- 1TeleSpecialists, LLC, Fort Myers, FL, United States
- 2Department of Mathematics, University of Tennessee at Chattanooga, Chattanooga, TN, United States
Introduction: Prompt treatment with IV thrombolytics (IVT) in acute ischemic stroke (AIS) patients is critical for improved recovery and survival. Recently, hospital systems have switched to the IVT tenecteplase (TNK) instead of the FDA-approved alteplase (tPA) for treatment. Multiple studies and meta-analyses evaluating the efficacy and safety of TNK demonstrate similar or superior outcomes when compared to tPA. TNK is not FDA-approved for treatment, which has led to hesitation in its use and increased attention on its complication profile, including the risk of intracranial hemorrhage (ICH).
Methods: Data from AIS consults conducted in the emergency departments of 220 facilities across 26 states, between 1 January 2022 and 31 May 2023, were extracted from the TeleCare by TeleSpecialists™ database. The encounters were reviewed for IVT candidates, door-to-needle (DTN) time, type of IVT administered, use of advanced imaging, presence of LVO, occurrence and type of complications, complication type, symptomatic ICH, and the ECASS II ICH score.
Results: A total of 2,305 TNK patients and 3,337 tPA patients were extracted. DTN times were faster (37 min vs. 42 min, p < 0.0001), and more total complications were observed in the TNK group (87 vs. 80, p = 0.0035). In non-LVO IVT patients, the TNK group had more complications (57 vs. 47, p = 0.0078), specifically ICH (48 vs. 35, p = 0.0036). No statistically significant difference in the incidence of ICH was observed between the TNK group and the tPA group (21 vs. 18, p = 0.07). In IVT patients not accepted for NIR, the TNK group had more complications (77 vs. 69, p = 0.005), specifically ICH (63 vs. 51, p = 0.0026). In IVT patients accepted for NIR, no significant differences were observed. There were no statistically significant differences in symptomatic ICH between the groups.
Conclusion: The TNK group was found to have significantly more complications, including ICH, than the tPA group driven by non-LVO patients. A closer analysis of the potential for increased risk to non-LVO patients is warranted based on this large, multistate, and multi-hospital system study.
Introduction
Stroke is one of the leading causes of death and disability in the United States and globally. Prompt recognition of symptoms and treatment with IV thrombolytics in acute ischemic stroke patients is critical for improved recovery and survival (1). Until recently, the gold standard of thrombolytic treatment was alteplase (tPA), which was approved by the FDA in 1996 and remains the only FDA-approved treatment for acute stroke patients in the United States. However, both small community hospitals and large tertiary care systems have been transitioning to the newer modified form of tPA, tenecteplase (TNK), for thrombolytic treatment due to its cost-effectiveness and ease of administration. TNK has a longer half-life than tPA; therefore, it can be administered in a single bolus within seconds rather than needing an hour-long continuous infusion. It also appears to have an increased specificity to fibrin within clots, theoretically leading to more effective thrombus lysis (2).
Multiple studies and meta-analyses evaluating the efficacy and safety of TNK demonstrate similar or superior outcomes when compared to tPA (3). The most recent acute stroke guidelines from the American Heart Association have also incorporated the use of tenecteplase in the setting where a mechanical thrombectomy is planned (4). Despite these studies, the lack of FDA approval for TNK has led to hesitation in its use and increased attention concerning its complication profile, particularly intracranial hemorrhage (ICH).
The purpose of this study was to compare TNK and tPA complications in the community setting to add to the data in randomized controlled trials, specifically to see whether stroke types such as large vessel occlusions (LVOs) or supplemental invasive treatment with neurointerventional radiology (NIR) affect bleeding rates.
Methods
Data from acute stroke consultations conducted in the emergency departments of 220 facilities across 26 states, between 1 January 2022 and 31 May 2023, were extracted from the TeleCare by TeleSpecialists™ database. This database comprises prospectively collected data that were retrospectively reviewed. The criteria for treatment with thrombolytic was limited to the 4.5-hour from last known normal time window for all the patients included in the study. The encounters were reviewed for age, gender, National Institutes of Health Stroke Scale (NIHSS) score, premorbid modified Rankin Score (pre-mRS), race, ethnicity, IV thrombolytic candidacy, door-to-needle (DTN) time, type of thrombolytics used, advanced imaging, presence of LVO, complication occurrence, type of complications, presence of ICH, symptomatic ICH, and the ECASS II ICH score. Once a hemorrhagic complication was identified, stroke neurologists reviewed images in PACS to validate the ECASS II ICH Score as part of the standard quality review process per the TeleSpecialists, LLC quality improvement project following the transition to TNK.
Statistical analysis
For the statistical analysis, this study compared demographics and clinical outcome variables between the two groups of simultaneously collected stroke patients who received either tPA or TNK as the sole thrombolytic agent, per facility protocol. A total of 5,642 patients were included (TNK: 2,305 and tPA: 3,337). Continuous variables (e.g., age and DTN times) were compared using Student’s t-test for normally distributed data and the Mann–Whitney U-test for non-normally distributed data. Categorical variables (e.g., complications and symptomatic intracranial hemorrhage [ICH]) were analyzed using Pearson’s chi-square test. Subgroup analyses were conducted based on the presence of large vessel occlusion (LVO) and candidacy for neurointervention (NIR). Patients were further categorized by LVO status within each group, allowing for comparisons of both continuous and categorical variables. Statistical significance was defined as a p-value <0.05. All analyses were performed using R version 4.3.1.
Results
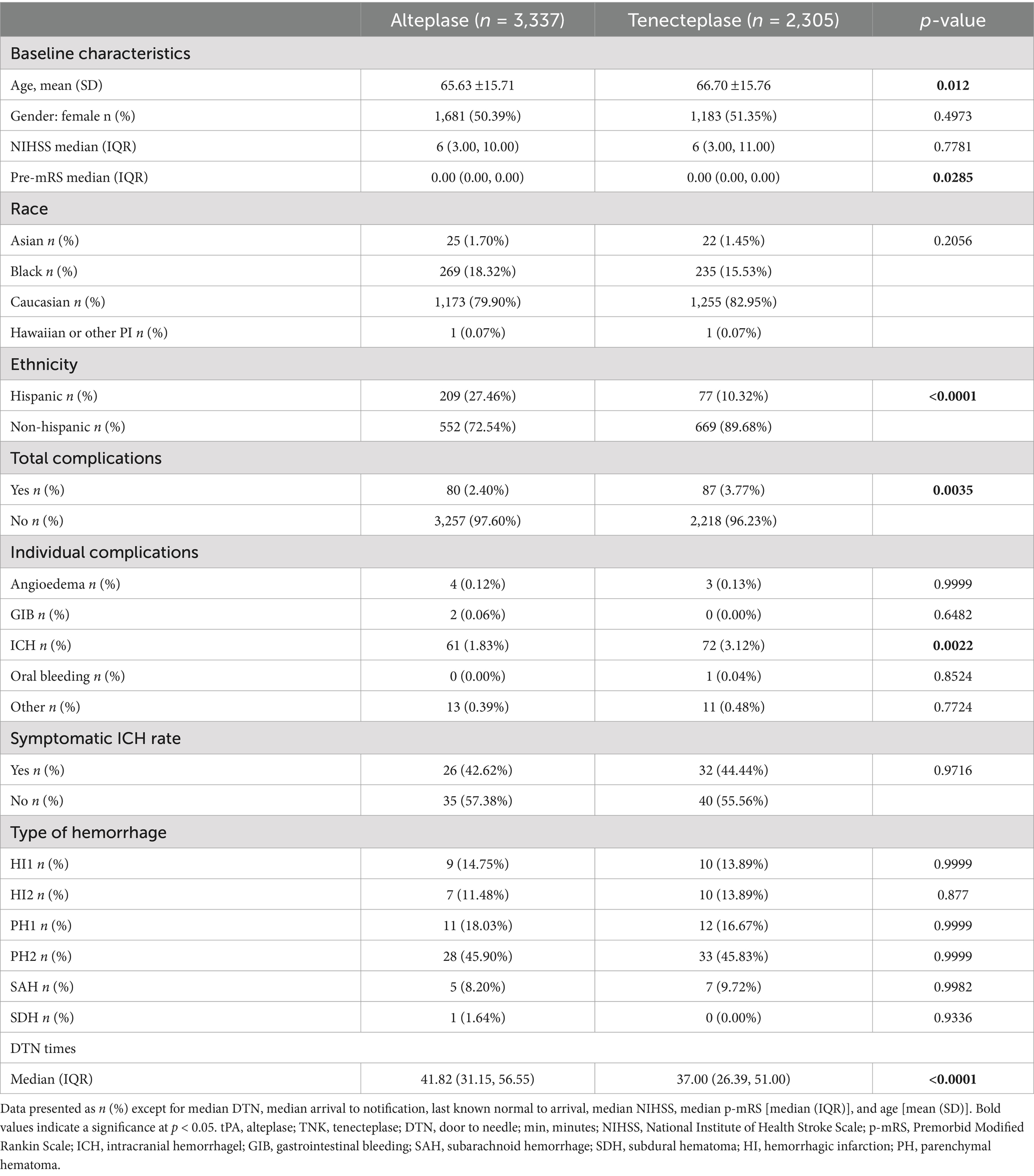
A total of 5,642 patients were included, with 2,305 in the TNK group and 3,337 in the tPA group (Figure 1). Within patient demographics, there was a statistically significant difference in age, with the TNK group being slightly older (Table 1). DTN times were significantly faster in the TNK group. However, significantly more total complications and more ICH were observed. There was no statistically significant difference in the rate of symptomatic ICH, which is consistent with previous smaller studies.
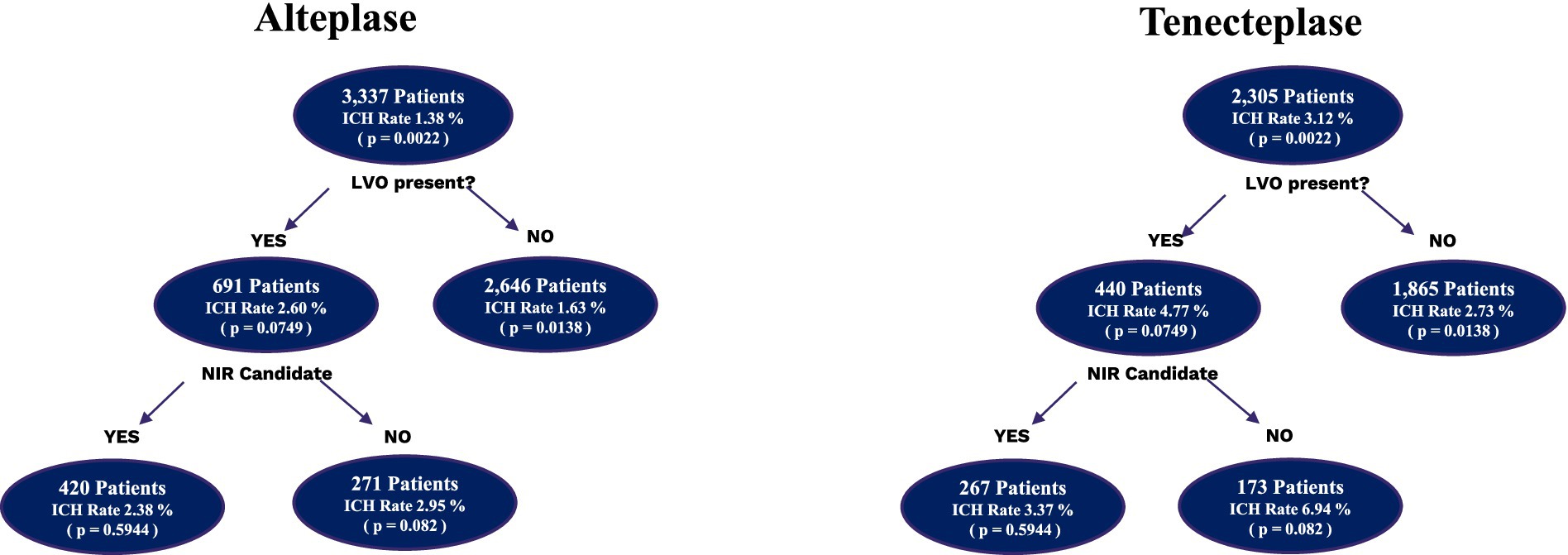
Figure 1. Subgroup analysis of intracranial hemorrhages in alteplase vs. tenecteplase patients. Depiction of the number of patients who received alteplase and tenecteplase subdivided by the presence of LVO and the intracranial hemorrhage rates. The LVO patients were then further subdivided into whether they were accepted for neurointervention or not and the associated intracranial hemorrhage rates. ICH, intracranial hemorrhage.
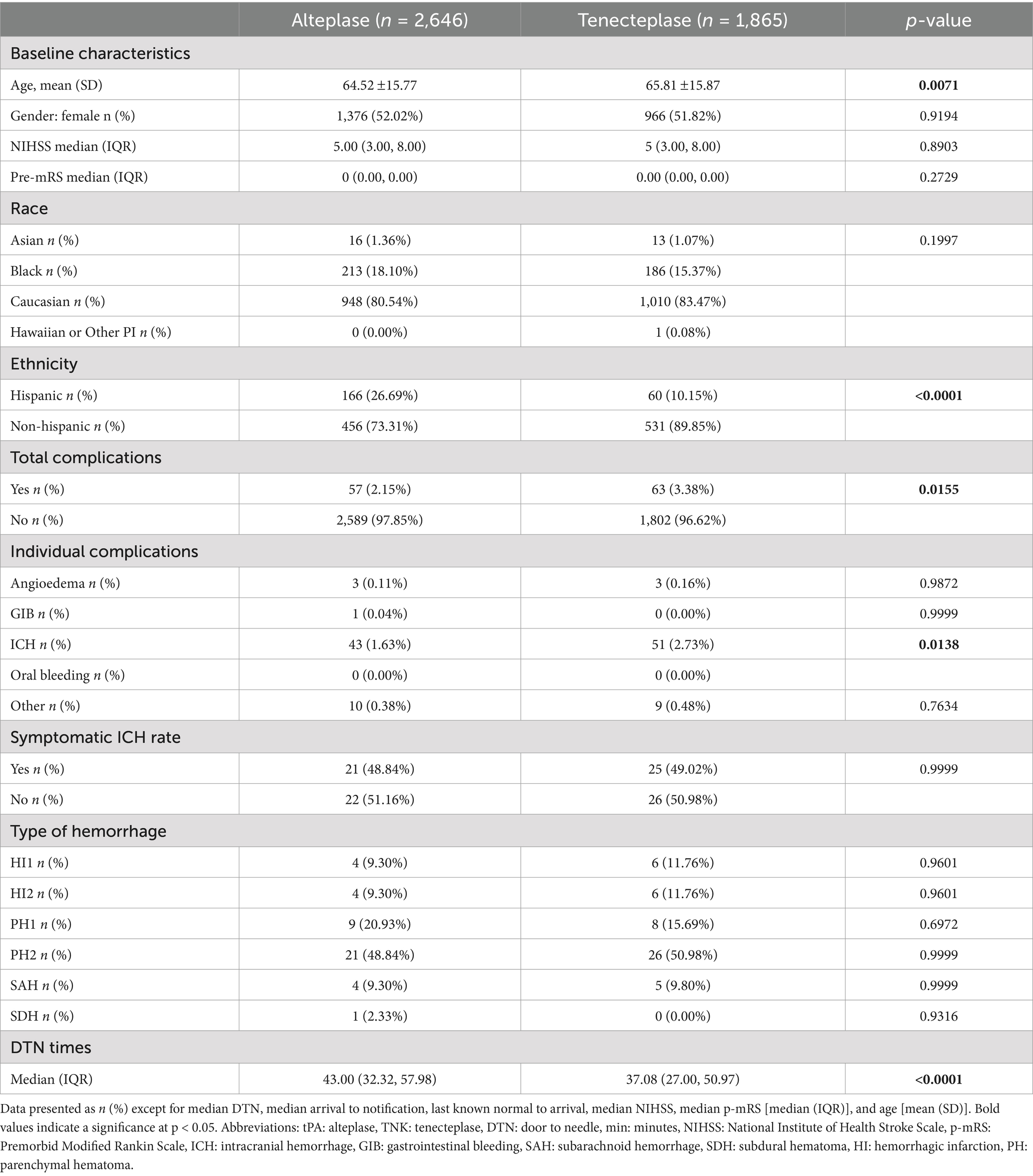
A total of 4,511 patients did not have an LVO present, with 1,865 in the TNK group and 2,646 in the tPA group. The baseline NIHSS score in both groups was 5. There were some statistical differences within the demographics of this cohort (Table 2), with older age in the TNK group and significantly more Hispanic patients in the tPA group. The DTN time was significantly faster in the TNK group. There were more total complications, specifically ICH, but no statistical difference in symptomatic ICH.
There were 1,131 patients identified with an LVO. Of these patients, 691 patients received tPA and 440 patients received TNK. The demographics showed (Table 3) that the tPA group had significantly more Hispanic patients. The baseline NIHSS score in both groups was 13. There were no significant differences in complications, ICH, or in DTN time. Of the 1,131 patients with an LVO, 687 patients were accepted for neurointervention, while 444 patients were not. A total of 420 NIR patients were administered tPA and 267 patients were administered TNK, and no significant differences in demographics, complications, or DTN time were observed (Table 4). The baseline NIHSS score in both groups was 14.
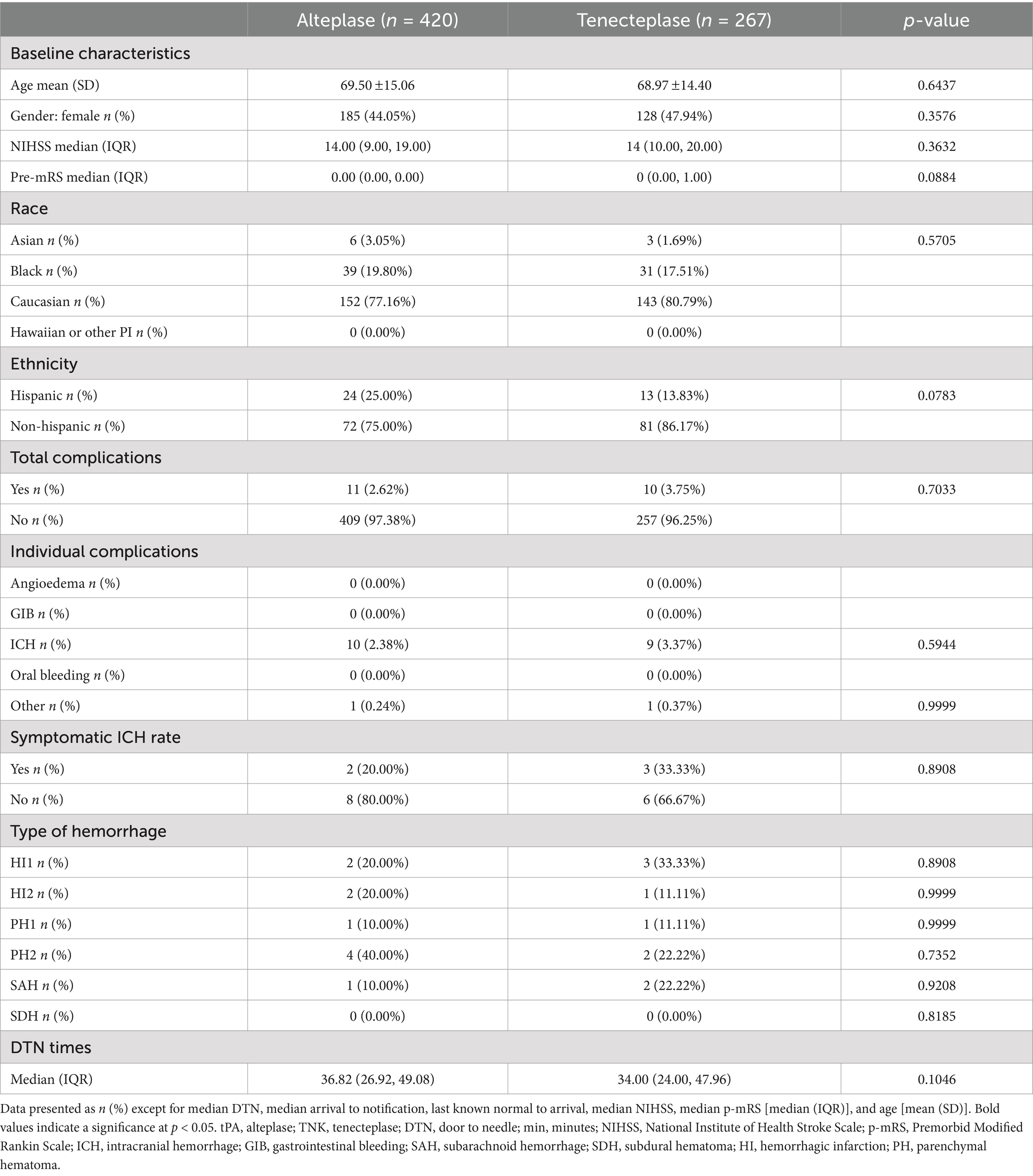
Table 4. Comparison of variables between alteplase and tenecteplase subgroups with LVO who were accepted for neurointervention.
In the 444 patients who had an LVO and were not candidates for NIR, 271 patients were administered tPA and 173 patients were administered TNK. TNK patients were older than tPA patients, and tPA patients were significantly more likely to be Hispanic (Table 5). The baseline NIHSS score in both groups was 10. There were no significant differences in complications, ICH, or in DTN time. There were no statistically significant differences in symptomatic ICH between all groups.
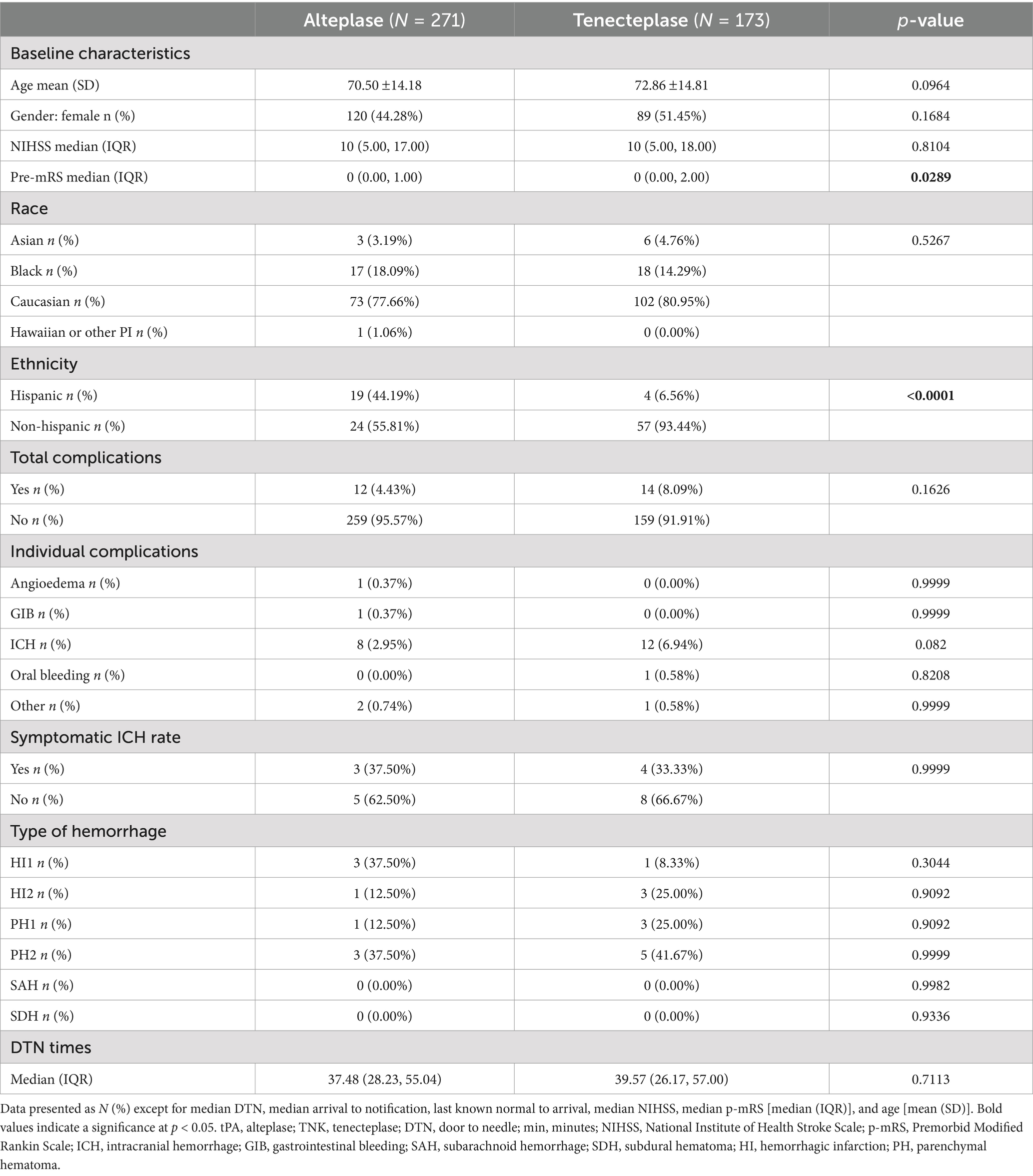
Table 5. Comparison of variables between alteplase and tenecteplase subgroups with LVO and no neurointervention.
Conclusion
This study is the largest, non-randomized, community-based study of the complications of tPA vs. TNK in the acute stroke setting. Overall, DTN times were significantly faster in the TNK group, which is advantageous when considering the importance of expedited treatment to improve patient outcomes (5). With studies showing non-inferiority and the utilization of a non-FDA-approved treatment when an FDA-approved treatment is available, it is important to have detailed data on the complication profile. This large sample demonstrated an increased rate of total complications, though it is important to note that the complication rates within both groups remained low (tPA 2.40% and TNK 3.77%) compared to the most cited complication rate of 6.4% (6). The only significant difference observed was in the rate of intracranial hemorrhages (tPA 1.83% and TNK 3.12%), with no significant differences in the rates of angioedema, GI bleeding, oral bleeding, or other complications. One of the most important findings is that despite the overall ICH rate being higher with TNK, there was no significant difference in symptomatic ICHs.
Randomized controlled trials have shown non-inferior outcomes and a trend toward more intracranial hemorrhages but not symptomatic hemorrhages (3). This large real-world study shows that the increased hemorrhage rate is statistically significant with a larger sample size, but there is still no significant difference in symptomatic hemorrhage. While these data do not include outcomes, they do provide unique insight into the complications profile.
The ability to compare the LVO vs. non-LVO groups, as well as those who were accepted for neurointervention vs. those who were not, shows some interesting insights. Surprisingly, the difference in ICH was caused by the increased rate of ICH in non-LVO patients receiving TNK. These patients tend to have lower NIHSS scores and smaller stroke volumes. This may explain why there are fewer symptomatic ICHs in this patient population. However, there was no significant difference in the ECASS II score assigned to the hemorrhages, and no cases of subarachnoid hemorrhages or subdural hematomas were observed in this group. There was no significant difference in hemorrhage grading volumes among the subgroups or the primary analysis.
With regard to symptomatic ICH, there is no significant difference between TNK and tPA, which is an important finding with the TNK transition trend. Although there were no significant differences in symptomatic intracranial hemorrhage between the groups, the increased ICH rate in non-LVO patients with TNK stands out in this study as a potentially pertinent finding. Future research targeting the non-LVO population receiving thrombolytics in the 4.5-h time window is warranted, focusing on outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
MF: Investigation, Methodology, Writing – original draft, Writing – review & editing. SC: Data curation, Writing – review & editing. OC: Formal analysis, Methodology, Writing – review & editing. LG: Data curation, Formal analysis, Methodology, Writing – review & editing. AA: Conceptualization, Methodology, Writing – review & editing. KD: Writing – review & editing. LM: Writing – review & editing. TS: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors acknowledge the physicians and quality team who helped to collect and verify the data in the TeleCare by TeleSpecialists™ database.
Conflict of interest
TS has fractional shares in Moderna stock. MF, TS, AA, and SC were employed by the company TeleSpecialists, LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dhar, N, Kumar, M, Tiwari, A, Desai, I, Madhaw, G, and Kumar, N. Tenecteplase and Alteplase for thrombolysis of acute ischemic stroke within 4.5 hours: an efficacy and safety study. Ann Indian Acad Neurol. (2022) 25:897–901. doi: 10.4103/aian.aian_1127_21
2. Singh, N, Almekhlafi, M, Bala, F, Ademola, A, Coutts, S, Deschaintre, Y, et al. Effect of time to thrombolysis on clinical outcomes in patients with acute ischemic stroke treated with Tenecteplase compared to Alteplase: analysis from the AcT randomized controlled trial. Stroke. (2023) 54:2766–75. doi: 10.1161/STROKEAHA.123.044267
3. Wang, Y, Li, S, Pan, Y, Li, H, Parsons, MW, Campbell, BCV, et al. TRACE-2 investigators. Tenecteplase versus alteplase in acute ischaemic cerebrovascular events (TRACE-2): a phase 3, multicentre, open-label, randomised controlled, non-inferiority trial. Lancet. (2023) 401:645–54. doi: 10.1016/S0140-6736(22)02600-9
4. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
5. Saver, JL, Fonarow, GC, Smith, EE, Reeves, MJ, Grau-Sepulveda, MV, Pan, W, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. (2013) 309:2480–8. doi: 10.1001/jama.2013.6959
Keywords: tenecteplase, alteplase, telestroke, acute stroke care, thrombolytics
Citation: Figurelle M, Corti S, Collins O, Gao L, Avila A, Delfino K, Mayer L and Sevilis T (2025) Safety of tenecteplase vs. alteplase in telestroke: a large multistate experience (STAT). Front. Neurol. 15:1514915. doi: 10.3389/fneur.2024.1514915
Edited by:
Cristina Tiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Hipólito Nzwalo, University of Algarve, PortugalPiotr Sobolewski, Jan Kochanowski University, Poland
Copyright © 2025 Figurelle, Corti, Collins, Gao, Avila, Delfino, Mayer and Sevilis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Theresa Sevilis, dHNldmlsaXNAdHN0ZWxlbWVkLmNvbQ==
 Morgan Figurelle
Morgan Figurelle Sandro Corti
Sandro Corti Oleg Collins2
Oleg Collins2 Lan Gao
Lan Gao Amanda Avila
Amanda Avila Kristie Delfino
Kristie Delfino Laurie Mayer
Laurie Mayer Theresa Sevilis
Theresa Sevilis