
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 07 January 2025
Sec. Movement Disorders
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1503342
 Ayesha Khan1†
Ayesha Khan1† Harsh Kumar1†
Harsh Kumar1† Kuldeep Dalpat Rai1†
Kuldeep Dalpat Rai1† Anzel Saeed1†
Anzel Saeed1† Jawad Ishtiaq1†
Jawad Ishtiaq1† Muhammad Tanveer Alam1
Muhammad Tanveer Alam1 Sakshi Chawla1†
Sakshi Chawla1† Md Ariful Haque2,3*†
Md Ariful Haque2,3*†Introduction: Restless Legs Syndrome (RLS), also known as Willis-Ekbom Disease (WED), is a sensorimotor disorder characterized by an uncontrollable urge to move the legs, typically accompanied by discomfort. Low iron levels, pregnancy, and age are some identified risk factors. RLS is treated using various pharmacological options, including dopamine agonists, benzodiazepines, anticonvulsants, opioids, and bupropion. Iron supplementation, particularly with intravenous Ferric carboxymaltose (FCM), has gained attention due to the role of iron deficiency in RLS pathophysiology. This meta-analysis evaluates the efficacy and safety of FCM in treating RLS symptoms.
Materials and methods: A systematic review and meta-analysis were conducted following the PRISMA guidelines, using databases such as PubMed, Google Scholar, and Cochrane. Studies involving intravenous FCM in patients diagnosed with RLS were included. Statistical analysis was performed using Review Manager 5.4.
Results: Seven studies involving 539 participants were analyzed. FCM significantly reduced IRLS scores (WMD = −5.77; 95% CI = [−8.85, −2.70]; p = 0.0002) and improved VAS and SF-36 scores compared to placebo. However, FCM did not significantly improve RLS quality of life scores. Adverse events were more common in the FCM group, particularly nausea, but no significant differences were found for severe adverse events.
Conclusion: In conclusion, intravenous ferric carboxymaltose significantly reduces Restless Legs Syndrome symptoms, especially in patients with confirmed iron deficiency. The treatment appears generally well-tolerated, with adverse effects being manageable. However, further long-term studies are needed to fully assess the safety profile and confirm sustained symptom improvement in a broader population.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42024585233.
Restless Legs Syndrome (RLS) is a neuromuscular disorder characterized by an uncomfortable and sometimes painful sensation during inactivity of the legs (1). These sensations are temporarily relieved by movement. The discomfort can manifest as crawling, tingling, or aching feelings in the legs and may cause significant disruption to sleep and daily activities. Over the last 10 years, there has been a high focus on studying Restless Legs Syndrome. Yet, there is still limited knowledge about this condition and countless individuals experience symptoms of RLS without receiving a diagnosis from healthcare professionals. A recent study found that despite 45% of patients showing moderate to severe RLS symptoms, none were diagnosed or treated (2). Only 6.2% of people experiencing RLS symptoms and seeking medical assistance stated that they had been diagnosed with RLS (3). The inadequate diagnosis given to patients with RLS highlights the lack of knowledge about this disorder.
The International RLS Study Group (IRLSSG) established the RLS standard diagnostic criteria in 2003. The key factors of RLS comprise: (a) a desire to move the legs, frequently coupled with discomfort in the legs or other areas of the body; (b) symptoms worsened during rest; (c) symptoms improved by movement and (d) symptoms that peak in the evening or night (3). There is also a 1995 IRLSSG diagnostic criteria for diagnosing RLS (4), used by studies before 2005. This criteria also suggests four essential characteristics for diagnosing the condition: (a) urge to move limbs, usually accompanied by abnormal sensations; (b) physical restlessness; (c) symptoms aggravated during rest but with temporary relief through activity and movement; and (d) symptom exacerbation in the evening or night.
Studies on the epidemiology of RLS suggest that the prevalence rates in the general population range from 1 to 15% in various populations (5–7). Previous studies have suggested that the actual prevalence of RLS could be underestimated due to patients being scared of social rejection in some cultures, not experiencing symptoms during testing, and the belief that it is a minor issue not requiring medical intervention (8). Numerous global studies show a higher occurrence of RLS in women, indicating that women are at a higher risk than men worldwide, regardless of location. A study proposed that a potential genetic pattern might contribute to women's higher prevalence of RLS (9).
The risk factors that might contribute to RLS include (a) pregnancy: studies found a higher rate of RLS in pregnant women and hypothesized that hormones might be the cause (10). (b) Low iron levels: the “Iron Hypothesis” explains the connection of low iron levels with RLS. RLS can hinder the typical age-related rise in brain iron, leading to decreased iron levels in specific brain regions and impacting brain function (11). (c) Lower socioeconomic status (12). (d) Poor health (13). (e) Old age: supported by the results from the REST study (2004), which concluded that the prevalence of RLS increased from a 1% prevalence in the 20–29 age group to a 4% prevalence in the 70–89-year-old groups. (f) Parkinson's disease, (g) psychiatric disorders, and (h) end-stage renal disease (14).
The possible treatments of RLS include dopamine agonists, benzodiazepines, anticonvulsants (such as carbamazepine or gabapentin), opioids (such as codeine or oxycodone), bupropion and iron supplementation (14). Multiple articles have covered various aspects of each of these treatments. Still, this article provides explicitly an analysis of clinical trials done to assess the efficacy and safety of the treatment of RLS with ferric carboxymaltose (FCM), an intravenous iron supplement.
This systematic review and meta-analysis fully comply with the preferred reporting items for the systematic review and meta-analysis (PRISMA) 2020 statement (15). The protocol is registered in PROSPERO with protocol ID: CRD42024585233.
We performed a literature search using the following electronic databases: PubMed (MEDLINE), Google Scholar, and Cochrane Central Register of Controlled Trials (The Cochrane Library) from inception to Aug 1 2024 for all relevant articles that used “Restless Legs Syndrome,” “RLS,” “Willis -Ekbom disease,” “ferric carboxymaltose,” “ferric compounds,” “injectafer,” and “FCM.” as keywords in the title, subject heading, and text word. The detailed database search strategy is shown in Supplementary Table 1. The search results were not filtered or restricted in any way. All potentially relevant studies, articles (including undocumented data and meta-analyses), and international guidelines were searched manually, and their references were cross-searched to identify additional suitable studies. Two independent investigators (JJ and KK) evaluated the published articles, and disagreements were fully discussed to reach a consensus.
The eligibility criteria included (1) Participants must have a diagnosis of RLS according to the international Restless Leg Syndrome Study Group (IRLS) criteria, with a score of 15 or higher. (2) The studies must evaluate clinical outcomes related to RLS, such as changes in IRLS scores, VAS pain levels, or quality of life (QoL) metrics. (3) The intervention arm must involve IV FCM as the iron replacement therapy for managing RLS symptoms.
Exclusion criteria consisted of (1) non-randomized studies, including case reports and observational research due to concerns about bias, (2) no control group in the trial, and (3) participants with RLS, which is secondary to any other disease condition. Supplementary Table 2 provides information on the study characteristics of the included studies.
Studies were independently screened and assessed by two reviewers (KD and JI). For each RCTS, data on study characteristics (e.g., author, year, sample size, and follow-up), patient demographics (age, sex), and intervention details (dose of IV FCM) were extracted. Information on clinical outcomes, including IRLS scores, VAS pain ratings, ferritin, Hb levels, and adverse events, was also collected. Additionally, the overall conclusion of each study regarding the effect of IV FCM on RLS was noted. Two reviewers independently extracted these data using predefined criteria. Primary authors of the selected publications were contacted when the relevant information was not reported. If the above data could not be found, items were designated as “N/A (not available).” In cases where a consensus could not be reached, the opinion of a third reviewer (AK) was sought.
The quality of included RCTs was independently assessed by two authors (AK AND AS) using RevMan 5.4.1 software according to the Cochrane Handbook, and the ROB-2 tool was used to assess the risk of bias in RCTs (10). In cases where a consensus could not be reached, the opinion of a third reviewer (SC) was sought. The risk-of-bias assessment included five items, as follows: (1) adequate sequence generation; (2) allocation concealment; (3) incomplete outcome data; (4) free of selective reporting; (5) free of other biases. The judgments were categorized as “yes” (low risk of bias), “no” (high risk of bias), or “unclear” (unclear risk of bias; Supplementary Figure 2). All analyses were based on previously published studies; thus, no ethical approval and patient consent were required. Figure 1 presents an assessment of the risk of bias.
The analysis was conducted using (RevMan) Review Manager Version 5 with a random effects model applied to account for variations between studies. The standard mean difference (SMD) and 95% confidence interval CI were calculated for continuous data. Relative risks (RR) or risk difference (RD) were used for dichotomous data. A p-value below 0.05 was considered statistically significant. Heterogeneity within the clinical trials was evaluated using the Higgins (I2) statistic, with interpretations as follows: 25–50% indicating low heterogeneity, 50–75% indicating moderate heterogeneity, and >75% indicating high heterogeneity. A sensitivity analysis was conducted to identify its source in cases of high heterogeneity.
Our initial search led to the identification of 1,280 records, which were reduced to 967 after the removal of duplicates. After a preliminary assessment of these records, 41 articles were shortlisted for further scrutiny. Six articles were selected based on matching inclusion and exclusion criteria (17–21). Of the 41 articles scrutinized, 16 were excluded due to irrelevant outcomes, eight due to add-on therapeutic agents, nine due to different control agents, and finally, two were secondary to incomplete data being present. The selection process is summarized in Figure 2.
The findings of our systematic review and meta-analysis are presented below, with primary outcomes comprising IRLS change from baseline and secondary outcomes being the RLS Qol, VAS score, SF-36, and safety outcomes.
Seven studies assessed the effect of FCM on the IRLS score. The pooled analysis of data from these studies revealed that FCM elicited a significant decrease in IRLS score compared to the placebo group (WMD = −5.77; 95% CI= [−8.85, −2.70]; p = 0.0002). Seventy-nine percent heterogeneity was observed (Figure 3A).
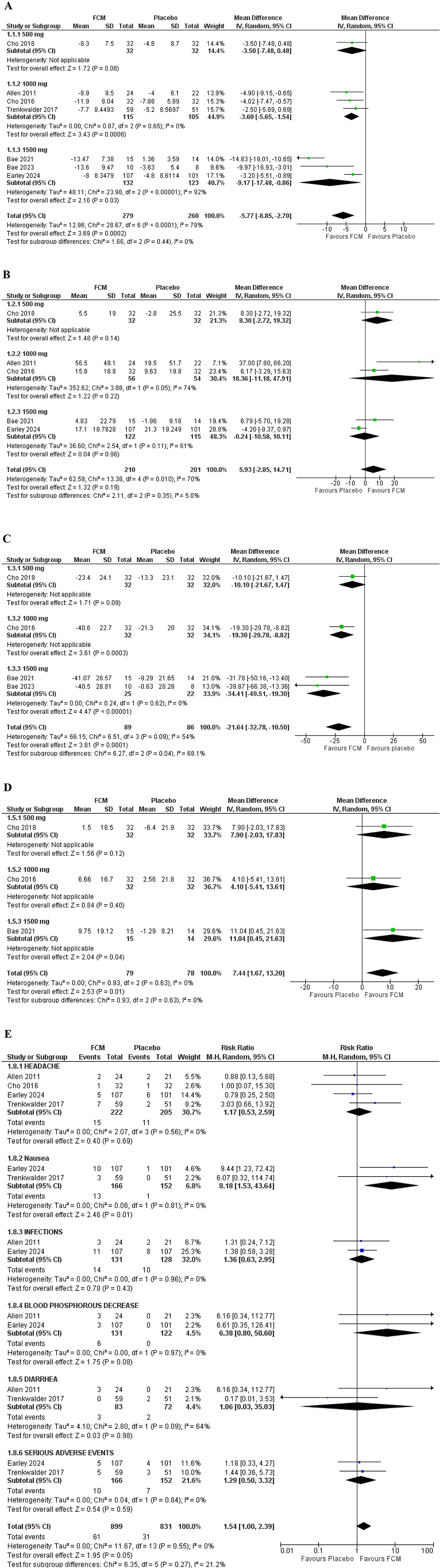
Figure 3. Forest plots; primary outcome (A) IRLS change from baseline. Secondary outcomes (B) RLS QOL (C) VAS score (D) SF-36 (E) adverse effects.
Five studies evaluated the RLS Quality of Life score. The pooled analysis of data from these three studies revealed that FCM elicited an insignificant improvement in QOL-PFD scores compared to the placebo group (WMD = 5.93; 95% CI = [−2.85, 14.71]; p = 0.19). Seventy-percent heterogeneity was observed (Figure 3B).
Four studies assessed the effect of FCM on VAS scores in total. The pooled data analysis from these studies revealed that FCM elicited a significant decrease in VAS score compared to the placebo group (WMD = −21.64; 95% CI = [−32.78, −10.50]; p = 0.0001). However, 54% heterogeneity was observed (Figure 3C).
Three studies evaluated the SF-36 score. The pooled analysis of data from these three studies revealed that FCM caused a significant improvement in SF-36 score compared to the placebo group (WMD = 7.44; 95% CI = [1.67, 13.20]; p = 0.01). There was 0% heterogeneity recorded (Figure 3D).
Four studies assessed the risk ratio of headaches in individuals with RLS treated with FCM compared to those treated with placebo. The risk ratio for headache was 1.17 (95% CI = [0.53, 2.59]; p = 0.69; I2 = 0%), indicating a non-significant difference between the two groups (Figure 3E).
Two studies assessed the risk ratio of nausea in individuals with RLS treated with FCM compared to those treated with placebo. The risk ratio for nausea was 8.18 (95% CI = [1.53, 43.64]; p = 0.01; I2 = 0%), indicating a significantly higher incidence of nausea in the FCM group compared to placebo.
Two studies assessed the risk ratio of infections in individuals with RLS treated with FCM compared to those treated with placebo. The infection risk ratio was 1.36 (95% CI = [0.63, 2.95]; p = 0.43; I2 = 0%), indicating a non-significant difference between the two groups.
Two studies assessed the risk ratio of Blood phosphorus decrease in individuals with RLS treated with FCM compared to those treated with placebo. The risk ratio for Blood phosphorus decrease was 6.38 (95% CI = [0.80, 50.60]; p = 0.08; I2 = 0%), indicating a non-significant difference between the two groups.
Two studies assessed the risk ratio of Diarrhea in individuals with RLS treated with FCM compared to those treated with placebo. The risk ratio for Diarrhea was 1.06 (95% CI = [0.03, 35.03]; p = 0.98; I2 = 0%), indicating a non-significant difference between the two groups.
Two studies assessed the risk ratio of serious adverse effects in individuals with RLS treated with FCM compared to those treated with placebo. The risk ratio for Serious adverse effects was 1.29 (95% CI = [0.50, 3.32]; p = 0.59; I2 = 0%), indicating a non-significant difference between the two groups.
A sensitivity analysis evaluated heterogeneity by excluding the Bae 21 study. This resulted in a reduction of heterogeneity to 0% (WMD = −3.70; 95% CI = [−5.10, −2.30]; p < 0.00001; I2 = 0%), illustrated by Supplementary Figure 2.
We conducted a sensitivity analysis to assess heterogeneity by excluding the Early 21 study. This exclusion reduced the heterogeneity to 24%, and a significant mean difference was observed between the two groups in the RLS-QoL score (WMD = 8.81; 95% CI = [1.49, 16.12]; p = 0.02; I2 = 24%), illustrated by Supplementary Figure 3.
This systematic review and meta-analysis includes most studies and patients of any previous study comparing FCM to placebo in patients with RLS. This meta-analysis includes seven studies with 539 participants, of which 279 patients received FCM, and 260 received a placebo. The pooled data analysis showed that compared to a placebo, FCM is a more effective intervention for alleviating the symptoms of Restless Legs Syndrome.
RLS has been associated with dopaminergic dysfunction in the brain. However, contrary to common belief, it is not solely attributable to this; the underlying pathophysiology is more intricate. Iron deficiency has been implicated in RLS, and it is primarily associated with brain iron deficiency rather than systemic iron deficiency, which has been proved by low iron levels in neuropathological samples, brain imaging studies, and cerebrospinal fluid analysis (16). Intravenous iron supplementation has emerged as an effective treatment for iron deficiency without causing harmful iron buildup in the brain. Studies in iron-deficient mice showed that this method could increase iron levels in the substantia nigra, a brain region important for movement, without affecting iron levels in other parts of the brain (17).
The pooled data analysis showed that treating individuals with FCM is associated with a significant reduction in IRLS scores compared to a placebo. These results were consistent with previous studies by Avni et al.; however, their findings were based on limited studies and a small patient population (18). With new literature available with a greater population size, our analysis showed that FCM decreased the IRLS score by 5 points (p < 0.05). A subgroup analysis using different dosages showed that 1,500 mg was associated with a more significant improvement in the IRLS scores than 500 and 1,000 mg.
Our analysis also showed that FCM was associated with significantly improved VAS and SF-36 scores. VAS is a commonly used tool to assess pain in chronic conditions (19), and SF-36 is a multi-item scale that evaluates several health concepts and general health perceptions, such as limitations in physical and social activities (20). This is the first meta-analysis that has compared these outcomes in individuals taking FCM for RLS.
On the other hand, the analysis of the RLS-QOL score outcome did not show any significant difference between the FCM and placebo groups. These findings were inconsistent with a previous meta-analysis by Avni et al. which showed that compared to placebo, FCM significantly increased the Qol score by 8 points (18). More studies are warranted to establish a more conclusive result.
To understand the safety profile of the drug, an analysis of adverse events such as headache, nausea, and diarrhea was done. Compared to the placebo, FCM showed no significant increase in adverse events except for nausea. These findings were similar to a retrospective study by Park et al., which showed no adverse effects with IV FCM infusion (21).
Although the systemic review and meta-analysis show promising results for FCM use in individuals with RLS, there were some limitations. The follow-up duration varied among some studies. Two of the included studies reported data after 4 weeks, while the others had a follow-up duration of 6 weeks. This change might impact our outcomes to a certain degree. Furthermore, the meta-analysis was conducted under the assumption that the baseline characteristics of all studies were similar. Some studies included a higher proportion of patients with anemia, which could have influenced the outcomes. Therefore, further studies are needed to understand the efficacy of FCM in RLS individuals.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
AK: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. HK: Investigation, Methodology, Visualization, Writing – review & editing. KR: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. AS: Data curation, Formal analysis, Methodology, Software, Writing – review & editing. JI: Data curation, Formal analysis, Investigation, Software, Writing – review & editing. MT: Investigation, Validation, Visualization, Writing – review & editing. SC: Investigation, Validation, Visualization, Writing – review & editing. MH: Investigation, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1503342/full#supplementary-material
1. Benes H, Walters AS, Allen RP, Hening WA, Kohnen R. Definition of restless legs syndrome, how to diagnose it, and how to differentiate it from RLS mimics. Mov Disord. (2007) 22:S401–8. doi: 10.1002/mds.21604
2. Hogl B, Kiechl S, Willeit J, Saletu M, Frauscher B, Seppi K, et al. Restless legs syndrome. Neurology. (2005) 64:1920–4. doi: 10.1212/01.WNL.0000163996.64461.A3
3. Allen RP, Walters AS, Montplaisir J, Hening W, Myers A, Bell TJ, et al. Restless legs syndrome prevalence and impact. Arch Intern Med. (2005) 165:1286. doi: 10.1001/archinte.165.11.1286
4. Ohayon MM, O'Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. (2012) 16:283–95. doi: 10.1016/j.smrv.2011.05.002
5. Tan EK, Seah A, See SJ, Lim E, Wong MC, Koh KK. Restless legs syndrome in an Asian population: a study in Singapore. Mov Disord. (2001) 16:577–9. doi: 10.1002/mds.1102
6. Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. (1994) 17:739–43.
7. Nichols DA, Allen RP, Grauke JH, Brown JB, Rice ML, Hyde PR, et al. Restless legs syndrome symptoms in primary care. Arch Intern Med. (2003) 163:2323. doi: 10.1001/archinte.163.19.2323
8. Winkler AS, Trendafilova A, Meindl M, Kaaya J, Schmutzhard E, Kassubek J. Restless legs syndrome in a population of northern Tanzania: a community-based study. Mov Disord. (2010) 25:596–601. doi: 10.1002/mds.22806
9. Bentley A, Rosman K, Mitchell D. Gender differences in the presentation of subjects with restless legs syndrome. Sleep Med. (2006) 7:37–41. doi: 10.1016/j.sleep.2005.03.004
11. Ekbom K, Ulfberg J. Restless legs syndrome. J Intern Med. (2009) 266:419–31. doi: 10.1111/j.1365-2796.2009.02159.x
12. Phillips B, Young T, Finn L, Asher K, Hening WA, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. (2000) 160:2137. doi: 10.1001/archinte.160.14.2137
13. Hening W. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. (2004) 5:237–46. doi: 10.1016/j.sleep.2004.03.006
14. Lyseng-Williamson KA, Keating GM. Ferric carboxymaltose. Drugs. (2009) 69:739–56. doi: 10.2165/00003495-200969060-00007
15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 2021:n71. doi: 10.1136/bmj.n71
16. Nanayakkara B, Di Michiel J, Yee BJ. Restless legs syndrome. Aust J Gen Pract. (2023) 52:615–21. doi: 10.31128/AJGP-02-23-6722
17. Unger EL, Earley CJ, Thomsen LL, Jones BC, Allen RP. Effects of IV iron isomaltoside-1000 treatment on regional brain iron status in an iron-deficient animal. Neuroscience. (2013) 246:179–85. doi: 10.1016/j.neuroscience.2013.04.049
18. Avni T, Reich S, Lev N, Gafter-Gvili A. Iron supplementation for restless legs syndrome - a systematic review and meta-analysis. Eur J Intern Med. (2019) 63:34–41. doi: 10.1016/j.ejim.2019.02.009
19. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R, editors. Handbook of Pain Assessment, 3rd Ed. New York, NY: Guilford Press (2011). p. 15–34.
20. Ware Jr JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. (1992). doi: 10.1037/t06708-000
Keywords: Restless Legs Syndrome, Willis-Ekbom Disease, ferric carboxymaltose, iron therapy, neuromuscular disorder
Citation: Khan A, Kumar H, Rai KD, Saeed A, Ishtiaq J, Tanveer Alam M, Chawla S and Haque MA (2025) Efficacy and safety of intravenous ferric carboxymaltose in the treatment of Restless Legs Syndrome: a systematic review and meta-analysis. Front. Neurol. 15:1503342. doi: 10.3389/fneur.2024.1503342
Received: 30 September 2024; Accepted: 16 December 2024;
Published: 07 January 2025.
Edited by:
Keisuke Suzuki, Dokkyo Medical University, JapanReviewed by:
Mushtaq Ahmad, Oujiang Laboratory, ChinaCopyright © 2025 Khan, Kumar, Rai, Saeed, Ishtiaq, Tanveer Alam, Chawla and Haque. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Md Ariful Haque, YXJpZnVsaGFxdWU1OEBnbWFpbC5jb20=
†ORCID: Ayesha Khan orcid.org/0009-0005-0444-5095
Harsh Kumar orcid.org/0009-0009-0339-7270
Kuldeep Dalpat Rai orcid.org/0009-0000-2135-0673
Anzel Saeed orcid.org/0009-0006-9602-4569
Jawad Ishtiaq orcid.org/0000-0002-4415-4453
Sakshi Chawla orcid.org/0009-0001-8835-027X
Md Ariful Haque orcid.org/0000-0003-4632-5153
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.