- 1West China School of Stomatology, Sichuan University, Chengdu, China
- 2State Key Laboratory of Oral Diseases, National Centre for Stomatology & National Clinical Research Centre for Oral Diseases, West China Hospital of Stomatology, Chengdu, Sichuan University, Chengdu, China
- 3Division of Dentistry, Ng Teng Fong General Hospital and Faculty of Dentistry, National University Health System, Singapore, Singapore
- 4National Dental Research Institute Singapore, National Dental Centre Singapore, Duke-NUS Medical School, Singapore Health Services, Singapore, Singapore
- 5Affiliated Hospital of North Sichuan Medical College, Nanchong, China
Aim: This study aimed to evaluate the factor structure of the Oral Behaviors Checklist (OBC) in Chinese temporomandibular disorder (TMDs) patients and compare the outcomes with those of Western patients. Additionally, it examined the correlations between different OBC subscale scoring methods.
Methods: A total of 869 patients completed a survey that included demographic information, the Symptom Questionnaire, and OBC. This was followed by a clinical examination and diagnosis based on the Diagnostic Criteria for TMDs (DC/TMDs). Exploratory factor analysis, along with confirmatory factor analysis, was applied to waking-state oral behaviors, revealing two key factors: Chinese non-functional (C-NFA) and functional (C-FA) oral activities. Items were contrasted with those of Italian TMDs patients (I-NFA and I-FA), and subscale scores were computed, compared, and correlated using Kruskal Wallis and Post-hoc and Spearman’s rank-order correlation (α = 0.05).
Results: Variations in NFA and FA items were observed between Chinese and Italian TMDs patients. For both NFA scoring methods, significant differences were noted between pain-related and intra-articular TMDs. The C-NFA and I-NFA, as well as C-FA and I-FA, scoring methods yielded scores with strong correlations (r > 0.8).
Conclusion: NFA and FA subscale items were determined for Chinese TMDs patients. Despite item discrepancies, C-NFA and C-FA scores were strongly correlated with I-NFA and I-FA scores, respectively. The OBC can be effectively simplified for use with Chinese TMDs patients. Developing and validating an East–West short-form version of the OBC should be prioritized, given the variations in oral behaviors across countries and cultures.
1 Introduction
Temporomandibular disorders (TMDs) represent the second most common musculoskeletal condition, following chronic low back pain, affecting approximately 34% of the general population (1–3). These disorders are associated with considerable pain and dysfunction within the masticatory system. Based on the Diagnostic Criteria for TMDs (DC/TMDs), TMDs can be categorized into three types: intra-articular (IT), pain-related (PT), and combined (CT) (1, 4). The features of TMDs include facial and preauricular pain, temporomandibular joint (TMJ) sounds, and episodes of both closed and open jaw joint locking (5–7). Furthermore, TMDs are frequently associated with primary headaches, with a high prevalence of comorbidity between the two conditions (8, 9).
Given the complex etiology of TMDs, the biopsychosocial model is essential for understanding TMDs. Supported by the Orofacial Pain Prospective and Risk Assessment (OPERA) studies, this approach also serves a key role in effectively managing TMDs (10, 11). The model proposes that TMDs emerge from the dynamic interaction of biological, psychological, social, and behavioral factors (12, 13).
Oral behaviors (OB) can occur during sleep or wakefulness, with waking-state oral activities being either functional, such as chewing and speaking, or non-functional (parafunctional), such as teeth grinding and jaw clenching (14, 15). Although these OBs are typically harmless, an increase in their frequency or intensity can exceed physiological tolerance and potentially cause adverse effects on the health of the stomatognathic system including tooth wear/fracture, restorative complications, and the development of TMDs (15, 16). However, the relationship between OBs and TMDs remains inconclusive. While most research indicates a higher prevalence of OBs (17) in individuals with TMDs compared to those without, some studies suggest the opposite (5, 18, 19).
The assessment of OBs can be subject-based, clinically-based, and/or instrumentally-based, with self-reported questionnaires being the most widely used subject-based method (17, 20). Among these, the Oral Behavior Checklist (OBC), which consists of two sleeping-state and nineteen waking-state items, is a well-established tool for identifying and quantifying ‘jaw overuse’ behaviors (1, 21–23). Although the OBC is an integral part of the DC/TMDs Axis II protocol (psychosocial and behavioral aspects), not all items in the OBC are pertinent or commonly observed in individuals with TMDs (14). Furthermore, in both clinical practice and research settings, patients and participants often lack the patience to meticulously complete all items of the OBC due to its length. Recently, Donnarumma et al. used exploratory factor analysis (EFA) to determine the factor structure of the OBC among Italian TMDs patients. Their analysis identified two distinct groups of OBs during wakefulness, specifically six non-functional (NFA) and six functional (FA) oral activities, resulting in a markedly streamlined OBC (19). Genetic factors, along with race, culture, and socio-environmental influences, can affect OBs during both sleep and wakefulness (24). Therefore, OBs prevalent in Western TMDs patients may differ from those of Eastern TMDs patients. Considering the limited research on OBs in Eastern TMDs samples, a similar methodology was applied to investigate the factor structure in Chinese TMDs patients. The aims of our study were: (1) to evaluate the factor structure of the OBC in Chinese TMDs patients, (2) to compare this factor structure with that of Western TMDs patients, and (3) to explore the correlations between different OBC subscales scoring methods. We hypothesize that the factor structure of the OBC in Chinese TMD patients is consistent with that observed in Italian TMD patients and that there is a significant correlation between the two OBC subscale scoring methods.
2 Methods
2.1 Study population
This study was carried out using data from questionnaires. Participants were recruited from consecutive patients seeking TMDs treatment at the West China Hospital of Stomatology, Sichuan University, from May 2022 to May 2024. This study has been ethically approved by the review board of the West China Hospital of Stomatology at Sichuan University and conducted in accordance with the Declaration of Helsinki, with the project identification number WCHSIRB-D-2022-212. A sample size of at least 300 participants was chosen for conducting exploratory (EFA) and confirmatory (CFA) factor analyses of the OBC, following the guidelines of Myers et al. (25). The inclusion criteria were: (1) age 18 years or older, and (2) at least one Axis I TMDs diagnosis according to the TMDs diagnostic criteria (DC/TMDs). The exclusion criteria were: (1) indeterminate diagnoses; (2) history of orofacial trauma and/or surgeries; (3) non-TMDs conditions; (4) cognitive impairments or illiteracy, and (5) incomplete questionnaires. Participants were provided with the study information and informed consent was duly obtained.
2.2 TMDs subgroups
TMDs diagnosis was determined using the DC/TMDs Axis I protocol, which includes a symptom questionnaire (SQ), physical examination, and, when applicable, supplementary diagnostic imaging. The short form version of Chinese in the DC-TMD Translations was retrieved from the following source: https://ubwp.buffalo.edu/rdc-tmdinternational/tmd-assessmentdiagnosis/dc-tmd-translations/. The physical examinations were conducted by three trained and calibrated specialists who were proficient in DC/TMDs procedures. Participants were subsequently categorized into IT, PT, and CT groups.
2.3 Assessment of OBs
The Chinese version of the OBC questionnaire, a self-reported instrument designed to identify and quantify the frequency of various obsessive behaviors, was sourced from www.rdc-tmdinternational.org.OBs over the past month were evaluated using the OBC, with items scored on a 5-point response scale, ranging from 0 points for “none of the time” to 4 points for “4–7 nights per week” or “all of the time.” The total OBC score (OBC-TS), representing the overall level of jaw overuse behavior, was obtained by summing the scores for all 21 items and categorized into three levels: normal (0 to 16 points), low (17 to 24 points), and high (25 to 84 points). Scores for waking-state and sleeping-state OBs were calculated by summing the nineteen items for wakefulness and the two items for sleep, respectively. As sleeping-state OBs only contained two items, they were excluded during the EFA. The Italian scoring method for the NFA subscale (I-NFA) involved summing the scores for items 3, 4, 5, 6, 7, and 11, while the Italian FA (I-FA) was based on the sum of items 12, 13, 17, 18, 19, and 20. The corresponding items from the Italian and Chinese NFA and FA subscales were eventually compared, and the resulting scores were correlated.
2.4 Statistical analysis
Statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS) software, version 27.0 (IBM Corporation, Armonk, NY, USA), with the significance level set at 0.05. EFA was conducted to reduce the dimensionality of the dataset and identify the underlying factors that influence the observed variables. The dataset was randomly divided, with two-thirds allocated to the EFA for exploration (n = 579) and one-third allocated to the CFA for validation (n = 290). The validity of the factor analysis models was assessed using two tests: the Kaiser-Meyer-Olkin (KMO) test and Bartlett’s test of sphericity. While the KMO test evaluates the level of multicollinearity, with values ideally exceeding 0.5, Bartlett’s test assesses the likelihood that the initial correlation matrix is an identity matrix, with a significant result (p < 0.05) indicating that the data is suitable for factor analysis. Given the ordinal nature of OBC responses, the polychoric covariance matrix and varimax rotation were applied to estimate the latent trait. Items with factor loadings under 0.5 were excluded to enhance the robustness of the analysis. Based on previous research, two factors were chosen (18).
To verify the factor structure, CFA was conducted, with specific criteria for model fit: a chi-square statistic to degrees of freedom ratio less than 3 (χ2/df < 3) with a p-value greater than 0.05, Root Mean Square Error of Approximation (RMSEA) below 0.10, Root Mean Square Residual (RMR) below 0.08, and Standardized Root Mean Square Residual (SRMR) below 0.08. These criteria collectively evaluated whether the model fitted the OBC data (26).
OBC data were presented as both means with standard deviations and medians with interquartile ranges (IQR). The Kolmogorov–Smirnov test indicated that the OBC data were not normally distributed. Therefore, the Kruskal-Wallis test, followed by post-hoc Mann–Whitney U tests, was used to examine the differences in OBC subscale scores among the various TMDs subgroups. Relationships between different subscale scoring methods of the OBs were assessed using Spearman’s rank-order correlation (27). The correlation coefficients (r) were classified into four categories: small (≥0.1), medium (≥0.3), large (≥0.5) (28).
3 Results
3.1 The sample of temporomandibular disorder patients
Out of 970 participants who initially met the inclusion criteria, 101 were omitted for meeting the specified exclusion criteria (Figure 1). The final sample of 869 participants, 78.83% of whom were female, had a mean age of 30.16 years (SD = 11). Among these, 35.1% (305) were diagnosed with IT, 22.6% (196) with PT, and 42.3% (368) with CT.
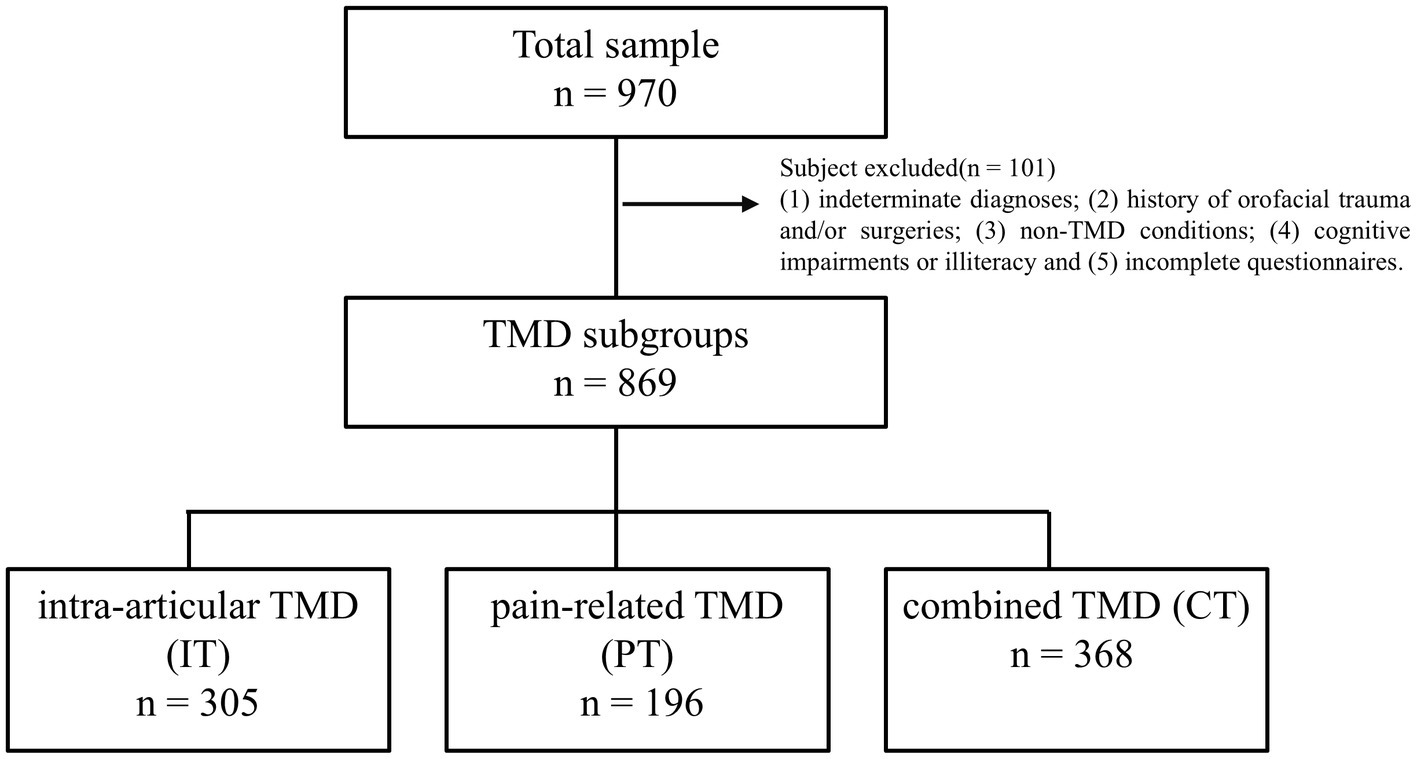
Figure 1. The sample of temporomandibular disorder divided three subgroups: intra-articular TMDs, pain-related TMD and combined TMD.
3.2 Exploratory factor analysis of OBC items in the waking state
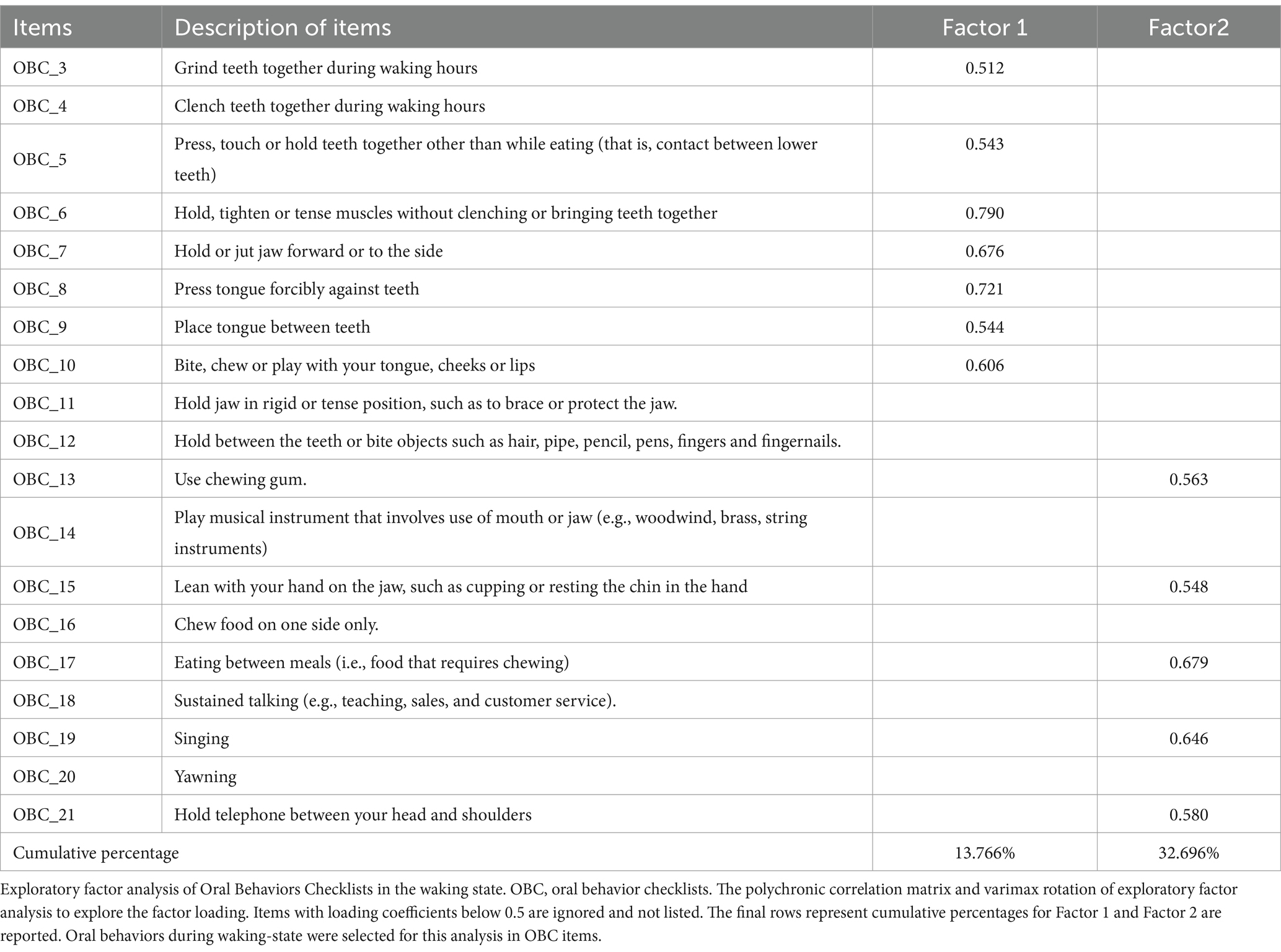
The KMO value of 0.832 and the significant result from Bartlett’s test of sphericity (p < 0.001) confirmed that the correlation matrices were suitable for factor analysis and supported its use for the OBC dataset. Two distinct factors were identified in the EFA (Table 1). The first, termed Chinese-NFA (C-NFA), includes seven items (3, 5–10) representing non-functional oral behaviors like teeth grinding and holding actions. The second factor, termed C-FA, consists of five items (13, 15, 17, 18 and 21). Related to functional oral activities, such as chewing and singing.
3.3 The scatterplot of exploratory factor analysis loading of OBC items
A scatterplot was used to visualize the associations between the OBC items and the two identified factors, with items 4, 11, 12, 14, 16, 18, and 20 excluded. These items were omitted because their factor loadings fell below the 0.5 threshold (Figure 2).
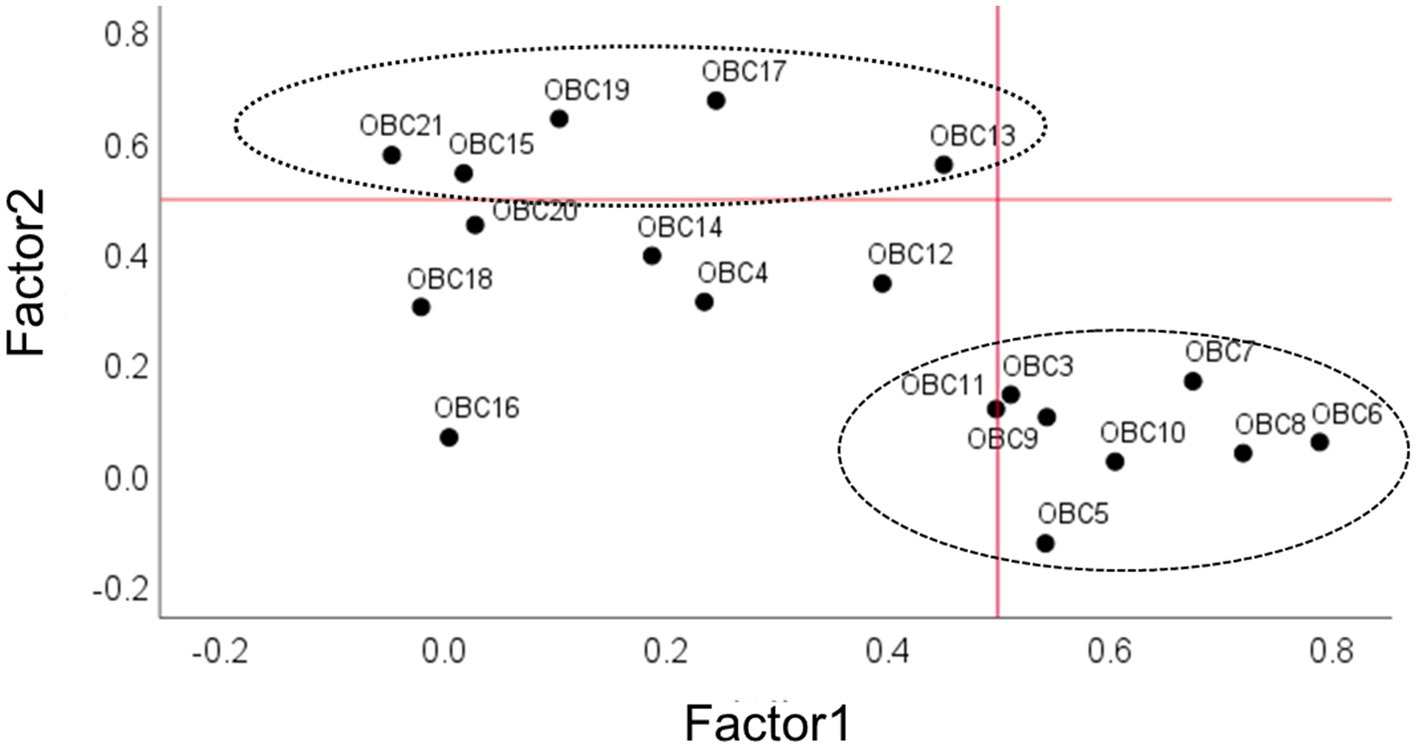
Figure 2. Scatterplot of exploratory factor analysis (EFA) loading of OBC items related to diurnal oral behaviors. On x-axis and y-axis, loadings are shown for factor 1 and factor 2. Numerals in the plot space refer to item numbers (see Table 2 for clarification). Circles with different line drawing represent OBC new scale (NFA and FA, see methods and results). Dotted line represents loading factor <0.5.
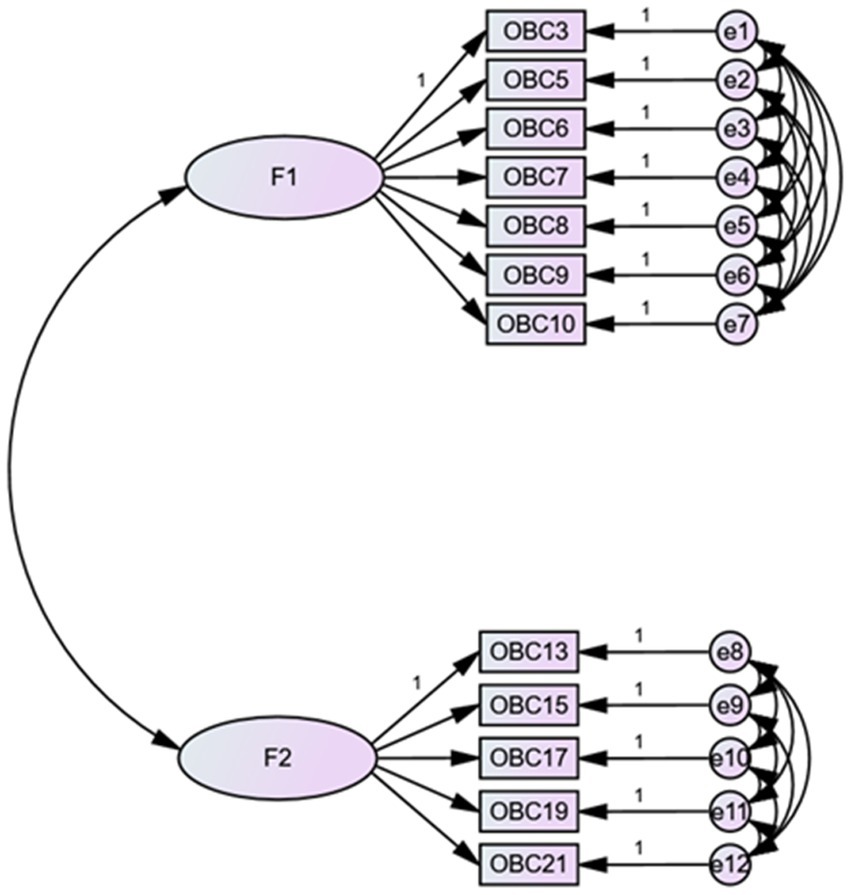
3.4 Confirmatory factor analysis of OBC items in TMDs patients
Figure 3 presents the CFA results with critical values indicating RMSEA <0.10, RMR <0.08, and SRMR <0.08, demonstrating a satisfactory goodness of fit.
3.5 The mean/median oral behaviors among three TMDs subgroups
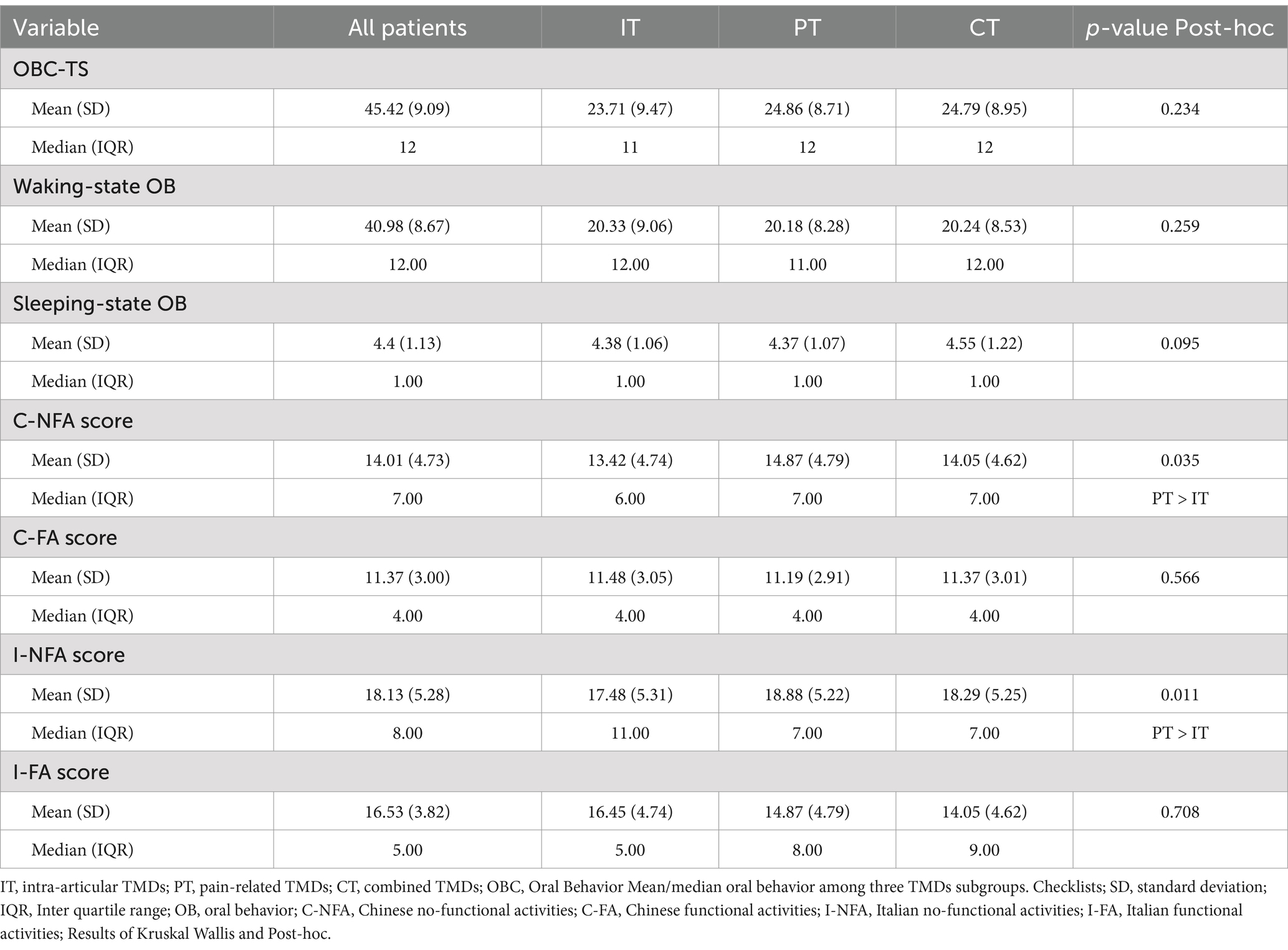
Table 2 shows the statistically significant differences in C-NFA scores (PT > IT) and I-NFA scores (PT > IT) among the TMDs subgroups. However, no significant differences were observed in OBC-TS, waking-state OBs, sleeping-state OBs, C-FA, or I-FA scores across the three TMDs groups.
3.6 The correlations among the different subscale scoring methods for oral behaviors in TMDs patients
Table 3 displays the results of the correlation analysis, indicating a large relationship between OBC-TS and waking-state OBs (r = 0.99). Additionally, OBC-TS had large correlations with C-NFA (r = 0.80), C-FA (r = 0.75), I-NFA (r = 0.87), and I-FA (r = 0.84). Similarly, the waking-state OB score was strongly associated with C-NFA (r = 0.82), C-FA (r = 0.73), I-NFA (r = 0.88), and I-FA (r = 0.83). The large correlations were observed between C-NFA and I-NFA (r = 0.95) and between C-FA and I-FA (r = 0.82). The association between C-NFA and C-FA was small (r = 0.36), while the correlation between I-NFA and I-FA scores was medium (r = 0.51).
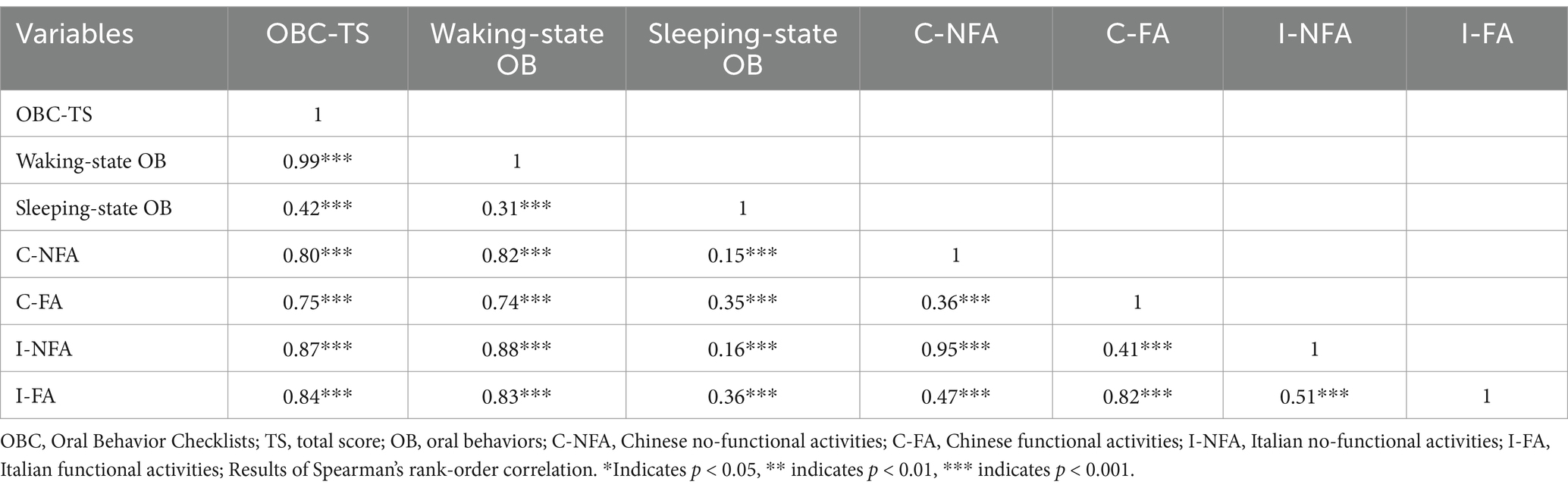
Table 3. The correlations among the different subscale scoring methods for oral behaviors in TMDs patients.
4 Discussion
We applied EFA and CFA to investigate the factorial structure of OBC in Chinese TMDs patients. The primary findings of our study are as follows: (1) The waking-state component of the OBC comprised two key factors, C-NFA and C-FA. However, the specific items contributing to these factors differed from those reported in Italian patients. (2) Patients with PT showed significantly higher C-NFA and I-NFA subscale scores compared to those with IT. No statistically significant differences were found in OBC-TS, waking-state OB, sleeping-state OB, C-FA, or I-FA scores among the TMDs subgroups. (3) The scoring methods for C-NFA and I-NFA, as well as C-FA and I-FA, yielded scores with significant and strong correlations. In light of the above, the first research hypothesis was partially supported, while the second was fully endorsed. The relationship between OBs and TMDs remains a contentious issue in current research. While most studies indicate a higher prevalence of OBs among TMDs patients compared to non-TMDs patients, as well as in patients with PT, some studies have reported no significant differences between the two groups (18, 19, 29). Part of these discrepancies may stem from racial and cultural differences that influence the types of OBs and the presentation of TMDs (2). Therefore, the factor structure and items reported for Italian patients must be evaluated in their Chinese counterparts.
4.1 EFA and CFA
Although EFA, corroborated by CFA, showed that waking-state OBs could be divided into two key factors for both Chinese and Italian patients, C-NFA and C-FA comprised seven and five items, respectively, while both I-NFA and I-FA contained six items each. In addition to differences in the number of items, the specific OBs also displayed slight variation, reflecting ethnocultural disparities in oral activity patterns. For NFA, four items overlapped between Chinese and Italian TMDs patients (items 3, 5, 6, and 7), whereas for FA, only three items were shared (items 13, 17, and 19). These seven waking-state items, along with the two sleeping-state items, could potentially serve as a “universal” East–West short-form OBC.
Five items excluded in Chinese TMDs patients (items 4, 11, 12, 18, and 20) were present in their Italian counterparts. Of particular interest is item 4, “clenching teeth together during waking hours,” which had the high factor loading in the I-NFA (19). This may be because Chinese TMDs patients were avoiding teeth clenching as well as other behaviors that could trigger or intensify pain (29). Item 12, concerning the behavior of “holding between the teeth or biting objects such as hair, pipes, pencils, pens, fingers, and fingernails,” was more prevalent among children, despite the study’s primary focus on an adult population. Notably, the items “playing musical instruments involving the mouth or jaw” (item 14) and “chewing food on one side only” (item 16) were absent in both patient groups. The exclusion of item 14 may be due to most patients not playing woodwind, brass, or relevant string instruments, while a lack of awareness about unilateral chewing may partially account for the omission of item 16.
4.2 Comparison among TMDs subgroups
Although no significant differences were observed in OBC-TS, waking/sleeping state, and FA scores, Chinese patients with PT exhibited substantially greater NFA scores than those with IT, regardless of the scoring methods used. These findings align with studies indicating that NFA may increase the risk of developing PT (29, 30). However, they contrast with results from Korean TMDs patients, where no significant differences in NFA scores were observed using the Italian scoring method, suggesting possible ethnocultural variances even within East Asian TMDs samples (17). Among the NFAs, “holding teeth together during activities other than eating” was especially common in Chinese TMDs patients, reported in approximately one-third of cases (29). The previously esteemed “biopsychosocial” model of TMDs etiology emphasized the multifactorial contributions of genetics, environmental conditions, gonadal hormones, overall health status, jaw trauma, oral parafunctional activities, somatization, depression, and anxiety (31, 32). Given the methodological parallels, it is reasonable to suggest that a combination of genetic and cultural influences might have played a role in the observed differences in the factor structure of OBC between the Chinese and Italian TMDs patients.
4.3 Correlations between Chinese and Italian scoring methods
The strong correlation between OBC-TS and waking-state OBs was expected, as they encompassed 90% of all items. Likewise, associations between OBC-TS and waking-state OB scores with C-NFA, C-FA, I-NFA, and I-FA scores were also anticipated. The large correlations between C-NFA and I-NFA, as well as between C-FA and I-FA, can be attributed to the considerable overlap in their items, specifically four items for NFA and three for FA. Given these findings, East Asian studies that have applied the Italian scoring method for NFA and FA can be regarded as valid (17). Notwithstanding, the 9-item East–West short-form OBC warrants further exploration and testing of its psychometric properties in both clinical and community settings. While the correlation between C-NFA and C-FA scores was small, that between I-NFA and I-FA scores was medium. The contrast can be attributed to the different items used in the two scoring methods. According to the results of the EFA, the two factors are separate and thus generally not expected to be related.
4.4 Study limitations
Despite its strengths, including a large sample size, the use of the DC/TMDs, and a robust statistical methodology, the study had several limitations. First, the study involved only Chinese TMDs patients, which may reduce the applicability of its findings to other racial groups. Replicating the study in more diverse global TMDs populations is necessary to confirm its generalizability. Moreover, the study should be extended to include community samples to capture a broader representation of oral behaviors in individuals both with and without TMDs. Second, the OBC relied on self-reporting, which can be subject to recall, social desirability, and other forms of information bias. The results could also be influenced by individual perception and reporting accuracy. However, the large sample size may help offset this effect. Lastly, the analysis does not adjust for potential confounders such as age, gender, and socioeconomic status, which can affect oral behaviors and TMDs.
5 Conclusion
In summary, NFA and FA subscale items of the OBC were determined for Chinese TMDs patients using EFA and CFA. While the C-NFA comprised seven items (3, 5–10), the C-FA contained five items (13, 15, 17, 18 and 21). In spite of item discrepancies, C-NFA and C-FA scores were strongly correlated with I-NFA and I-FA scores, respectively. The OBC can be effectively simplified for use with Chinese TMDs patients. Developing and validating a “universal” East–West short-form version of the OBC should be prioritized, given the variations in oral behaviors across countries and cultures. Additionally, the “universal” short-form OBC needs to be verified in community samples. This approach would significantly enhance its applicability and relevance across diverse populations, fostering a deeper understanding and more effective assessment of oral behaviors and TMDs globally.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of the West China Hospital of Stomatology at Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
TL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – review & editing, Software, Visualization. AY: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft. YS: Formal analysis, Methodology, Resources, Validation, Data curation, Software, Writing – review & editing. YZ: Data curation, Investigation, Resources, Writing – review & editing. TW: Data curation, Investigation, Resources, Writing – review & editing. SZ: Data curation, Investigation, Resources, Writing – review & editing. ZL: Data curation, Investigation, Resources, Writing – review & editing, Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Validation. XX: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Clinical Research Project of West China Hospital of Stomatology, Sichuan University (LCYJ-2023-YY-2) and the National Natural Science Foundation of China (82301129).
Acknowledgments
We thank all the participants in the study. We also acknowledge the reviewers and editors for viewing our work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schiffman, E, Ohrbach, R, Truelove, E, Look, J, Anderson, G, Goulet, J-P, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network and orofacial pain special interest group. J Oral Facial Pain Headache. (2014) 28:6–27. doi: 10.11607/jop.1151
2. Zieliński, G, Pająk-Zielińska, B, and Ginszt, M. A Meta-analysis of the global prevalence of temporomandibular disorders. J Clin Med. (2024) 13:1365. doi: 10.3390/jcm13051365
3. Bond, EC, Mackey, S, English, R, Liverman, CT, and Yost, O. Temporomandibular disorders: Priorities for research and care. Washington, DC: National Academies Press (2020).
4. Yap, AU, Lei, J, Fu, KY, Kim, SH, Lee, B, and Park, JW. DC/TMD Axis I diagnostic subtypes in TMD patients from Confucian heritage cultures: a stratified reporting framework. Clin Oral Invest. (2023) 27:4459–70. doi: 10.1007/s00784-023-05067-2
5. Costanti Vilela Campos, M, Simoes Velloso Schuler, S, de Barros, MP, Cátia Mazzoni, A, Cristina da Silva, F, Domingues Martins, M, et al. The effect of systemic versus local transcutaneous laser therapy on tension-type cephalea and orofacial pain in post-COVID-19 patients: a pragmatic randomized clinical trial. Medicine (Baltimore). (2022) 101:e31218. doi: 10.1097/MD.0000000000031218
6. Do, TP, Heldarskard, GF, Kolding, LT, Hvedstrup, J, and Schytz, HW. Myofascial trigger points in migraine and tension-type headache. J Headache Pain. (2018) 19:84. doi: 10.1186/s10194-018-0913-8
7. Kuć, J, Szarejko, KD, and Gołȩbiewska, M. Smiling, yawning, jaw functional limitations and Oral behaviors with respect to general health status in patients with temporomandibular disorder-myofascial pain with referral. Front Neurol. (2021) 12:646293. doi: 10.3389/fneur.2021.646293
8. Durham, J, Newton-John, TRO, and Zakrzewska, JM. Temporomandibular disorders. BMJ. (2015) 350:h1154–4. doi: 10.1136/bmj.h1154
9. Yıldırım, G, Erol, F, Güven, MC, and Şakar, O. Evaluation of the effects of bruxism on oral health-related quality of life in adults. Cranio. (2023) 41:230–7. doi: 10.1080/08869634.2020.1853308
10. Fillingim, RB, Slade, GD, Greenspan, JD, Dubner, R, Maixner, W, Bair, E, et al. Long-term changes in biopsychosocial characteristics related to temporomandibular disorder: findings from the OPPERA study. Pain. (2018) 159:2403–13. doi: 10.1097/j.pain.0000000000001348
11. Ernberg, M, Jasim, H, Wåhlén, K, and Ghafouri, B. Altered plasma proteins in Myogenous temporomandibular disorders. J Clin Med. (2022) 11:2777. doi: 10.3390/jcm11102777
12. Di Paolo, C, Falisi, G, Panti, F, Di Giacomo, P, and Rampello, A. “RA.DI.CA.” splint for the Management of the Mandibular Functional Limitation: a retrospective study on patients with anterior disc displacement without reduction. Int J Environ Res Public Health. (2020) 17:9057. doi: 10.3390/ijerph17239057
13. Ostrc, T, Frankovič, S, Pirtošek, Z, and Rener-Sitar, K. Headache because of problems with teeth, mouth, jaws, or dentures in chronic temporomandibular disorder patients: a case-control study. Int J Environ Res Public Health. (2022) 19:3052. doi: 10.3390/ijerph19053052
14. Ohrbach, R, Markiewicz, MR, and McCall, WD Jr. Waking-state oral parafunctional behaviors: specificity and validity as assessed by electromyography. European J Oral Sci. (2008) 116:438–44. doi: 10.1111/j.1600-0722.2008.00560.x
15. Ohrbach, R, and Michelotti, A. The role of stress in the etiology of Oral parafunction and myofascial pain. Oral Maxillofac Surg Clin North Am. (2018) 30:369–79. doi: 10.1016/j.coms.2018.04.011
16. Ohrbach, R, Fillingim, RB, Mulkey, F, Gonzalez, Y, Gordon, S, Gremillion, H, et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. J Pain. (2011) 12:T27–45. doi: 10.1016/j.jpain.2011.09.001
17. Manfredini, D, Ahlberg, J, Aarab, G, Bracci, A, Durham, J, Ettlin, D, et al. Towards a standardized tool for the assessment of bruxism (STAB)—overview and general remarks of a multidimensional bruxism evaluation system. J of Oral Rehabilitation. (2020) 47:549–56. doi: 10.1111/joor.12938
18. Donnarumma, V, Ohrbach, R, Simeon, V, Lobbezoo, F, Piscicelli, N, and Michelotti, A. Association between waking-state oral behaviours, according to the oral behaviors checklist, and TMD subgroups. J Oral Rehabil. (2021) 48:996–1003. doi: 10.1111/joor.13221
19. Yap, AU, Kim, S, Lee, B, Jo, JH, and Park, JW. Sleeping and waking-state oral behaviors in TMD patients: their correlates with jaw functional limitation and psychological distress. Clin Oral Invest. (2024) 28:332. doi: 10.1007/s00784-024-05730-2
20. Lobbezoo, F, Ahlberg, J, Raphael, KG, Wetselaar, P, Glaros, AG, Kato, T, et al. International consensus on the assessment of bruxism: report of a work in progress. J Oral Rehabil. (2018) 45:837–44. doi: 10.1111/joor.12663
21. Leketas, M, Šaferis, V, Kubilius, R, Cervino, G, Bramanti, E, and Cicciù, M. Oral behaviors and parafunctions: comparison of temporomandibular dysfunction patients and controls. J Craniofacial Surg. (2017) 28:1933–8. doi: 10.1097/SCS.0000000000003945
22. Fernandes, G, van Selms, MKA, Gonçalves, D, Lobbezoo, F, and Camparis, CM. Factors associated with temporomandibular disorders pain in adolescents. J Oral Rehabil. (2015) 42:113–9. doi: 10.1111/joor.12238
23. Ohrbach, R, Bair, E, Fillingim, RB, Gonzalez, Y, Gordon, SM, Lim, P-F, et al. Clinical orofacial characteristics associated with risk of first-onset TMD: the OPPERA prospective cohort study. J Pain. (2013) 14:18. doi: 10.1016/j.jpain.2013.07.018
24. Oporto, GH, Bornhardt, T, Iturriaga, V, and Salazar, LA. Single nucleotide polymorphisms in genes of dopaminergic pathways are associated with bruxism. Clin Oral Invest. (2018) 22:331–7. doi: 10.1007/s00784-017-2117-z
25. Myers, ND, Ahn, S, and Jin, Y. Sample size and power estimates for a confirmatory factor analytic model in exercise and sport: a Monte Carlo approach. Res Q Exerc Sport. (2011) 82:412–23. doi: 10.1080/02701367.2011.10599773
26. Zucoloto, ML, Maroco, J, and Campos, JADB. Psychometric properties of the Oral health impact profile and new methodological approach. J Dent Res. (2014) 93:645–50. doi: 10.1177/0022034514533798
27. Ju, X, Ribeiro Santiago, PH, Do, L, and Jamieson, L. Validation of a 4-item child perception questionnaire in Australian children. PLoS One. (2020) 15:e0239449. doi: 10.1371/journal.pone.0239449
28. Gignac, GE, and Szodorai, ET. Effect size guidelines for individual differences researchers. Personal Individ Differ. (2016) 102:74–8. doi: 10.1016/j.paid.2016.06.069
29. Yap, AU, and Marpaung, C. Personality, psychosocial and oral behavioural risk factors for temporomandibular disorder symptoms in Asian young adults. J Oral Rehabil. (2023) 50:931–9. doi: 10.1111/joor.13527
30. Kaplan, S, and Ohrbach, R. Self-report of waking-state Oral parafunctional behaviors in the natural environment. J Oral Facial Pain Headache. (2016) 30:107–19. doi: 10.11607/ofph.1592
31. Kmeid, E, Nacouzi, M, Hallit, S, and Rohayem, Z. Prevalence of temporomandibular joint disorder in the Lebanese population, and its association with depression, anxiety, and stress. Head Face Med. (2020) 16:19. doi: 10.1186/s13005-020-00234-2
Keywords: factor structure, oral behaviors, temporomandibular disorders, exploratory factor analysis, confirmatory factor analyses
Citation: Liu T, Yap AU, Sun Y, Zheng Y, Wang T, Zeng S, Liu Z and Xiong X (2024) Oral behaviors in Chinese temporomandibular disorder patients: insights from exploratory and confirmatory factor analyses. Front. Neurol. 15:1522057. doi: 10.3389/fneur.2024.1522057
Edited by:
Liliana Szyszka-Sommerfeld, Pomeranian Medical University, PolandReviewed by:
Grzegorz Zieliński, Medical University of Lublin, PolandRenata Samulak, Pomeranian Medical University, Poland
Copyright © 2024 Liu, Yap, Sun, Zheng, Wang, Zeng, Liu and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Liu, bHp5czE5NzZAMTYzLmNvbQ==; Xin Xiong, ZHJ4aW9uZ3hpbkBzY3UuZWR1LmNu
 Tiqian Liu
Tiqian Liu Adrian Ujin Yap1,3,4
Adrian Ujin Yap1,3,4 Yunhao Zheng
Yunhao Zheng Tianqi Wang
Tianqi Wang Xin Xiong
Xin Xiong