- 1Department of Development and Regeneration, KU Leuven, Leuven, Belgium
- 2Department of Pediatrics, University Hospitals Leuven, Leuven, Belgium
- 3Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium
- 4Department of Pediatrics, Pediatric Cardiology Unit, University Hospitals Leuven, Leuven, Belgium
- 5Department of Pediatrics, Neonatal Intensive Care Unit, University Hospitals Leuven, Leuven, Belgium
- 6Department of Pediatrics, Pediatric Neurology Unit, University Hospitals Leuven, Leuven, Belgium
The increased risk of neurodevelopmental impairment in children with congenital heart disease (CHD) has been established, but the search for targeted neurological predictors of adverse outcome is ongoing. This systematic review reports on the utility of three functional neuromonitoring modalities, Near-infrared Spectroscopy (NIRS), electroencephalography (EEG) and biochemical biomarkers, in predicting either clinical neurodevelopmental outcome or structural brain abnormalities after pediatric CHD surgery. Medline, Embase, CENTRAL, Web of Science, clinicaltrials.gov and ICTRP were systematically searched for eligible articles. Original research articles, written in English, published before November 2023 and reporting on perioperative NIRS, EEG or biomarkers and their association with clinical neurodevelopmental outcome or neuroimaging in children <17 years undergoing surgery for CHD were included. The search yielded 11,367 citations, of which 40 papers were included in the final review: sixteen articles (n = 908 cases) reported on NIRS, twelve (n = 1,163) on EEG and fifteen (n = 903) on biochemical biomarkers. Three papers reported on a combination of modalities. Median age at time of surgery was 9 (IQR 7–57) days. Postoperative MRI was performed before discharge at varying timepoints. Median age at clinical outcome assessment was 15 (IQR 12–24) months. Limited evidence supports an association of cerebral oxygen extraction, cerebral desaturation and cerebral autoregulation with outcome, but there was significant heterogeneity in results. Perioperative electroencephalographic ictal discharges and abnormal background were associated with impaired neurological outcome and abnormal neuroimaging. Numerous biochemical biomarkers have been reported but showed no consistent relationship with outcome, except for lactate, which could serve as a predictor of poor outcome. There is a need for larger homogeneous cohorts of children with CHD to determine which perioperative modalities might serve as predictors of neurodevelopmental outcome or neuroimaging abnormalities.
Systematic review registration: http://www.crd.york.ac.uk/PROSPERO, CRD42023479344
1 Introduction
Congenital heart disease (CHD) is the most common birth defect with a prevalence of about 8 (1–8) per 1,000 live births (9). Around 25–50% of patients with CHD require neonatal cardiac intervention. Medical and surgical advancements have significantly decreased mortality and morbidity, although, survivors of critical CHD remain at risk of neurodevelopmental impairments in several domains, including overall intellectual functioning, speech, language, executive and memory function, gross, fine motor and visual spatial skills (10). Additionally, around a third of children with congenital heart disease have brain abnormalities on preoperative Magnetic Resonance Imaging (MRI), with an additional third acquiring new or increased injury postoperatively (11). The predominant lesions visualized in postoperative patients with CHD are stroke and white matter injury (1, 12, 13). In addition, children without overt lesions have structural abnormalities such as altered brain volumes and cortical folding on fetal and neonatal brain MRI (2–4). Reviews have shown that perioperative cerebral findings can be associated with neurodevelopmental outcome (NDO) although abnormal neuroimaging is not always proportionally associated with clinical outcome and should be interpreted with caution (5–7). The cause of this neurological risk is considered multi-factorial. Inherent disease- (type of cardiopathy, cyanosis) and patient-specific factors such as genetic syndromes or extracardiac anomalies contribute to the neurological risk profile of these infants (8, 14), in addition to the inherent risk of surgical techniques such as atrial septostomy, cardiopulmonary bypass (CPB) or deep hypothermic circulatory arrest (DHCA) (15, 16), postoperative critical illness and low cardiac output syndrome (17).
Because of this inherent risk, the American Heart Association issued recommendations for the follow-up of neurodevelopment in children with CHD (18). As neurodevelopmental follow-up is a resource-intensive practice, it is most important to identify CHD patients at highest risk for impaired neurodevelopment. Current practices focus largely on clinical development, which is only a late predictor, or on brain MRI which can be performed early but is resource-intensive and not easily accessible in an intensive-care setting. In order to allocate these resources to the patients in greatest need, it is necessary to explore different predictive strategies differentiating subsequent neurodevelopmental risk in CHD patients.
Neuromonitoring practices in a pediatric cardiac intensive-care setting vary widely (19). A recent European survey (20) showed similar variety in perioperative neuromonitoring/neuroimaging after pediatric congenital heart disease surgery: near-infrared spectroscopy (NIRS) was most commonly used, with 64% of centers indicating preoperative, 80% intraoperative and 72% postoperative use. Amplitude-integrated electroencephalography (aEEG) was used in 32% of participating centers, and 20% performed postoperative aEEG. Twelve percent of centers performed preoperative continuous EEG (cEEG), none used it in the postoperative period. Twenty percent of centers measured biochemical biomarkers in the postoperative period. Half of the participating centers indicated having a follow-up program in place for children with CHD.
This systematic review provides a comprehensive overview of the available evidence on three perioperative neuromonitoring modalities, NIRS, EEG and biochemical biomarkers, and their association with subsequent clinical neurological outcome or neuroimaging.
2 Methods
2.1 Design
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (21). The search strategy was created with help of the biomedical reference librarians of the KU Leuven Libraries – 2Bergen. The protocol was prospectively registered in the international prospective register of systematic reviews (PROSPERO) database (Registration number: CRD42023479344, http://www.crd.york.ac.uk/PROSPERO).
2.2 Eligibility criteria
Studies eligible for inclusion reported on the use of neuromonitoring modalities (EEG, NIRS and/or non-invasive biochemical biomarkers) in pediatric patients with CHD necessitating surgical intervention (excluding catheter interventions), and their association with either clinical neurodevelopmental outcome (NDO) evaluated with a validated scale, instrument or test, or postoperative brain MRI evaluating either brain damage or brain maturation. Studies were excluded if the full text was not available in English. Case reports, case series, conference abstracts and review papers were excluded from the analysis.
2.3 Search strategy and data sources
We comprehensively searched Medline, Embase, CENTRAL, Web of Science, clinicaltrials.gov and the International Clinical Trials Registry Platform (ICTRP) for eligible studies on November 28th, 2023. The full search strategy can be found in the Supplementary materials. Additionally, we hand-searched references of included studies for relevant publications. References for the selected studies were managed in the Rayyan© software.
2.4 Data extraction
After removal of duplicates, all studies were screened based on title and abstract by 2 reviewers (LVL, KJ). Subsequently, the full text of the remaining articles was examined in parallel by 2 reviewers (LVL, KJ) to determine if all inclusion criteria were met. Additionally, the reference list of included articles was manually checked for additional studies. Disagreement was resolved through discussion until consensus was achieved.
One reviewer (LVL) performed data extraction from the manuscripts. The extracted data was summarized in a data extraction sheet. If insufficient data was available from the manuscript, an attempt was made to contact corresponding authors to obtain additional information.
2.5 Data analysis
We summarized data on study design, patient characteristics and type of interventions. Primary outcome was either neurodevelopment assessed with standardized neurodevelopmental testing using a validated test, or postoperative neuroimaging using MRI. Papers who did not report standardized assessments (e.g., chart review for neurodevelopmental impairment) were excluded.
For synthesis of the results, studies were grouped by the different neuromonitoring modalities utilized: EEG, NIRS and biochemical biomarkers. It was not possible to perform a meta-analysis due to the large heterogeneity in interventions, reported outcomes and statistical analyses.
2.6 Assessment of risk of bias and grading of evidence
Individual studies were assessed for risk of bias using the validated Risk Of Bias In Non-randomized Studies – of Exposure (ROBINS-E) tool for observational data (22). The quality of evidence was assessed for each outcome using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (23), rating the quality of evidence as high, moderate, low or very low in five areas: risk of bias, inconsistency, indirectness, imprecision and publication bias. We aimed to minimize reporting bias by searching clinical trial registries to incorporate unpublished reports.
3 Results
3.1 Study selection
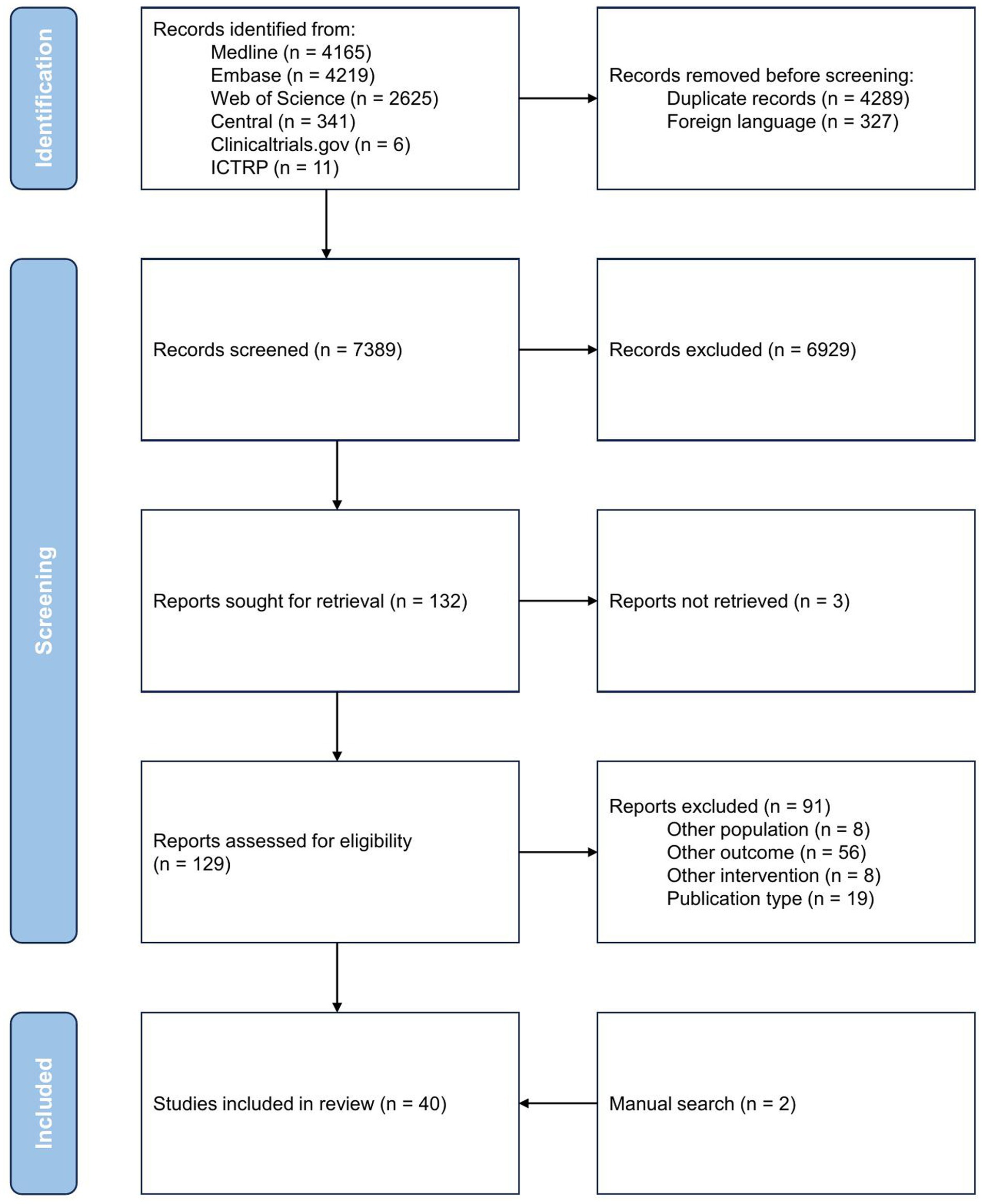
The study selection process is presented in the PRISMA flow diagram (Figure 1). Of the 7,389 records screened, 129 were assessed for eligibility based on full text. We excluded 91 further records: 59 reported on outcomes other than specified in the inclusion criteria (e.g., nonstandardized neurodevelopmental outcome, neuroimaging other than brain MRI), eight articles included different populations (e.g., cardiopathy other than congenital, adults), eight compared interventions than NIRS, EEG or biochemical biomarkers, and 19 articles used non-suitable publication formats (e.g., case reports, case series, review articles). Ultimately, 40 studies met all inclusion criteria and were included in this systematic review (24–63).
Williams et al. (64) performed neonatal high-density, 128-lead, EEG (hdEEG) measurements in children undergoing neonatal cardiac surgery for CHD and measured power (measure of local neural synchrony) and coherence (measure of functional connectivity) as measures of cortical function. While seemingly meeting the inclusion criteria, we excluded this article as the hdEEG is predominantly used in research settings and not deemed feasible in daily clinical practice.
3.2 Characteristics of included studies
All studies were observational. Twenty five studies reported prospectively collected data (24–26, 29, 32–35, 37, 41–43, 45–48, 50, 51, 53–56, 58–60, 63). In 14 studies, data was collected retrospectively (27, 28, 30, 31, 36, 38–40, 44, 49, 52, 57, 61, 62).
Most studies had an upper limit for age at inclusion, varying from 30 days to 17 years, median age at surgery was 9 (IQR 7–57) days. 36/40 studies only included patients with critical CHD whereas 4/40 studies included patients with varying disease severity. 25/40 studies specified the necessity for CPB as an inclusion criterium (24, 26–29, 32, 34, 36–39, 41, 43, 45–47, 50, 54, 56, 57, 59–63). In five studies, measurements were performed surrounding a specific procedure (e.g., stage 1 palliation) and five other studies included only specific cardiac diagnoses [mostly hypoplastic left heart syndrome (HLHS) or dextrotransposition of the great arteries (D-TGA)]. All but one study (28) excluded patients with pre-existing neurologic or genetic comorbidities in order to minimize the influence of other causes of neurodevelopmental impairment.
Sixteen studies reported on NIRS as a predictor, with eight studies reporting clinical neurodevelopmental testing (24–31), four studies using MRI as an outcome marker (32–35), and four studies using a combination of both (36–39). aEEG was utilized in five studies (40–44) and seven studies used cEEG (45–51). Of these, outcome variables were clinical neurodevelopment in nine studies (40–43, 45–49), MRI in two (44, 51) and a combination of both in one study (50). Biochemical biomarkers were compared to clinical NDO in 13 studies (24, 29, 47, 52–61), to brain MRI in one study (63) and to both in one study (62). In total, 2,846 individual cases were assessed, of which 908 with NIRS, 1163 with EEG and 903 with biochemical biomarkers.
As a clinical outcome measure, the Bayley Scales of Infant Development (BSID-II or III) was reported most frequently (n = 25). Other studies reported Pediatric Cerebral Performance Category (PCPC) (n = 2), Pediatric Stroke Outcome Measure (PSOM) (n = 1), age-appropriate IQ testing (Wechsler IQ scales or similar) (n = 5), Denver Developmental Screening test (DDST) (n = 1) or Vineland Adaptive Behavior Scale (VABS) (n = 3). Median age at clinical developmental testing was 15 (IQR 12–24) months.
For MRI-based outcomes, 10 studies used brain injury (stroke, hemorrhage and white matter injury) as outcome measures (32, 34–36, 38, 44, 50, 51, 62, 63), whereas others performed brain volumetry (33, 37, 39). Postoperative MRI was performed before discharge at varying timepoints.
All studies were executed in tertiary or quaternary hospital settings. Sixteen studies took place in the United States (24, 27, 28, 30, 31, 34, 36, 38, 45, 46, 48–50, 57, 59, 62) and 15 in European countries (25, 26, 29, 32, 33, 37, 39, 43, 44, 53, 55, 56, 60, 61, 63). The remaining 9 studies were conducted in Australia, New Zealand, China, Canada and Israel (35, 40–42, 47, 51, 52, 54, 58). No studies were conducted in Low- or Middle-Income Countries.
3.3 Results of included studies
An overview of the study characteristics, inclusion criteria, inclusion period and main results, can be found in Tables 1–3.
3.3.1 Association of NIRS with neurological outcome
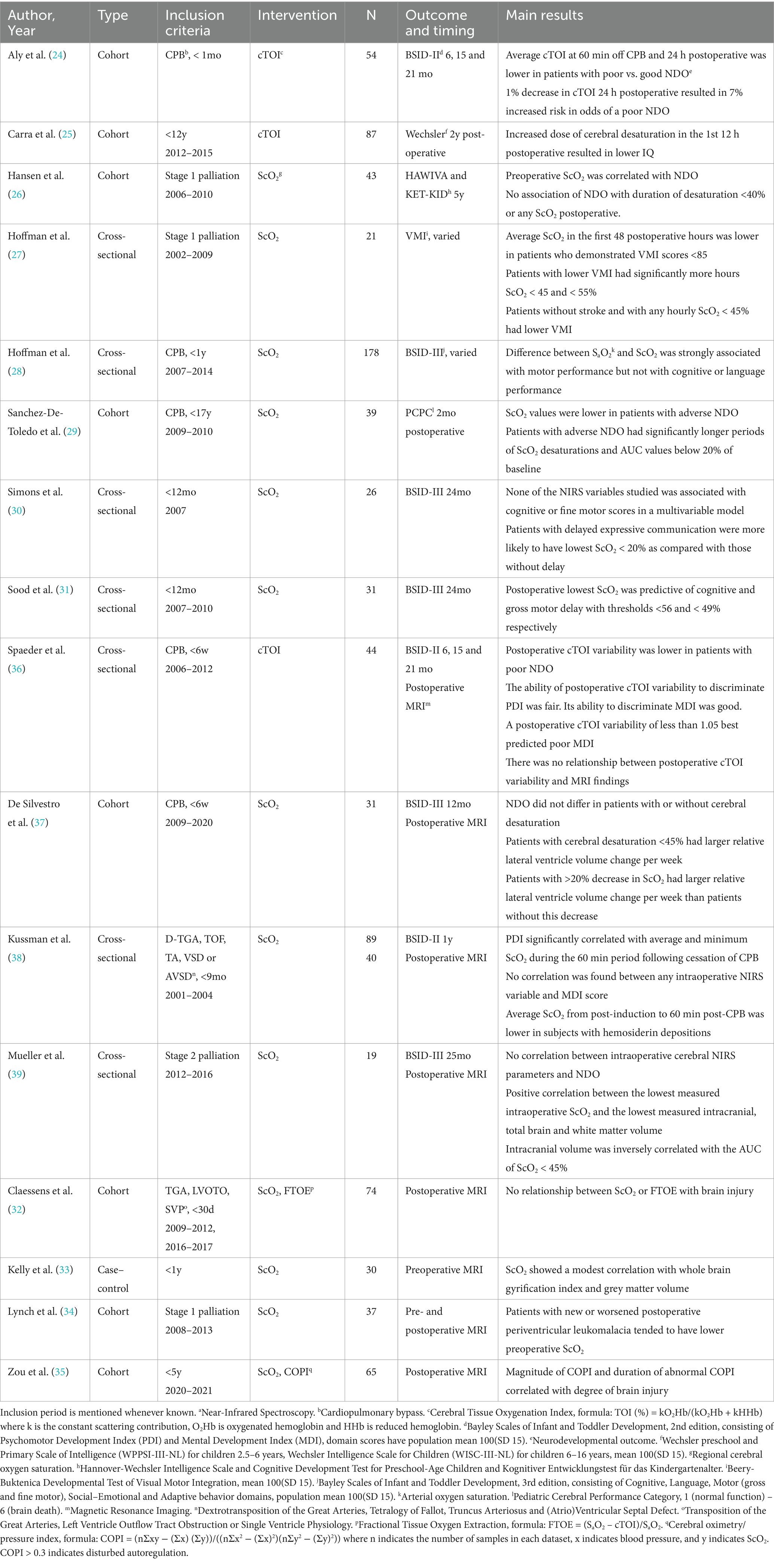
Cerebral oximetry using NIRS is a non-invasive monitoring technique based on detection of hemoglobin oxygenation using near-infrared light, from which parameters such as Cerebral Oxygen Saturation (ScO2), Cerebral Tissue Oxygenation Index (cTOI) and Fractional Tissue Oxygen Extraction (FTOE) can be derived. Combining NIRS parameters with heart rate or blood pressure measurements allows for the measurement of cerebral autoregulation, for example by calculating Cerebral Oximetry/Pressure Index (COPI), as COPI >0.3 indicates disturbed autoregulation.
3.3.1.1 Association with clinical NDO
Twelve articles examined the association of NIRS variables with clinical NDO (Table 1).
Pre-operative ScO2 before stage 1 palliation was associated with cognitive scores in the report by Hansen et al. (26). Intraoperatively, a retrospective study of children with biventricular CHD without aortic arch obstruction, found a correlation of intraoperative ScO2 immediately post-CPB surgery with Psychomotor Developmental Index (PDI) but not with Mental Developmental Index (MDI) subscores of the BSID-II (38). In contrast, Mueller et al. did not report any associations between NIRS values during stage 2 palliation and BSID scores (39).
Postoperatively, cTOI after neonatal CPB surgery significantly predicted mortality and poor BSID scores (24), and postoperative cTOI variability was able to discriminate between poor and good BSID (36). Hoffman et al. reported associations of average postoperative ScO2 with NDO after stage 1 palliation (27). Postoperative cerebral desaturation predicted poorer NDO in three mixed CHD populations (25, 28, 31). Conversely, Hansen et al. did not report any association of NDO with NIRS after stage 1 palliation (26).
Some studies reported on findings in the entire perioperative period rather than specific timepoints. Both perioperative ScO2 and cerebral desaturation were associated with NDO in children undergoing CPB surgery in childhood (29). Comparable results on cerebral desaturation were reported in children below 1 year of age, although they only reached significance in the expressive communication BSID subscore (30). Hoffman et al. described an association of cerebral desaturation and the difference between arterial and cerebral oxygenation with NDO in two retrospective cohorts (27, 28). Contrarily, De Silvestro et al. did not find an association between cerebral desaturation around neonatal CPB surgery and BSID scores at 1 year (37).
3.3.1.2 Association with brain MRI
The eight studies reporting on the association of NIRS and MRI are summarized in Table 1.
Pre-operative ScO2 values were associated with brain injury after stage 1 palliation in one study (34). Two studies researched intraoperative ScO2: one found an association with both lower brain volumetry in hypoplastic left heart syndrome (HLHS) and the other with brain injury in a mixed cohort (38, 39). Spaeder et al. found no relationship between postoperative cTOI variability and MRI findings (36).
Again, perioperative timepoints were not always specified. An association between perioperative ScO2 and lower brain volumes was found in a mixed cohort by Kelly et al. (33), whereas cerebral desaturation significantly correlated with lower volumetry in the report on HLHS by Mueller et al. and the study on neonatal CPB surgery by De Silvestro et al. (37, 39). Zou et al. found that the magnitude and duration of abnormal COPI correlated with degree of brain injury (35). On the other hand, Claessens et al. did not find an association between ScO2 or FTOE surrounding CPB surgery and brain injury (32).
3.3.2 Association of EEG with neurological outcome
EEG electrodes are placed on the scalp for detection of the spontaneous electrical activity of the brain. The EEG can be utilized for different indications, such as the detection of subclinical, electrographic seizures and the review of background activity and sleep–wake cycling. cEEG uses the full array of scalp electrodes according to the international 10–20 system (modified for neonates) and provides detailed information on the temporospatial occurrence of electrical potentials. In contrast, aEEG is a simplified method using a more limited number of electrodes (up to 4) and provides a time-compressed signal based on the amplitude of the electrographic signal. It is useful in background detection and detection of sleep–wake cycling, and has its value in seizure detection although with less sensitivity compared to cEEG, and with loss of temporospatial information.
For this review, we included studies reporting on both cEEG and aEEG, as the outcomes of interest should be detectable on both modalities.
3.3.2.1 Association with clinical NDO
Ten studies examined the association of EEG variables with clinical NDO (Table 2). Four studies used aEEG and six used cEEG.
Using aEEG, Latal et al. showed lower cognitive BSID subscores in patients with electrographic seizures after CPB surgery before 3 months of age (43) whereas Gunn et al. did not report any association between perioperative seizures and BSID scores (41, 42).
Using cEEG, The Boston Circulatory Arrest group showed that electrographic seizures during the arterial switch operation were associated with lower psychomotor BSID subscores at 1 year of age (50). Gaynor et al. found lower cognitive BSID scores with frontal-onset seizures at 1 year of age in children undergoing CPB surgery before 6 months (45) and executive dysfunction at 4 years of age in patients experiencing perioperative electrographic seizures (46). Robertson et al. did not find any association between seizures measured with cEEG and BSID scores (47).
Both Gui et al. and Vaughan et al. described an association of preoperative background pattern with BSID scores (40, 49), whereas two others did not confirm this association (41, 47). All four studies included mixed CHD types. Postoperatively, an abnormal background pattern was consistently associated with poor NDO in 5 studies: in particular, delayed recovery of sleep–wake cycling, duration of the isoelectric state and prolonged discontinuity were associated with poor NDO (40–43, 48).
3.3.2.2 Association with brain MRI
Three studies report on the association of postoperative EEG variables with brain injury on MRI (Table 2). Claessens et al. describe both postoperative abnormal background and ictal discharges on aEEG as risk factors for new-onset brain injury (44). The association with electrographic seizures on cEEG is also reported in the Boston Circulatory Arrest cohort (50). In addition, Lin et al. found that electrographic abnormalities seen on cEEG were associated with brain injury, with patients with longer isoelectric traces or not recovering to normal background and sleep–wake cycling by 48 h having worse degree of injury (51).
3.3.3 Association of biochemical biomarkers with clinical NDO or brain MRI
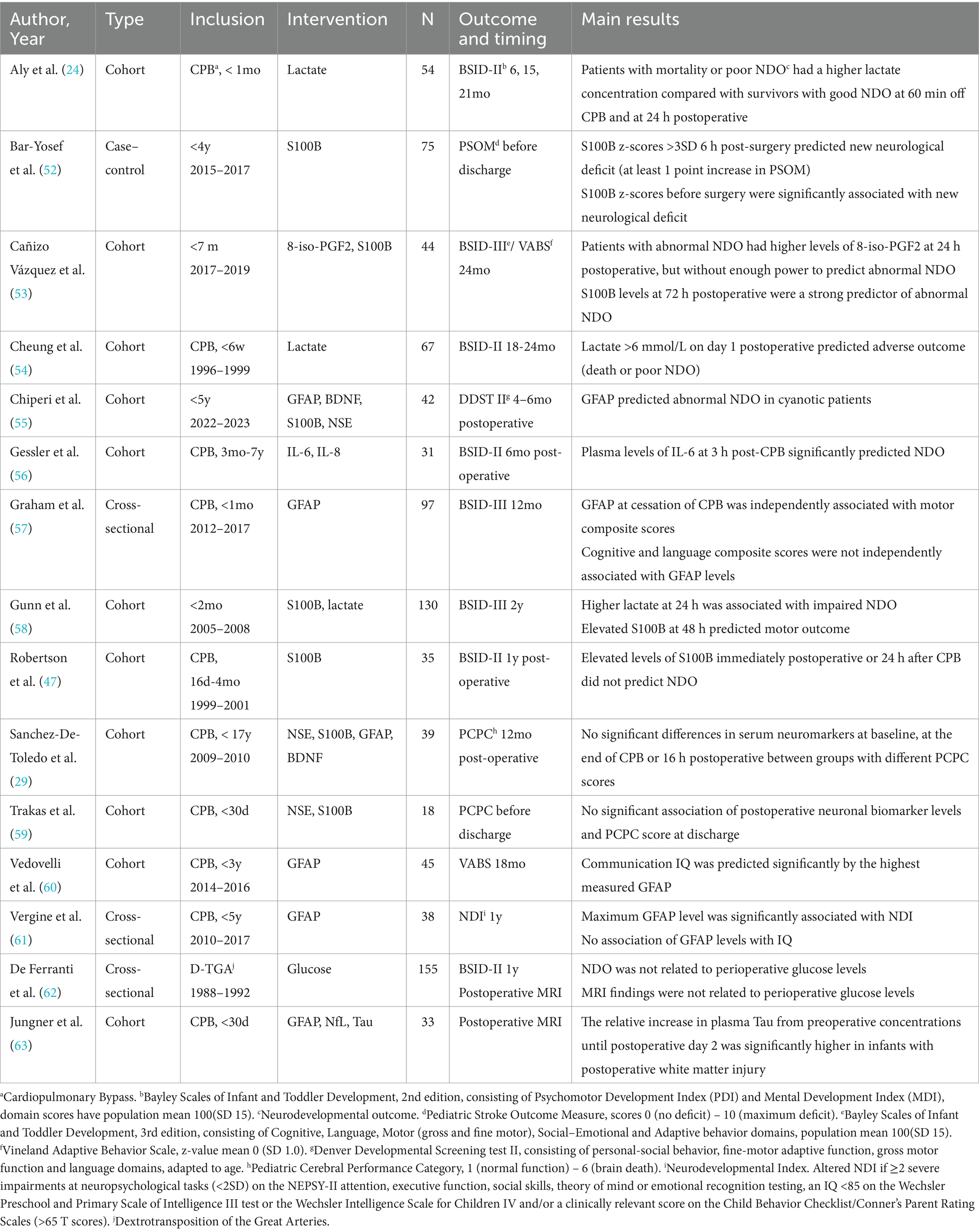
A large variety of biochemical biomarkers of brain injury are being studied in relation to neurological outcome. The 15 included studies are summarized in Table 3. S100 calcium-binding protein B (S100B) was most studied in association with NDO. Of the seven studies reporting this outcome, four did not find anycorrelation (29, 47, 55, 59). One study found that elevated S100B at 48 h after CHD surgery before 2 months of age predicted BSID motor outcome at 2 years, and another found associations between S100B at 72 h postoperative and BSID at 2 years (53, 58). In addition, In children undergoing CHD surgery before 4 years of age, elevated S100B was associated with new neurological deficit upon discharge (52).
Six studies studied glial fibrillary acidic protein (GFAP) as a predictive biomarker. Chiperi et al. report its predictive ability for DDST scores in cyanotic patients undergoing surgery before 5 years of age (55), while Graham et al. found an association with BSID motor but not with cognitive and language scores in infants undergoing CPB surgery in their first month (57). The highest measured GFAP was able to predict communication intelligence quotient using the VABS at 18 months in the study by Vedovelli et al. (60). In contrast, Vergine et al. did not find an association of GFAP levels during CPB surgery with cognitive abilities, although did relate to the composite neurodevelopmental scoring system used in their study (61). Sanchez-De-Toledo et al. did not did not report significant associations with PCPC scores 12 months after CPB surgery in childhood (29). In addition to GFAP, the report by Jungner et al. examined Neurofilament light polypeptide (NfL) and Tau and described that patients with postoperative white matter injury on brain MRI had a significantly higher increase in plasma Tau levels, but no associations with GFAP or NfL were found (63).
PCPC scores at discharge were not related to levels of neuron-specific enolase (NSE) or brain-derived neurotropic factor (BDNF) in infants undergoing CPB surgery before 30 days, and neither were NSE and PCPC scores at 12 months postoperative in pediatric CPB surgery for CHD (29, 59). Another study reporting DDST scores 4–6 months after surgery, did not find an association with NSE or BDNF levels during CHD surgery (55). Three studies reported that perioperative lactate elevations significantly predicted poor BSID scores (24, 58, 65). Cañizo Vàzquez et al. reported increased levels of 8-iso-prostaglandin F2α (8-iso-PGF2), a urinary biomarker for oxidative stress, in patients with abnormal BSID or VABS scores. In an article by Gessler et al., plasma IL-6 at 3 h post-CPB significantly predicted NDO (56). Lastly, one article explored perioperative glucose levels during the arterial switch operation, but found no association with clinical NDO or MRI (62).
3.4 Risk of bias
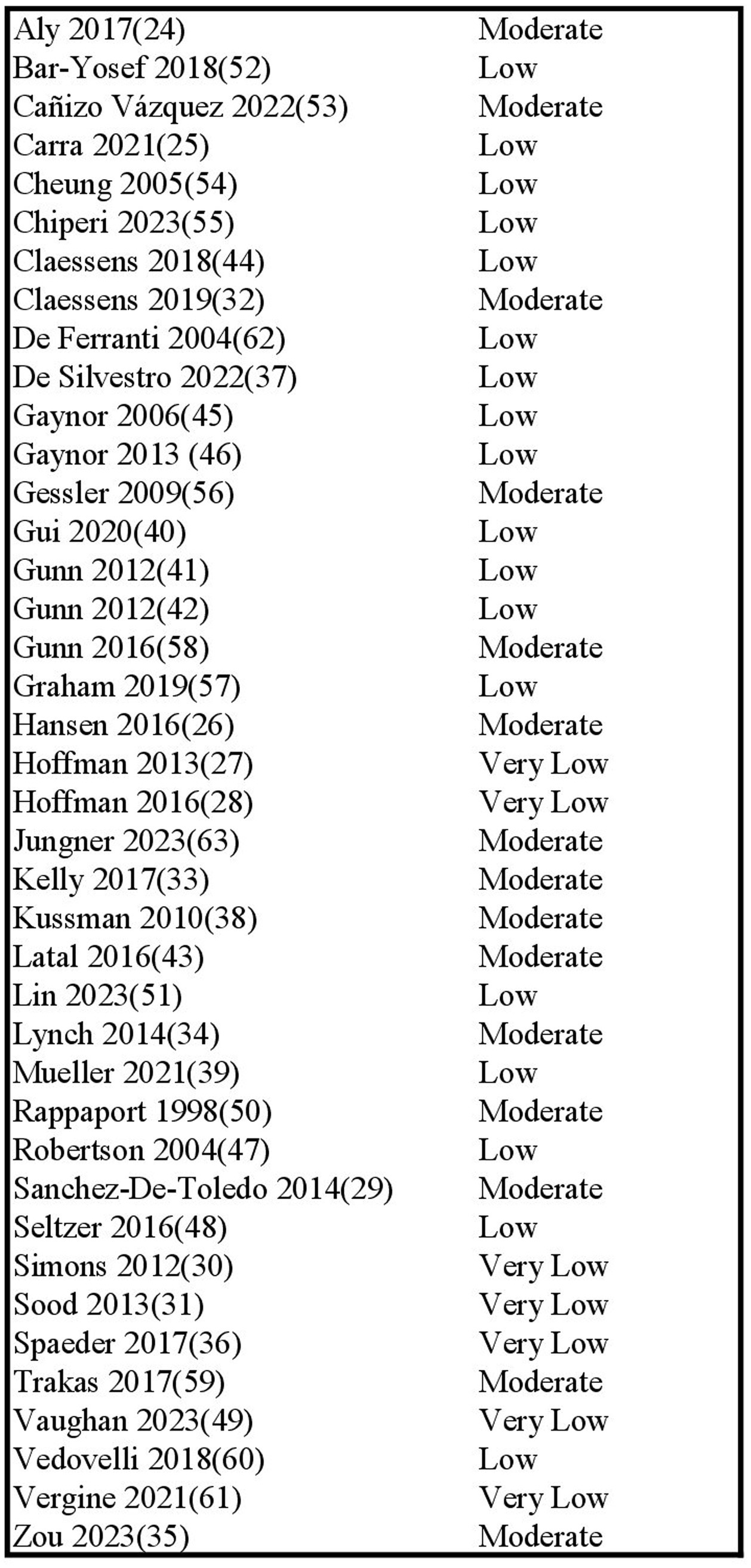
Risk of bias across studies was assessed using the ROBINS-E tool. Results are presented in Figure 2. Risk of bias was overall low to moderate, with concerns mostly due to the risk of confounding, inherent to the observational study design. The risk of selection of reported results seemed low overall, given that the reported outcomes were consistent with preregistered protocols and also non-significant data was reported.
Four studies were judged to have high risk of bias due to the large proportion of missing data (27, 28, 30, 49). Additionally, the studies by Hoffman et al. (27, 28) selected patients based on referral for neurodevelopmental testing (possibly due to suspicion of developmental impairment). Two studies also included patients with genetic syndromes, likely influencing neurodevelopmental outcome scores (28, 46). Two studies (48, 60) posed some concern for bias because their outcomes were (in part) based on subjective parent-based questionnaires, although this was accounted for by the use of a validated scoring system (VABS).
3.5 Quality of evidence
We rated the quality of evidence using the GRADE approach (23), results are presented in Figure 3. The included reports provided mostly low-quality evidence due to their observational nature. Studies were upgraded to moderate-level evidence if they had strong designs, were well conducted, and had few major flaws. Studies were downgraded if there was risk of bias or inconsistency in results.
4 Discussion
This review article summarizes existing data regarding neuromonitoring using perioperative NIRS, EEG, and serum brain biomarkers and their association with neurodevelopmental outcome, brain maturation and brain injury on MRI after surgery for congenital heart disease. This type of data is potentially valuable in risk stratification, in light of recent guidelines supporting neurodevelopmental follow-up for certain subgroups of CHD patients, which is resource-intensive and of variable availability.
First, the majority of studies evaluating NIRS suggest an association between cerebral oxygenation measurements (cTOI, ScO2, cerebral desaturation) and the pre-specified neurological outcomes (either clinical or neuroimaging). It is important to note however, that there was large variability in the thresholds to define cerebral desaturation, as well as the exposure measures (binary outcome versus time below a certain threshold). Interestingly, disturbed cerebral autoregulation seemed to correlate with neurological outcome.
Secondly, electrographic seizures, either measured with cEEG or aEEG, were independently associated with poorer neurological outcome in the majority of reports and patients with prolonged abnormal background patterns or delayed return of sleep–wake cycling, were at risk for adverse outcome.
Thirdly, the predictive ability of biomarkers such as S100B and GFAP is not sufficiently convincing. Postoperatively increased lactate however, was associated with neurodevelopment, in addition to its known associations with short-term adverse outcomes (66, 67). According to the included studies, significant elevation >4–6 mmol/L or persistent elevation beyond 24 h seemed predictive of outcome. This is an important finding as most cardiac centers already routinely measure lactate levels as an indicator of the hemodynamic and metabolic condition of the patient. For the other neuronal markers mentioned in this study, evidence is too sparse to draw a sound conclusion. The ability to draw stronger conclusions and perform meta-analyses was limited by the inconsistency and heterogeneity of the findings. This review provides an update to existing literature by comprehensively and systematically summarizing available data on perioperative neuromonitoring and its association with outcomes. These findings build on recent publications showing that patients with CHD show abnormal electroencephalographic activity even in the preoperative period (68, 69), and impaired perioperative autoregulation which cannot simply be explained by differences in blood pressure (70). In addition, the perioperative period is characterized by significant hemodynamic disturbances and might require even more from the autoregulatory mechanisms of the brain. Moreover, new information on the disrupted autonomic regulation and altered circadian rhythms in CHD is arising.
Knowledge on these neuromonitoring modalities is steadily expanding in different fields of medicine, and it might be possible to extrapolate, without generalizing to the unique group of neonates with CHD. Cerebral NIRS monitoring has been utilized to guide treatment in the preterm population as a predictor of neurodevelopmental impairment in preterm neonates (71, 72), although in a large randomized trial on treatment guided by cerebral oximetry, no difference in serious adverse events was found (73). Secondly, in neonatal and pediatric patients undergoing extracorporeal life support (ECLS), the prognostic value of cerebral oximetry on neurodevelopment was demonstrated (74, 75), and a pilot study highlighted the importance of impaired cerebral autoregulation in patients with acute neurological events (76). aEEG and cEEG are widely used in preterm babies as a biomarker of brain injury, as a modality to monitor brain maturation and as a prognostic tool for subsequent neurodevelopment (77, 78). Fogtmann et al. performed a systematic review on this topic which demonstrated the good predictive value of EEG for NDO after prematurity (79). In hypoxic–ischemic encephalopathy, the prognostic value of NIRS and EEG has repeatedly been demonstrated (80, 81). Specifically, studies showed that that prolonged abnormal background on EEG was associated with impaired neurodevelopment (82–84), and seizure burden was predictive of neurodevelopmental outcome (85–87). EEG seizure burden and asymmetric EEG background correlated with brain injury in two pediatric ECLS populations (88, 89), while background abnormalities predicted poor NDO with good specificity (90). The utility of different neuromonitoring modalities in ECLS is summarized in the review by Felling et al. (91). The information extrapolated from these patient groups could aid in the comprehension of underlying autoregulation mechanisms in patients with CHD. The field of biochemical neurological biomarkers has predominantly been studied in adult settings and evidence from pediatric populations is only recently emerging. For example, a wide array of biomarkers is being assessed for outcome prediction after ischemic stroke in adults (92, 93). In pediatric traumatic brain injury, S100B levels correlated to the extent of brain injury (94). In pediatric ECLS patients, elevation of plasma brain injury biomarkers was associated with unfavorable NDO and brain imaging abnormalities (95). Even though the underlying conditions differ from CHD, they face many similar difficulties concerning hemodynamic disturbances, inflammation, altered cerebral perfusion and reperfusion injury (96), which makes it plausible that neuromonitoring modalities used in these clinical settings could potentially be of use in the setting of CHD. In addition, Chiperi et al. (97) recently published a systematic review on the use of biochemical biomarkers in pediatric CHD surgery: they reported poor predictive value of NSE and BDNF and conflicting results on S100B, but suggested GFAP as a possible biomarker for brain injury.
4.1 Limitations
The results of this analysis should be interpreted with necessary caution. Whereas the single studies are overall of good quality, although observational in design, the studies included relatively small and diverse groups of patients, with different cardiac diagnoses, age ranges and treatment strategies. Even though most studies only included patients with normal neurologic exams before surgery, pre-existing comorbidities may be unaccounted for. Studies were conducted in different epochs causing differences in surgical and medical management strategies. Additionally, there was large variability in reported NIRS variables across studies, rendering a quantitative meta-analysis impossible. A possible explanation is that patients with varying CHD types have altered baseline ScO2 from the healthy population, making current definitions of cerebral desaturation (typically ScO2 < 45 or < 65%) less relevant (98). In future analyses, a relative decrease in ScO2 from baseline may be more valuable than absolute values. The duration of EEG monitoring was variable (pre- vs. postoperative, duration of postoperative monitoring) and authors did not control for the effects of sedative medications on EEG traces, possibly introducing bias as the most unstable patients likely received more suppressive medication.
There was large variability in timing of outcome measurements, which were mostly short-term, and instruments for clinical assessment of neurological outcome focused on different neurodevelopmental domains. For example, postoperative PCPC or PSOM may indicate global neurological outcome before discharge, whereas comprehensive neurodevelopmental assessment at preschool age is the preferred method for detailed evaluation of long-term neurological outcomes. No studies were conducted in Low or Middle-Income Countries, limiting generalizability to these countries.
Should we aim to establish guidelines surrounding effective perioperative neuromonitoring, there is a need for larger, more definitive studies with increased consistency in the application of these modalities and definition of clinically useful outcomes. In addition, patient-specific (e.g., socio-economic status, (epi)genetics) and medical factors (e.g., surgical technique, length of stay, ventilation time, sedative use) should be considered as they impact the cerebral compensatory mechanisms and might better explain the variability in neurological outcome in this population. Longer-term data using outcome measures encompassing different neurodevelopmental domains (cognitive, psychomotor, visuospatial, executive functioning) is necessary to more accurately predict the long-term prognosis of these patients.
4.2 Conclusion
To conclude, there is some evidence indicating an association between perioperative non-invasive neuromonitoring modalities and neurological outcome in the CHD population, most convincingly cerebral desaturation and autoregulation measured with NIRS, electrographic seizures, and prolonged background abnormalities on EEG, and elevated lactate in the perioperative period. These results should be interpreted with caution however, and in further research, the standardization of perioperative monitoring application and outcome determination is necessary before clinical implementation of these strategies for neurological prognostication.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. BC: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. AD: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. KJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors wish to thank Chayenne Van Meel, biomedical reference librarian of the KU Leuven Libraries – 2Bergen – learning Centre Désiré Collen (Leuven, Belgium), for their help in conducting the systematic literature search.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1502762/full#supplementary-material
Abbreviations
aEEG, Amplitude-integrated electroencephalography; BSID-II or III, Bayley scales of infant development; BDNF, Brain-derived neurotropic factor; COPI, Cerebral oximetry/pressure index; ScO2, Cerebral oxygen saturation; cTOI, Cerebral tissue oxygenation index; CHD, Congenital heart disease; cEEG, Continuous electroencephalography; CPB, Cardiopulmonary bypass; DHCA, Deep hypothermic circulatory arrest; DDST, Denver developmental screening test; FTOE, Fractional tissue oxygen extraction; GRADE, Grading of recommendations assessment, development and evaluation; GFAP, Glial fibrillary acidic protein; hdEEG, High-density EEG; HLHS, Hypoplastic left heart syndrome; MRI, Magnetic resonance imaging; MDI, Mental developmental index; NIRS, Near-infrared spectroscopy; NDO, Neurodevelopmental outcome; NfL, Neurofilament light polypeptide; NSE, Neuron-specific enolase; PCPC, Pediatric cerebral performance category; PSOM, Pediatric stroke outcome measure; PDI, Psychomotor developmental index; ROBINS-E, Risk of bias in non-randomized studies – of exposure; S100B, S100 calcium-binding protein B; VABS, Vineland adaptive behavior scale.
References
1. Chen, J, Zimmerman, RA, Jarvik, GP, Nord, AS, Clancy, RR, Wernovsky, G, et al. Perioperative stroke in infants undergoing open heart operations for congenital heart disease. Ann Thorac Surg. (2009) 88:823–9. doi: 10.1016/j.athoracsur.2009.03.030
2. Schellen, C, Ernst, S, Gruber, GM, Mlczoch, E, Weber, M, Brugger, PC, et al. Fetal MRI detects early alterations of brain development in tetralogy of Fallot. Am J Obstet Gynecol. (2015) 213:392. doi: 10.1016/j.ajog.2015.05.046
3. Sun, L, Macgowan, CK, Sled, JG, Yoo, SJ, Manlhiot, C, Porayette, P, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. (2015) 131:1313–23. doi: 10.1161/CIRCULATIONAHA.114.013051
4. Von Rhein, M, Buchmann, A, Hagmann, C, Dave, H, Bernet, V, Scheer, I, et al. Severe congenital heart defects are associated with global reduction of neonatal brain volumes. J Pediatr. (2015) 167:1259–63.e1. doi: 10.1016/j.jpeds.2015.07.006
5. Dijkhuizen, EI, de Munck, S, de Jonge, RCJ, Dulfer, K, van Beynum, IM, Hunfeld, M, et al. Early brain magnetic resonance imaging findings and neurodevelopmental outcome in children with congenital heart disease: a systematic review. Dev Med Child Neurol. (2023) 65:1557–72. doi: 10.1111/dmcn.15588
6. Mebius, MJ, Kooi, EMW, Bilardo, CM, and Bos, AF. Brain injury and neurodevelopmental outcome in congenital heart disease: a systematic review. Pediatrics. (2017) 140:55. doi: 10.1542/peds.2016-4055
7. Phillips, K, Callaghan, B, Rajagopalan, V, Akram, F, Newburger, JW, and Kasparian, NA. Neuroimaging and neurodevelopmental outcomes among individuals with complex congenital heart disease: JACC state-of-the-art review. J Am Coll Cardiol. (2023) 82:2225–45. doi: 10.1016/j.jacc.2023.09.824
8. Gaynor, JW, Stopp, C, Wypij, D, Andropoulos, DB, Atallah, J, Atz, AM, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. (2015) 135:816–25. doi: 10.1542/peds.2014-3825
9. Hoffman, JIE, and Kaplan, S. The incidence of congenital heart disease. J Am Coll Cardiol. (2002) 39:1890–900. doi: 10.1016/S0735-1097(02)01886-7
10. Peyvandi, S, Latal, B, Miller, SP, and McQuillen, PS. The neonatal brain in critical congenital heart disease: insights and future directions. NeuroImage. (2019) 185:776–82. doi: 10.1016/j.neuroimage.2018.05.045
11. McQuillen, PS, Barkovich, AJ, Hamrick, SE, Perez, M, Ward, P, Glidden, DV, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. (2007) 38:736–41. doi: 10.1161/01.STR.0000247941.41234.90
12. Galli, KK, Zimmerman, RA, Jarvik, GP, Wernovsky, G, Kuypers, MK, Clancy, RR, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. (2004) 127:692–704. doi: 10.1016/j.jtcvs.2003.09.053
13. Miller, SP, McQuillen, PS, Hamrick, S, Xu, D, Glidden, DV, Charlton, N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. (2007) 357:1928–38. doi: 10.1056/NEJMoa067393
14. Investigators ICCoNI. Impact of operative and postoperative factors on neurodevelopmental outcomes after cardiac operations. Ann Thorac Surg. (2016) 102:843–9. doi: 10.1016/j.athoracsur.2016.05.081
15. Mahle, WT, and Wernovsky, G. Long-term developmental outcome of children with complex congenital heart disease. Clin Perinatol. (2001) 28:235–47. doi: 10.1016/S0095-5108(05)70077-4
16. McQuillen, PS, Hamrick, SE, Perez, MJ, Barkovich, AJ, Glidden, DV, Karl, TR, et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. (2006) 113:280–5. doi: 10.1161/CIRCULATIONAHA.105.566752
17. Claessens, NHP, Chau, V, de Vries, LS, Jansen, NJG, Au-Young, SH, Stegeman, R, et al. Brain injury in infants with critical congenital heart disease: insights from two clinical cohorts with different practice approaches. J Pediatr. (2019) 215:75–82.e2. doi: 10.1016/j.jpeds.2019.07.017
18. Marino, BS, Lipkin, PH, Newburger, JW, Peacock, G, Gerdes, M, Gaynor, JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. (2012) 126:1143–72. doi: 10.1161/CIR.0b013e318265ee8a
19. Kirschen, MP, LaRovere, K, Balakrishnan, B, Erklauer, J, Francoeur, C, Ganesan, SL, et al. A survey of Neuromonitoring practices in north American pediatric intensive care units. Pediatr Neurol. (2022) 126:125–30. doi: 10.1016/j.pediatrneurol.2021.11.002
20. Feldmann, M, Hagmann, C, de Vries, L, Disselhoff, V, Pushparajah, K, Logeswaran, T, et al. Neuromonitoring, neuroimaging, and neurodevelopmental follow-up practices in neonatal congenital heart disease: a European survey. Pediatr Res. (2023) 93:168–75. doi: 10.1038/s41390-022-02063-2
21. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
22. Rooney, A, Taylor, K, Thayer, K, Silva, R, Lemeris, C, Akl, A, et al. Risk of Bias in non-randomized studies - of exposure (ROBINS-E). (2023).
23. Schünemann, H, Brożek, J, Guyatt, G, and Oxman, A. GRADE handbook for grading quality of evidence and strength of recommendations (2013).
24. Aly, SA, Zurakowski, D, Glass, P, Skurow-Todd, K, Jonas, RA, and Donofrio, MT. Cerebral tissue oxygenation index and lactate at 24 hours postoperative predict survival and neurodevelopmental outcome after neonatal cardiac surgery. Congenit Heart Dis. (2017) 12:188–95. doi: 10.1111/chd.12426
25. Carra, G, Flechet, M, Jacobs, A, Verstraete, S, Vlasselaers, D, Desmet, L, et al. Postoperative cerebral oxygen saturation in children after congenital cardiac surgery and long-term Total intelligence quotient: a prospective observational study. Crit Care Med. (2021) 49:967–76. doi: 10.1097/CCM.0000000000004852
26. Hansen, JH, Rotermann, I, Logoteta, J, Jung, O, Dütschke, P, Scheewe, J, et al. Neurodevelopmental outcome in hypoplastic left heart syndrome: impact of perioperative cerebral tissue oxygenation of the Norwood procedure. J Thorac Cardiovasc Surg. (2016) 151:1358–66. doi: 10.1016/j.jtcvs.2016.02.035
27. Hoffman, GM, Brosig, CL, Mussatto, KA, Tweddell, JS, and Ghanayem, NS. Perioperative cerebral oxygen saturation in neonates with hypoplastic left heart syndrome and childhood neurodevelopmental outcome. J Thorac Cardiovasc Surg. (2013) 146:1153–64. doi: 10.1016/j.jtcvs.2012.12.060
28. Hoffman, GM, Brosig, CL, Bear, LM, Tweddell, JS, and Mussatto, KA. Effect of Intercurrent operation and cerebral oxygenation on developmental trajectory in congenital heart disease. Ann Thorac Surg. (2016) 101:708–16. doi: 10.1016/j.athoracsur.2015.08.059
29. Sanchez-de-Toledo, J, Chrysostomou, C, Munoz, R, Lichtenstein, S, Sao-Avilés, CA, Wearden, PD, et al. Cerebral regional oxygen saturation and serum neuromarkers for the prediction of adverse neurologic outcome in pediatric cardiac surgery. Neurocrit Care. (2014) 21:133–9. doi: 10.1007/s12028-013-9934-y
30. Simons, J, Sood, ED, Derby, CD, and Pizarro, C. Predictive value of near-infrared spectroscopy on neurodevelopmental outcome after surgery for congenital heart disease in infancy. J Thorac Cardiovasc Surg. (2012) 143:118–25. doi: 10.1016/j.jtcvs.2011.09.007
31. Sood, ED, Benzaquen, JS, Davies, RR, Woodford, E, and Pizarro, C. Predictive value of perioperative near-infrared spectroscopy for neurodevelopmental outcomes after cardiac surgery in infancy. J Thorac Cardiovasc Surg. (2013) 145:438–45. doi: 10.1016/j.jtcvs.2012.10.033
32. Claessens, NHP, Jansen, NJG, Breur, J, Algra, SO, Stegeman, R, Alderliesten, T, et al. Postoperative cerebral oxygenation was not associated with new brain injury in infants with congenital heart disease. J Thorac Cardiovasc Surg. (2019) 158:867–77.e1. doi: 10.1016/j.jtcvs.2019.02.106
33. Kelly, CJ, Makropoulos, A, Cordero-Grande, L, Hutter, J, Price, A, Hughes, E, et al. Impaired development of the cerebral cortex in infants with congenital heart disease is correlated to reduced cerebral oxygen delivery. Sci Rep. (2017) 7:15088. doi: 10.1038/s41598-017-14939-z
34. Lynch, JM, Buckley, EM, Schwab, PJ, McCarthy, AL, Winters, ME, Busch, DR, et al. Time to surgery and preoperative cerebral hemodynamics predict postoperative white matter injury in neonates with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. (2014) 148:2181–8. doi: 10.1016/j.jtcvs.2014.05.081
35. Zou, M, Yu, L, Lin, R, Feng, J, Zhang, M, Ning, S, et al. Cerebral autoregulation status in relation to brain injury on electroencephalogram and magnetic resonance imaging in children following cardiac surgery. J Am Heart Assoc. (2023) 12:e028147. doi: 10.1161/JAHA.122.028147
36. Spaeder, MC, Klugman, D, Skurow-Todd, K, Glass, P, Jonas, RA, and Donofrio, MT. Perioperative near-infrared spectroscopy monitoring in neonates with congenital heart disease: relationship of cerebral tissue oxygenation index variability with neurodevelopmental outcome. Pediatr Crit Care Med. (2017) 18:213–8. doi: 10.1097/PCC.0000000000001056
37. De Silvestro, AA, Krüger, B, Steger, C, Feldmann, M, Payette, K, Krüger, J, et al. Cerebral desaturation during neonatal congenital heart surgery is associated with perioperative brain structure alterations but not with neurodevelopmental outcome at 1 year. Eur J Cardiothorac Surg. (2022) 62:138. doi: 10.1093/ejcts/ezac138
38. Kussman, BD, Wypij, D, Laussen, PC, Soul, JS, Bellinger, DC, DiNardo, JA, et al. Relationship of intraoperative cerebral oxygen saturation to neurodevelopmental outcome and brain magnetic resonance imaging at 1 year of age in infants undergoing biventricular repair. Circulation. (2010) 122:245–54. doi: 10.1161/CIRCULATIONAHA.109.902338
39. Mueller, M, Zajonz, T, Mann, V, Koerner, C, Akintuerk, H, Yoerueker, U, et al. Interrelations of intraoperative changes in cerebral tissue oxygen saturation with brain volumes and neurodevelopment outcome after the comprehensive stage II procedure in infants with Hypoplastic left heart syndrome: a retrospective cohort study. J Cardiothorac Vasc Anesth. (2021) 35:2907–12. doi: 10.1053/j.jvca.2020.12.013
40. Gui, J, Liang, S, Sun, Y, Liu, Y, Chen, C, Wang, B, et al. Effect of perioperative amplitude-integrated electroencephalography on neurodevelopmental outcomes following infant heart surgery. Exp Ther Med. (2020) 20:2879–87. doi: 10.3892/etm.2020.9004
41. Gunn, JK, Beca, J, Hunt, RW, Olischar, M, and Shekerdemian, LS. Perioperative amplitude-integrated EEG and neurodevelopment in infants with congenital heart disease. Intensive Care Med. (2012) 38:1539–47. doi: 10.1007/s00134-012-2608-y
42. Gunn, JK, Beca, J, Penny, DJ, Horton, SB, d’Udekem, YA, Brizard, CP, et al. Amplitude-integrated electroencephalography and brain injury in infants undergoing Norwood-type operations. Ann Thorac Surg. (2012) 93:170–6. doi: 10.1016/j.athoracsur.2011.08.014
43. Latal, B, Wohlrab, G, Brotschi, B, Beck, I, Knirsch, W, and Bernet, V. Postoperative amplitude-integrated electroencephalography predicts four-year neurodevelopmental outcome in children with complex congenital heart disease. J Pediatr. (2016) 178:55–60.e1. doi: 10.1016/j.jpeds.2016.06.050
44. Claessens, NHP, Noorlag, L, Weeke, LC, Toet, MC, Breur, J, Algra, SO, et al. Amplitude-integrated electroencephalography for early recognition of brain injury in neonates with critical congenital heart disease. J Pediatr. (2018) 202:199–205.e1. doi: 10.1016/j.jpeds.2018.06.048
45. Gaynor, JW, Jarvik, GP, Bernbaum, J, Gerdes, M, Wernovsky, G, Burnham, NB, et al. The relationship of postoperative electrographic seizures to neurodevelopmental outcome at 1 year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. (2006) 131:181–9. doi: 10.1016/j.jtcvs.2005.08.062
46. Gaynor, JW, Jarvik, GP, Gerdes, M, Kim, DS, Rajagopalan, R, Bernbaum, J, et al. Postoperative electroencephalographic seizures are associated with deficits in executive function and social behaviors at 4 years of age following cardiac surgery in infancy. J Thorac Cardiovasc Surg. (2013) 146:132–9. doi: 10.1016/j.jtcvs.2013.04.002
47. Robertson, DR, Justo, RN, Burke, CJ, Pohlner, PG, Graham, PL, and Colditz, PB. Perioperative predictors of developmental outcome following cardiac surgery in infancy. Cardiol Young. (2004) 14:389–95. doi: 10.1017/S104795110400407X
48. Seltzer, L, Swartz, MF, Kwon, J, Burchfiel, J, Cholette, JM, Wang, H, et al. Neurodevelopmental outcomes after neonatal cardiac surgery: role of cortical isoelectric activity. J Thorac Cardiovasc Surg. (2016) 151:1137–44. doi: 10.1016/j.jtcvs.2015.10.065
49. Vaughan, T, Hammoud, MS, Pande, A, Chu, L, Cummins, K, McCloskey, O, et al. Can perioperative electroencephalogram and adverse hemodynamic events predict neurodevelopmental outcomes in infants with congenital heart disease? J Thorac Cardiovasc Surg. (2023) 168:342–52. doi: 10.1016/j.jtcvs.2023.10.063
50. Rappaport, LA, Wypij, D, Bellinger, DC, Helmers, SL, Holmes, GL, Barnes, PD, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation. (1998) 97:773–9. doi: 10.1161/01.CIR.97.8.773
51. Lin, R, Du, N, Feng, J, Li, J, Li, L, Cui, Y, et al. Perioperative EEG background and discharge abnormalities in children undergoing cardiac surgery: a prospective single-Centre observational study. Br J Anaesth. (2023) 131:360–72. doi: 10.1016/j.bja.2023.04.042
52. Bar-Yosef, O, Greidinger, D, Iskilova, M, Hemi, R, Tirosh, T, and Vardi, A. Neurological deficit is predicted by S100B in children after cardiac surgery. Clin Chim Acta. (2018) 481:56–60. doi: 10.1016/j.cca.2018.02.032
53. Cañizo Vázquez, D, Hadley, SM, Pérez Ordóñez, M, Lopez-Abad, M, Valls, A, Viñals, ML, et al. Oxidative stress and indicators of brain damage following pediatric heart surgery. Antioxidants (Basel). (2022) 11:489. doi: 10.3390/antiox11030489
54. Cheung, PY, Chui, N, Joffe, AR, Rebeyka, IM, and Robertson, CM. Postoperative lactate concentrations predict the outcome of infants aged 6 weeks or less after intracardiac surgery: a cohort follow-up to 18 months. J Thorac Cardiovasc Surg. (2005) 130:837–43. doi: 10.1016/j.jtcvs.2005.04.029
55. Chiperi, LE, Huţanu, A, Tecar, C, and Muntean, I. Serum markers of brain injury in pediatric patients with congenital heart defects undergoing cardiac surgery: diagnostic and prognostic role. Clin Pract. (2023) 13:1253–65. doi: 10.3390/clinpract13050113
56. Gessler, P, Schmitt, B, Prètre, R, and Latal, B. Inflammatory response and neurodevelopmental outcome after open-heart surgery in children. Pediatr Cardiol. (2009) 30:301–5. doi: 10.1007/s00246-008-9354-5
57. Graham, EM, Martin, RH, Atz, AM, Hamlin-Smith, K, Kavarana, MN, Bradley, SM, et al. Association of intraoperative circulating-brain injury biomarker and neurodevelopmental outcomes at 1 year among neonates who have undergone cardiac surgery. J Thorac Cardiovasc Surg. (2019) 157:1996–2002. doi: 10.1016/j.jtcvs.2019.01.040
58. Gunn, JK, Beca, J, Hunt, RW, Goldsworthy, M, Brizard, CP, Finucane, K, et al. Perioperative risk factors for impaired neurodevelopment after cardiac surgery in early infancy. Arch Dis Child. (2016) 101:1010–6. doi: 10.1136/archdischild-2015-309449
59. Trakas, E, Domnina, Y, Panigrahy, A, Baust, T, Callahan, PM, Morell, VO, et al. Serum neuronal biomarkers in neonates with congenital heart disease undergoing cardiac surgery. Pediatr Neurol. (2017) 72:56–61. doi: 10.1016/j.pediatrneurol.2017.04.011
60. Vedovelli, L, Padalino, M, Suppiej, A, Sartori, S, Falasco, G, Simonato, M, et al. Cardiopulmonary-bypass glial fibrillary acidic protein correlates with neurocognitive skills. Ann Thorac Surg. (2018) 106:792–8. doi: 10.1016/j.athoracsur.2018.03.083
61. Vergine, M, Vedovelli, L, Simonato, M, Tonazzo, V, Correani, A, Cainelli, E, et al. Perioperative glial fibrillary acidic protein is associated with long-term neurodevelopment outcome of infants with congenital heart disease. Children (Basel). (2021) 8:655. doi: 10.3390/children8080655
62. de Ferranti, S, Gauvreau, K, Hickey, PR, Jonas, RA, Wypij, D, du Plessis, A, et al. Intraoperative hyperglycemia during infant cardiac surgery is not associated with adverse neurodevelopmental outcomes at 1, 4, and 8 years. Anesthesiology. (2004) 100:1345–52. doi: 10.1097/00000542-200406000-00005
63. Jungner, Å, Lennartsson, F, Björkman-Burtscher, I, Blennow, K, Zetterberg, H, and Ley, D. Perioperative brain injury marker concentrations in neonatal open-heart surgery: a prospective observational study. Front Pediatr. (2023) 11:1186061. doi: 10.3389/fped.2023.1186061
64. Williams, IA, Tarullo, AR, Grieve, PG, Wilpers, A, Vignola, EF, Myers, MM, et al. Fetal cerebrovascular resistance and neonatal EEG predict 18-month neurodevelopmental outcome in infants with congenital heart disease. Ultrasound Obstet Gynecol. (2012) 40:304–9. doi: 10.1002/uog.11144
65. Cheung, EW, Chay, GW, Ma, ES, and Cheung, YF. Systemic oxygen saturation and coagulation factor abnormalities before and after the fontan procedure. Am J Cardiol. (2005) 96:1571–5. doi: 10.1016/j.amjcard.2005.07.074
66. Valencia, E, Staffa, SJ, Nathan, M, Smith-Parrish, M, Kaza, AK, DiNardo, JA, et al. Hyperlactataemia as a predictor of adverse outcomes post-cardiac surgery in neonates with congenital heart disease. Cardiol Young. (2021) 31:1401–6. doi: 10.1017/S1047951121000263
67. Reitz, JG, Zurakowski, D, Kuhn, VA, Murnick, J, Donofrio, MT, d’Udekem, Y, et al. Brain injury and neurodevelopmental outcomes in children undergoing surgery for congenital heart disease. JTCVS Open. (2024) 17:229–47. doi: 10.1016/j.xjon.2023.11.018
68. Hermans, T, Thewissen, L, Gewillig, M, Cools, B, Jansen, K, Pillay, K, et al. Functional brain maturation and sleep organisation in neonates with congenital heart disease. Eur J Paediatr Neurol. (2022) 36:115–22. doi: 10.1016/j.ejpn.2021.12.008
69. ter Horst, HJ, Mud, M, Roofthooft, MT, and Bos, AF. Amplitude integrated electroencephalographic activity in infants with congenital heart disease before surgery. Early Hum Dev. (2010) 86:759–64. doi: 10.1016/j.earlhumdev.2010.08.028
70. Votava-Smith, JK, Statile, CJ, Taylor, MD, King, EC, Pratt, JM, Nelson, DP, et al. Impaired cerebral autoregulation in preoperative newborn infants with congenital heart disease. J Thorac Cardiovasc Surg. (2017) 154:1038–44. doi: 10.1016/j.jtcvs.2017.05.045
71. Katheria, AC, Stout, J, Morales, AL, Poeltler, D, Rich, WD, Steen, J, et al. Association between early cerebral oxygenation and neurodevelopmental impairment or death in premature infants. J Perinatol. (2021) 41:743–8. doi: 10.1038/s41372-021-00942-w
72. Tombolini, S, De Angelis, F, Correani, A, Marchionni, P, Monachesi, C, Ferretti, E, et al. Is low cerebral near infrared spectroscopy oximetry associated with neurodevelopment of preterm infants without brain injury? J Perinat Med. (2022) 50:625–9. doi: 10.1515/jpm-2021-0498
73. Hansen, ML, Pellicer, A, Hyttel-Sørensen, S, Ergenekon, E, Szczapa, T, Hagmann, C, et al. Cerebral oximetry monitoring in extremely preterm infants. N Engl J Med. (2023) 388:1501–11. doi: 10.1056/NEJMoa2207554
74. Tsou, PY, Garcia, AV, Yiu, A, Vaidya, DM, and Bembea, MM. Association of Cerebral Oximetry with outcomes after extracorporeal membrane oxygenation. Neurocrit Care. (2020) 33:429–37. doi: 10.1007/s12028-019-00892-4
75. Clair, MP, Rambaud, J, Flahault, A, Guedj, R, Guilbert, J, Guellec, I, et al. Prognostic value of cerebral tissue oxygen saturation during neonatal extracorporeal membrane oxygenation. PLoS One. (2017) 12:e0172991. doi: 10.1371/journal.pone.0172991
76. Joram, N, Beqiri, E, Pezzato, S, Moscatelli, A, Robba, C, Liet, J-M, et al. Continuous monitoring of cerebral autoregulation in children supported by extracorporeal membrane oxygenation: a pilot study. Neurocrit Care. (2021) 34:935–45. doi: 10.1007/s12028-020-01111-1
77. Pavlidis, E, Lloyd, RO, and Boylan, GB. EEG - a valuable biomarker of brain injury in preterm infants. Dev Neurosci. (2017) 39:23–35. doi: 10.1159/000456659
78. Hayashi-Kurahashi, N, Kidokoro, H, Kubota, T, Maruyama, K, Kato, Y, Kato, T, et al. EEG for predicting early neurodevelopment in preterm infants: an observational cohort study. Pediatrics. (2012) 130:e891–7. doi: 10.1542/peds.2012-1115
79. Fogtmann, EP, Plomgaard, AM, Greisen, G, and Gluud, C. Prognostic accuracy of electroencephalograms in preterm infants: a systematic review. Pediatrics. (2017) 139:1951. doi: 10.1542/peds.2016-1951
80. Niezen, CK, Bos, AF, Sival, DA, Meiners, LC, and Ter Horst, HJ. Amplitude-integrated EEG and cerebral near-infrared spectroscopy in cooled, asphyxiated infants. Am J Perinatol. (2018) 35:904–10. doi: 10.1055/s-0038-1626712
81. Chock, VY, Rao, A, and Van Meurs, KP. Optimal neuromonitoring techniques in neonates with hypoxic ischemic encephalopathy. Front Pediatr. (2023) 11:1138062. doi: 10.3389/fped.2023.1138062
82. Glass, HC, Numis, AL, Comstock, BA, Gonzalez, FF, Mietzsch, U, Bonifacio, SL, et al. Association of EEG background and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy receiving hypothermia. Neurology. (2023) 101:e2223–33. doi: 10.1212/WNL.0000000000207744
83. Chandrasekaran, M, Chaban, B, Montaldo, P, and Thayyil, S. Predictive value of amplitude-integrated EEG (aEEG) after rescue hypothermic neuroprotection for hypoxic ischemic encephalopathy: a meta-analysis. J Perinatol. (2017) 37:684–9. doi: 10.1038/jp.2017.14
84. Bourel-Ponchel, E, Querne, L, Flamein, F, Ghostine-Ramadan, G, Wallois, F, and Lamblin, MD. The prognostic value of neonatal conventional-EEG monitoring in hypoxic-ischemic encephalopathy during therapeutic hypothermia. Dev Med Child Neurol. (2023) 65:58–66. doi: 10.1111/dmcn.15302
85. Kharoshankaya, L, Stevenson, NJ, Livingstone, V, Murray, DM, Murphy, BP, Ahearne, CE, et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev Med Child Neurol. (2016) 58:1242–8. doi: 10.1111/dmcn.13215
86. Rutherford, M, Ramenghi, LA, Edwards, AD, Brocklehurst, P, Halliday, H, Levene, M, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic–ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. (2010) 9:39–45. doi: 10.1016/S1474-4422(09)70295-9
87. Basti, C, Maranella, E, Cimini, N, Catalucci, A, Ciccarelli, S, Del Torto, M, et al. Seizure burden and neurodevelopmental outcome in newborns with hypoxic-ischemic encephalopathy treated with therapeutic hypothermia: a single center observational study. Seizure. (2020) 83:154–9. doi: 10.1016/j.seizure.2020.10.021
88. Lin, JJ, Banwell, BL, Berg, RA, Dlugos, DJ, Ichord, RN, Kilbaugh, TJ, et al. Electrographic seizures in children and neonates undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med. (2017) 18:249–57. doi: 10.1097/PCC.0000000000001067
89. Hanalioglu, D, Temkit, MH, Hildebrandt, K, MackDiaz, E, Goldstein, Z, Aggarwal, S, et al. Neurophysiologic features reflecting brain injury during pediatric ECMO support. Neurocrit Care. (2023) 40:759–68. doi: 10.1007/s12028-023-01836-9
90. Chahine, A, Chenouard, A, Joram, N, Berthomieu, L, Du Pont-Thibodeau, G, Leclere, B, et al. Continuous amplitude-integrated electroencephalography during neonatal and pediatric extracorporeal membrane oxygenation. J Clin Neurophysiol. (2023) 40:317–24. doi: 10.1097/WNP.0000000000000890
91. Felling, RJ, Kamerkar, A, Friedman, ML, Said, AS, LaRovere, KL, Bell, MJ, et al. Neuromonitoring during ECMO support in children. Neurocrit Care. (2023) 39:701–13. doi: 10.1007/s12028-023-01675-8
92. Huang, Y, Wang, Z, Huang, ZX, and Liu, Z. Biomarkers and the outcomes of ischemic stroke. Front Mol Neurosci. (2023) 16:1171101. doi: 10.3389/fnmol.2023.1171101
93. Dassan, P, Keir, G, and Brown, MM. Criteria for a clinically informative serum biomarker in acute ischaemic stroke: a review of S100B. Cerebrovasc Dis. (2009) 27:295–302. doi: 10.1159/000199468
94. Kövesdi, E, Lückl, J, Bukovics, P, Farkas, O, Pál, J, Czeiter, E, et al. Update on protein biomarkers in traumatic brain injury with emphasis on clinical use in adults and pediatrics. Acta Neurochir. (2010) 152:1–17. doi: 10.1007/s00701-009-0463-6
95. Bembea, MM, Rizkalla, N, Freedy, J, Barasch, N, Vaidya, D, Pronovost, PJ, et al. Plasma biomarkers of brain injury as diagnostic tools and outcome predictors after extracorporeal membrane oxygenation. Crit Care Med. (2015) 43:2202–11. doi: 10.1097/CCM.0000000000001145
96. Sood, BG, McLaughlin, K, and Cortez, J. Near-infrared spectroscopy: applications in neonates. Semin Fetal Neonatal Med. (2015) 20:164–72. doi: 10.1016/j.siny.2015.03.008
97. Chiperi, LE, Tecar, C, and Toganel, R. Neuromarkers which can predict neurodevelopmental impairment among children with congenital heart defects after cardiac surgery: a systematic literature review. Dev Neurorehabil. (2023) 26:206–15. doi: 10.1080/17518423.2023.2166618
Keywords: neuromonitoring, neurodevelopment, neuroimaging, congenital heart disease, near-infrared spectroscopy, electroencephalography, biomarkers
Citation: Van Loo L, Cools B, Dereymaeker A and Jansen K (2024) Neuromonitoring modalities predicting neurological impairment in pediatric congenital heart disease: a systematic review. Front. Neurol. 15:1502762. doi: 10.3389/fneur.2024.1502762
Edited by:
Igor Nestrasil, University of Minnesota Health Twin Cities, United StatesReviewed by:
Arif Somani, University of Minnesota Twin Cities, United StatesBrian Mendel, National Cardiovascular Center Harapan Kita, Indonesia
Copyright © 2024 Van Loo, Cools, Dereymaeker and Jansen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liselotte Van Loo, bGlzZWxvdHRlLnZhbmxvb0B1emxldXZlbi5iZQ==
†ORCID: Liselotte Van Loo, http://orcid.org/0000-0002-6712-2489
Bjorn Cools, http://orcid.org/0000-0003-3633-4216
Anneleen Dereymaeker, http://orcid.org/0000-0003-3923-1298
Katrien Jansen, http://orcid.org/0000-0002-5503-529X
 Liselotte Van Loo
Liselotte Van Loo Bjorn Cools
Bjorn Cools Anneleen Dereymaeker
Anneleen Dereymaeker Katrien Jansen
Katrien Jansen