- 1Department of Pediatrics in the College of Medicine, University of Florida, Gainesville, FL, United States
- 2Metabolic Unit, Department of Translational Medical Sciences, University of Naples Federico II, Naples, Italy
- 3Telethon Institute of Genetics and Medicine, Pozzuoli, Italy
- 4Friedrich-Baur-Institute, Department of Neurology, LMU University Hospital, LMU Munich, Munich, Germany
- 5Erasmus MC University Medical Center, Rotterdam, Netherlands
- 6M6P-Therapeutics, St Louis, MO, United States
- 7Amicus Therapeutics, Inc., Princeton, NJ, United States
- 8Metrum Research Group, Tariffville, CT, United States
- 9Incyte Corporation, Wilmington, DE, United States
- 10Department of Neurology, University of California, Irvine, Irvine, CA, United States
A corrigendum on
Cipaglucosidase alfa plus miglustat: linking mechanism of action to clinical outcomes in late-onset Pompe disease
by Byrne, B. J., Parenti, G., Schoser, B., van der Ploeg, A. T., Do, H., Fox, B., Goldman, M., Johnson, F. K., Kang, J., Mehta, N., Mondick, J., Sheikh, M. O., Sitaraman Das, S., Tuske, S., Brudvig, J., Weimer, J. M., and Mozaffar. T. (2024). Front. Neurol. 15:1451512. doi: 10.3389/fneur.2024.1451512
In the published article, there was an error in Figure 13 as published. The error relates to the positive/negative value of some of the numbers on the axes for the lower MMT score and the GSGC total score in Figure 13A. For both lower MMT score and GSGC total score, the axis scale incorrectly read −3, −2, 1, 0, −1, −2, −3 from left to right, whereas the axis scale for lower MMT score should have been −3, −2, −1, 0, 1, 2, 3 and the axis scale for GSGC total score should have been 3, 2, 1, 0, −1, −2, −3. The corrected Figure 13 and its caption appear below.
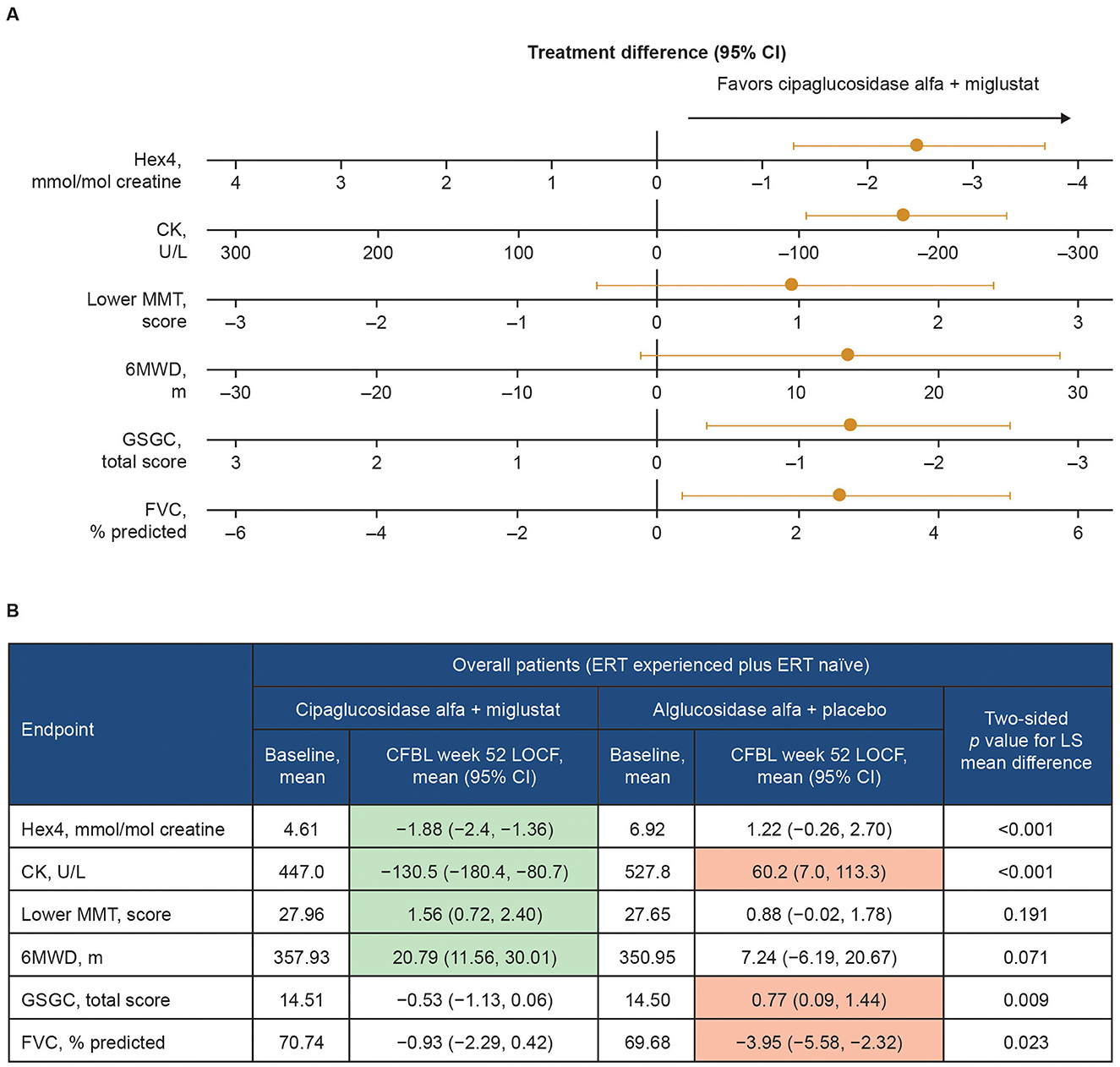
Figure 13. Change from baseline at week 52 of PROPEL—effect of cipaglucosidase alfa plus miglustat compared with alglucosidase alfa plus placebo in key efficacy outcomes. (A) Forest plot illustrating mean estimated treatment differences between cipaglucosidase alfa plus miglustat versus alglucosidase alfa plus placebo and corresponding 95% CIs are shown for the combined PROPEL study population for each outcome, with units as indicated on the x-axes. For all outcomes, right-sided directionality of treatment differences indicates favorable outcomes for cipaglucosidase alfa plus miglustat compared with alglucosidase alfa plus placebo. (B) The table shows baseline mean values and Week 52 CFBL values for cipaglucosidase alfa plus miglustat and alglucosidase alfa plus placebo. Shaded CFBL indicates nominally significant improvement (green) or nominally significant worsening (red) from baseline (i.e., the 95% CI does not include zero) within each treatment group. The p-values (two-tailed LS mean difference) shown in the far-right column are for the between-group treatment differences illustrated in the forest plot.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: Pompe disease, glycogen storage disease type II, lysosomal storage disorders, enzyme replacement therapy, n-butyldeoxynojirimycin
Citation: Byrne BJ, Parenti G, Schoser B, van der Ploeg AT, Do H, Fox B, Goldman M, Johnson FK, Kang J, Mehta N, Mondick J, Sheikh MO, Sitaraman Das S, Tuske S, Brudvig J, Weimer JM and Mozaffar T (2025) Corrigendum: Cipaglucosidase alfa plus miglustat: linking mechanism of action to clinical outcomes in late-onset Pompe disease. Front. Neurol. 15:1540452. doi: 10.3389/fneur.2024.1540452
Received: 05 December 2024; Accepted: 12 December 2024;
Published: 03 January 2025.
Edited and reviewed by: Edoardo Malfatti, Hôpitaux Universitaires Henri Mondor, France
Copyright © 2025 Byrne, Parenti, Schoser, van der Ploeg, Do, Fox, Goldman, Johnson, Kang, Mehta, Mondick, Sheikh, Sitaraman Das, Tuske, Brudvig, Weimer and Mozaffar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barry J. Byrne, YmFycnkuYnlybmVAdWZsLmVkdQ==
 Barry J. Byrne
Barry J. Byrne Giancarlo Parenti
Giancarlo Parenti Benedikt Schoser
Benedikt Schoser Ans T. van der Ploeg5
Ans T. van der Ploeg5 Brian Fox
Brian Fox Franklin K. Johnson
Franklin K. Johnson Jia Kang
Jia Kang Nickita Mehta
Nickita Mehta M. Osman Sheikh
M. Osman Sheikh Steven Tuske
Steven Tuske Jon Brudvig
Jon Brudvig