- 1Department of Interdisciplinary Medicine-Intensive Care Unit Section, University of Bari Aldo Moro, Bari, Italy
- 2Department of Intensive Care, Hôpital Universitaire de Bruxelles (HUB), Université Libre de Bruxelles (ULB), Brussels, Belgium
- 3UOC Anesthesia and Intensive Care II, IRCCS Casa Sollievo Della Sofferenza Viale Cappuccini, San Giovanni Rotondo, Italy
- 4Department of Biomedical Sciences, Humanitas University, Via Rita Levi Montalcini, Milan, Italy
- 5Department of Anesthesia and Intensive Care, Humanitas Gavazzeni, Bergamo, Italy
- 6Department of Neurosurgery, Hôpital Universitaire de Bruxelles (HUB), Université Libre de Bruxelles (ULB), Brussels, Belgium
Background: Approximately one-third of trauma-related deaths are due to traumatic brain injury (TBI), particularly among young adults and elderly patients. Management strategies may vary across different age groups, potentially influencing short-term neurological outcomes. This study aims to investigate age-related disparities in treatment approaches and 3-month neurological outcomes among TBI patients.
Methods: We conducted a retrospective study on TBI patients requiring Intensive Care Unit (ICU) admission from January 1, 2015, to January 1, 2024, in a tertiary University hospital. Patient demographics, major comorbidities, ICU admission parameters, interventions and ICU complications were collected. An unfavorable neurological outcome at 3 months (UO) was defined as a Glasgow Outcome Scale (GOS) score of 1–3. A high therapy intensity level (TIL) was defined as a TIL basic of 3–4. A multivariable logistic regression model and a Cox proportional Hazard Regression model were used to assess the association of age and TIL with neurological outcome and mortality. A sensitivity analysis on low TIL (0–2) and high TIL subgroups was also conducted.
Results: We enrolled 604 TBI patients, of which 240 (40%) had UO. The highest prevalence of UO was found in patients aged ≥80 years (53/94, 56%), followed by patients aged 50–79 years (104/255, 41%). The age group 35–49 years had the lowest rate of UO (38/127, 30%). Older patients (age ≥ 80 years) received less frequently high TIL than others (p = 0.03). In the multivariable analysis, age ≥ 80 years [OR: 3.42 (95% CI 1.72–6.81)] was independently associated with UO, while age ≥ 80 years [HR 5.42 (95% CI 3.00–9.79)] and age 50–79 years [HR 2.03, (95% CI 1.19–3.48)] were independently associated with mortality. Although there was no interaction between age groups and TIL on outcome, an exploratory analysis showed that in the high TIL subgroup of patients, age had no independent impact on the outcome, whereas, in the low TIL group, age ≥ 80 years was independently associated with UO [OR: 3.65 (95% CI: 1.64–8.14)].
Conclusion: Older age, especially in the setting of low intensity treatment, may impact short-term neurological outcome of traumatic brain-injured patients.
1 Introduction
Traumatic Brain Injury (TBI) poses a significant health burden worldwide, affecting approximately 70 million individuals annually, regardless of the underlying mechanisms of injury (1). Considering all ages and severities in Europe, the crude incidence of TBI varies from 47.3 per 100,000 to 694 per 100,000 inhabitants in country-level studies (2). TBI exhibits two distinct incidence peaks: in children and young adults (typically aged 18–40 years) and older adults (over 60 years). The first peak primarily results from high-risk behaviors and activities, such as motor vehicle accidents and sports injuries, while the second peak is mainly due to falls, which are more common in the elderly population (3, 4). These age-related patterns underscore the need for targeted prevention strategies to reduce TBI risk in these vulnerable groups (5, 6).
Several studies have revealed that older age is a significant negative prognostic factor after TBI (7–11). These issues justify the need for further research using standardized protocols, comprehensive assessments, longer follow-up periods, and larger sample sizes to understand better how age affects TBI outcomes, ultimately leading to better use of resources and improved patient care. Furthermore, diagnostic testing and treatment interventions may vary among different age groups, and a lower level of management intensity could explain the association between older age and poor outcomes (12). Indeed, elderly TBI patients are less likely to receive the activation of trauma teams and advanced imaging, both crucial for early and accurate diagnosis and intervention (13). This conservative approach extends to critical care procedures, where ventilator support and intracranial pressure (ICP) monitoring decrease significantly with older age (13). Surgical treatments, which can be life-saving, are also less frequently performed in elderly patients. Decompressive craniectomy, in particular, is rarely conducted in individuals over 50 despite similar injury severities observed in younger cohorts (12, 14, 15). This decline in treatment intensity is often driven by assumptions about the frailty and limited recovery potential of older patients, which can lead to poorer outcomes.
However, not all TBI patients can be considered using such assumptions. For instance, if adequate acute and post-acute care is provided, a mild TBI may have a much better outcome than moderate and severe TBI, even when older age is considered (13, 16). In fact, inadequate therapy may lessen the chances of survival and recovery for older TBI patients, blunting the potential recovery of such patients, especially the so-called “younger” elderly (17, 18).
The therapy intensity level (TIL) is a composite score that assesses the graded interventions for treating intracranial pressure (ICP) in TBI, with a higher TIL indicating a higher tier of treatments in managing ICP (19, 20). The TIL evaluation includes different treatments such as sedation, osmotic therapy, hypoventilation, hypothermia, cerebrospinal fluid drainage, and surgery. The rationale for using this score is that interpreting ICP is impossible without understanding the therapy’s efforts (20). While prior studies (7–11) have shown that older age is a significant predictor of poor outcomes in TBI patients, few have examined how varying levels of treatment intensity influence these outcomes, especially in elderly patients. This study addresses this critical gap in the literature by investigating how varying levels of TIL affect neurological outcomes in TBI patients across different age groups.
While it is well established that older age is associated with worse outcomes, our study is one of the first to explore whether high-intensity treatment can mitigate these negative effects, particularly in the elderly. By focusing on the interaction between age and therapy intensity, we offer new insights into how personalized, aggressive treatment can improve outcomes even in patients traditionally considered high-risk due to their age.
2 Materials and methods
2.1 Study design
A monocentric retrospective cohort study was conducted in the Intensive Care Unit of the Hôpital Universitaire de Bruxelles (HUB), Brussels, Belgium. The local ethics committee approved the study protocol (P2020/252), which waived the need for informed written consent because of its retrospective design and since all interventions were part of the standard patients’ care. The study was performed in accordance with the ethical standards of the Declaration of Helsinki.
2.2 Study population
We enrolled all adult (>18 years) patients consecutively admitted to our hospital from January 2015 to December 2023 due to TBI, with an available cerebral CT-scan on admission and who stayed at least 24 h in the ICU. We excluded pregnant women and patients transferred from other hospitals.
2.3 Traumatic brain injury management
Our center adheres to the current TBI management guidelines as outlined by the Brain Trauma Foundation (21, 22). The management of elevated ICP in TBI includes general measures, such as elevating the head of the bed, sedation, maintaining normothermia, and fluid resuscitation to ensure adequate cerebral perfusion pressure (CPP). Specific interventions involve hyperosmolar therapy using mannitol or hypertonic saline, cautious hyperventilation for acute ICP crises, cerebrospinal fluid drainage, and barbiturate therapy for refractory cases. Continuous ICP monitoring is essential, with thresholds typically set between 15 and 25 mmHg, and maintaining CPP between 60 and 70 mmHg is crucial. Decompressive craniectomy may be considered for refractory intracranial hypertension, while corticosteroids are generally not recommended due to potential adverse effects.
We utilize a tiered algorithm for treating elevated ICP in severe TBI: tier zero: General clinical management, including basic supportive measures; tier one: sedatives, analgesics, hypertonic solutions, and cerebrospinal fluid (CSF) drainage; tier two: more intensive treatments, including neuromuscular blockade and controlled hyperventilation; tier three: high-dose barbiturate therapy, mild hypothermia, and/or decompressive craniectomy.
This structured approach ensures progressively more aggressive management of ICP, tailored to the patient’s response and the severity of their condition. Although the COVID-19 pandemic led to widespread changes in healthcare delivery across the globe, including reduced in-person consultations and shifts in treatment protocols (23), we found no significant differences in the treatment approaches for TBI patients in our cohort during 2020 compared to previous years. Consequently, data from 2020 were included in the analysis without adjustments for pandemic-related changes.
2.4 Data collection and definitions
We collected patients’ comorbidities and demographic data. Patients were divided into four age groups: 18–34 years, 35–49 years, 50–79 years, and ≥ 80 years. Severity scores on admission, including the Sequential Organ Failure Assessment (SOFA) (24), the Glasgow Coma Scale (GCS) (25), and the Marshall score (26), were recorded. Additionally, we collected data on admission glycemia, pre-hospital hypoxemia, hypotension on admission, pupillary light reflex, and the presence of extracranial injuries.
We documented the use of various interventions during the ICU stay, such as mechanical ventilation, osmotic agents, inotropic agents, vasopressors, sedatives, invasive and non-invasive neuromonitoring, and all interventions aimed at controlling intracranial hypertension, including hyperventilation, barbiturates, hypothermia, and decompressive craniectomy. We also recorded medical, neurological, and clinical complications during the ICU stay, such as all forms of shock (e.g., vasopressor therapy for more than 6 consecutive hours and lactate levels >2.0 mmol/L), clinical seizures, and ICP exceeding 20 mmHg for more than 5 min and requiring treatment (e.g., intracranial hypertension).
All interventional variables were used to assess the intensity of ICP treatment to compute the basic TIL; the highest TIL basic for each patient was calculated for any day during the ICU stay. The TIL scale, as defined by Maas et al. (27), in its simplified version, assigns TIL 0 to no specific ICP-directed therapy, TIL 1 to basic ICU care (e.g., sedation, head-up positioning, and normocapnia), TIL 2 to mild therapy (e.g., low-dose osmotic therapy, mild hypocapnia, and CSF drainage), TIL 3 to moderate therapy (e.g., higher dose osmotic therapy, moderate hypocapnia, and higher CSF drainage), and TIL 4 to extreme therapy (e.g., profound hypocapnia, hypothermia, metabolic suppression for ICP control, and surgery for refractory ICP). In our study, a high TIL was defined as TIL 3–4, while a low TIL was defined as TIL 0–2.
TBI severity was categorized using the GCS: mild TBI as a GCS score of 13–15, with loss of consciousness (LOC) up to 30 min and post-traumatic amnesia (PTA) less than 24 h; moderate TBI as a GCS score of 9–12, LOC between 30 min and 24 h, and PTA from 24 h to 7 days; severe TBI as a GCS score of 8 or below, LOC over 24 h, and PTA more than 7 days (28). ICU mortality and hospital mortality were also recorded. Neurological outcome was assessed at 3 months using the Glasgow Outcome Scale (29). An unfavorable neurological outcome was defined as a GOS of 1 to 3 (UO; death, vegetative state, and severe disability, respectively). A favorable neurological outcome was defined as a GOS of 4 or 5 (moderate disability, mild disability or asymptomatic).
2.5 Outcome assessment
The study’s primary analysis was to investigate the association between age and UO considering TIL. The secondary analysis of the study was to investigate the association between age and in-hospital mortality, taking into account TIL.
2.6 Statistical analysis
We conducted a descriptive statistical analysis using the Medcalc program for Windows v20.217. For continuous variables, data are expressed as mean (±SD) or median and interquartile ranges (IQR), according to the distribution of variables. Categorical variables are described as count and percentage. Categorical variables were compared using the Chi-square or Fisher exact test. Continuous variables were compared using t-Student test or Mann–Whitney test according to their distribution. We performed a univariate and multivariate logistic regression to study the association between the age groups and neurological outcome, adjusted for high intensity of treatment and the following predefined variables which are known factors associated with outcome in the literature: GCS on admission, Marshall CT score on admission, pupillary light reflex on admission, pre hospital/admission hypotension, prehospital/admission hypoxemia, seizures, shock during ICU stay, traumatic subarachnoid hemorrhage. We assessed the interaction between these variables and age groups. We tested all variables for multicollinearity to avoid strong correlations. All variables were tested for multicollinearity before modeling. We also assessed the interaction between age groups and TIL on measured outcomes (p value for interaction). We reported odds ratio (OR) and 95% Confidence Intervals (CI) for all variables included in the model. Similarly, we performed a Cox regression analysis to assess the association between age and in-hospital mortality adjusted for high intensity of treatment and for the same predefined variables that have previously been reported in the literature as factors associated with hospital death. We reported hazard ratios (HR) and 95% confidence intervals (CI) for all variables included in the model. As described above, we also performed a exploratory subgroup analysis of patients according to the TIL score.
Furthermore, In the case of our study, logistic regression was selected over Poisson regression due to the binary nature of the primary outcomes (e.g., mortality and neurological outcome). Logistic regression is the standard approach for analyzing binary outcomes and provides easily interpretable odds ratios, which are commonly used in TBI research. While Poisson regression with robust standard errors is appropriate for common outcomes to obtain prevalence ratios, logistic regression remains a well-validated method for binary data and is consistent with the methodology of similar studies in the field, such as in Dhandapani et al. (30). Finally, given the relatively small sample size in certain subgroups (e.g., patients over 80 years old), logistic regression was preferred due to its robustness in handling such data. We acknowledge the potential for odds ratio overestimation, but logistic regression allows for comparability with other TBI studies and remains a valid analytical choice.
3 Results
3.1 Study population
We identified 623 patients admitted to the ICU due to TBI with an initial cerebral CT-scan showing signs of head trauma, of whom 19 were excluded due to loss of follow-up, for a total of 604 included patients in the final analysis. Among them, 193 (32%) had severe TBI on admission. The mean age of the population was 56 (± 21) years, and the male sex was predominant (65%). Falls (n = 427–70%) were the most frequent injury mechanism within all age groups. The median GCS on admission was 14 (6–15). Intracranial hypertension was diagnosed in 191/604 patients (32%) during the ICU stay. The characteristics of the study population are reported in Table 1. A total of 144 (24%) patients died during the hospital stay, and 240 (40%) of patients had unfavorable neurological outcome.
3.2 Characteristics of the population according to different age groups
The characteristics of the study population according to different age groups are described in Table 1. The majority of patients (254, 42%) were aged between 50 and 79 years. The median GCS score on admission, Marshall score, and the percentage of severe TBI were not significantly different between the age groups. The mean TIL basic was also not different between groups; however, patients aged ≥80 years had a lower prevalence of high TIL compared to other groups. Additionally, patients aged ≥80 years, as well as those aged 50–79 years, had higher mortality rates than younger patients. Furthermore, patients aged ≥80 years had a higher rate of UO compared to the other age groups.
3.3 Age groups and neurological outcome
Patients with UO were older, had lower GCS scores on admission, a higher incidence of organ dysfunction, and received a higher TIL compared to those with favorable outcomes (Supplementary Table S1). Furthermore, epidural hematoma and traumatic subarachnoid hemorrhage were more frequently present in the UO group. In the multivariate logistic regression model, age ≥ 80 years [OR 3.42 (95% CI 1.72–6.81)] was independently associated with UO (Table 2). There was no interaction between age and TIL groups on UO (High TIL* age 35–49 years, p = 0.47; High*TIL age 50–79 years, p = 0.98; High TIL* age ≥ 80 years, p = 0.87).
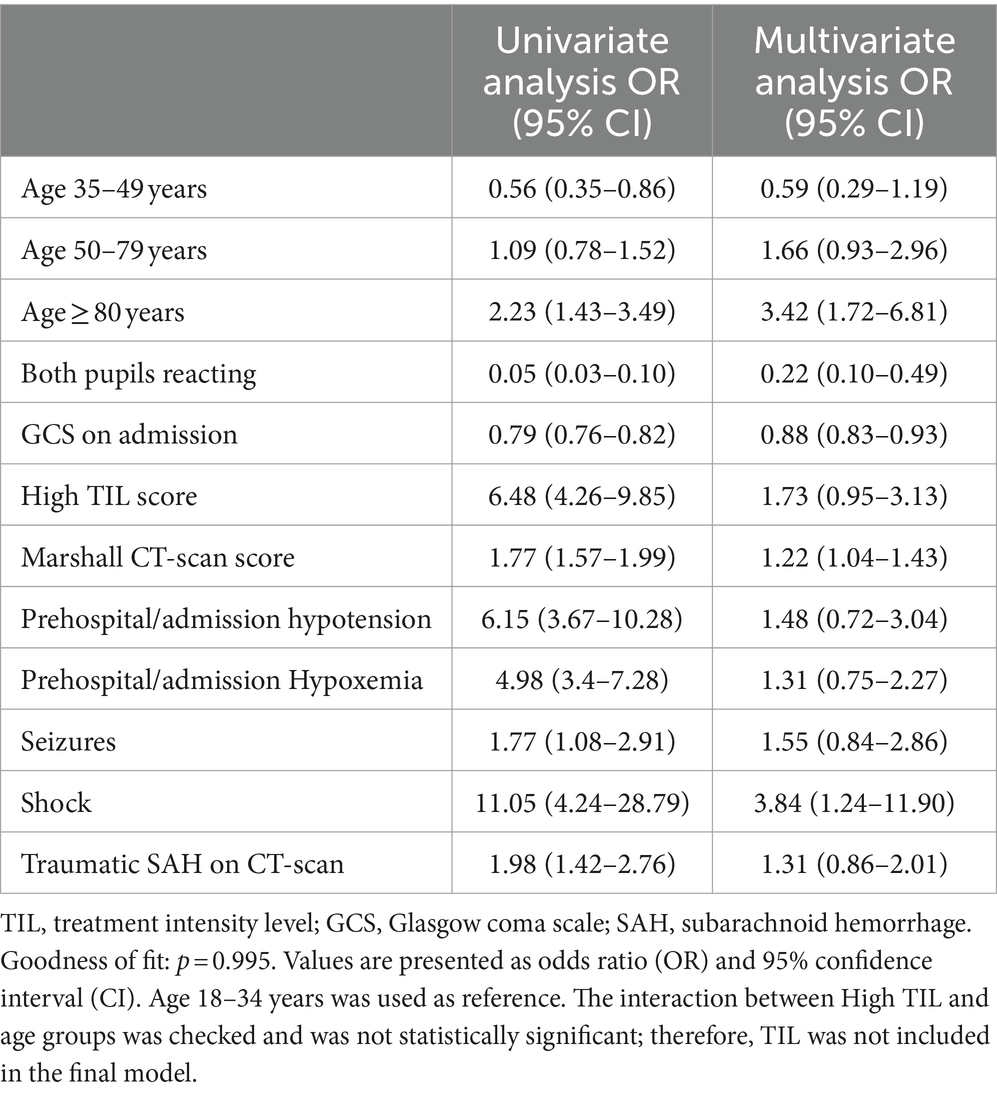
Table 2. Univariate and multivariate logistic regression of factors associated with unfavorable neurological outcome at 3 months.
3.4 Age groups and in-hospital mortality
Non-survivors were older, had lower GCS scores, worse Marshall scores, a higher frequency of hypoxemia and hypotension on admission, and received a higher TIL compared to survivors (Supplementary Tables S2, S3). In an adjusted Cox proportional hazard regression analysis, we found that age ≥ 80 years [HR 5.42 (95% CI 3.00–9.79)] and the age group 50–79 years [HR 2.03 (95% CI 1.19–3.48)] were both associated with a higher risk of in-hospital death (Table 3 and Figure 1). There was no interaction between age and TIL groups on in-hospital mortality (High TIL* age 35–49 years, p = 0.47; High*TIL age 50–79 years, p = 0.08; High TIL* age ≥ 80 years, p = 0.08).
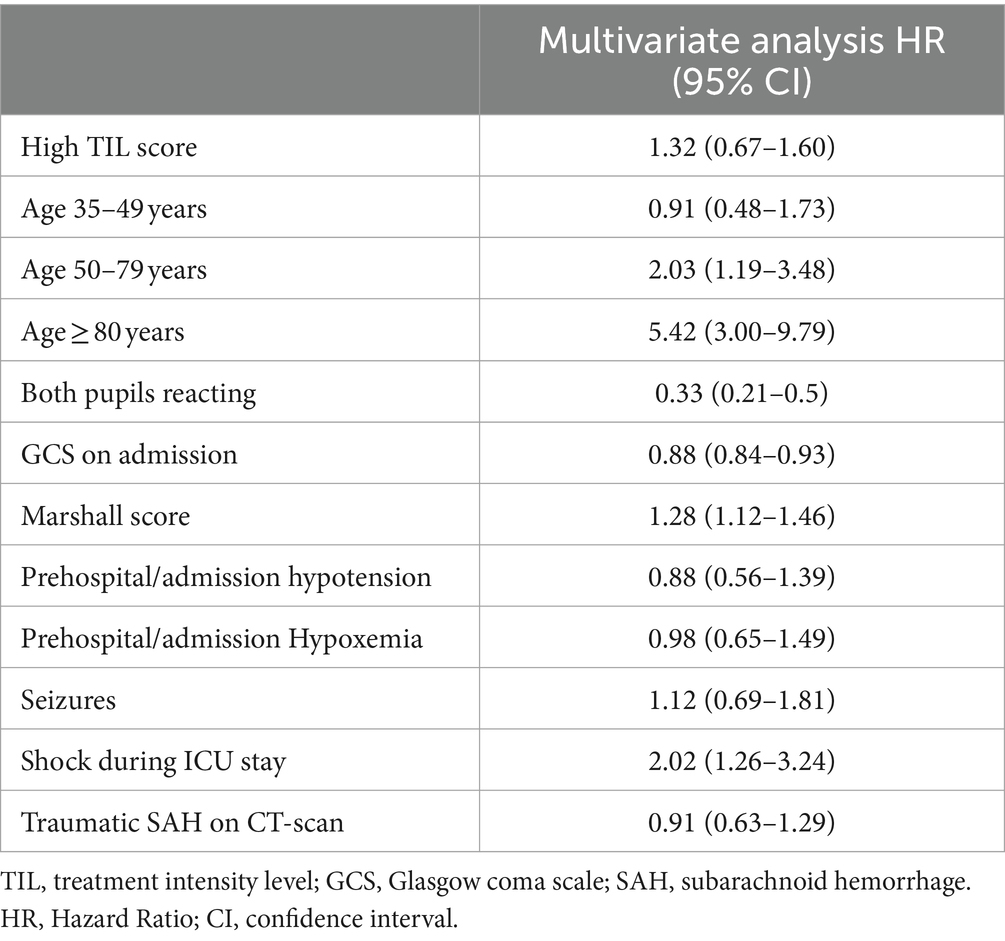
Table 3. Multivariate cox proportional hazard regression analysis of factors associated with in hospital death.
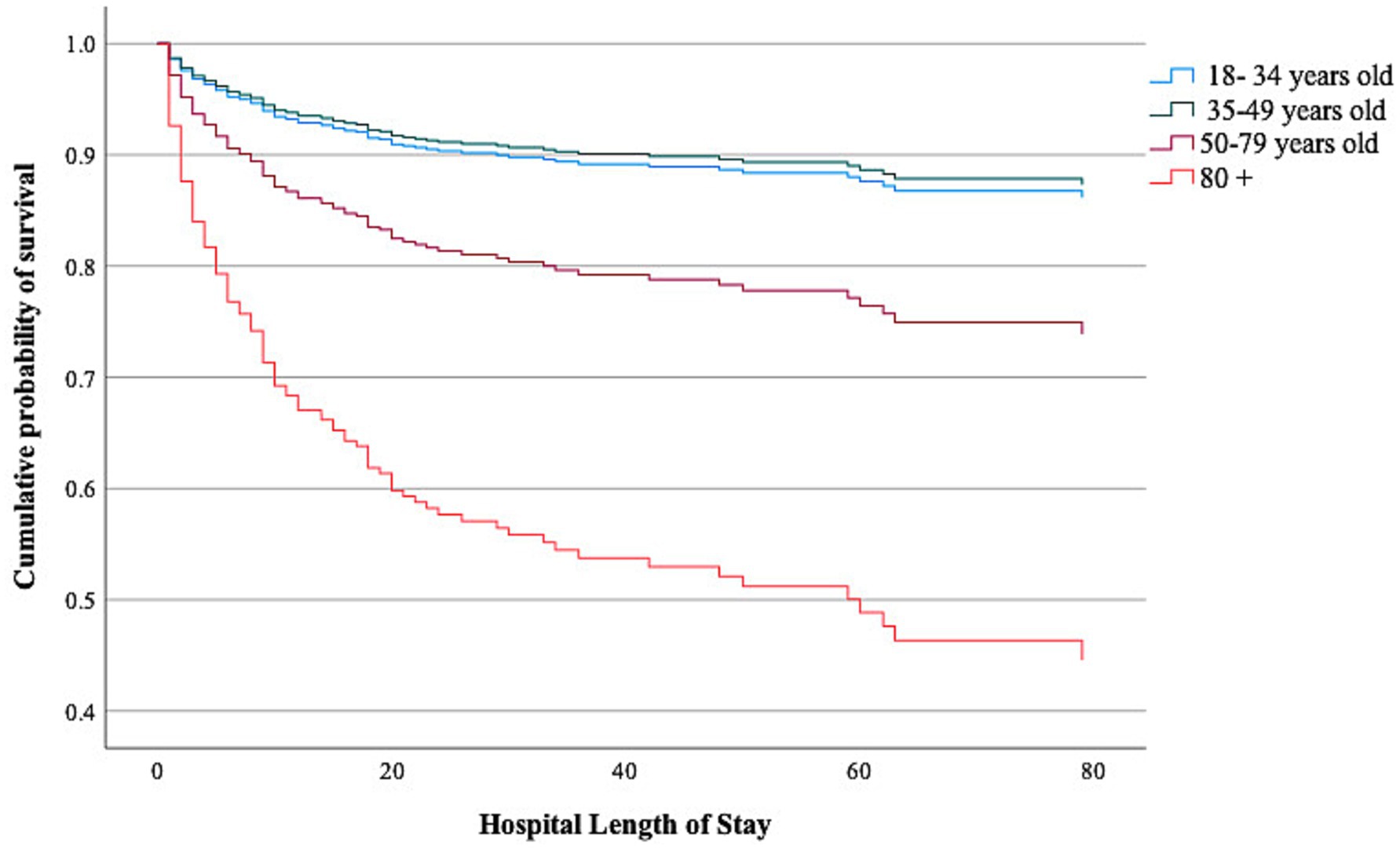
Figure 1. Cumulative incidence of survival over time according to age groups. p-value was calculated using a Cox proportion hazard regression model adjusted for High TIL, GCS on admission, pupil reactivity on admission, Marshall CT score on admission, Prehospital/admission hypotension, Prehospital/admission hypoxemia, traumatic SAH.
3.5 Exploratory subgroup analysis
Patients with high TIL (n = 144, 24%) were younger, had more organ dysfunction, higher Marshall CT scores, lower GCS on admission, and worse neurological outcomes compared to others (Supplementary Table S4). In the high TIL subgroup, multivariate logistic regression analysis found no association between age and UO (Supplementary Table S5). In the low TIL group, age ≥ 80 years [OR 3.65 (95% CI 1.64–8.14)] was independently associated with UO (Supplementary Table S6). Similarly, a multivariate Cox proportional hazard regression analysis found that in the high TIL subgroup, age was not associated with in-hospital mortality (Supplementary Table S7), while age 50–79 years [HR 6.31 (95% CI 1.83–21.75)] and age ≥ 80 years [HR 21.25 (95% CI 5.88–76.74)] were independently associated with in-hospital mortality in the low TIL group (Supplementary Table S8).
4 Discussion
In this study, we found that age ≥ 80 years was independently associated with worse neurological outcomes and that age ≥ 50 years was associated with increased in-hospital mortality. However, in a exploratory hypothesis generating subgroup analysis considering patients who received high-intensity treatment, age did not increase the likelihood of poor outcomes at 3 months or in-hospital mortality. In contrast, among patients who received lower-intensity therapy, age ≥ 80 years was independently associated with poor outcomes and age ≥ 50 years was independently associated with in-hospital mortality.
Currently, the rate of hospital admissions due to TBI is higher in the elderly population (≥ 65 years old) than in other age groups (31). This patient population is more susceptible to falls, which usually cause mass effect injuries, and they have more comorbidities and severe baseline cognitive or functional deficits (32), increasing the risk of post-recovery functional decline compared to younger patients (33). Indeed, older age has consistently been reported as a factor associated with unfavorable outcomes (34–37) and mortality (33, 38–40) in this setting. However, this association may be impacted by frailty, which represents the cumulative decline in physiological systems over a lifetime (13), making patients more vulnerable to further insults such as TBI and susceptible to poorer outcomes. A recent study has shown that older patients have higher frailty scores, and frailty was an independent determinant of poor outcomes (41). Previous studies have also demonstrated that older TBI patients, especially those without significant pre-existing comorbid conditions (42–44), can survive and recover, reinforcing that factors other than age are important when prognosticating these patients (14, 18, 45). Moreover, a study from the CENTER-TBI database recently highlighted that patients receiving high-intensity treatments may have better outcomes despite the initial severity of the illness (46). This finding has also been confirmed in the SYNAPSE-ICU study, which showed that ICP monitoring and higher TIL were associated with lower mortality, despite older patients not being specifically analyzed in detail for outcomes, based on age groups (47). Similarly, Skaansar et al. demonstrated that a low management intensity strategy correlated with higher 30-day mortality in elderly patients (12).
Despite these findings, older TBI patients are less frequently admitted to the ICU, receive less intensive monitoring (such as invasive ICP), and undergo fewer life-saving interventions like mechanical ventilation or surgery, as highlighted in previous studies (11, 48–51). For instance, randomized clinical trials of surgical interventions in TBI, such as decompressive craniectomy, often exclude patients over the age of 60–65 years (52, 53). The clinical implications of this study, particularly for elderly patients, deserve close attention. Aggressive interventions such as decompressive craniectomy in this population carry inherent risks due to frailty, pre-existing comorbidities, and polypharmacy. These factors likely contribute to the exclusion of older patients from clinical trials, as studies have demonstrated higher mortality and complication rates in this age group (52, 53). Frailty and functional status play a pivotal role in the recovery potential of elderly patients and must be carefully balanced against the potential benefits of invasive procedures (54–56). Geriatric trauma outcome scores (GTOS) and tools like the GERtality Score can help quantify these risks (54–56). These scoring systems, validated in predicting mortality and poor outcomes in elderly trauma patients, aid clinicians in identifying which patients might benefit from high-intensity treatments (57). Additionally, socioeconomic status (SES) has been shown to significantly influence healthcare access, with lower SES often correlating with poorer outcomes due to limited access to post-acute care and rehabilitation services (58).
Additionally, patients included in the BEST-TRIP randomized clinical trial that assessed the impact of ICP monitoring on the outcome of TBI patients were young, with a median age of 29 years, despite not having an upper age limit as an exclusion criterion, further demonstrating that elderly patients are often not offered specific modalities of monitoring and treatment (59). Nevertheless, successful rehabilitation and community reintegration are possible for older TBI patients when adequately treated (49, 60–62).
When assessing the prognosis of brain-injured patients, a so-called “self-fulfilling prophecy” occurs, e.g., when a comatose patient is given a poor prognosis, leading to the withholding of life-sustaining care based on that prediction, which directly results in the patient’s poor outcome (e.g., death) (63). This phenomenon is common in the elderly population but can also be observed in younger patients. A recent study found that among 1,400 patients with severe TBI, some who were taken off life support may have survived and regained some level of independence a few months later, as predicted by a complex mathematical model (64). Neurological recovery and improvement of disorders of consciousness require time and intensive rehabilitation (65) in both elderly and young patients (66). This situation points to a cyclical, self-fulfilling prophecy where clinicians predict poor outcomes based on previously published data in the context of limited therapy, including ICU admission for cases deemed unsalvageable. This assumption leads to withdrawing life support, which increases the likelihood of poor outcomes and further decisions to remove life support (64). In our exploratory analysis, the differences between high and low TIL subgroups may suggest a benefit in treating implementing high intensity treatment to an elderly population. However, this was an exploratory analysis of a retrospective registry and we are unable to ascertain what were the criteria used to decide the intensity of treatment applied to some elderly patients but not others, which may have introduced bias. Further studies are therefore required to investigate this hypothesis. In fact, whether a more proactive therapeutic approach could potentially decrease the high mortality rate and improve neurological outcomes among older TBI patients remains to be validated.
One might speculate that high-tier treatments for TBI patients are costly and not generally worth it compared to comfort care. However, this hypothesis has been contradicted by a study in which researchers developed a decision-analytical model to compare aggressive care, routine care, and comfort care. The study showed that aggressive care may be significantly less expensive up to the age of 80, after which it becomes more expensive than routine care (67). Nonetheless, even for 80-year-old patients, aggressive care may be reasonable depending on the patient’s baseline functional status. Although the p-value for interaction was not significantly different when analyzing the impact of age and TIL on measured outcomes, subgroup analyses were performed, as suggested in previous publications (68, 69). These analyses suggested the importance of age on outcomes in relation to the intensity of therapies used to lower ICP.
The advantage of our research lies in its detailed examination of how TIL interacts with age to influence outcomes in TBI patients. While many studies have considered age an independent predictor of worse outcomes, few have explored the mitigating effects of aggressive treatment, especially in older populations. Our findings indicate that high-intensity treatment can improve neurological outcomes and survival even in elderly patients traditionally considered high-risk due to their age. These results challenge conservative treatment practices and suggest that age should not be the sole factor in determining treatment intensity. This study’s focus on the interaction between age and TIL provides new insights into optimizing care for elderly TBI patients and supports a more personalized, aggressive treatment approach.
Our study has several limitations. The retrospective design may introduce biases and limit the ability to establish causal relationships between the identified predictors and clinical outcomes. Additionally, it restricted our analysis to short-term outcomes, which are not ideal for the neurocritical population. Despite our large sample size, the results may lack generalizability as it is a single-center study. Furthermore, the assessment of TIL may be subject to variability in measurement techniques and lacks standardized cut-offs for defining high and low TIL subgroups. The TIL score refers primarily to the intensity of treatment focused on intracranial hypertension, which, while an essential cause of mortality and unfavorable outcomes in TBI patients, does not account for other complications, such as hospital-acquired infections that could have impacted our results. Importantly, we did not evaluate the economic burden associated with patient care across different age groups. Such an analysis requires specialized expertise and falls beyond the scope of our study. Finally, we were unable to assess mortality due to withdrawal of care, which may have influenced our findings, and we did not retrieve the SES of patients. Additionally, we did not evaluate patients’ functional status before admission or their frailty score. Our study also had a limited number of patients aged 80 years or more, which may have reduced the power of the study to detect significant outcomes in this age group and adequately evaluate the interaction between age categories and TIL.
5 Conclusion
Our findings suggested that while advanced age is independently associated with worse outcomes in TBI patients, treatment intensity can modify this relationship. High-intensity therapy could lead to better outcomes, even in patients aged 80 years or older, suggesting that age alone should not be a limiting factor in treatment decisions. A personalized, potentially aggressive approach to improve survival and neurological outcomes across age groups, particularly for elderly needs to be further investigated.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Brussels Erasme Hospital EC, Belgium. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
AC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. AF: Data curation, Writing – review & editing. ST: Data curation, Writing – review & editing. RZ: Data curation, Writing – review & editing. AI: Data curation, Writing – review & editing. LP: Writing – review & editing. MS: Writing – review & editing. CF: Data curation, Writing – review & editing. LC: Data curation, Writing – review & editing. MP: Data curation, Writing – review & editing. EV: Data curation, Writing – review & editing. SS: Writing – review & editing. FT: Conceptualization, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – review & editing. EB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CPP, cerebral perfusion pressure; FO, Favor neurological outcome; GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Scale; GTOS, Geriatric Trauma Outcome Scores; ICP, Intracranial Pressure; LOC, loss of consciousness; TBI, Traumatic Brain Injury; PTA, Post-traumatic amnesia; TIL, Therapy intensity level; UO, Unfavorable Outcome; SFP, Self-fulfilling prophecy; SAH, Subarachnoid Hemorrhage; SES, Socioeconomic Status; SOFA, Sequential Organ Failure Assessment; WHO, World Health Organization.
References
1. Dewan, MC, Rattani, A, Gupta, S, Baticulon, RE, Hung, YC, Punchak, M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. (2018) 130:1080–97. doi: 10.3171/2017.10.JNS17352
2. Brazinova, A, Rehorcikova, V, Taylor, MS, Buckova, V, Majdan, M, Psota, M, et al. Epidemiology of traumatic brain injury in Europe: a living systematic review. J Neurotrauma. (2021) 38:1411–40. doi: 10.1089/neu.2015.4126
3. Nguyen, R, Fiest, KM, McChesney, J, Kwon, CS, Jette, N, Frolkis, AD, et al. The international incidence of traumatic brain injury: a systematic review and Meta-analysis. Can J Neurol Sci. (2016) 43:774–85. doi: 10.1017/cjn.2016.290
4. Galgano, M, Toshkezi, G, Qiu, X, Russell, T, Chin, L, and Zhao, LR. Traumatic brain injury. Cell Transplant. (2017) 26:1118–30. doi: 10.1177/0963689717714102
5. Rutland-Brown, W, Langlois, JA, Thomas, KE, and Xi, YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. (2006) 21:544–8. doi: 10.1097/00001199-200611000-00009
6. CDC . Report to congress on traumatic brain injury in the United States: Epidemiology and rehabilitation. (2024). Available at: https://stacks.cdc.gov/view/cdc/29215 (Accessed May 30, 2024).
7. Gómez, PA, Lobato, RD, Boto, GR, De la Lama, A, González, PJ, and de la Cruz, J. Age and outcome after severe head injury. Acta Neurochir. (2000) 142:373–81. doi: 10.1007/s007010050445
8. Narayan, RK, Greenberg, RP, Miller, JD, Enas, GG, Choi, SC, Kishore, PR, et al. Improved confidence of outcome prediction in severe head injury. A comparative analysis of the clinical examination, multimodality evoked potentials, CT scanning, and intracranial pressure. J Neurosurg. (1981) 54:751–62. doi: 10.3171/jns.1981.54.6.0751
9. Braakman, R, Gelpke, GJ, Habbema, JD, Maas, AI, and Minderhoud, JM. Systematic selection of prognostic features in patients with severe head injury. Neurosurgery. (1980) 6:362–70.
10. Teasdale, G, Skene, A, Parker, L, and Jennett, B. Age and outcome of severe head injury. Acta Neurochir Suppl (Wien). (1979) 28:140–3. doi: 10.1007/978-3-7091-4088-8_33
11. Røe, C, Skandsen, T, Anke, A, Ader, T, Vik, A, Lund, SB, et al. Severe traumatic brain injury in Norway: impact of age on outcome. J Rehabil Med. (2013) 45:734–40. doi: 10.2340/16501977-1198
12. Skaansar, O, Tverdal, C, Rønning, PA, Skogen, K, Brommeland, T, Røise, O, et al. Traumatic brain injury—the effects of patient age on treatment intensity and mortality. BMC Neurol. (2020) 20:376. doi: 10.1186/s12883-020-01943-6
13. Maas, AIR, Menon, DK, Manley, GT, Abrams, M, Åkerlund, C, Andelic, N, et al. Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. (2022) 21:1004–60. doi: 10.1016/S1474-4422(22)00309-X
14. De Bonis, P, Pompucci, A, Mangiola, A, D’Alessandris, QG, Rigante, L, and Anile, C. Decompressive craniectomy for the treatment of traumatic brain injury: does an age limit exist? J Neurosurg. (2010) 112:1150–3. doi: 10.3171/2009.7.JNS09505
15. Ratha Krishnan, R, Ting, SWX, Teo, WS, Lim, CJ, and Chua, KSG. Rehabilitation of older Asian traumatic brain injury inpatients: a retrospective study comparing functional Independence between age groups. Life. (2023) 13:2047. doi: 10.3390/life13102047
16. Hume, CH, Wright, BJ, and Kinsella, GJ. Systematic review and Meta-analysis of outcome after mild traumatic brain injury in older people. J Int Neuropsychol Soc. (2022) 28:736–55. doi: 10.1017/S1355617721000795
17. Mak, CHK, Wong, SKH, Wong, GK, Ng, S, Wang, KKW, Lam, PK, et al. Traumatic brain injury in the elderly: is it as bad as we think? Curr Transl Geriatr Exp Gerontol Rep. (2012) 1:171–8. doi: 10.1007/s13670-012-0017-2
18. Lilley, EJ, Williams, KJ, Schneider, EB, Hammouda, K, Salim, A, Haider, AH, et al. Intensity of treatment, end-of-life care, and mortality for older patients with severe traumatic brain injury. J Trauma Acute Care Surg. (2016) 80:998–1004. doi: 10.1097/TA.0000000000001028
19. Bhattacharyay, S, Beqiri, E, Zuercher, P, Wilson, L, Steyerberg, EW, Nelson, DW, et al. Therapy intensity level scale for traumatic brain injury: Clinimetric assessment on neuro-monitored patients across 52 European intensive care units. J Neurotrauma. (2023) 41:887–909. doi: 10.1089/neu.2023.0377
20. Meyfroidt, G, Bouzat, P, Casaer, MP, Chesnut, R, Hamada, SR, Helbok, R, et al. Management of moderate to severe traumatic brain injury: an update for the intensivist. Intensive Care Med. (2022) 48:649–66. doi: 10.1007/s00134-022-06702-4
21. Carney, N, Totten, AM, O’Reilly, C, Ullman, JS, Hawryluk, GWJ, and Bell, MJ. et al, Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. (2017) 80:6–15. doi: 10.1227/NEU.0000000000001432
22. Brain Trauma Foundation. Guidelines for the Management of Severe TBI, 3rd edition. (2024). (cited Jul 21, 2024).
23. Cutler, DM . Health system change in the wake of COVID-19. JAMA Health Forum. (2023) 4:e234355. doi: 10.1001/jamahealthforum.2023.4355
24. Vincent, JL, Moreno, R, Takala, J, Willatts, S, De Mendonça, A, Bruining, H, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
25. Teasdale, G, and Jennett, B. Assessment of coma and impaired consciousness: a practical scale. Lancet. (1974) 304:81–4. doi: 10.1016/S0140-6736(74)91639-0
26. Marshall, LF, Marshall, SB, Klauber, MR, Clark, MVB, Eisenberg, HM, Jane, JA, et al. A new classification of head injury based on computerized tomography. J Neurosurg. (1991) 75:S14–20. doi: 10.3171/sup.1991.75.1s.0s14
27. Maas, AIR, Harrison-Felix, CL, Menon, D, Adelson, PD, Balkin, T, Bullock, R, et al. Standardizing data collection in traumatic brain injury. J Neurotrauma. (2011) 28:177–87. doi: 10.1089/neu.2010.1617
28. Silver, JM, McAllister, TW, and Arciniegas, DB. Textbook of traumatic brain injury. 3rd Edn. Washington, DC: American Psychiatric Association Publishing, pp. 3–22. (2018).
29. Jennett, B, and Bond, M. Assessment of outcome after severe brain damage: a practical scale. Lancet. (1975) 305:480–4. doi: 10.1016/S0140-6736(75)92830-5
30. Dhandapani, S, Manju, D, Sharma, B, and Mahapatra, A. Prognostic significance of age in traumatic brain injury. J Neurosci Rural Pract. (2012) 3:131–5. doi: 10.4103/0976-3147.98208
31. Majdan, M, Plancikova, D, Brazinova, A, Rusnak, M, Nieboer, D, Feigin, V, et al. Epidemiology of traumatic brain injuries in Europe: a cross-sectional analysis. Lancet Public Health. (2016) 1:e76–83. doi: 10.1016/S2468-2667(16)30017-2
32. Gardner, RC, Dams-O’Connor, K, Morrissey, MR, and Manley, GT. Geriatric traumatic brain injury: epidemiology, outcomes, knowledge gaps, and future directions. J Neurotrauma. (2018) 35:889–906. doi: 10.1089/neu.2017.5371
33. Dams-O’Connor, K, Ketchum, JM, Cuthbert, JP, Corrigan, J, Hammond, FM, Krupa, JH, et al. Functional outcome trajectories following inpatient rehabilitation for TBI in the United States: a NIDILRR TBIMS and CDC interagency collaboration. J Head Trauma Rehabil. (2020) 35:127–39. doi: 10.1097/HTR.0000000000000484
34. Cuthbert, JP, Harrison-Felix, C, Corrigan, JD, Kreider, S, Bell, JM, Coronado, VG, et al. Epidemiology of adults receiving acute inpatient rehabilitation for a primary diagnosis of traumatic brain injury in the United States. J Head Trauma Rehabil. (2015) 30:122–35. doi: 10.1097/HTR.0000000000000012
35. Mosenthal, AC, Livingston, DH, Lavery, RF, Knudson, MM, Lee, S, Morabito, D, et al. The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. J Trauma. (2004) 56:1042–8. doi: 10.1097/01.TA.0000127767.83267.33
36. Thompson, HJ, Dikmen, S, and Temkin, N. Prevalence of comorbidity and its association with traumatic brain injury and outcomes in older adults. Res Gerontol Nurs. (2012) 5:17–24. doi: 10.3928/19404921-20111206-02
37. Stocchetti, N, Paternò, R, Citerio, G, Beretta, L, and Colombo, A. Traumatic brain injury in an aging population. J Neurotrauma. (2012) 29:1119–25. doi: 10.1089/neu.2011.1995
38. Ramanathan, DM, McWilliams, N, Schatz, P, and Hillary, FG. Epidemiological shifts in elderly traumatic brain injury: 18-year trends in Pennsylvania. J Neurotrauma. (2012) 29:1371–8. doi: 10.1089/neu.2011.2197
39. McIntyre, A, Mehta, S, Aubut, J, Dijkers, M, and Teasell, RW. Mortality among older adults after a traumatic brain injury: a meta-analysis. Brain Inj. (2013) 27:31–40. doi: 10.3109/02699052.2012.700086
40. Coronado, VG, Thomas, KE, Sattin, RW, and Johnson, RL. The CDC traumatic brain injury surveillance system: characteristics of persons aged 65 years and older hospitalized with a TBI. J Head Trauma Rehabil. (2005) 20:215–28. doi: 10.1097/00001199-200505000-00005
41. Galimberti, S, Graziano, F, Maas, AIR, Isernia, G, Lecky, F, Jain, S, et al. Effect of frailty on 6-month outcome after traumatic brain injury: a multicentre cohort study with external validation. Lancet Neurol. (2022) 21:153–62. doi: 10.1016/S1474-4422(21)00374-4
42. Raj, R, Mikkonen, ED, Kivisaari, R, Skrifvars, MB, Korja, M, and Siironen, J. Mortality in elderly patients operated for an acute subdural hematoma: a surgical case series. World Neurosurg. (2016) 88:592–7. doi: 10.1016/j.wneu.2015.10.095
43. Harvey, LA, Mitchell, R, Brodaty, H, Draper, B, and Close, JC. Comparison of fall-related traumatic brain injury in residential aged care and community-dwelling older people: a population-based study. Australas J Ageing. (2017) 36:144–50. doi: 10.1111/ajag.12422
44. Valadka, AB, and Sprunt, JM. Craniotomy for acute subdural hematoma in the elderly: not as bad as you thought. World Neurosurg. (2012) 78:231–2. doi: 10.1016/j.wneu.2011.12.067
45. Taussky, P, Hidalgo, ET, Landolt, H, and Fandino, J. Age and salvageability: analysis of outcome of patients older than 65 years undergoing craniotomy for acute traumatic subdural hematoma. World Neurosurg. (2012) 78:306–11. doi: 10.1016/j.wneu.2011.10.030
46. Huijben, JA, Dixit, A, Stocchetti, N, Maas, AIR, Lingsma, HF, van der Jagt, M, et al. Use and impact of high intensity treatments in patients with traumatic brain injury across Europe: a CENTER-TBI analysis. Crit Care. (2021) 25:78. doi: 10.1186/s13054-020-03370-y
47. Robba, C, Graziano, F, Rebora, P, Elli, F, Giussani, C, Oddo, M, et al. Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): an international, prospective observational cohort study. Lancet Neurol. (2021) 20:548–58. doi: 10.1016/S1474-4422(21)00138-1
48. Bus, S, Verbaan, D, Kerklaan, BJ, Sprengers, MES, Vandertop, WP, Stam, J, et al. Do older patients with acute or subacute subdural hematoma benefit from surgery? Br J Neurosurg. (2019) 33:51–7. doi: 10.1080/02688697.2018.1522418
49. Dijkers, M, Brandstater, M, Horn, S, Ryser, D, and Barrett, R. Inpatient rehabilitation for traumatic brain injury: the influence of age on treatments and outcomes. NeuroRehabilitation. (2013) 32:233–52. doi: 10.3233/NRE-130841
50. Hernesniemi, J . Outcome following head injuries in the aged. Acta Neurochir. (1979) 49:67–79. doi: 10.1007/BF01809175
51. Petridis, AK, Dörner, L, Doukas, A, Eifrig, S, Barth, H, and Mehdorn, M. Acute subdural hematoma in the elderly; clinical and CT factors influencing the surgical treatment decision. Cent Eur Neurosurg. (2009) 70:73–8. doi: 10.1055/s-0029-1224096
52. Cooper, DJ, Rosenfeld, JV, Murray, L, Arabi, YM, Davies, AR, D’Urso, P, et al. Decompressive Craniectomy in diffuse traumatic brain injury. N Engl J Med. (2011) 364:1493–502. doi: 10.1056/NEJMoa1102077
53. Hutchinson, PJ, Kolias, AG, Timofeev, IS, Corteen, EA, Czosnyka, M, Timothy, J, et al. Trial of decompressive Craniectomy for traumatic intracranial hypertension. N Engl J Med. (2016) 375:1119–30. doi: 10.1056/NEJMoa1605215
54. Ravindranath, S, Ho, KM, Rao, S, Nasim, S, and Burrell, M. Validation of the geriatric trauma outcome scores in predicting outcomes of elderly trauma patients. Injury. (2021) 52:154–9. doi: 10.1016/j.injury.2020.09.056
55. Scherer, J, Kalbas, Y, Ziegenhain, F, Neuhaus, V, Lefering, R, Teuben, M, et al. The GERtality score: the development of a simple tool to help predict in-hospital mortality in geriatric trauma patients. J Clin Med. (2021) 10:1362. doi: 10.3390/jcm10071362
56. Zhuang, Y, Feng, Q, Tang, H, Wang, Y, Li, Z, and Bai, X. Predictive value of the geriatric trauma outcome score in older patients after trauma: a retrospective cohort study. Int J Gen Med. (2022) 15:4379–90. doi: 10.2147/IJGM.S362752
57. El-Qawaqzeh, K, Anand, T, Alizai, Q, Colosimo, C, Hosseinpour, H, Spencer, A, et al. Trauma in the geriatric and the super-geriatric: should they be treated the same? J Surg Res. (2024) 293:316–26. doi: 10.1016/j.jss.2023.09.015
58. Braveman, P, Egerter, S, and Williams, DR. The social determinants of health: coming of age. Annu Rev Public Health. (2011) 32:381–98. doi: 10.1146/annurev-publhealth-031210-101218
59. Chesnut, RM, Temkin, N, Carney, N, Dikmen, S, Rondina, C, Videtta, W, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. (2012) 367:2471–81. doi: 10.1056/NEJMoa1207363
60. Tokutomi, T, Miyagi, T, Ogawa, T, Ono, J, Kawamata, T, Sakamoto, T, et al. Age-associated increases in poor outcomes after traumatic brain injury: a report from the Japan Neurotrauma data Bank. J Neurotrauma. (2008) 25:1407–14. doi: 10.1089/neu.2008.0577
61. Balandin, AA, Balandina, IA, and Pankratov, MK. Effectiveness of treatment of elderly patients with traumatic brain injury complicated by subdural hematoma. Adv Gerontol Uspekhi Gerontol. (2021) 34:461–5.
62. Rothweiler, B, Temkin, NR, and Dikmen, SS. Aging effect on psychosocial outcome in traumatic brain injury. Arch Phys Med Rehabil. (1998) 79:881–7. doi: 10.1016/S0003-9993(98)90082-X
63. Mertens, M, King, OC, Van, PMJAM, and Boenink, M. Can we learn from hidden mistakes? Self-fulfilling prophecy and responsible neuroprognostic innovation. J Med Ethics. (2022) 48:922–8. doi: 10.1136/medethics-2020-106636
64. Sanders, WR, Barber, JK, Temkin, NR, Foreman, B, Giacino, JT, Williamson, T, et al. Recovery potential in patients who died after withdrawal of life-sustaining treatment: a TRACK-TBI propensity score analysis. J Neurotrauma. (2024) 41:2336–48. doi: 10.1089/neu.2024.0014
65. Edlow, BL, Claassen, J, Schiff, ND, and Greer, DM. Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies. Nat Rev Neurol. (2021) 17:135–56. doi: 10.1038/s41582-020-00428-x
66. McCrea, MA, Giacino, JT, Barber, J, Temkin, NR, Nelson, LD, Levin, HS, et al. Functional outcomes over the first year after moderate to severe traumatic brain injury in the prospective, longitudinal TRACK-TBI study. JAMA Neurol. (2021) 78:982–92. doi: 10.1001/jamaneurol.2021.2043
67. Whitmore, RG, Thawani, JP, Grady, MS, Levine, JM, Sanborn, MR, and Stein, SC. Is aggressive treatment of traumatic brain injury cost-effective? J Neurosurg. (2012) 116:1106–13. doi: 10.3171/2012.1.JNS11962
68. Williamson, SF, Grayling, MJ, Mander, AP, Noor, NM, Savage, JS, Yap, C, et al. Subgroup analyses in randomized controlled trials frequently categorized continuous subgroup information. J Clin Epidemiol. (2022) 150:72–9. doi: 10.1016/j.jclinepi.2022.06.017
Keywords: traumatic brain injury, age, treatment, mortality, neurological outcome, TBI, TIL
Citation: Corriero A, Fornaciari A, Terrazzino S, Zangari R, Izzi A, Peluso L, Savi M, Faso C, Cavallini L, Polato M, Vitali E, Schuind S, Taccone FS and Bogossian EG (2024) The impact of age and intensity of treatment on the outcome of traumatic brain injury. Front. Neurol. 15:1471209. doi: 10.3389/fneur.2024.1471209
Edited by:
Mark M. Walsh, Saint Joseph Regional Medical Center, United StatesReviewed by:
Edoardo Picetti, University Hospital of Parma, ItalyRohadi Muhammad Rosyidi, University of Mataram, Indonesia
Copyright © 2024 Corriero, Fornaciari, Terrazzino, Zangari, Izzi, Peluso, Savi, Faso, Cavallini, Polato, Vitali, Schuind, Taccone and Bogossian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto Corriero, YWxiZXJ0by5jb3JyaWVyb0BnbWFpbC5jb20=
 Alberto Corriero
Alberto Corriero Anna Fornaciari
Anna Fornaciari Samuel Terrazzino2
Samuel Terrazzino2 Rossella Zangari
Rossella Zangari Lorenzo Peluso
Lorenzo Peluso Marzia Savi
Marzia Savi Chiara Faso
Chiara Faso Laura Cavallini
Laura Cavallini Eva Vitali
Eva Vitali Fabio Silvio Taccone
Fabio Silvio Taccone Elisa Gouvêa Bogossian
Elisa Gouvêa Bogossian