng X, Shen T, Sun C, Yu X, Lu J and Yang W (2025) Association of ADHD and LD with vision abnormalities among the children and adolescents in US, NHANES 1999–2004. Front. Neurol. 16:1465444. doi: 10.3389/fneur.2025.1465444
Edited by:
Thiago P. Fernandes, Federal University of Paraíba, BrazilReviewed by:
Gabriella Medeiros Silva, Federal University of Paraíba, BrazilJunbin Tian, Peking University Sixth Hospital, China
Copyright © 2025 Lu, Zhou, Liang, Li, Zhong, Zeng, Shen, Sun, Yu, Lu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenhan Yang, d2VuaGFuLXlhbmdAZ2RwdS5lZHUuY24=; Jinhua Lu, amluaHVhLmx1QGJpZ2NzLm9yZw==
†These authors have contributed equally to this work and share first authorship
 Jiamin Lu
Jiamin Lu Kefan Zhou1†
Kefan Zhou1† Xia Zeng
Xia Zeng Tianran Shen
Tianran Shen Xinping Yu
Xinping Yu Jinhua Lu
Jinhua Lu Wenhan Yang
Wenhan Yang