- 1Department of Otorhinolaryngology, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany
- 2Department of Neuroradiology, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Objectives: Multiple studies have described the onset and variable incidence of postoperative acute vertigo following cochlear implant (CI) surgery. However, postoperative imaging has not yet been specifically evaluated with special focus on vertigo. The aim of this study is to assess the incidence and causes of new-onset, acute postoperative vertigo following CI surgery using cone beam computed tomography (CBCT).
Materials and methods: This is a retrospective study involving ten patients who experienced postoperative dizziness and ten matched controls without dizziness. All patients received a cochlear implant (CI) between 2020 and 2024. The matching analysis was performed based on the implant, electrode array, and access to the cochlear. We analyzed the postoperative CBCT scans for changes suspicious of air trapping, a so-called pneumolabyrinth in the vestibule using minimal Hounsfield Units (HU).
Results: We compared postoperative CBCT images for electrode array position monitoring in ten patients with vertigo versus ten patients without vertigo after CI surgery. Among the ten patients with postoperative dizziness, six showed suspicious changes in the vestibule consistent with the presence of air. These air-related changes were observed in the vestibule and, in one patient, additionally in the horizontal semicircular canal. Minimal HU were significantly different and confirmed the suspicion of intravestibular air.
Conclusion: This is the first study to describe the suspicion of intravestibular air in CI patients with postoperative vertigo. Therefore, suctioning after the fenestration of the round window membrane or the endosteum after cochleostomy, as well as actions such as bending, pressing, and nose-blowing by the patient, should be strictly avoided. Furthermore, this finding highlights the importance of carefully sealing the electrode array at the cochleostomy site with connective tissue. Risk factors for the development of a pneumolabyrinth with air in the vestibule include intralabyrinthine or intracranial pressure changes, large cochleostomies or a second cochleostomy and electrode placement in the scala tympani.
Introduction
The occurrence of acute postoperative vertigo is recognized following Cochlear Implantation (CI) and has been reported with variable incidence. Studies have reported postoperative dizziness rates as up to 60% (1–5). Some studies have suggested that membrane damage of the sacculus is associated with a loss of function in cervical vestibular evoked myogenic potentials (cVEMPs) and with acute postoperative vertigo (1, 6, 7). In their meta-analysis, Hänsel et al. (8), reported new-onset postoperative vertigo in 202 out of 1743 patients (17.4%) following CI surger. Martin et al. (9), 16.9% postoperative vertigo in 71 included CI patients, along with associated risk factors such as straight lateral wall electrode arrays and surgical trauma. They evaluated cochlear coverage and scalar position via cone beam computed tomography (CBCT) and found no significant difference in postoperative vertigo for either cochlear coverage or scalar position.
Previous studies have described a lower occurrence of acute postoperative vertigo in patients with round window-inserted CIs, but these findings were based on small study cohorts (8, 10, 11). Karimi et al. (12), reported acute postoperative vertigo in patients with possible perilymphatic leakage and observed the disappearance of postoperative dizziness following the resealing of the inserted electrode array. Nevertheless, postoperative imaging of the vestibule has not yet been evaluated in terms of acute postoperative vertigo.
The aim of this study is to assess on air suspicious changes in the vestibule by CBCT in acute postoperative vertigo following CI surgery. The occurrence of air in the labyrinth, the so called pneumolabyrinth, is described amongst others following trauma with temporal bone fracture (13), barotrauma (14, 15), Eustachian tube air inflation (16), and stapes surgery (17). According to a review by Botti et al. (18), the most common site of air entrapment is the vestibule, followed by the cochlea and the most common causes were head trauma with temporal bone fracture followed by stapes surgery. Clinical symptoms of a pneumolabyrinth are hearing loss and vestibular symptoms (18). The pneumolabyrinth directly after CI surgery or in the course can be another cause, so far this cause has mainly been described in case reports (19–25). The pneumolabyrinth can be diagnosed by CT or CBCT scans. In this case, air appears hypodense on imaging. The radiodensity is described in Hounsfield units (HU). Water has an attenuation of 0 HU, whereas air has typically an HU number of −1,000 (26).
Methods
We conducted a retrospective study on CI patients enrolled between 2020 and 2024. Ten patients who reported dizziness during hospitalization after CI surgery were included in the study and one matched control without dizziness for each vertigo patient, so that a total 20 patients were included. The matching was performed with regard to the implant, electrode array, cochlear access and age. Twelve patients underwent imaging through cone beam computed tomography (CBCT) and eight patients high resolution computed tomography (HRCT) before surgery.
Postoperatively, we performed CBCT (NewTom 5G, Cefla s.c., Imola, Italy) one to three days after surgery in all patients. All pre- and postoperative CBCT scans were evaluated by two experienced CI surgeons and a neuroradiologist using the DeepUnity Diagnost 1.2.0.3 program (DH Healthcare GmbH, Germany). The minimal Hounsfield units (HU) in the vestibule in the area of the suspected air were noted. The sectional plane was chosen so that the part of the labyrinth where the cochlea, the lateral semicircular canal and the vestibule are sliced is shown. A region of interest (ROI) with a diameter of 4–5 mm was then placed in the vestibule and the minimal HU was noted (Figure 1). We performed a video head impulse test (vHIT) preoperatively. Postoperatively, we conducted vHIT 4 to 6 weeks following CI surgery in the matching cohort and in patients with vertigo.

Figure 1. CBCT scan of the labyrinth postoperatively after CI surgery with region of interest (ROI; yellow circle) in the area of the vestibule. The diameter (d) of the ROI is 4.4 mm, the minimal Hounsfield unit (HU) is at - 506 HU.
Statistical analysis was done with Prism 7 software (GraphPad Software, Inc., La Jolla, CA, USA). We used the nonparametric Kruskal–Wallis test for statistical analysis. The significance level was set to p < 0.05. Significances were differentiated into (***) for p < 0.001, (**) for p < 0.01, and (*) for p < 0.05.We received ethical approval from the Hospital’s Ethics Committee following the Declaration of Helsinki guidelines (Washington, 2002) (Ethics Committee approval number: 129/19; amendment 240,022) and registered this study in the German Clinical Trials Register (www.drks.de / DRKS00034647).
Results
All patients received a unilateral cochlear implant and met the indication criteria. The average age of the patients was 61.3 years (19 to 76 years) in the vertigo group and 54.9 years (21 to 72 years) in the control group (Tab. 1). Patients were free to choose the CI manufacturer. Middle ear access was achieved after mastoidectomy via a posterior tympanotomy. One patient had a radical cavity (patient B) and in two patients an intracochlear schwannoma was removed during CI surgery by a second cochleostomy in the second turn of the cochlea (patients E and F). Electrode insertion was performed via the round window in 14 patients and via cochleostomy in six patients (Table 1). In all patients, following the electrode insertion, the round window or cochleostomy was sealed with connective tissue.
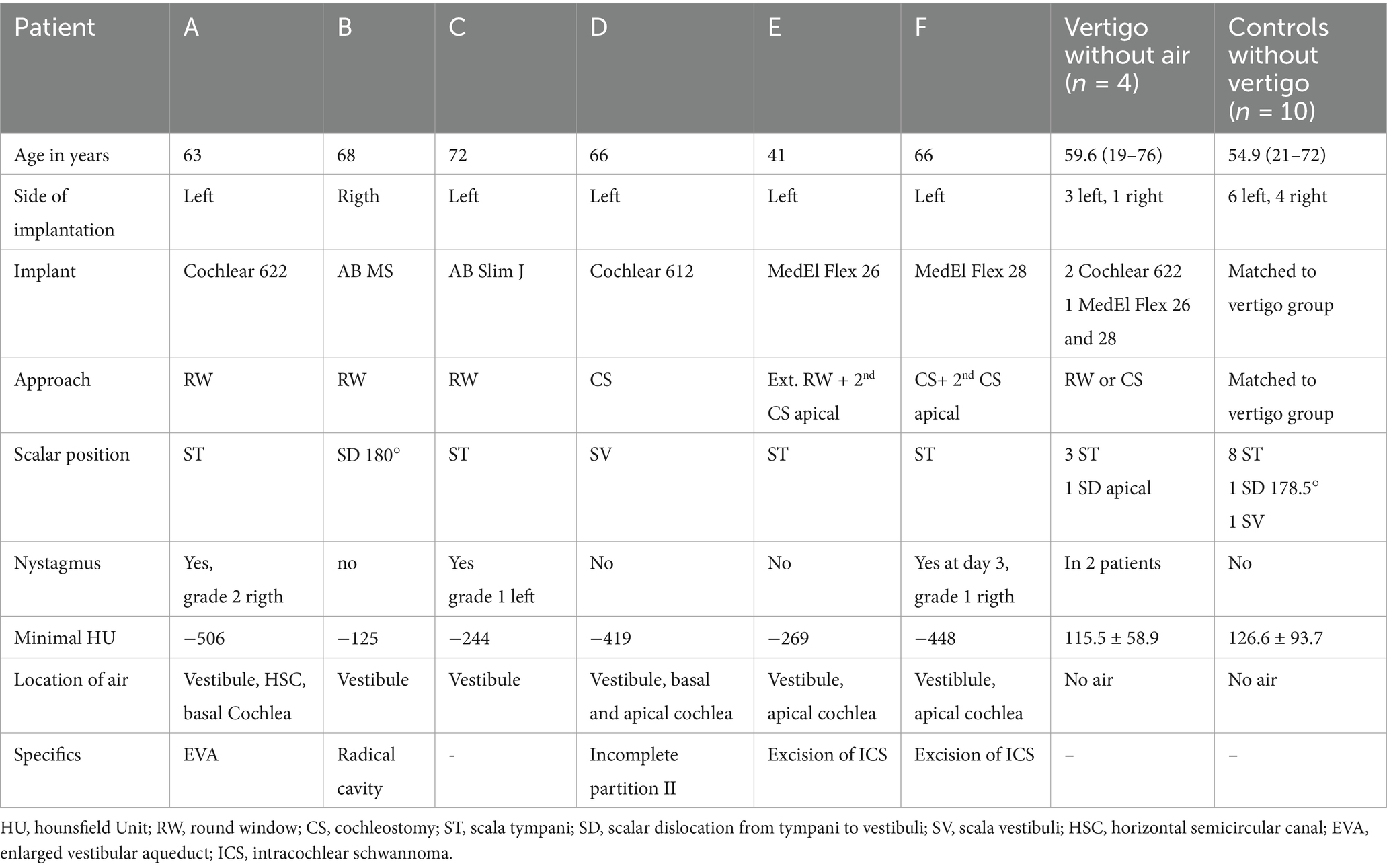
Table 1. Characteristics of the six patients with changes in the vestibule suspicious for air (A-F), four patients with vertigo without air in the vestibule and 10 controls.
We compared postoperative CBCT images for electrode position monitoring of ten patients who suffered from vertigo after CI surgery with ten patients who had no vertigo after CI surgery. In the CBCT scans, attention was focused on suspicious changes in the vestibule, the semicircular canals, and the cochlea. Of the ten patients who had dizziness post-operatively, six patients had suspicious changes in the vestibule on air (see Figure 2 and Table 1). The changes suspicious for air were observed in the vestibule and, in one patient (A), additionally in the horizontal semicircular canal and the cochlea (Figures 2A,A´). The clearest indication of air was observed in patient A of all six patients who showed suspicious changes in the vestibule. Post-operatively, the patient did not experience any dizziness. However, after bending down, sudden dizziness with nystagmus occurred. Additionally, the patient had an enlarged vestibular aqueduct (EVA), but during the cochlear implant surgery, there was no gusher (Table 1). In a revision surgery, which was performed after 2 days, there was no leakage of fluid in the area of the round window. Nevertheless, the area was sealed again and the dizziness improved over time.
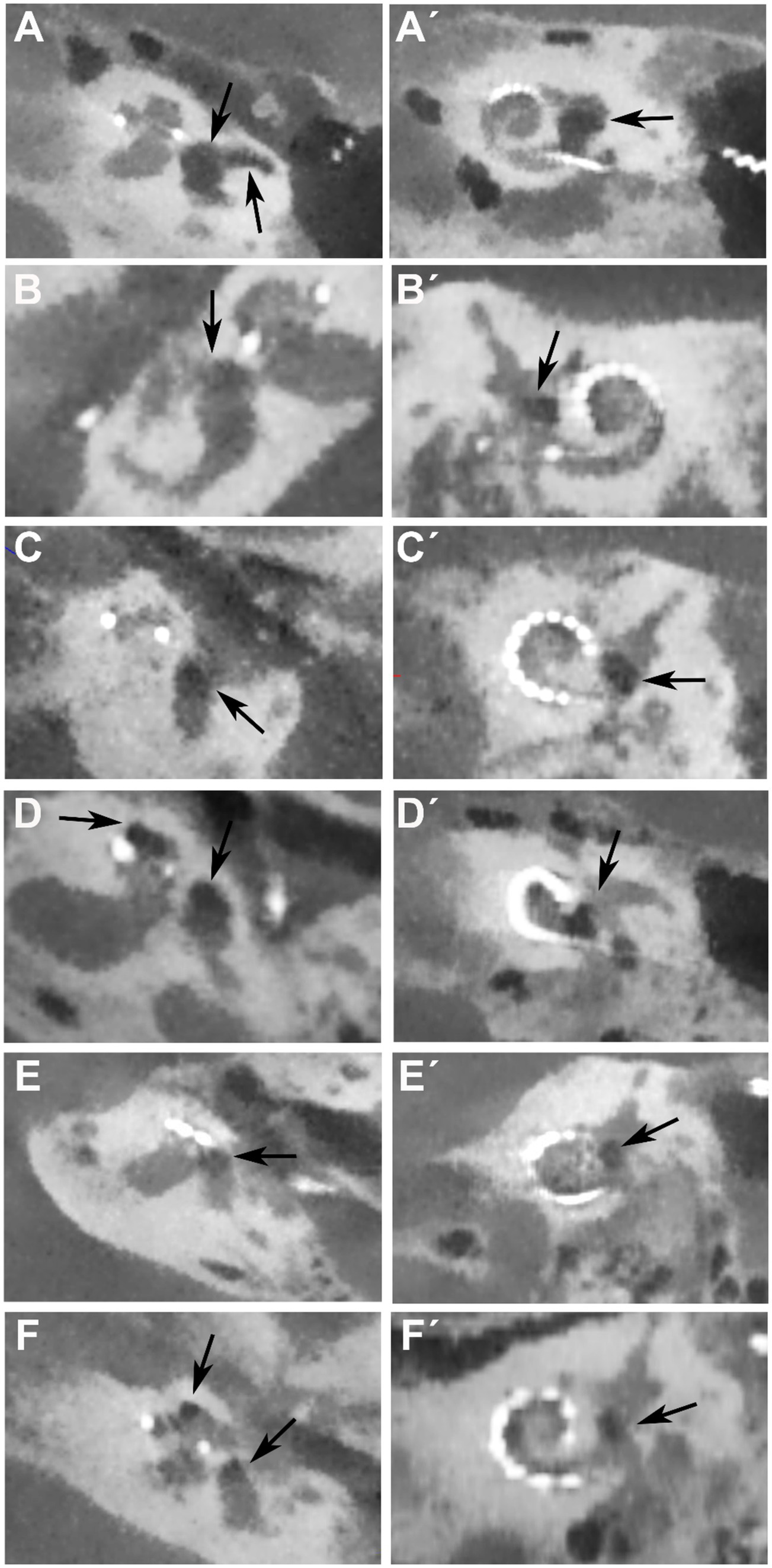
Figure 2. Postoperative CBCT images for electrode position control in cochlea view of the six patients (A–F) with vertigo and on air suspected changes in the vestibulum after CI surgery. Arrow shows localization of air in the vestibule in the axial view (A–F) and coronal view (A´-F´). In patient (A) there is additional air in the lateral semicircular canal and in patients (D,F) additional air is found in the apical turn of the cochlea (second arrows).
In three patients additional air was seen in the cochlea (patients D, E, F; Figure 2), in two of these patients (E and F) a second cochleostomy was performed in the apical turn of the cochlea and an intracochlear schwannoma was removed. The second cochleostomy was also sealed by connective tissue.
Air was also found intracochlearly in patient D. In this patient, a cochlear abnormality of an incomplete partition II was found and therefore a bigger cochleostomy was performed.
The scalar electrode position was mostly the scala tympani (ST). In the vertigo group with air in four patients the electrode was placed in the ST (patients A, C, E, and F; Table 1), in one patient there was a scalar dislocation (SD) from the ST to the SV (scala vestibuli) at 180° (patient B). In one Patient (D) the electrode was in the SV. In the vertigo group without air in three patients the electrode was located in the ST and in one patient an apical SD was found (Table 1). In the control group, the electrodes were in the ST in eight cases, in the SV in one case and in one case there was a dislocation from ST to SV at 178.5° (Table 1).
In three patients in the group with suspicious changes of air postoperatively a preoperative CBCT did not show any suspicious changes in the vestibule and the minimal HU ranged from −12 to 94. In the other three patients in that group HRCT was performed preoperatively so that it was not possible compare the pre- and postoperative scans using the HUs, but no air was visualized.
The matched study patients did not demonstrate suspicious differences in vHIT or VNG preoperatively versus postoperatively. Patients with vertigo and pneumolabyrinth did not demonstrate highly suspicious vHIT postoperatively, except patient A, where air was also found in the ipsilateral horizontal semicircular canal and in the vestibule (Table 2).
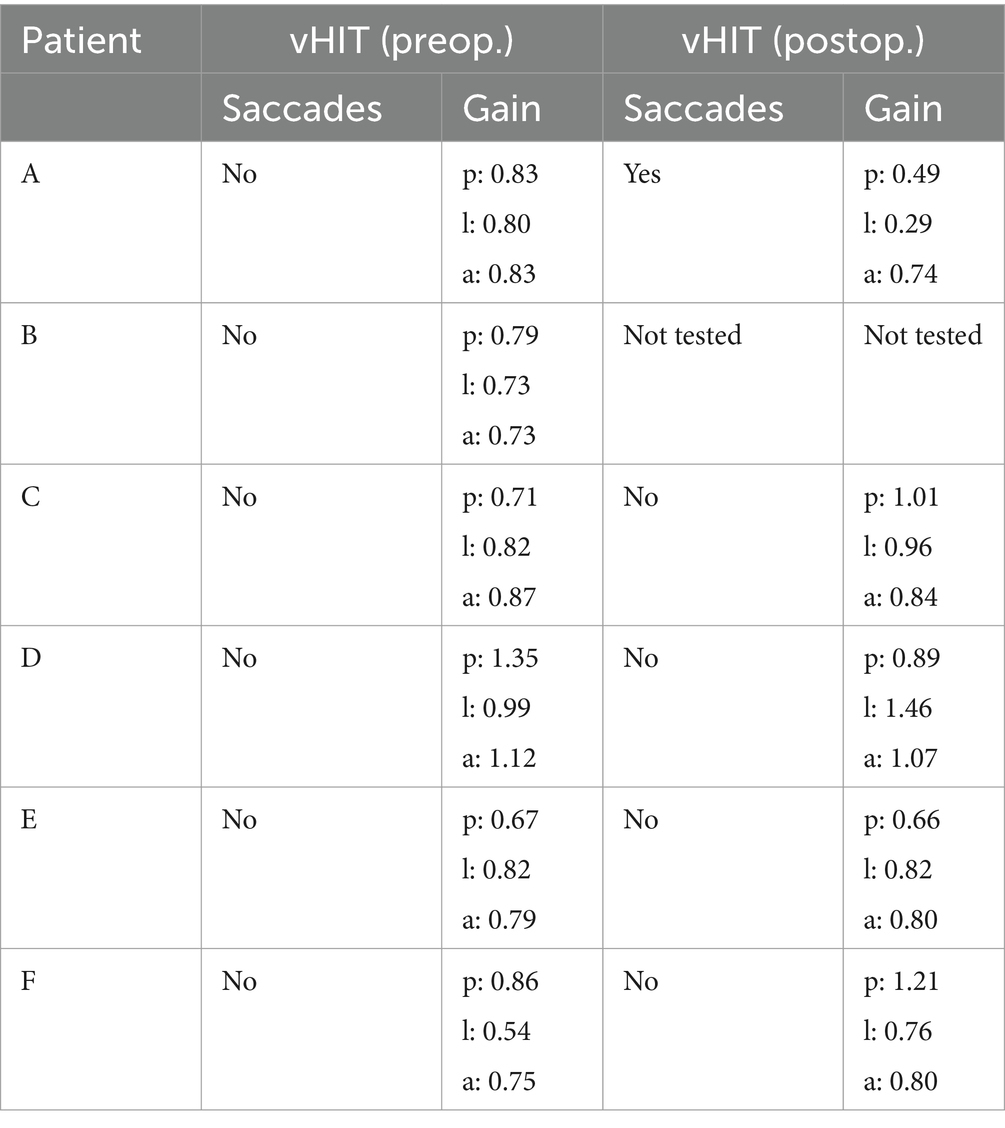
Table 2. video assisted head impuls test (vHIT) preoperatively and postoperatively of patients A to F [p = posterior, l = lateral (=horizontal), a = anterior semicircular canal].
Minimal HUs ranged from −125 to −506 (mean − 335.2 ± 132.8) in the vertigo-group with changes in the vestibule and from 51 to 182 (mean 115.5 ± 58.9) in the vertigo-group without changes in the vestibule (Table 1 and Figure 3). In the control group (patients with no vertigo post-operatively) minimal HU ranged from 11 to 305 (mean 126.6 ± 93.7; Table 1 and Figure 3). There was a significant difference in the minimal HU between the patients with CBCT scans suspicious for air in the vestibule and those with dizziness but not such changes in the vestibule (p = 0.029; Figure 3). In addition, a significant difference between the patients with suspicious changes and controls was observed (p = 0.003; Figure 3). However, such a difference was not found in patients suffering from dizziness but without changes in the vestibule and controls (p > 0.999; Figure 3).
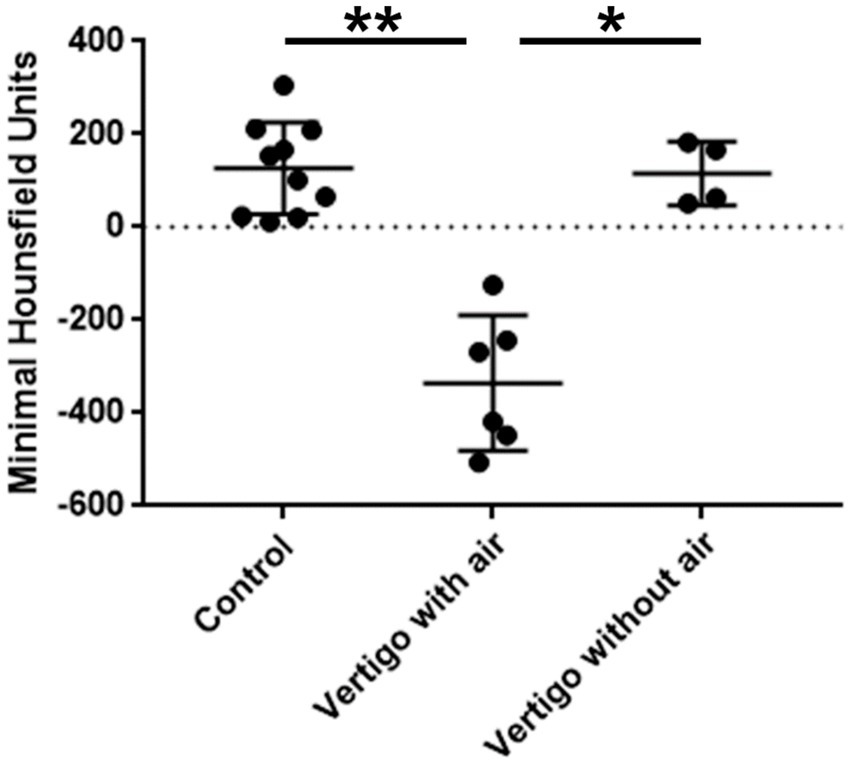
Figure 3. Comparison of the minimal Hounsfield units between the groups vertigo with air, vertigo without air and control. Significant differences were found between the vertigo with air and vertigo without air group and the control group. There was no difference between the vertigo without air group and the control group.
Discussion
Following numerous studies that examined the incidence of acute postoperative dizziness following cochlear implant (CI) surgery and described a wide range of its occurrence (1–5), this study focused on identifying abnormalities in postoperative dizziness in patients through CBCT. A total of ten patients with acute postoperative dizziness were investigated and matched with ten CI patients each who did not exhibit dizziness. To our knowledge, there is only one study to date that focused on pneumolabyrinth after CI surgery. Here, however, air was mainly found in the cochlea of every implanted ear and only in one of 53 patients in the vestibule. The volume of the pneumolabyrinth in the cochlea was significant larger in patients with postoperative dizziness (27).
Although several studies (10, 11) have described cochleostomy as a risk factor for acute postoperative dizziness, our cohort of ten patients with dizziness included four patients who underwent cochleostomy and six patients who had round window insertions. Since most of our CI patients are implanted via the round window and therefore the number of these patients is significantly larger, the ratio of 6:4 could suggest the assumption that a cochleostomy is associated with an increased risk of postoperative vertigo (Table 1).
In six of the ten patients with dizziness who were suitable for analysis, we identified suspicious changes in the vestibule, which appeared to be indicative of air on HU, despite all patients being sealed with connective tissue post-insertion. The fact that the minimal HUs are not around −1,000 but on average around −335 HUs can be explained by the small volume of the vestibule and the resulting possible artifact overlays. Thus, the pathway by which the air entered the vestibule remains to be discussed. The long route via the cochlea from the ST to the SV and then to the vestibule appears unlikely. Other possibilities include passage through the scala media and then the ductus reuniens, potentially due to injuries to the basilar membrane during implantation or through existing fissures or diffusion, followed by pressure increases, such as when blowing the nose or bending over, as described in patient A. The pathophysiology of a pneumolabyrinth discussed in literature are external forces on the oval or round window, fistula ante fenestram, Hyrtl’s fissure, and micofissures that allow air entering into the perilymphatic space (18). In general, pressure changes or large accesses into the cochlea appear to be associated with an increased risk of a pneumolabyrinth. In the existing case reports on a pneumolabyrinth, the patients had a ventriculoperitoneal (VP) shunt which partially did not function (19, 22, 23), an enlarged vestibular aqueduct syndrome (EVA) (24, 25) or a large cochleostomy due to the electrode size (20). In one case, an electrode position in the SV was suspected, but the CT image in the publication also seems to show an EVA (21). In five of our six patients with air in the vestibule, there were also abnormalities in the sense of an EVA (patient A) or large or a second accesses to the cochlea (patient D large cochleostomy for malformation of the cochlea and patients E and F second cochleostomy in the apical turn for removal of intracochlear schwannoma). Patient D exhibited an incomplete partition II with an enlarged cochleostomie. In patient B a scalar dislocation at 180° was found and in patient D the electrode position was in the SV. Only in patient C no anomalies were found. It can be concluded from this that the risk of a pneumolabyrinth is very low in the case of “normal” anatomy and insertion of the electrode via the round window without electrode dislocation.
Some studies have shown that CI surgery leads to shifts of the amplitude in the VEMPs, but a clear cause for this is not yet known (for review (see 8, 28)). One possible explanation for the observed changes in cVEMPs could be the air inclusions we have identified in the vestibule. Since we did not examine VEMPs, this would be interesting for a follow-up study. Especially in cases with preoperative abnormalities such as EVA, intracochlear schwannomas, or cochlear malformations, this examination could be conducted both pre- and postoperatively.
To date, there is no standard treatment for pneumolabyrinth after CI surgery. Depending on the symptoms, cause and extent of air, a conservative approach with vertigo exercises and physiotherapy or surgical exploration with re-sealing can be discussed.
A limitation of this study is the small cohort size. Additionally, the cohort is not particularly homogeneous, as it includes one patient with a radical cavity, one patient with EVA, one patient with incomplete partition II and two others with an intralabyrinthine schwannoma. Nevertheless, to the best of our knowledge, this is the first study to describe intravestibular air in CI patients with postoperative vertigo.
In summary, there are various risk factors for postoperative dizziness with pneumolabyrinth. These risk factors include intralabyrinthine and intracranial pressure changes, e.g., due to EVA or the presence of a VP shunt. Other risk factors that favor a pneumolabyrinth are large CS or a second CS in intralabyrinthine schwannomas and an electrode location in the SV or a dislocation of the electrode from the ST to the SV.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethikkommission Universität Freiburg. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FE: Data curation, Methodology, Writing – review & editing. NL: Formal analysis, Validation, Writing – review & editing. A-KR: Data curation, Methodology, Project administration, Writing – review & editing. AA: Conceptualization, Formal analysis, Project administration, Resources, Writing – review & editing. SA: Methodology, Writing – review & editing. TJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the Fördergesellschaft “Taube Kinder lernen Hören e.V.,” which has supported the cochlear implant rehabilitation center in Freiburg for the past several years.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Basta, D, Todt, I, Goepel, F, and Ernst, A. Loss of saccular function after cochlear implantation: the diagnostic impact of intracochlear electrically elicited vestibular evoked myogenic potentials. Audiol Neurootol. (2008) 13:187–92. doi: 10.1159/000113509
2. Brey, R, Facer, GW, Trine, MB, Lynn, SG, Peterson, AM, and Suman, VJ. Vestibular effects associated with implantation of a multiple channel cochlear prosthesis. Am J Otol. (1995) 16:424–30.
3. Fina, M, Skinner, M, Goebel, JA, Piccirillo, JF, Neely, JG, and Black, O. Vestibular dysfunction after cochlear implantation. Otol Neurotol. (2003) 24:234–42; discussion 242. doi: 10.1097/00129492-200303000-00018
4. Huygen, PL, van den Broek, P, Spies, TH, Mens, LH, and Admiraal, RJ. Does intracochlear implantation jeopardize vestibular function? Ann Otol Rhinol Laryngol. (1994) 103:609–14. doi: 10.1177/000348949410300805
5. Klenzner, T, Neumann, M, Aschendorff, A, and Laszig, R. Thermische Erregbarkeit des Vestibularorgans nach Cochlear-implantation. Laryngorhinootologie. (2004) 83:659–64. doi: 10.1055/s-2004-825678
6. Krause, E, Louza, JPR, Hempel, J-M, Wechtenbruch, J, Rader, T, and Gürkov, R. Effect of cochlear implantation on horizontal semicircular canal function. Eur Arch Otorrinolaringol. (2009) 266:811–7. doi: 10.1007/s00405-008-0815-5
7. Krause, E, Wechtenbruch, J, Rader, T, and Gürkov, R. Influence of cochlear implantation on sacculus function. Otolaryngol Head Neck Surg. (2009) 140:108–13. doi: 10.1016/j.otohns.2008.10.008
8. Hänsel, T, Gauger, U, Bernhard, N, Behzadi, N, Romo Ventura, ME, Hofmann, V, et al. Meta-analysis of subjective complaints of vertigo and vestibular tests after cochlear implantation. Laryngoscope. (2018) 128:2110–23. doi: 10.1002/lary.27071
9. Matin, F, Krueger, C, Avallone, E, Rossberg, W, Demyanchuk, A, Guenther, A, et al. Influence of the electrode Array design on incidence of Vertigo symptoms and vestibular function after Cochlear implantation. Ear Nose Throat J. (2023) 102:701–8. doi: 10.1177/01455613211022075
10. Adunka, OF, Dillon, MT, Adunka, MC, King, ER, Pillsbury, HC, and Buchman, CA. Cochleostomy versus round window insertions: influence on functional outcomes in electric-acoustic stimulation of the auditory system. Otol Neurotol. (2014) 35:613–8. doi: 10.1097/MAO.0000000000000269
11. Todt, I, Basta, D, and Ernst, A. Does the surgical approach in cochlear implantation influence the occurrence of postoperative vertigo? Otolaryngol Head Neck Surg. (2008) 138:8–12. doi: 10.1016/j.otohns.2007.09.003
12. Karimi, D, Mittmann, P, Ernst, A, and Todt, I. Surgical treatment of vertigo in cochlear implantees by electrode resealing. Acta Otolaryngol. (2017) 137:1031–4. doi: 10.1080/00016489.2017.1331045
13. Choi, JW, Lyu, A-R, Ryu, KA, Kim, D, and Park, Y-H. Detection of pneumolabyrinth after temporal bone trauma using computed tomography. Acta Otolaryngol. (2016) 136:682–6. doi: 10.3109/00016489.2016.1157266
14. Immordino, A, Lorusso, F, Sireci, F, and Dispenza, F. Acute pneumolabyrinth: a rare complication after cochlear implantation in a patient with obstructive sleep apnoea on CPAP therapy. BMJ Case Rep. (2023) 16:e254069. doi: 10.1136/bcr-2022-254069
15. McGhee, MA, and Dornhoffer, JL. A case of barotrauma-induced pneumolabyrinth secondary to perilymphatic fistula. Ear Nose Throat J. (2000) 79:456–9. doi: 10.1177/014556130007900611
16. Yanagihara, N, Hyodo, J, Takagi, D, and Miuchi, S. A case of pneumolabyrinth induced by Eustachian tube air inflation. Otol Neurotol. (2012) 33:1408–11. doi: 10.1097/MAO.0b013e31826a50dc
17. Vandevoorde, A, Williams, MT, Ukkola-Pons, E, Daval, M, and Ayache, D. Early postoperative imaging of the labyrinth by cone beam CT after stapes surgery for Otosclerosis with correlation to Audiovestibular outcome. Otol Neurotol. (2017) 38:168–72. doi: 10.1097/MAO.0000000000001306
18. Botti, C, Castellucci, A, Crocetta, FM, Fornaciari, M, Giordano, D, Bassi, C, et al. Pneumolabyrinth: a systematic review. Eur Arch Otorrinolaringol. (2021) 278:4619–32. doi: 10.1007/s00405-021-06827-0
19. Hallin, K, Stillesjö, F, Sundblom, J, and Danckwardt-Lillieström, N. Pneumolabyrinth following cochlear implantation resolved after shunt adjustment. Acta Oto-Laryngologica Case Reports. (2020) 5:81–5. doi: 10.1080/23772484.2020.1838906
20. Hempel, J-M, Jäger, L, Baumann, U, Krause, E, and Rasp, G. Labyrinth dysfunction 8 months after cochlear implantation: a case report. Otol Neurotol. (2004) 25:727–9. doi: 10.1097/00129492-200409000-00014
21. Karataş, E, Toplu, Y, Gündüz, E, and Demir, İ. Severe Vertigo after Cochlear implantation: acute Pneumolabyrinth. Balkan Med J. (2018) 35:408–9. doi: 10.4274/balkanmedj.2017.1088
22. McKinnon, BJ, and Watts, T. Subcutaneous emphysema and pneumolabyrinth plus pneumocephalus as complications of middle ear implant and cochlear implant surgery. Ear Nose Throat J. (2013) 92:298–300. doi: 10.1177/014556131309200707
23. Moteki, H, Fujinaga, Y, Goto, T, and Usami, S-I. Pneumolabyrinth, intracochlear and vestibular fluid loss after cochlear implantation. Auris Nasus Larynx. (2018) 45:1116–20. doi: 10.1016/j.anl.2018.03.004
24. Ott, IO, Schwab, B, Becker, H, and Issing, PR. Pneumolabyrinth after cochlear implantation in large vestibular aqueduct syndrome. Otol Neurotol. (2008) 29:1037–8. doi: 10.1097/mao.0b013e318164cb6b
25. Rother, T, Albrecht, C, and Issing, PR. Pneumolabyrinth after cochlear implantation in large vestibular aqueduct syndrome: a case report. Am J Otolaryngol. (2011) 32:430–2. doi: 10.1016/j.amjoto.2010.07.011
26. Spratt, JD, Salkowski, LR, Loukas, M, Turmezei, T, Weir, J, and Abrahams, PH. Weir & Abrahams' imaging atlas of human anatomy. Philadelphia: Elesevier (2017).
27. Im, SY, Kim, M-K, Lee, S, Chung, J-H, and Choi, JW. Pneumolabyrinth as an early computed tomographic finding after Cochlear implantation and its clinical significance. Otol Neurotol. (2022) 43:e38–44. doi: 10.1097/MAO.0000000000003345
Keywords: cochlear implant, Vertigo, Pneumolabyrinth, dizziness, surgery, hearing loss
Citation: Ketterer MC, Everad F, Lützen N, Rauch A-K, Aschendorff A, Arndt S and Jakob TF (2024) Postoperative dizziness after cochlear implant surgery: can it be caused by air? Front. Neurol. 15:1520472. doi: 10.3389/fneur.2024.1520472
Edited by:
Brian John McKinnon, University of Texas Medical Branch at Galveston, United StatesReviewed by:
Andrea Canale, University of Turin, ItalyHan Matsuda, Saitama Medical University, Japan
Copyright © 2024 Ketterer, Everad, Lützen, Rauch, Aschendorff, Arndt and Jakob. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Till F. Jakob, dGlsbC5qYWtvYkB1bmlrbGluaWstZnJlaWJ1cmcuZGU=
 Manuel Christoph Ketterer
Manuel Christoph Ketterer Friederike Everad
Friederike Everad Niklas Lützen2
Niklas Lützen2 Ann-Kathrin Rauch
Ann-Kathrin Rauch Antje Aschendorff
Antje Aschendorff Till F. Jakob
Till F. Jakob