- 1UPMC Stroke Institute and Department of Neurology, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States
- 2Department of Neurology, Comprehensive Stroke Center, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Background: Renal dysfunction is a known predictor of long-term functional dependency after anterior circulation large vessel occlusion (ACLVO) stroke. However, the impact of renal dysfunction on early infarct growth rate (IGR) has not been previously demonstrated. The objective of this study was to define the association of creatinine-based renal biomarkers with fast or slow progressor phenotypes and related clinical outcomes in ACLVO stroke.
Methods: This retrospective study examined patients with acute intracranial internal carotid artery or middle cerebral artery-M1 occlusions admitted between 2014 and 2019. Patients were included if they received baseline CT perfusion (CTP) or MRI on presentation within 24 h of estimated stroke onset. Infarct growth rate (IGR) was determined by ischemic core volume on CTP or MRI divided by time from stroke onset to imaging. IGR was used to stratify fast progressor (IGR ≥10 mL/h) and slow progressor (IGR < 10 mL/h) status. Renal dysfunction was assessed based on serum creatinine and estimated glomerular filtration rate (eGFR) on presenting laboratories. Logistic regression models, adjusted for significant covariates, identified independent associations between renal dysfunction biomarkers, progressor status, and clinical outcomes based on modified Rankin Scale (mRS) at 90 days.
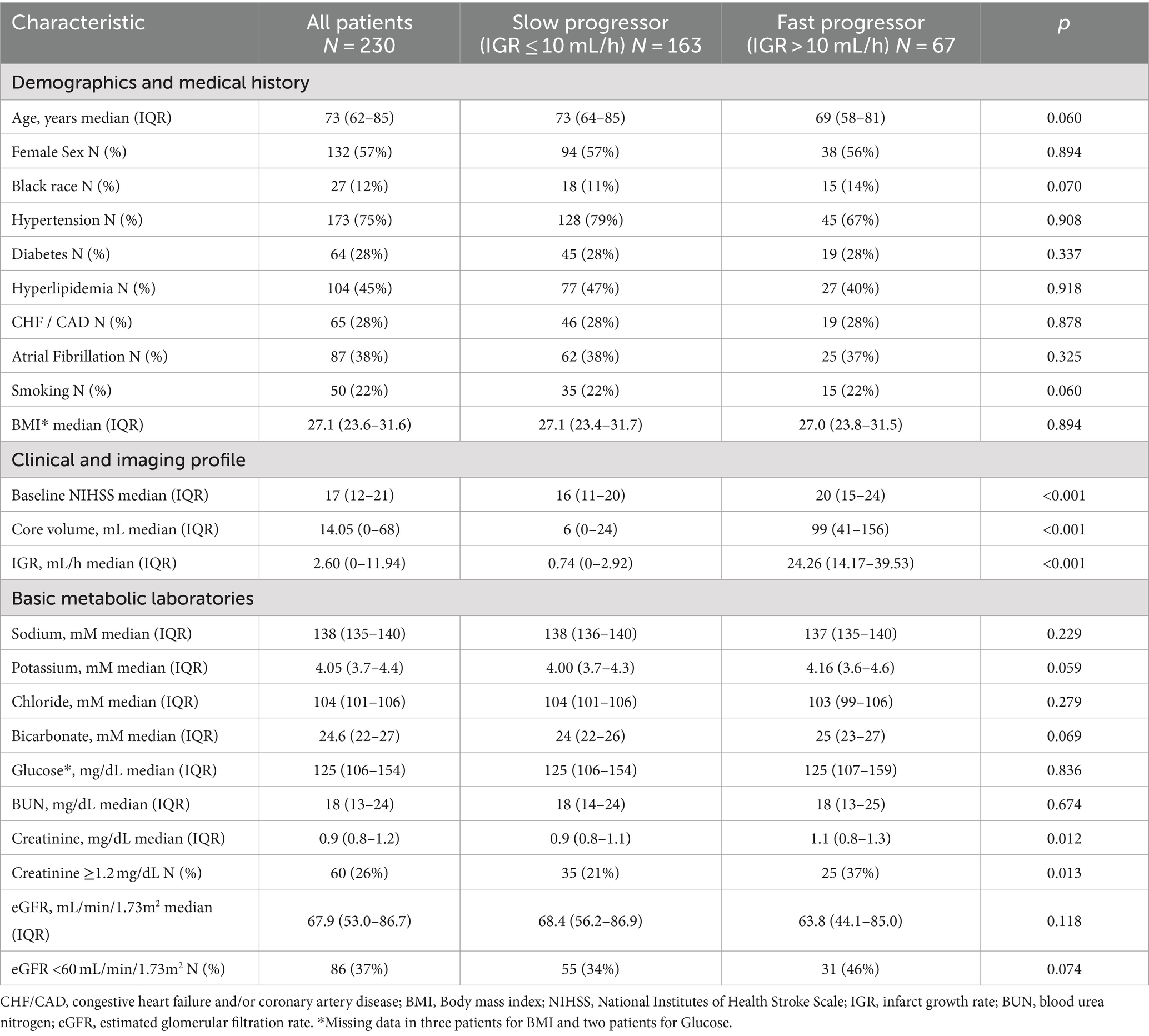
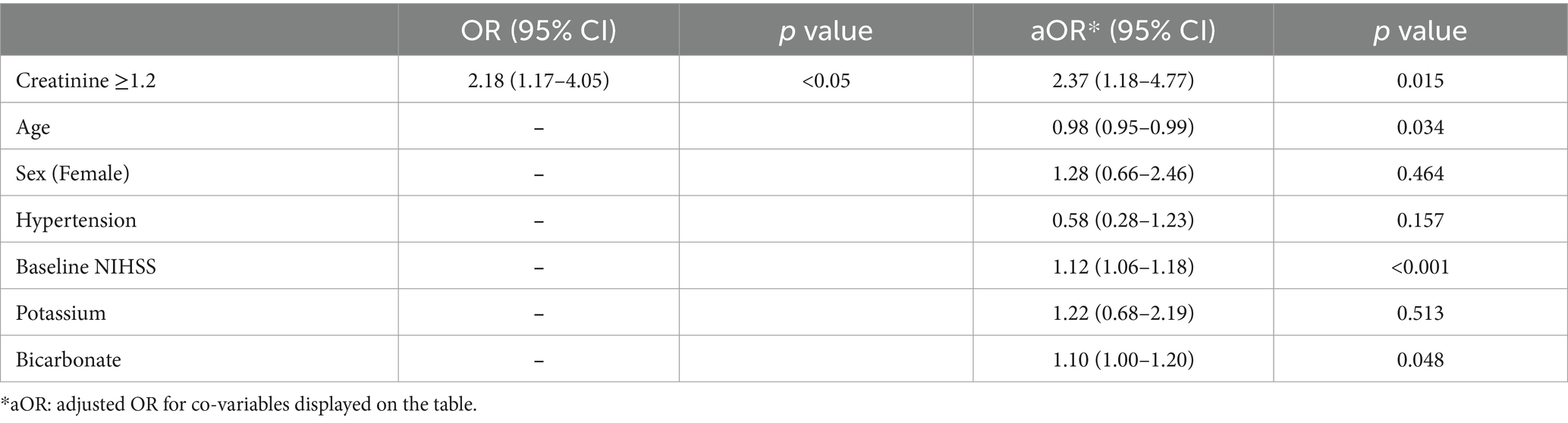
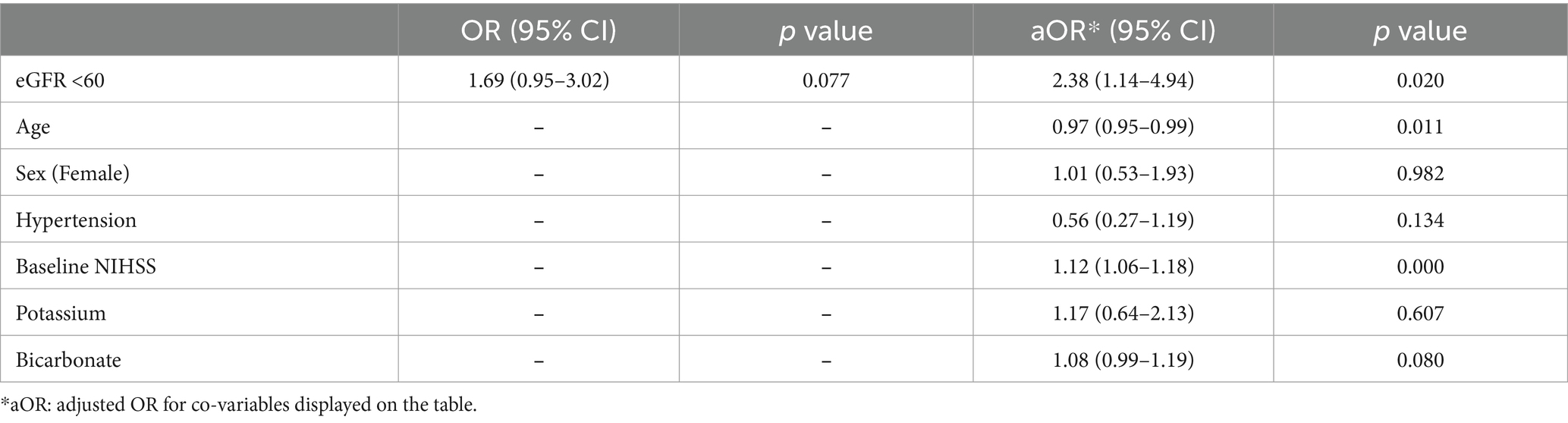
Results: Among 230 patients with ACLVO, 29% were fast progressors, with median serum creatinine levels higher than slow progressors (1.1 vs. 0.9 mg/dL, p < 0.05) and lower median eGFR (66.2 vs. 69.0 mL/min/1.73m2, p < 0.05). Elevated creatinine (≥1.2 mg/dL) was independently associated with fast progressor status (adjusted OR 2.37, 95% CI 1.18–4.77), worse 90-day mRS (adjusted OR 1.88, 95% CI 1.01–3.51) and mortality (adjusted OR 2.57, 95% CI 1.14–5.79). Reduced eGFR (<60 mL/min/1.73m2) was independently associated with fast progressor status (adjusted OR 2.38, 95% CI 1.14–4.94), but not with 90-day mRS or mortality.
Conclusion: Serum creatinine-based biomarkers of renal dysfunction were associated with fast progressor phenotype of ACLVO stroke, and worse clinical outcomes, which may help identify such patients earlier during emergency evaluation for expedited access to EVT. Future prospective studies are warranted to confirm and test implementation of these findings.
Introduction
The systemic determinants of early infarct growth rate (IGR) and fast versus slow progressor phenotypes in large vessel occlusion (LVO) stroke are incompletely defined. Collateral blood flow is a key predictor of IGR in acute LVO, being modulated by native arteriolar anatomy and hemodynamic factors which vary widely across individuals (1). Age, metabolic syndrome, baseline atherosclerosis, and anemia have also been associated with modulation of collateral capacity and IGR (2–5). Good collateral capacity is strongly associated with slower IGR and improved clinical outcomes (6, 7). Systemic co-morbidities such as hypertension and diabetes are associated with a higher risk of stroke and worse outcomes (8, 9), potentially due to microvascular disease mediated by inflammation and impaired endothelial function (10, 11), representing potential additional mechanisms to early ischemic penumbra loss and core growth in LVO stroke.
Chronic kidney disease (CKD) is an important systemic condition that is prevalent in individuals with hypertension and diabetes due to end-organ damage from microvascular injury (11, 12). Previous research indicates that serum biomarkers of renal dysfunction have also been associated with cerebral microvascular injury and greater incidence of stroke (13, 14). Serum creatinine and estimated glomerular filtration rate (eGFR) are established laboratory indicators of renal insufficiency or prediction of CKD (9, 15). Numerous studies have demonstrated that renal dysfunction, indicated by elevated creatinine and reduced eGFR, is associated with higher stroke severity (16, 17), and worse long-term morbidity and mortality (18). Renal function is often assessed during acute evaluation of stroke patients, particularly those being considered for time-sensitive reperfusion therapies, such as anterior circulation LVO (ACLVO) patients.
Renal dysfunction has been previously associated with cerebral microangiopathy (13), poor collateral flow (19) and higher risk of mortality after endovascular therapy (EVT) for ACLVO (20). This study aimed to determine whether renal dysfunction biomarkers are independently associated with fast or slow progressor phenotypes of ACLVO stroke and related clinical outcomes. Determining whether renal function is associated with early infarct growth during acute ACLVO stroke presentation has the potential to help identify fast progressors on the field and provide novel insight into the pathophysiology of individual tolerance to focal cerebral ischemia.
Materials and methods
Population and study design
This was a chart review-based retrospective study of patients with acute intracranial internal carotid artery (ICA) or middle cerebral artery (MCA) M1 segment occlusion who were admitted between 2014 and 2019 at two academic comprehensive stroke centers (Supplementary Figure 1). Patients were included if they underwent baseline magnetic resonance imaging (MRI) or computed tomography perfusion (CTP) on presentation within 24 h after stroke onset, irrespective of subsequent EVT or medical management alone. Patients with missing advanced imaging or serum creatinine data were excluded.
Data collection and management
Demographics, medical history, National Institutes of Health Stroke Scale (NIHSS) score, basic metabolic laboratories, neuroimaging, treatment, and outcome data were collected by trained stroke researchers. The serum creatinine was obtained upon arrival to the comprehensive stroke center where advanced imaging was performed. The cutoff for abnormally elevated serum creatinine was arbitrarily set at 1.2 mg/dL based on an average threshold for standard female and male laboratory values (15, 21). The creatinine-based eGFR was calculated using the 2021 CKD-EPI equation, which has been extensively validated (22–24). The predictor group for lower glomerular function was defined as eGFR <60 mL/min/1.73m2, since it is the main criteria for Stage 3 CKD according to international guidelines (9).
Progressor phenotypes and key clinical variables
Ischemic core volume was measured using automated RAPID software (iSchemaView; Menlo Park), with thresholds set for CTP (regional cerebral blood flow <30%) or MRI (apparent diffusion coefficient < 620 μm2/s). The documented time of last known well was used as a surrogate for time of stroke onset. The IGR was determined by dividing the ischemic core volume (mL) by the time from estimated stroke onset to imaging acquisition (hours). Fast and slow progressor groups were stratified using an IGR cutoff of 10 mL/h as previously determined (6). Patients with IGR ≥10 mL/h were defined as fast progressors, and those with IGR <10 mL/h were defined as slow progressors. Chi-square analysis assessed if witnessed or unwitnessed stroke onset would affect the proportions of fast and slow progressors. The modified Rankin Scale (mRS) was used to measure functional outcomes on discharge and at 90 days post-stroke. Functional independence was defined as mRS 0–2 after stroke. Missing mRS scores at 90 days were imputed from the discharge mRS scores (14% of observations).
Statistical analysis
All statistical analyses were conducted using STATA 18.5 (StataCorp, College Station, TX). Baseline characteristics between groups were compared using chi-square and Mann–Whitney U tests where appropriate. The model assumptions were verified using the Ramsey RESET test, the Breusch-Pagan test, and the Shapiro–Wilk test, leading to the selection of non-linear models accordingly. Bivariate and multivariate logistic regression modeling was used to identify associations with outcome variables of fast progressor status, functional independence (mRS 0–2) or mortality. Ordinal logistic regression was used for analyses of shift in mRS outcome. Additional regression analyses were conducted to explore the association of BUN-Creatinine ratio greater than 20 (BCr ratio > 20) with progressor status. All multivariate regression models were adjusted for age, sex, NIHSS and covariates with a p-value <0.1 in unadjusted group comparisons. An alpha level of 0.05 was considered statistically significant.
Results
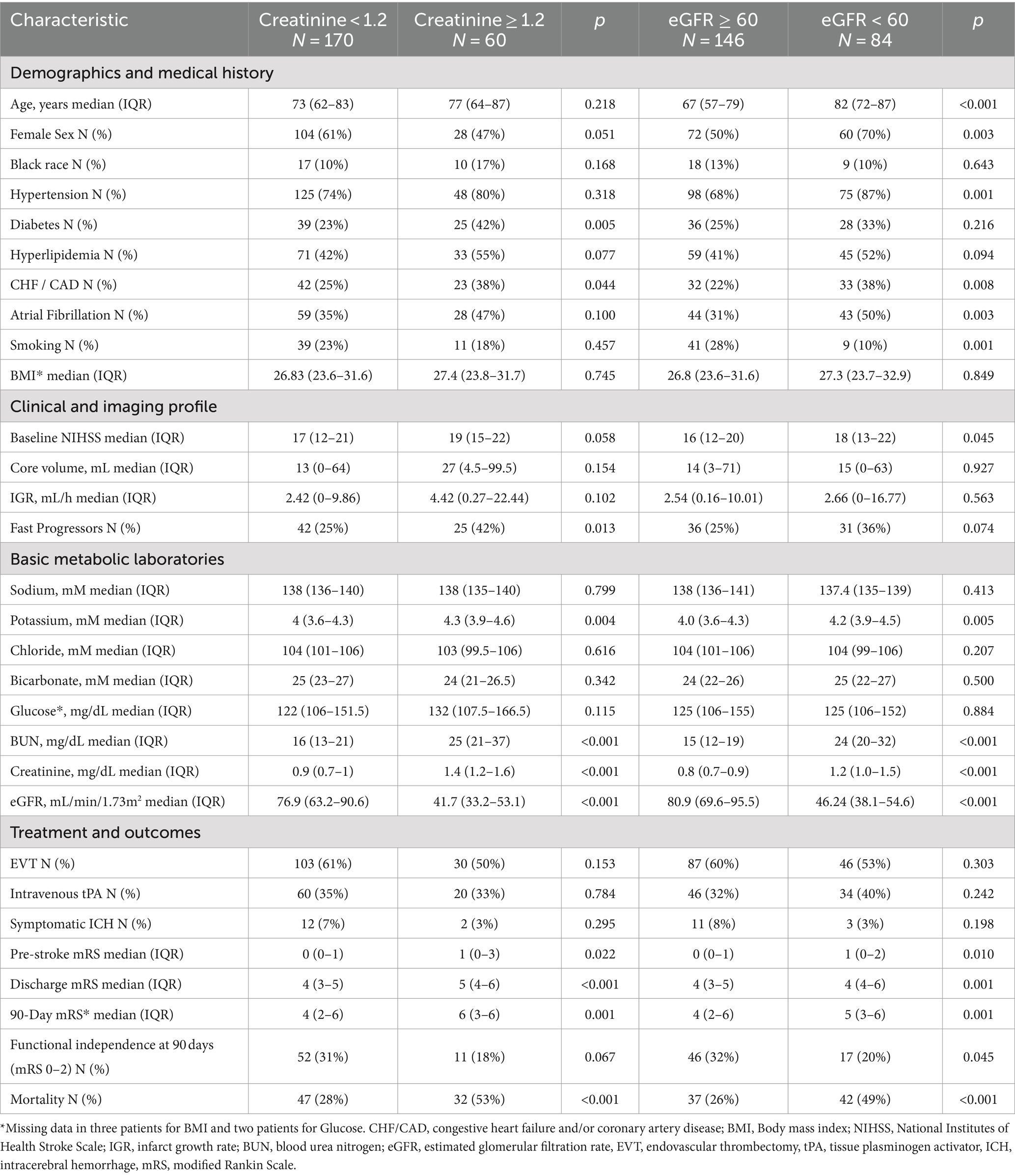
There were 230 patients with acute intracranial ICA or MCA occlusion who met pre-defined inclusion criteria, including 163 (71%) slow progressors and 67 (29%) fast progressors. Demographic, clinical, imaging, and basic metabolic laboratory in the overall population, slow and fast progressor groups are presented in Table 1. In the overall population, the median age was 73 years, 57% were female, 12% were black, and the median body mass index (BMI) was 27.1 kg/m2. The median time of last known well to advanced imaging was 6.4 h in the overall cohort, and a sensitivity analysis showed that the prevalence of slow and fast progressors was similar irrespective of witnessed or unwitnessed stroke onset (Supplementary Table 1).
Race, diabetes, hyperlipidemia, atrial fibrillation, smoking, and BMI were similarly distributed across fast and slow progressor groups, except for known hypertension which was numerically more prevalent in slow progressors (79%) than fast progressors (67%). In comparison to slow progressors, fast progressors had higher baseline NIHSS (median: 20 vs. 16), larger core volume (median: 99 mL vs. 6 mL) and higher IGR (median: 24.3 mL/h vs. 0.74 mL/h). Serum creatinine was higher in fast progressors (median, 1.1 mg/dL) compared to slow progressors (median, 0.9 mg/dL), with a greater proportion of fast progressors having creatinine levels ≥1.2 (37% vs. 21%, p < 0.05). Estimated GFR was numerically lower in fast compared to slow progressors (median: 63.8 vs. 68.4 mL/min/1.73m2), with greater prevalence of fast progressors having reduced eGFR (46% vs. 34%, p = 0.07). Median potassium and bicarbonate levels were numerically higher in fast progressors (4.2 and 25 mM) than slow progressors (4.0 and 24 mM).
In regression analysis, elevated serum creatinine (≥1.2 mg/dL) was independently associated with fast progressor status (OR 2.18, 95% CI 1.17–4.05, and adjusted OR 2.37, 95% CI 1.18–4.77; Table 2). The association was adjusted for age, sex, hypertension, baseline NIHSS, potassium, and bicarbonate levels. Similarly, reduced eGFR (< 60 mL/min/1.73m2) was independently associated with fast progressor status (OR 1.69, 95% CI 0.95–3.02; adjusted OR 2.38, 95% CI 1.14–4.94; Table 3). These data support the independent relationship between creatinine-based markers of renal dysfunction and fast progressor status.
In exploratory analysis, we tested whether the observed association between renal dysfunction markers and fast progressor status was driven by pre-renal acute kidney injury which is typically observed with a serum BCr ratio > 20. The analyses (Supplementary Table 2) showed that elevated creatinine remained significantly associated with fast progressor status (adjusted OR 2.33, 95% CI, 1.15–4.69), independently of serum BCr ratio > 20 (OR 0.79, 95% CI, 0.39–1.57). Similarly, eGFR <60 (Supplementary Table 3) remained significantly associated with fast progressor status (adjusted OR 2.32, 95% CI, 1.11–4.84), independently of BCr ratio > 20 (OR 0.80, 95% CI, 0.40–1.59).
In terms of treatments and outcomes, EVT was performed more frequently in slow progressors (65% vs. 40%, p < 0.01), while intravenous tPA was more commonly administered in fast progressors (49% vs. 29%, p < 0.01; Supplementary Table 4). At 90 days, fast progressors had a higher mRS score than slow progressors (median: 6 vs. 3, p < 0.001). In addition (Table 4), patients with creatinine ≥1.2 had a higher 90-day mRS score than those with creatinine <1.2 (median: 6 vs. 4, p < 0.01), and patients with eGFR <60 had a higher mRS score than those with eGFR ≥60 (median: 5 vs. 4, p < 0.01). Overall, patients with elevated serum creatinine (Figure 1) or lower eGFR (Figure 2) exhibited a higher proportion of severe disability and mortality compared to those with normal renal function. This trend was similarly observed in both fast and slow progressor groups suggesting an additive effect of renal dysfunction on long-term outcomes. Notably, the proportion of mortality in the fast progressor groups with preserved renal function (creatinine <1.2 or eGFR ≥60) was comparable to the percentage of patients with impaired renal function (creatinine ≥1.2 or eGFR <60) in slow progressor groups.
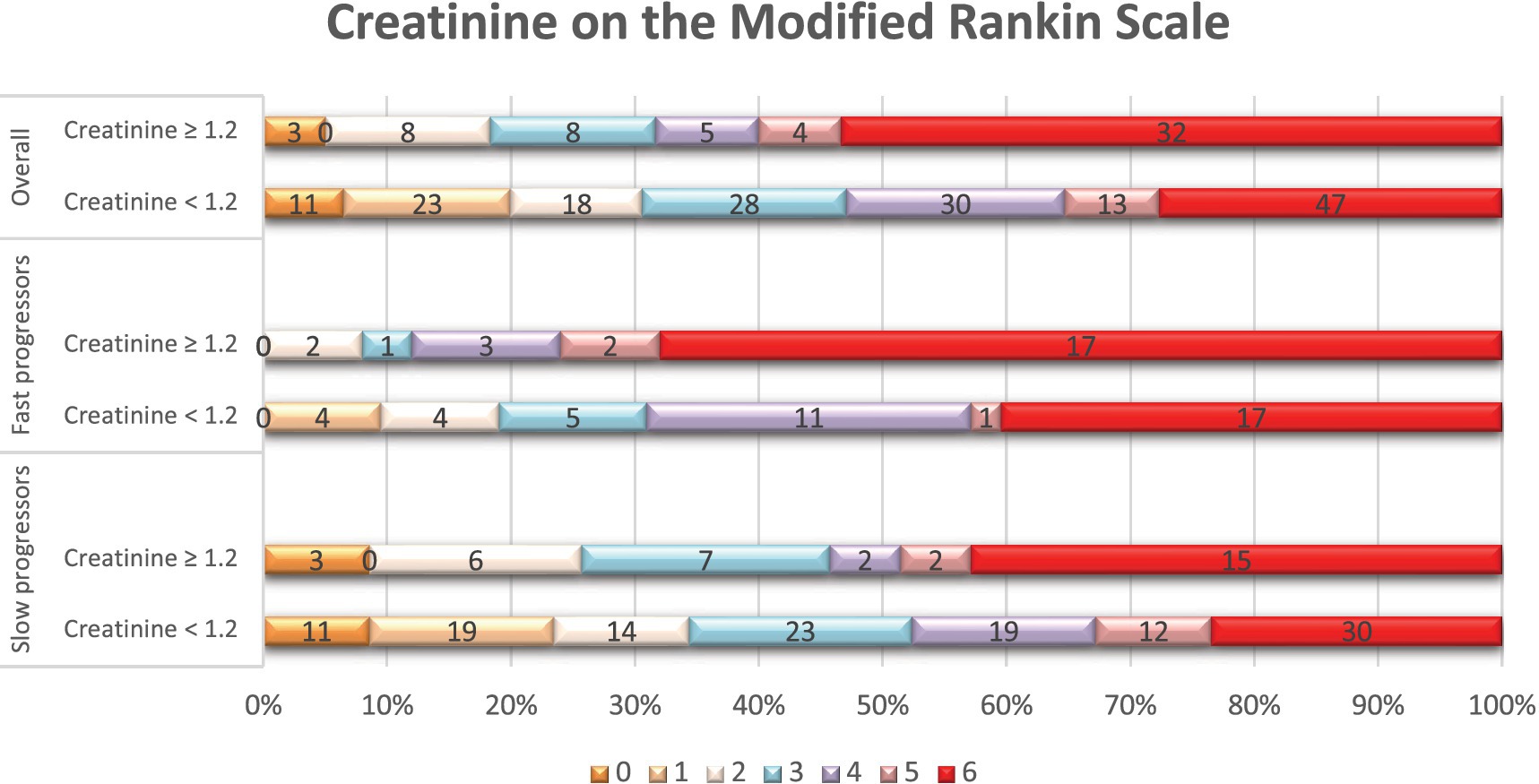
Figure 1. Distribution of scores on the modified Rankin scale at 90 days per serum creatinine status. Shown is the distribution of scores for disability on the modified Rankin scale (mRS) at 90 days among patients with elevated serum creatinine levels (≥ 1.2 mg/dL) and those with normal creatinine levels (< 1.2 mg/dL), in the overall population, fast, and slow progressor strata. The mRS ranges from 0 to 6, with higher scores indicating more severe disability. The numbers in the bars represent the absolute number of patients who had each score; the vertical lines represent percentages at the bottom of the figure.
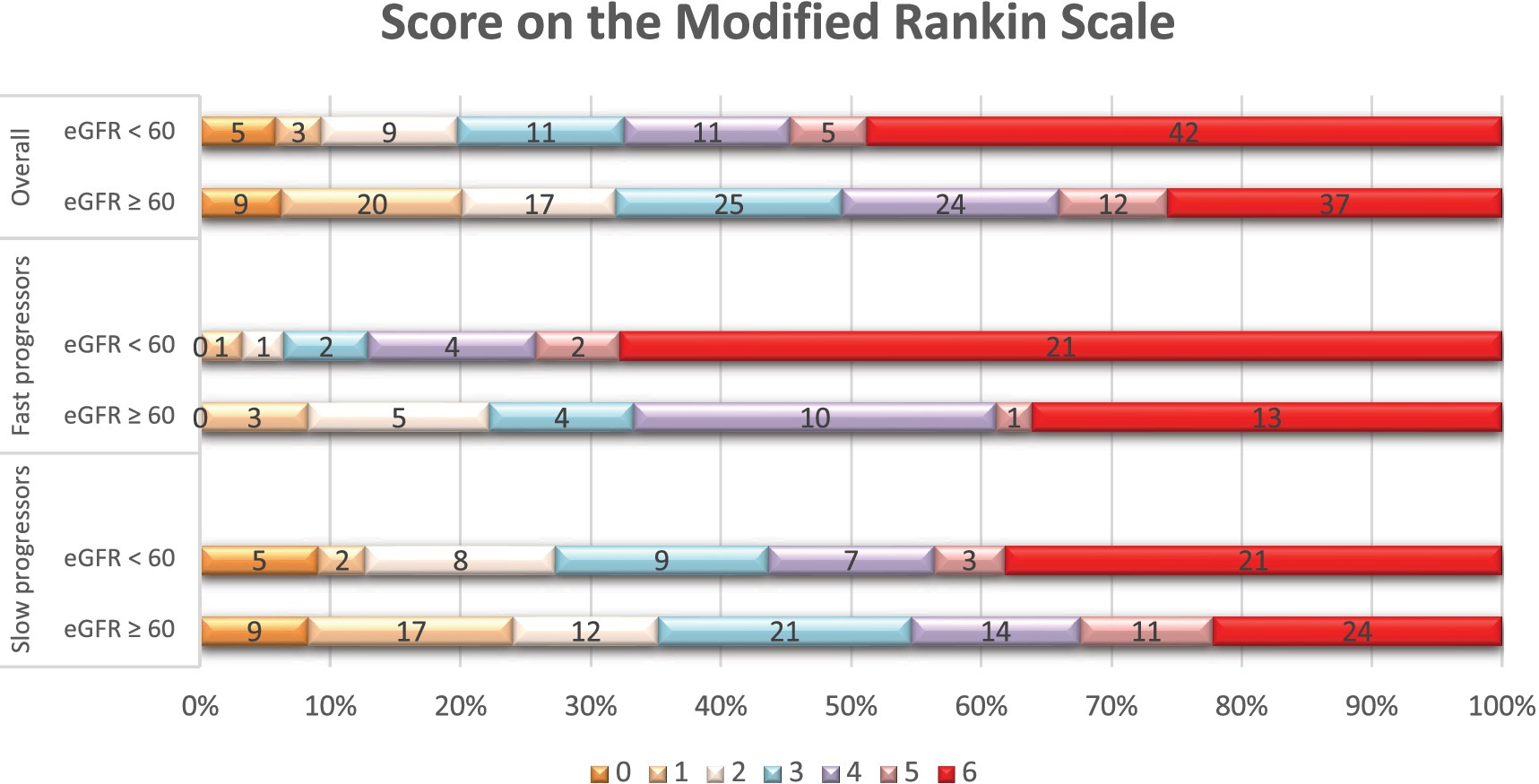
Figure 2. Distribution of scores on the modified Rankin scale at 90 days per eGFR status. Shown is the distribution of scores for disability on the modified Rankin scale (mRS) at 90 days among patients with reduced eGFR (< 60 mL/min/1.73m2) and those with normal eGFR (≥ 60 mL/min/1.73m2), in the overall population, fast, and slow progressor strata. The mRS ranges from 0 to 6, with higher scores indicating more severe disability. The numbers in the bars represent the absolute number of patients who had each score; the vertical lines represent percentages at the bottom of the figure.
The independent association of serum creatinine and eGFR with clinical outcomes at 90 days was further evaluated. Overall, patients with either creatinine ≥1.2 or eGFR <60 were also older, had greater prevalence of hypertension, diabetes, hyperlipidemia, heart disease, atrial fibrillation, and smoking. After adjusting for significant demographic and vascular risk factors (Table 5), serum creatinine ≥1.2 mg/dL remained associated with worse mRS at 90 days (OR 2.57, 95% CI 1.47–4.47; adjusted OR 1.88, 95% CI 1.01–3.51). Additionally, elevated creatinine was associated with higher mortality (OR 2.99, 95% CI 1.63–5.50; adjusted OR 2.57, 95% CI 1.14–5.79) but not with functional independence (OR 0.51, 95% CI 0.25–1.06; adjusted OR 0.93, 95% CI 0.36–2.39). Lower glomerular filtration (eGFR <60) was similarly associated with worse ordinal mRS scores at 90 days (OR 2.30, 95% CI 1.40–3.76), although it was not significant after adjustment (adjusted OR 1.09, 95% CI 0.60–1.98). Additionally, patients with eGFR <60 had higher mortality (OR 2.76, 95% CI, 1.57–4.85) and lower functional independence (OR 0.52, 95% CI 0.28–0.99). However, these associations did not reach statistical significance after adjustment for other co-variables (adjusted OR 1.46, 95% CI 0.67–3.17 for mortality; adjusted OR 1.16, 95% CI 0.47–2.88, respectively).
Discussion
The main finding of our study is that creatine-based biomarkers of renal dysfunction are independently associated with the fast progressor phenotype and worse clinical outcomes in acute ACLVO stroke. In the overall study cohort, approximately 1 in 3 ACLVO patients presented with impaired eGFR (< 60 mL/min/1.73 m2), similar to data reported in other LVO stroke cohorts (25, 26). The relationship between reduced eGFR and worse clinical outcomes in ACLVO patients after EVT has been previously well demonstrated in a recent meta-analysis of 11 international studies (27). Our data support and extend these earlier findings by indicating a novel association between creatinine-based biomarkers of renal dysfunction with rapid early ischemic core growth during ACLVO stroke, which is a known predictor of worse clinical outcomes in this population (6).
Since emergency point-of-care determination of serum creatinine is commonly performed during acute stroke evaluation before contrast-based CT studies, biomarkers of renal dysfunction could potentially aid in the early identification of ACLVO patients with fast progressor phenotype before advanced imaging is available. Therefore, serum creatinine-based biomarkers may have practical implications in the pre-hospital or primary stroke center when hemodynamic management and time-sensitive transfers of fast progressors directly to the neuro-angiography suite may have significant benefit. Early recognition of fast progressors may also have potential use to help enrollment in future clinical trials of hyper-acute neuroprotection targeting patients with large ischemic core. However, data from our study is limited to serum creatinine levels available at the comprehensive stroke center where advanced imaging was obtained, and future studies examining renal dysfunction in the pre-hospital or primary stroke center setting are needed to confirm these contentions.
Possible explanations for the association between renal dysfunction and faster early IGR include CKD-related white matter microangiopathy (11) and reduced leptomeningeal collateral recruitment during ACLVO stroke (28). Castro et al. (13) also found that acute MCA territory stroke patients with CKD had significant impairment in dynamic cerebral autoregulation and increased burden of white matter hyperintensities relative to non-CKD controls. Therefore, patients with renal dysfunction could be potentially more vulnerable to faster early infarct growth due to impaired cerebral autoregulation causing reduced collateral capacity (19) and lower tolerance to acute ischemia due to baseline white matter disease burden (11). Further investigation is needed to elucidate the pathophysiological mechanisms linking renal dysfunction, early infarct growth rate, and individual ischemic tolerance to ACLVO stroke.
In support of previous reports, our data also demonstrated that lower renal function was associated with higher mortality and worse functional outcomes independently of reperfusion therapies. Notably, patients with markers of renal dysfunction consistently experienced worse outcomes in studies that did not consider baseline fast or slow progressor status (18, 27). In our unadjusted analysis, we found that baseline renal dysfunction markers and fast progressor status may have an incrementally worse effect on functional dependency and mortality after ACLVO stroke (Figures 1, 2). It is possible that renal dysfunction also reduces odds of longer-term stroke recovery due to association with pre-morbid burden of microvascular dysfunction and white matter hyperintensities (11, 28). However, our study was not designed to study these potential mechanisms which will need further investigation.
Age and common vascular risk factors such as hypertension and diabetes are also known predictors of worse functional outcomes after EVT for ACLVO stroke (29). Dawod et al. (12) argued that the presence of CKD may be a biomarker of end-organ vascular injury in stroke populations due to underlying hypertension or diabetes, rather than an independent stroke risk factor. Our study found older age and increased prevalence of hypertension, diabetes, hyperlipidemia, coronary disease, and atrial fibrillation in patients with creatinine, eGFR or both criteria for renal dysfunction on hospital presentation. However, abnormally elevated serum creatine conferred a 2-fold increase in odds of worse mRS at 90 days and a 2.5-fold increase in odds of mortality despite adjustment for age and significant vascular risk factors in our cohort. These findings are consistent with other studies demonstrating that renal dysfunction at the time of ACLVO stroke is an independent predictor of worse long-term functional outcomes via yet poorly defined mechanisms (27).
Other studies have further compared the impact of AKI and CKD on long-term outcomes after EVT. In particular, Fandler-Hofler et al. found that development of AKI during the hospitalization rather than pre-morbid CKD was a significant predictor of unfavorable prognosis after EVT (26). Moreover, acute hypovolemic renal insufficiency measured by a high BUN-creatine ratio has been weakly associated with poor outcomes in ischemic stroke (30, 31). In contrast to these previous reports, our supplementary analysis showed that elevated serum creatinine and reduced eGFR remained associated with fast progressor status after adjustment for BUN-Creatinine ratio > 20 (Supplementary Tables 2, 3). Therefore, we stipulate that the observed association between renal dysfunction markers and early rapid infarct growth in ACLVO stroke is more likely due to intrinsic renal impairment rather than acute prerenal azotemia from hypovolemia. In addition to hypovolemia, contrast induced nephropathy is a common contributor to acute renal insufficiency in stroke patients undergoing EVT (32, 33). Future studies are needed to clarify whether the association of creatine-based markers of renal dysfunction on hospital presentation and fast progressor phenotypes are due to acute, chronic, or acute on chronic nephropathy.
Our study has limitations. First, its retrospective design is intrinsically prone to selection bias. Some ACLVO stroke patients were excluded over the study period due to the absence of advanced imaging or laboratory data for serum creatinine measurement on hospital presentation. Second, we used a pre-specified laboratory cut-off point for serum creatinine of 1.2 mg/dL as an acceptable indicator of impaired renal function as previously determined (15, 21, 32). However, serum creatinine and eGFR can vary according to biological sex, race (23), and BMI, particularly in patients with higher muscle mass (9). To control for these potential confounders, our regression models were adjusted for sex and race, but not for BMI since it was similarly distributed across progressor status, creatine, and eGFR strata. Third, recorded serum creatinine values were only available at a single time point rather than over a prolonged period which would have been necessary to confirm CKD (9) and chart diagnosis of pre-morbid CKD were not available. However, the prevalence of patients with eGFR <60 mL/min/1.73 m2 in our population is similar to that from other similar cohorts that used alternative formulas for GFR calculation (27), supporting the validity of our estimates of renal dysfunction during acute stroke presentation.
Conclusion
This study found that biomarkers of renal dysfunction (serum creatinine ≥1.2 mg/dL or eGFR <60 mL/min/1.73m2) were associated with a faster progressor phenotype of ACLVO stroke, and worse clinical outcomes, suggesting that serum creatinine could serve as an adjunct in early identification of higher risk ACLVO patients. These findings have potential implications in early management and prioritized transfer of fast progressor ACLVO patients to EVT, and for enrollment in future clinical trials of bridge neuroprotective therapies. Future prospective studies are required in larger and more diverse populations to confirm and test implementation of these findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Pittsburgh Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LR: Writing – original draft, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – review & editing. MK: Data curation, Formal analysis, Writing – review & editing. BM: Data curation, Writing – review & editing. AA-Q: Data curation, Writing – review & editing, Methodology. MD: Methodology, Writing – review & editing. AA-B: Writing – review & editing. NB: Methodology, Writing – review & editing. MS: Writing – review & editing, Methodology. SS: Data curation, Writing – review & editing, Methodology. RN: Writing – review & editing, Methodology. MR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. MR received grant funding from NIH P30AG24827 and NIH 2U24 NS107216.
Conflict of interest
RGN reports consulting fees for advisory roles with Anaconda, Biogen, Cerenovus, Genentech, Philips, Hybernia, Imperative Care, Medtronic, Phenox, Philips, Prolong Pharmaceuticals, Stryker Neurovascular, Shanghai Wallaby, Synchron, and stock options for advisory roles with Astrocyte, Brainomix, Cerebrotech, Ceretrieve, Corindus Vascular Robotics, Vesalio, Viz-AI, RapidPulse, and Perfuze. RGN is one of the principal investigators of the “Endovascular Therapy for Low NIHSS Ischemic Strokes (ENDOLOW)” trial. Funding for this project is provided by Cerenovus. RGN is the principal investigator of the “Combined Thrombectomy for Distal MediUm Vessel Occlusion StroKe (DUSK)” trial. Funding for this project is provided by Stryker Neurovascular. RGN is an investor in Viz-AI, Perfuze, Cerebrotech, Reist/Q’Apel Medical, Truvic, Vastrax, and Viseon.
The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1475135/full#supplementary-material
References
1. Rocha, M, and Jovin, TG. Fast versus slow Progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke. (2017) 48:2621–7. doi: 10.1161/STROKEAHA.117.017673
2. Rocha, M, Desai, S, Son, J, Tonetti, DA, Jovin, T, and Jadhav, AP. Clinical characteristics of fast and slow progressors of infarct growth in anterior circulation large vessel occlusion stroke. J Cereb Blood Flow Metab. (2021) 41:1517–22. doi: 10.1177/0271678X211015068
3. Rocha, M, Desai, SM, Jadhav, AP, and Jovin, TG. Prevalence and temporal distribution of fast and slow Progressors of infarct growth in large vessel occlusion stroke. Stroke. (2019) 50:2238–40. doi: 10.1161/STROKEAHA.118.024035
4. Menon, BK, Smith, EE, Coutts, SB, Welsh, DG, Faber, JE, Goyal, M, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. (2013) 74:241–8. doi: 10.1002/ana.23906
5. Seo, WK, Liebeskind, DS, Yoo, B, Sharma, L, Jahan, R, Duckwiler, G, et al. Predictors and functional outcomes of fast, intermediate, and slow progression among patients with acute ischemic stroke. Stroke. (2020) 51:2553–7. doi: 10.1161/STROKEAHA.120.030010
6. Sarraj, A, Hassan, AE, Grotta, J, Blackburn, S, Day, A, Abraham, M, et al. Early infarct growth rate correlation with endovascular Thrombectomy clinical outcomes: analysis from the SELECT study. Stroke. (2021) 52:57–69. doi: 10.1161/STROKEAHA.120.030912
7. Borggrefe, J, Gluck, B, Maus, V, Onur, O, Abdullayev, N, Barnikol, U, et al. Clinical outcome after mechanical Thrombectomy in patients with diabetes with major ischemic stroke of the anterior circulation. World Neurosurg. (2018) 120:e212–20. doi: 10.1016/j.wneu.2018.08.032
8. Egle, M, Wang, WC, Fann, YC, Johansen, MC, Lee, JT, Yeh, CH, et al. Sex differences in the role of multimorbidity on Poststroke disability: the Taiwan stroke registry. Neurology. (2024) 102:e209140. doi: 10.1212/WNL.0000000000209140
9. Webster, AC, Nagler, EV, Morton, RL, and Masson, P. Chronic kidney disease. Lancet. (2017) 389:1238–52. doi: 10.1016/S0140-6736(16)32064-5
10. Masi, S, Rizzoni, D, Taddei, S, Widmer, RJ, Montezano, AC, Luscher, TF, et al. Assessment and pathophysiology of microvascular disease: recent progress and clinical implications. Eur Heart J. (2021) 42:2590–604. doi: 10.1093/eurheartj/ehaa857
11. Khatri, M, Wright, CB, Nickolas, TL, Yoshita, M, Paik, MC, Kranwinkel, G, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the northern Manhattan study (NOMAS). Stroke. (2007) 38:3121–6. doi: 10.1161/STROKEAHA.107.493593
12. Dawod, J, and Coull, BM. Chronic kidney disease is a biomarker rather than a risk factor for stroke. J Stroke Cerebrovasc Dis. (2021) 30:105869. doi: 10.1016/j.jstrokecerebrovasdis.2021.105869
13. Castro, P, Azevedo, E, Rocha, I, Sorond, F, and Serrador, JM. Chronic kidney disease and poor outcomes in ischemic stroke: is impaired cerebral autoregulation the missing link? BMC Neurol. (2018) 18:21. doi: 10.1186/s12883-018-1025-4
14. Shlipak, MG, Fried, LF, Crump, C, Bleyer, AJ, Manolio, TA, Tracy, RP, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. (2003) 107:87–92. doi: 10.1161/01.CIR.0000042700.48769.59
15. Couchoud, C, Pozet, N, Labeeuw, M, and Pouteil-Noble, C. Screening early renal failure: cut-off values for serum creatinine as an indicator of renal impairment. Kidney Int. (1999) 55:1878–84. doi: 10.1046/j.1523-1755.1999.00411.x
16. Akemokwe, FM, Adejumo, OA, Odiase, FE, Okaka, EI, Imarhiagbe, FA, and Ogunrin, OA. Relationship between kidney dysfunction, stroke severity, and outcomes in a Nigerian tertiary hospital: a prospective study. Niger J Clin Pract. (2023) 26:1742–9. doi: 10.4103/njcp.njcp_369_23
17. Guettier, S, Cogez, J, Bonnet, AL, Dean, P, Apoil, M, Tchoumi, T, et al. Factors associated with timing of early neurological improvement after thrombolysis for ischaemic stroke. Eur J Neurol. (2016) 23:664–7. doi: 10.1111/ene.12943
18. Hayden, D, McCarthy, C, Akijian, L, Callaly, E, Ni Chroinin, D, Horgan, G, et al. Renal dysfunction and chronic kidney disease in ischemic stroke and transient ischemic attack: a population-based study. Int J Stroke. (2017) 12:761–9. doi: 10.1177/1747493017701148
19. Xiao, L, Ma, M, Gu, M, Han, Y, Wang, H, Zi, W, et al. Renal impairment on clinical outcomes following endovascular recanalization. Neurology. (2020) 94:e464–73. doi: 10.1212/WNL.0000000000008748
20. Laible, M, Mohlenbruch, MA, Pfaff, J, Jenetzky, E, Ringleb, PA, Bendszus, M, et al. Influence of renal function on treatment results after stroke Thrombectomy. Cerebrovasc Dis. (2017) 44:351–8. doi: 10.1159/000481147
21. Hosten, AO. BUN and creatinine In: HK Walker, WD Hall, and JW Hurst, editors. Clinical methods: The history, physical, and laboratory examinations. 3rd ed. Boston: Butterworths (1990)
22. Matsushita, K, Mahmoodi, BK, Woodward, M, Emberson, JR, Jafar, TH, Jee, SH, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. (2012) 307:1941–51. doi: 10.1001/jama.2012.3954
23. Inker, LA, Eneanya, ND, Coresh, J, Tighiouart, H, Wang, D, Sang, Y, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. (2021) 385:1737–49. doi: 10.1056/NEJMoa2102953
24. Umeukeje, EM, Koonce, TY, Kusnoor, SV, Ulasi, II, Kostelanetz, S, Williams, AM, et al. Systematic review of international studies evaluating MDRD and CKD-EPI estimated glomerular filtration rate (eGFR) equations in black adults. PLoS One. (2022) 17:e0276252. doi: 10.1371/journal.pone.0276252
25. Toyoda, K, and Ninomiya, T. Stroke and cerebrovascular diseases in patients with chronic kidney disease. Lancet Neurol. (2014) 13:823–33. doi: 10.1016/S1474-4422(14)70026-2
26. Fandler-Hofler, S, Odler, B, Kneihsl, M, Wunsch, G, Haidegger, M, Poltrum, B, et al. Acute and chronic kidney dysfunction and outcome after stroke Thrombectomy. Transl Stroke Res. (2021) 12:791–8. doi: 10.1007/s12975-020-00881-2
27. Wang, R, Xie, Z, Li, B, and Zhang, P. Renal impairment and the prognosis of endovascular thrombectomy: a meta-analysis and systematic review. Ther Adv Neurol Disord. (2022) 15:17562864221083620. doi: 10.1177/17562864221083620
28. Nannoni, S, Cereda, CW, Sirimarco, G, Lambrou, D, Strambo, D, Eskandari, A, et al. Collaterals are a major determinant of the core but not the penumbra volume in acute ischemic stroke. Neuroradiology. (2019) 61:971–8. doi: 10.1007/s00234-019-02224-x
29. Venema, E, Roozenbeek, B, Mulder, M, Brown, S, Majoie, C, Steyerberg, EW, et al. Prediction of outcome and endovascular treatment benefit: validation and update of the MR PREDICTS decision tool. Stroke. (2021) 52:2764–72. doi: 10.1161/STROKEAHA.120.032935
30. Wu, FF, Hung, YC, Tsai, YH, Yang, JT, Lee, TH, Liow, CW, et al. The influence of dehydration on the prognosis of acute ischemic stroke for patients treated with tissue plasminogen activator. BMC Cardiovasc Disord. (2017) 17:154. doi: 10.1186/s12872-017-0590-6
31. Deng, L, Wang, C, Qiu, S, Bian, H, Wang, L, Li, Y, et al. Association between blood urea nitrogen-to-creatinine ratio and three-month outcome in patients with acute ischemic stroke. Curr Neurovasc Res. (2019) 16:166–72. doi: 10.2174/1567202616666190412123705
32. Lameire, N, Adam, A, Becker, CR, Davidson, C, McCullough, PA, Stacul, F, et al. Baseline renal function screening. Am J Cardiol. (2006) 98:21K–6K. doi: 10.1016/j.amjcard.2006.01.021
Keywords: ischemic tolerance, large vessel occlusion, anterior circulation, renal function, stroke outcomes
Citation: Rios Rocha L, Kayyali MN, Mahat BC, Al-Qudah A, Doheim MF, Al-Bayati AR, Bhatt NR, Starr MT, Song SS, Nogueira RG and Rocha M (2024) Association of renal biomarkers with fast progressor phenotype and related outcomes in anterior circulation large vessel occlusion stroke. Front. Neurol. 15:1475135. doi: 10.3389/fneur.2024.1475135
Edited by:
Cheng-Yang Hsieh, Tainan Sin Lau Hospital, TaiwanReviewed by:
Darko Quispe-Orozco, Texas Tech University, United StatesMichael Valente, Monash Health, Australia
Copyright © 2024 Rios Rocha, Kayyali, Mahat, Al-Qudah, Doheim, Al-Bayati, Bhatt, Starr, Song, Nogueira and Rocha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcelo Rocha, cm9jaGFtQHVwbWMuZWR1
 Lucas Rios Rocha
Lucas Rios Rocha Mohammad N. Kayyali
Mohammad N. Kayyali Bishow C. Mahat1
Bishow C. Mahat1 Abdullah Al-Qudah
Abdullah Al-Qudah Mohamed F. Doheim
Mohamed F. Doheim Alhamza R. Al-Bayati
Alhamza R. Al-Bayati Nirav R. Bhatt
Nirav R. Bhatt Matthew T. Starr
Matthew T. Starr Marcelo Rocha
Marcelo Rocha