- 1Norton Neuroscience Institute, Norton Healthcare, Louisville, KY, United States
- 2Neurodiagnostic Center of Louisville, Louisville, KY, United States
Objectives: Proximal median nerve (PMN) neuropathies are caused by lesions proximal to the carpal tunnel, which include the forearm, elbow, upper arm, and brachial plexus. Differentiating between carpal tunnel syndrome and PMN neuropathies is important to guide management and is based on clinical, electrodiagnostic (EDX), and ultrasound (US) findings. This study describes the clinical, EDX, and US features in 62 patients with PMNs.
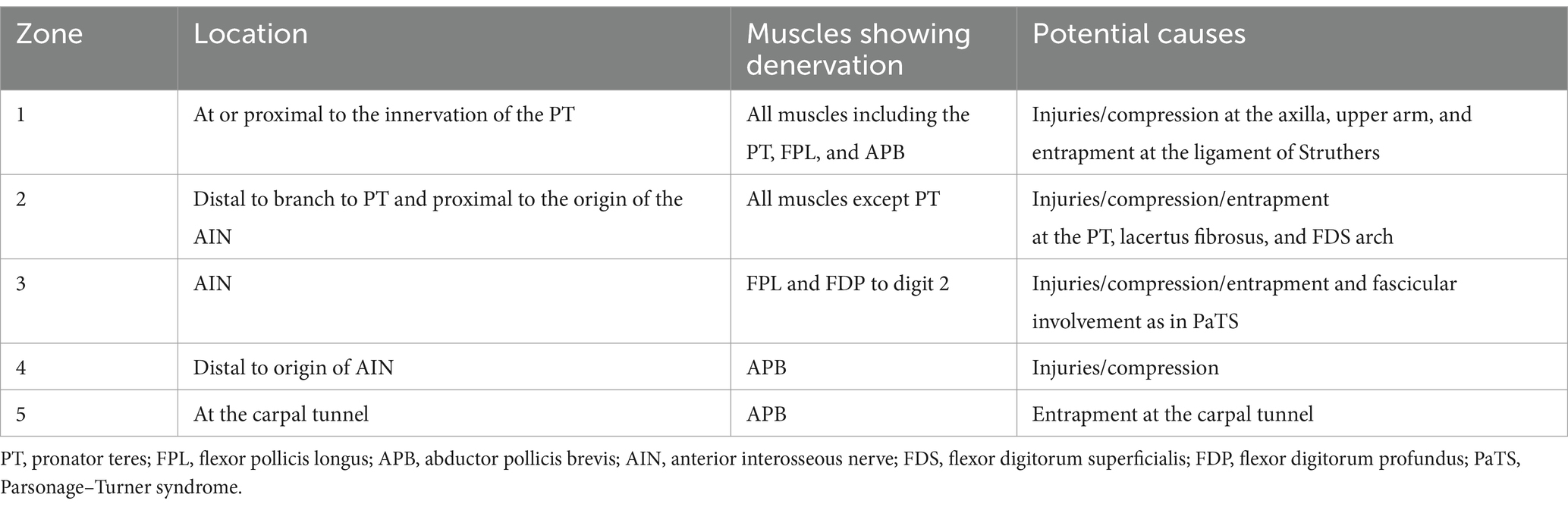
Methods: All patients underwent EDX studies, and 52 (83.9%) had a US study. The patients were assigned to one of the following four localization zones of PMN neuropathies based on clinical and EDX criteria: Zone 1: extends from the fascicles in the brachial plexus contributing to the median nerve to the innervation of the pronator teres (PT); Zone 2: distal to the branch to the PT and proximal to the origin of the anterior interosseous nerve (AIN); Zone 3: involves the origin of the AIN; and Zone 4: distal to the origin of the AIN and proximal to the carpal tunnel. The localization was based on the pattern of muscle weakness, topography of EMG abnormalities, and US study findings.
Results: The anatomical locations of the PMN neuropathies based on clinical, EDX, and US findings were as follows: Zone 1 in 38 patients (61.3%), Zone 2 in 6 patients (9.7%), Zone 3 in 7 patients (11.3%), and Zone 4 in 11 patients (17.7%). The most common etiology among all 62 patients was iatrogenic injury (30 [48.4%]), followed by non-iatrogenic trauma (20 [32.2%]). The following EDX findings were noted: prolonged distal motor latency (29 [46.8%]), decreased motor nerve conduction velocity in the forearm (22 [35.5%]), low amplitude or absent compound muscle action potentials (50 [80.6%]), and abnormal or absent sensory nerve action potentials (50 [80.6%]). Of the 52 (83.9%) patients who underwent US studies, a total of 22 (42.3%) patients showed an increased cross-sectional area of the median nerve. A neuroma was observed in 9 patients (17.4%).
Conclusion: It is often possible to localize the site of the median nerve involvement and gain insight into the underlying cause based on clinical and EMG findings, but in certain cases, a US study may be necessary to confirm the location.
1 Introduction
The median nerve arises from the medial and lateral cords of the brachial plexus and has contributions from C5-T1 nerve roots (1–8). It descends lateral to the brachial artery and between the biceps brachii and brachialis muscles. The median nerve then traverses the antecubital fossa and runs deep to the bicipital aponeurosis and anterior to the brachialis muscle. It then passes between the superficial and deep heads of the pronator teres (PT). In the forearm, the median nerve travels between the flexor digitorum superficialis and profundus muscle bellies. The most common median nerve neuropathy is carpal tunnel syndrome (CTS), where the median nerve is compressed under the flexor retinaculum (4–6). Proximal median nerve (PMN) neuropathies denote lesions of the median nerve at locations proximal to the carpal tunnel, which include the forearm, elbow, upper arm, and brachial plexus. They are often underdiagnosed or misdiagnosed as CTS due to the similarity of symptoms and overlapping pathologies, which may co-exist in the same patient (4, 9).
Several proximal median nerve entrapment sites have been identified (from proximal to distal): (1) beneath the ligament of Struthers, which extends from the supracondylar process of the distal humerus; (2) bicipital aponeurosis (lacertus fibrosus); (3) proximal insertion of the PT humeral head; (4) passage between the two heads of the PT; (5) dysplasia of the deep PT head; (6) common arcade between the PT and flexor digitorum superficialis (FDS); (7) fibrous arch/arcade of the FDS; and (8) Gantzer muscle (1, 5, 7, 9–14). At several entrapment sites, mechanical compression causing a compromised arterial supply to the median nerve may lead to PMN neuropathies (10). At the level of the axilla, the nerve is vulnerable to compression in the medial fascial compartment (15).
Both PT and anterior interosseous nerve (AIN) syndromes involve compression/entrapment of the median nerve proximal to the carpal tunnel (4, 7, 14, 16). In 1951, Seyffarth described a case of median nerve compression in the proximal forearm and coined the term “pronator syndrome” (17). PT syndrome is considered to be a compressive/entrapment neuropathy of the median nerve in the proximal forearm with symptoms and signs that are similar to those of CTS (1, 10, 11, 18–20). AIN syndrome is a pure motor palsy resulting from compression/entrapment of the AIN close to its origin, most often by the deep and superficial heads of the PT or fibrous arcade of the FDS (10, 16).
The median nerve may be injured by either iatrogenic (excessive traction or use of retractors during surgery) or non-iatrogenic causes (direct penetrating trauma by a gunshot wound, knife wound, or broken glass) (3). EDX and US studies are invaluable in identifying the lesion site and distinguishing between the different underlying pathologies (16). These studies, especially US, are valuable to ascertain the etiology of the PMN neuropathies, which can potentially direct medical or surgical management (5).
A large series of patients with PMN neuropathies that includes EDX and US data is lacking in the literature. In this report, we describe 62 patients with PMN neuropathies, confirmed by EDX studies, 52 (83.9%) of whom also had US studies. The clinical, EDX, and US findings are presented. The etiologies and mechanisms of PMN neuropathies based on the specific anatomical zone of involvement are discussed. The significance of US in complementing the EDX studies for localization and determining the underlying cause is also highlighted.
2 Materials and methods
2.1 Electrodiagnostic studies and ultrasound studies
Under an Institutional Review Board (IRB)-approved protocol, we performed a 14½-year (October 2010–June 2024) retrospective analysis of patients referred to our neurodiagnostic center for EDX studies to evaluate PMN neuropathies. In each patient, PMN neuropathy was assigned a specific anatomic zone based on the localization of median nerve involvement by EMG and US findings (Figure 1; Table 1). The patients underwent a focused neurological examination of the upper extremities, followed by nerve conduction velocity (NCV) and needle EMG studies. The EDX studies were performed in our American Association of Neuromuscular & Electrodiagnostic Medicine (AANEM)-accredited facility using the standard protocol of our laboratory (21).
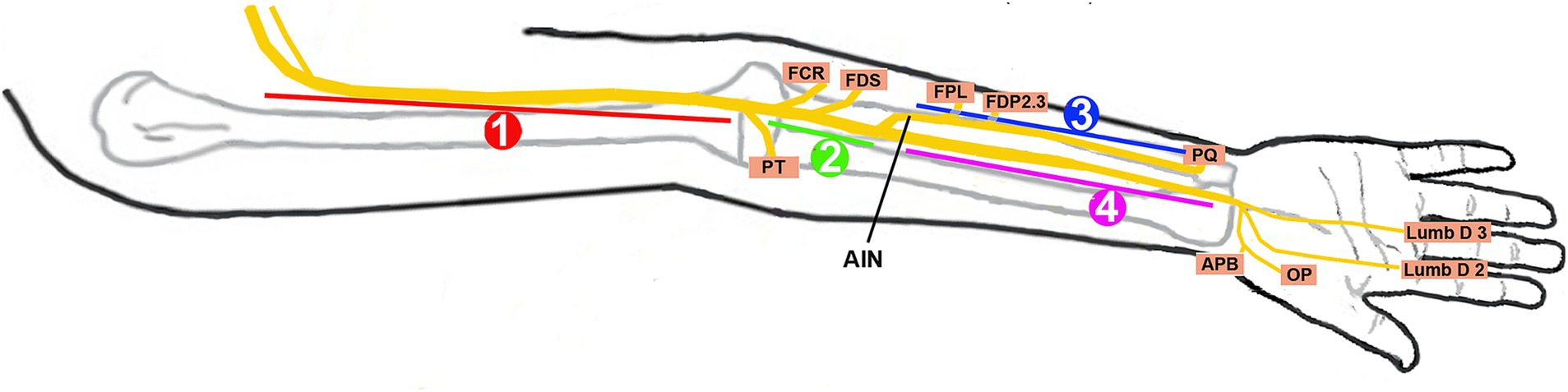
Figure 1. Localization zones of proximal median neuropathy. Zone 1, at or proximal to the branch to the pronator teres; Zone 2, distal to the branch to the pronator teres and proximal to the origin of the anterior interosseous nerve (AIN); Zone 3, involving the AIN; Zone 4, distal to the origin of the AIN and proximal to the carpal tunnel; FCR, flexor carpi radialis; FDS, flexor digitorum superficialis; FPL, flexor pollicis longus; FDP 2 and 3, flexor digitorum profundus to the second and third digits; PQ, pronator quadratus; APB, abductor pollicis brevis; OP, opponens pollicis; Lumb D 2 and 3, lumbricals of the second and third digits.
The standard protocol in our lab is to stimulate the median nerve at the wrist and the elbow with the recording electrodes over the abductor pollicis brevis (APB) (second lumbrical, if no response is seen over the APB). Additional studies were performed when indicated: (1) if the clinical findings suggested Zone 1 and intact CMAP were recorded over the APB, stimulation was extended to the upper arm and axilla to detect the site of conduction block in the proximal portions of the median nerve and (2) if the findings suggested the AIN as the site of involvement, the recording electrodes were placed over the pronator quadratus (PQ) to calculate the motor conduction velocity in the AIN (22, 23). A comparison study was performed on the unaffected side.
Needle EMG was performed in a “topographic mapping sequence,” starting at the APB, followed by flexor pollicis longus (FPL) and PT. Based on the initial findings, additional muscles such as the flexor carpi radialis (FCR), FDS, flexor digitorum profundus (FDP), and PQ were studied to get more precise localization and to identify selective fascicular involvement—common in Parsonage-Turner syndrome (PaTS). A proximal inflammatory (or even partial trauma) lesion can potentially cause distal slowing of motor conduction velocity due to anterograde demyelination and loss of fast conduction axons; this can also be caused by slow conduction in regenerating axons.
A US study was conducted using a GE Logiq E system with a 5–16 and/or 12–18 MHz probe, tracking the median nerve. The cross-sectional area (CSA) of the nerve was measured along the course of the median nerve at the wrist, proximal forearm, elbow, and upper arm.
2.2 Categorization of inflammatory AIN lesions
While PaTS is considered an immune-mediated acute brachial plexus neuropathy, the majority of patients present with symptoms and signs of single nerve involvement, most often anterior or posterior interosseous, axillary, or suprascapular nerves. MR neurography has shown changes in the brachial plexus but more often hourglass appearance in fascicles, which finally become one of the above-mentioned nerves (24). In our study, we classified PMN neuropathies into zones based on clinical/EMG patterns. If the pattern suggested location to the AIN in patients with a clinical picture of PaTS, they were included in Zone 3. Patients with a clinical diagnosis of PaTS but showed changes in the PT were included in Zone 1. Patients clinically diagnosed with PaTS (acute onset of scapular pain followed by muscle weakness in the distribution of one or more nerves derived from the brachial plexus) may have features of Zone 1, 2, or 3.
The diagnosis of PaTS is based on the typical clinical picture, specifically the acute onset of pain in the scapular area, followed by the onset of muscle weakness in the distribution of usually a single nerve derived from the brachial plexus. By the time the patients were referred to our lab, it was too late to perform MR neurography of the brachial plexus.
2.3 Inclusion and exclusion criteria
The inclusion criteria were (1) patients with clinical features of PMN neuropathies (Zones 1–4) and (2) EDX findings confirming PMN neuropathies. Patients with evidence of distal median neuropathy at the carpal tunnel (Zone 5) were excluded from the study. Several metrics were collected including age, sex, symptom laterality (right/left), hand dominance (right/left/ambidextrous), clinical history, specific zone of PMN neuropathies, etiology (trauma [iatrogenic vs. non-iatrogenic] or other causes), symptom onset (acute versus gradual), EDX findings, and US features. Specific NCV data that were compiled included distal motor latency, motor nerve conduction velocity (MNCV) in the forearm, amplitude of the compound muscle action potentials (CMAPs), and latency and amplitude of sensory nerve action potentials (SNAPs). Needle EMG was recorded from the APB, FPL, and PT muscles. US findings collected include CSA of the median nerve at different locations and altered echogenicity.
2.4 Institutional review board approval of research
Informed consent was obtained from all patients. The IRB determined that our study was exempt according to 45 CFR 46.101(b) under Category 4. The IRB number is 22.1060.
3 Results
3.1 Demographics and specific proximal median nerve localization
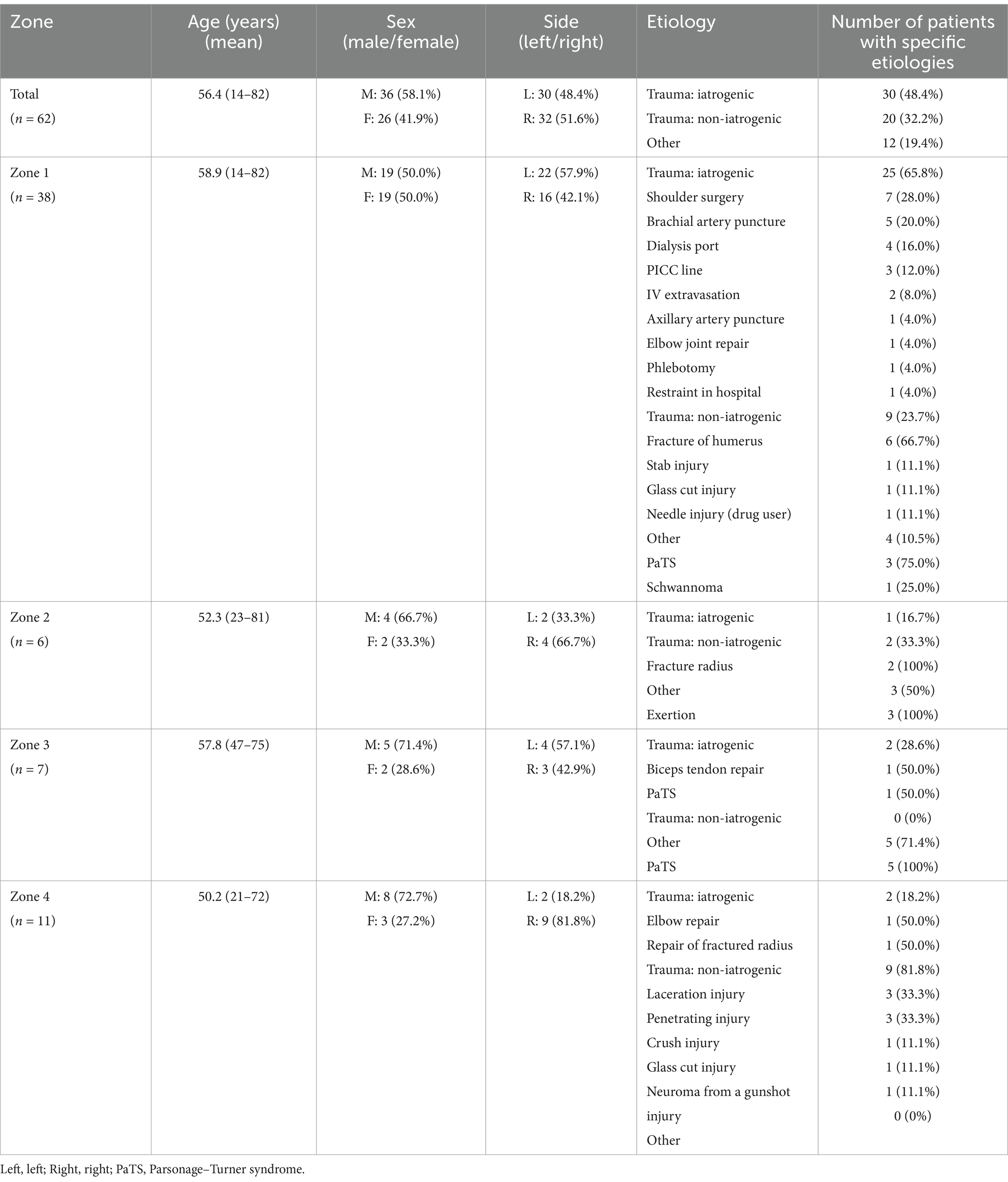
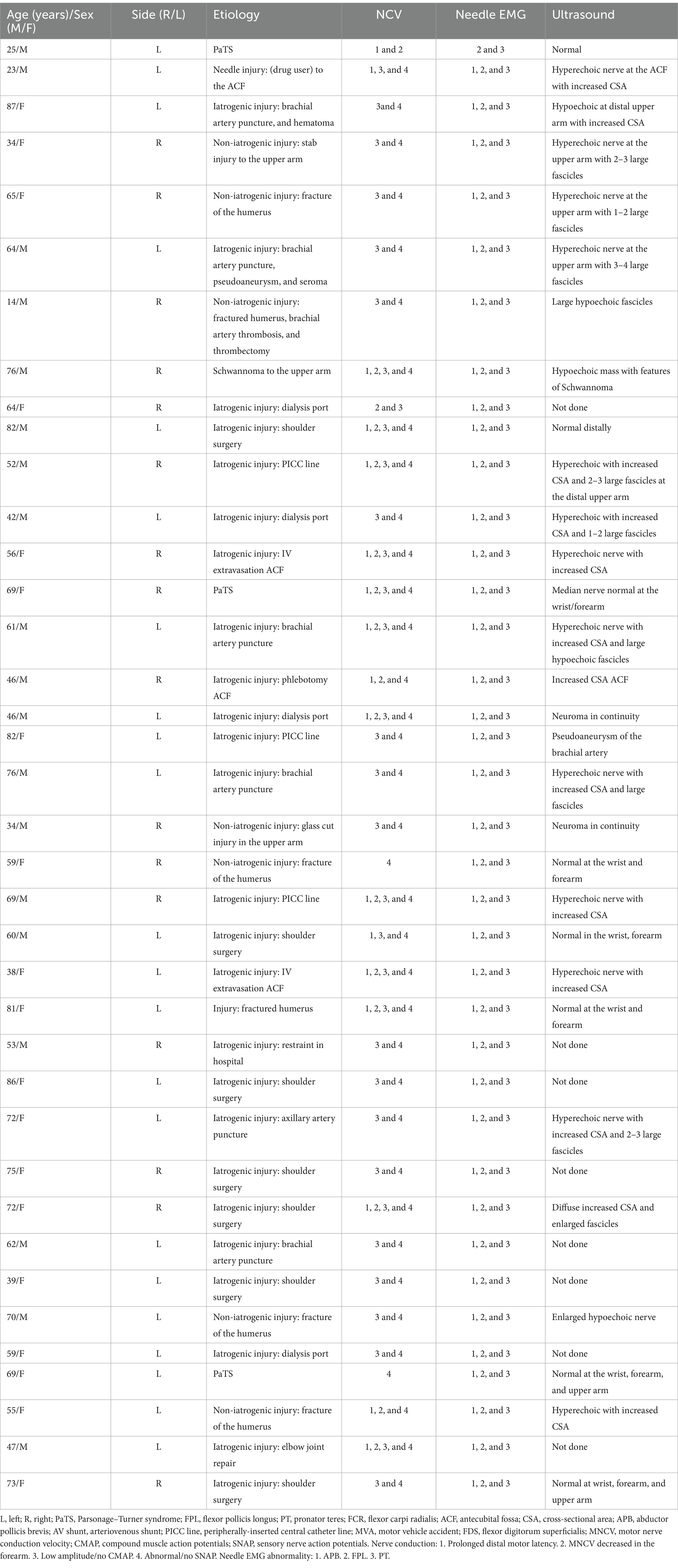
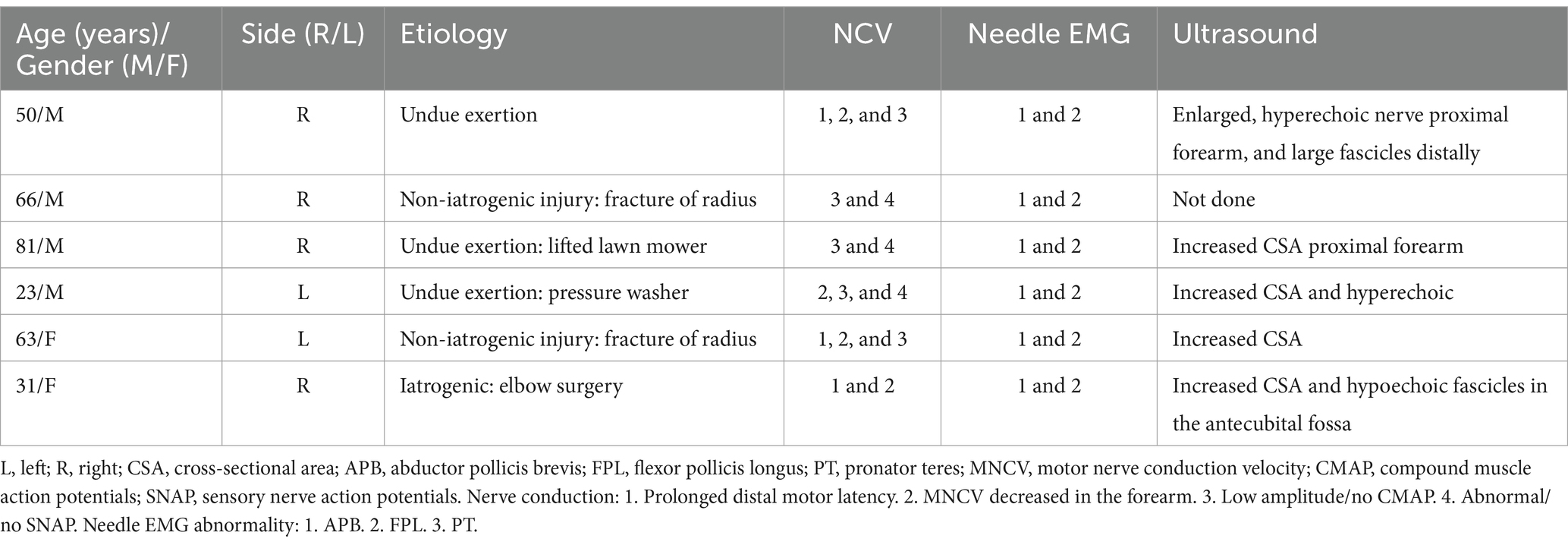
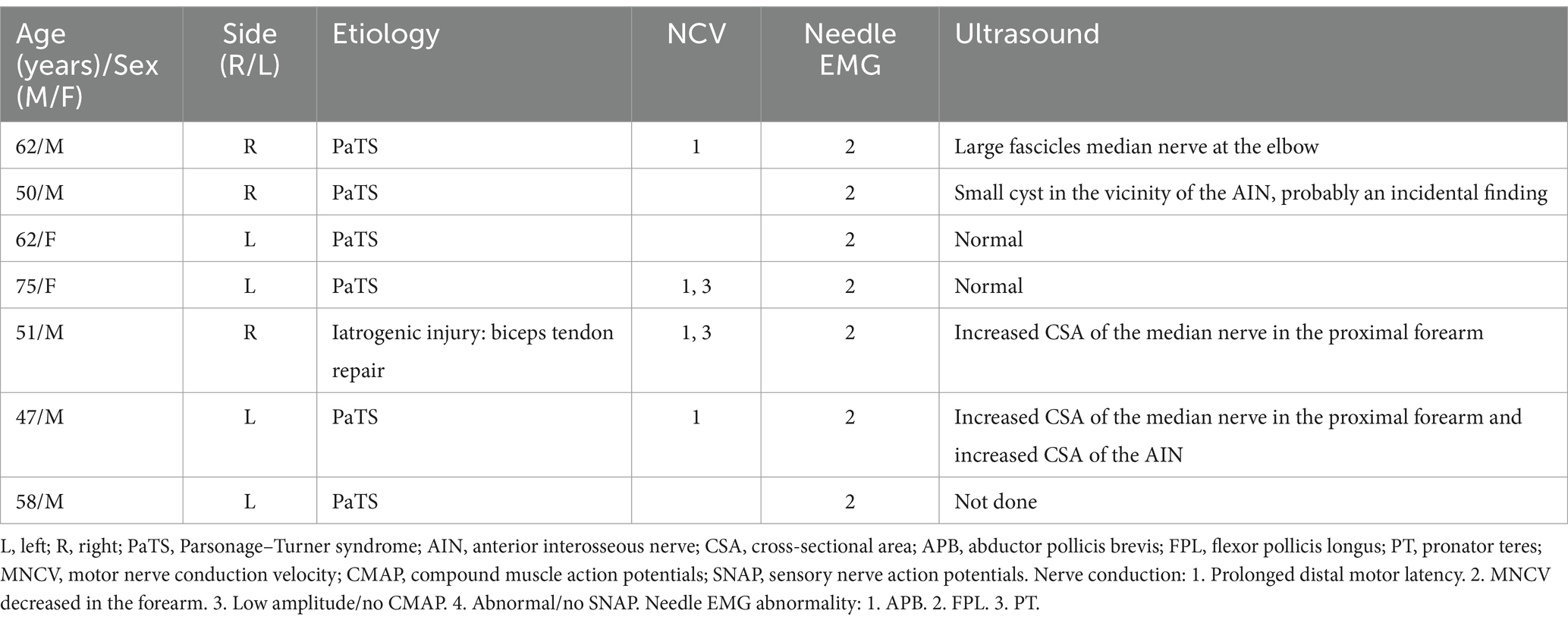
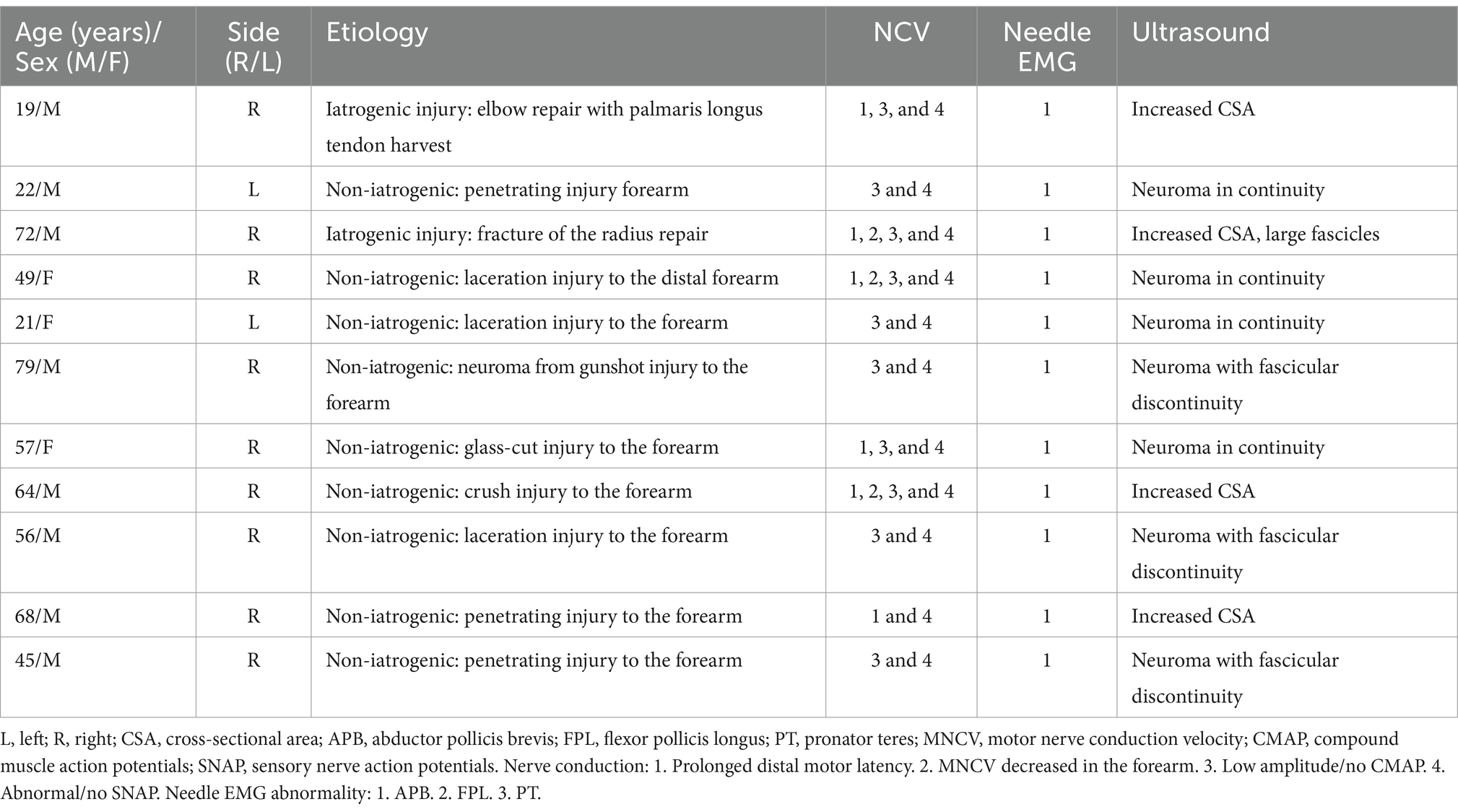
A total of 62 patients were diagnosed with PMN neuropathies based on EDX and US studies (Tables 2–7). The mean age was 56.4 years (range: 14–82 years), and the majority (36 [58.1%]) of the patients were male. The occurrence of PMN neuropathy was more common on the right side (32 [51.6%]). Fifty-four patients (87.1%) were right-hand dominant, 5 (8.1%) were left-hand dominant, and 3 (4.8%) were ambidextrous. The symptomatic side corresponded to hand dominance in 34 patients (54.8%). The PMN neuropathy localization was as follows: Zone 1 (38 patients [61.3%]), Zone 2 (6 patients [9.7%]), Zone 3 (7 patients [11.3%]), and Zone 4 (11 patients [17.7%]). Fifty-nine (95.2%) patients had an acute onset of symptoms, while 3 (4.8%) experienced gradual onset (Figures 2A–C).

Figure 2. Acute onset of pronator teres syndrome. (A) Positive O sign on the right. (B) Weakness of the flexor pollicis longus on the right. (C) Ultrasound study showing enlarged median nerve (encircled by yellow dots, arrow) with a few large fascicles.
3.2 Etiologies by localization to specific zones of PMN neuropathy
The most common etiology for all 62 patients was iatrogenic (30 [48.4%]), followed by non-iatrogenic trauma (20 [32.2%]) (Table 2). In Zone 1, iatrogenic injury was the most frequent etiology (25 [65.8%] patients), primarily due to shoulder surgery (7 patients [28.0%]) and brachial artery puncture (5 patients [20.0%]) (Figures 3A–C, 4A–E). The etiology of PMN neuropathies was excessive or undue exertion in 3 (50.0%) patients in Zone 2, PaTS in 5 patients (71.4%) in Zone 3, and non-iatrogenic trauma in 9 patients (81.8%) in Zone 4 (3 [33.3%] patients with a laceration injury and 3 [33.5%] patients with a penetrating injury).
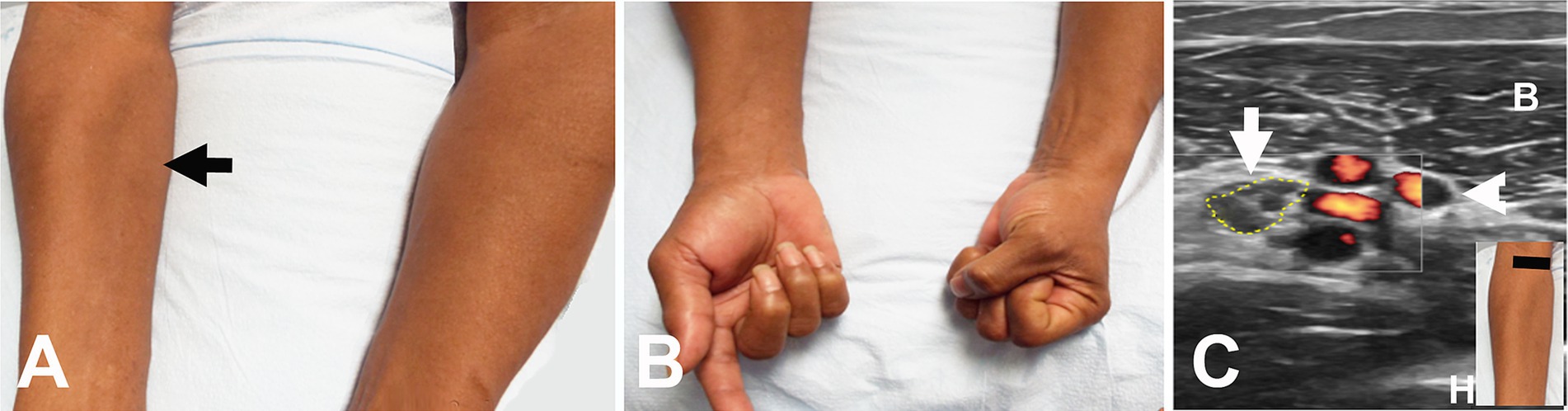
Figure 3. Acute onset of symptoms following intravenous access for a transjugular procedure. (A) Wasting of the pronator teres on the right (arrow). (B) Weakness of the flexor pollicis longus and flexor digitorum profundus on the right. (C) Short axis view at the distal right upper arm showing enlarged median nerve (encircled by yellow dots) adjacent to the brachial artery and vein (arrowhead). The black line in the insert indicates the level of ultrasound. B, biceps muscle; H, humerus.
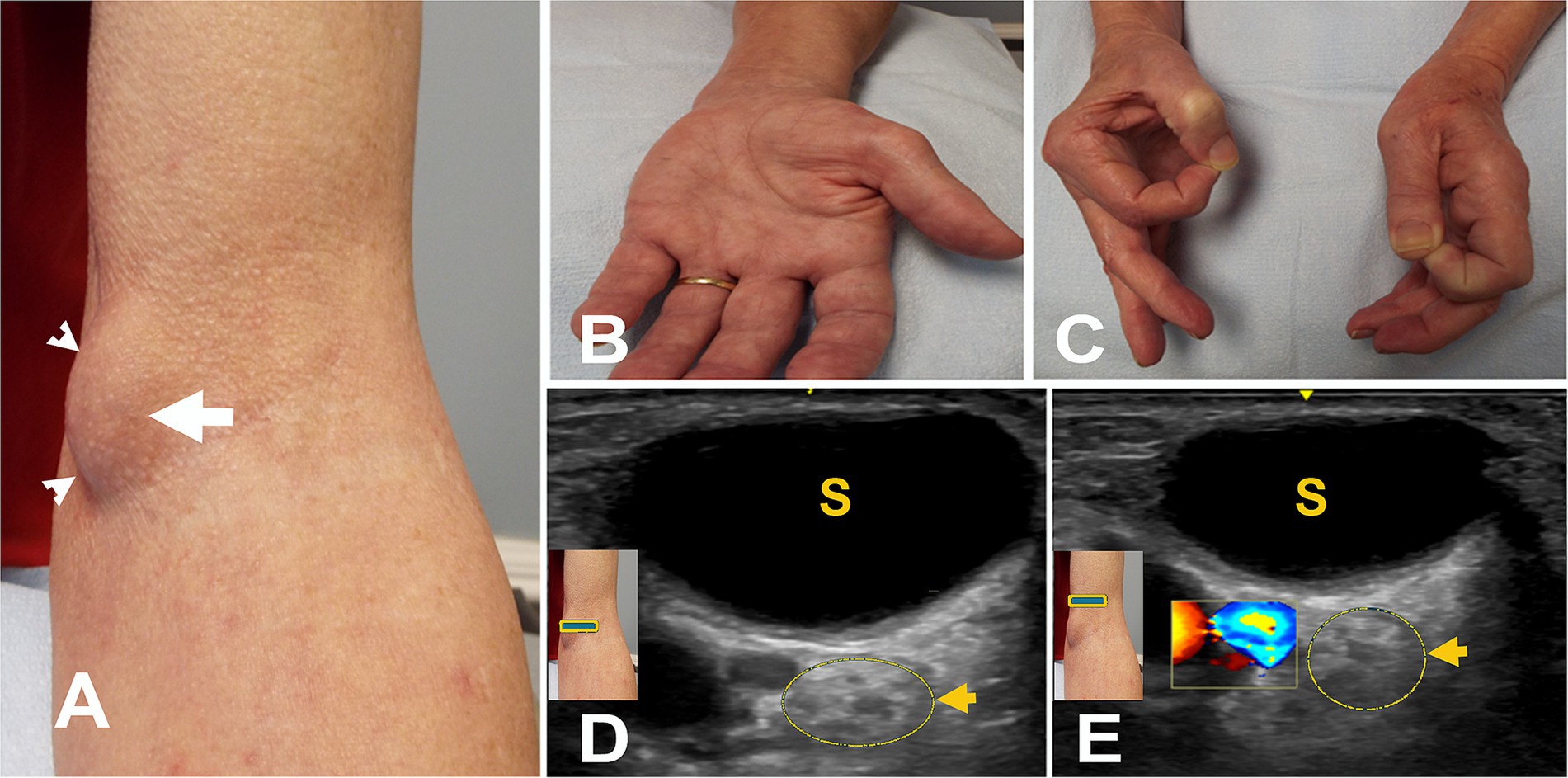
Figure 4. (A) Mass after brachial artery cannulation (arrowheads). (B) Atrophy of left thenar muscles. (C) Positive O sign on the left. (D) Short axis view at the elbow with color Doppler over the mass (square box) showing no blood flow. The arrow points to the hyperechoic enlarged median nerve with a few fascicles. (E) Short axis view with color Doppler (square box) 5 mm proximal to “A” showing the brachial artery with hyperechoic enlarged median nerve with a few fascicles (arrow). S, seroma.
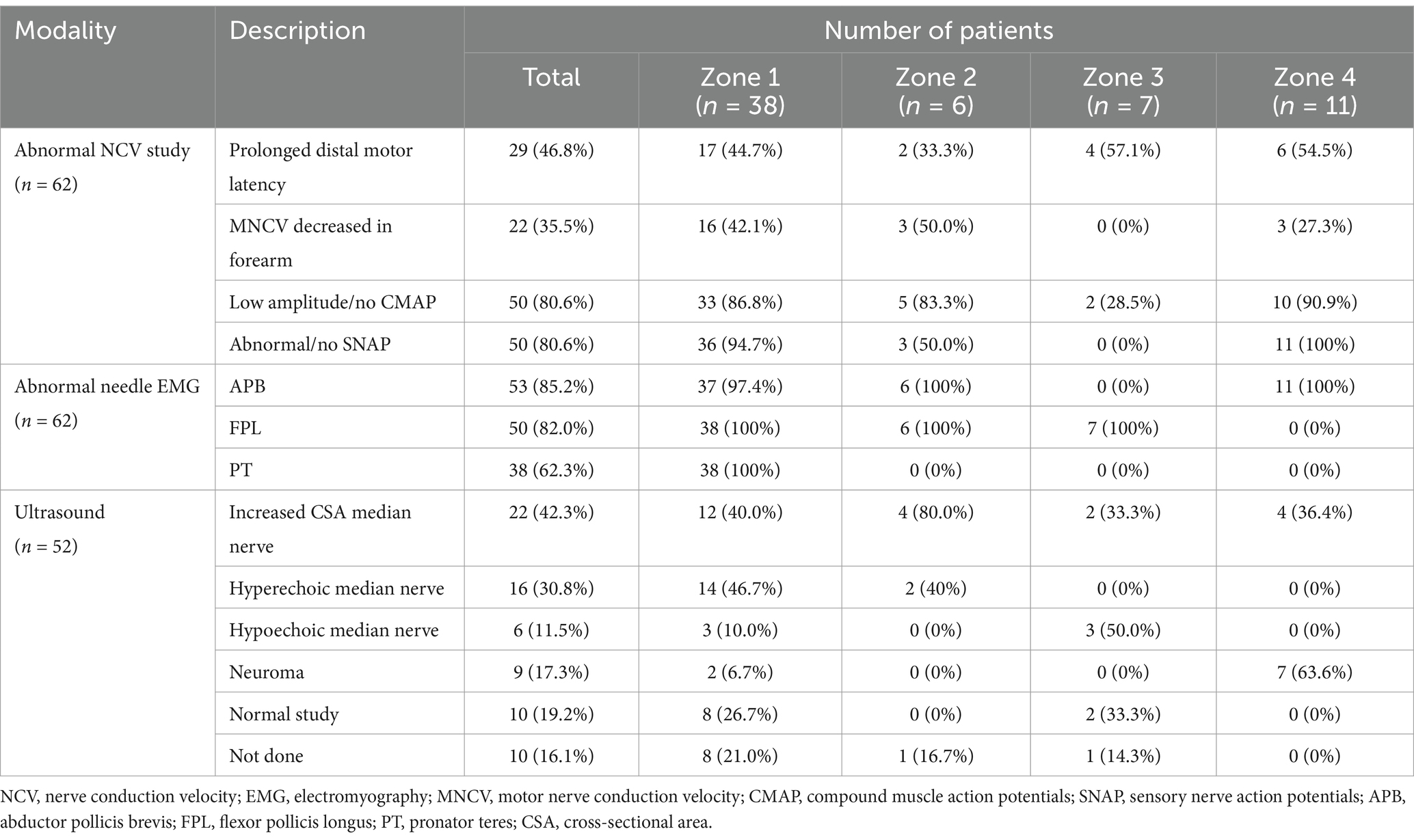
3.3 Electrodiagnostic studies
The findings of the EDX studies are summarized in Table 3. The following EDX findings were observed: prolonged distal motor latency (29 [46.8%]), decreased motor nerve conduction velocity in the forearm (22 [35.5%]), low amplitude or absent compound muscle action potentials (50 [80.6%]), and abnormal or absent sensory nerve action potentials (50 [80.6%]). In Zone 1, 36 (94.7%) patients had either abnormal or no SNAP, 33 (86.8%) had either low amplitude or no CMAP, and 17 (44.7%) had prolonged distal motor latency. Low amplitude or no CMAPs were detected in 5 (83.3%) patients in Zone 2, a prolonged distal motor latency was observed in 4 (57.1%) patients in Zone 3, and abnormal/no SNAPs were identified in 11 (100%) patients in Zone 4. In Zone 1, the needle EMG for the FPL and PT was abnormal in all 38 (100%) patients, and the APB was abnormal in 37 (97.4%). A needle EMG abnormality was observed in 6 patients in Zone 2 (APB and FPL), 7 patients in Zone 3 (FPL), and 11 patients in Zone 4 (APB).
3.4 Ultrasound studies
Fifty-two (83.9%) patients underwent US studies (Table 3; Figure 5). A total of 23 (42.3%) patients showed an increased CSA of the median nerve. The median nerve was hyperechoic in 16 (30.8%) patients and hypoechoic in 6 (11.5%) patients. A neuroma was observed in 9 (17.3%) patients (with continuity in 7 [77.8%] patients and fascicular discontinuity in 2 [22.2%] patients) (Figures 6A,B). The US studies were normal in 10 (19.2%) patients. A hyperechoic (14 [46.7%]) median nerve with an increased CSA (12 [40.0%]) was commonly observed in Zone 1. Seven (63.6%) patients in Zone 4 had evidence of a neuroma.
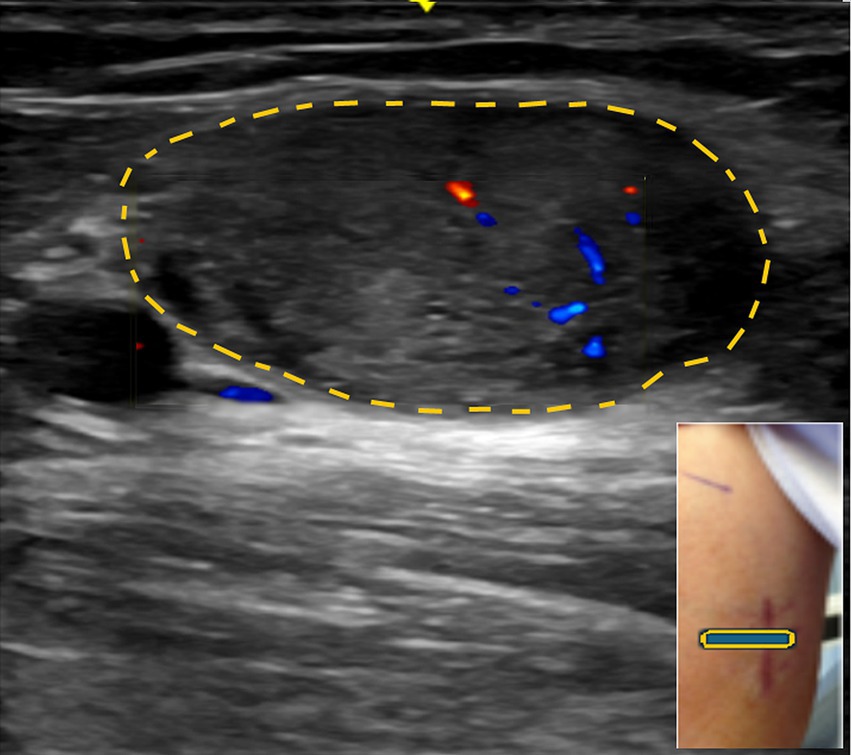
Figure 5. Short axis view with color Doppler at the mid-upper arm showing a schwannoma in the median nerve.

Figure 6. (A) Swelling of the right anterior forearm, representing the neuroma (arrow). (B) Long axis view at the distal forearm showing median nerve with neuroma in continuity encircled by yellow dots.
4 Discussion
The differential diagnoses of PMN neuropathy include CTS, thoracic outlet syndrome (TOS), other brachial plexopathies, and cervical radiculopathy (1). EDX studies are the primary modality for confirming anatomical localization of the site of PMN and determining the underlying pathology and its severity (16). Slowing of motor and sensory conduction, along with alterations in the morphology and amplitude of CMAP and SNAP are metrics used in this assessment (6, 20). EDX studies are useful in differentiating PMN neuropathy from CTS, although they are usually inconclusive or negative in clinically diagnosed PT syndrome (1, 16, 19). The needle EMG should include muscles innervated through the entire length of the median nerve so that the topography of abnormal muscles can be mapped (14). Denervation/reinnervation changes are identified in the median nerve-innervated muscles at and distal to the lesion site (10).
When the lesion is in Zone 1 (involving fascicles in the brachial plexus that form the median nerve and the median nerve itself up to the branch to the PT), precise localization by EDX studies can be challenging. The addition of US can provide further insight. High-resolution US provides high soft-tissue and spatial resolution, cost-effectiveness, safety, ready accessibility, easy portability, and real-time and dynamic nerve imaging in a timely manner (2, 5, 8, 12). US complements EDX studies in providing clues to localizing the lesion and insight into the underlying pathology in patients with PMN neuropathy. There are several scenarios where US is invaluable for the localization of median nerve neuropathy. In Zone 1, nerve conduction studies (NCS) and needle EMG do not always provide adequate findings for localization. Denervation in the PT (the most proximal muscle innervated by the median nerve) makes it difficult to differentiate between lesions at the site of the branch to the PT and more proximal lesions in the upper arm and axilla. US can locate the lesion in most circumstances. Similarly in Zone 4 lesions (in the forearm distal to the origin of the AIN) with denervation of the APB, differentiation from entrapment at the carpal tunnel is difficult. When motor conduction is slow proximal to the wrist, it is not necessarily a conclusive finding as retrograde slowing of motor conduction can occur in longstanding entrapment at the carpal tunnel (25). Precise localization may also be challenging when only the sensory fascicles are affected. While an inching study across the carpal tunnel can be helpful, detecting lesions in the distal forearm may be problematic. US can be useful in detecting such lesions in the forearm. When no CMAPs are detected over the APB and second lumbrical and no SNAPs are recordable over the digits, US is crucial for localization.
US is also useful in identifying the underlying pathology in two specific situations: (1) detection of lesions such as a schwannoma or other tumors/cysts compressing the nerve and (2) distinction between neurotmesis and axonotmesis in cases of nerve injuries. After Wallerian degeneration occurs distal to the site of injury, no conduction can be documented distal to the injury (unlike in a conduction block). While documentation of neurotmesis dictates quick surgical repair, in cases of axonotmesis, one could wait to see if reinnervation occurs within the expected period, which also depends on the length of the injured segment. EMG cannot make the distinction between total axonotmesis and neurotmesis, while US is able to in the majority of circumstances.
Fascicular lesions are particularly important in cases of inflammatory changes. A fascicular somatotopia can be seen early on in the nerve, which may explain the pattern of muscle paresis and sensory disturbance of more proximally located lesions (26). The fascicular anatomy of the median nerve has been well-described, and precise localization by nerve conduction and extensive needle EMG may fail. In PaTS, fascicular involvement proximal to the location suggested by the clinical picture has been reported (26), and our patients did not undergo MR neurography to localize the lesion.
The majority of the studies of PMN neuropathy have focused on entrapment/injury at the elbow and proximal forearm (13, 14, 27). In the study by Sos et al., 53 patients (55 cases, including 2 bilateral) with median nerve entrapment syndrome in the elbow and proximal forearm underwent surgical release of the median nerve and AIN around the elbow and/or proximal forearm. The most common compressed structure was isolated proximal PT insertion (14). The mean age at diagnosis was 56 years with a male-to-female ratio of 1:1 (29 male patients and 26 female patients). The symptom onset was gradual in 32 (58%) patients and acute in 23 (42%) patients. EMG studies demonstrated reduced motor conduction velocity in 38 (56%) patients, conduction block in 5 (9%) patients, and median nerve territory EMG abnormalities in 47 (85%) patients. Three patients had no EMG abnormalities. In Olehnik and colleagues’ study of 36 patients (39 limbs) who underwent surgical decompression of the median nerve in the proximal forearm, the site of compression was the FDS in 22 patients, PT in 13 patients, and both in 4 patients (13). The average age was 39 years, and 29 (80%) were female. Of the 37 limbs that underwent motor and sensory nerve conduction tests, 12 showed an abnormal nerve conduction velocity from the elbow to the wrist. Motor and sensory conduction abnormalities were detected in 12 and 2 limbs, respectively. In Gross and Jones’ study of 17 patients with PMN neuropathy who underwent EMG, the cause of neuropathy was trauma in 5, overuse of the PT in 3 patients, post-infection in 2 patients, secondary to a congenital lesion in 1, and undetermined in 6 (27). The main branch of the median nerve at or proximal to the PT was identified in 14 patients and the AIN in 3. The EMG demonstrated that median nerve compression by the PT caused denervation of the PT and distal muscles. EMG was unable to differentiate a median nerve lesion at the PT from a more proximal lesion (27). Our study concurs with the study by Sos et al. with respect to a similar mean age of patients (56.7 years). However, our study features a higher percentage of male patients (58.1%), acute symptom onset (95.2%), and iatrogenic etiology (48.4%). Our study was also the only one that presented US findings that revealed 42.3% of patients with an increased CSA of the median nerve and 30.2% of patients with a hyperechoic median nerve. All patients in our study underwent EDX studies, which showed low amplitude or no CMAP in 82.2% of the patients and abnormal/no SNAP in 79.0% of the patients.
Traumatic neuropathies were referred for EMG at our neurodiagnostic center after several weeks to months, and, therefore, we could classify them into only conduction block or axonal injury. US was helpful in showing neuroma in continuity versus neurotmesis. However, we were not successful in detecting hourglass fascicular lesions with US in our study.
4.1 Strengths and limitations
The strength of the current study is the large number of patients with PMN neuropathy, confirmed by EDX studies. A large percentage of patients in this series also underwent US studies. By determining the etiology of all patients with PMN neuropathy neuropathy, the most effective treatment course may be pursued. Limitations of the present study include its retrospective nature and lack of follow-up after the EDX and US studies as the majority of patients were evaluated only once.
5 Conclusion
Neurologists should be aware of the four localization zones of the PMN neuropathy with their distinctive etiologies and utilize additional investigations to confirm the diagnosis and formulate appropriate management. EDX and US studies are important techniques that complement each other in determining the specific localization zone for the PMN neuropathy and providing valuable clues to the underlying pathology in patients with PMN neuropathy. The classification into the four localization zones should be based on the final localization. The suspected lesion may initially be established by the clinical findings, with subsequent details offered by the EDX studies, and final exact localization with the assistance of the US studies. This tiered approach emphasizes the importance of the different modalities. Further studies are warranted to determine the contribution of inflammatory changes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the University of Louisville Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. VI: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. YZ: Resources, Software, Validation, Visualization, Writing – review & editing. CS: Conceptualization, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We acknowledge Norton Healthcare for their continued support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Adler, JA, and Wolf, JM. Proximal median nerve compression: pronator syndrome. J Hand Surg Am. (2020) 45:1157–65. doi: 10.1016/j.jhsa.2020.07.006
2. Brown, JM, Yablon, CM, Morag, Y, Brandon, CJ, and Jacobson, JA. US of the peripheral nerves of the upper extremity: a landmark approach. Radiographics. (2016) 36:452–63. doi: 10.1148/rg.2016150088
3. Isaacs, J, and Ugwu-Oju, O. High median nerve injuries. Hand Clin. (2016) 32:339–48. doi: 10.1016/j.hcl.2016.03.004
4. Lopponen, P, Hulkkonen, S, and Ryhanen, J. Proximal median nerve compression in the differential diagnosis of carpal tunnel syndrome. J Clin Med. (2022) 11:11. doi: 10.3390/jcm11143988
5. Mangi, MD, Zadow, S, and Lim, W. Nerve entrapment syndromes of the upper limb: a pictorial review. Insights Imaging. (2022) 13:166. doi: 10.1186/s13244-022-01305-5
6. Meyer, P, Lintingre, PF, Pesquer, L, Poussange, N, Silvestre, A, and Dallaudiere, B. The median nerve at the carpal tunnel …and elsewhere. J Belg Soc Radiol. (2018) 102:17. doi: 10.5334/jbsr.1354
7. Miller, TT, and Reinus, WR. Nerve entrapment syndromes of the elbow, forearm, and wrist. AJR Am J Roentgenol. (2010) 195:585–94. doi: 10.2214/AJR.10.4817
8. Nulle, K, and Jaudzema, A. Ultrasonographic evaluation of the median nerve: normal and variant anatomy and appearance. J Ultrason. (2021) 21:e318–25. doi: 10.15557/JoU.2021.0053
9. Cline, JA, Frantz, LM, Adams, JM, and Hearon, BF. Experience with proximal median nerve entrapment by the lacertus fibrosus. Hand. (2023) 19:904–11. doi: 10.1177/15589447231153233
10. Bair, MR, Gross, MT, Cooke, JR, and Hill, CH. Differential diagnosis and intervention of proximal median nerve entrapment: a resident's case problem. J Orthop Sports Phys Ther. (2016) 46:800–8. doi: 10.2519/jospt.2016.6723
11. Fitzmaurice, M. J. (2021). Endoscopic pronator release (proximal forearm median nerve decompression). In: ed. T. H. Lui Springer, Singapore: Arthroscopy and Endoscopy of the Hand, Wrist and Elbow.
12. Kara, M, Yalcin, S, Tiftik, T, and Ozcakar, L. Proximal median nerve entrapment caused by a distal biceps tendon cyst: an ultrasonographic diagnosis. Am J Phys Med Rehabil. (2013) 92:942–3. doi: 10.1097/PHM.0b013e3182a45c52
13. Olehnik, WK, Manske, PR, and Szerzinski, J. Median nerve compression in the proximal forearm. J Hand Surg Am. (1994) 19:121–6. doi: 10.1016/0363-5023(94)90235-6
14. Sos, C, Roulet, S, Lafon, L, Corcia, P, Laulan, J, and Bacle, G. Median nerve entrapment syndrome in the elbow and proximal forearm. Anatomic causes and results for a 55-case surgical series at a mean 7years' follow-up. Orthop Traumatol Surg Res. (2021) 107:102825. doi: 10.1016/j.otsr.2021.102825
15. Tsao, BE, and Wilbourn, AJ. The medial brachial fascial compartment syndrome following axillary arteriography. Neurology. (2003) 61:1037–41. doi: 10.1212/01.WNL.0000089488.35632.08
16. Barnett, SA, Shah, SA, and Ahmad, RI. Endoscopic proximal median nerve decompression: an alternative treatment for pronator syndrome. J Hand Surg Glob Online. (2021) 3:210–4. doi: 10.1016/j.jhsg.2021.03.006
17. Seyffarth, H. Primary myoses in the M. Pronator teres as cause of lesion of the N. Medianus (the pronator syndrome). Acta Psychiatr Neurol Scand Suppl. (1951) 74:251–4.
18. Delzell, PB, and Patel, M. Ultrasound-guided perineural injection for pronator syndrome caused by median nerve entrapment. J Ultrasound Med. (2020) 39:1023–9. doi: 10.1002/jum.15166
19. El-Haj, M, Ding, W, Sharma, K, Novak, C, Mackinnon, SE, and Patterson, JMM. Median nerve compression in the forearm: a clinical diagnosis. Hand. (2021) 16:586–91. doi: 10.1177/1558944719874137
20. Neal, S, and Fields, KB. Peripheral nerve entrapment and injury in the upper extremity. Am Fam Physician. (2010) 81:147–55.
21. Iyer, VG. Understanding nerve conduction and electromyographic studies. Hand Clin. (1993) 9:273–87. doi: 10.1016/S0749-0712(21)00968-9
22. Nakano, KK, Lundergran, C, and Okihiro, MM. Anterior interosseous nerve syndromes. Diagnostic methods and alternative treatments. Arch Neurol. (1977) 34:477–80. doi: 10.1001/archneur.1977.00500200037007
23. Seror, P. Anterior interosseous nerve syndrome. Electromyographic characteristics (2 cases) and review of the literature (62 cases). Rev Electroencephalogr Neurophysiol Clin. (1986) 16:153–63. doi: 10.1016/S0370-4475(86)80007-7
24. Sneag, DB, Urban, C, Li, TY, Colucci, PG, Pedrick, EG, Nimura, CA, et al. Hourglass-like constrictions on MRI are common in electromyography-confirmed cases of neuralgic amyotrophy (parsonage-turner syndrome): a tertiary referral center experience. Muscle Nerve. (2024) 70:42–51. doi: 10.1002/mus.27961
25. Iyer, VG. Role of ultrasound in the EMG lab. Open access. J Neurol Neurosurg. (2021) 15:15. doi: 10.19080/OAJNN.2021.15.555910
26. Pham, M, Baumer, P, Meinck, HM, Schiefer, J, Weiler, M, Bendszus, M, et al. Anterior interosseous nerve syndrome: fascicular motor lesions of median nerve trunk. Neurology. (2014) 82:598–606. doi: 10.1212/WNL.0000000000000128
Keywords: neurology, proximal median nerve neuropathy, iatrogenic nerve injury, electrodiagnostic studies, ultrasound study of nerve
Citation: Shields LBE, Iyer VG, Zhang YP and Shields CB (2024) Proximal median nerve neuropathy: electrodiagnostic and ultrasound findings in 62 patients. Front. Neurol. 15:1468813. doi: 10.3389/fneur.2024.1468813
Edited by:
Jian-Quan Shi, Nanjing Medical University, ChinaReviewed by:
Giovanna Squintani, Integrated University Hospital Verona, ItalyNatalie Winter, Hertie Institute for Clinical Brain Research (HIH), Germany
Copyright © 2024 Shields, Iyer, Zhang and Shields. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher B. Shields, Y2JzaGllbGRzMUBnbWFpbC5jb20=
 Lisa B. E. Shields
Lisa B. E. Shields Vasudeva G. Iyer
Vasudeva G. Iyer Yi Ping Zhang
Yi Ping Zhang Christopher B. Shields
Christopher B. Shields