- 1Department of Neurology, Deyang People’s Hospital, Deyang, China
- 2Sichuan Clinical Research Center for Neurological Diseases, Deyang, China
Objectives: Patients with minor ischemic stroke (MIS) have substantial disability rates at 90 days. Our study aimed to explore the association between the systemic inflammation response index (SIRI) and 3-month functional outcomes in patients with MIS.
Methods: We conducted a prospective observational study in patients with MIS [defined as a National Institutes of Health Stroke Scale (NIHSS) score of 0–3] admitted within 24 h from symptoms onset. Blood samples for the SIRI measurement were collected on admission. The primary outcome measure was poor outcomes at 90 days (defined as a modified Rankin Scale score of 2–6). Univariate and multivariate logistic analyses were performed to assess the association between the SIRI and the risk of 3-month poor outcomes.
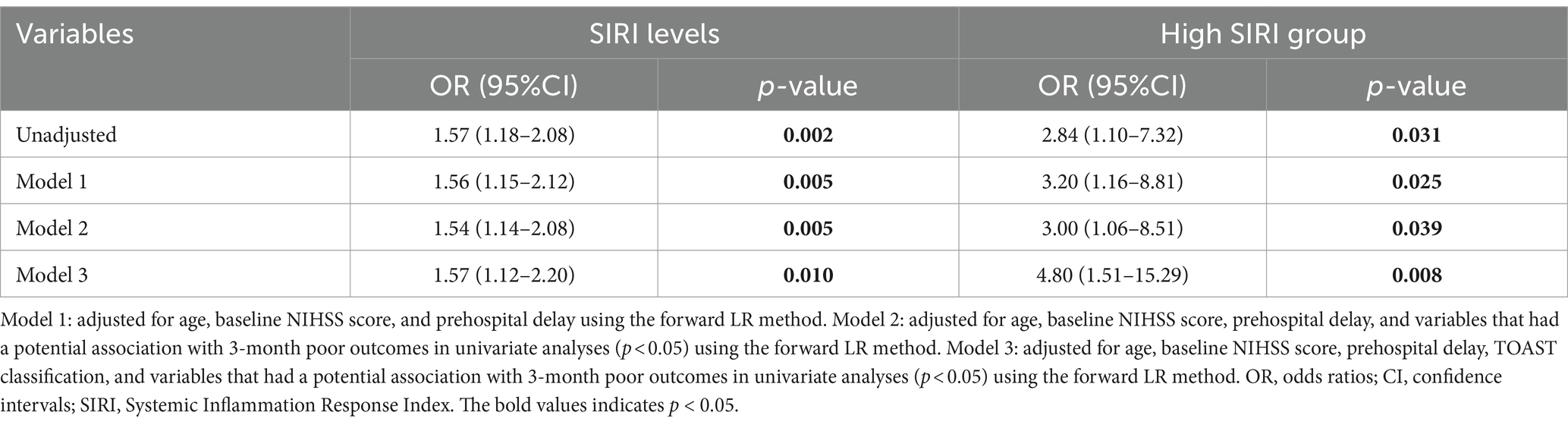
Results: A total of 152 patients with MIS were enrolled, of which 24 cases (15.8%) had poor outcomes at 90 days. The median SIRI level was 1.27 [interquartile range (IQR), 0.77–1.92, ×10^9 /L] on admission. MIS patients with poor outcomes had higher levels of the SIRI than patients with good outcomes (poor outcomes: median, 1.93, IQR: 1.17–3.28, ×10^9 /L; good outcomes: median, 1.21, IQR: 0.71–1.80, ×10^9 /L; p = 0.003). The high SIRI level group (SIRI >1.27 × 10^9 /L) had significantly higher rates of poor outcomes at 90 days (22.4% vs. 9.2%, p = 0.026). After adjusting for age, baseline NIHSS score, prehospital delay, Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification, and other confounders in multivariate analyses, an elevated SIRI level remained independently associated with an increased risk of poor outcomes in patients with MIS [odds ratio (OR): 1.57, 95% confidence interval (CI): 1.12–2.20; p = 0.010]. Meanwhile, a high level of the SIRI (>1.27 × 10^9/L) was still an independent risk factor for 3-month poor outcomes (OR: 4.80, 95%CI: 1.51–15.29; p = 0.008) in MIS patients.
Conclusion: Disability at 90 days was common in patients with MIS. An elevated SIRI was associated with poor outcomes in MIS patients. The SIRI might be a promising biomarker candidate that can help identify high-risk MIS patients with poor outcomes for reaching individual therapeutic decisions in clinical trials.
Introduction
Minor ischemic stroke (MIS) is fairly common and accounts for approximately 30% of all strokes (1). Although the outcomes of most patients with MIS are favorable, there are still a few individuals who suffer poor outcomes (2). Several studies suggested that approximately 11–34% of patients with MIS had significant disability at hospital discharge (3–6). It also has been demonstrated that patients with MIS have substantial rates (29%) of disability at 90 days (7). Therefore, it is essential to identify the MIS patients who are at a higher risk of poor outcomes in the early stage of clinical practice.
It has been demonstrated that brain inflammation might continuously shape pathophysiological processes after cerebral ischemic injury and is closely linked to the development, progression, and outcome of acute ischemic stroke (AIS). Inflammation can induce secondary brain injury by exacerbating blood–brain barrier damage, leukocyte infiltration, secretion of multiple inflammatory mediators, microvascular failure, brain edema, and neuronal cell death (8–11). Therefore, inflammation is currently considered to be one of the major targets for developing new stroke therapies, with broad application prospects (12, 13). According to a recent systematic review, blood-based biomarkers of inflammation are expected to be one of the most promising biomarkers to predict functional outcomes in stroke patients (14). As inflammatory indicators composed of the ratios of blood cell subgroups, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio have been extensively studied as prognostic tools (15–18); however, the results of some studies were inconsistent (17, 18). The systemic inflammation response index (SIRI) is a new and more comprehensive marker based on the composition ratio of peripheral blood neutrophil, monocyte, and lymphocyte counts (calculated by neutrophil count × monocyte count/lymphocyte count) (19). Several studies have investigated the relationship between the SIRI and the outcomes of patients with AIS (19–25). A retrospective study based on the Medical Information Mart for Intensive Care IV (MIMIC-IV) database found that an elevated SIRI was associated with a higher risk of mortality among intensive care unit patients with AIS (19). Some studies have suggested that higher admission SIRI was associated with poor functional outcomes of AIS (20–24), especially for those AIS patients who were treated with intravenous thrombolysis or endovascular therapy (20–23), but another study has reached a different conclusion (25). Fewer studies have attempted to investigate the association between the SIRI and the prognosis in patients with MIS.
To date, whether SIRI levels are associated with functional outcomes in patients with MIS has not been elucidated. Therefore, the aim of the current study was to explore the potential association between SIRI levels and functional outcomes in patients with MIS.
Methods
Study design and subjects
A total of 152 consecutive MIS patients who had magnetic resonance diffusion-weighted imaging (DWI) evidence of new-onset cerebral infarction and were hospitalized within 24 h from symptoms onset were enrolled in this observational study from 1 March 2020 to 31 June 2021. MIS was defined as a baseline total National Institutes of Health Stroke Scale (NIHSS) score of ≤3 (26). All patients received an extensive stroke etiologic workup and were routinely followed up via telephone or mail after 3 months. We excluded cases with incomplete hospital records or missing imaging data. We also excluded cases with a pre-stroke modified Rankin Scale (mRS) score of ≥2 and those who lived with disabilities (27). Meanwhile, patients who had premorbid conditions such as infections, connective tissue diseases, malignancies, or other disorders that might affect the blood system were excluded as well. Detailed methods for including and excluding patients and the flow diagram have been described in our previous study (28). The study protocol was approved by the Ethics Committee of Deyang People’s Hospital (Reference No. 2019–01-142-K01) and registered (unique Identifier: ChiCTR2000029902).1 Written informed consent was obtained from all patients before they were enrolled.
Data collection
We collected patient data using a standardized form that included age, sex, prehospital delay, baseline NIHSS score, systolic and diastolic blood pressure on admission, baseline serum glucose, vascular risk factors, and potential stroke etiology, which have been elaborated in our previous study (29). In-hospital treatments analyzed in our study included intravenous thrombolysis, antiplatelet therapy, antihypertensives, antidiabetics, and statins. Intravenous thrombolysis was performed according to the Chinese guidelines, which had similar inclusion and exclusion criteria compared to the American guidelines (30, 31). The final treatment decision was made in consultation with a neurologist and the patient’s family. Antiplatelet therapies were administered at the physicians’ discretion. Patients included in the present study received either (1) aspirin or clopidogrel only, or (2) clopidogrel plus aspirin (dual antiplatelet therapy) at admission.
Laboratory measurements and SIRI levels
Blood samples were collected on admission from the cubital vein from each patient before initial treatment. The absolute counts of white blood cell subgroups such as lymphocytes, monocytes, and neutrophils were assessed, as well as C-reactive protein (CRP). We calculated the level of SIRI as follows: SIRI = neutrophil count × monocyte count/lymphocyte count (19).
Assessment of clinical outcomes
The degree of disability was measured by using a modified Rankin Scale (mRS) score at 90 days after admission (27). The primary outcome of the current study was poor outcomes at 90 days, defined as a mRS score of 2–6 (7). The secondary outcome was 3-month mortality and recurrent ischemic stroke during the first 3 months after admission.
Statistical analyses
Continuous variables are presented as means with standard deviations (SD) or medians with interquartile ranges (IQR), and categorical variables are presented as frequencies with percentages. The normality of data was tested using a Shapiro–Wilk test. The χ2 tests or Fisher’s exact tests were used for differences in categorical data, while Student’s t-tests or the Mann–Whitney U-test were used for differences in continuous data. Baseline characteristics, laboratory values, and in-hospital treatment were compared between MIS patients with good or poor outcomes.
Univariate analyses comparing the baseline characteristics and clinical outcomes between low SIRI and high SIRI level groups were performed. Multivariate logistic regression analyses were performed to identify the association between the SIRI and 3-month poor outcomes of MIS patients in three different models. Model 1 was adjusted for age, baseline NIHSS score, and prehospital delay using the forward logistic regression (LR) method. Model 2 was adjusted for variables in model 1 and variables that had a potential association with 3-month poor outcomes in univariate analyses (p < 0.05). Model 3 was adjusted for variables in model 1, TOAST classification, and variables that had a potential association with 3-month poor outcomes in univariate analyses (p < 0.05).
All statistical analyses were performed using SPSS v21.0 (IBM, Chicago, IL, United States), the statistical software packages R (The R Foundation, version 3.4.3),2 and EmpowerStats (X&Y Solutions, Inc., Boston, MA, United States),3 which have been described in our previous study (32). A two-sided p-value of <0.05 was considered to be statistically significant.
Results
During the study period, 798 AIS patients without a pre-stroke mRS score of ≥2 were consecutively registered. Of these, 152 (19.0%) who were admitted within 24 h were enrolled in the study [mean age: 67.7 ± 11.1 years; 104 (68.4%) male; median baseline NIHSS score: 2, IQR, 1–3]. The median SIRI level was 1.27 [interquartile range (IQR), 0.77–1.92] on admission. Overall, 30 (19.7%) cases were treated with intravenous thrombolysis, and 109 (71.7%) cases were treated with dual antiplatelet therapy after admission. All enrolled patients completed a 3-month follow-up. Three (2.0%) patients died, and 24 cases (15.8%) had poor outcomes (mRS score of 2–6) at 90 days.
Baseline characteristics and in-hospital treatment between MIS patients with good or poor outcomes
The baseline characteristics, in-hospital treatment, and the median SIRI levels in MIS patients with good or poor outcomes are summarized in Table 1. MIS patients with poor outcomes had higher levels of the SIRI than patients with good outcomes (poor outcomes: median, 1.93, IQR, 1.17–3.28; good outcomes: median, 1.21, IQR, 0.71–1.80; p = 0.003). The SIRI levels between groups are shown as violin plots in Figure 1. Meanwhile, as compared to the good outcome group, patients with poor outcomes were older (73.4 ± 11.1 vs. 66.6 ± 10.9, p = 0.006) and less frequently received dual antiplatelet therapy (50.0% vs. 75.8%, p = 0.010). There was no difference in the sex, prehospital delay, the NIHSS score on admission, baseline systolic and diastolic blood pressure, baseline serum glucose, vascular risk factors, TOAST classification, other laboratory values, and other in-hospital treatments between the two groups (all p > 0.05).
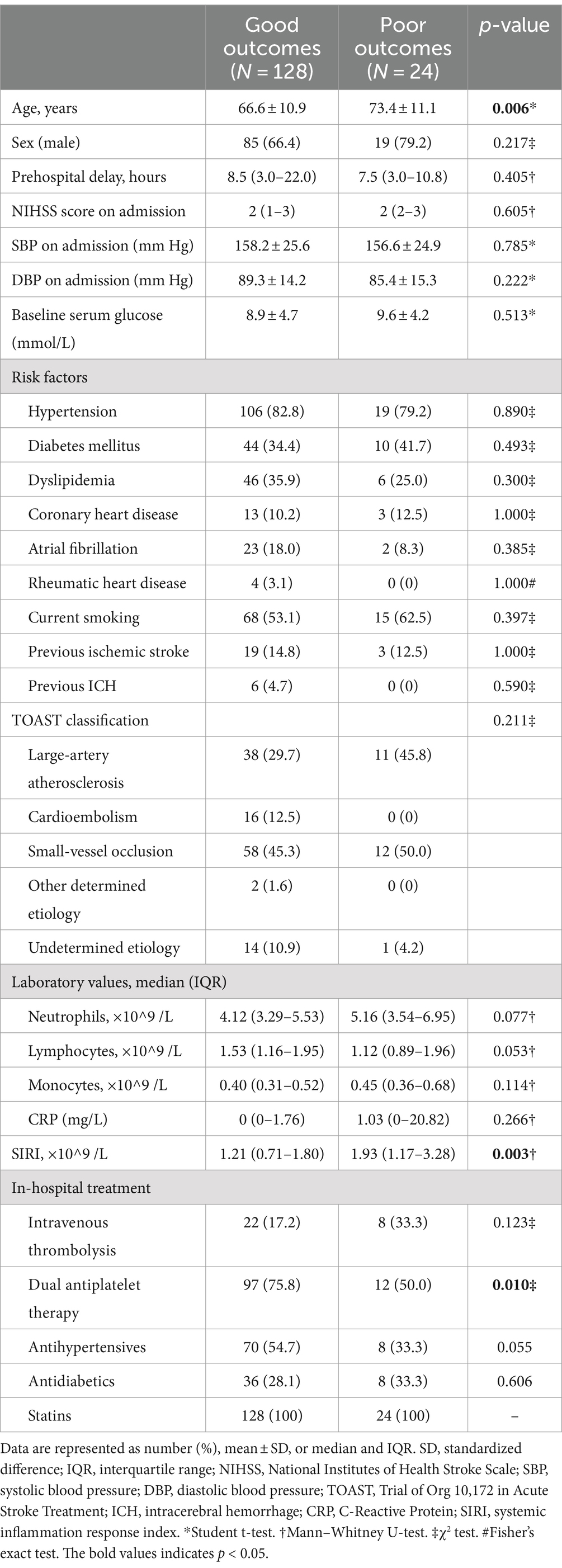
Table 1. Baseline characteristics and in-hospital treatment between MIS patients with good or poor outcomes.
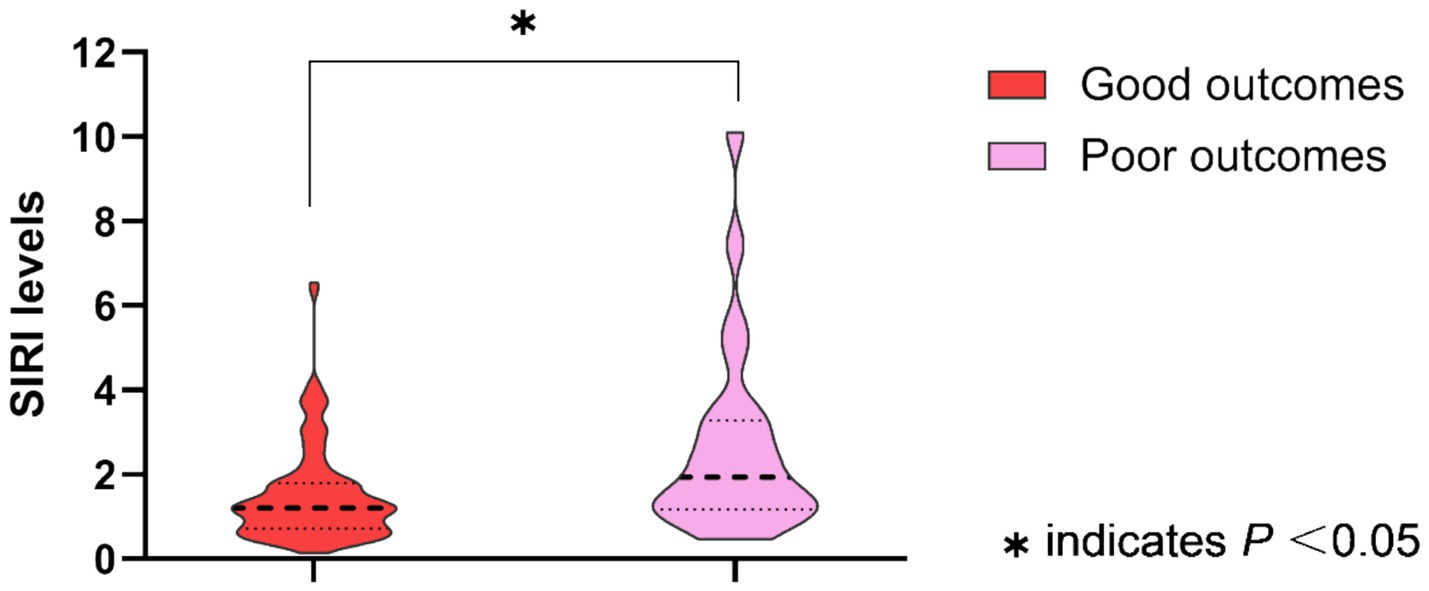
Figure 1. Systemic inflammation response index (SIRI) levels between groups are shown as violin plots (MIS patients with good outcomes vs. poor outcomes, p = 0.003).
Baseline characteristics and clinical outcomes between low SIRI and high SIRI level groups
As shown in Table 2, MIS patients were divided into two groups according to the baseline SIRI levels. The high SIRI level group was defined as cases who had baseline SIRI levels greater than the median SIRI levels (>1.27), while the low SIRI level group was defined as cases who had baseline SIRI levels less than or equal to the median SIRI levels (≤1.27). Compared to the low SIRI level group, patients in the high SIRI level group had longer prehospital delay (11.5 vs. 6.0 h, p < 0.001). As for the laboratory data, the high SIRI level group had higher levels of neutrophil counts and monocyte counts, and lower levels of lymphocyte counts (all p-values <0.001). There was no difference in age, sex, initial NIHSS score on admission, baseline systolic and diastolic blood pressure, baseline serum glucose, vascular risk factors, TOAST classification, CRP levels, or in-hospital treatment between the high and low SIRI level groups (all p > 0.05). For the clinical outcomes, the high SIRI level group (SIRI >1.27) had a significantly higher rate of poor outcomes at 90 days (22.4% vs. 9.2%, p = 0.026), and there was no difference in the incidence rate of recurrent ischemic stroke or 3-month mortality between the two groups (all p > 0.05). The distributions of the mRS score at 3 months for MIS patients between low SIRI and high SIRI level groups are displayed in Figure 2.
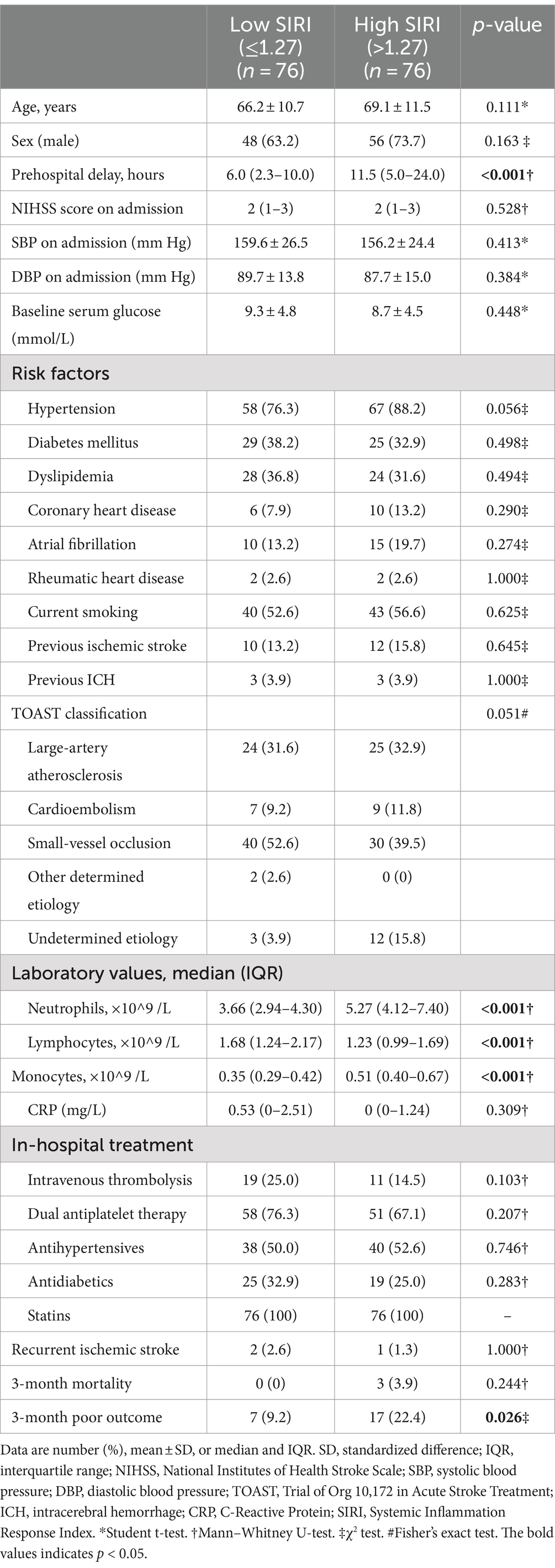
Table 2. Baseline characteristics and clinical outcomes between low SIRI and high SIRI level groups.
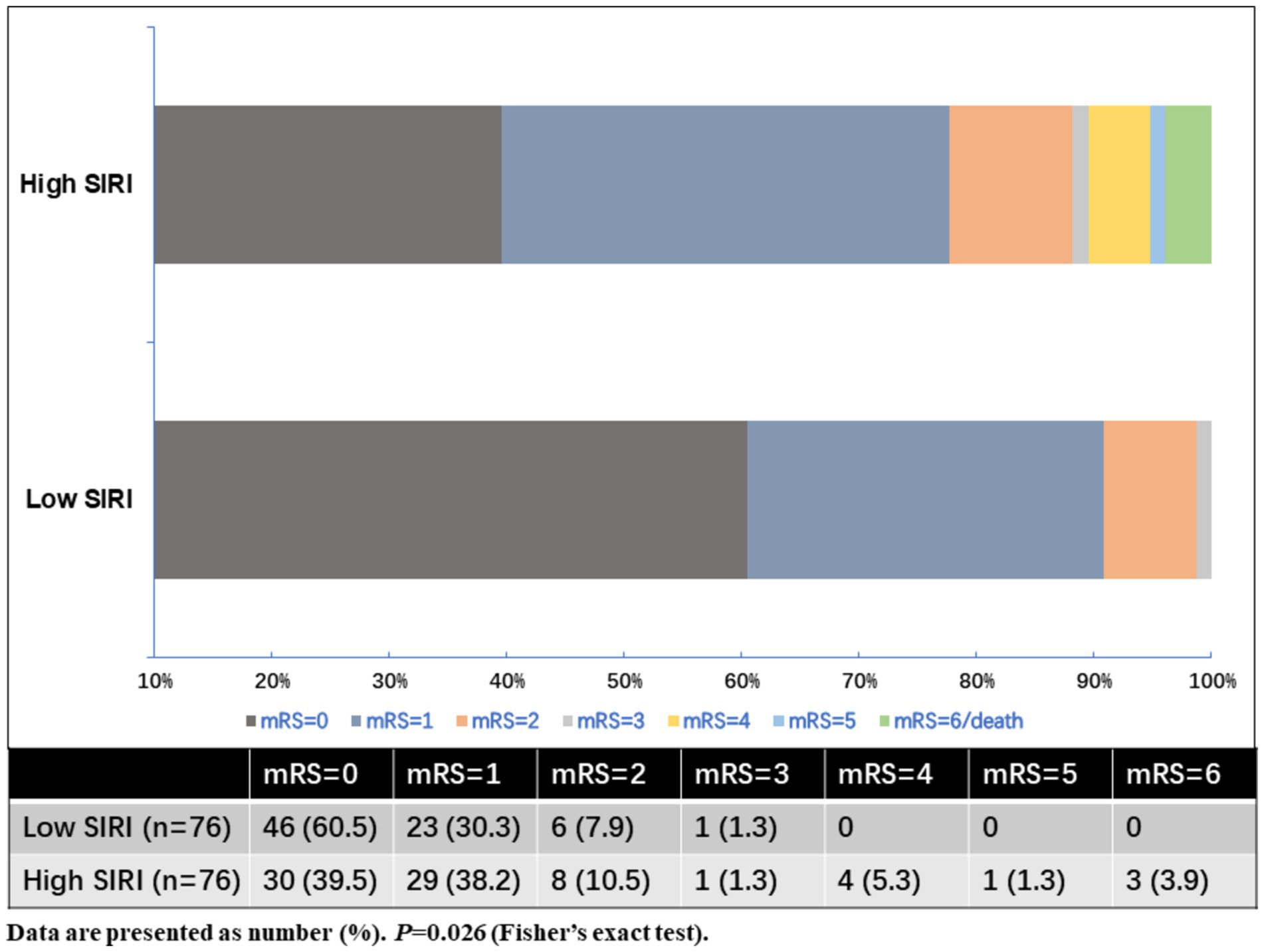
Figure 2. Distributions of the mRS score at 3 months for MIS patients between low SIRI and high SIRI level groups.
Multivariate analyses for the association between SIRI and 3-month poor outcomes in MIS patients
Multivariate logistic regression analyses were performed to identify the association between SIRI and 3-month poor outcomes of MIS patients in three different models, as shown in Table 3. After adjusting for age, baseline NIHSS score, and prehospital delay (model 1), both elevated SIRI level (p = 0.005) and high SIRI level groups (p = 0.025) were significantly associated with 3-month poor outcomes in MIS patients. When variables that had a potential association with 3-month poor outcomes in univariate analyses were further added in the multivariate logistic regression (model 2), both elevated SIRI level (p = 0.005) and high SIRI level groups (p = 0.039) were also significantly associated with 3-month poor outcomes. After adjusting for age, baseline NIHSS score, prehospital delay, TOAST classification, and other potential confounders (model 3), elevated SIRI level remained independently associated with an increased risk of poor outcomes [odds ratio (OR): 1.57, 95% confidence interval (CI): 1.12–2.20; p = 0.010]. Meanwhile, a high level (>1.27) of SIRI was still an independent risk factor for 3-month poor outcomes (OR: 4.80, 95%CI: 1.51–15.29; p = 0.008).
Discussion
In China, there are approximately 3 million new-onset cases of stroke each year, with approximately 1 million of these cases being MIS (33, 34). Since the initial stroke severity assessed by the NIHSS score is the most crucial prognostic indicator for stroke patients, the prognosis of MIS patients is generally good (2). However, prospective observational studies have shown that 4.5 to 26.4% of MIS patients experience early neurological deterioration and develop disability (7, 35–37). In addition, approximately 11 to 34% of MIS patients had significant disability at the time of discharge (3–6). It also has been demonstrated that the incidence rate of disability in MIS patients can be as high as 29% at 90 days (7, 38, 39). Our study provides further evidence that disability at 90 days was common in patients with MIS. The incidence rate of disability at 90 days in patients with MIS is 15.8% in our cohort. The differences in the incidence of poor outcomes in patients with MIS may reflect the heterogeneity in the demographics of enrolled patients (age, sex, and race), prehospital delay, the definition of MIS, and the way in which poor outcomes of MIS patients are defined, highlighting the need for a standardized definition of MIS. Regarding the risk factors associated with unfavorable outcomes in MIS patients, several factors have been proposed, including advanced age, female sex, increased baseline NIHSS score, stroke etiology, early neurological deterioration, acute infarct growth, the penumbra volume (>5 cm3) on computed tomography perfusion, and medication adherence (7, 38–42). However, to date, the risk factors associated with poor outcomes in patients with MIS have not been clearly elucidated. A proper understanding of the factors associated with unfavorable outcomes in patients with MIS could provide valuable insights for close monitoring of MIS patients. Moreover, early targeting of patients at a higher risk of poor outcomes is of great importance for improving the outcome of MIS.
In the present study, we found that MIS patients with poor outcomes had a higher level of SIRI than patients with good outcomes (1.93 vs. 1.21, ×10^9 /L). The high SIRI level group (defined as cases who had a baseline SIRI level greater than the median SIRI level, >1.27 × 10^9/L) had a significantly higher rate of poor outcomes at 90 days (22.4% vs. 9.2%). The SIRI is a new and more comprehensive inflammatory marker based on the composition ratio of peripheral blood neutrophil, monocyte, and lymphocyte counts (19). In our study, the high SIRI level group had higher levels of neutrophil counts and monocyte counts and lower levels of lymphocyte counts. After the onset of ischemic stroke, the neutrophils are the first innate immune cells infiltrating into the brain lesions through adhesion to the endothelial cells and migration (43). Neutrophils can aggravate the inflammation of the brain by releasing a variety of pro-inflammatory mediators, leading to secondary injury of brain tissue (44). Cerebral ischemia and hypoxia injury can stimulate monocytes to generate interleukin-6 (IL-6), tumor necrosis factor (TNF), and other inflammatory mediators, which further aggravate cerebral ischemia and hypoxia. Monocytes can also activate platelets to become platelet–monocyte aggregates and promote thrombosis and cerebral vascular occlusion, causing hemodynamic changes and exacerbating cerebral ischemia injury (45). Lymphocytes are involved in coordinating the inflammatory response. The role of lymphocytes in stroke is complicated. T regulatory cells (Tregs) are usually involved in inhibiting inflammation and regulating and maintaining peripheral immune tolerance and homeostasis. Moreover, Tregs secreting cytokine IL-10 have a protective effect on stroke. Experiments have demonstrated that animals with increased numbers of Tregs after stroke show better outcomes (46). The results of our study suggested that inflammation in the early stage of stroke might play an important role in the development of poor functional outcomes in MIS. Our findings also support the view that inflammation is one of the major targets for developing new stroke treatments in MIS patients in the future. As we know, a recently published randomized controlled trial (Colchicine in High-risk Patients with Acute Minor-to-moderate Ischemic Stroke or Transient Ischemic Attack, CHANCE-3) showed that the anti-inflammatory agent colchicine could not reduce the risk of subsequent stroke and poor functional outcomes (mRS > 1) within 90 days among patients with acute non-cardioembolic minor-to-moderate ischemic stroke or transient ischemic attack (47). The CHANCE-3 trial included patients with a baseline concentration for high-sensitivity C-reactive protein at least 2 mg/L (47). The negative results of the CHANCE-3 trial suggested that the optimal anti-inflammatory strategy in MIS and the biomarkers most suitable for screening high-risk MIS patients with poor outcomes still need to be further explored.
A recently published systematic review suggests that blood-based inflammatory markers might be among the most promising biomarkers for predicting functional outcomes in stroke patients (14). The SIRI, which is a more comprehensive inflammatory indicator based on the composition ratio of blood cell subgroups, has lately been explored as a novel prognostic marker for stroke (19–25, 48–49). Several studies have suggested an association between baseline SIRI level and functional outcome in patients with AIS (20–24, 49), especially for those cases treated with intravenous thrombolysis or endovascular therapy (20–23, 49), but another study came to a different conclusion (25). As a comprehensive, easily accessible, and inexpensive inflammatory marker, the SIRI might be a suitable candidate biomarker of clinical outcomes in MIS patients. However, fewer studies have attempted to elucidate the association between SIRI and outcomes in MIS. In our study, after adjusting for age, baseline NIHSS score, prehospital delay, TOAST classification, and other potential confounders in multivariate logistic regression analyses, elevated SIRI levels remained independently associated with an increased risk of poor outcomes in patients with MIS (OR: 1.57, 95% CI: 1.12–2.20). Meanwhile, a high level of the SIRI (>1.27 × 10^9/L) was still an independent risk factor for 3-month poor outcomes (OR: 4.80, 95%CI: 1.51–15.29). Our study suggested that elevated SIRI levels were associated with poor outcomes in Chinese patients with MIS. As a result, the SIRI might be a promising biomarker candidate that can help identify high-risk MIS patients with poor outcomes for reaching individual therapeutic decisions in clinical trials. Further studies with large sample sizes are needed to determine the optimal cutoff value of the SIRI as an indicator for poor outcomes in MIS and validate the SIRI as a biomarker for disability in patients with MIS.
Limitations
There are still several limitations in the present study. Thus, the results of our study should be interpreted with caution. First, it was a single hospital-based study conducted in China; the results of our study might not be generalizable to diverse populations with different genetic, demographic, and socioeconomic backgrounds. Second, the sample size of our study was small, and finally, only 24 cases suffered poor outcomes at 90 days. Although the findings are statistically significant, the small sample size may limit the generalizability and robustness of the results. Further multicenter studies with larger sample sizes are needed to confirm these findings and determine the best cutoff value of the SIRI as a predictor of poor outcomes in patients with MIS. Third, the SIRI levels may change dynamically after acute ischemic stroke. In the current study, the SIRI level was tested only one time at baseline. We did not have longitudinal data on SIRI levels during their 90-day follow-up. Further studies are needed to evaluate the association between the dynamic changes in SIRI levels and poor outcomes in MIS patients. In addition, several studies have shown an association between inflammatory biomarkers, such as high-sensitivity C-reactive protein, cytokines, and other acute-phase proteins, and poor outcomes in AIS. However, these biomarkers were not measured in the present study. In addition, our study focused only on the 90-day outcomes, but long-term follow-up would help to understand the full impact of the SIRI on disability and survival in patients with MIS. Moreover, we performed the follow-up by telephone interviews or mailed questionnaires instead of clinic visits, which might result in reporting bias. Finally, our study was only an observational study; no causal link could be drawn. Thus, well-designed multicenter studies with large sample sizes are needed to validate our findings.
Conclusion
Despite the above limitations, we conducted a prospective observational study in MIS patients hospitalized within 24 h from symptoms onset and identified that disability at 90 days was common in patients with MIS. An elevated SIRI was associated with poor outcomes in MIS patients. SIRI might be a promising biomarker candidate that can help identify high-risk MIS patients with poor outcomes for reaching individual therapeutic decisions in clinical trials.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Deyang People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. PZ: Data curation, Formal analysis, Funding acquisition, Project administration, Writing – original draft, Investigation. HC: Data curation, Funding acquisition, Project administration, Writing – original draft, Investigation. YW: Data curation, Project administration, Writing – review & editing. YH: Investigation, Supervision, Writing – review & editing. CW: Investigation, Supervision, Writing – review & editing. XY: Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Research Foundation of Chinese Stroke Association (2020017), the Science and Technology Research Foundation of Deyang City (2020SZZ069), and the Research Foundation of Chengdu University of Traditional Chinese Medicine (LH202402029) in China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MIS, minor ischemic stroke; SIRI, systemic inflammation response index; NIHSS, National Institutes of Health Stroke Scale; IQR, interquartile range; OR, odds ratio; CI, confidence interval; AIS, acute ischemic stroke; MIMIC-IV, Medical Information Mart for Intensive Care IV; DWI, diffusion-weighted imaging; mRS, modified Rankin Scale; CRP, C-reactive protein; CHANCE-3, Colchicine in High-risk Patients with Acute Minor-to-moderate Ischemic Stroke or Transient Ischemic Attack; SD, standardized difference; SBP, systolic blood pressure; DBP, diastolic blood pressure; ICH, intracerebral hemorrhage; TOAST, Trial of Org 10,172 in Acute Stroke Treatment.
Footnotes
References
1. Ayis, SA, Coker, B, Rudd, AG, Dennis, MS, and Wolfe, CDA. Predicting independent survival after stroke: a European study for the development and validation of standardised stroke scales and prediction models of outcome. J Neurol Neurosurg Psychiatry. (2013) 84:288–96. doi: 10.1136/jnnp-2012-303657
2. Adams, HP Jr, Davis, PH, Leira, EC, Chang, KC, Bendixen, BH, Clarke, WR, et al. Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the trial of org 10172 in acute stroke treatment (TOAST). Neurology. (1999) 53:126–31. doi: 10.1212/WNL.53.1.126
3. Barber, PA, Zhang, J, Demchuk, AM, Hill, MD, and Buchan, AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. (2001) 56:1015–20. doi: 10.1212/WNL.56.8.1015
4. Smith, EE, Abdullah, AR, Petkovska, I, Rosenthal, E, Koroshetz, WJ, and Schwamm, LH. Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke. Stroke. (2005) 36:2497–9. doi: 10.1161/01.STR.0000185798.78817.f3
5. van den Berg, JS, and de Jong, G. Why ischemic stroke patients do not receive thrombolytic treatment: results from a general hospital. Acta Neurol Scand. (2009) 120:157–60. doi: 10.1111/j.1600-0404.2008.01140.x
6. Hills, NK, and Johnston, SC. Why are eligible thrombolysis candidates left untreated? Am J Prev Med. (2006) 31:S210–6. doi: 10.1016/j.amepre.2006.08.004
7. Khatri, P, Conaway, MR, and Johnston, KC. Acute stroke accurate prediction study (ASAP) investigators. Ninety-day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke. (2012) 43:560–2. doi: 10.1161/STROKEAHA.110.593897
8. Candelario-Jalil, E, Dijkhuizen, RM, and Magnus, T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. (2022) 53:1473–86. doi: 10.1161/STROKEAHA.122.036946
9. De Meyer, SF, Langhauser, F, and Haupeltshofer, S. Thromboinflammation in brain ischemia: recent updates and future perspectives. Stroke. (2022) 53:1487–99. doi: 10.1161/STROKEAHA.122.038733
10. Boltze, J, and Perez-Pinzon, MA. Focused update on stroke Neuroimmunology: current Progress in preclinical and clinical research and recent mechanistic insight. Stroke. (2022) 53:1432–7. doi: 10.1161/STROKEAHA.122.039005
11. Anrather, J, and Iadecola, C. Inflammation and stroke: an overview. Neurotherapeutics. (2016) 13:661–70. doi: 10.1007/s13311-016-0483-x
12. Chamorro, Á, Dirnagl, U, Urra, X, and Planas, AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. (2016) 15:869–81. doi: 10.1016/S1474-4422(16)00114-9
13. Maida, CD, Norrito, RL, Daidone, M, Tuttolomondo, A, and Pinto, A. Neuroinflammatory mechanisms in ischemic stroke: focus on Cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci. (2020) 21:6454. doi: 10.3390/ijms21186454
14. Montellano, FA, Ungethüm, K, Ramiro, L, Nacu, A, Hellwig, S, Fluri, F, et al. Role of blood-based biomarkers in ischemic stroke prognosis: a systematic review. Stroke. (2021) 52:543–51. doi: 10.1161/STROKEAHA.120.029232
15. Goyal, N, Tsivgoulis, G, Chang, JJ, Malhotra, K, Pandhi, A, Ishfaq, MF, et al. Admission neutrophil to-lymphocyte ratio as a prognostic biomarker of outcomes in large vessel occlusion strokes. Stroke. (2018) 49:1985–7. doi: 10.1161/STROKEAHA.118.021477
16. Ren, H, Han, L, Liu, H, Wang, L, Liu, X, and Gao, Y. Decreased lymphocyte-to-monocyte ratio predicts poor prognosis of acute ischemic stroke treated with thrombolysis. Med Sci Monit. (2017) 23:5826–33. doi: 10.12659/MSM.907919
17. Gong, P, Liu, Y, Gong, Y, Chen, G, Zhang, X, Wang, S, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. (2021) 18:51. doi: 10.1186/s12974-021-02090-6
18. Sun, YY, Wang, MQ, Wang, Y, Sun, X, Qu, Y, Zhu, HJ, et al. Platelet-to-lymphocyte ratio at 24h after thrombolysis is a prognostic marker in acute ischemic stroke patients. Front Immunol. (2022) 13:1000626. doi: 10.3389/fimmu.2022.1000626
19. Dang, H, Mao, W, Wang, S, Sha, J, Lu, M, Cong, L, et al. Systemic inflammation response index as a prognostic predictor in patients with acute ischemic stroke: a propensity score matching analysis. Front Neurol. (2023) 13:1049241. doi: 10.3389/fneur.2022.1049241
20. Chen, YF, Qi, S, Yu, ZJ, Li, JT, Qian, TT, Zeng, Y, et al. Systemic inflammation response index predicts clinical outcomes in patients with acute ischemic stroke (AIS) after the treatment of intravenous thrombolysis. Neurologist. (2023) 28:355–61. doi: 10.1097/NRL.0000000000000492
21. Chu, M, Luo, Y, Wang, D, Liu, Y, Wang, D, Wang, Y, et al. Systemic inflammation response index predicts 3-month outcome in patients with mild acute ischemic stroke receiving intravenous thrombolysis. Front Neurol. (2023) 14:1095668. doi: 10.3389/fneur.2023.1095668
22. Lattanzi, S, Norata, D, Divani, AA, di Napoli, M, Broggi, S, Rocchi, C, et al. Systemic inflammatory response index and futile recanalization in patients with ischemic stroke undergoing endovascular treatment. Brain Sci. (2021) 11:1164. doi: 10.3390/brainsci11091164
23. Yi, HJ, Sung, JH, and Lee, DH. Systemic inflammation response index and systemic immune-inflammation index are associated with clinical outcomes in patients treated with mechanical Thrombectomy for large artery occlusion. World Neurosurg. (2021) 153:e282–9. doi: 10.1016/j.wneu.2021.06.113
24. Zhou, Y, Zhang, Y, Cui, M, Zhang, Y, and Shang, X. Prognostic value of the systemic inflammation response index in patients with acute ischemic stroke. Brain Behav. (2022) 12:e2619. doi: 10.1002/brb3.2619
25. Huang, L . Increased systemic immune-inflammation index predicts disease severity and functional outcome in acute ischemic stroke patients. Neurologist. (2023) 28:32–8. doi: 10.1097/NRL.0000000000000464
26. Fischer, U, Baumgartner, A, Arnold, M, Nedeltchev, K, Gralla, J, Marco de Marchis, G, et al. What is a minor stroke? Stroke. (2010) 41:661–6. doi: 10.1161/STROKEAHA.109.572883
27. de Haan, R, Limburg, M, Bossuyt, P, van der Meulen, J, and Aaronson, N. The clinical meaning of Rankin ‘handicap’ grades after stroke. Stroke. (1995) 26:2027–30. doi: 10.1161/01.STR.26.11.2027
28. Li, J, Zhang, P, Zhu, Y, Duan, Y, Liu, S, Fan, J, et al. Serum neurofilament light chain levels are associated with early neurological deterioration in minor ischemic stroke. Front Neurol. (2023) 14:1096358. doi: 10.3389/fneur.2023.1096358
29. Li, J, Wang, D, Tao, W, Dong, W, Zhang, J, Yang, J, et al. Early consciousness disorder in acute ischemic stroke: incidence, risk factors and outcome. BMC Neurol. (2016) 16:140. doi: 10.1186/s12883-016-0666-4
30. Qiu, S, and Xu, Y. Guidelines for acute ischemic stroke treatment. Neurosci Bull. (2020) 36:1229–32. doi: 10.1007/s12264-020-00534-2
31. Powers, WJ, Rabinstein, AA, and Ackerson, TAmerican Heart Association Stroke Council. Guidelines for the early Management of Patients with Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018. (2018) 49:e46–e110. doi: 10.1161/STR.0000000000000158
32. Li, J, Gao, L, Zhang, P, Liu, Y, Zhou, J, Yi, X, et al. Vulnerable plaque is more prevalent in male individuals at high risk of stroke: a propensity score-matched study. Front Physiol. (2021) 12:642192. doi: 10.3389/fphys.2021.642192
33. Wang, YL, Wu, D, Liao, X, Zhang, W, Zhao, X, and Wang, YJ. Burden of stroke in China. Int J Stroke. (2007) 2:211–3. doi: 10.1111/j.1747-4949.2007.00142.x
34. Zhao, D, Liu, J, Wang, W, Zeng, Z, Cheng, J, Liu, J, et al. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing project. Stroke. (2008) 39:1668–74. doi: 10.1161/STROKEAHA.107.502807
35. Kim, JT, Heo, SH, Yoon, W, Choi, KH, Park, MS, Saver, JL, et al. Clinical outcomes of patients with acute minor stroke receiving rescue IA therapy following early neurological deterioration. J Neurointerv Surg. (2016) 8:461–5. doi: 10.1136/neurintsurg-2015-011690
36. Yi, X, Han, Z, and Zhou, Q. 20-Hydroxyeicosatetraenoic acid as a predictor of neurological deterioration in acute minor ischemic stroke. Stroke. (2016) 47:3045–7. doi: 10.1161/STROKEAHA.116.015146
37. Ferrari, J, Knoflach, M, Kiechl, S, Willeit, J, Schnabl, S, Seyfang, L, et al. Austrian stroke unit registry collaborators. Early clinical worsening in patients with TIA or minor stroke: the Austrian stroke unit registry. Neurology. (2010) 74:136–41. doi: 10.1212/WNL.0b013e3181c9188b
38. Sato, S, Uehara, T, Ohara, T, Suzuki, R, Toyoda, K, Minematsu, K, et al. Factors associated with unfavorable outcome in minor ischemic stroke. Neurology. (2014) 83:174–81. doi: 10.1212/WNL.0000000000000572
39. Ton, MD, Phuong, DV, Thom, VT, Dung, NT, Tho, PQ, Thuan, LD, et al. Factors related to unfavorable outcome in minor ischemic stroke. J Stroke Cerebrovasc Dis. (2023) 32:107203. doi: 10.1016/j.jstrokecerebrovasdis.2023.107203
40. Liao, CH, Liao, NC, Chen, WH, Chen, HC, Chang, MH, Tsuei, YS, et al. Penumbra volume predicts unfavorable outcome in patients with acute minor stroke or transient ischemic attack. J Chin Med Assoc. (2020) 83:551–6. doi: 10.1097/JCMA.0000000000000342
41. Tan, Y, Pan, Y, Liu, L, Wang, Y, Zhao, X, Wang, Y, et al. One-year outcomes and secondary prevention in patients after acute minor stroke: results from the China National Stroke Registry. Neurol Res. (2017) 39:484–91. doi: 10.1080/01616412.2017.1322804
42. Kim, YJ, Choi, SH, Kim, TY, Park, HM, Shin, DJ, and Shin, DH. Factors associated with functional disability in patients with acute stroke excluded from alteplase administration due to minor non-disabling neurological deficits. Front Neurol. (2022) 13:1062721. doi: 10.3389/fneur.2022.1062721
43. Wang, Y, Jin, H, Wang, W, Wang, F, and Zhao, H. Myosin1f-mediated neutrophil migration contributes to acute neuroinflammation and brain injury after stroke in mice. J Neuroinflammation. (2019) 16:77. doi: 10.1186/s12974-019-1465-9
44. Cai, W, Liu, S, Hu, M, Huang, F, Zhu, Q, Qiu, W, et al. Functional dynamics of neutrophils after ischemic stroke. Transl Stroke Res. (2020) 11:108–21. doi: 10.1007/s12975-019-00694-y
45. Kanazawa, M, Ninomiya, I, Hatakeyama, M, Takahashi, T, and Shimohata, T. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int J Mol Sci. (2017) 18:2135. doi: 10.3390/ijms18102135
46. Selvaraj, UM, and Stowe, AM. Long-term T cell responses in the brain after an ischemic stroke. Discov Med. (2017) 24:323–33. doi: 10.26226/morressier.58e389b0d462b80292384db3
47. Li, J, Meng, X, Shi, FD, Jing, J, Gu, HQ, Jin, A, et al. CHANCE-3 investigators. Colchicine in patients with acute ischaemic stroke or transient ischaemic attack (CHANCE-3): multicentre, double blind, randomised, placebo controlled trial. BMJ. (2024) 385:e079061. doi: 10.1136/bmj-2023-079061
48. Zhang, Y, Xing, Z, Zhou, K, and Jiang, S. The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin Interv Aging. (2021) 16:1997–2007. doi: 10.2147/CIA.S339221
Keywords: minor ischemic stroke, prognosis, disability, systemic inflammation response index, odds ratio
Citation: Li J, Zhang P, Chen H, Wang Y, Han Y, Wang C and Yi X (2024) Elevated systemic inflammation response index is associated with poor outcomes in minor ischemic stroke. Front. Neurol. 15:1492224. doi: 10.3389/fneur.2024.1492224
Edited by:
Marzia Baldereschi, National Research Council (CNR), ItalyReviewed by:
Faheem Shehjar, University of Toledo Medical Center, United StatesAlessandro Sodero, Careggi University Hospital, Italy
Copyright © 2024 Li, Zhang, Chen, Wang, Han, Wang and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Li, bGlqaWU4NjAxMTRAMTYzLmNvbQ==
 Jie Li
Jie Li Ping Zhang
Ping Zhang Hong Chen
Hong Chen Yanfen Wang1,2
Yanfen Wang1,2 Chun Wang
Chun Wang Xingyang Yi
Xingyang Yi