- 1Department of Neurology, Taizhou Hospital of Zhejiang Province, Affiliated to Wenzhou Medical University, Linhai, Zhejiang, China
- 2Department of Neurology, Saarland University, Homburg, Germany
Background: Perioperative ischemic stroke is a rare but devastating complication. Mechanical thrombectomy is a promising therapeutic method, but very little data is available on its efficacy and safety. This study aims to answer this question by comparing the clinical outcomes of perioperative and community-onset stroke patients after endovascular therapy.
Methods: A retrospective cohort study was conducted on a total of 35 perioperative and 584 community-onset acute ischemic stroke patients who underwent endovascular thrombectomy at our hospital over the past 3.5 years. The recanalization rate, clinical recovery and cerebral hemorrhage within 90 days after therapy were compared between these two patient groups.
Results: Endovascular thrombectomy provided perioperative and community-onset ischemic stroke patients with comparable rates of successful reperfusion (mTICI ≥2b grade) (97.1% vs. 97.3%; p = 0. 967) and favorable functional recovery (mRS ≤ 2) (51.4% vs. 43.3%, p = 0.348), with no increase in severe intracranial hemorrhage (0% vs. 2.6 and 1.0%, for hematoma ≥30% of infarcted tissue and intraventricular hemorrhage, respectively) within 90 days. In addition, perioperative stroke patients had higher prevalence of atrial fibrillation (42.9% vs. 26.7%; p = 0.038) and intracranial cerebral artery stenosis without clear embolism (17.1% vs. 3.8%; p < 0.001) than community-onset stroke patients.
Conclusion: Endovascular thrombectomy is an effective and safe therapeutic approach for patients with perioperative ischemic stroke, although the results need to be validated by further studies with larger populations. Atrial fibrillation and large artery stenosis may contribute to the pathogenesis of perioperative ischemic stroke.
Introduction
Perioperative stroke is defined as any embolic, thrombotic or hemorrhagic cerebrovascular incident within 30 days of an operation (1). Similar to community-onset stroke, the majority of perioperative strokes are ischemic rather than hemorrhagic (2). Although perioperative stroke is a rare complication (2–6), it is devastating for both patients and the treating surgeons/anesthesiologists, because the 30-day mortality rate for patients who suffer a perioperative stroke after non-cardiac/major-vascular/neurologic surgery is up to 8 times higher than for stroke patients unrelated to surgery (3, 6). The length of hospitalization and the likelihood of discharge to a long-term care facility are also increased in patients with a perioperative stroke. In patients undergoing cardiac surgery, emboli are frequently identified as a primary cause of perioperative stroke (1, 3). In patients undergoing non-cardiac/vascular surgery, there may be multiple pathogenic mechanisms that lead to perioperative stroke, which include cerebral hypotension/low-flow states and previously undetected stenosis of the large arteries (1). Since intracranial atherosclerotic stenosis is more common in Asian people than in the Western population (7), the mechanism of large artery stenosis in perioperative stroke should be considered in Chinese hospitals.
Therapy for perioperative stroke is challenging. Intravenous thrombolysis is usually avoided in cases of perioperative stroke due to surgery itself being a contraindication for thrombolysis, leading to an elevated risk of bleeding (2). Mechanical endovascular thrombectomy (EVT) has become a novel therapeutic approach for perioperative stroke over the past 15 years (8). A retrospective study showed that EVT can achieve comparable success in cerebral reperfusion in patients with perioperative stroke and stroke unrelated to surgery, although mortality within 3 months after EVT is higher in the former than in the latter (33.3% vs. 4.2%) (9). However, in a retrospective study comparing patients with in-hospital and community-onset stroke, EVT resulted in a lower recanalization rate and poorer recovery in in-hospital stroke patients (10). We remain optimistic about EVT therapy in perioperative stroke as surgical procedures continue to evolve and minimally invasive procedures in particular is becoming more common (11), which means that the pathophysiology of current perioperative stroke patients may be different from that in the past. Further research is needed to evaluate the benefits and safety of EVT in perioperative stroke.
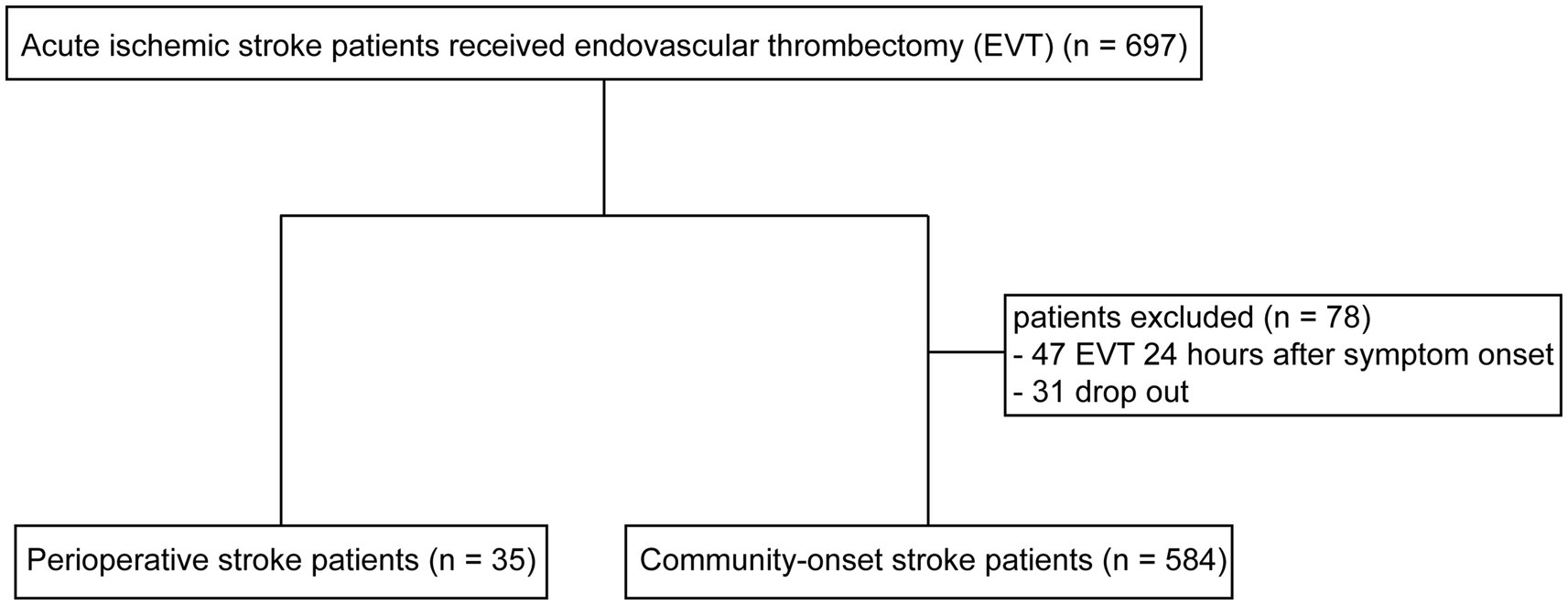
In our research, we conducted a retrospective analysis of all 697 patients with acute ischemic stroke (AIS) who underwent EVT at our hospital over the past 3.5 years, and compared cerebral reperfusion post therapy, clinical recovery within 3 months, and incidence of cerebral bleeding between patients with perioperative and community-onset stroke. The older version of this manuscript has been released as a pre-print at medRxiv (12).
Methods
Study design
Our project was a retrospective cohort study. The study protocol was approved by the Ethics Committee of Taizhou Hospital, Zhejiang Province, China (Registration number: K20181204). Written consent was waived due to the retrospective design according to “World Medical Association Declaration of Helsinki” (Paragraph 32) (13). Between January 2020 and June 2023, total 697 patients aged ≥18 years suffering from AIS (including 35 patients with perioperative stroke) at our hospital received EVT according to the international and Chinese guidelines (14, 15). Perioperative stroke was defined as AIS occurring during or within 30 days following the operative procedure (6). Inclusion criteria were as follows: Age ≥ 18 years; diagnosis of acute ischemic stroke with proven arterial occlusion confirmed by computed tomography (CT) or magnetic resonance imaging (MRI); at least one attempt of EVT; EVT performed within 24 h of symptom onset. A CT perfusion scan was required for stroke patients admitted 6 to 24 h after the onset of symptoms or with unclear time of symptom onset (e.g., wake-up stroke). Patients participating in this study must have (1) infarct core <70 mL, (2) a ratio of ≥1.8 between the volumes of Tmax >6 s and ischemic core, and (3) an absolute mismatch volume (penumbra) ≥ 15 mL. Exclusion criteria was the presence of severe medical conditions, such as advanced heart failure, end-stage kidney disease, or terminal illness, which needed more urgent management.
EVT and post-operative management
Endovascular therapy included stent retriever, aspiration catheter, balloon angioplasty and combination of different techniques. Balloon angioplasty was performed as the therapeutic method of first choice for recanalization in AIS patients who exhibited the “first-pass effect” of the microcatheter, in which blood was already flowing through the occluded vessel when the microcatheter was first passed and then withdrawn to the proximal side of the occluded vessel during EVT (16). Acute intracranial stenting has not been performed along with angioplasty in any case in this study. The number of passes of catheter needed to achieve recanalization or until the end of procedure and the location of endovascular therapy were documented. A maximum of 3 passes could be attempted during EVT. At the end of EVT, the interventional neurologist determined a modified Thrombolysis in Cerebral Infarction (mTICI) score (17). Twenty-four hours after EVT or at any time within 24 h when neurologic deficits progressed, patients were reexamined by head CT and scored using the National Institute of Health Stroke Scale (NIHSS). In the absence of hemorrhagic side effects, regular treatment consisted of antiplatelet agents and anticoagulants started 24 h after EVT. Other treatment strategies included the therapy for causative surgeries in perioperative AIS patients, and statins, blood glucose and blood pressure control or combinations of these treatments according to the Chinese guidelines for the early treatment of AIS patients (15).
Data collection
The aim of this study was to compare the efficacy and safety of EVT in patients with perioperative and community-onset ischemic stroke. The primary efficacy outcome was the recovery of functional outcome as shown in the modified Rankin Scale (mRS) at 90 days after EVT. Good functional outcome was defined as mRS score ≤ 2. The secondary efficacy outcome was the attenuation of neurological deficits as assessed by NIHSS scores at 24 h, and 7 days after EVT (or at the hospital discharge). The third efficacy outcome was recanalization of the occluded cerebral artery. Successful reperfusion was defined as mTICI score of 2b, 2c or 3 and unsuccessful revascularization as mTICI score of 0, 1 or 2a after EVT (17).
The primary safety outcome was intracranial hemorrhage as reflected by CT scanning or MRI, which were typically conducted within 7 days after EVT, with the timing determined by the individual patient’s situation and the examination capacity of our neuroradiology department. Upon neurological deterioration, a head CT was immediately performed. The Heidelberg Bleeding Classification was used to categorize the hemorrhage (18). The second safety outcome was all-cause mortality within 90 days after EVT.
Baseline demographic, clinical information, and laboratory findings within 24 h after EVT were collected for all enrolled patients. Stroke subtypes were classified according to the Trial of ORG 10172 in the Acute Stroke Treatment classification (TOAST) (19). The Alberta stroke program early CT score (ASPECTS) was performed for both anterior and posterior circulations on the CT scan at admission (20, 21).
Statistical analysis
The statistical analysis was conducted using SPSS software for Windows (Version 26.0, IBM, Armonk, USA). The data for continuous variables were described as mean ± SD, and categorical variables were presented as frequencies. Continuous variables were compared between independent groups by T-test or Mann–Whitney U test depending on whether the continuous variables were normally distributed. Categorical variables were compared by Pearson χ2 test. p < 0.05 was considered statistically significant.
Results
Patient data
From January 2020 to June 2023, 35 perioperative AIS patients and 662 community-onset AIS patients underwent EVT in our hospital according to the international and Chinese guidelines (14, 15). The causative surgeries of 35 perioperative stroke patients are listed in Supplementary Table 1, of whom 28 (80.0%) developed ischemic stroke minimally 1 day (1–28 days with a median duration of 2 days) after surgery and 10 (28.6%) had undergone cardiovascular surgery. After discharge from hospital, all AIS patients were followed up for more than 3 months by face-to-face examination or by telephone. Of community-onset AIS patients, 47 patients underwent EVT 24 h after symptom onset due to the presence of a penumbra on CT or MRI imaging; and 31 AIS patients dropped out during the 90-day follow-up period. These 78 patients were not included in this study. Finally, 35 perioperative and 584 community-onset AIS patients were analyzed in this study (Figure 1).
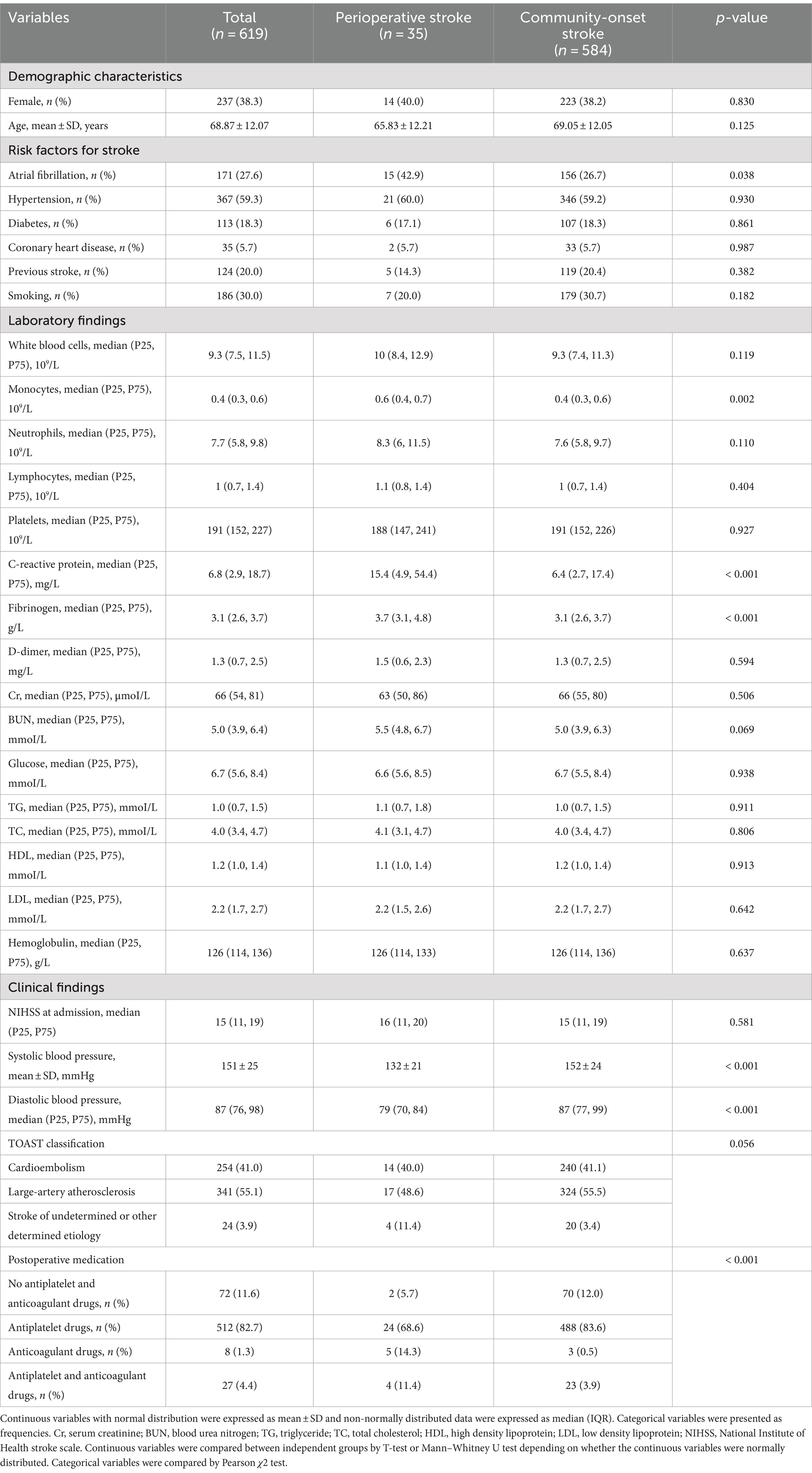
The demographic and baseline characteristics of perioperative and community-onset AIS patients were shown in Table 1. The atrial fibrillation was more frequent in perioperative group than in community-onset group (42.9% vs. 26.7%; χ2 (1) = 4.305, p = 0.038). However, both the systolic and diastolic blood pressures were lower in perioperative than in community-onset stroke patients (p < 0.001). The number of monocytes, concentrations of C-reactive protein and fibrinogen in the blood were higher in perioperative than in community-onset AIS patients (p < 0.05). Due to the causative surgeries, significantly more perioperative AIS patients than community-onset patients received anticoagulant or together with antiplatelet therapy after EVT (χ2 (3) = 54.721, p < 0.001). All other analyzed parameters, including age, sex, pre-stroke risk factors, laboratory findings 24 h after EVT, and TOAST classification were not different between these two groups. Of note, there were no significant differences between the perioperative and community-onset groups in the neurological severity of stroke patients as shown by NIHSS scores before EVT (median value 17.4 ± 10.1 vs. 15.9 ± 7.6; Mann–Whitney-U-Test, Z = −0.553, p = 0.580). Thus, these 2 comparable groups of AIS patients were suitable for analysis of the efficacy and safety of EVT treatments in perioperative stroke.
EVT provided perioperative AIS patients with a comparable reperfusion rate and 3-month rehabilitation, and even a faster recovery within 24 h compared to community-onset AIS patients
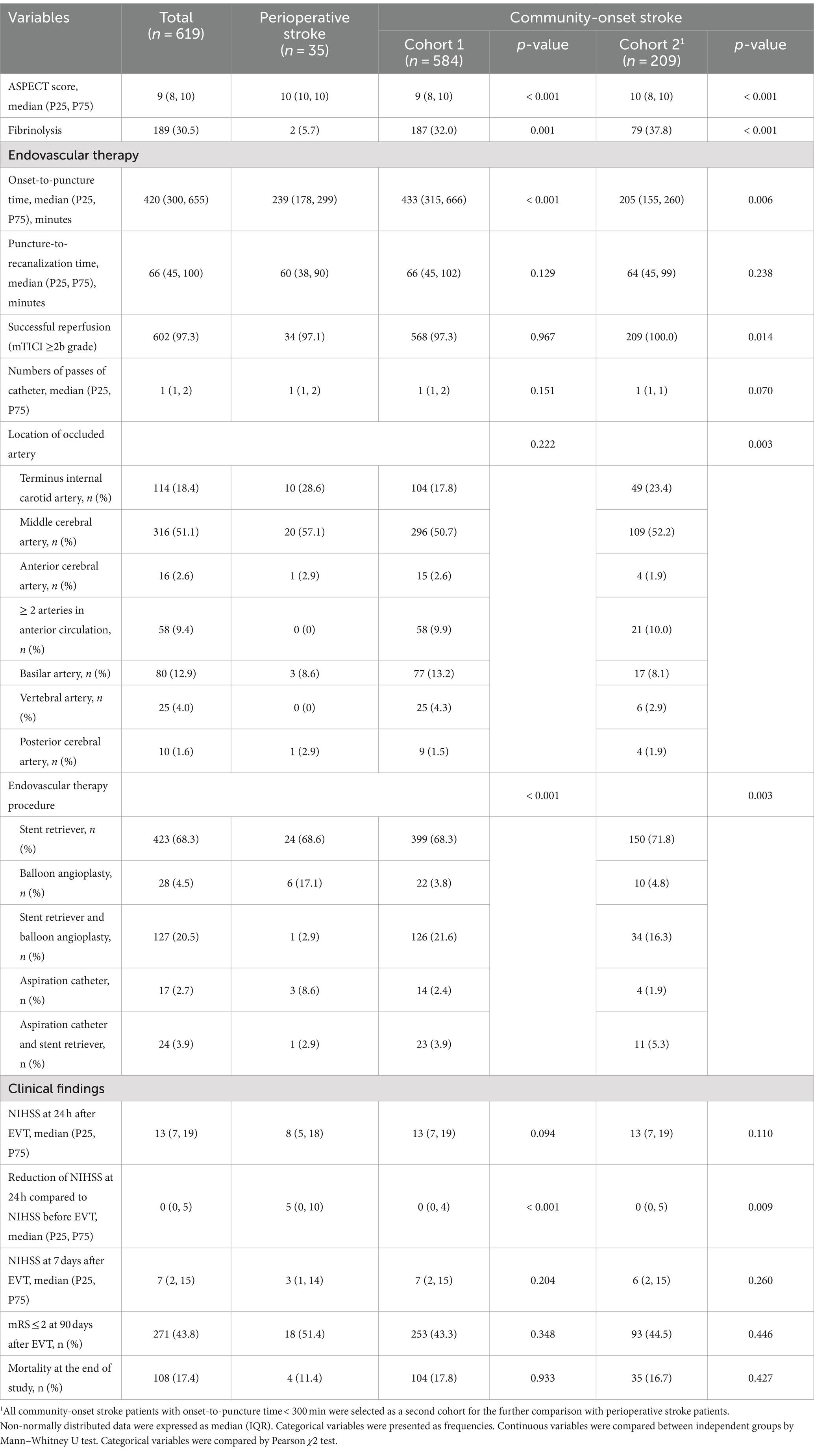
There was no significant difference in the rate of successful reperfusion [mTICI ≥2b grade] between perioperative and community-onset AIS patients (Table 2, Cohort 1; 97.1% vs. 97.3%; χ2 (1) = 0.002, p = 0.967). Similarly, the distribution of mTICI scores (1 = mTICI 0, 1 or 2a, 2 = mTICI 2b, and 3 = mTICI 3), which categorize the level of successful reperfusion, did not show any significant differences between these two AIS patient groups (Figure 2; χ2 (2) = 0.035, p = 0.983). It was also found that the causative surgeries did not complicate the EVT procedure, as the number of catheter passes required for recanalization did not vary significantly between these two groups of patients (1.6 ± 0.9 vs. 1.4 ± 0.8; Mann–Whitney-U-Test, Z = −1.436, p = 0.151). In addition, perioperative and community-onset AIS patients did not differ in operative time of EVT and the site of arterial occlusion.
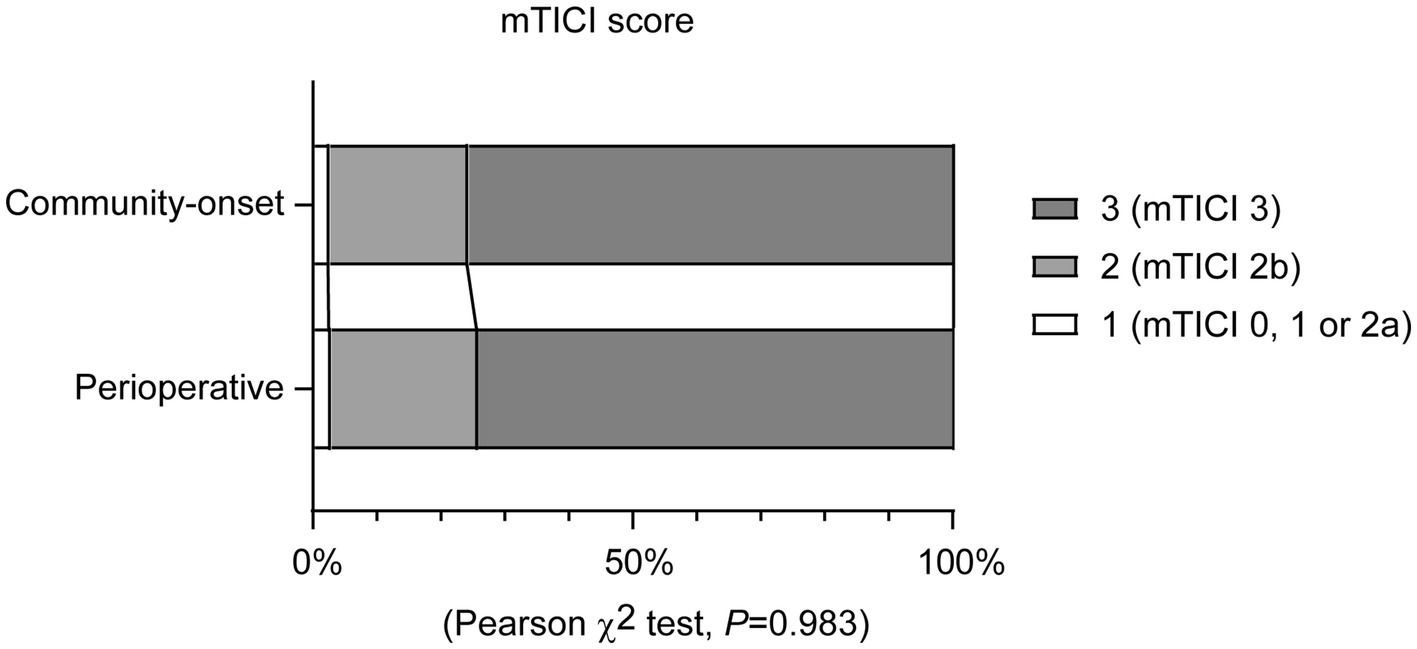
Figure 2. Endovascular thrombectomy leads to a comparable recanalization rate in perioperative and community-onset stroke patients. The degree of blood reperfusion in the brain was visualized using the modified Thrombolysis in Cerebral Infarction (mTICI) score. The distribution of patients with different mTICI scores (1 = mTICI 0, 1 or 2a, 2 = mTICI 2b, and 3 = mTICI 3) did not differ between perioperative and community-onset stroke groups. χ2 test; n = 35 and 584 for perioperative and community-onset stroke groups, respectively.
Interestingly, there were more patients in the perioperative stroke group than in the community-onset stroke group (Table 2, Cohort 1; 17.1% vs. 3.8%; χ2 (1) = 13.680, p < 0.001), who had the “first-pass effect” of the microcatheter, which suggests severe stenosis in the intracranial cerebral arteries and not severe embolism-related vascular occlusion (22), and whose cerebral blood flow could be sufficiently improved by balloon angioplasty alone.
Possibly due to the professional medical care in the hospital, patients with perioperative stroke were much easier to recognize than patients with community-onset stroke. As shown in Table 2, Cohort 1, the time from onset of symptoms to puncture was significantly shorter (239 vs. 433 min median time; Mann–Whitney-U-Test, Z = −6.553, p < 0.001), and the ASPECT score was significantly higher (9.9 ± 0.7 vs. 8.5 ± 1.8; Mann–Whitney-U-Test, Z = −5.454, p < 0.001) in perioperative AIS patients than in the community-onset AIS controls. The improvement in neurological deficits shown by the reduction in NIHSS score 24 h after EVT compared with NIHSS score before EVT was significantly more pronounced in perioperative stroke patients than in community-onset stroke patients (Table 2; ΔNIHSS score: 5.5 ± 8.9 vs. 1.56 ± 6.0; Mann–Whitney-U-Test, Z = −3.052, p = 0.002). Unfortunately, this favorable recovery after EVT was lost in perioperative AIS patients compared to community-onset AIS patients in the following 7 days, as the NIHSS scores did not differ between perioperative and community-onset AIS patients 7 days after EVT (Table 2, Cohort 1; 9.4 ± 9.4 vs. 11.0 ± 12.0; Mann–Whitney-U-Test, Z = −1.271, p = 0.204).
To assess the functional recovery of all AIS patients under study, mRS scores were evaluated 90 days after EVT. The analysis revealed no significant differences in the distribution of mRS scores between patients with perioperative and community-onset AIS (Figure 3; χ2 (6) = 5.923, p = 0.432). The percentage of patients achieving a favorable outcome (mRS ≤ 2) in perioperative group was comparable with that in community-onset AIS group (Table 2, Cohort 1; 51.4% vs. 43.3%, χ2 (1) = 0.882, p = 0.348). The mortalities during the entire course of the study were also not significantly different between perioperative and community-onset AIS patients (Table 2, Cohort 1; 11.4% vs. 17.8%, χ2 (1) = 0.933, p = 0.334).
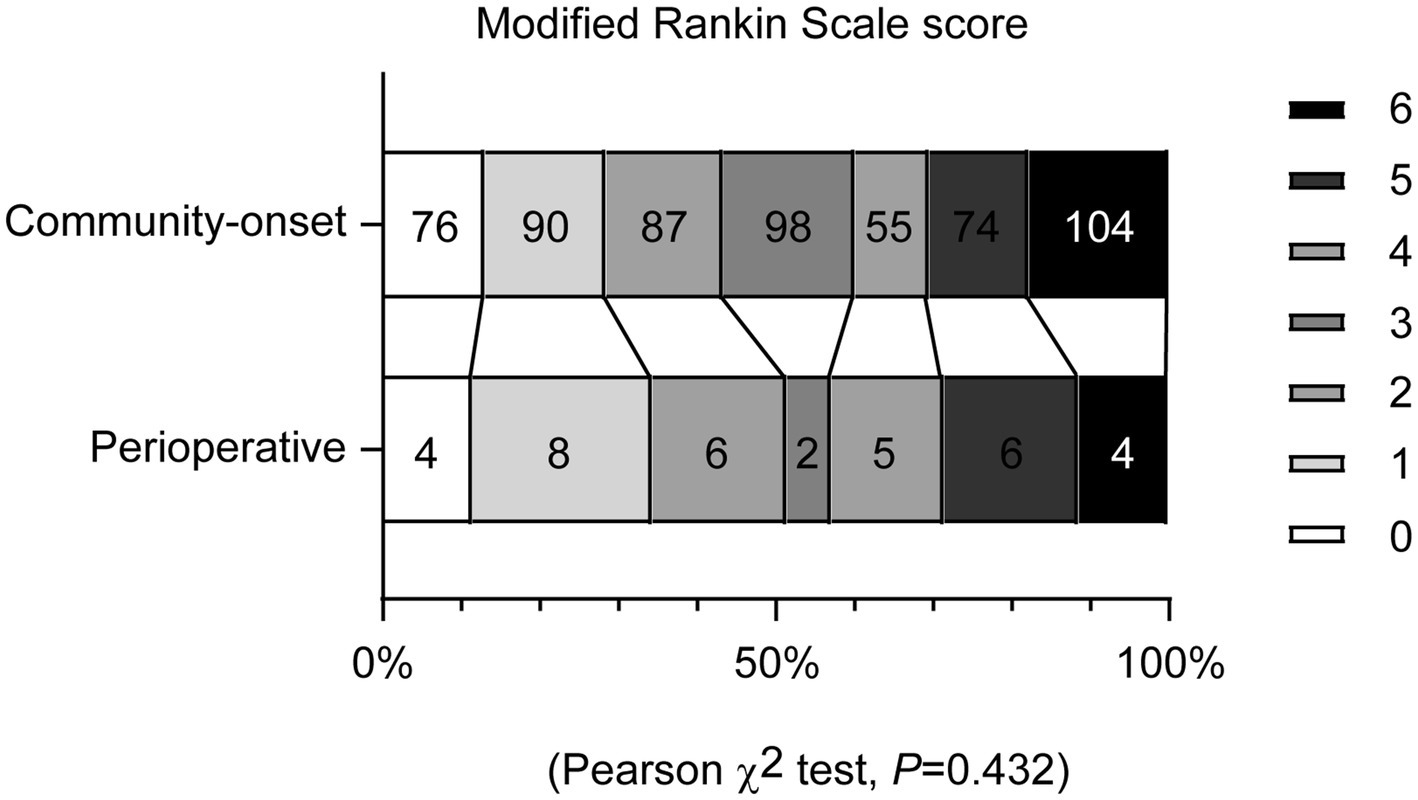
Figure 3. Endovascular thrombectomy achieves comparable functional recovery in perioperative and community-onset stroke patients. Functional recovery of patients with acute ischemic stroke was assessed using the modified Rankin Scale (mRS) at 90 days after endovascular thrombectomy. The mRS scores range from 0 to 6, with score 0 indicating no symptoms, to score 5 indicating severe disability and score 6 indicating death. The distribution of patients with different mRS scores did not differ between the perioperative and community-onset stroke patient groups. χ2 test, n = 35 and 584 for perioperative and community-onset stroke groups, respectively.
It should be noted that the time from symptom onset to puncture is closely associated with the likelihood of functional recovery in AIS patients (23, 24). We wondered whether the functional recover in perioperative AIS patients was actually worse than in community-onset AIS patients, because the onset-to-puncture time was shorter in the former group than the later. We formed Cohort 2 of community-onset AIS patients by excluding patients with onset-to-puncture time ≥ 300 min, in whom the median time (205 min) from symptom onset to puncture was even shorter than in perioperative AIS patients (Mann–Whitney-U-Test, Z = −2.761, p = 0.006). The results in terms of reduction in NIHSS within 24 h, NIHSS on the seventh day and mRS at 90 days after EVT were the same as in the analysis of Cohort 1 (Table 2, Cohort 2). However, the rate of successful reperfusion (mTICI ≥2b) was slightly but significantly lower in perioperative AIS patients than in community-onset AIS patients when the onset-to-puncture time was comparable between these groups (Table 2, Cohort 2; 97.1% vs. 100%, χ2 (1) = 5.996, p = 0.014), which is consistent with the previous study (10).
Similarly, the ASPECTS score is a strong predictor of clinical outcome after EVT (25). The significantly higher ASPECTS score in perioperative AIS patients compared to community-onset AIS patients may have biased the evaluation of the therapeutic efficacy of EVT in perioperative stroke. Therefore, we created a third cohort (Cohort 3) of community-onset AIS patients with ASPECTS score 10, in which the median score (10) was higher than that of perioperative AIS patients, including 1 case with score 7, 1 case with score 8 and 2 cases with score 9 (Mann–Whitney-U-Test, Z = −3.436, p = 0.001). Again, EVT resulted in similar functional recovery in perioperative and community-onset AIS patients, as shown by the percentage of patients with an mRS ≤ 2 90 days after EVT (Supplementary Table 2; 51.4% vs. 44.7%, χ2 (1) = 0.555, p = 0.456).
EVT did not increase a hemorrhagic risk for perioperative AIS patients compared with community-onset AIS patients
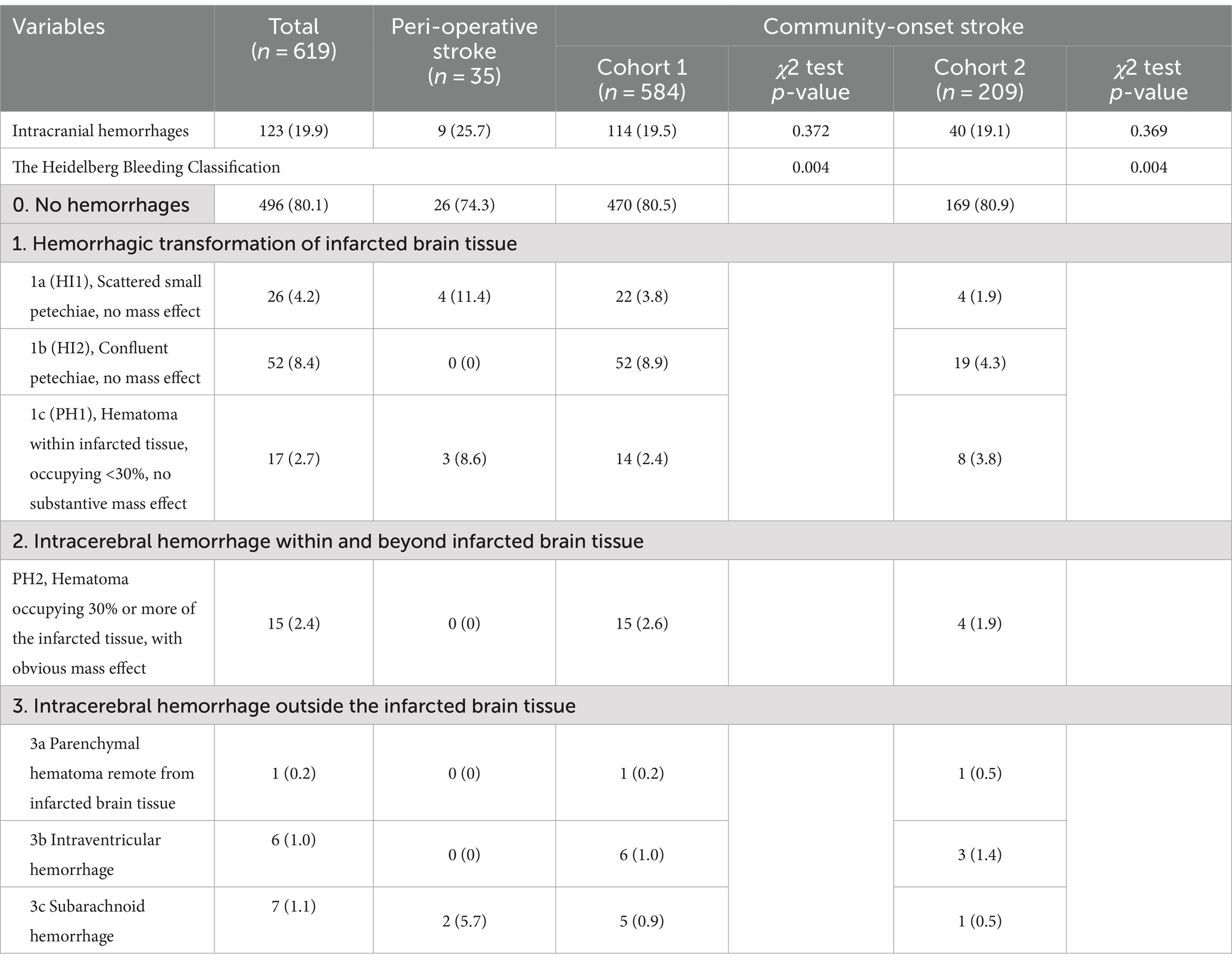
Hemorrhagic transformation is the most important adverse event after EVT therapy in AIS patients. The percentage of patients diagnosed with overall intracranial hemorrhage within 7 days after EVT was comparable in the perioperative and in either Cohort 1 including all community-onset AIS patients recruited or in Cohort 2 of community-onset AIS patients with onset-to-puncture time < 300 min (Table 3; 25.7% vs. 19.5% or 19.1%; χ2 (1) = 0.796 or 0.808, p = 0.372 or 0.369). After dividing the AIS patients into subgroups according to the Heidelberg Bleeding Classification (18), we found that perioperative and community-onset AIS patients in Cohort 1 differed significantly in terms of intracranial hemorrhage (Table 3; χ2 (5) = 16.299, p = 0.004). However, the difference was limited in the subgroups of HI1 (11.4% vs. 3.8; χ2 (1) = 4.817, p = 0.028) and PH1-type (8.6% vs. 2.4%; χ2 (1) = 4.713, p = 0.030) hemorrhages, and subarachnoid hemorrhage (5.7% vs. 0.9%; χ2 (1) = 6.970, p = 0.008) (Table 3). There were no perioperative AIS patients in the subgroups of PH2, parenchymal hematoma remote from infarcted brain tissue, and intraventricular hemorrhage, perhaps due to the limited sample size (Table 3). In Cohort 2, the same results were observed as in Cohort 1 (Table 3).
Discussion
Intravenous thrombolysis is often not feasible in perioperative AIS patients as it increases the risk of bleeding (2, 6). EVT is the most useful method for recanalization of cerebral arteries in perioperative AIS patients and is increasingly practiced in many hospitals (8). However, the efficacy of EVT in improving patient outcomes remains uncertain. Our study showed that EVT in perioperative AIS patients resulted in a similar reperfusion rate and 3-month recovery without an increased risk of bleeding and death compared to patients with community-onset stroke.
In our study, we used the entire cohort (Cohort 1) of community-onset stroke patients (although we also formed Cohort 2 by selecting patients with a comparable onset-to-puncture time with perioperative AIS patients, which will be discussed later), rather than selecting specific matched AIS patients as a control group for perioperative AIS patients, which we believe may limit sample bias and better represent real-world information. The NIHSS score at admission is higher in perioperative AIS patients than in community-onset AIS patients, even though there is no statistical difference, which demonstrates the efficacy of EVT in perioperative stroke. We found that atrial fibrillation was significantly more common in perioperative AIS patients than in community-onset AIS patients, which is consistent with previous studies that perioperative or postoperative atrial fibrillation was associated with an increased risk of both early and long-term ischemic stroke, especially in patients undergoing non-cardiac surgery (26, 27). Our study seems to show that undetected stenosis of the intracranial arteries is a pathogenic mechanism of perioperative stroke. We observed more AIS patients in the perioperative stroke group than in the community-onset stroke group (17.1% vs. 3.8%) who had atherosclerotic stenosis in the cerebral arteries without clear thrombi or emboli, in whom balloon angioplasty alone was able to restore blood flow to the brain tissue. Intracranial atherosclerotic stenoses pose a challenge for EVT in AIS patients as they increase intraprocedural re-occlusion (28). A recent study in AIS patients with intracranial atherosclerosis-related large vessel occlusion showed that balloon angioplasty as a first-choice recanalization strategy has a higher efficiency in recanalization and better functional outcomes at 90 days compared to thrombectomy (16).
Our study showed that the recanalization rate after EVT did not differ between perioperative and community-onset AIS stroke patients, which corroborates previous studies on stroke patients after both cardiovascular and non-cardiovascular surgeries (9, 29). Similarly, EVT provided perioperative and community-onset AIS patients with comparable functional recovery and mortality 3 months after EVT, although it is different from a previous observation that perioperative AIS patients had a higher rate of death within 3 months of EVT than community-onset AIS patients (9). Not surprisingly, the underlying diseases requiring surgeries and comorbidities influence the outcome of perioperative AIS patients after EVT. In the previous study (9), there were 68% perioperative AIS patients receiving cardiovascular surgery and 12% receiving neurosurgery, while we had only 29% patients in the perioperative AIS group, who had undergone cardiovascular procedure. In studies of the therapeutic efficacy of EVT in in-hospital and community-onset AIS patients, the former generally had poorer recovery and higher mortality, which was correlated with the modified Charlson Comorbidity Index (mCCI), incorporating 7 comorbidities, age, diabetes, anemia, active cancer, myocardial infarction, congestive heart disease, and ulcer disease into the model (10, 30). We suppose that the widely used mini-invasive surgery in our studying cohort may also favor the functional recovery of perioperative AIS patients. Compared to open surgical procedures such as sternotomy or open thoracotomy, minimally invasive techniques offer advantages to patients, such as a lower risk of surgical and postoperative complications, shorter recovery times and a reduction in postoperative pain, which can reduce systemic inflammation and hemodynamic changes (31).
Consistent with previous studies (9, 10, 29, 30), EVT did not lead to an increase in total intracranial hemorrhage in perioperative AIS patients compared to community-onset AIS patients. However, when hemorrhages with different subtypes according to the Heidelberg hemorrhage classification were considered, there were significantly more perioperative AIS patients with HI1 and PH1 type hemorrhages and subarachnoid hemorrhage than community-onset AIS patients. Nevertheless, these types of intracranial hemorrhage were generally thought to have little impact on patient outcome. There were no perioperative AIS patients with PH2-type hemorrhage and intravascular hemorrhage, which often lead to symptomatic intracranial hemorrhage and poorer prognosis (18, 32).
However, it should be noted that the symptom onset-to-groin puncture time is strongly associated with better clinical outcome, e.g., functional independence at discharge or 90 days after recanalization treatment (23, 24). In our study, the time from symptom onset to groin puncture was significantly shorter in perioperative stroke patients than in community-onset AIS stroke patients (median time, 239 vs. 433 min), while mRS scores did not differ between these two groups, calling into question the actual benefit of EVT in AIS patients with perioperative stroke. Interestingly, the analysis of Cohort 2 of community-onset AIS patients, in whom the symptom onset-to-puncture time was shorter than that of perioperative AIS patients, confirmed the results of the entire cohort (Cohort 1) of community-onset AIS patients. Similarly, the ASPECTS score predicts the clinical outcome of AIS patients after EVT (25). To avoid ASPECTS score-induced bias in the evaluation of the therapeutic efficacy of EVT in perioperative stroke. We created Cohort 3 of community-onset AIS patients with ASPECTS score 10, which was higher than that of perioperative stroke patients. Functional recovery 90 days after EVT was still comparable between perioperative and community-onset AIS patients. Thus, our study indicated that EVT is an effective therapy for perioperative ischemic stroke.
Intravenous thrombolysis and EVT have been reported to have complementary benefits for AIS patients (33). In our study, the thrombolysis rate in perioperative stroke patients was significantly lower than in community-onset stroke patients. However, the clinical recovery of AIS patients in the latter group after EVT therapy was not better than that of AIS patients in the former group. One possible reason for this could be the different pathogenic mechanism, e.g., there is no severe thrombus or embolism in perioperative stroke patients as we discussed above, so intravenous thrombosis is actually unnecessary in perioperative stroke patients. Moreover, a recent study has shown that intravenous thrombosis only adds benefit to EVT in AIS patients with anterior-circulation large-vessel occlusion if therapy is started within 2 h and 20 min of symptom-onset (33). We wondered whether intravenous thrombosis contributed to therapeutic efficacy in our community-onset stroke patients, as the median time from symptom onset to puncture was 205 min, which was very close to the time delay for thrombolysis therapy.
Obviously, our study has a limitation. The study population was recruited from a single institution with a limited number of patients. The patients cannot be further subdivided to evaluate the therapeutic efficacy and safety of EVT in perioperative AIS patients with cardiovascular or non-cardiovascular procedures. The incidence of PH2-type intracranial hemorrhage and intravascular hemorrhage in perioperative AIS patients after EVT also remains unclear.
Conclusion
Our study shows that endovascular thrombectomy or balloon angioplasty may be an effective and safe therapeutic method for the treatment of perioperative strokes with large vessel occlusions. It is helpful for clinicians to make treatment decisions for perioperative stroke patients. Our study also supports the hypothesis that atrial fibrillation and intracranial cerebral artery stenosis contribute to the occurrence of perioperative stroke. However, our results need to be validated by further studies with larger populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Taizhou Hospital, Zhejiang Province, China. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin due to the retrospective nature of the study.
Author contributions
FW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. XX: Data curation, Writing – review & editing. LZ: Data curation, Writing – review & editing. JZ: Data curation, Writing – review & editing. EW: Data curation, Investigation, Resources, Writing – review & editing. YL: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. SK: Conceptualization, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from Zhejiang Provincial Basic and Public Welfare Research Program (No. LGF21H020005 to Feng Wang); Zhejiang Provincial Medicine and Health Research Foundation (grant number: 2021RC141 to Feng Wang), and Saarland University through Anschubfinazierung 2024 (to Yang Liu).
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1489296/full#supplementary-material
References
1. Benesch, C, Glance, LG, Derdeyn, CP, Fleisher, LA, Holloway, RG, Messé, SR, et al. Perioperative neurological evaluation and management to lower the risk of acute stroke in patients undergoing noncardiac, nonneurological surgery: a scientific statement from the American Heart Association/American Stroke Association. Circulation. (2021) 143:e923–46. doi: 10.1161/CIR.0000000000000968
2. Mashour, GA, Moore, LE, Lele, AV, Robicsek, SA, and Gelb, AW. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: consensus statement from the Society for Neuroscience in anesthesiology and critical care*. J Neurosurg Anesthesiol. (2014) 26:273–85. doi: 10.1097/ANA.0000000000000087
3. Mashour, GA, Shanks, AM, and Kheterpal, S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. (2011) 114:1289–96. doi: 10.1097/ALN.0b013e318216e7f4
4. Vasivej, T, Sathirapanya, P, and Kongkamol, C. Incidence and risk factors of perioperative stroke in noncardiac, and nonaortic and its major branches surgery. J Stroke Cerebrovasc Dis. (2016) 25:1172–6. doi: 10.1016/j.jstrokecerebrovasdis.2016.01.051
5. Bateman, BT, Schumacher, HC, Wang, S, Shaefi, S, and Berman, MF. Perioperative acute ischemic stroke in noncardiac and nonvascular surgery: incidence, risk factors, and outcomes. Anesthesiology. (2009) 110:231–8. doi: 10.1097/ALN.0b013e318194b5ff
7. Kim, JS, Kim, YJ, Ahn, SH, and Kim, BJ. Location of cerebral atherosclerosis: why is there a difference between east and west? Int J Stroke. (2018) 13:35–46. doi: 10.1177/1747493016647736
8. Shah, S, Hatfield, J, Fuller, M, Ohnuma, T, Luke James, M, Bartz, RR, et al. Mechanical thrombectomy for perioperative ischemic stroke following elective inpatient surgery in the United States. J Clin Neurosci. (2022) 101:100–5. doi: 10.1016/j.jocn.2022.05.009
9. Premat, K, Clovet, O, Frasca Polara, G, Shotar, E, Bartolini, B, Yger, M, et al. Mechanical Thrombectomy in perioperative strokes: a case-control study. Stroke. (2017) 48:3149–51. doi: 10.1161/STROKEAHA.117.018033
10. Mönch, S, Lehm, M, Maegerlein, C, Hedderich, D, Berndt, M, Boeckh-Behrens, T, et al. Worse endovascular mechanical recanalization results for patients with in-hospital onset acute ischemic stroke. J Neurol. (2018) 265:2525–30. doi: 10.1007/s00415-018-9035-0
11. Siddaiah-Subramanya, M, Tiang, KW, and Nyandowe, M. A new era of minimally invasive surgery: Progress and development of major technical innovations in general surgery over the last decade. Surg J. (2017) 3:e163–6. doi: 10.1055/s-0037-1608651
12. Wang, F, Xu, X, Zheng, L, Zhong, J, Wang, E, Liu, Y, et al. Endovascular thrombectomy: an effective and safe therapy for perioperative ischemic stroke. medRxiv :24307153. (2024). doi: 10.1101/2024.05.09.24307153
13. World Medical A. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
14. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
15. Liu, L, Chen, W, Zhou, H, Duan, W, Li, S, Huo, X, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol. (2020) 5:159–76. doi: 10.1136/svn-2020-000378
16. Zhang, L, He, X, Li, K, Ling, L, Peng, M, Huang, L’, et al. Balloon angioplasty as first-choice recanalization strategy for intracranial atherosclerosis-related emergent large vessel occlusion with small clot burden. Neuroradiology. (2024) 66:399–407. doi: 10.1007/s00234-023-03278-8
17. Higashida, RT, Furlan, AJ, Roberts, H, Tomsick, T, Connors, B, Barr, J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. (2003) 34:e109–37. doi: 10.1161/01.STR.0000082721.62796.09
18. von Kummer, R, Broderick, JP, Campbell, BC, Demchuk, A, Goyal, M, Hill, MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46:2981–6. doi: 10.1161/STROKEAHA.115.010049
19. Adams, HP Jr, Bendixen, BH, Kappelle, LJ, Biller, J, Love, BB, Gordon, DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.str.24.1.35
20. Barber, PA, Demchuk, AM, Zhang, J, and Buchan, AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS study group. Alberta stroke Programme early CT score. Lancet. (2000) 355:1670–4. doi: 10.1016/s0140-6736(00)02237-6
21. Puetz, V, Sylaja, PN, Coutts, SB, Hill, MD, Dzialowski, I, Mueller, P, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. (2008) 39:2485–90. doi: 10.1161/STROKEAHA.107.511162
22. Yi, TY, Chen, WH, Wu, YM, Zhang, MF, Zhan, AL, Chen, YH, et al. Microcatheter "first-pass effect" predicts acute intracranial artery atherosclerotic disease-related occlusion. Neurosurgery. (2019) 84:1296–305. doi: 10.1093/neuros/nyy183
23. Jahan, R, Saver, JL, Schwamm, LH, Fonarow, GC, Liang, L, Matsouaka, RA, et al. Association between time to treatment with endovascular reperfusion therapy and outcomes in patients with acute ischemic stroke treated in clinical practice. JAMA. (2019) 322:252–63. doi: 10.1001/jama.2019.8286
24. Mulder, M, Jansen, IGH, Goldhoorn, RB, Venema, E, Chalos, V, Compagne, KCJ, et al. Time to endovascular treatment and outcome in acute ischemic stroke: MR CLEAN registry results. Circulation. (2018) 138:232–40. doi: 10.1161/CIRCULATIONAHA.117.032600
25. Goyal, M, Menon, BK, van Zwam, WH, Dippel, DW, Mitchell, PJ, Demchuk, AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
26. Siontis, KC, Gersh, BJ, Weston, SA, Jiang, R, Kashou, AH, Roger, VL, et al. Association of new-Onset Atrial Fibrillation after Noncardiac Surgery with Subsequent Stroke and Transient Ischemic Attack. JAMA. (2020) 324:871–8. doi: 10.1001/jama.2020.12518
27. Lin, MH, Kamel, H, Singer, DE, Wu, YL, Lee, M, and Ovbiagele, B. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke. (2019) 50:1364–71. doi: 10.1161/STROKEAHA.118.023921
28. Tsang, ACO, Orru, E, Klostranec, JM, Yang, IH, Lau, KK, Tsang, FCP, et al. Thrombectomy outcomes of intracranial atherosclerosis-related occlusions. Stroke. (2019) 50:1460–6. doi: 10.1161/STROKEAHA.119.024889
29. Bay, B, Gloyer, NO, Remmel, M, Schell, M, Zelenak, K, Seiffert, M, et al. Mechanical thrombectomy in ischemic stroke after cardiovascular procedures: a propensity-matched cohort analysis. J Neurointerv Surg. (2023) 15:e129–35. doi: 10.1136/jnis-2022-019152
30. Jung, JW, Kim, KH, Yun, J, Nam, HS, Heo, JH, Baik, M, et al. Effectiveness of endovascular treatment for in-hospital stroke vs. community-onset stroke: a propensity score-matched analysis. J Neurol. (2024) 271:2684–93. doi: 10.1007/s00415-024-12232-4
31. Mohiuddin, K, and Swanson, SJ. Maximizing the benefit of minimally invasive surgery. J Surg Oncol. (2013) 108:315–9. doi: 10.1002/jso.23398
32. Hall, E, Ullberg, T, Andsberg, G, and Wasselius, J. Incidence of intracranial hemorrhagic complications after anterior circulation endovascular thrombectomy in relation to occlusion site: a nationwide observational register study. J Neurointerv Surg. (2023) 16:1088–93. doi: 10.1136/jnis-2023-020768
Keywords: perioperative stroke, community-onset stroke, endovascular thrombectomy, intracranial hemorrhage, prognosis
Citation: Wang F, Xu X, Zheng L, Zhong J, Wang E, Liu Y and Ke S (2024) Endovascular thrombectomy: an effective and safe therapy for perioperative ischemic stroke. Front. Neurol. 15:1489296. doi: 10.3389/fneur.2024.1489296
Edited by:
Shakir Husain Hakim, University Hospital Zürich, SwitzerlandReviewed by:
Amy Susan George, Baby Memorial Hospital, IndiaAchmad Firdaus Sani, Airlangga University, Indonesia
Copyright © 2024 Wang, Xu, Zheng, Zhong, Wang, Liu and Ke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Liu, YS5saXVAbXgudW5pLXNhYXJsYW5kLmRl; Shaofa Ke, a2VzZkBlbnplbWVkLmNvbQ==
†These authors have contributed equally to this work
‡These authors share senior authorship
§ORCID: Yang Liu, orcid.org/0000-0002-7614-4233
 Feng Wang1†
Feng Wang1† Xiaoping Xu
Xiaoping Xu Yang Liu
Yang Liu