- 1Department of Neurological Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 2Department of Bioengineering, University of Pittsburgh Medical Center, Pittsburgh, PA, United States
Minimally invasive surgical techniques, such as MR-guided laser interstitial thermal therapy (LITT), have emerged as promising alternatives to open disconnective surgeries in drug-resistant epilepsy (DRE). This review synthesizes current literature on the application of LITT for corpus callosal disconnection and functional hemispheric disconnection. Studies highlight LITT's effectiveness for achieving seizure control and functional outcomes, often with reduced complications compared to traditional open procedures. Challenges include technical limitations to achieving total disconnection and adequate assessment of disconnection postoperatively. The literature is largely composed of observational studies and there is a need for rigorous, multi-center trials to establish robust guidelines and improve generalizability in clinical practice. There is also a need for a more robust exploration of how patient-specific factors contribute to response or nonresponse to intervention.
1 Introduction
Up to 40% of patients with epilepsy have drug resistant epilepsy (DRE) and should be referred for surgical evaluation (1, 2). However, due to a myriad of barriers, epilepsy surgery continues to be underutilized (3). Importantly, one source of underutilization is the stigma of epilepsy surgery and patient or caregiver resistance to open cranial epilepsy surgeries and associated risks (1). Uncontrolled epilepsy is associated with decreased quality of life, neurodevelopmental regression, physical injury, and death (4–7). Meanwhile, surgical intervention for DRE is associated with significant improvements in quality of life (8) and cognitive development (9–11).
To address the morbidity of epilepsy surgery, several minimally invasive techniques have been developed. Endoscopic approaches are less invasive than open surgery, but require craniotomy and interhemispheric dissection with the associated inherent risks (12, 13). Gamma knife radiosurgery is the least invasive option, but it comes with the potential for radiation necrosis and is not immediately effective (14, 15). Finally, Laser Interstitial Thermal Therapy (LITT) eliminates the need for craniotomy (16) and avoids radiation-related complications (e.g., radiation necrosis and mutagenic risks), which is a significant advantage for use in pediatric populations (17, 18).
LITT is a minimally invasive neurosurgical technique used to treat a variety of brain pathologies via targeted thermal ablation (19). It was first described for treatment of seizure foci in 2012 and is now used for various focal epilepsy etiologies (20). During the procedure a laser probe is placed along a planned trajectory using stereotactic technique (Figure 1). Light energy emitted by the laser converts to heat, inducing coagulation necrosis to ablate the tissue (19). Compared to open surgical techniques, LITT is associated with a more favorable complication profile (21–37), shorter hospital stays, and non-inferior seizure outcomes in epilepsy surgery (22–33). However, many studies, including recent prospective observational non-inferiority analyses, suggest that LITT is less effective than traditional epilepsy surgery (21, 38–41). Therefore, the role of LITT relative to traditional approaches is an open debate and remains to be fully evaluated. However, when used in certain disconnective contexts—such as corpus callosotomy—LITT seems to have a similar effectiveness to open microsurgical corpus callosotomy based on recent systematic reviews (42).
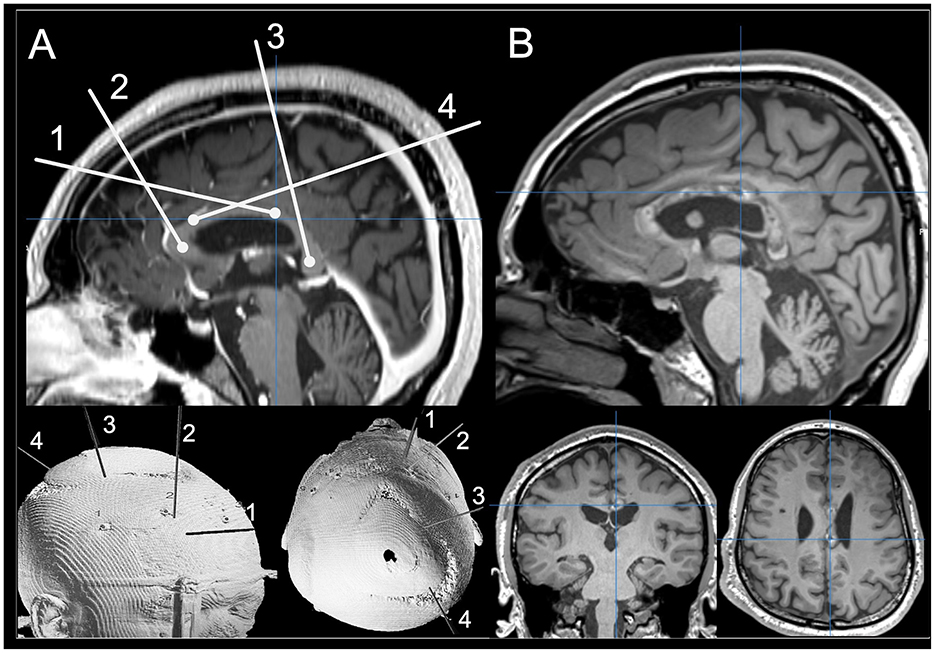
Figure 1. (A) Preoperative planning photo for a complete CCA using mid-sagittal T1-weighted MRI images and 3D rendering for the patient's skull. (B) Postoperative T1-weighted MRI images scans showing ablated tissue on mid-sagittal T1-weighted MRI images with coronal and axial views.
For patients with DRE who experience drop seizures, when resection of an epileptogenic focus is not possible vagus nerve stimulation (VNS) or disconnective surgery are alternative treatment options. VNS is widely used globally as an adjunctive therapy to reduce seizure frequency in both adults and children (43). Its safety and effectiveness have been well-established across many epilepsy centers (44–47) and it is associated with significant improvements in health-related quality of life and reduced use of hospital services among DRE patients including those with drop seizures (48, 49). Furthermore, a retrospective single-center study found that 57.8% of VNS patients experienced reduced overall seizure frequency or total seizure freedom at last follow-up (50) and a 2022 meta-analysis of 5,223 pediatric and adult patients demonstrated increased effectiveness of VNS over time, with responder rates improving from 42.1% at 3 month to 50.8% at 60 months (51).
Despite VNS being generally well-tolerated and effective, patients are significantly more likely to achieve a >50% reduction in overall seizure frequency with CC than with VNS (88.6 vs. 52.5%). This trend is particularly pronounced with drop seizures, the most injurious seizure type, with 58.0% of callosotomy patients achieving drop seizure freedom compared to only 21.1% of VNS patients. In addition, adverse events (hoarseness and voice changes) were more common with VNS though they are typically milder than those associated with corpus callosotomy (e.g., disconnection syndrome) (52).
Disconnective techniques such as corpus callosotomy and functional hemispherotomy control seizures by surgically interrupting fibers in an epileptic network (53). In the case of callosotomy, this disconnection slows the rapid spread of ictal activity between the two cerebral hemispheres that leads to drop seizures—characterized by sudden loss of motor tone, leading to falls and traumatic injuries (54). In the case of hemispherotomy, disconnection completely disconnects a hemispheric seizure focus. LITT has emerged as a minimally invasive alternative or adjunct to these open procedures. However, much like LITT as a whole relative to open surgery, the contemporary clinical applications and limitations of LITT in disconnective epilepsy surgeries remain unclear. In this review we explore the use of LITT for corpus callosum ablation and hemispherotomy.
2 Literature search and screening methodology for this narrative review
On September 24, 2024 a database search of PubMed (National Library of Medicine), Embase (Elsevier), and Cochrane Library (Wiley) was performed by F.G. using the following search line:
(epilepsy OR refractory epilepsy OR lennox-gastaut syndrome OR drug resistant epilepsy OR generalized epilepsy OR intractable epilepsy OR seizures OR drop seizures OR drop attacks OR atonic seizures) AND (laser interstitial thermal therapy OR MRgLITT OR LITT OR laser ablation OR laser callosotomy, OR MRI-guided OR stereotactic functional neurosurgery OR laser OR MR-guided) AND (disconnective surgery OR epilepsy surgery OR disconnection OR redisconnection OR reoperation OR hemispherectomy OR hemispherotomy OR anatomic hemispherectomy OR functional hemispherectomy OR reoperative hemispherectomy OR corpus callosotomy OR CC OR callosotomy OR robotic-assisted callosotomy OR corpus callosum)
The results of the database searches were downloaded and compiled in excel (version 16.78.3) and a manual screening process was performed. First, records were sorted by their digital object identifier and titles and duplicates were removed. Then, titles were screened, followed by abstracts. Finally, articles were retrieved and assessed for eligibility. One study was identified through citation mining and manually added following the initial search (Figure 2).
Articles that reported on the wrong seizure semiologies (e.g., glioma, tuberous sclerosis, focal epilepsies) or the wrong procedure (e.g., gamma knife, ultrasound, stereoelectroencephalography, vagus nerve stimulation, amygdalohippocampectomy, etc.) were excluded. Nonsystematic reviews and systematic reviews published before 2023 were also excluded. To be eligible for this study articles had to report on human subjects only and include patients with drug resistant epilepsy who underwent LITT for either corpus callosum ablation or hemispherotomy (including functional hemispherotomy and completion of a prior disconnection). Studies included in this review are recorded in Tables 1, 2 for corpus callosum ablation and hemispherotomy, respectively.
3 Applications of LITT to corpus callosotomy
3.1 Surgeries performed
First described by Van Wagenen in the 1940s, corpus callosotomy is one of the most common and effective treatments for drop seizures; it also leads to significant reductions in tonic seizures and partial seizures with rapid secondary generalization (55–57). While less effective than callosotomy, many patients undergo VNS first, likely because it seems less invasive (58). Recently, LITT corpus callosum ablation (CCA) emerged a minimally invasive alternative to traditional open corpus callosotomy (23, 31, 35, 59, 60). In a 2024 retrospective cohort study, Phillips et al. reported significantly lower prior VNS (47%) in CCA patients compared to open callosotomy patients (80%) (60). This suggests that the minimally invasive nature of LITT CCA may make patients and their families more amenable to pursuing it as an initial procedure.
Similar seizure outcomes have been reported for CCA and open callosotomy. In their 2023 meta-analysis, Wu et al. reported total seizure freedom rates of 12.38% and freedom from drop seizures of 61.86% in patients with at least 1 year of follow-up after callosotomy (61). Similarly, Hect et al. found overall seizure freedom rates of 18.87% and drop seizure freedom of 46.28% in patients with at least 6 months follow-up after CCA (42). These findings should be considered cautiously as long-term follow-up data is limited, and the timing to achieve seizure freedom varies: some patients experience immediate reduction or freedom from drop seizures, while others respond gradually over weeks to months. Also, some studies report increased seizure burden or new semiologies post-CCA. Finally, as with open callosotomy, CCA's effectiveness in reducing atonic seizures appears to diminish over time. Nevertheless, there may still be long-term improvements in patient quality of life even in light of continued seizure activity: Phillips et al. found that medication burden remained decreased or unchanged in 83% of patients at longest follow-up post-CCA (60).
The most common applications of LITT for corpus callosum ablation has been for complete CCA (27.78%), anterior two-thirds CCA (38.89%), and posterior one-third CCA for completion of a prior partial CCA (22.22%) (42). Upfront complete CCAs were often performed in the setting of prior VNS implantation with insufficient seizure control with rates ranging from 20 to 100% (22, 23, 25, 26, 37, 60) and Hect et al. found that by last follow-up, 62.50% of patients had undergone VNS placement. Taken together with the 2020 meta-analysis by Ye et al., these findings suggest that VNS may enhance seizure control in conjunction with CCA and that some patients may be poor responders to VNS and would benefit from earlier or initial intervention with CCA (62).
Of the 45 patients, reported by Hect et al., with complete anatomical CCA at the most recent follow-up, one-quarter required additional surgery as anterior two-thirds disconnection was insufficient for seizure freedom (42). Numerous studies suggest that single stage complete corpus callosotomy yields better outcomes for drop seizures than anterior two-thirds callosotomy (55, 63–71). However, due to severe functional losses associated with complete callosotomy, partial callosotomy is preferred in patients without significant developmental delays and those with intact language abilities and ambulation (26, 54, 62, 72). It is worth considering the effects that repeat procedures and prolonged seizure burden can have on patient and family quality of life, as well as the long-term impact on development. Unfortunately, not all patients who respond poorly to anterior two-thirds callosotomy/CCA show significant improvement beyond their initial seizure control after follow-up posterior disconnection (54), suggesting that increasing the rate of upfront complete CCA without careful consideration is not advisable. This highlights the need for improved methods to identify patients who are responders and non-responders to anterior CCA to optimize patient outcomes.
3.2 Surgical complications
While the rates of seizure freedom are comparable, and complication rates are similar, open corpus callosotomy carries a far less favorable complication profile. This includes all the risks of cranial surgery as well as the risk for complications such as hydrocephalus or persistent CSF leak requiring a shunt, disconnection syndrome, retraction injury to the cingulate gyrus, and vascular injury to the pericallosal arteries (23, 73). Beyond having a more favorable complication profile, CCA has also been associated with lower blood loss and shorter hospital stays (42, 54).
The two most common surgical complications associated with CCA are probe malpositioning and hemorrhage: out of 90 CCA operations, Hect et al. documented six cases of probe malpositioning and five cases of hemorrhages (42). Accurate probe placement is critical for completeness of CCA and effective seizure control (22). Lehner et al. reported misplacing two of three catheters in a case which resulted in incomplete ablation of the corpus callosum and necessitated a follow-up procedure (30). Beyond technical errors, a thin or tortuous corpus callosum can complicate laser targeting (35).
The documented hemorrhages occurred in five patients across five institutions, usually at the probe entry site or along its trajectory. However, Pruitt et al. has described a case where hemorrhage occurred upon removal of a laser probe likely caused by a bent probe that overheated and charred surrounding tissue (33). None of the hemorrhages required surgical intervention or led to permanent neurological deficit. Other documented complications include ablation of a non-target area due to extension of the heat applied from the splenium into the left thalamus, infection, and CSF leak, with no reported patient deaths during or after CCA.
In general, complications can potentially be avoided using MR guidance (Figure 3) and stereotactic planning with preoperative CT angiography merged with T1-weighted MRI (31, 74). Furthermore, to maximize targeting accuracy Ball et al. suggests minimizing the time interval between the removal of the titanium mandrel and reinsertion of the ablating probe; surgeons should be careful not to damage to the probe, though, because it could lead to insufficient cooling and cause overheating of the surrounding brain tissue (25). Also, given that the probes are relatively blunt, inserting them into the corpus callosum in a more perpendicular fashion—rather than a shallow angle— can reduce the of probe deflection due to the density of white matter tracts.
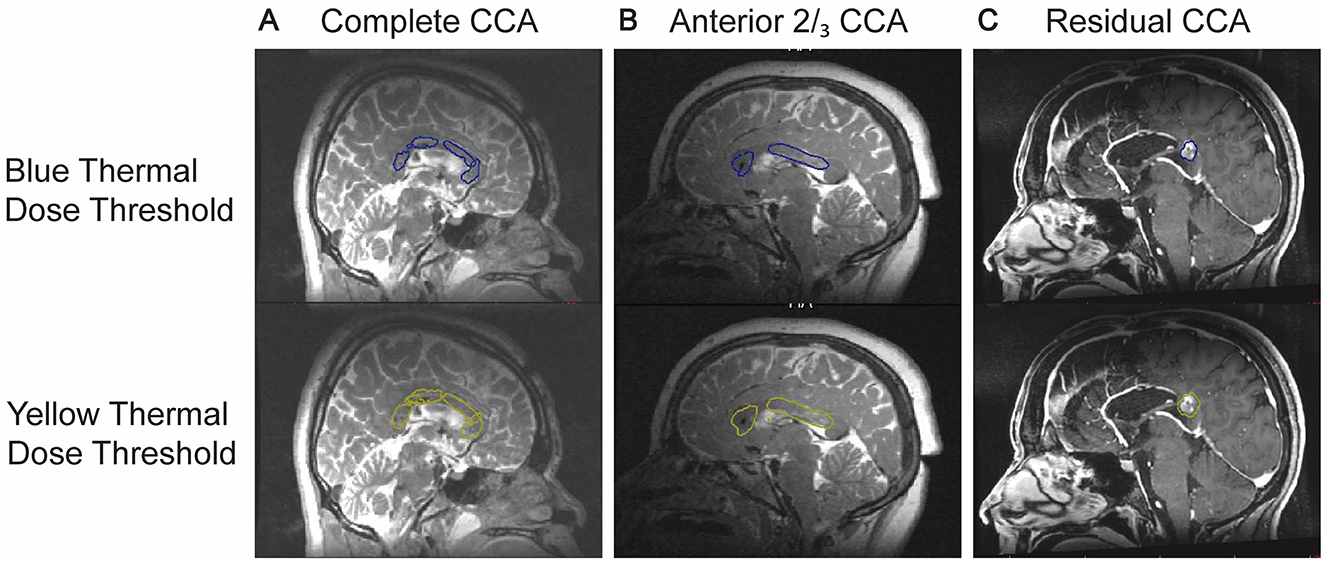
Figure 3. Sample images showing real time thermography when targeting the corpus callosum for (A) complete CCA, (B) anterior 2/3rds CCA, and (C) residual CCA.
Neurological deficits can occur in patients undergoing CCA. Hect et al. found that 18.82% of patients experienced transient neurological deficits and 4.71% experienced permanent deficits (42). The most common transient deficits were transient hemiparesis, often occurring without damage to the supplemental motor area, and disconnection syndrome. Potential causes of supplemental motor area -like syndromes post-CCA include probe malpositioning leading to off target ablation or hemorrhage causing cingulate gyrus edema (75). Other temporary deficits reported include truncal ataxia and imbalance with one patient experiencing permanent dysarthria after complete CCA (31, 34, 37).
3.3 Optimizing patient selection
The major indications for CCA are similar to that of open callosotomy. Institutions consistently required evaluation by a multidisciplinary team, diagnosis with drug resistant epilepsy (evidenced by failure of two or more antiepileptics) with documentation of drop seizures, electrophysiological testing, and a neuropsychological evaluation to decide on appropriateness and extent of CCA. As noted by Aum et al. and others, LITT was especially useful for CCA when applied for patients with multiple medical comorbidities, higher risks associated with surgery, severe or complex epilepsy for which the potential benefit of callosotomy was less clear, or those who wished to avoid open operations (54).
Two major considerations for CCA candidacy are the shape of the corpus callosum and the number of trajectories needed for complete ablation (26). Thin corpus callosums pose a challenge due to their small size: the heat sink properties of cerebrospinal fluid (CSF) can result in less effective ablation. Best et al. recommends against attempting ablations in a corpus callosum that is “not much thicker than the laser catheter being used” (26). Additionally, achieving complete ablation may be challenging in patients with tortuous corpus callosums, requiring multiple trajectories and increasing the risk of complications from probe malpositioning. However, it is important to note that because the lateral corpus callosum “fans out” and becomes wider (76), a functional disconnection may still be safely achieved in some patients even with a thin corpus callosum. Other considerations for CCA candidacy include the absence of implants that could interfere with laser fiber placement (e.g., MRI-incompatible vagus nerve stimulators) and the ability to obtain an MRI scan under general anesthesia (54).
3.4 Limitations in the literature
The literature on CCA is limited by the predominance of observational studies and significant heterogeneity across patient populations. Variations in the extent of CCA, history of other surgeries or concurrent neuromodulation, types and etiology of epilepsy, pharmacologic management, methods for reporting seizure outcomes, and age at CCA limit the generalization of results (42).
4 Application of MRgLITT to hemispherotomy
4.1 Completion of prior disconnective surgery for refractory epilepsy
Residual connections after functional hemispherotomy is one of the most common reasons for requiring reoperation (77–80). Reoperation of failed disconnective surgeries is necessary to achieve seizure control but presents significant challenges and risks, including hydrocephalus, stroke, and infection (81–84). In 2023, Candela-Cantó et al. published a case series in which LITT was used to complete disconnective surgery post-hemispherectomy (four patients) or post- temporal occipital parietal disconnection (two patients) to address persistent or recurring seizures or to facilitate antiepileptic drug withdrawal (24). Four of the six patients were seizure free after the initial LITT operation; the remaining two patients (one temporal occipital parietal disconnection and one hemispherotomy patient) required reoperation. No complications were reported in any of these cases.
Similarly, Ravindra et al. published a single-center case series in 2023 involving five pediatric patients who underwent LITT for completion of hemispherotomy (85). These patients exhibited recurrent or persistent seizures, which were believed to result from incomplete disconnection. The mean daily seizure frequencies were as follows: 11.25 ± 5.2 before the first open surgery, 8.6 ± 9.5 after open surgery, and 1.03 ± 1.98 after LITT completion. Four out of five patients showed improvements in neuropsychiatric functioning and speech performance. Notably, none of the patients required shunt placement for hydrocephalus, which is often necessary in open reoperations.
These studies indicate that LITT is a safe and effective method for completing previous disconnective surgeries when dealing with persistent seizure semiologies. The deep-seated and small nature of persistent connections makes them particularly amenable to LITT. However, a higher amount of energy is required to ablate residual fibers: this is likely due to the cooling effect of the surrounding CSF (24).
4.2 Complete functional hemispherotomy
Chua et al. and Mendoza-Elias et al. reported on the use of LITT for performing functional hemispherotomies in three patients (86, 87).
Chua et al. described a 5-year-old patient with medically refractory hemiclonic seizures following a hemispheric infarction. Due to multiple comorbidities, including congenital heart disease and end-stage renal failure, open craniotomy was deemed too risky. The surgery was performed pre-transplant to avoid complications related to posttransplant immunosuppression. The patient experienced transient increased intracranial pressure and vomiting, managed with CSF drainage and dexamethasone. Postoperatively, there was an expected worsening of left hemiparesis, but the patient regained ambulation and was discharged to rehabilitation 16 days postop with no documented seizures. Within the first 5 months postoperatively she had three episodes of possible seizures at home but was seizure free at 16 months follow-up with continued moderate levetiracetam and oxcarbazepine therapy.
Chua et al. noted that the patient was an ideal candidate due to prior stroke-induced encephalomalacia of the insula which caused subinsular disconnection and generalized hemispheric volume loss, which facilitated near-complete cortical and subcortical disconnections. Despite the inability to complete tractography postoperatively, the patient's favorable seizure outcome indicated successful functional disconnection.
Mendoza et al. reported on two 11-year-old patients with epilepsy secondary to perinatal stroke, who underwent LITT hemispherotomy individually tailored using preoperative tractography. Both patients experienced postoperative hemorrhage without permanent neurological damage and were seizure-free at 9 months postoperatively, with significant improvements in quality of life. Postoperative tractography revealed preserved basal frontal and callosal streamlines. Seizure freedom despite residuals might be attributed to fake streamlines or the non-involvement of residual regions in the preoperative epileptogenic zone.
These findings suggest that LITT functional hemispherotomy could be a viable minimally invasive alternative to open procedures, potentially reducing complication rates and making the surgery more accessible. However, it may be more difficult to achieve the same level of complete 3D disconnection as in open surgery.
4.3 Limitations in the literature
The application of LITT in hemispherotomies is still underexplored, with existing studies primarily comprising case series. Therefore, the results cannot be generalized. Further research, including larger, more diverse patient populations, is necessary to validate these findings.
5 Confirming ablation of target tracts
Various methods have been employed to verify adequate ablation following application of LITT for disconnective surgeries and to explore the relationship between postoperative changes in connectivity. These methods include MRI scans performed intraoperatively (25), immediate confirmation postoperatively using T1-weighted or FLAIR images (88), MRI scans obtained more than 3 months after the LITT procedure (26), and diffusion-weighted imaging (28). Additionally, multimodal approaches integrating electrophysiology-based methods have been utilized (30). Despite the diversity in approaches, there continues to be loose, inconsistent correlations between extent of ablation and patient outcomes.
6 Discussion
The literature supports the safe and effective application of LITT to disconnective epilepsy surgeries. This shift toward minimally invasive approaches potentially increases accessibility for patients and families hesitant to undergo traditional open brain surgeries. Unfortunately, the existing literature primarily consists of observational studies, characterized by considerable variation in patient populations, methodologies for assessing patient outcomes, and criteria for assessing surgical success. This complicates generalizability. Moving forward there is a critical need for multi-center, large-scale studies with uniform protocols for patient selection and outcome measurement. Such efforts are essential to address these gaps and provide robust evidence to guide clinical practice effectively.
In addition to the need for improved studies, there is a need for further investigation into the role of VNS and seizure control. VNS devices are being implanted before, simultaneously with, and after performing CCA (42) which complicates our ability to understand the impact of either procedure on seizure control. For example, Ordaz et al. reported that five out of 11 patients had VNS before undergoing CCA procedure (22). Among these patients, four experienced a 100% decrease in drop seizures while one had no decrease in drop seizures. This positive effect of preoperative VNS was not seen as strongly in the open corpus callosotomy cohort. Paired with the all-or-nothing response to CCA, this suggests that there are both patient-specific and intervention-specific factors contributing to seizure control but with the current data available we are unable to identify them.
A better understanding of how patient characteristics and intervention modalities synergistically improve seizure outcomes can significantly enhance both surgical decision-making and patient comfort. The high prevalence of dual VNS and CCA interventions, coupled with unclear guidelines for selecting patients who would actually benefit from both, may lead to unnecessary procedures and increased time away from school and work. Furthermore, it is important to consider the financial impact of these surgical redundancies, especially if VNS is being performed in patients that would have otherwise achieved total drop seizure freedom with CCA alone. This high prevalence of dual procedures may be due to the relative novelty LITT as it was first applied to CCA in 2016. Over time, as CCA becomes more widely available, there may be a downwards trend in prior VNS.
Another significant gap in the literature is an insufficient understanding of the structural components to epileptogenic networks and how macro- and microstructural changes in the corpus callosum affect said network and, subsequently, patient outcomes. Various methods for assessing extent of ablation have been employed. Best et al. used measurements from T1 postcontrast and diffusion-weighted imaging (26), Caruso et al. used MRI scans (23), and Ordaz et al. used free tracing of T1 weighted images (22). While all authors found that there were high percentages of ablation, and therefore some degree of correlation between ablation and seizure freedom, the correlation was not strong, and the sample sizes were too small to perform rigorous statistical analyses. These findings suggests that the percent of disconnection alone may not be a specific enough measure to predict seizure freedom. Therefore, more systematic investigations into patient-specific macro-and microstructural anatomy are necessary to better understand structural connectivity within the epileptogenic network. Such research could illuminate how surgical interventions succeed or fail in disrupting said networks and contribute to seizure freedom.
Author contributions
FG: Conceptualization, Writing – original draft, Writing – review & editing. JH: Conceptualization, Visualization, Writing – review & editing. TA: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Engel J. The current place of epilepsy surgery. Curr Opin Neurol. (2018) 31:192–7. doi: 10.1097/WCO.0000000000000528
2. Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. (2001) 345:311–8. doi: 10.1056/NEJM200108023450501
3. Kaiboriboon K, Malkhachroum AM, Zrik A, Daif A, Schiltz NM, Labiner DM, et al. Epilepsy surgery in the United States: Analysis of data from the National Association of Epilepsy Centers. Epilepsy Res. (2015) 116:105–9. doi: 10.1016/j.eplepsyres.2015.07.007
4. Giussani G, Falcicchio G, Neve AL, Costagliola G, Striano P, Scarabello A, et al. Sudden unexpected death in epilepsy: a critical view of the literature. Epilepsia Open. (2023) 8:728–57. doi: 10.1002/epi4.12722
5. van Rijckevorsel K. Cognitive problems related to epilepsy syndromes, especially malignant epilepsies. Seizure. (2006) 15:227–34. doi: 10.1016/j.seizure.2006.02.019
6. Nguyen R, Téllez Zenteno JF. Injuries in epilepsy: a review of its prevalence, risk factors, type of injuries and prevention. Neurol Int. (2009) 1:e20. doi: 10.4081/ni.2009.e20
7. Sutula TP, Hagen J, Pitkänen A. Do epileptic seizures damage the brain? Curr Opin Neurol. (2003) 16:189–95. doi: 10.1097/01.wco.0000063770.15877.bc
8. Iwasaki M, Uematsu M, Hino-Fukuyo N, Osawa S-i, Shimoda Y, Jin K, et al. Clinical profiles for seizure remission and developmental gains after total corpus callosotomy. Brain Dev. (2016) 38:47–53. doi: 10.1016/j.braindev.2015.04.010
9. Ukishiro K, Osawa S-I, Iwasaki M, Kakisaka Y, Jin K, Uematsu M, et al. Age-related recovery of daily living activity after 1-stage complete corpus callosotomy: a retrospective analysis of 41 cases. Neurosurgery. (2022) 90:547–51. doi: 10.1227/neu.0000000000001871
10. Honda R, Baba H, Adachi K, Koshimoto R, Ono T, Toda K, et al. Developmental outcome after corpus callosotomy for infants and young children with drug-resistant epilepsy. Epilepsy Behav. (2021) 117:107799. doi: 10.1016/j.yebeh.2021.107799
11. Lassonde M, Sauerwein C. Neuropsychological outcome of corpus callosotomy in children and adolescents. J Neurosurg Sci. (1997) 41:67–73.
12. Sood S, Marupudi NI, Asano E, Haridas A, Ham SD. Endoscopic corpus callosotomy and hemispherotomy. J Neurosurg Pediatr. (2015) 16:681–6. doi: 10.3171/2015.5.PEDS1531
13. Sood S, Asano E, Altinok D, Luat A. Endoscopic posterior interhemispheric complete corpus callosotomy. J Neurosurg Pediatr. (2016) 18:689–92. doi: 10.3171/2016.6.PEDS16131
14. Eder HG, Feichtinger M, Pieper T, Kurschel S, Schroettner O. Gamma knife radiosurgery for callosotomy in children with drug-resistant epilepsy. Childs Nerv Syst. (2006) 22:1012–7. doi: 10.1007/s00381-006-0138-z
15. Feichtinger M, Schröttner O, Eder H, Holthausen H, Pieper T, Unger F, et al. Efficacy and safety of radiosurgical callosotomy: a retrospective analysis. Epilepsia. (2006) 47:1184–91. doi: 10.1111/j.1528-1167.2006.00592.x
16. Hoppe C, Witt JA, Helmstaedter C, Gasser T, Vatter H, Elger CE. Laser interstitial thermotherapy (LiTT) in epilepsy surgery. Seizure. (2017) 48:45–52. doi: 10.1016/j.seizure.2017.04.002
17. Guadix SW, Pandey A, Gundlach C, Walsh M, Moss NS, Souweidane MM. Laser interstitial thermal therapy as a radiation-sparing approach for central nervous system tumors in children with cancer predisposition syndromes: report of a child with Li-Fraumeni syndrome. Illustrative case. J Neurosurg Case Lessons. (2024) 7:CASE23595. doi: 10.3171/CASE23595
18. Cuschieri A, Borg M, Levinskaia E, Zammit C. LITT for biopsy proven radiation necrosis: a qualitative systematic review. J Clin Neurosci. (2023) 116:69–78. doi: 10.1016/j.jocn.2023.08.020
20. Curry DJ, Gowda A, McNichols RJ, Wilfong AA. MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav. (2012) 24:408–14. doi: 10.1016/j.yebeh.2012.04.135
21. Yossofzai O, Stone S, Madsen J, Moineddin R, Wang S, Ragheb J, et al. Seizure outcome of pediatric magnetic resonance-guided laser interstitial thermal therapy versus open surgery: a matched noninferiority cohort study. Epilepsia. (2023) 64:114–26. doi: 10.1111/epi.17451
22. Ordaz JD, Vishnubhotla R, Alfonso A, Budnick H, Wen Q, Radhakrishnan R, et al. Single-institution comparative study of magnetic resonance–guided laser interstitial thermal therapy and open corpus callosotomy. World Neurosurg. (2023) 175:e326–35. doi: 10.1016/j.wneu.2023.03.082
23. Caruso JP, Janjua MB, Dolce A, Price AV. Retrospective analysis of open surgical versus laser interstitial thermal therapy callosotomy in pediatric patients with refractory epilepsy. J Neurosurg Pediatr. (2021) 27:420–8. doi: 10.3171/2020.7.PEDS20167
24. Candela-Cantó S, Muchart J, Valera C, Jou C, Culebras D, Alamar M, et al. Completion of disconnective surgery for refractory epilepsy in pediatric patients using robot-assisted MRI-guided laser interstitial thermal therapy. J Neurosurg Pediatr. (2023) 31:61–70. doi: 10.3171/2022.9.PEDS22258
25. Ball T, Sharma M, White AC, Neimat JS. Anterior corpus callosotomy using laser interstitial thermal therapy for refractory epilepsy. Stereotact Funct Neurosurg. (2018) 96:406–11. doi: 10.1159/000495414
26. Best BJ, Kim I, Lew SM. Magnetic resonance imaging–guided laser interstitial thermal therapy for complete corpus callosotomy: technique and 1-year outcomes. Patient series. J Neurosurg Case Lessons. (2022) 4:CASE22364. doi: 10.3171/CASE22364
27. Ho AL, Miller KJ, Cartmell S, Inoyama K, Fisher RS, Halpern CH. Stereotactic laser ablation of the splenium for intractable epilepsy. Epilepsy Behav Case Rep. (2016) 5:23–6. doi: 10.1016/j.ebcr.2015.12.003
28. Huang Y, Yecies D, Bruckert L, Parker JJ, Ho AL, Kim LH, et al. Stereotactic laser ablation for completion corpus callosotomy. J Neurosurg Pediatr. (2019) 24:433–41. doi: 10.3171/2019.5.PEDS19117
29. Karsy M, Patel DM, Halvorson K, Mortimer V, Bollo RJ. Anterior two-thirds corpus callosotomy via stereotactic laser ablation. Neurosurg Focus. (2018) 44(VideoSuppl2):V2. doi: 10.3171/2018.4.FocusVid.17721
30. Lehner KR, Yeagle EM, Argyelan M, Klimaj Z, Du V, Megevand P, et al. Validation of corpus callosotomy after laser interstitial thermal therapy: a multimodal approach. J Neurosurg. (2019) 131:1095–105. doi: 10.3171/2018.4.JNS172588
31. Mallela AN, Hect JL, Abou-Al-Shaar H, Akwayena E, Abel TJ. Stereotactic laser interstitial thermal therapy corpus callosotomy for the treatment of pediatric drug-resistant epilepsy. Epilepsia Open. (2022) 7:75–84. doi: 10.1002/epi4.12559
32. Palma AE, Wicks RT, Popli G, Couture DE. Corpus callosotomy via laser interstitial thermal therapy: a case series. J Neurosurg Pediatr. (2018) 23:303–7. doi: 10.3171/2018.10.PEDS18368
33. Pruitt R, Gamble A, Black K, Schulder M, Mehta AD. Complication avoidance in laser interstitial thermal therapy: lessons learned. J Neurosurg. (2017) 126:1238–45. doi: 10.3171/2016.3.JNS152147
34. Rich CW, Fasano RE, Isbaine F, Saindane AM, Qiu D, Curry DJ, et al. MRI-guided stereotactic laser corpus callosotomy for epilepsy: distinct methods and outcomes. J Neurosurg. (2021) 135:770–82. doi: 10.3171/2020.7.JNS20498
35. Roland JL, Akbari SHA, Salehi A, Smyth MD. Corpus callosotomy performed with laser interstitial thermal therapy. J Neurosurg. (2019) 134:314–22. doi: 10.3171/2019.9.JNS191769
36. Tao JX, Satzer D, Issa NP, Collins J, Wu S, Rose S, et al. Stereotactic laser anterior corpus callosotomy for Lennox-Gastaut syndrome. Epilepsia. (2020) 61:1190–200. doi: 10.1111/epi.16535
37. Ung TH, Kahn L, Hirt L, Chatain G, Humes E, David-Gerecht P, et al. Using a robotic-assisted approach for stereotactic laser ablation corpus callosotomy: a technical report. Stereotact Funct Neurosurg. (2022) 100:61–6. doi: 10.1159/000518109
38. Alomar SA, Moshref RH, Moshref LH, Sabbagh AJ. Outcomes after laser interstitial thermal ablation for temporal lobe epilepsy: a systematic review and meta-analysis. Neurosurg Rev. (2023) 46:261. doi: 10.1007/s10143-023-02164-4
39. Brotis AG, Giannis T, Paschalis T, Kapsalaki E, Dardiotis E, Fountas KN, et al. A meta-analysis on potential modifiers of LITT efficacy for mesial temporal lobe epilepsy: seizure-freedom seems to fade with time. Clin Neurol Neurosurg. (2021) 205:106644. doi: 10.1016/j.clineuro.2021.106644
40. Patel A, Dawit S, Mastorakos G, Zimmerman R, Crepeau A. Long-term outcomes in patients with intractable mesial temporal lobe epilepsy who undergo laser ablation (P5.5-019). Neurology. (2019) 92(15_supplement):P5.5-019. doi: 10.1212/WNL.92.15_supplement.P5.5-019
41. Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. (2016) 57:325–34. doi: 10.1111/epi.13284
42. Hect JL, Harford E, Maroufi SF, Klem ML, Mansouri A, Abel TJ. Clinical outcomes of MR-guided laser interstitial thermal therapy corpus callosum ablation in drug-resistant epilepsy: a systematic review and meta-analysis. J Neurosurg Pediatr. (2023) 33:12–21. doi: 10.3171/2023.9.PEDS23326
43. Fisher B, DesMarteau JA, Koontz EH, Wilks SJ, Melamed SE. Responsive vagus nerve stimulation for drug resistant epilepsy: a review of new features and practical guidance for advanced practice providers. Front Neurol. (2021) 11:610379. doi: 10.3389/fneur.2020.610379
44. Kawai K, Tanaka T, Baba H, Bunker M, Ikeda A, Inoue Y, et al. Outcome of vagus nerve stimulation for drug-resistant epilepsy: the first three years of a prospective Japanese registry. Epileptic Disord. (2017) 19:327–38. doi: 10.1684/epd.2017.0929
45. Bodin E, Le Moing AG, Bourel-Ponchel E, Querne L, Toussaint P, Berquin P. Vagus nerve stimulation in the treatment of drug-resistant epilepsy in 29 children. Eur J Paediatr Neurol. (2016) 20:346–51. doi: 10.1016/j.ejpn.2016.01.011
46. Pakdaman H, Harandi AA, Abbasi M, Karimi M, Arami MA, Mosavi SA, et al. Vagus nerve stimulation in drug-resistant epilepsy: the efficacy and adverse effects in a 5-year follow-up study in Iran. Neurol Sci. (2016) 37:1773–8. doi: 10.1007/s10072-016-2661-3
47. Kayyali H, Abdelmoity S, Bansal L, Kaufman C, Smith K, Fecske E, et al. The efficacy and safety of rapid cycling vagus nerve stimulation in children with intractable epilepsy. Pediatr Neurol. (2020) 109:35–8. doi: 10.1016/j.pediatrneurol.2020.04.003
48. Soleman J, Stein M, Knorr C, Datta AN, Constantini S, Fried I, et al. Improved quality of life and cognition after early vagal nerve stimulator implantation in children. Epilepsy Behav. (2018) 88:139–45. doi: 10.1016/j.yebeh.2018.09.014
49. Englot DJ, Hassnain KH, Rolston JD, Harward SC, Sinha SR, Haglund MM. Quality-of-life metrics with vagus nerve stimulation for epilepsy from provider survey data. Epilepsy Behav. (2017) 66:4–9. doi: 10.1016/j.yebeh.2016.10.005
50. Shan M, Mao H, Xie H, Gan Y, Wu D, Song J, et al. Vagus nerve stimulation for drug resistant epilepsy: clinical outcome, adverse events, and potential prognostic factors in a single center experience. J Clin Med. (2022) 11:7536. doi: 10.3390/jcm11247536
51. Mao H, Chen Y, Ge Q, Ye L, Cheng H. Short- and long-term response of vagus nerve stimulation therapy in drug-resistant epilepsy: a systematic review and meta-analysis. Neuromodulation Technol Neural Interface. (2022) 25:327–42. doi: 10.1111/ner.13509
52. Rolston JD, Englot DJ, Wang DD, Garcia PA, Chang EF. Corpus callosotomy versus vagus nerve stimulation for atonic seizures and drop attacks: a systematic review. Epilepsy Behav. (2015) 51:13–7. doi: 10.1016/j.yebeh.2015.06.001
53. Kamalboor H, Alhindi H, Alotaibi F, Althubaiti I, Alkhateeb M. Frontal disconnection surgery for drug-resistant epilepsy: outcome in a series of 16 patients. Epilepsia Open. (2020) 5:475–86. doi: 10.1002/epi4.12424
54. Aum DJ, Reynolds RA, McEvoy S, Tomko S, Zempel J, Roland JL, et al. Surgical outcomes of open and laser interstitial thermal therapy approaches for corpus callosotomy in pediatric epilepsy. Epilepsia. (2023) 64:2274–85. doi: 10.1111/epi.17679
55. Graham D, Tisdall MM, Gill D. Corpus callosotomy outcomes in pediatric patients: a systematic review. Epilepsia. (2016) 57:1053–68. doi: 10.1111/epi.13408
56. Cendes F, Ragazzo PC, da Costa V, Martins LF. Corpus callosotomy in treatment of medically resistant epilepsy: preliminary results in a pediatric population. Epilepsia. (1993) 34:910–7. doi: 10.1111/j.1528-1157.1993.tb02111.x
57. Wong TT, Kwan SY, Chang KP, Hsiu-Mei W, Yang TF, Chen YS, et al. Corpus callosotomy in children. Childs Nerv Syst. (2006) 22:999–1011. doi: 10.1007/s00381-006-0133-4
58. Roth J, Bergman L, Weil AG, Brunette-Clement T, Weiner HL, Treiber JM, et al. Added value of corpus callosotomy following vagus nerve stimulation in children with Lennox–Gastaut syndrome: a multicenter, multinational study. Epilepsia. (2023) 64:3205–12. doi: 10.1111/epi.17796
59. Badger CA, Lopez AJ, Heuer G, Kennedy BC. Systematic review of corpus callosotomy utilizing MRI guided laser interstitial thermal therapy. J Clin Neurosci. (2020) 76:67–73. doi: 10.1016/j.jocn.2020.04.046
60. Phillips HW, Hect JL, Harford E, Pan E, Abel TJ. Comparison of magnetic resonance-guided laser interstitial thermal therapy corpus callosum ablation to open microsurgical corpus callosotomy: a single-center retrospective cohort study. Epilepsia Open. (2023) 9:96–105. doi: 10.1002/epi4.12835
61. Wu X, Ou S, Zhang H, Zhen Y, Huang Y, Wei P, et al. Long-term follow-up seizure outcomes after corpus callosotomy: a systematic review with meta-analysis. Brain Behav. (2023) 13:e2964. doi: 10.1002/brb3.2964
62. Thohar Arifin M, Muttaqin Z, Bakhtiar Y, Andar E, Priambada D, Kurnia H, et al. Seizure outcomes in patients with complete versus anterior corpus callosotomy: analysis of outcome. Int J Gen Med. (2020) 13:105–10. doi: 10.2147/IJGM.S247438
63. Chan AY, Rolston JD, Lee B, Vadera S, Englot DJ. Rates and predictors of seizure outcome after corpus callosotomy for drug-resistant epilepsy: a meta-analysis. J Neurosurg. (2018) 130:1193–202. doi: 10.3171/2017.12.JNS172331
64. Gazzaniga MS, Risse GL, Springer SP, Clark E, Wilson DH. Psychologic and neurologic consequences of partial and complete cerebral commissurotomy. Neurology. (1975) 25:10–10. doi: 10.1212/WNL.25.1.10
65. Gordon HW, Bogen JE, Sperry RW. Absence of deconnexion syndrome in two patients with partial section of the neocommissures. Brain. (1971) 94:327–36. doi: 10.1093/brain/94.2.327
66. Jalilian L, Limbrick DD, Steger-May K, Johnston J, Powers AK, Smyth MD. Complete versus anterior two-thirds corpus callosotomy in children: analysis of outcome: clinical article. J Neurosurg Pediatr. (2010) 6:257–66. doi: 10.3171/2010.5.PEDS1029
67. Jea A, Vachhrajani S, Widjaja E, Nilsson D, Raybaud C, Shroff M, et al. Corpus callosotomy in children and the disconnection syndromes: a review. Childs Nerv Syst. (2008) 24:685–92. doi: 10.1007/s00381-008-0626-4
68. Risse GL. Interhemispheric transfer in patients with incomplete section of the corpus callosum: anatomic verification with magnetic resonance imaging. Arch Neurol. (1989) 46:437. doi: 10.1001/archneur.1989.00520400097026
69. Shim KW, Lee YM, Kim HD, Lee JS, Choi JU, Kim DS. Changing the paradigm of 1-stage total callosotomy for the treatment of pediatric generalized epilepsy. J Neurosurg Pediatr. (2008) 2:29–36. doi: 10.3171/PED/2008/2/7/029
70. Spencer SS. Corpus callosum section and other disconnection procedures for medically intractable epilepsy. Epilepsia. (1988) 29:S85–99. doi: 10.1111/j.1528-1157.1988.tb05802.x
71. Kasasbeh AS, Smyth MD, Steger-May K, Jalilian L, Bertrand M, Limbrick DD. Outcomes after anterior or complete corpus callosotomy in children. Neurosurgery. (2014) 74:17–28. doi: 10.1227/NEU.0000000000000197
72. Rossi GF, Colicchio G, Marchese E, Pompucci A. Callosotomy for severe epilepsies with generalized seizures: outcome and prognostic factors. Acta Neurochir. (1996) 138:221–7. doi: 10.1007/BF01411365
73. Ye VC, Mansouri A, Warsi NM, Ibrahim GM. Atonic seizures in children: a meta-analysis comparing corpus callosotomy to vagus nerve stimulation. Childs Nerv Syst. (2021) 37:259–67. doi: 10.1007/s00381-020-04698-0
74. Hect JL, Alattar AA, Harford EE, Reecher H, Fernandes DT, Esplin N, et al. Stereotactic laser interstitial thermal therapy for the treatment of pediatric drug-resistant epilepsy: indications, techniques, and safety. Childs Nerv Syst. (2022) 38:961–70. doi: 10.1007/s00381-022-05491-x
75. Kim YH, Kim CH, Kim JS, Lee SK, Han JH, Kim CY, et al. Risk factor analysis of the development of new neurological deficits following supplementary motor area resection. J Neurosurg. (2013) 119:7–14. doi: 10.3171/2013.3.JNS121492
76. Carmichael O, Sakhanenko L. Estimation of integral curves from high angular resolution diffusion imaging (HARDI) data. Linear Algebra Its Appl. (2015) 473:377–403. doi: 10.1016/j.laa.2014.12.007
77. Goel K, Phillips HW, Chen J-S, Ngo J, Edmonds B, Ha PX, et al. Hemispheric epilepsy surgery for hemimegalencephaly: the UCLA experience. Epilepsia. (2024) 65:57–72. doi: 10.1111/epi.17807
78. Kurwale NS, Patil SB, Jagtap SA, Joshi A, Nilegaonkar S, Bapat D, et al. Failed hemispherotomy: insights from our early experience in 40 patients. World Neurosurg. (2021) 146:e685–90. doi: 10.1016/j.wneu.2020.10.170
79. Lew SM, Koop JI, Mueller WM, Matthews AE, Mallonee JC. Fifty consecutive hemispherectomies: outcomes, evolution of technique, complications, and lessons learned. Neurosurgery. (2014) 74:182–94; discussion 195. doi: 10.1227/NEU.0000000000000241
80. Kossoff EH, Vining EPG, Pillas DJ, Pyzik PL, Avellino AM, Carson BS, et al. Hemispherectomy for intractable unihemispheric epilepsy etiology vs outcome. Neurology. (2003) 61:887–90. doi: 10.1212/01.WNL.0000090107.04681.5B
81. Bartoli A, Hassani YE, Jenny B, Momjian S, Korff CM, Seeck M, et al. What to do in failed hemispherotomy? Our clinical series and review of the literature. Neurosurg Rev. (2018) 41:125–32. doi: 10.1007/s10143-017-0888-y
82. Vadera S, Moosa ANV, Jehi L, Gupta A, Kotagal P, Lachhwani D, et al. Reoperative hemispherectomy for intractable epilepsy: a report of 36 patients. Neurosurgery. (2012) 71:388–92; discussion 392–3. doi: 10.1227/NEU.0b013e31825979bb
83. Chen S, Guan Y, Liu C, Du X, Zhang Y, Chen S, et al. Treatment for patients with recurrent intractable epilepsy after primary hemispherectomy. Epilepsy Res. (2018) 139:137–42. doi: 10.1016/j.eplepsyres.2017.11.021
84. Lew SM, Matthews AE, Hartman AL, Haranhalli N. Post-Hemispherectomy Hydrocephalus Workgroup. Posthemispherectomy hydrocephalus: results of a comprehensive, multiinstitutional review. Epilepsia. (2013) 54:383–9. doi: 10.1111/epi.12010
85. Ravindra VM, Ruggieri L, Gadgil N, Addison AP, Patino I, Gonda DD, et al. An initial experience of completion hemispherotomy via magnetic resonance-guided laser interstitial therapy. Stereotact Funct Neurosurg. (2023) 101:179–87. doi: 10.1159/000528452
86. Chua MMJ, Bushlin I, Stredny CM, Madsen JR, Patel AA, Stone S. Magnetic resonance imaging-guided laser-induced thermal therapy for functional hemispherotomy in a child with refractory epilepsy and multiple medical comorbidities. J Neurosurg Pediatr. (2020) 27:30–5. doi: 10.3171/2020.6.PEDS20455
87. Mendoza-Elias N, Satzer D, Henry J, Nordli DR, Warnke PC. Tailored hemispherotomy using tractography-guided laser interstitial thermal therapy. Oper Neurosurg Hagerstown Md. (2023) 24:e407–13. doi: 10.1227/ons.0000000000000650
Keywords: epilepsy, corpus callosotomy, hemispherotomy, tractography, pediatric neurosurgery
Citation: Gaba F, Hect JL and Abel TJ (2024) Applications of magnetic resonance-guided laser interstitial thermal therapy in disconnective epilepsy surgery. Front. Neurol. 15:1484263. doi: 10.3389/fneur.2024.1484263
Received: 21 August 2024; Accepted: 11 November 2024;
Published: 02 December 2024.
Edited by:
Daichi Sone, Jikei University School of Medicine, JapanReviewed by:
Kapil Gururangan, Northwestern University, United StatesDebayan Dasgupta, National Hospital for Neurology and Neurosurgery (NHNN), United Kingdom
Copyright © 2024 Gaba, Hect and Abel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taylor J. Abel, YWJlbHRqQHVwbWMuZWR1
 Fidelia Gaba
Fidelia Gaba Jasmine L. Hect
Jasmine L. Hect Taylor J. Abel
Taylor J. Abel