
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 08 January 2025
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1482119
This article is part of the Research TopicIntracranial aneurysms, AVM and other vascular malformations, and connective tissue disorders as potential causes of stroke: Advances in diagnosis and therapeutics including novel neurosurgical techniquesView all 15 articles
Objective: To evaluate the clinical utility of improved machine learning models in predicting poor prognosis following endovascular intervention for intracranial aneurysms and to develop a corresponding visualization system.
Methods: A total of 303 patients with intracranial aneurysms treated with endovascular intervention at four hospitals (FuShun County Zigong City People's Hospital, Nanchong Central Hospital, The Third People's Hospital of Yibin, The Sixth People's Hospital of Yibin) from January 2022 to September 2023 were selected. These patients were divided into a good prognosis group (n = 207) and a poor prognosis group (n = 96). An improved machine learning model was employed to analyze patient clinical data, aiding in the construction of a prediction model for poor prognosis in intracranial aneurysm endovascular intervention. This model simultaneously performed feature selection and weight determination. Logistic multivariate analysis was used to validate the selected features. Additionally, a visualization system was developed to automatically calculate the risk level of poor prognosis.
Results: In the training set, the improved machine learning model achieved a maximum F1 score of 0.8633 and an area under the curve (AUC) of 0.9118. In the test set, the maximum F1 score was 0.7500, and the AUC was 0.8684. The model identified 10 key variables: age, hypertension, preoperative aneurysm rupture, Hunt-Hess grading, Fisher score, ASA grading, number of aneurysms, intraoperative use of etomidate, intubation upon leaving the operating room, and surgical time. These variables were consistent with the results of logistic multivariate analysis.
Conclusions: The application of improved machine learning models for the analysis of patient clinical data can effectively predict the risk of poor prognosis following endovascular intervention for intracranial aneurysms at an early stage. This approach can assist in formulating intervention plans and ultimately improve patient outcomes.
Intracranial aneurysms are protrusions resulting from congenital defects in the cerebral artery walls or increased intraluminal pressure, and they are a primary cause of subarachnoid hemorrhage. Clinically, these aneurysms often present with symptoms such as vomiting, severe headache, and visual field disturbances. Without timely intervention, ruptured aneurysms can lead to intracranial hemorrhage, posing significant threats to patient survival. The associated mortality and disability rates are notably high, leading to poor prognoses (1, 2).
Endovascular intervention is the primary treatment for intracranial aneurysms, offering advantages in reducing trauma for patients with ruptured aneurysms and promoting postoperative recovery. However, the factors influencing prognosis following this treatment remain unclear (3, 4). Traditional logistic regression models rely heavily on selected independent factors to predict patient outcomes, but they often miss critical clinical information, significantly reducing data utilization and overall predictive performance (5).
In recent years, machine learning, a branch of artificial intelligence, has shown great promise in the medical field. It can develop predictive models by analyzing clinical data and extracting case characteristics for accurate diagnosis and prognosis. Machine learning also allows for iterative improvements during validation, offering high efficiency and rapid results (6). Numerous studies have explored the use of machine learning models to predict outcomes following endovascular intervention for intracranial aneurysms. For instance, several studies have employed models such as support vector machines, random forests, and neural networks to enhance prediction accuracy and clinical decision-making (7–9).
Despite these advancements, there is still a lack of comprehensive research that integrates various machine learning techniques to improve the prediction of poor prognosis specifically for intracranial aneurysm endovascular intervention. Furthermore, existing studies often do not provide user-friendly visualization systems that can aid clinicians in interpreting the results and making informed decisions.
This study aims to bridge these gaps by evaluating the clinical value of using an improved machine learning model to predict poor prognosis in intracranial aneurysm endovascular intervention. Additionally, we aim to develop a visualization system to enhance the interpretability and usability of the predictive model for clinical practitioners. By doing so, we hope to provide a more robust and practical tool for improving patient outcomes.
This study included 303 patients with intracranial aneurysms who underwent endovascular interventions at four hospitals (FuShun County Zigong City People's Hospital, Nanchong Central Hospital, The Third People's Hospital of Yibin, The Sixth People's Hospital of Yibin) from January 2022 to September 2023. The patients were divided into two groups based on their prognosis: the good prognosis group (n = 207) and the poor prognosis group (n = 96). Prognosis was evaluated at a 6-month follow-up using the Glasgow Outcome Scale (GOS) score (10), with scores of 4–5 indicating a good prognosis and scores of 1–3 indicating a poor prognosis. This retrospective study was approved by the Ethics Committee of Fushun County People's Hospital (Approval Number: 2023-077), and patient informed consent was waived. All patient data were anonymized and analyzed.
Inclusion criteria: (1) head CT, MRI, cerebral angiography, and other examinations clearly for intracranial aneurysm; (2) endovascular interventional therapy; (3) complete clinical data.
Exclusion criteria: (1) pseudoaneurysm, non-aneurysmal subarachnoid hemorrhage; (2) other cerebrovascular diseases; (3) intolerance of surgical treatment.
Clinical data were collected for each patient, including age, gender, history of hypertension, preoperative aneurysm rupture status, Hunt-Hess classification, Fisher score, ASA classification, number of aneurysms, intraoperative use of etomidate, intubation status upon leaving the operating room, operation time, body weight, platelet count, platelet distribution, prothrombin time, partial activated prothrombin time, thrombin time, fibrinogen. Prior to model construction, all data were standardized using the Z-score to eliminate the influence of dimensional differences.
The Automatic Feature Filtering and Weight Determination Integrated (AFFWDI) model is an innovative framework designed to enhance the prediction accuracy and generalization ability of machine learning models. The core of the AFFWDI model utilizes swarm intelligence algorithms, inspired by natural behaviors such as bird flocking and ant foraging, to solve optimization problems. The methodology of the AFFWDI model can be divided into two main steps.
(1) Initialization: Randomly generate a population of solutions, each representing a subset of possible features. (2) Adaptation Assessment: Assign a fitness value to each feature subset, usually based on its performance in a predictive model. In this study, the fitness is measured by the accuracy of cross-validation. (3) Search for Updates: Update each solution, i.e., a subset of features, according to the rules of the swarm intelligence algorithm. (4) Termination Conditions: Repeat the iterations until the termination conditions are met, such as reaching the maximum number of iterations or when the fitness value no longer shows significant improvement.
(1) Weight initialization: After determining the optimal subset of features, initial weights are assigned to these features. (2) Weight Optimization: The same swarm intelligence algorithm is used to optimize the weights, thereby improving the overall prediction performance of the model. (3) Synergistic Optimization: The optimization of feature subsets and weights is not conducted independently but synchronously, ensuring that the synergistic effect of feature screening and weight assignment is maximized.
This study employs a swarm intelligence optimization algorithm to handle the complex tasks of feature screening and weight determination. To ensure superior global optimization capability, we introduce an improved Prairie Dog Optimization Algorithm (IPDO). The original Prairie Dog Optimization Algorithm (PDO) divides the behavior of prairie dogs into two stages: global exploration and local exploitation. However, previous studies have indicated a risk of the algorithm falling into local optima (11). To address this, the IPDO enhances exploration and convergence performance through Tent chaotic initialization and t-distribution perturbation variation. Detailed descriptions of these enhancements are as follows.
Tent mapping is a simple yet effective chaotic mapping method with excellent non-linear and traversal properties. By using Tent chaotic initialization, the algorithm can generate a more diversified and uniformly distributed solution space in the initial stage, which enhances the global search capability and prevents the algorithm from prematurely falling into local optima (12). During the algorithm's initialization phase, each prairie dog's location is no longer randomly generated but is determined by a Tent chaotic sequence, thus ensuring better diversity and coverage of the initial population.
The t-distribution (Student's t-distribution) is a probability distribution whose shape is controlled by the degrees of freedom parameter. When the degrees of freedom are low, the t-distribution has a thicker tail, which allows for generating more significant variances with smaller probabilities when creating perturbations, thereby increasing the algorithm's ability to escape local optima (13). In the position updating stage, in addition to the traditional PDO position updating rules, a perturbation factor generated by the t-distribution is introduced to randomly perturb the position of an individual. This variation enhances the algorithm's local search ability, explores a broader search space to some extent, and improves the probability of finding the optimal global solution.
The optimization performance of the IPDO algorithm was tested using 23 standard test functions, each designed for minimization problems and varying in dimensions and complexity. Key characteristics of these functions include search space boundary range, function dimensionality, function category, and optimal solutions. The test functions were classified into unimodal (U) and multimodal (M) types. Unimodal functions assess local exploitation capability, while multimodal functions evaluate global exploration capability. To ensure a fair comparison, the population size was set at 30, the number of iterations at 200, and the average convergence curves were plotted after 30 repetitions.
SPSS 25.0 software was used to analyze the data, and the count data were expressed as [n (%)] and compared with theχ2-test; the normally distributed measure data were expressed as (x̄ ± s) and compared with t-test; the analysis of influencing factors was performed by multifactorial Logistic regression analysis, and the independent variables were entered into the regression equations by stepwise method. P < 0.05 was used to indicate statistically significant differences in two-sided tests.
The results show that the overall convergence speed and global optimization-seeking ability of the IPDO algorithm are significantly improved compared to the pre-improvement period (Figure 1).
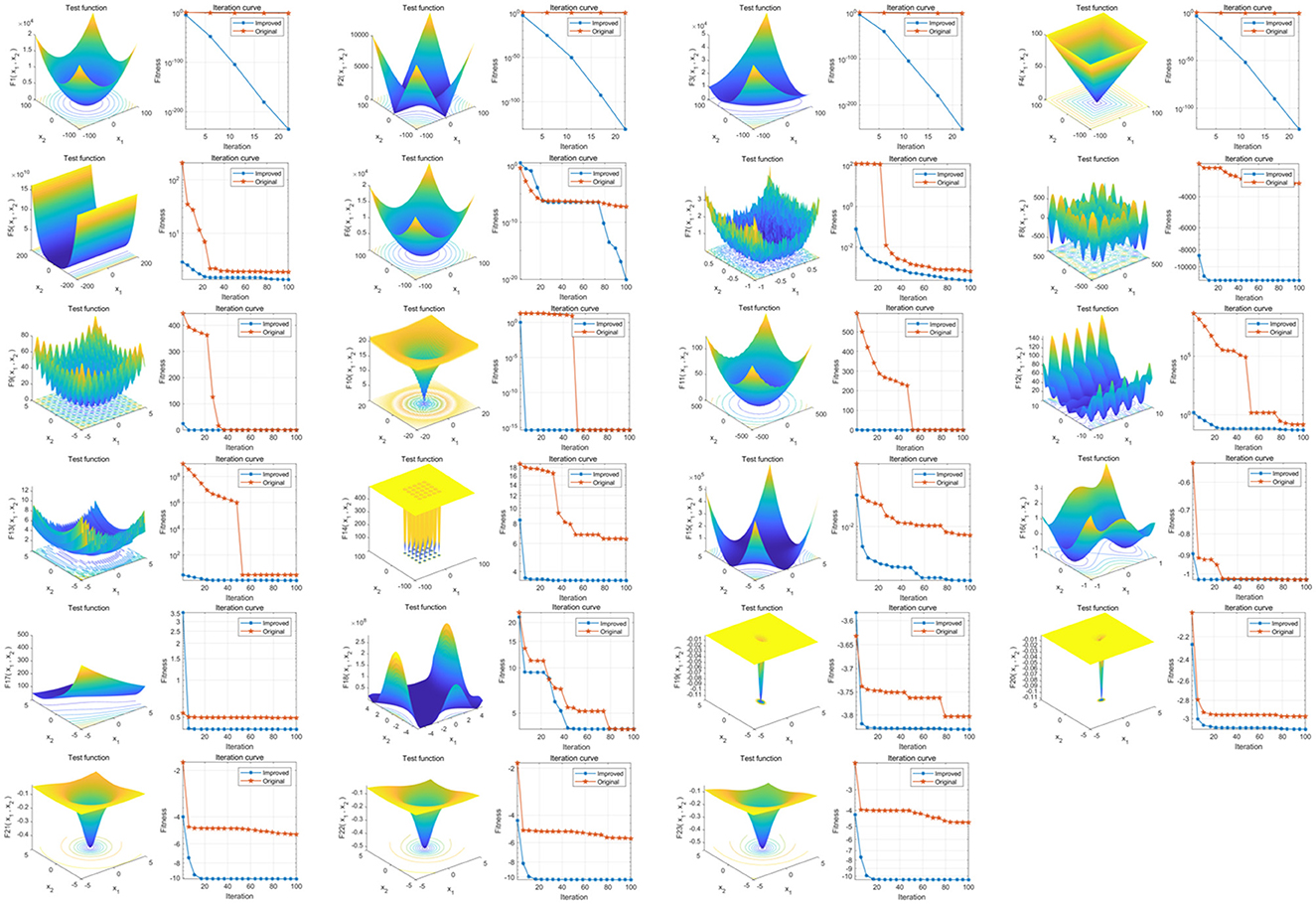
Figure 1. Comparison of optimization-seeking capability before and after PDO improvement. The three-dimensional surface plots in the figure show the two-dimensional search space of each benchmark function; the convergence curves show the convergence trend of the first solution in the first dimension of each benchmark function, and the trends of PDO and IPDO are compared. The red convergence curve in the figure corresponds to the original PDO algorithm, and the blue convergence curve corresponds to the improved IPDO algorithm.
Eighty percent of the dataset is randomly selected as the training set, cross-validation is executed, IPDO is utilized to find the optimal combination of features and hyper-parameters, and four types of base learners are selected; namely, LR, SVM, Back Propagation Neural Network (BPNN), and XGBoost, and the final model training results show that the AFFWDI model with XGBoost as the base learner performs optimally (Table 1, Figure 2).
The remaining 20% of the dataset was used as a test set to examine the generalization ability of each model. The results showed that the AFFWDI model with XGBoost as the base learner had the best performance, and 10 variables were screened for age, comorbid hypertensive disorders, ruptured aneurysm preoperatively, Hunt-Hess classification, Fisher score, ASA classification, number of aneurysms, and intraoperative use of etomidate, intubation on leaving the operating room, and length of surgery (Table 2, Figure 3).
All 10 variables the improved machine learning model screened had statistically significant differences between the two groups (P < 0.05), coinciding with the logistic multifactor analysis results (Tables 3, 4).
In the application of the visualization system, the user only needs to enter 10 specific values in the “Characteristic Input” field, including “age, combined hypertension, preoperative aneurysm rupture, Hunt-Hess classification, Fisher score, ASA classification, number of aneurysms, intraoperative use of etomidate, intubation, length of surgery.” The system automatically calculates the risk of poor prognosis for a patient by assigning specific values to each of the 10 characteristics: age, Hunt-Hess classification, Fisher score, ASA classification, number of aneurysms, intraoperative use of etomidate, time of intubation, and length of surgery (Figure 4).
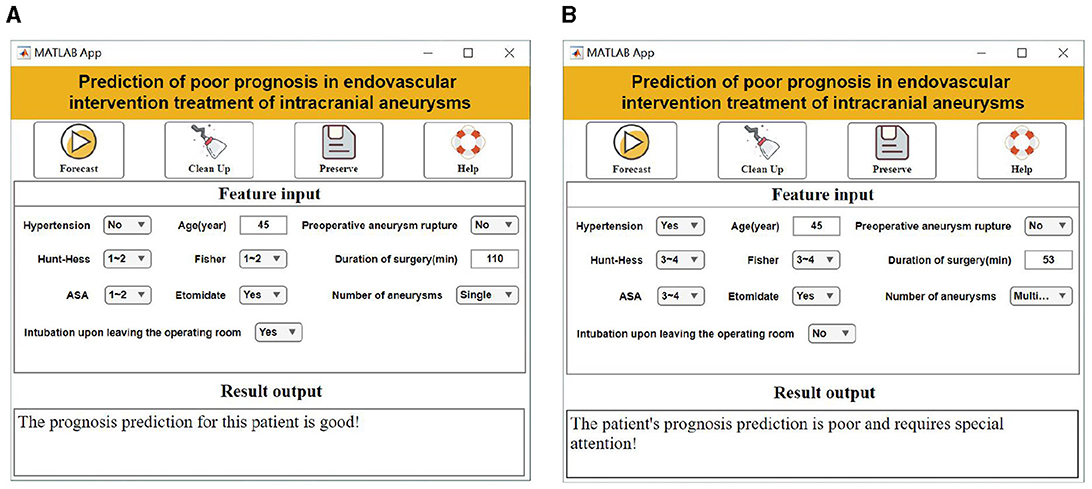
Figure 4. Visualization system interface display. (A) Good prognostic forecasting demonstration. (B) Demonstration of poor prognostic prediction.
Intracranial aneurysms are vascular abnormalities characterized by the abnormal bulging of intracranial artery walls, and their rupture is associated with high rates of disability and mortality (14, 15). Therefore, preventing aneurysm rebleeding is crucial. Endovascular intervention has increasingly been recommended as the preferred treatment for intracranial aneurysms due to its advantages such as reduced trauma, shorter operation times, and minimal damage to brain tissues. However, patients undergoing this procedure are susceptible to cerebral vasospasm and face a high rate of postoperative recurrence, both of which contribute to poor prognosis (16, 17). Consequently, analyzing the risk factors that influence the poor prognosis of endovascular intervention for intracranial aneurysms is vital for improving patient outcomes.
Machine learning has shown significant promise in enhancing clinical prediction efficacy by analyzing clinical data and applying specific algorithms to predict various outcomes (18, 19). By learning from multiple data modules, machine learning effectively identifies variables associated with patient outcomes, accurately predicts relevant risk factors, explores patterns, and builds mathematical models from complex data. It can also be iteratively calibrated during validation (20, 21). The Prairie Dog Optimization (PDO) algorithm, inspired by the behavior of prairie dogs, offers advantages such as easy implementation and balanced exploration and exploitation capabilities (22). However, it faces challenges such as slow convergence speed and low optimization accuracy. To address these issues, we implemented Tent chaotic initialization and t-distribution perturbation variation to initialize the population. Additionally, we incorporated suboptimal individual guidance strategies, natural enemy avoidance strategies, and adaptive probability threshold guidance strategies. These enhancements improve the algorithm's ability to avoid local optima and ensure higher solution accuracy and faster convergence speeds for the PDO algorithm (23).
In this study, we developed an improved machine learning model to predict poor prognosis in patients undergoing endovascular intervention for intracranial aneurysms. By adjusting relevant parameters for different algorithms and employing 5-fold cross-validation, we minimized the effect of randomness and prevented overfitting. Model performance on the test set was further enhanced through pruning, leading to the identification of 10 key variables: age, comorbid hypertension, preoperative aneurysm rupture, Hunt-Hess classification, Fisher score, ASA classification, number of aneurysms, intraoperative use of etomidate, intubation status upon leaving the operating room, and procedure duration.
The identified variables provide valuable insights into the factors influencing poor prognosis. For instance. Age: Older patients show decreased vascular elasticity and repair capabilities, increasing the risk of complications and recurrence post-intervention (24). Hypertension: Hypertension exacerbates damage to the intracranial vascular wall and alters hemodynamics, leading to higher risks during and after the procedure (25, 26). Preoperative Aneurysm Rupture: Ruptured aneurysms complicate the intervention due to fragile vasculature and increased aneurysm numbers, leading to higher recurrence and poor prognosis (27, 28). Hunt-Hess Classification and Fisher Score: Higher scores indicate severe intracranial hemorrhage and increased risk of complications like vasospasm and edema, which adversely affect outcomes (29–31). ASA Classification: Higher ASA scores reflect severe underlying conditions and lower surgical tolerance, impacting recovery and prognosis (32, 33).
Compared to traditional logistic regression models, our machine learning model leverages a broader range of clinical data and advanced algorithms to enhance predictive accuracy. Previous studies have utilized various machine learning techniques such as support vector machines and neural networks for similar purposes (7–9). However, our approach integrates the Prairie Dog Optimization (PDO) algorithm with enhancements like Tent chaotic initialization and t-distribution perturbation, which improve convergence speed and solution accuracy. The development of a visualization system based on our model allows clinicians to input specific patient data and receive immediate risk assessments and recommendations. This tool has significant potential for both public health research and clinical practice, aiding in early intervention and personalized treatment planning.
Although our study aims to construct a prediction and visualization system for poor prognosis in intracranial aneurysm endovascular treatment based on an improved machine learning model, we acknowledge several limitations.
Firstly, the predictive capability of our model is constrained by the quality and scale of the currently available dataset. A larger and more comprehensive dataset could potentially enhance the model's accuracy and stability. Secondly, our research focuses on specific treatment methods and populations. Future studies should consider a broader range of treatment methods and population factors to increase generalizability. Additionally, the interpretability and clinical applicability of the model require further optimization and improvement.
Future research can expand in several directions. Firstly, we can further optimize our machine learning model by exploring more advanced algorithms and technologies to enhance predictive performance and improve interpretability. Secondly, incorporating additional clinical variables and imaging features into the model could enhance the accuracy and comprehensiveness of prognosis prediction. Furthermore, integrating other advanced technologies, such as deep learning and natural language processing, could enrich the functionality and efficacy of the predictive model. Finally, integrating our system with actual clinical practice and conducting large-scale validation and application will ensure its effectiveness and reliability in real clinical settings.
By continuously refining our research methods and technologies, we are confident that future studies will provide more accurate and reliable support for prognosis prediction and clinical decision-making in intracranial aneurysm endovascular intervention treatment. This will offer greater hope and opportunities for patient treatment and recovery.
In summary, applying improved machine learning models to analyze patients' clinical data can help clinics predict the risk of poor prognosis following endovascular intervention for intracranial aneurysms at an early stage. This can assist in developing intervention programs to improve patient outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by the Ethics Committee of Fushun County People's Hospital (Approval Number: 2023-077). The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee waived the requirement of written informed consent from the participants or the participants' legal guardians/next of kin because of the retrospective nature of the study.
CL: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. AF: Data curation, Investigation, Writing – review & editing. BL: Investigation, Writing – review & editing. SZ: Investigation, Writing – review & editing. JL: Investigation, Writing – review & editing. YC: Investigation, Writing – original draft. BZ: Conceptualization, Methodology, Project administration, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sharma T, Datta KK, Kumar M, Dey G, Khan AA, Mangalaparthi KK, et al. Intracranial aneurysm biomarker candidates identified by a proteome-wide study. Omics. (2020) 24:483–92. doi: 10.1089/omi.2020.0057
2. Mormina E, Musumeci O, Tessitore A, Ciranni A, Tavilla G, Pitrone A, et al. Intracranial aneurysm management in patients with late-onset Pompe disease (LOPD). Neurol Sci. (2021) 42:2411–9. doi: 10.1007/s10072-020-04819-2
3. Ghanaati H, Rahmatian A, Amiri-Nikpour MR, Altafi D, Taheri M, Siroos SB, et al. Outcome after neuro-interventional treatment of intracranial aneurysm (as a first treatment modality). Prague Med Rep. (2024) 125:15–33. doi: 10.14712/23362936.2024.2
4. Yamamoto K, Shiomi H, Morimoto T, Miyazawa A, Watanabe H, Nakamura S, et al. Dual antiplatelet therapy duration after multivessel optimal intravascular ultrasound-guided percutaneous coronary intervention. Circ J. (2023) 87:1661–71. doi: 10.1253/circj.CJ-23-0141
5. Algra AM, Lindgren A, Vergouwen MDI, Greving JP, van der Schaaf IC, van Doormaal TPC, et al. Procedural clinical complications, case-fatality risks, and risk factors in endovascular and neurosurgical treatment of unruptured intracranial aneurysms: a systematic review and meta-analysis. J Am Med Assoc Neurol. (2019) 76:282–93. doi: 10.1001/jamaneurol.2018.4165
6. Rojas JC, Fahrenbach J, Makhni S, Cook SC, Williams JS, Umscheid CA, et al. Framework for integrating equity into machine learning models: a case study. Chest. (2022) 161:1621–7. doi: 10.1016/j.chest.2022.02.001
7. Stumpo V, Staartjes VE, Esposito G, Serra C, Regli L, Olivi A, et al. Machine learning and intracranial aneurysms: from detection to outcome prediction. Acta Neurochir Suppl. (2022) 134:319–31. doi: 10.1007/978-3-030-85292-4_36
8. Xiong W, Chen T, Li J, Xiang L, Zhang C, Xiang L, et al. Interpretable machine learning model to predict rupture of small intracranial aneurysms and facilitate clinical decision. Neurol Sci. (2022) 43:6371–9. doi: 10.1007/s10072-022-06351-x
9. Shu Z, Chen S, Wang W, Qiu Y, Yu Y, Lyu N, et al. Machine learning algorithms for rupture risk assessment of intracranial aneurysms: a diagnostic meta-analysis. World Neurosurg. (2022) 165:e137–47. doi: 10.1016/j.wneu.2022.05.117
10. Wilson L, Boase K, Nelson LD, Temkin NR, Giacino JT, Markowitz AJ, et al. A manual for the glasgow outcome scale-extended interview. J Neurotrauma. (2021) 38:2435–46. doi: 10.1089/neu.2020.7527
11. Ezugwu A E, Agushaka J O, Abualigah L, Mirjalili S, Gandomi AH. Prairie dog optimization algorithm. Neural Comput Appl. (2022) 34:20017–65. doi: 10.1007/s00521-022-07530-9
12. De-Simone SG, Napoleão-Pêgo P, Lechuga GC, Carvalho JPRS, Gomes LR, Cardozo SV, et al. High-throughput IgG epitope mapping of tetanus neurotoxin: implications for immunotherapy and vaccine design. Toxins. (2023) 15:239. doi: 10.3390/toxins15040239
13. Thompson GZ, Maitra R, Meeker WQ, Bastawros AF. Classification with the matrix-variate-t distribution. J Comput Graph Stat. (2020) 29:668–74. doi: 10.1080/10618600.2019.1696208
14. Finsterer J. Familial intracranial aneurysm requires not only whole-exome sequencing, but also mitochondrial DNA sequencing. Korean J Radiol. (2022) 23:566–7. doi: 10.3348/kjr.2022.0029
15. Juchler N, Schilling S, Bijlenga P, Morel S, Rüfenacht D, Kurtcuoglu V, et al. Shape irregularity of the intracranial aneurysm lumen exhibits diagnostic value. Acta Neurochir. (2020) 162:2261–70. doi: 10.1007/s00701-020-04428-0
16. Sakakura K, Ito Y, Shibata Y, Okamura A, Kashima Y, Nakamura S, et al. Clinical expert consensus document on rotational atherectomy from the Japanese association of cardiovascular intervention and therapeutics. Cardiovasc Interv Ther. (2021) 36:1–18. doi: 10.1007/s12928-020-00715-w
17. Kuno T, Kiyohara Y, Maehara A, Ueyama HA, Kampaktsis PN, Takagi H, et al. Comparison of intravascular imaging, functional, or angiographically guided coronary intervention. J Am Coll Cardiol. (2023) 82:2167–76. doi: 10.1016/j.jacc.2023.09.823
18. Díaz-Eufracio BI, Medina-Franco JL. Machine learning models to predict protein-protein interaction inhibitors. Molecules. (2022) 27:7986. doi: 10.3390/molecules27227986
19. Mahecha D, Nuñez H, Lattig MC, Duitama J. Machine learning models for accurate prioritization of variants of uncertain significance. Hum Mutat. (2022) 43:449–60. doi: 10.1002/humu.24339
20. Ritchie F, Tilbrook A, Cole C, Jefferson E, Krueger S, Mansouri-Benssassi E, et al. Machine learning models in trusted research environments - understanding operational risks. Int J Popul Data Sci. (2023) 8:2165. doi: 10.23889/ijpds.v8i1.2165
21. Galuzio PP, Cherif A. Recent advances and future perspectives in the use of machine learning and mathematical models in nephrology. Adv Chronic Kidney Dis. (2022) 29:472–9. doi: 10.1053/j.ackd.2022.07.002
22. Yu H, Wang Y, Jia H, Abualigah L. Modified prairie dog optimization algorithm for global optimization and constrained engineering problems. Math Biosci Eng. (2023) 20:19086–132. doi: 10.3934/mbe.2023844
23. Sahoo GK, Choudhury S, Rathore RS, Bajaj M. A novel prairie dog-based meta-heuristic optimization algorithm for improved control, better transient response, and power quality enhancement of hybrid microgrids. Sensors. (2023) 23:5973. doi: 10.3390/s23135973
24. Dawod G, Henkel ND, Salahuddin H, Castonguay AC, Koneru S, Mugge L, et al. Outcomes of treatment modalities for ruptured intracranial aneurysms based on age - a meta-analysis. J Stroke Cerebrovasc Dis. (2020) 29:105039. doi: 10.1016/j.jstrokecerebrovasdis.2020.105039
25. Quintana EM, García AG, Valdés PV, Deza EM, Augusto DE, Soria LV, et al. Combined surgery and embolization to treat ruptured cerebral aneurysms with cerebral hematoma and intracranial hypertension: a retrospective analysis and review of the literature. Radiologia. (2019) 61:42–50. doi: 10.1016/j.rxeng.2018.12.004
26. Furukawa H, Wada K, Tada Y, Kuwabara A, Sato H, Ai J, et al. Mast cell promotes the development of intracranial aneurysm rupture. Stroke. (2020) 51:3332–9. doi: 10.1161/STROKEAHA.120.030834
27. Zuurbier CCM, Bourcier R, Beaufils PCD, Redon R, Desal H, Investigators I, et al. Number of affected relatives, age, smoking, and hypertension prediction score for intracranial aneurysms in persons with a family history for subarachnoid hemorrhage. Stroke. (2022) 53:1645–50. doi: 10.1161/STROKEAHA.121.034612
28. Etminan N, Dörfler A, Steinmetz H. Unruptured intracranial aneurysms- pathogenesis and individualized management. Dtsch Arztebl Int. (2020) 117:235–42. doi: 10.3238/arztebl.2020.0235
29. Doron O, McLellan R, Vranic JE, Regenhardt RW, Stapleton CJ, Patel AB, et al. stent for acute flow diversion in ruptured cerebral aneurysms arising from small-caliber vessels: a clinical case series. Neurosurg Focus. (2023) 54:6. doi: 10.3171/2023.2.FOCUS22645
30. Shetova IM, Shtadler VD, Aronov MS, Piradov MA, Krylov VV. Otdalennye rezul'taty khirurgicheskogo lecheniya patsientov s arterial'nymi anevrizmami golovnogo mozga [Long-term results of surgical treatment of patients with cerebral arterial aneurysms]. Zh Nevrol Psikhiatr Im S S Korsakova. (2023) 123:41–9. doi: 10.17116/jnevro202312303241
31. Perez MA, Hellstern V, Candel CS, Wendl C, Bäzner H, Gansladt O, et al. Use of pCONUS HPC for the treatment of unruptured wide-necked bifurcation aneurysms: early clinical experience using single antiplatelet therapy. Stroke Vasc Neurol. (2021) 6:57–64. doi: 10.1136/svn-2020-000399
32. Bhamidipati CM, Tohill BC, Robe C, Reid KJ, Eglitis NC, Farber MA, et al. Physiologic risk stratification is important to long-term mortality, complications, and readmission in thoracic endovascular aortic repair. J Vasc Surg Cases Innov Tech. (2023) 9:101174. doi: 10.1016/j.jvscit.2023.101174
Keywords: improve machine learning models, intracranial aneurysm, intravascular intervention therapy, poor prognosis, visualization system
Citation: Lei C, Fu A, Li B, Zhou S, Liu J, Cao Y and Zhou B (2025) Construction of a poor prognosis prediction and visualization system for intracranial aneurysm endovascular intervention treatment based on an improved machine learning model. Front. Neurol. 15:1482119. doi: 10.3389/fneur.2024.1482119
Received: 17 August 2024; Accepted: 16 December 2024;
Published: 08 January 2025.
Edited by:
Luis Rafael Moscote-Salazar, Colombian Clinical Research Group in Neurocritical Care, ColombiaReviewed by:
Luis Alberto Camputaro, Specialized Institute “Hospital El Salvador”, El SalvadorCopyright © 2025 Lei, Fu, Li, Zhou, Liu, Cao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Zhou, eGlheXV5b3VxaW5ndGlhbkAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.