- Neuroscience and Ophthalmology, Department of Inflammation and Ageing, School of Infection, Inflammation and Immunology, College of Medicine and Health, University of Birmingham, Birmingham, United Kingdom
Background: Photobiomodulation (PBM), using red- or near-infrared light, has been used to treat tendinopathies, nerve injuries, osteoarthritis and wounds and evaluated in experimental allergic encephalomyelitis (EAE). To date, only a few studies have been performed in EAE but surprisingly, a few clinical studies in humans have already been performed, despite the paucity of preclinical evidence.
Objective: Therefore, this study systematically reviewed the usefulness of PBM in ameliorating the clinical signs of EAE, a commonly used animal model of multiple sclerosis, and determine if there is enough evidence to warrant human studies.
Methods: PubMed, EMBASE and Web of Science were searched in July 2024 for studies relating to PBM and EAE without any language restrictions. Since only three studies have been published, all studies were included in the systematic review and data related to clinical signs of EAE was pooled together to conduct a meta-analysis. Non-homogenous data was also reported and thematically synthesized.
Results: A meta-analysis of the pooled data from the three included studies demonstrated a significant reduction of the clinical severity of EAE, with a mean reduction of 1.44, 95% CI (−2.45, −0.42), p = 0.006. PBM also significantly reduced other parameters such as infiltration of mononuclear cells, CNS demyelination, apoptosis markers and pro-inflammatory cytokines. However, there was an overall high risk of bias in all of the studies.
Conclusion: The meta-analysis supports the use of PBM to ameliorate the symptoms of EAE, but the paucity of studies and the high risk of bias in the included studies warrants further preclinical investigation before conducting human studies.
1 Introduction
Multiple sclerosis (MS) is an inflammatory autoimmune neurodegenerative condition of the human central nervous system (CNS) that is caused by Th1 and Th17 lymphocytes, which induce a response to components of the degenerating myelin (1–3). In the CNS, ongoing inflammation, reactive gliosis (response of glial cells to injury or disease) and axonal injury followed by demyelination characterise the disease, with progressive worsening and increasing disability. MS typically occurs in patients between the ages of 20 to 40 years and affects 2:1 women than men (4, 5). The eventual symptoms of MS include visual loss, spasticity, weakness, impaired walking and coordination, ataxia and bladder problems (5, 6). Fatigue, neuropathic pain and cognitive issues are also common in affected patients and usually manifest before a definitive diagnosis of MS. (7, 8)
Low level laser therapy (LLLT) has been used to treat a variety of tissues such as tendons, nerves, skin, bones, muscle and the CNS (9–12). LLLT modulates cellular processes such as cell and tissue death, promotes wound healing, reduces pain, swelling and inflammation (9, 13–15). The effects of LLLT are mainly through photobiomodulation (PBM), where red- or near-infrared light (range 600–1,000 nm) can be absorbed by the main light sensitive chromophore in mitochondria, cytochrome c oxidase (the terminal electron acceptor of the respiratory chain in mitochondria). This leads to cellular respiration, formation of ATP, modulation of oxidative stress and production of nitric oxide (NO) which can trigger cell signalling and gene expression transcription. PBM applied transcranially to the brain, has been shown to regulate a variety of processes including regulation of microglial function through Src kinases (a non-receptor tyrosine kinase that is activated by oxidative events) (16). PBM also promotes neuronal survival and axon regeneration after spinal cord injury (12) and reduces long-term neurological deficits after traumatic brain injury (17).
Experimental allergic encephalomyelitis (EAE) is the most well-established animal model of MS, caused by CD4+ T-cell-mediated inflammatory demyelination of the CNS in rodents (18). Much of the understanding of the pathogenesis of MS is based on studies using the EAE model and it is generally accepted that autoreactive, myelin-specific T cells (T cells which migrate across the blood-brain barrier and mediate damage of neurons and their myelin sheaths) initiate the disease and progression occurs through secretion of proinflammatory cytokines that includes interferon-gamma (IFN-γ), interleukin (IL)-17 and tumor necrosis factor alpha (TNF-α) (19). Recovery from EAE and disease amelioration is thought to occur through anti-inflammatory cytokines such as IL-10 and IL-4 (20). There are a just a few studies of the use of PBM in EAE. However, at present, there are no systematic reviews on the efficacy of PBM therapy in EAE that can be used to support further translational studies or progression into clinical trials. Therefore, the aim of this study was to systematically review the data regarding the use of PBM in EAE and determine its benefits in ameliorating the clinical signs of disease.
2 Materials and methods
2.1 Search strategy
This systematic review and meta-analysis was performed under the preferred reporting of items for systematic reviews and meta-analysis (PRISMA) statement guidelines (21). A comprehensive search was conducted in PubMed, Scopus, EMBASE and Web of Science in July 2024. The following search strategy along with Boolean and MeSH terms were used for the search: (photobiomodulation OR low-level light therapy OR low-level laser therapy OR red-light therapy OR LLLT OR PBM) AND (multiple sclerosis OR MS OR experimental allergic encephalomyelitis OR EAE OR experimental autoimmune encephalomyelitis). Moreover, a manual search of references was used to expand the yield of further relative studies.
2.2 Eligibility and study selection
Since there were only three studies evaluating PBM in EAE, all studies were included without language restrictions. Literature reviews, commentaries, conference papers or editorials were all excluded.
2.3 Data extraction
A predesigned Excel sheet was used to capture data from animal studies, including information regarding: study characteristics (author, year of publication, country) and study details (e.g., number of animals, EAE induction protocol) as well as PBM treatment regimen. Primary outcomes related to amelioration of clinical scores of EAE by PBM therapy whilst secondary outcomes included data collection on infiltration of mononuclear cells into the CNS, levels of nitric oxide in spinal cords, TUNEL+ cell counts and changes in pro- and anti-inflammatory cytokines.
2.4 Risk of bias assessment
Risk of bias in included studies was analysed using the SYRCLE risk of bias assessment tool (22), which is specific to animal studies and has been adapted from the Cochrane Collaboration’s tool for assessing risk of bias in RCTs (23), and used by us previously (24–26).
2.5 Data synthesis and analysis
A meta-analysis was conducted with pooled data from all three studies related to clinical scores of EAE where homogenous numerical data was available. The meta-analysis was carried out using RevMan version 5.4.1 software from Cochrane Informatics & Technology, using a random effects model and reporting mean differences and 95% confidence intervals. Other data presented in the three studies were heterogenous and hence a thematic, narrative synthesis of all data within each study is presented.
3 Results
3.1 Study selection
A total of three studies were identified as a result of the comprehensive searches in the three databases as well as searching through the reference list of publications. There were no duplicates to remove, and all three articles were included after the screening process and full-text reading (Figure 1).
3.2 Characterization of the included studies
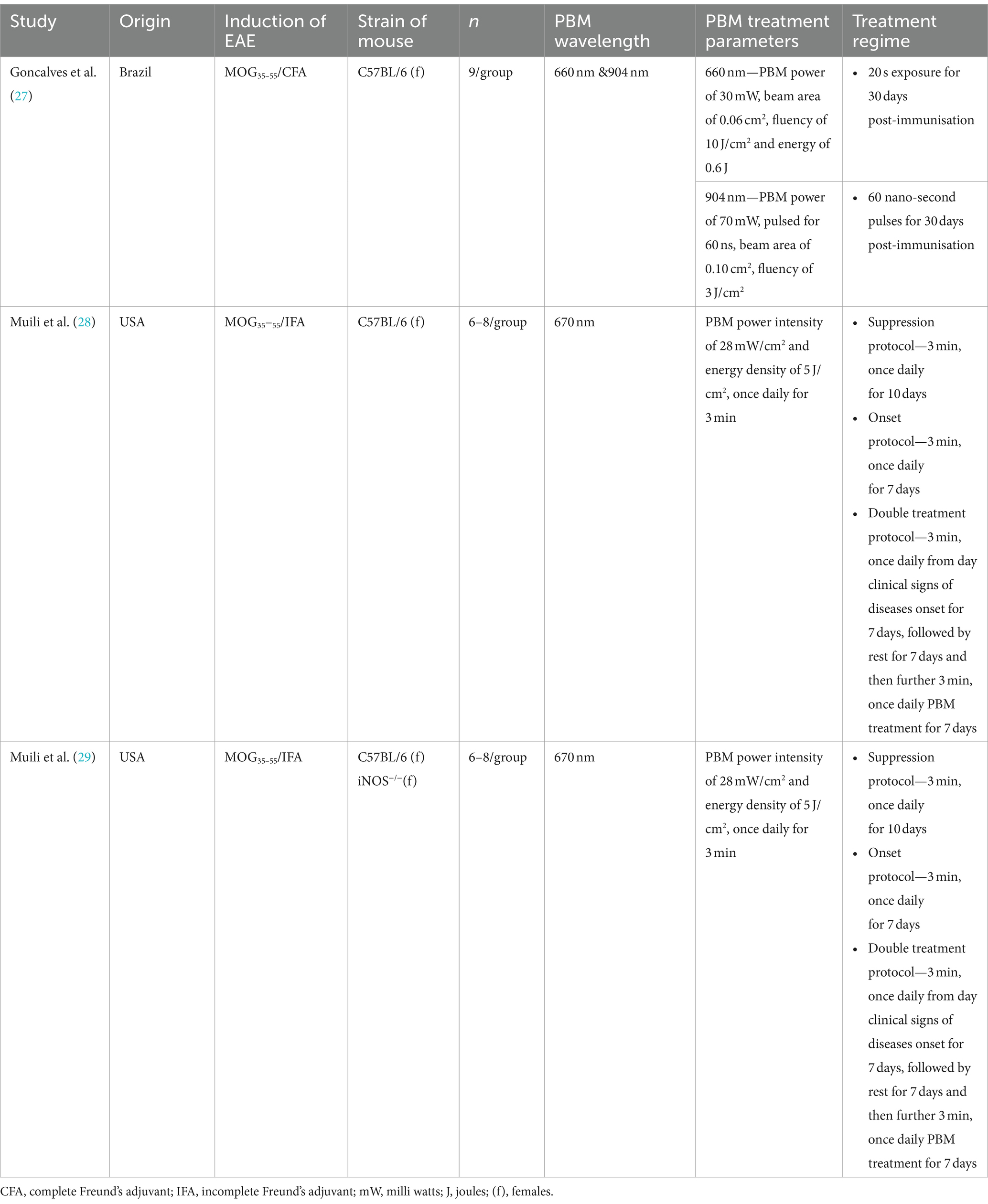
The characteristics of the studies are shown in Table 1. One study was from Brazil (27) whilst the other two studies were from the same lab in the US (28, 29), but all three studies used mouse models of EAE in females. For the EAE induction protocol, one study used MOG35–55/complete Freund’s adjuvant (CFA) (27) whilst the other two studies used MOG35–55/incomplete Freund’s adjuvant (IFA) followed by either 500 μg or 200 μg of Mycobacterium tuberculosis, respectively. All three studies used 660/670 nm PBM wavelength (27–29), whilst one study also used a wavelength of 904 nm (27). There were slight differences in the treatment regimen between the three studies with the two studies from the same lab using the same protocol, including PBM power, fluence and timings (28, 29). For example, PBM parameters in these two studies included a power intensity of 28 mW/cm2, energy density of 5 J/cm2 and once daily for 3-min exposure times.
PBM therapy was delivered by either focussing the laser on the spinal cord at an angle of 90° to the skin, timed to contact at six points on the spinal cord, each 0.5 cm apart (27), or by placing mice in a polypropylene restraint device (12.7 × 9 × 7.6 cm) and PBM delivered using an LED array positioned directly over the animal at a distance of 2 cm, covering the entire chamber and exposing the entire dorsal surface (28, 29). In addition, two studies also employed PBM therapy to several groups of mice in different treatment protocols: suppression protocol (once daily treatment for 10 days starting at 24 h after immunisation), onset protocol (once daily treatment for 7 days starting at the day of onset of clinical signs (score 1.0)) and double treatment protocol (once daily for 7 days on the day of onset of clinical signs (score 1.0)) followed by rest for seven days and then a subsequent seven days of once daily treatments (28, 29).
3.3 PBM ameliorates clinical signs of EAE
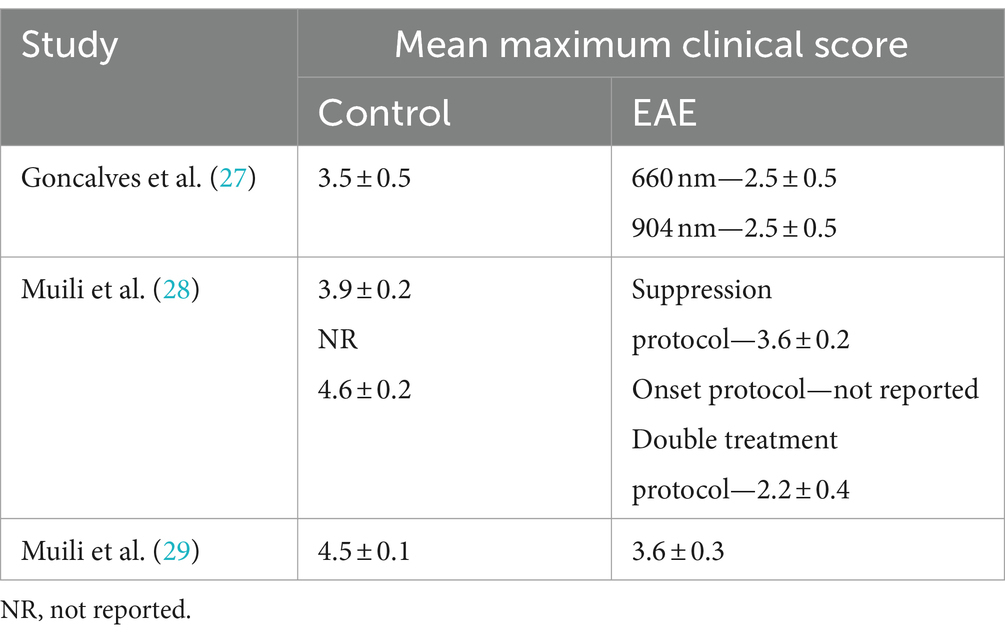
The clinical signs of EAE were recorded daily using a standard 0–5 scale: 0 = healthy; 1 = loss of tail tone; 2 = hind limb weakness; 3 = paresis or paralysis of one hind limb; 4 = paralysis of both hind limbs and 5 = dead or moribund. PBM therapy significantly ameliorated the severity of EAE in all animals tested, reducing them all back down to scores of 2.2–2.5 (i.e., hind limb weakness/paresis of one hind limb) (Table 2). Use of 904 nm wavelength PBM had the same effect at ameliorating EAE disease severity as 660/670 nm (27).
A meta-analysis of the pooled data from all three studies demonstrated that amelioration of EAE clinical scores by PBM therapy was significant, with a mean difference of −1.44, 95% CI (−2.45, −0.42), p = 0.006 (Figure 2).
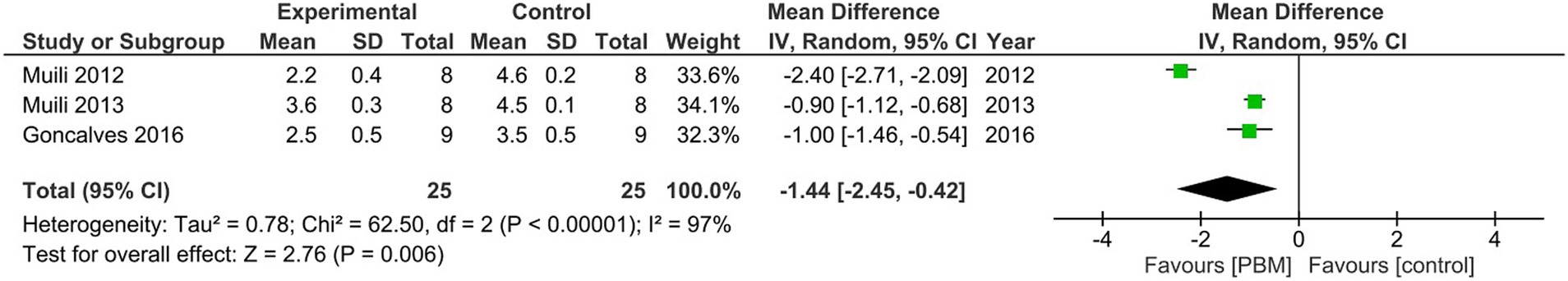
Figure 2. Meta-analysis of the pooled data from the three studies to show significant effects of PBM therapy on clinical score of EAE.
3.4 Other effects of PBM therapy in EAE
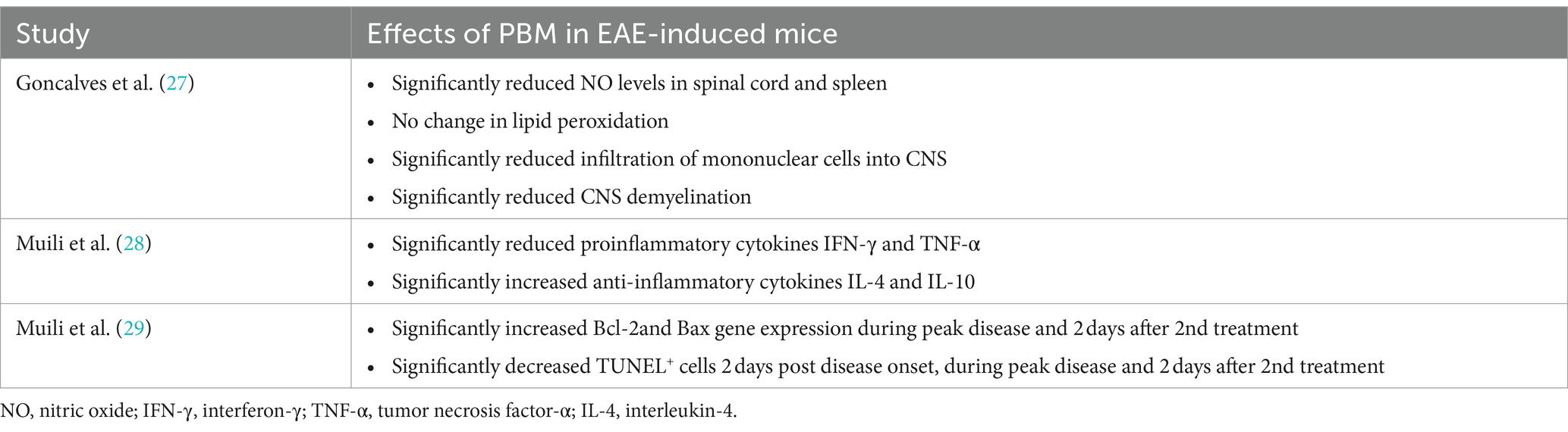
PBM therapy significantly reduced the levels of nitric oxide (NO) in the spinal cord and spleen, infiltration of mononuclear cells into the CNS and CNS demyelination without affecting lipid peroxidation in EAE treated mice (27) (Table 3). PBM also significantly reduced Bcl-2 and Bax gene expression as well as TUNEL+ cells during peak disease and after double treatment of EAE and reduced pro-inflammatory cytokines (IFN-γ and TNF-α) and increased anti-inflammatory cytokines (IL-4 and IL-10) (28, 29) (Table 3).
3.5 Risk of bias in included studies
Analysis of the risk of bias in the included studies using the SYRCLE tool for animal studies showed evidence of high risk of bias in all three studies such that domains including sample size calculation, reporting of incomplete data, blinding of outcome assessment and random outcome assessment were all judged as “no or not reported” (Figures 3A,B). Two of the three studies blinded the participants and personnel and only one study concealed the treatment group allocation and generated a random sequence to treat. However, all three studies specified the primary outcome and described baseline characteristics (Figures 3A,B). In summary, there was an overall high risk of bias in all of the studies.
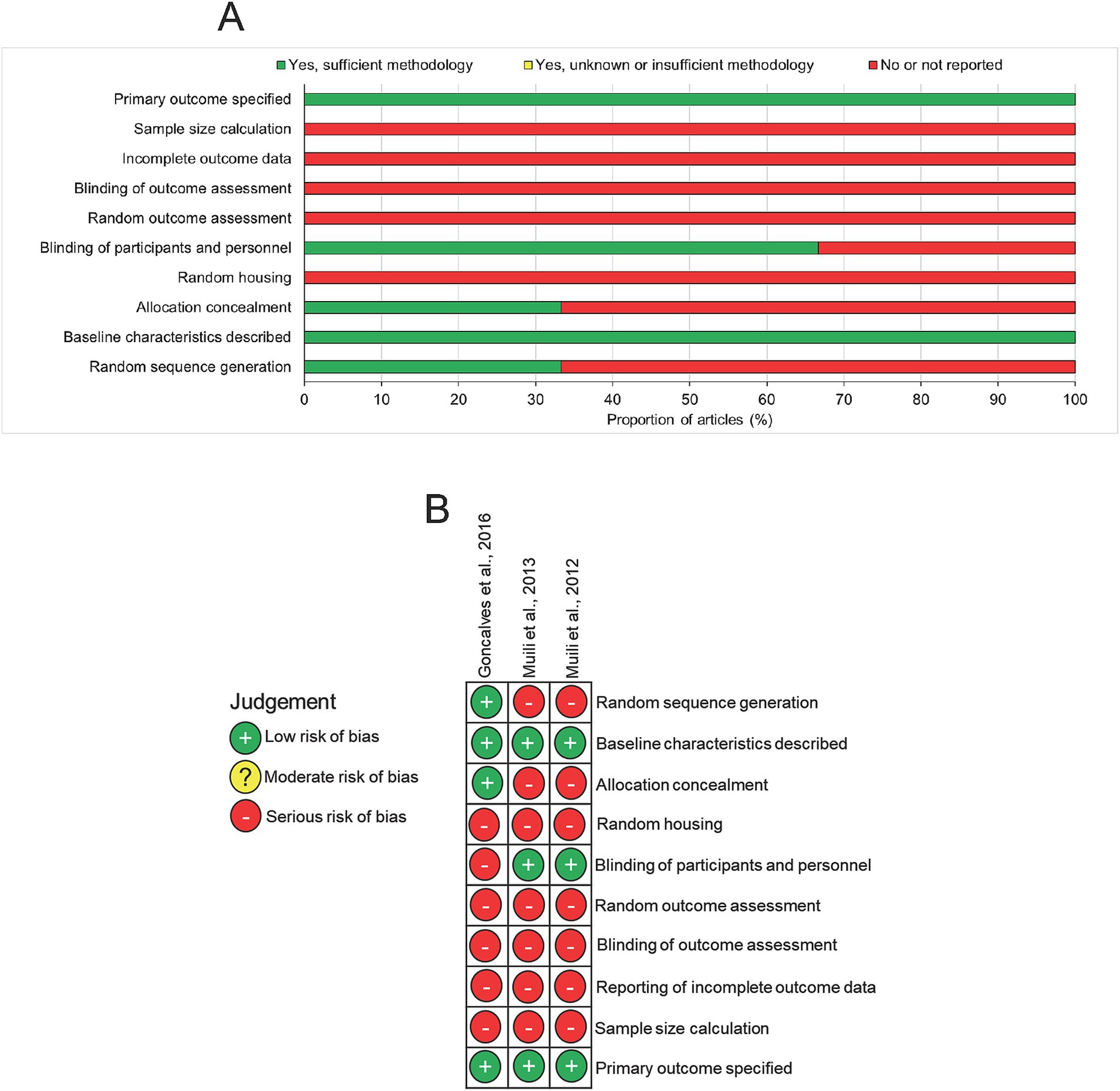
Figure 3. Risk of bias assessment in included studies. (A) Summary diagram and (B) risk of bias in individual studies.
4 Discussion
This systematic search in three databases found only three published studies, which evaluated the effects of PBM in the progression of EAE. All of the studies were performed in female mice using similar EAE induction protocols. We found that PBM can ameliorate the clinical signs of EAE, reducing clinical scores back down to 2.2–2.5. Meta-analysis showed that this reduction in clinical scores was significant. PBM also suppressed NO and infiltration of mononuclear cells into the CNS, reduced markers of cell death and reduced pro-inflammatory cytokines whilst increased anti-inflammatory cytokines. However, the overall risk of bias was judged as high since many of the domains assessed did not report adherence to robust experimental design.
To our knowledge this is the first systematic review and meta-analysis of the effects of PBM therapy on EAE. The fact that we could only find three studies in EAE demonstrates how relatively unexplored this area of research is, given that the first studies in EAE were conducted over a decade ago. There has also been no other reported study in the EAE model since 2016 and hence the use of PBM therapy remains a largely unexplored area of research in EAE. This paucity of studies in the EAE model limits the usefulness of this systematic review but highlights the need for further studies looking into this area of research. We also found another study of the effects of PBM, but in a cuprizone model of demyelination where six sessions of transcranial PBM were delivered to cuprizone-treated mice on three consecutive days, during the third and fourth weeks using 36 J/cm2, 50 mW and 0.028 cm2 spot area (30). The study found that PBM-treated mice presented with improved motor performance, attenuated demyelination, increased number of oligodendrocyte precursor cells, reduced microglial and astrocyte activation and a milder toxicity to cuprizone (30).
We are unsure why this work has not been followed up by more studies, given the initial promising results in all three studies and the relatively safe, efficacious and non-invasive way of delivering PBM in animal models. The significant reduction of clinical scores of EAE by all three studies points to a potential benefit of PBM in MS as well as reductions in NO in the spinal cord and spleen, which are normally enhanced in MS (31) as well as reduced demyelination, reduced cell death and reduction in pro-inflammatory cytokines and increases in anti-inflammatory cytokines all point to the benefits of PBM to multiple signalling pathways. A significant worry of all the animal studies in our systematic review is the high risk of bias. Whether this is from lack of reporting by authors or a failure to give importance to these parameters in animal studies remains to be determined. However, the high risk of bias in all included studies, especially in domains such as sample size calculation, blinding of outcome assessment, random outcome assessment and reporting of how incomplete outcome data, if any, was handled, means that conclusions from this systematic review must be made with caution. The high risk of bias in animal studies can be avoided by adhering to the standardised techniques in animal experiments based on the Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines (32, 33).
In support of PBM, two studies from the same authors showed benefits of PBM in suppressing IFN-γ and increasing IL-10 production by MS patient-derived peripheral blood mononuclear cells (PBMCs) and CD4+ T cells as well as reduced levels of nitrite that correlated with increased production of IL-10 and reduced production of IFN-γ (34). In a randomised controlled trial involving 14 patients with relapsing-remitting MS, PBM therapy to the sublingual region or over the radial artery using 808 nm wavelength and output power of 100 mW for 360 s, twice weekly for 24 sessions, led to significant increases in levels of anti-inflammatory cytokine, IL-10, in peripheral blood from both treatment groups but the levels of nitrites, which are metabolites of nitric oxide and are released by microglia and contribute to oxidative stress and demyelination, were not modulated in either treatment group (35). In two studies on the effects of PBM on muscle function in MS patients: one study shows that PBM treatment had no significant benefits on fatigue, one of the main symptoms of MS which has a negative impact on quality of life and few treatment options (36); whilst another study showed PBM therapy, administered to the belly of the tibialis anterior muscle, improved muscle force recovery and muscle strength in individuals with mild–moderate MS. (37) These studies suggest that there are benefits of PBM in MS patients and further high-quality studies are required to explore these benefits.
One significant advantage of PBM is that it can be delivered non-invasively and shown to have positive benefits. For example, in the EAE studies, PBM was delivered transcutaneously at several spots focussed on the spinal cord or over whole dorsal surface (27–29). In humans with MS, PBM has been delivered sublingually, over the radial artery or to the belly of the tibialis anterior muscle, all non-invasive methods of PBM delivery. There is significant interest in PBM dosimetry since inconsistencies in clinical outcomes of PBM are mainly due to problems in reporting PBM dosing and delivery (38). Therefore, for greater efficacy, other PBM delivery methods may need to be investigated, including implantable PBM devices (12). There is, however, a precedence to use PBM since it is already approved in the UK by the National Institute of Clinical Excellence (NICE) for use in the prevention or treatment of oral mucositis (39).
4.1 Limitations
The main limitation of this systematic review was the paucity of studies evaluating the efficacy of PBM in EAE. We found only three studies in animal models of MS that used PBM to monitor benefits in EAE models. Of these three studies, two were from the same lab and hence is an additional limitation. Another limitation is that the treatment regimen was different between studies with the two studies from the same laboratory reporting the same treatment regimen whilst the third study used a different treatment regimen. Finally, the high risk of bias in the three studies means that definitive conclusions from these studies on the usefulness of PBM in EAE models cannot be reliably made.
5 Conclusion
This systematic review shows that PBM can significantly reduce clinical signs of EAE in mice with associated benefits in terms of suppressing pro- and increasing anti-inflammatory cytokines, along with reduced demyelination, NO levels and markers of apoptosis. However, the study is limited in only three studies and the high risk of bias makes it difficult to make definitive conclusions about PBM and its usefulness in EAE. There is some additional supporting evidence from a limited number of human studies in MS patients, beginning to reveal some positive benefits to MS patients. These show that PBM not only suppresses pro-inflammatory cytokines and increases anti-inflammatory cytokines, which may help to counter ongoing disease, but also reduces neuron and axon damaging nitrites. To realise the full benefits of PBM however, further studies in the EAE model and more rigorous studies in MS are warranted.
Author contributions
ZA: Methodology, Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Haki, M, Al-Biati, HA, Al-Tameemi, ZS, Ali, IS, and Al-Hussaniy, HA. Review of multiple sclerosis: epidemiology, etiology, pathophysiology, and treatment. Medicine. (2024) 103:e37297. doi: 10.1097/MD.0000000000037297
2. Haase, S, and Linker, RA. Inflammation in multiple sclerosis. Ther Adv Neurol Disord. (2021) 14:17562864211007687. doi: 10.1177/17562864211007687
3. Ramaglia, V, Rojas, O, Naouar, I, and Gommerman, JL. The ins and outs of central nervous system inflammation-lessons learned from multiple sclerosis. Annu Rev Immunol. (2021) 39:199–226. doi: 10.1146/annurev-immunol-093019-124155
4. Kappos, L, O’Connor, PW, Polman, CH, Vermersch, P, Wiendl, H, Pace, A, et al. Clinical effects of natalizumab on multiple sclerosis appear early in treatment course. J Neurol. (2013) 260:1388–95. doi: 10.1007/s00415-012-6809-7
5. McGinley, MP, Goldschmidt, CH, and Rae-Grant, AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. (2021) 325:765–79. doi: 10.1001/jama.2020.26858
6. Ghasemi, N, Razavi, S, and Nikzad, E. Multiple sclerosis: pathogenesis, symptoms, diagnoses and cell-based therapy. Cell J. (2017) 19:1–10. doi: 10.22074/cellj.2016.4867
7. Noseworthy, JH, Lucchinetti, C, Rodriguez, M, and Weinshenker, BG. Multiple sclerosis. N Engl J Med. (2000) 343:938–52. doi: 10.1056/NEJM200009283431307
8. Chalah, MA, Riachi, N, Ahdab, R, Creange, A, Lefaucheur, JP, and Ayache, SS. Fatigue in multiple sclerosis: neural correlates and the role of non-invasive brain stimulation. Front Cell Neurosci. (2015) 9:460. doi: 10.3389/fncel.2015.00460
9. Mansouri, V, Arjmand, B, Rezaei Tavirani, M, Razzaghi, M, Rostami-Nejad, M, and Hamdieh, M. Evaluation of efficacy of low-level laser therapy. J Lasers Med Sci. (2020) 11:369–80. doi: 10.34172/jlms.2020.60
10. Stevens, AR, Hadis, M, Milward, M, Ahmed, Z, Belli, A, Palin, W, et al. Photobiomodulation in acute traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. (2023) 40:210–27. doi: 10.1089/neu.2022.0140
11. Davies, DJ, Hadis, M, Di Pietro, V, Lazzarino, G, Forcione, M, Harris, G, et al. Photobiomodulation reduces hippocampal apoptotic cell death and produces a Raman spectroscopic “signature”. PLoS One. (2022) 17:e0264533. doi: 10.1371/journal.pone.0264533
12. Stevens, AR, Mohammed, H, Phillips, A, Thareja, A, Milward, M, Belli, A, et al. Implantable and transcutaneous photobiomodulation promote neuroregeneration and recovery of lost function after spinal cord injury. Bioeng Transl Med. (2024) 2024:e10674. doi: 10.1002/btm2.10674
13. Vaghardoost, R, Momeni, M, Kazemikhoo, N, Mokmeli, S, Dahmardehei, M, Ansari, F, et al. Effect of low-level laser therapy on the healing process of donor site in patients with grade 3 burn ulcer after skin graft surgery (a randomized clinical trial). Lasers Med Sci. (2018) 33:603–7. doi: 10.1007/s10103-017-2430-4
14. Kazemikhoo, N, Vaghardoost, R, Dahmardehei, M, Mokmeli, S, Momeni, M, Nilforoushzadeh, MA, et al. Evaluation of the effects of low level laser therapy on the healing process after skin graft surgery in burned patients (a randomized clinical trial). J Lasers Med Sci. (2018) 9:139–43. doi: 10.15171/jlms.2018.26
15. Gur, A, Karakoc, M, Cevik, R, Nas, K, Sarac, AJ, and Karakoc, M. Efficacy of low power laser therapy and exercise on pain and functions in chronic low back pain. Lasers Surg Med. (2003) 32:233–8. doi: 10.1002/lsm.10134
16. Cardoso, FDS, Salehpour, F, Coimbra, NC, Gonzalez-Lima, F, and Gomes da Silva, S. Photobiomodulation for the treatment of neuroinflammation: a systematic review of controlled laboratory animal studies. Front Neurosci. (2022) 16:1006031. doi: 10.3389/fnins.2022.1006031
17. Lim, L . Traumatic brain injury recovery with Photobiomodulation: cellular mechanisms, clinical evidence, and future potential. Cells. (2024) 13:385. doi: 10.3390/cells13050385
18. Steinman, L, Patarca, R, and Haseltine, W. Experimental encephalomyelitis at age 90, still relevant and elucidating how viruses trigger disease. J Exp Med. (2023) 220:e2022132. doi: 10.1084/jem.20221322
19. Constantinescu, CS, Farooqi, N, O’Brien, K, and Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. (2011) 164:1079–106. doi: 10.1111/j.1476-5381.2011.01302.x
20. Dai, H, Ciric, B, Zhang, GX, and Rostami, A. Interleukin-10 plays a crucial role in suppression of experimental autoimmune encephalomyelitis by Bowman–Birk inhibitor. J Neuroimmunol. (2012) 245:1–7. doi: 10.1016/j.jneuroim.2012.01.005
21. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
22. Hooijmans, CR, Rovers, MM, de Vries, RB, Leenaars, M, Ritskes-Hoitinga, M, and Langendam, MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
23. Higgins, JP, Altman, DG, Gotzsche, PC, Juni, P, Moher, D, Oxman, AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
24. Thareja, A, Hughes, H, Alvarez-Lorenzo, C, Hakkarainen, JJ, and Ahmed, Z. Penetration enhancers for topical drug delivery to the ocular posterior segment—a systematic review. Pharmaceutics. (2021) 13:276. doi: 10.3390/pharmaceutics13020276
25. Masterman, E, and Ahmed, Z. Experimental treatments for oedema in spinal cord injury: a systematic review and meta-analysis. Cells. (2021) 10:2682. doi: 10.3390/cells10102682
26. Lelyte, I, Ahmed, Z, Kaja, S, and Kalesnykas, G. Structure-function relationships in the rodent streptozotocin-induced model for diabetic retinopathy: a systematic review. J Ocul Pharmacol Ther. (2022) 38:271–86. doi: 10.1089/jop.2021.0128
27. Goncalves, ED, Souza, PS, Lieberknecht, V, Fidelis, GS, Barbosa, RI, Silveira, PC, et al. Low-level laser therapy ameliorates disease progression in a mouse model of multiple sclerosis. Autoimmunity. (2016) 49:132–42. doi: 10.3109/08916934.2015.1124425
28. Muili, KA, Gopalakrishnan, S, Meyer, SL, Eells, JT, and Lyons, JA. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by photobiomodulation induced by 670 nm light. PLoS One. (2012) 7:e30655. doi: 10.1371/journal.pone.0030655
29. Muili, KA, Gopalakrishnan, S, Eells, JT, and Lyons, JA. Photobiomodulation induced by 670 nm light ameliorates MOG35–55 induced EAE in female C57BL/6 mice: a role for remediation of nitrosative stress. PLoS One. (2013) 8:e67358. doi: 10.1371/journal.pone.0067358
30. Duarte, KCN, Soares, TT, Magri, AMP, Garcia, LA, Le Sueur-Maluf, L, Renno, ACM, et al. Low-level laser therapy modulates demyelination in mice. J Photochem Photobiol B. (2018) 189:55–65. doi: 10.1016/j.jphotobiol.2018.09.024
31. Koch, M, Ramsaransing, GS, Arutjunyan, AV, Stepanov, M, Teelken, A, Heersema, DJ, et al. Oxidative stress in serum and peripheral blood leukocytes in patients with different disease courses of multiple sclerosis. J Neurol. (2006) 253:483–7. doi: 10.1007/s00415-005-0037-3
32. Percie du Sert, N, Hurst, V, Ahluwalia, A, Alam, S, Avey, MT, Baker, M, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. (2020) 18:e3000410. doi: 10.1371/journal.pbio.3000410
33. Percie du Sert, N, Ahluwalia, A, Alam, S, Avey, MT, Baker, M, Browne, WJ, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. (2020) 18:e3000411. doi: 10.1371/journal.pbio.3000411
34. Tolentino, M, Cho, CC, and Lyons, JA. Photobiomodulation at 830 nm reduced nitrite production by peripheral blood mononuclear cells isolated from multiple sclerosis subjects. Photobiomodul Photomed Laser Surg. (2022) 40:480–7. doi: 10.1089/photob.2021.0170
35. Silva, T, Fragoso, YD, Destro Rodrigues, MFS, Gomes, AO, da Silva, FC, Andreo, L, et al. Effects of photobiomodulation on interleukin-10 and nitrites in individuals with relapsing-remitting multiple sclerosis—randomized clinical trial. PLoS One. (2020) 15:e0230551. doi: 10.1371/journal.pone.0230551
36. Silva, T, Alencar, RC, de Souza Silva, BC, Viana, E, Fragoso, YD, Gomes, AO, et al. Effect of photobiomodulation on fatigue in individuals with relapsing-remitting multiple sclerosis: a pilot study. Lasers Med Sci. (2022) 37:3107–13. doi: 10.1007/s10103-022-03567-3
37. Rouhani, M, Tolentino, M, Lyons, JA, and Ng, AV. Effects of photobiomodulation therapy on muscle function in individuals with multiple sclerosis. Mult Scler Relat Disord. (2024) 86:105598. doi: 10.1016/j.msard.2024.105598
38. Gobbo, M, Merigo, E, Arany, PR, Bensadoun, RJ, Santos-Silva, AR, Gueiros, LA, et al. Quality assessment of PBM protocols for oral complications in head and neck cancer patients: part 1. Front Oral Health. (2022) 3:945718. doi: 10.3389/froh.2022.945718
Keywords: multiple sclerosis, photobiomodulation, experimental allergic encephalomyelitis, clinical signs, tumor necrosis factor-alpha, interleukin-10
Citation: Ahmed Z (2024) The benefits of photobiomodulation in animal models of multiple sclerosis: a systematic review and meta-analysis. Front. Neurol. 15:1482096. doi: 10.3389/fneur.2024.1482096
Edited by:
Omid Mirmosayyeb, University at Buffalo, United StatesReviewed by:
Luis Del Carpio-Orantes, Mexican Social Security Institute, MexicoOscar Kurt Bitzer Quintero, Centro de Investigación Biomédica de Occidente (CIBO), Mexico
Reza Kord, University of Cincinnati, United States
Copyright © 2024 Ahmed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zubair Ahmed, ei5haG1lZC4xQGJoYW0uYWMudWs=
†ORCID: Zubair Ahmed, http://orcid.org/0000-0001-6267-6442
 Zubair Ahmed
Zubair Ahmed