- 1Department of Engineering and Geology, University “G. d’Annunzio” of Chieti-Pescara, Pescara, Italy
- 2Department of Oral Medical Science and Biotechnology, Physical and Rehabilitation Medicine, BIND, CARES, University “G. d’Annunzio” of Chieti-Pescara, Chieti, Italy
- 3Department of Life, Health and Environmental Sciences, University of L’Aquila, L’Aquila, Italy
- 4San Raffaele Institute of Sulmona, Sulmona, Italy
- 5Department of Medical and Surgical Specialties and Dentistry, University of Campania Luigi Vanvitelli, Napels, Italy
- 6Multidisciplinary Department of Medical and Surgical Specialties and Dentistry, University of Campania “Luigi Vanvitelli”, Naples, Italy
- 7Department of Mental and Physical Health and Preventive Medicine, University of Campania “Luigi Vanvitelli”, Naples, Italy
- 8Scientific Institute for Research Hospitalisation and Health Care IRCCS Santa Lucia Foundation, Rome, Italy
Introduction: Cerebral palsy (CP) is a group of permanent disorders of movement development that may cause activity limitations. In this context, robot-assisted therapy might play a key role in clinical management. This comprehensive systematic review aimed to investigate the efficacy of robotic systems in improving upper limb (UL) functions in children with CP.
Methods: PubMed, EMBASE, Scopus, and PEDro were searched from inception to February 2024. The risk of bias was assessed with the Joanna Briggs Institute critical appraisal tools battery.
Results: Of 756 articles identified, 14 studies involving 193 children with CP with a judged to be of good methodological quality, but with a lack in the study design, were included in the final synthesis. In the included studies a wide range of devices was used, both exoskeletons and end-effectors, both wearable and non-wearable. The CP children who underwent robot-assisted therapy reported a significant overall increase in clinical assessment, specifically in UL movements and manual dexterity. The clinical improvement was often accompanied by a gain also in instrumental assessments (i.e., kinematic analysis, EMG).
Discussion: The present review suggested that robot-assisted therapy can improve UL motor functions in children with CP. Moreover, the availability of different devices with adjustable parameters can represent an important resource in proposing patient-centered-personalized rehabilitation protocols to enhance the efficacy of rehabilitation and integration into daily life. However, the limited sample size and lack of standardized and clearly reproducible protocols impose to recommend the use of robot-assisted therapy as an integration to usual rehabilitation and not as a replacement.
Systematic review registration: https://osf.io/a78zb/.
1 Introduction
Cerebral palsy, which occurs in two to three out of 1,000 live births (1), has multiple etiologies resulting in brain injury that affects movement, posture, and balance. Movement disorders can be categorized as spasticity, dyskinesia, ataxia, or mixed, and spasticity is the most common movement disorder, occurring in 80% of CP (2). According to this evidence, CP is classified into spastic (80%), dyskinetic (15%), and ataxic (5%) forms. The focus of rehabilitation treatment (3–5) has recently shifted to neurological rehabilitation in response to increasing evidence for neuroplasticity. This approach aims to improve development and function by enhancing the capacity of the central nervous system to change and adapt throughout the patient’s life. As the life expectancy of CP children generally approaches that of the overall population, rehabilitative therapies must be developed with the aim to address the needs of children first and then adults aging with disability and provide a guide for developing shared goals with families considering the six “Fs” framework: function, family, fitness, fun, friends, and future (4, 6). Robotic systems are new devices that are becoming increasingly popular as a part of the treatment for CP (7) to improve gross motor function of the upper limbs (UL) and the fine motor function of the hand. Traditionally, based on the International Classification of Functioning, Disability, and Health, Children and Youth (ICF-CY) conceptual framework, constraint-induced movement therapy (mCIMT) and bimanual training (BIT) are the main physiotherapeutic techniques used in the recovery of UL function in children with CP (8, 9). Rehabilitation robots can provide “controlled, intensive task-specific training that is goal directed and cognitively engaging,” a concept consistent with the current emphasis on therapeutic interventions for UL function in children with CP in a successful association or alternation with traditional physiotherapy (7). Findings of recent research support robotic systems in combination with Virtual Reality (VR) settings to enhance UL rehabilitation in CP with the motivational features built into the interactive VR games (10). Robotic interfaces allow multiple methods to shape UL movement patterns (11, 12) and could represent a facilitation in the complex reaching movements with finalized game-like virtual simulations that can accomplish the repetition, attention, and ecological validity required for effective massed practice also for assisted play for functional and playful activities. The robotic system with virtual assistance has been clinically validated to be significantly more effective, compared to both unassisted and typical approaches, by increasing the hand controllability, reducing the physical load, and increasing the easiness of maintaining movements within the lines. Participating in a robot-mediated play activity has demonstrated to increase children’s motivation and, at the same time, engagement with the important resource for home-based implementation of the technology to promote manual play activities for children with disabilities (13). Robot-assisted training (RAT) provides a promising alternative; however, there is a need for solutions that specifically target children and their needs, especially on improving UL function and fine hand movement. However, the paucity of group design studies summons the need for more rigorous research before conclusive recommendations can be made (14, 15). Another important aspect of robot training is the mixed use of RAT with biofeedback systems. Biofeedback techniques play a significant role in enhancing motor skills through various mechanisms. Studies have shown that neurofeedback training (NFT) can effectively improve motor performance by allowing individuals to manipulate their brain activity with sensory feedback (16). Additionally, biofeedback relaxation training has been found to enhance athletes’ motor skills by improving the balance between sympathetic and parasympathetic nerves, increasing heart rate variability, and promoting psychological relaxation. Furthermore, multimodal biofeedback, involving EEG, ECG, PPG, and RSP signals, has demonstrated promising results in improving both motor and nonmotor symptoms in patients with Parkinson’s disease, highlighting the potential of biofeedback in enhancing motor functions through signal regulation (16). These findings collectively emphasize the valuable contribution of biofeedback techniques in optimizing motor skills and performance across different populations and contexts. Combining biofeedback with robotic training shows promise in enhancing UL rehabilitation for children with CP. Studies have highlighted the effectiveness of integrating biofeedback systems within wearable robotic devices to improve user engagement and muscle activity (17, 18). Additionally, research on RAT combined with transcranial direct current stimulation (tDCS) in children with CP suggests feasibility and safety, emphasizing the importance of careful participant selection and protocol adaptations for successful outcomes (19). These findings underscore the potential of feedback and biofeedback-enhanced RAT (20) to optimize UL rehabilitation outcomes in CP patients. This offers a novel approach to improving motor functionality and quality of life in this population. Therefore, the objective of this study was to review the effectiveness of robotic systems either as a therapy alone or in combination with the physiotherapy treatments of CP children in improving UL function as primary outcomes and their autonomy and quality of life as secondary ones.
2 Materials and methods
We conducted a comprehensive systematic review to explore the effect of RAT for upper limb recovery in children with CP. We summarized the results of all published studies following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (21). The protocol was recorded in the Open Science Framework (OSF) register.1
2.1 PICO question
We used the PICO model (Population, Intervention, Comparison, Outcome) to define the search strategy and report the results (22). The PICO tool was allowed to respond to the research question: “Is robot-assisted therapy effective in promoting upper limb motor recovery in CP patients?” (the PICO strategy is reported in Table 1).
2.2 Search strategy and eligibility criteria
A systematic search was conducted for all peer-reviewed articles published from inception to the first of January 2024, using the following databases: PubMed, EMBASE, Scopus, and PEDro. Mesh terms and free terms about the topic have been used: “cerebral palsy,” “robot-assisted therapy,” and “motor recovery” (the complete search strategy is reported in the Supplementary Appendix A). We included all studies involving children with CP subjected to RAT. Specifically, the inclusion criteria were: (i) children with CP (pediatric population (<18 years) affected by CP); (ii) RAT (alone or combined with other rehabilitation approaches); (iii) written in English language; and (iv) published in a peer-reviewed journal. We have excluded articles describing theoretical models, methodological approaches, algorithms, and basic technical descriptions. We excluded: (i) animal studies; (ii) conference proceedings and review; (iii) studies involving healthy children; (iv) single sessions investigations; and (v) feasibility studies. Considering the limited literature available, we included multiple study designs for qualitative synthesis: (i) Randomized Controlled Trial (RCT); (ii) Controlled trial; (iii) Longitudinal study (iv) case series; and (v) case study.
2.3 Data extraction and analysis
All search results were imported into an online database (RYYAN) (23) and screened by two blinded reviewers (F. D. and A. C.). In the first screening, duplicated articles were removed. After that, both reviewers screened the title and abstract to select eligible articles according to the inclusion/exclusion criteria. After screening based on titles and abstracts, the blind was opened, and the cases of disagreement were discussed with a third reviewer to allow a consensus (A. M. C.). After full-text selection, the data extraction from the included studies was reported on a sheet. Data from the fully read articles were extracted by the two reviewers (A. M. C. and A. B.). The population, age, type of robotic systems (intervention), outcome measures, and follow-ups were registered in a synoptic table. When there was any form of disagreement, a third reviewer was consulted (T. P.).
2.4 Risk of bias
The risk of bias of the individual studies was assessed with the Joanna Briggs Institute critical appraisal tools battery (24, 25). JBI is used to evaluate trustworthiness, relevance, and results via a specific tool for each study design and is useful in the case of comprehensive reviews with heterogeneous design (25–27). The risk of bias was assessed by two independent reviewers (A. B. and A. M. C.). Potential discrepancies in quality assessment were resolved through consensus or through discussion with a third reviewer (T. P.).
3 Results
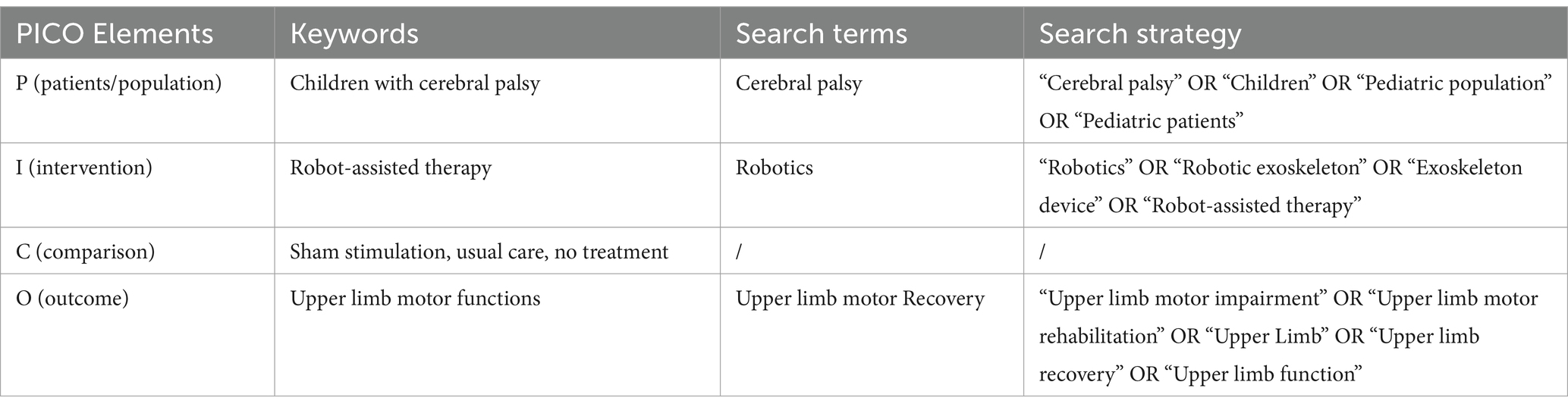
In total, 756 articles were found. After duplicate removal (137), 619 articles were screened by title and abstract and 582 were excluded because did not met the inclusion/exclusion criteria. Finally, 37 studies were extracted for the full-text analysis. Of the 37 full-text screened, 14 studies (10, 11, 28–39) were included in the synthesis (Figure 1). We found 2 RCTs, 9 longitudinal studies, 2 case series, and a single case. The included studies were published from 2008 (11) to 2022 (36). About 42.9% of studies were conducted in the USA, whereas 21.4% in Italy (see Table 2).
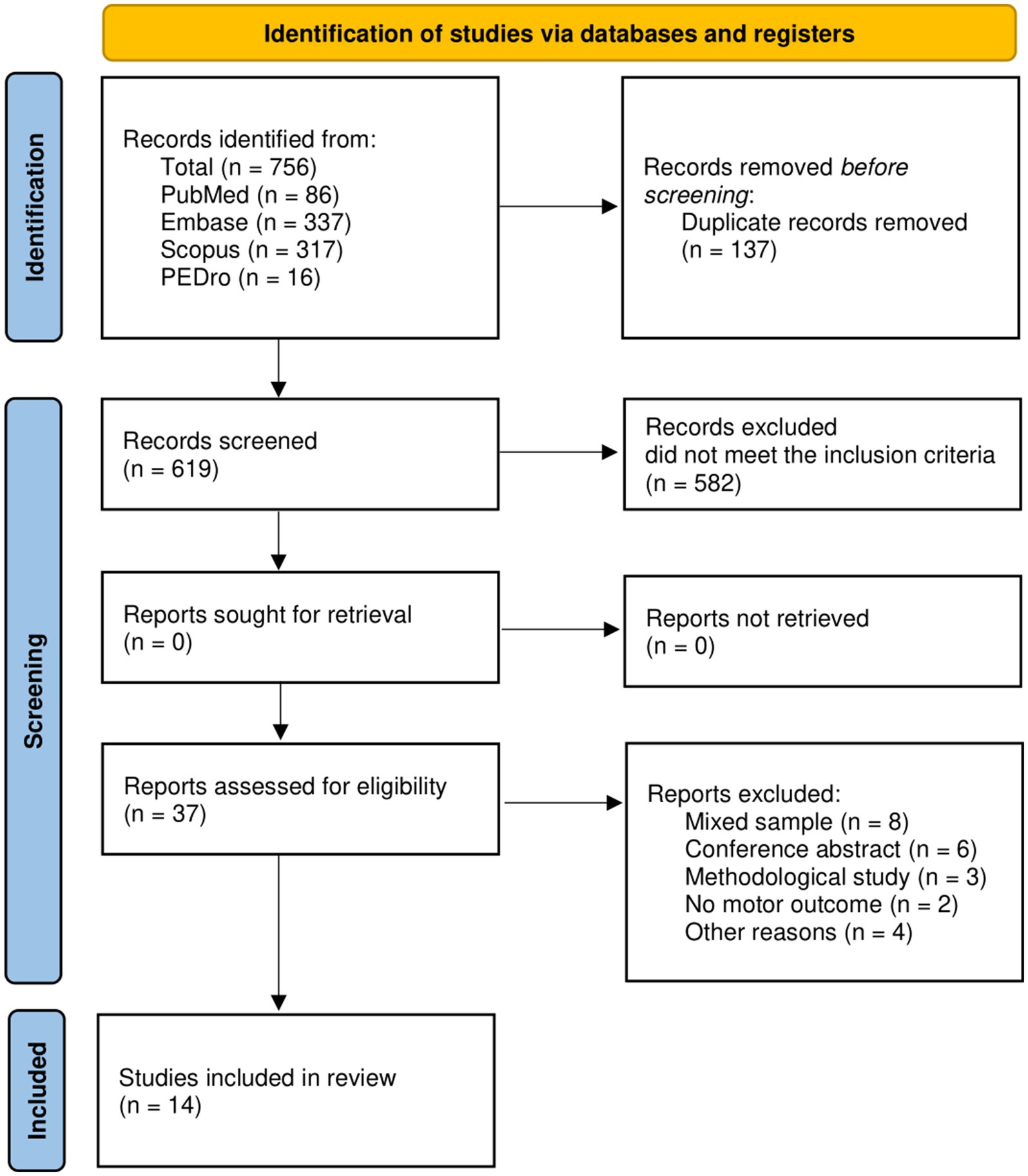
Figure 1. PRISMA Flow-diagram of studies selection. Source: Page et al. (63).
3.1 Population
The population of included studies involved a total of 214 children, 193 children with CP, aged between 4.5 months (33) to 16 years (36), and 21 healthy controls. In the ten studies that reported the gender (10, 11, 28–35), there were 87 males (64%) and 49 females (36%). Three studies reported the CP sub-types, two of these included children with spastic CP (30, 36) and the other one included all CP subtypes (33). Concerning the classification, eight studies reported the severity of UL impairment using the Manual Ability Classification System (MACS) (28, 30, 31, 33, 34, 36–38) or the Functional Level of Hemiplegia (FLH) (10). Only two studies (28, 35) reported a baseline cognitive assessment of total intelligence quotient and nonverbal intelligence, respectively.
3.2 Intervention
The included studies performed a RAT protocol of at least 3 sessions (32) to 40 sessions (28) with a mean of ~17 sessions. The duration of each session ranged from 15 min (36) to 70 min (32) with a mean of ~47 min. Four studies performed an hour of training (10, 11, 35, 39) and the other four performed 45 min of training (28–31). Regarding the total duration of RAT protocol, the range was between 10 days (36) to 32 weeks (36) with an average of ~7.6 weeks. The most recurrent total duration was 8 weeks (11, 30, 35, 39). Finally, the frequency (sessions/week) ranged from 0.25 (36) to 10 (28) with a mean of ~4 sessions per week. In seven studies, two or three sessions per week is the most adopted frequency (10, 11, 29, 35, 36, 38, 39).
3.2.1 Devices characteristics and setting
About the device used, in the included studies 50% used an exoskeleton device, 42.9% used an end-effector device and Kolobe et al. (33) used an integration of robotics and sensor technologies designed to capture and influence movement effort as infants learn prone locomotion. Specifically, five studies (28, 30, 32, 36, 37) used the Armeo® Spring device, three studies (11, 29, 35) used the InMotion2 device, two studies (10, 38) used the NJIT-RAVE System, and the other four studies (30, 31, 33, 36) used: the REAPlan; the Self-Initiated Prone Progression Crawler (SIPPC) device; The Gloreha Sinfonia with a dynamic support system; and a Hybrid Assistive Limb (HAL) with an Integrated Volitional Control Electrical Stimulation (Figure 2). Relatively to the settings, most of the studies were clinical (85.71%), and only two studies were nonclinical longitudinal (33, 39). Two studies used wearable devices (33) for flexion-extension of the elbow and for prone locomotion. However, only Kuroda et al. (36) used the wearable device in an environmental setting (home or childcare center). None of the cited works using wearable devices involved remote monitoring from home. Roberts et al. (38) and a subgroup of children (group III) of the study by Fluet et al. (10) performed RAT protocol in a clinical camp during a scheduled daily routine.
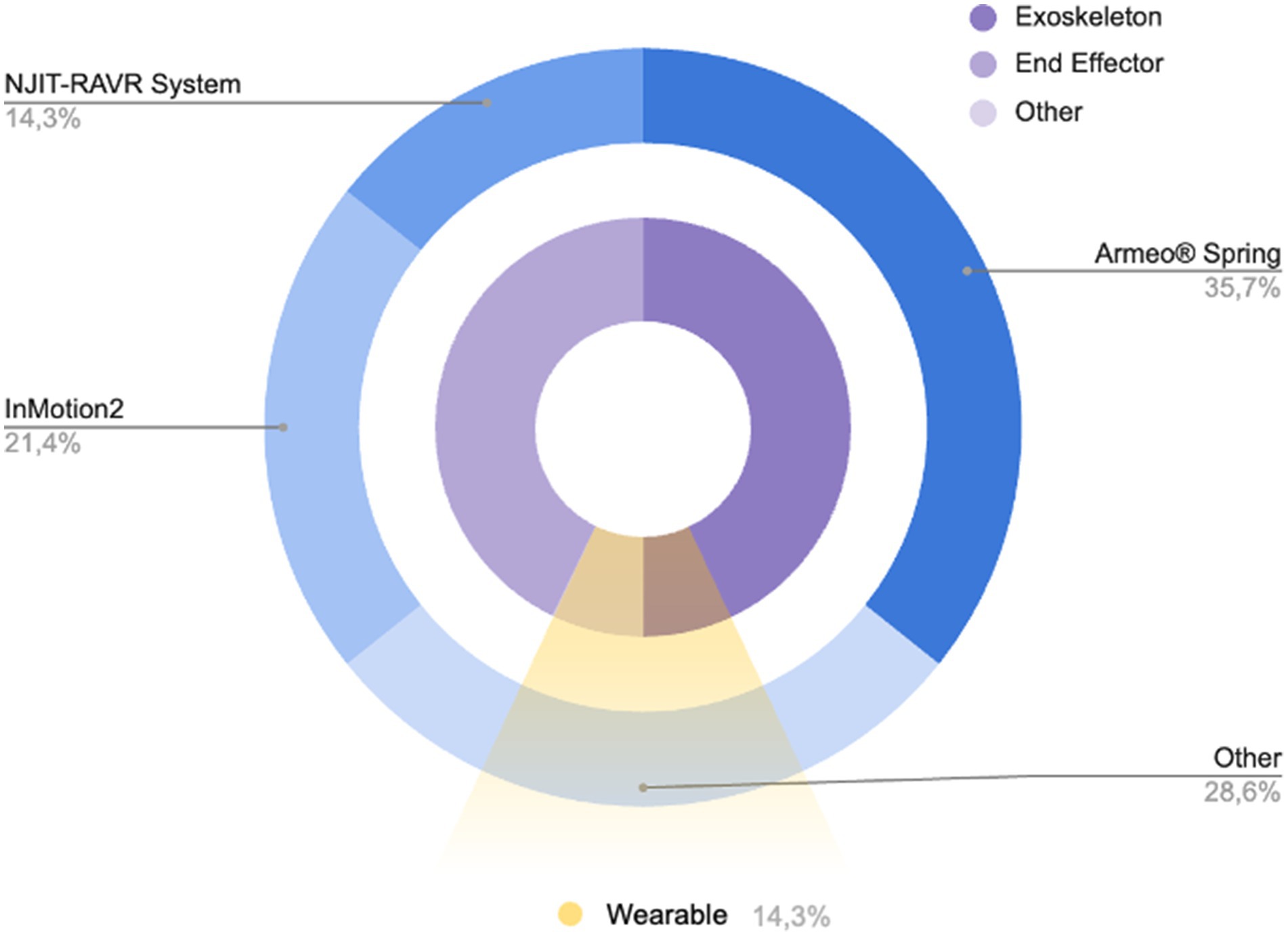
Figure 2. Pie chart showing the distribution of devices used in the included studies (blue pie chart) and their main characteristics (purple chart and yellow slice).
3.2.2 Feedback and biofeedback
Twelve studies (10, 11, 28–36, 38) used an integrated feedback system via audio-visual and/or haptic stimuli often in the form of exergaming. Fluet et al. (10) used stereoscopic glasses to enhance depth perception, which increases the sense of immersion. Few studies used an immersive virtual reality environment during RAT. In the other two studies (33, 36) functional tasks were performed during RAT (reaching/grasping and prone locomotion).
3.2.3 Combination with other therapies
In two studies (11, 29) the RAT protocol was administered after Botulinum Toxin Type-A injection (3 out of 5 children for (29)). In the study of Kuroda et al. (36) RAT therapy was coupled with online neuromuscular electrical stimulation. In two studies (10, 38) the RAT was administered inside a daily routine of other rehabilitative activities (i.e., CIMT and intensive bimanual therapeutic interventions) in a clinical camp. In one RCT study (30) the patients allocated in the experimental group underwent a mixed program of 40 sessions subdivided into 16 RAT sessions and 24 sessions of usual care. Other studies investigated exclusively the effect of RAT without other concomitant therapies.
3.3 Comparison
Only two studies compared the effect of RAT with respect to control groups subjected to conventional therapy (30, 31); another two (33, 36) compared the kinematics data of RAT in CP children with respect to healthy controls (15 and 6, respectively). Moreover, Kolobe et al. (33) split the CP sample into two RAT subgroups subjected to reinforcement (9 children) and reinforcement plus error-based learning (14 children). Fluet et al. (10) divided the children into 3 subgroups, the first two differing for the range of motion of prono-supination, the last one (4 children) performed the same number of sessions as group 1 and 2 (9 sessions) but with a frequency of 3 sessions per week plus 5 h daily of other rehabilitative activities (for more detail see Paragraph 3.2.3). Both RCTs (30, 31) performed the same dose of exercises, in terms of intensity, duration, and frequencies, in both experimental and control groups. El-Shamy (30) subjected the RAT group to 40 sessions of treatment composed of 16 sessions of RAT and 24 sessions of conventional therapy, while the control group performed 40 sessions of conventional therapy only. In the three studies (30, 31, 36), no declared statistical differences at the baseline were reported between groups except for Gilliaux et al. (31) and Kolobe et al. (33) which reported differences in the coefficient of variation straightness (6.9 and 3.7%, p = 0.03) and in the MOCS scores between the two CP subgroups (16.84 and 11.55, p = 0.02), respectively.
3.4 Outcome
All included studies used clinical scales and tests to assess the changes in UL functions after RAT. The most used clinical scales were: the Melbourne Assessment (MA) (10, 11, 32, 33, 36, 38), the Quality of Upper Extremity Skills Test (QUEST) (11, 31, 33–36), and the Fugl-Meyer Assessment scale for Upper Extremity (FMA-UE) (28, 33, 35). About clinical tests, the most used was the Box and Block test (BBT) (31, 33, 35). Ten studies (10, 28, 31–38)also reported instrumented evaluation including kinematics features, electromyography activity, or grip force results. Specifically, nine studies (10, 28, 31–36, 38) performed kinematics analysis, eight of these (10, 28, 32–36, 38) using robotics output collected on the same devices used for the training, and the other one (31) with an optoelectronic system with passive markers (SMART-DX, BTS, Milan, Italy) and a video system synchronized with the optoelectronic system (BTS, Milan, Italy). Kuo et al. (35) used a surface EMG (sEMG) on brachioradialis and extensor digitorum communis muscles during reaching tasks. Finally, four studies (10, 11, 28, 35) evaluated the force using a manual dynamometer for grip or elbow strength. Eleven studies (10, 28, 31–38) performed statistical analysis to interpret the results. For the rest, two studies (32, 37) reported descriptive statistics and the last one (10) used the minimal detectable change to discuss the RAT effects. Only two studies (28, 35) used the international classification of functioning (ICF) in the choice of outcome measurements.
3.4.1 Change in clinical outcome
In the two RCTs included (30, 31) a statistically significant increase in the RAT group with respect to the control group was observed in manual dexterity, spasticity, movement patterns, and hand function. All other studies (10, 11, 28, 31–36, 38) reported an improvement in the within-group/within-subject analysis performed on clinical data except for one (33). Specifically, four studies (32–34, 38) reported an improvement in all clinical scales and tests, and the other seven studies (10, 11, 28, 31, 35–37) in at least one clinical outcome. About clinical tests, four studies administered the BBT (28, 31, 33, 35) but only two of these (31, 35) reported a significant improvement in manual dexterity.
3.4.2 Change in instrumental outcome
In the eight studies that investigated UL kinematics (10, 28, 30, 31, 33–35, 38), seven studies (10, 28, 30, 31, 33, 35, 38) reported an overall improvement after RAT treatment in the kinematics features or in their derived indices. Most recurrent changes were observed in smoothness (10, 28, 30, 33, 38) and speed (10, 28, 30, 31). Kolobe et al. (33) reported an improvement in the rotational amplitude, but not in the wrist path length, foot path length, and linear path length. Regarding sEMG changes, Kuo et al. (35) observed a statistically significant improvement in the brachioradialis activity in the co-contraction ratio and in the electrical agonist–antagonist muscle ratio. Finally, in the force evaluation, only one study (10) out of four studies (10, 30, 31, 35) reported an improvement in grip strength after RAT treatment.
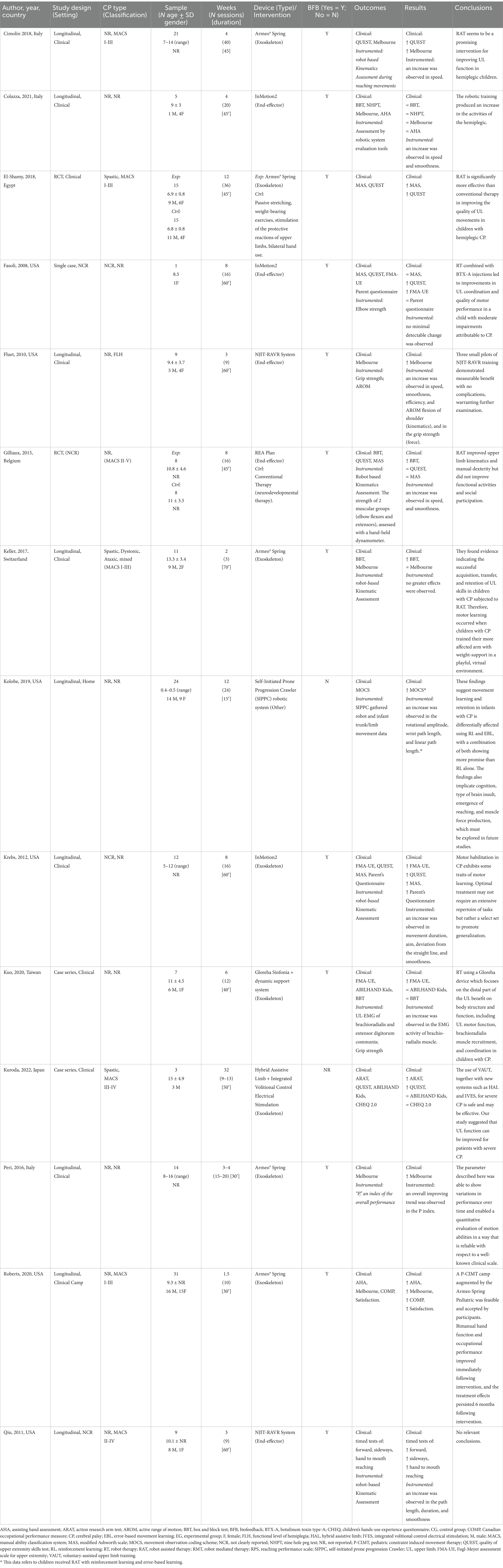
3.5 Risk of bias
Eight out of the fourteen included studies were evaluated to have a low risk of bias (10, 28, 31–35, 38). Five studies were judged to have a moderate risk of bias (11, 30, 31, 36, 37). Finally, one study was considered to have a high risk of bias (33). In the RCTs, the impact on the judgment was mainly attributable to the blinding items. In the other studies, the issues included consecutive inclusion, unclear patient history, and missing conflict of interest, which affected the overall judgment (Table 3).
4 Discussion
In this review, the efficacy of robotic devices, either as standalone therapy or in conjunction with physiotherapy, in improving UL function in children diagnosed with cerebral palsy CP is examined. However, the results should be interpreted with caution due to the heterogeneity, and generalization is not possible because of the limited number of RCTs with adequate sample sizes, which affects the inferential statistics. Nevertheless, the available data can be valuable in summarizing the initial evidence on the use of RAT in the rehabilitation of UL function in children with CP. From the literature investigation, RAT has been proven to be potentially effective for rehabilitating UL. Its objectives include enhancing the amount and intensity of therapy while also standardizing the treatment process (40). This approach employs rigorous, repeated, interactive, and personalized exercises to enhance motor learning (41). This kind of therapy is administered through both end-effector and/or exoskeleton devices. End-effector robots engage with patients via a solitary point, whereas exoskeletons contain several contact points that closely mimic the anatomy of the human limb (42). The variation in contact points might potentially impact the way the devices interact with the patient’s limb throughout the rehabilitation process. Exoskeletons provide a more extensive and evenly distributed support to the upper limb during training compared to end-effector robots, thanks to their many contact points. Exoskeletons are defined by their wearable design, which closely imitates the shape of the human limb, providing more natural and instinctive contact during rehabilitation sessions (42). This design element has the potential to increase patient involvement and comfort during treatment, which might result in better results for CP patients undergoing upper limb rehabilitation. Conversely, end-effector robots, which interact via a single point, may provide benefits in terms of simplicity and user-friendliness during training sessions. The exact contact point may enable targeted workouts and meticulous control over certain motions, which might be advantageous for addressing specific rehabilitation objectives in people with CP. Ultimately, both end-effector and exoskeleton devices have distinct features and benefits in upper limb rehabilitation for CP patients. However, exoskeletons with multiple contact points and wearable designs may provide a more comprehensive and natural interaction experience, potentially resulting in improved rehabilitation outcomes. Conversely, end-effector robots provide focused and accurate manipulation of motions, addressing rehabilitation requirements. Importantly, it is possible to integrate robotic therapy administered through both end-effectors or exoskeleton with biofeedback, which is essential to increase patients’ motivation (43). Several examples of feedback and biofeedback have been implemented so far with the aim of making the rehabilitation process more effective. For instance, the trajectory data from robot motions during rehabilitation exercises is sent to a computer’s virtual scene, which aids in movement coordination and improves the rehabilitation process (44). Moreover, a study on the use of biofeedback-enhanced therapeutic exercise video games for young people with CP demonstrated that the implementation of biofeedback improved engagement by offering autonomy and varying feedback presentation (43). This approach increased the efficiency, effectiveness, and engagement of the therapy, highlighting the positive impact of biofeedback on patient involvement. Additionally, the use of EMG biofeedback has been suggested as a training tool to improve muscle activation and decrease spasticity in children with CP, leading to increased cooperation and compliance with treatment (45). By providing visual feedback through EMG biofeedback, children can actively participate in rehabilitation, promoting rapid recovery and enhancing engagement in the therapy process. Importantly, biofeedback has the capacity to enhance central nervous system (CNS) activity, thereby impacting a range of physiological responses. Research conducted by Stubberud et al. (46) has shown that EMG biofeedback may decrease the level of cortical excitability and influence the resonance and oscillations of crucial feedback loops in the CNS. Likewise, kinematic biofeedback therapies have been proposed to impact the activity of the CNS allowing patients to consciously manage hidden physiological reactions by making these responses more apparent (47). In addition, motion compensation control systems based on electromyography (EMG) are often designed to enhance the active involvement of patients in rehabilitation training, with the goal of improving results (48). These mechanisms offer visual cues for muscle activation, allowing patients to visualize and adjust their muscle activation patterns during therapy, which can enhance engagement and motivation in rehabilitation sessions (49).
Moreover, biofeedback treatments have been associated with the regulation of autonomic nervous system activity. Research has shown that biofeedback training may improve baroreflexes and increase heart rate variability, suggesting a direct impact on autonomic nervous system function via central processes (50). The impact of employing biofeedback has been proved not only in outpatient environments but also for training done at home (51). This training can make use of telemedicine and telerehabilitation methods. These evaluations are vital for tracking progress and customizing rehabilitation therapies with precision. Moreover, the use of virtual reality therapy in conjunction with biofeedback has shown efficacy in enhancing UL functionality in individuals diagnosed with CP, including children and young adults (52). This can lead to long-term enhancements in motor function and overall quality of life (53).
In summary, comparing the considered studies, all of them found evidence in the improvement of skills, such as dexterity, and motor abilities. One study (11) showed that RT combined with BTX-A injections led to improvements in UL coordination and quality of motor performance. Another study (38) focused also on the mid-term efficiency of RAT, showing that the treatment effects persisted 6 months following intervention. However, overall results consisted in better outcomes in terms of clinical scales.
4.1 Limitations
The review of the literature regarding the employment of robotic therapy for the treatment of upper limbs impairment in children with CP revealed significant heterogeneity in study design. Due to the limited number of available RCTs, to broaden the synthesis of results and enhance the robustness of the findings, the inclusion criteria were expanded from the initial protocol to cover a broader age range, from birth to 18 years (previously 4–18 years). The criteria were also modified to include a wider variety of study designs focused on robotic rehabilitation protocols for the upper limb, while excluding single-session studies, theoretical models, methodological approaches, algorithms, basic technical descriptions, conference proceedings, and reviews. This variability includes differences in the type and intensity of interventions, the duration and frequency of therapy sessions, and the metrics used to assess outcomes. Such heterogeneity poses a potential limitation, as it complicates the comparison and synthesis of results across studies. Additionally, we did not select a specific measure for the primary outcome but focused on overall functional improvement of the upper limb. The goal was to assess any positive changes in the functioning of the affected limb, without restricting the measure to a particular aspect such as strength, dexterity, or functional ability. This broader approach allowed us to capture a more comprehensive view of therapeutic effects across various domains of upper limb function. A notable concern is the lack of standardized dosage of therapy, which is further complicated by the variability in the severity of symptoms among patients. This inconsistency highlights the need for the development and implementation of standardized protocols to ensure more reliable and generalizable findings in future research. Besides, the method used to assess robotic therapy (RAT) performance is not uniquely defined. In the included studies, kinematic assessment was performed using the kinematics output of the robot device, and only one study used an external system such as pre-post measures. The use of the robot’s kinematic output for evaluating the change in upper limb (UL) motor gain is controversial (54, 55). Other approaches (i.e., inertial measurement units, optoelectronic systems, and stereophotogrammetry) can support the instrumented evaluation to objectify the results (56) both in clinical and home-based environments (57). Another significant limitation identified in the review is the limited sample size of the studies. Collectively, the studies have investigated fewer than 200 patients, which restricts the generalizability and robustness of the findings. Small sample sizes can lead to reduced statistical power, increasing the likelihood of Type II errors and limiting the ability to detect meaningful differences or effects. Furthermore, larger sample sizes are crucial for the application of advanced statistical analysis methods, including those based on artificial intelligence (AI). AI-based methods, such as machine learning algorithms, require extensive data to accurately identify patterns, make predictions, and generalize findings to broader populations (58). A larger dataset would enable the application of these sophisticated techniques, potentially leading to more precise and individualized therapeutic approaches. Therefore, future research should aim to include larger cohorts to enhance the reliability of results and fully leverage the potential of AI in analyzing and optimizing RAT for upper limb rehabilitation in patients with CP. Several studies employed mixed approaches, incorporating additional therapies alongside robotic interventions, thus limiting the ability to isolate and evaluate the effectiveness of RAT as a stand-alone treatment (8). This methodological choice complicates the interpretation of results, as the observed outcomes cannot be solely attributed to RAT. The concurrent use of various therapeutic modalities, such as conventional physical therapy, occupational therapy, or pharmacological treatments, introduces confounding variables that obscure the specific impact of robotic intervention. Consequently, the mixed-method approach reduces the clarity and precision in determining the efficacy of robotic therapy independently. To address this limitation, future research should prioritize the design of studies that evaluate RAT in isolation, ensuring a clearer understanding of its direct effects and therapeutic potential for upper limb rehabilitation in patients with CP. This will facilitate more accurate conclusions and inform clinical practice and guidelines more effectively.
Another limitation is the lack of flexibility and adaptability in existing UL exoskeleton rehabilitation robots, making it challenging to meet the diverse needs of patients with CP and address individualized rehabilitation requirements effectively (59). Furthermore, studies have indicated that patients undergoing RAT may not necessarily exhibit significant improvements in upper limb movement concerning daily living activities, suggesting a gap in translating the benefits of robotic therapy into functional improvements in real-world scenarios for individuals with CP (60). This discrepancy between the outcomes of RAT and the actual performance in daily activities highlights a limitation in the effectiveness of current biofeedback-enhanced robotic rehabilitation approaches. In this context, more in-depth research is needed to assess the association between individual differences in clinical functionality and/or genomics and the predisposition of patients to achieve better results with RAT. Of course, it is a false generalization that RAT is for all CP patients. For individuals with severe spasticity, contractures, or joint deformities, robotic therapy may be limited or require significant accommodations. Coexisting medical conditions (e.g., epilepsy, cardiovascular problems) or complications such as skin breakdown may sometimes limit the use of robotic devices. Diagnostic neuroimaging techniques (such as fNIRS, fMRI) could increase the knowledge of how robotic therapy can affect improvement, in parallel with a genetic analysis of the specific phenotype of each individual patient. This would be in the perspective of precision and individualized medicine that is highly desired recently.
Moreover, the complexity and cost associated with long-term hospitalization for the rehabilitation of patients with CP pose significant challenges, indicating a barrier to widespread adoption of biofeedback-integrated RAT in real-world clinical settings. The need for continuous monitoring, adjustment, and personalized feedback in RAT for CP patients may also present logistical challenges in terms of resource allocation (61) and clinical implementation (18). Clear determination of positive results on the functionality and abilities of patients with CP could be the basis for a huge investment of funds by the world health system on RAT. After more in-depth studies on a large cohort of patients with CP with RAT methodology, it will be necessary to draw up guidelines that allow the use of this therapy focusing only on patients who could potentially benefit significantly from it.
Finally, it is of fundamental importance to evaluate that general improvements in clinical assessments do not always translate directly into significant functional improvements in the daily life of rehabilitated patients (62). It should be emphasized that patients must be periodically stimulated and simultaneously monitored if the best results on their abilities and performances are to be obtained.
4.2 Future perspectives
Future research directions for the employment of robotic therapy in the treatment of CP patients should focus on several key areas to enhance efficacy and integration into daily life. Firstly, studies should aim to standardize therapy protocols, including dosage, duration, and frequency, to enable more consistent and comparable outcomes. Additionally, increasing sample sizes will allow for the application of advanced statistical methods and artificial intelligence to optimize treatment plans and personalize therapy based on individual patient profiles. Moreover, the integration of wearable devices that provide biofeedback (63) can be explored to extend the benefits of RAT beyond clinical settings. These devices, connected through the Internet of Things (IoT), can offer continuous monitoring and real-time feedback, empowering patients to practice therapeutic exercises in their daily routines and ensuring adherence to prescribed therapy. In addition, more studies are needed also to further investigate the combination of RAT with the Botulinum toxin type-A injection. Some studies included in the present review already combined the two therapies to reduce spasticity and parallelly improve motor outcome (64–66) but future investigations are however needed to confirm the effect in CP children. Furthermore, while standardizing protocols is essential for consistency, it is equally important to customize therapy to meet individual patient needs. Providing biofeedback information to robotic devices enable dynamic modulation of the robot’s behavior in real-time, tailored to the patient’s specific requirements. This approach ensures that the therapy is not only standardized but also adaptable, optimizing the therapeutic outcomes for each patient. Telemedicine can facilitate this personalized approach, allowing healthcare professionals to remotely supervise and adjust the therapy based on the data collected by wearable devices, ensuring that each patient receives the most effective and individualized care. Emphasizing these future directions will enhance the effectiveness, accessibility, and personalization of RAT for CP patients.
5 Conclusion
The results of the present review suggested that RAT can be used to improve UL functions in children with CP. Specifically in a considerable subset of the included studies, the use of RAT increased UL movements and manual dexterity such as recorded via clinical scales and tests. Moreover, in some studies the movement gain was coupled with a reduction of spasticity. The clinical improvement was also confirmed by the instrumented evaluation where an increase in many kinematics parameters was observed. Despite the heterogeneity of devices (i.e., exoskeleton or end-effectors) can represent a limitation for the synthesis about RAT efficacy, on the other hand this broad range of devices, and the possibility to modulate its parameters, can permitted a personalization of therapy according to children’s development and ability. This characteristic of RAT can be useful in children with cognitive and learning difficulties to correctly adapt the proposal aiming for a tailored rehabilitative approach. Finally, the RAT feedback provided in the form of exergaming seems to have increased participation and therapy satisfaction. However, the limited sample size, the heterogeneity of protocols (in terms of dose, duration and type of device), and the unsatisfactory study designs brings about the necessity of further randomized controlled trials to confirm our deductions. Considering strengths and limitations of RAT, to date, the use of RAT can be recommended as add-on to conventional rehabilitation to improve UL functions in children with CP, but never as a replacement of it.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
DC: Writing – review & editing. DP: Writing – original draft. MN: Writing – review & editing. ArM: Writing – review & editing. GM: Writing – review & editing. IC: Writing – review & editing. AnM: Writing – review & editing. FG: Writing – review & editing. AC: Writing – review & editing. FF: Writing – review & editing. AM: Writing – original draft. TP: Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the European Union - Next Generation EU, Mission 4, Component 1, CUP D53D23021770001, Project Name: AID2GAIT.
Acknowledgments
We thank for their collaboration Emanuele F. Russo and Maria T. La Gatta affiliated to “Gli Angeli di Padre Pio” Foundation San Giovanni Rotondo – Italy for participation on PRIN “Biofeedback based system to enhance robotic assisted gait training in children with cerebral palsy (AID2GAIT),” and A. Bisirri affiliated to “Villa Sandra Institute” for the support in the risk-of-bias assessment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1499249/full#supplementary-material
Footnotes
References
1. Paul, S, Nahar, A, Bhagawati, M, and Kunwar, AJ. A review on recent advances of cerebral palsy. Oxidative Med Cell Longev. (2022) 2022:1–20. doi: 10.1155/2022/2622310
3. Aisen, ML, Kerkovich, D, Mast, J, Mulroy, S, Wren, TAL, Kay, RM, et al. Cerebral palsy: clinical care and neurological rehabilitation. Lancet Neurol. (2011) 10:844–52. doi: 10.1016/S1474-4422(11)70176-4
4. Graham, D, Paget, SP, and Wimalasundera, N. Current thinking in the health care management of children with cerebral palsy. Med J Aust. (2019) 210:129–35. doi: 10.5694/mja2.12106
5. Reid, LB, Pagnozzi, AM, Fiori, S, Boyd, RN, Dowson, N, and Rose, SE. Measuring neuroplasticity associated with cerebral palsy rehabilitation: an MRI based power analysis. Int J Dev Neurosci. (2017) 58:17–25. doi: 10.1016/j.ijdevneu.2017.01.010
6. Castelli, E, and Fazzi, E. Recommendations for the rehabilitation of children with cerebral palsy. Eur J Phys Rehabil Med. (2015) 52:691–703.
7. Llamas-Ramos, R, Sánchez-González, JL, and Llamas-Ramos, I. Robotic systems for the physiotherapy treatment of children with cerebral palsy: a systematic review. Int J Environ Res Public Health. (2022) 19:5116. doi: 10.3390/ijerph19095116
8. Bingöl, H, and Günel, MK. Comparing the effects of modified constraint-induced movement therapy and bimanual training in children with hemiplegic cerebral palsy mainstreamed in regular school: a randomized controlled study. Arch Pediatr. (2022) 29:105–15. doi: 10.1016/j.arcped.2021.11.017
9. Houx, L, Pons, C, Saudreau, H, Dubois, A, Creusat, M, le Moine, P, et al. No pain, no gain? Children with cerebral palsy and their experience with physiotherapy. Ann Phys Rehabil Med. (2021) 64:101448. doi: 10.1016/j.rehab.2020.10.002
10. Fluet, GG, Qiu, Q, Kelly, D, Parikh, HD, Ramirez, D, Saleh, S, et al. Interfacing a haptic robotic system with complex virtual environments to treat impaired upper extremity motor function in children with cerebral palsy. Dev Neurorehabil. (2010) 13:335–45. doi: 10.3109/17518423.2010.501362
11. Fasoli, SE, Fragala-Pinkham, M, Hughes, R, Krebs, HI, Hogan, N, and Stein, J. Robotic therapy and botulinum toxin type a: a novel intervention approach for cerebral palsy. Am J Phys Med Rehabil. (2008) 87:1022–6. doi: 10.1097/PHM.0b013e31817fb346
12. Frascarelli, F, Masia, L, di Rosa, G, Cappa, P, Petrarca, M, Castelli, E, et al. The impact of robotic rehabilitation in children with acquired or congenital movement disorders. Eur J Phys Rehabil Med. (2009) 45:135–41.
13. Jafari, N, Adams, K, Tavakoli, M, Wiebe, S, and Janz, H. Usability testing of a developed assistive robotic system with virtual assistance for individuals with cerebral palsy: a case study. Disabil Rehabil Assist Technol. (2018) 13:517–22. doi: 10.1080/17483107.2017.1344884
14. Chen, Y-P, and Howard, AM. Effects of robotic therapy on upper-extremity function in children with cerebral palsy: a systematic review. Dev Neurorehabil. (2016) 19:64–71. doi: 10.3109/17518423.2014.899648
15. Krebs, HI, Ladenheim, B, Hippolyte, C, Monterroso, L, and Mast, J. Robot-assisted task-specific training in cerebral palsy. Develop Med Child Neurol. (2009) 51:140–5. doi: 10.1111/j.1469-8749.2009.03416.x
16. Riahi, N, Ruth, W, D’Arcy, RC, and Menon, C. A method for using neurofeedback to guide mental imagery for improving motor skill. IEEE Trans Neural Syst Rehabil Eng. (2022) 31:130–8. doi: 10.1109/TNSRE.2022.3218514
17. Li, H, Fu, X, Lu, L, Guo, H, Yang, W, Guo, K, et al. Upper limb intelligent feedback robot training significantly activates the cerebral cortex and promotes the functional connectivity of the cerebral cortex in patients with stroke: a functional near-infrared spectroscopy study. Front Neurol. (2023) 14:1042254. doi: 10.3389/fneur.2023.1042254
18. Ibrahim, HA, Ammar, HH, and Shalaby, R. A review of upper limb robot assisted therapy techniques and virtual reality applications. IAES Int J Artif Intelligence. (2022) 11:613. doi: 10.11591/ijai.v11.i2.pp613-623
19. Raess, L, Hawe, RL, Metzler, M, Zewdie, E, Condliffe, E, Dukelow, SP, et al. Robotic rehabilitation and transcranial direct current stimulation in children with bilateral cerebral palsy. Front Rehabil Sci. (2022) 3:843767. doi: 10.3389/fresc.2022.843767
20. Morone, G, Ghanbari Ghooshchy, S, Palomba, A, Baricich, A, Santamato, A, Ciritella, C, et al. Differentiation among bio- and augmented- feedback in technologically assisted rehabilitation. Expert Rev Med Devices. (2021) 18:513–22. doi: 10.1080/17434440.2021.1927704
21. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGP. Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
22. Eriksen, MB, and Frandsen, TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Medical Library Assoc. (2018) 106:420–31. doi: 10.5195/jmla.2018.345
23. Ouzzani, M, Hammady, H, Fedorowicz, Z, and Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. (2016) 5:1–10. doi: 10.1186/s13643-016-0384-4
24. Martino Cinnera, A, Bisirri, A, Chioccia, I, Leone, E, Ciancarelli, I, Iosa, M, et al. Exploring the potential of immersive virtual reality in the treatment of unilateral spatial neglect due to stroke: a comprehensive systematic review. Brain Sci. (2022) 12:1589. doi: 10.3390/brainsci12111589
25. Barker, TH, Stone, JC, Sears, K, Klugar, M, Tufanaru, C, Leonardi-Bee, J, et al. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evidence Synthesis. (2023) 21:494–506. doi: 10.11124/JBIES-22-00430
26. Lockwood, C, Munn, Z, and Porritt, K. Qualitative research synthesis: methodological guidance for systematic reviewers utilizing meta-aggregation. JBI Evidence Implement. (2015) 13:179–87. doi: 10.1097/XEB.0000000000000062
27. Munn, Z, Barker, TH, Moola, S, Tufanaru, C, Stern, C, McArthur, A, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evidence Synthesis. (2020) 2020:2127. doi: 10.11124/JBISRIR-D-19-00099
28. Cimolin, V, Germiniasi, C, Galli, M, Condoluci, C, Beretta, E, and Piccinini, L. Robot-assisted upper limb training for hemiplegic children with cerebral palsy. J Dev Phys Disabil. (2019) 31:89–101. doi: 10.1007/s10882-018-9632-y
29. Colazza, A, Celletti, C, Cordone, G, Mariani, G, Frascarelli, F, Petrarca, M, et al. Ten months follow up after upper limb robotic training in five children with hemiplegia. Gait Posture. (2021) 90:31–2. doi: 10.1016/j.gaitpost.2021.09.016
30. El-Shamy, SM. Efficacy of Armeo® robotic therapy versus conventional therapy on upper limb function in children with hemiplegic cerebral palsy. Am J Phys Med Rehabil. (2018) 97:164–9. doi: 10.1097/PHM.0000000000000852
31. Gilliaux, M, Renders, A, Dispa, D, Holvoet, D, Sapin, J, Dehez, B, et al. Upper limb robot-assisted therapy in cerebral palsy: a single-blind randomized controlled trial. Neurorehabil Neural Repair. (2015) 29:183–92. doi: 10.1177/1545968314541172
32. Keller, JW, and Van Hedel, HJA. Weight-supported training of the upper extremity in children with cerebral palsy: a motor learning study. J NeuroEngineering Rehabil. (2017) 14:87. doi: 10.1186/s12984-017-0293-3
33. Kolobe, THA, and Fagg, AH. Robot reinforcement and error-based movement learning in infants with and without cerebral palsy. Phys Ther. (2019) 99:677–88. doi: 10.1093/ptj/pzz043
34. Krebs, HI, Fasoli, SE, Dipietro, L, Fragala-Pinkham, M, Hughes, R, Stein, J, et al. Motor learning characterizes habilitation of children with hemiplegic cerebral palsy. Neurorehabil Neural Repair. (2012) 26:855–60. doi: 10.1177/1545968311433427
35. Kuo, F-L, Lee, H-C, Hsiao, H-Y, and Lin, J-C. Robotic-assisted hand therapy for improvement of hand function in children with cerebral palsy: a case series study. Eur J Phys Rehabil Med. (2020) 56:237–42. doi: 10.23736/s1973-9087.20.05926-2
36. Kuroda, MM, Iwasaki, N, Yoshikawa, K, Takeuchi, R, Mataki, Y, Nakayama, T, et al. Voluntary-assisted upper limb training for severe cerebral palsy using robotics devices and neuromuscular electrical stimulation: three case reports. PRM. (2022) 7:n/a. doi: 10.2490/prm.20220050
37. Peri, E, Biffi, E, Maghini, C, Servodio Iammarrone, F, Gagliardi, C, Germiniasi, F, et al. Quantitative evaluation of performance during robot-assisted treatment. Methods Inf Med. (2016) 55:84–8. doi: 10.3414/ME14-01-0126
38. Roberts, H, Shierk, A, Clegg, NJ, Baldwin, D, Smith, L, Yeatts, P, et al. Constraint induced movement therapy camp for children with hemiplegic cerebral palsy augmented by use of an exoskeleton to play games in virtual reality. Phys Occup Ther Pediatr. (2020) 41:150–65. doi: 10.1080/01942638.2020.1812790
39. Qiu, Q, Adamovich, S, Saleh, S, Lafond, I, Merians, AS, and Fluet, GG. A comparison of motor adaptations to robotically facilitated upper extremity task practice demonstrated by children with cerebral palsy and adults with stroke. IEEE Int Conf Rehabil Robot. (2011) 2011:5975431. doi: 10.1109/ICORR.2011.5975431
40. Germanotta, M, Cortellini, L, Insalaco, S, and Aprile, I. Effects of upper limb robot-assisted rehabilitation compared with conventional therapy in patients with stroke: preliminary results on a daily task assessed using motion analysis. Sensors. (2023) 23:3089. doi: 10.3390/s23063089
41. Constantino, C, May, E, Flanagan, A, Altiok, H, and Harris, GD. Myoelectric elbow-wrist-hand orthosis for an adolescent with hemiparesis: a case report. J Prosthetics Orthotics. (2020) 34:e99–e102. doi: 10.1097/jpo.0000000000000330
42. Gupta, A, Mondal, AK, and Gupta, M. Kinematic, dynamic analysis and control of 3 DOF upper-limb robotic exoskeleton. J Eur Des Systèmes Automatisés. (2019) 52:297–304. doi: 10.18280/jesa.520311
43. MacIntosh, A, Lam, E, Vigneron, V, Vignais, N, and Biddiss, E. Biofeedback interventions for individuals with cerebral palsy: a systematic review. Disabil Rehabil. (2018) 41:2369–91. doi: 10.1080/09638288.2018.1468933
44. Su, Y, and Liang, S. Intelligent medical rehabilitation training instrument based on movement coordination. IEEE Access. (2020) 8:73373–87. doi: 10.1109/access.2020.2986800
45. Liu, Q, Xue, J, Zhao, P, Ling, Y, Liu, S, du, Y, et al. Effect of Electromyographic biofeedback therapy on muscle strength recovery in children with Guillain–Barré syndrome. J Healthcare Engin. (2021) 2021:1–6. doi: 10.1155/2021/1220368
46. Stubberud, A, Varkey, E, McCrory, DC, Pedersen, SA, and Linde, M. Biofeedback as prophylaxis for pediatric migraine: a Meta-analysis. Pediatrics. (2016) 138. doi: 10.1542/peds.2016-0675
47. Hawksley, J, Cavanna, AE, and Nagai, Y. The role of the autonomic nervous system in Tourette syndrome. Front Neurosci. (2015) 9:117. doi: 10.3389/fnins.2015.00117
48. Meng, Q, Yue, Y, Li, S, and Yu, H. Electromyogram-based motion compensation control for the upper limb rehabilitation robot in active training. Mech Sci. (2022) 13:675–85. doi: 10.5194/ms-13-675-2022
49. Sturma, A, Hruby, LA, Prahm, C, Mayer, JA, and Aszmann, OC. Rehabilitation of upper extremity nerve injuries using surface EMG biofeedback: protocols for clinical application. Front Neurosci. (2018) 12:906. doi: 10.3389/fnins.2018.00906
50. Lehrer, PM, and Eddie, D. Dynamic processes in regulation and some implications for biofeedback and biobehavioral interventions. Appl Psychophysiol Biofeedback. (2013) 38:143–55. doi: 10.1007/s10484-013-9217-6
51. Beani, E, Menici, V, Ferrari, A, Cioni, G, and Sgandurra, G. Feasibility of a home-based action observation training for children with unilateral cerebral palsy: an explorative study. Front Neurol. (2020) 11:16. doi: 10.3389/fneur.2020.00016
52. Burin-Chu, S. Effectiveness of virtual reality interventions of the upper limb in children and young adults with cerebral palsy: a systematic review with Meta-analysis. Clin Rehabil. (2023) 38:15–33. doi: 10.1177/02692155231187858
53. Caikovska, K, and Lauznis, J. Visualisation method of electromyogram in rehabilitation and training of patients with cerebral palsy. Technol Comput Control. (2015) 16:45. doi: 10.7250/tcc.2015.006
54. Iosa, M, Martino Cinnera, A, Capone, F, Cruciani, A, Paolucci, M, di Lazzaro, V, et al. Clinical interpretation of working volume and weight support in upper limb robotic neurorehabilitation after stroke. Appl Sci. (2021) 11. doi: 10.3390/app112412123
55. Galeoto, G, Berardi, A, Mangone, M, Tufo, L, Silvani, M, González-Bernal, J, et al. RETRACTED: assessment capacity of the Armeo® power: cross-sectional study. Technologies. (2023) 11. doi: 10.3390/technologies11050125
56. Francisco-Martínez, C, Prado-Olivarez, J, Padilla-Medina, JA, Díaz-Carmona, J, Pérez-Pinal, FJ, Barranco-Gutiérrez, AI, et al. Upper limb movement measurement Systems for Cerebral Palsy: a systematic literature review. Sensors. (2021) 21. doi: 10.3390/s21237884
57. Martino Cinnera, A, Picerno, P, Bisirri, A, Koch, G, Morone, G, and Vannozzi, G. Upper limb assessment with inertial measurement units according to the international classification of functioning in stroke: a systematic review and correlation meta-analysis. Top Stroke Rehabil. (2024) 31:66–85. doi: 10.1080/10749357.2023.2197278
58. He, Y, Cai, S, Peng, T, Qiao, Y, Wu, N, and Xu, K. Machine learning enables update to pediatric neurorehabilitation. Pediatr Investig. (2024) 8:237–239. doi: 10.1002/ped4.12418
59. Zheng, K, and Zhang, Q. Research on configuration design and intelligent compliance control of reconfigurable modular flexible upper limb rehabilitation robot. Int J Adv Robot Syst. (2023) 20:172988062311756. doi: 10.1177/17298806231175600
60. Fraile, JC, Pérez-Turiel, J, Baeyens, E, Viñas, P, Alonso, R, Cuadrado, A, et al. E2Rebot: a robotic platform for upper limb rehabilitation in patients with Neuromotor disability. Adv Mech Eng. (2016) 8:168781401665905. doi: 10.1177/1687814016659050
61. Martino cinnera, A, Palomba, A, Paci, M, Marino, D, la Rosa, G, Gimigliano, F, et al. A three-year update on guidelines for upper limb robotic rehabilitation after stroke. Eur J Phys Rehabil Med. (2024) 60:556–8. doi: 10.23736/S1973-9087.24.08451-X
62. Lang, CE, Holleran, CL, Strube, MJ, Ellis, TD, Newman, CA, Fahey, M, et al. Improvement in the capacity for activity versus improvement in performance of activity in daily life during outpatient rehabilitation. JNPT. (2023) 47:16–25. doi: 10.1097/NPT.0000000000000413
63. Lupo, A, Cinnera, AM, Pucello, A, Iosa, M, Coiro, P, Personeni, S, et al. Effects on balance skills and patient compliance of biofeedback training with inertial measurement units and exergaming in subacute stroke: a pilot randomized controlled trial. Funct Neurol. (2018) 33:131–6.
64. Hyakutake, K, Morishita, T, Saita, K, Fukuda, H, Abe, H, Ogata, T, et al. Effect of robot-assisted rehabilitation to botulinum toxin a injection for upper limb disability in patients with chronic stroke: a case series and systematic review. Neurol Med Chir. (2022) 62:35–44. doi: 10.2176/nmc.oa.2020-0408
65. Martino Cinnera, A, Pucello, A, Lupo, A, Gimigliano, F, Mammucari, E, Cicero, DL, et al. Upper limb motor improvement in chronic stroke after combining botulinum toxin a injection and multi-joints robot-assisted therapy: a case report. Oxf Med Case Rep. (2019) 2019:omz097. doi: 10.1093/omcr/omz097
66. Hung, J-W, Yen, CL, Chang, KC, Chiang, WC, Chuang, IC, Pong, YP, et al. A pilot randomized controlled trial of botulinum toxin treatment combined with robot-assisted therapy, Mirror therapy, or active control treatment in patients with spasticity following stroke. Toxins. (2022) 14:415. doi: 10.3390/toxins14060415
Keywords: rehabilitation, robotics, hemiplegia, upper extremities, exoskeleton, endeffector
Citation: Cardone D, Perpetuini D, Di Nicola M, Merla A, Morone G, Ciancarelli I, Moretti A, Gimigliano F, Cichelli A, De Flaviis F, Martino Cinnera A and Paolucci T (2025) Robot-assisted upper limb therapy for personalized rehabilitation in children with cerebral palsy: a systematic review. Front. Neurol. 15:1499249. doi: 10.3389/fneur.2024.1499249
Edited by:
Pasquale Striano, Giannina Gaslini Institute (IRCCS), ItalyReviewed by:
Agata Polizzi, University of Catania, ItalyMariachiara Patelli, Giannina Gaslini Institute (IRCCS), Italy
Copyright © 2025 Cardone, Perpetuini, Di Nicola, Merla, Morone, Ciancarelli, Moretti, Gimigliano, Cichelli, De Flaviis, Martino Cinnera and Paolucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alex Martino Cinnera, YS5tYXJ0aW5vQGhzYW50YWx1Y2lhLml0
 Daniela Cardone
Daniela Cardone David Perpetuini
David Perpetuini Marta Di Nicola
Marta Di Nicola Arcangelo Merla1
Arcangelo Merla1 Giovanni Morone
Giovanni Morone Irene Ciancarelli
Irene Ciancarelli Antimo Moretti
Antimo Moretti Alice Cichelli
Alice Cichelli Alex Martino Cinnera
Alex Martino Cinnera