- 1Department of Neurology, NHO Osaka National Hospital, Osaka, Japan
- 2Department of Neurology, Osaka University Graduate School of Medicine, Osaka, Japan
- 3Department of Data Science, National Cerebral and Cardiovascular Center, Osaka, Japan
- 4Department of Cerebrovascular Medicine, National Cerebral and Cardiovascular Center, Osaka, Japan
- 5Department of Stroke and Cerebrovascular Medicine, Kyorin University School of Medicine, Tokyo, Japan
- 6Department of Neurology, The Jikei University School of Medicine, Tokyo, Japan
- 7Department of Neurology, Osaka General Medical Center, Osaka, Japan
- 8Department of Neurology, National Cerebral and Cardiovascular Center, Osaka, Japan
- 9Department of Cardiovascular Medicine, National Cerebral and Cardiovascular Center, Osaka, Japan
- 10Department of Neurosurgery, Seijinkai Shimizu Hospital, Kyoto, Japan
- 11Division of Stroke Prevention and Treatment, Institute of Medicine, University of Tsukuba, Ibaraki, Japan
Background: The addition of antiplatelet therapy to anticoagulant therapy in patients with stroke with non-valvular atrial fibrillation (NVAF) and atherothrombotic disease may increase bleeding risk without reducing recurrent stroke risk.
Aims: To evaluate the clinical benefits of anticoagulant monotherapy compared to combination therapy with anticoagulants and antiplatelet agents.
Methods and design: This is an investigator-initiated prospective multicenter, randomized, open-label, parallel-group clinical trial. Patients with NVAF and atherothrombotic disease who have had a recent ischemic stroke or transient ischemic attack will be eligible to participate in this trial.
Study outcomes: The primary outcome is a composite of ischemic cardiovascular events, including cardiovascular death, ischemic stroke, myocardial infarction, systemic embolism, ischemic events requiring urgent revascularization, and major bleeding events within 2 years after randomization.
Sample size estimates: This study will enroll 400 patients, 200 receiving anticoagulant monotherapy and 200 receiving combination therapy. This sample size will provide 90% power (one-sided p = 0.025) to detect a risk reduction in outcome events within 2 years, assuming event rates of 13 and 27% for each group, respectively, and a 10% loss to follow-up at a 2.5% significance level with one-sided log-rank tests at an interim analysis and a final analysis.
Discussion: This will be the first study to assess the net clinical benefit of oral anticoagulant monotherapy in ischemic stroke patients with NVAF and atherothrombosis.
Clinical trial registration: https://clinicaltrials.gov/study/NCT03062319, NCT03062319; https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000029222, UMIN000025392; https://jrct.niph.go.jp/latest-detail/jRCTs051180202, jRCTs051180202.
1 Introduction
Non-valvular atrial fibrillation (NVAF) and atherothrombotic disease frequently coexist, particularly in aging populations with shared risk factors such as hypertension and diabetes mellitus (1). Patients with both NVAF and atherothrombotic disease have an elevated risk of ischemic events, including stroke and myocardial infarction, as well as bleeding complications due to antithrombotic therapy. The current guidelines recommend oral anticoagulant therapy (OAC) for the prevention of stroke in patients with non-valvular atrial fibrillation (NVAF) (2, 3) and antiplatelet therapy for patients with non-cardiogenic stroke including large artery atherosclerosis and small vessel disease (4, 5). However, the optimal antithrombotic management for patients with both conditions remains uncertain due to limited evidence.
The combination of OAC and antiplatelet agents is often considered to reduce the risk of recurrent ischemic events (6, 7). However, recent studies have raised concerns regarding the safety and efficacy of this approach. The addition of antiplatelet agents to OAC has been associated with an increased risk of major bleeding (8). The AFIRE trial demonstrated that rivaroxaban monotherapy was non-inferior to combination therapy in terms of efficacy while significantly reducing bleeding risks in patients with NVAF and stable coronary artery disease (9). More recently, the EPIC-CAD trial also demonstrated that edoxaban monotherapy had higher clinical benefit than combination therapy in patients with NVAF and stable coronary artery disease (10). Meta-analyses provide further support for these findings, indicating that combination therapy may not provide a net clinical benefit over anticoagulant monotherapy (11). According to these results, the recent guidelines emphasize the importance of individualized antithrombotic therapy in patients with NVAF and atherothrombotic disease (12).
Furthermore, patients with a history of stroke or transient ischemic attack (TIA) who are receiving antiplatelet therapy are at an even higher risk of major bleeding when treated with OAC (8). Observational studies have demonstrated that the concomitant use of antiplatelet agents with OAC increases the composite outcome of cardiovascular and bleeding events without reducing recurrent stroke in patients with NVAF (13, 14). However, these findings are derived from non-randomized studies, and randomized controlled trials are required to establish the clinical benefit of OAC monotherapy in this context.
The optimal Antithrombotic Therapy in Ischemic Stroke patients with Non-Valvular Atrial Fibrillation and atherothrombosis (ATIS-NVAF) trial is designed to address this evidence gap. This investigator-initiated, prospective, multicenter, randomized control trial aims to evaluate the clinical benefits of OAC monotherapy compared to combination therapy with OAC and antiplatelet agents in patients who have experienced a recent ischemic stroke or TIA and have both NVAF and atherothrombotic disease. The results of this study could have significant implications for clinical practice, potentially informing guideline recommendations and optimizing antithrombotic therapy to improve patient outcomes.
2 Methods
2.1 Design
This is a protocol for an investigator-initiated, multicenter, prospective, randomized, open-label, parallel-group phase IV study.
2.2 Patient population
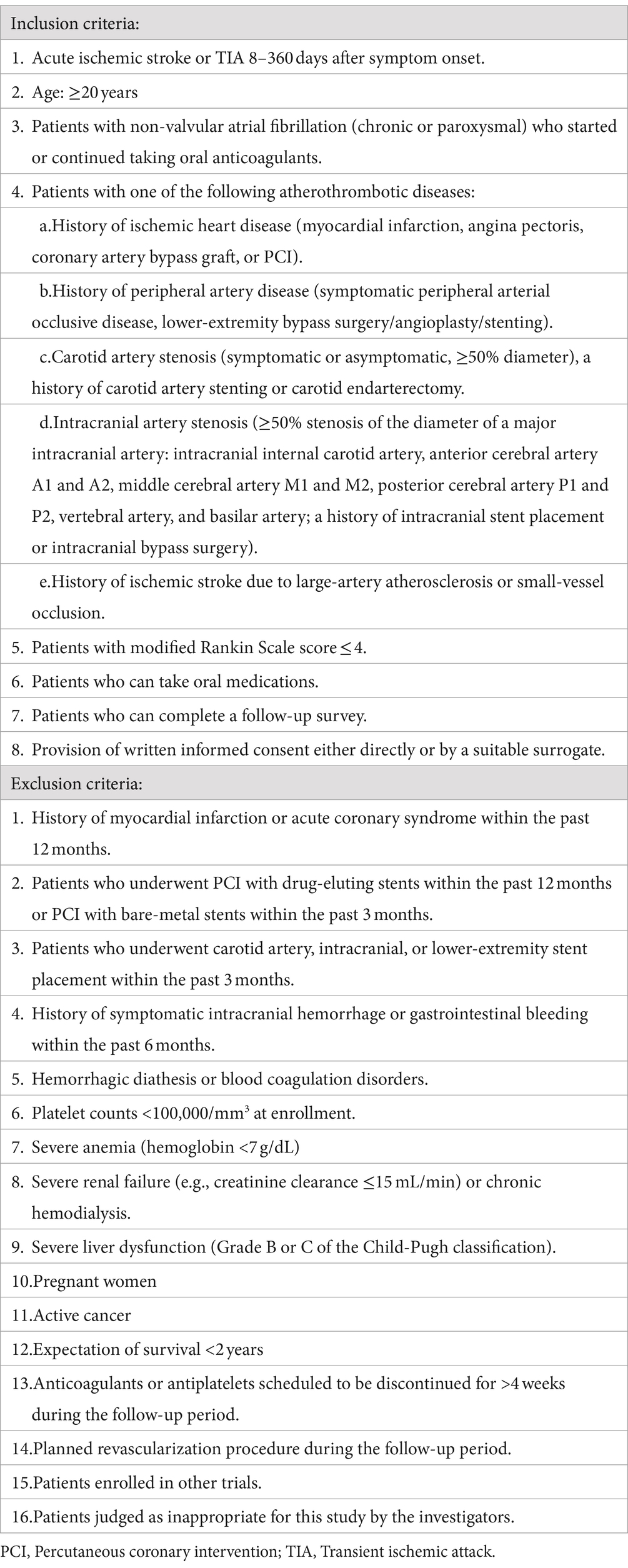
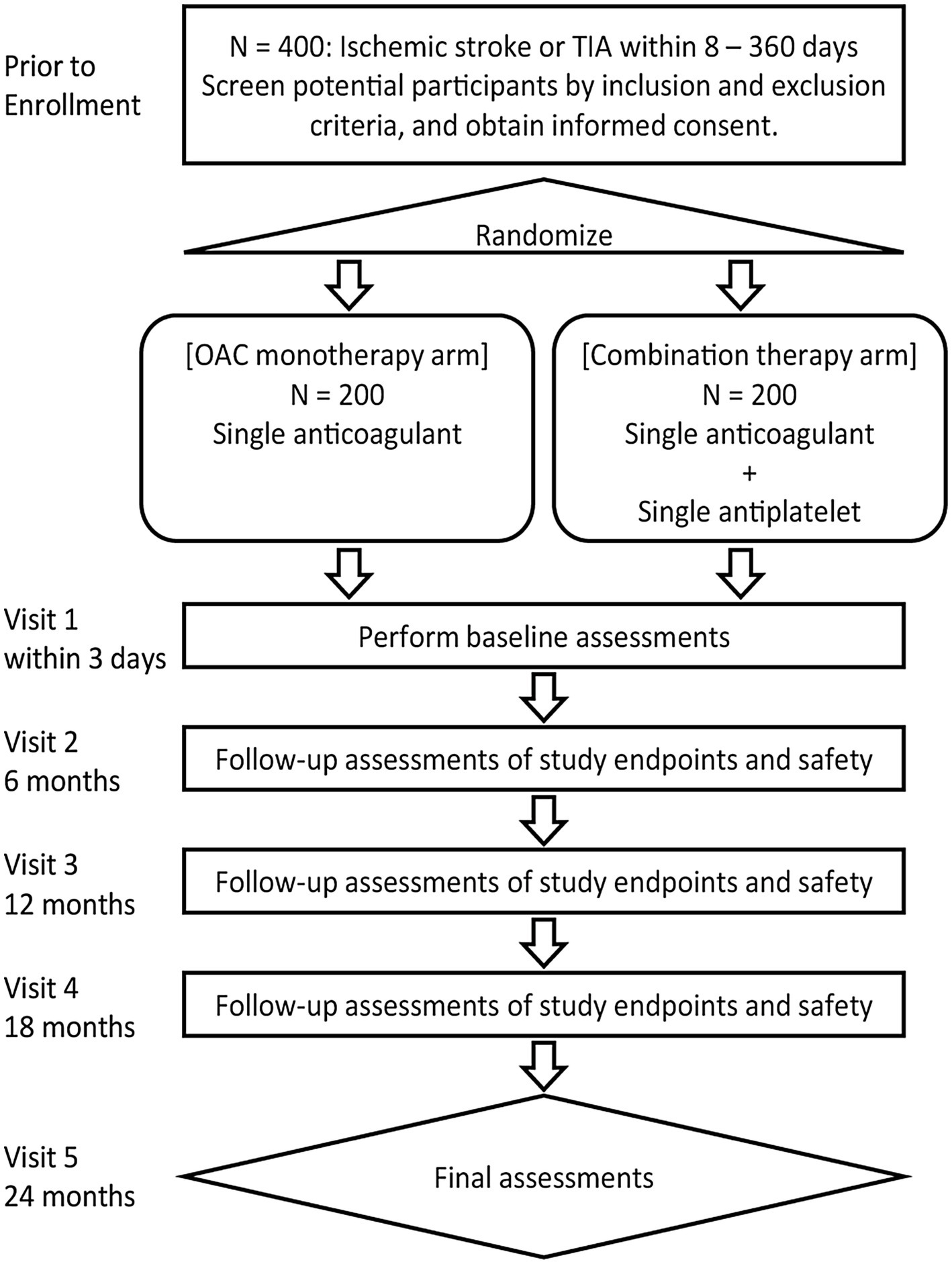
This trial will be conducted at 41 sites in Japan. The inclusion and exclusion criteria for study enrollment are listed in Table 1. Eligible patients will be identified by their physicians during routine consultations. Figure 1 shows the flowchart of the study design.
2.3 Randomization
All patients who consent to participate and fulfill the eligibility criteria will be randomized to either the OAC monotherapy group or the combination therapy group with a 1:1 allocation according to a computer-generated randomization schedule using the Pocock-Simon minimization method, with adjustment for sex, history of ischemic heart disease, and history of acute ischemic stroke.
The participants will be randomized using the cloud-based electronic data capture service DDworks 21 (Fujitsu Ltd., Tokyo, Japan), an online central randomization service. Allocation concealment will be ensured because the service will not release the randomization code until the patient has been recruited into the trial, which takes place after all baseline measurements have been completed. Clinical events will be assessed by a blinded Independent Central Review Committee. Additionally, all other individuals involved in the study will be blinded to the treatment allocation.
2.4 Intervention
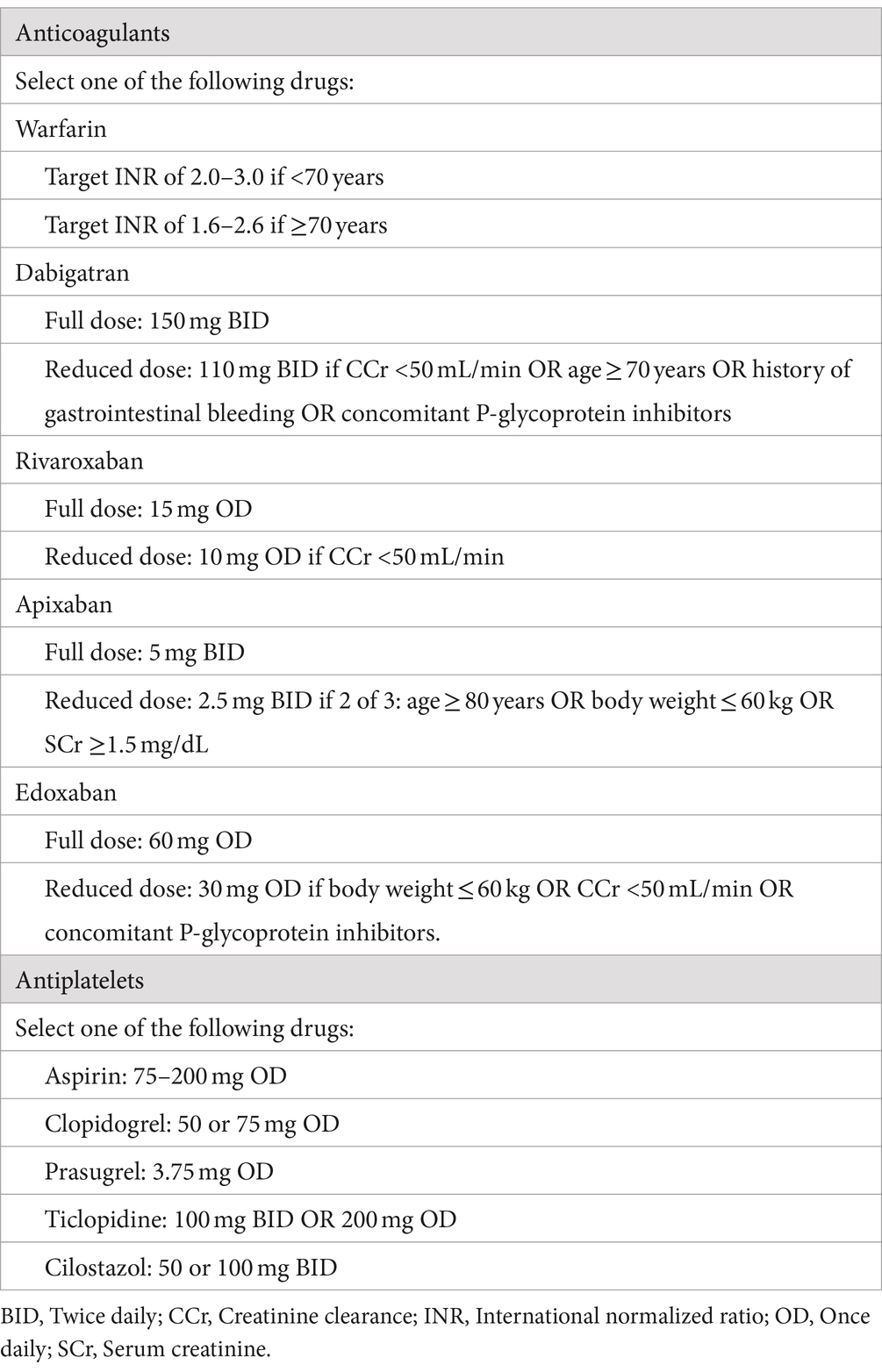
Patients will receive either OAC monotherapy or OAC plus antiplatelet therapy at the discretion of the treating physician, in accordance with the protocols outlined in Table 2. The dose of each drug is consistent with the approved dose in Japan. Details of the drugs to be used in both groups are shown in Table 2. Treatment will start within 3 days after randomization and continue for 2 years. If the treating physician needs to change the OAC or antiplatelet drug during the study, the choice of drug may be changed based on the predefined drugs for each group (Table 2). Temporary interruption of study treatment is allowed during invasive procedures such as surgery but cannot exceed 4 weeks. Patients in both treatment groups will be treated according to the relevant guidelines for the prevention of stroke or systemic embolism.
2.5 Study outcomes
Follow-up visits will be scheduled at 6 ± 2, 12 ± 2, 18 ± 2, and 24 ± 2 months. Each follow-up visit will be conducted by the treating physician. Blood pressure measurements and laboratory examinations will be performed. Patients will be asked about any outcomes or adverse events that occurred before the follow-up visit.
The primary outcome is a composite of ischemic cardiovascular events, including cardiovascular death, ischemic stroke, myocardial infarction, systemic embolism, ischemic events requiring urgent revascularization, and significant bleeding, as defined by the International Society of Thrombosis and Hemostasis (ISTH) criteria (15) within 2 years after randomization.
Secondary outcomes include incidences of the following events within 2 years after randomization: (1) all-cause mortality; (2) ischemic cardiovascular events (cardiovascular death, ischemic stroke, myocardial infarction, systemic embolism, and ischemic events requiring urgent revascularization); (3) all ischemic cardiovascular events (ischemic cardiovascular events, TIA, unstable angina pectoris, and progression of symptomatic peripheral artery disease); and (4) ischemic stroke; and (5) myocardial infarction and cardiovascular death.
Safety outcomes include incidences of the following events within 2 years after randomization: (1) ISTH major bleeding; (2) ISTH major bleeding and clinically relevant non-major bleeding; (3) intracranial hemorrhage.
2.6 Sample size estimates
The sample size was calculated based on the primary hypothesis and the results of our previous study. A retrospective study of 277 patients with NVAF and atherothrombotic disease demonstrated that the combination therapy group had a higher incidence of ischemic cardiovascular events and major bleeding than the OAC monotherapy group (33% vs. 19%/2 years, log-rank p = 0.04; unpublished data). Based on this result, we calculated that a maximum total sample size of 370 (equally-sized groups and the maximum number of events is 74) would be required to provide a power of 90% to detect a risk reduction in outcome events within 2 years, assuming lower event rates of 13 and 27% for each group, respectively, at an overall significance level of 2.5% with one-sided log-rank tests at an interim analysis and a final analysis (EAST® version 6.4, Cytel, Boston, MA, United States). An interim analysis was planned to assess the efficacy and futility when half the required number of events was observed. The critical values for the analyses were determined based on the Lan-DeMets error-spending method using an O’Brien-Fleming-type function with a non-binding boundary for futility. With a proposed 10% dropout rate, 400 participants will be recruited for this study.
2.7 Statistical analyses
Analyses will be performed based on the intention-to-treat (ITT) principle. The data will be summarized using descriptive statistics. For the primary endpoint, survival curves will be estimated using the Kaplan–Meier method and compared between the two groups using the log-rank test. Hazard ratios with 95% confidence intervals will be calculated using a proportional hazards model. A per-protocol analysis will be performed to support the conclusions derived from the ITT-based analysis. Additionally, homogeneity of the treatment effects will be exploratively evaluated with the following subgroups: age (<80 vs. ≥80), sex, anticoagulant drug (warfarin vs. direct OACs), CHADS2 score (≤3 vs. 4 vs. ≥ 5), body weight (<60 kg vs. ≥60 kg), creatinine clearance (<30 mL/min vs. 30 ~ 50 mL/min vs. ≥50 mL/min), history of ischemic stroke and major bleeding, and prevalence of carotid or intracranial artery stenosis, hypertension, diabetes mellitus, dyslipidemia, and congestive heart failure.
Secondary endpoints will be analyzed in the same manner as the primary endpoints, except for the calculation of p values. Safety data will be analyzed descriptively for the treated set, which consists of all randomized patients who received at least one study treatment. The detailed plan for the interim and final analyses will be prespecified in the statistical analysis plan and finalized before the database lock. All statistical analyses will be conducted at the data center in the National Cerebral and Cardiovascular Center.
2.8 Safety and data monitoring body
Data management, statistical analysis, and monitoring will be performed by the Department of Data Science at the National Cerebral and Cardiovascular Center in Suita, Japan. An independent Data Safety Monitoring Board (DSMB), comprising two stroke neurologists and a biostatistician, will be created to monitor the safety and efficacy of the study. The DSMB will recommend trial discontinuation/continuation or protocol amendment based on annual reviews of patient accrual, the incidence of adverse events, and interim efficacy analyses comparing the two treatment arms.
2.9 Study organization and funding
This study is partially supported by the Japan Thrombosis Investigator Initiated Research Program (JRISTA), funded by Bristol-Myers Squibb (BMS) and Pfizer. Bristol-Myers Squibb (BMS) and Pfizer were not involved in the planning, data management, analysis, or discussion of the study results. The final trial protocol was prepared by the Protocol Committee. An independent data-monitoring committee will adjudicate new outcomes at regular intervals. The committee receives outcome information and is blinded to patient identifiers and treatment allocation.
3 Discussion
The ATIS-NVAF is the first randomized trial to evaluate the safety and efficacy of OAC monotherapy in a population at risk for both cardiogenic embolism and atherothrombotic disease. The data obtained from this trial will be crucial for identifying optimal medical treatment for these patients.
The study design focuses on comparing OAC monotherapy with combination therapy of OAC and antiplatelet agents, without imposing any specific antiplatelet or OAC regimens, to reflect real-world situations. Since the safety profile of direct OACs in patients with end-stage renal failure has not been established (16), warfarin or warfarin and antiplatelet therapy are often used in clinical practice for patients with cardioembolic stroke, renal failure, and ischemic heart disease. This study allows the use of warfarin in the anticoagulation group to explore optimal antithrombotic treatment strategies for high-risk patients.
Recent evidence highlights that inflammation and endothelial dysfunction are key contributors to both atrial fibrillation and atherosclerosis (17, 18). In atrial fibrillation, endothelial injury promotes thrombogenesis (17). Similarly in atherosclerosis, endothelial dysfunction increases the risk of thrombotic events (19). These shared mechanisms emphasize the necessity for optimized antithrombotic therapy in patients with both conditions.
Although the ATIS-NVAF trial is designed to reflect real-world clinical practice by allowing flexibility in treatment selection, this approach introduces certain limitations. First, the open-label design of the study may introduce bias, as both patients and physicians are aware of the assigned treatments. This awareness may potentially influence patient adherence, reporting of outcomes, and clinical decision-making by physicians. Second, the selection of specific OACs and antiplatelet agents, as well as their dosage, is left to the discretion of the treating physicians within the predefined options. This variability may lead to heterogeneity in the interventions, which could affect the internal validity of the study. These limitations should be considered when interpreting the results of the ATIS-NVAF trial. Despite these challenges, the pragmatic design enhances the external validity and applicability of the findings to routine clinical practice, which is a significant strength of the study.
In conclusion, this study will be the first to assess the net clinical benefit of OAC monotherapy in patients with ischemic stroke with NVAF and atherothrombosis.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the National Cerebral and Cardiovascular Center, and the National Hospital Organization Review Board for Clinical Trials. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SO: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Data curation, Conceptualization. HaY: Writing – review & editing, Supervision, Formal analysis, Data curation. KA: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. KO: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. HM: Writing – review & editing, Formal analysis, Data curation. KTa: Writing – review & editing, Writing – original draft, Project administration, Investigation, Data curation, Conceptualization. SY: Writing – review & editing, Methodology, Data curation. TH: Writing – review & editing, Methodology. YI: Writing – review & editing, Methodology. MS: Writing – review & editing, Methodology. MK: Writing – review & editing, Methodology. MI: Writing – review & editing, Methodology. KTo: Writing – review & editing, Methodology. TN: Writing – review & editing, Methodology. NS: Writing – review & editing, Methodology. HiY: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study is partially supported by the Japan Thrombosis Investigator Initiated Research Program (JRISTA), funded by Bristol-Myers Squibb (BMS) and Pfizer (REQ-0000001583). The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
Hiroshi Yamagami received research grants from Bristol-Myers Squibb and lecturer fees from Daiichi Sankyo, Bristol-Myers Squibb, Otsuka Pharmaceuticals, Stryker, Medtronic, Boston Scientific Japan, and Abbott Medical Japan.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Odutayo, A, Wong, CX, Hsiao, AJ, Hopewell, S, Altman, DG, and Emdin, CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. (2016) 354:i4482. doi: 10.1136/bmj.i4482
2. Ntaios, G, Papavasileiou, V, Diener, HC, Makaritsis, K, and Michel, P. Nonvitamin-K-antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and previous stroke or transient ischemic attack: an updated systematic review and meta-analysis of randomized controlled trials. Int J Stroke. (2017) 12:589–96. doi: 10.1177/1747493017700663
3. Saxena, R, and Koudstaal, P. Anticoagulants versus antiplatelet therapy for preventing stroke in patients with nonrheumatic atrial fibrillation and a history of stroke or transient ischemic attack. Cochrane Database Syst Rev. (2004) 18:CD000187. doi: 10.1002/14651858.CD000187.pub2
4. Mohr, JPP, Thompson, JLPL, Lazar, RMM, Levin, B, Sacco, RLL, Furie, KLL, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. (2001) 345:1444–51. doi: 10.1056/NEJMoa011258
5. Greving, JP, Diener, H-C, Reitsma, JB, Bath, PM, Csiba, L, Hacke, W, et al. Antiplatelet therapy after noncardioembolic stroke. Stroke. (2019) 50:1812–8. doi: 10.1161/STROKEAHA.118.024497
6. Eikelboom, JW, and Hirsh, J. Combined antiplatelet and anticoagulant therapy: clinical benefits and risks. J Thromb Haemost. (2007) 5:255–63. doi: 10.1111/j.1538-7836.2007.02499.x
7. Paikin, JS, Wright, DS, and Eikelboom, JW. Effectiveness and safety of combined antiplatelet and anticoagulant therapy: a critical review of the evidence from randomized controlled trials. Blood Rev. (2011) 25:123–9. doi: 10.1016/j.blre.2011.01.007
8. Hylek, EM, Held, C, Alexander, JH, Lopes, RD, De Caterina, R, Wojdyla, DM, et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: the ARISTOTLE trial (Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation): predictors, characteristics, and clinical outcomes. J Am Coll Cardiol. (2014) 63:2141–7. doi: 10.1016/j.jacc.2014.02.549
9. Yasuda, S, Kaikita, K, Akao, M, Ako, J, Matoba, T, Nakamura, M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. (2019) 381:1103–13. doi: 10.1056/NEJMoa1904143
10. Cho, MS, Kang, D-Y, Ahn, J-M, Yun, S-C, Oh, Y-S, Lee, CH, et al. Edoxaban antithrombotic therapy for atrial fibrillation and stable coronary artery disease. N Engl J Med. (2024):1–13. doi: 10.1056/NEJMoa2407362
11. Ullah, W, Sattar, Y, Shaukat, M, and Fischman, DL. Safety and efficacy of anticoagulant monotherapy in atrial fibrillation and stable coronary artery disease: a systematic review and meta-analysis. Eur J Intern Med. (2020) 81:54–9. doi: 10.1016/j.ejim.2020.06.035
12. Byrne, RA, Rossello, X, Coughlan, JJ, Barbato, E, Berry, C, Chieffo, A, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. (2023) 44:3720–826. doi: 10.1093/eurheartj/ehad191
13. RAF and RENO-EXTEND Investigators. Risk of recurrent stroke in patients with atrial fibrillation treated with oral anticoagulants alone or in combination with anti-platelet therapy. Eur Stroke J. (2023) 8:722–30. doi: 10.1177/23969873231183211
14. Kim, TJ, Lee, JS, Yoon, JS, Oh, MS, Kim, JW, Park, SH, et al. Optimal use of antithrombotic agents in ischemic stroke with atrial fibrillation and large artery atherosclerosis. Int J Stroke. (2023) 18:812–20. doi: 10.1177/17474930231158211
15. Schulman, S, and Kearon, C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. (2005) 3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x
16. Kimachi, M, Furukawa, TA, Kimachi, K, Goto, Y, Fukuma, S, and Fukuhara, S. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. (2017) 2017:CD011373. doi: 10.1002/14651858.CD011373.pub2
17. Guazzi, M, and Arena, R. Endothelial dysfunction and pathophysiological correlates in atrial fibrillation. Heart. (2009) 95:102–6. doi: 10.1136/hrt.2007.135277
18. Issac, TT, Dokainish, H, and Lakkis, NM. Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data. J Am Coll Cardiol. (2007) 50:2021–8. doi: 10.1016/j.jacc.2007.06.054
Keywords: antiplatelet, anticoagulant, nonvalvular atrial fibrillation (NVAF), atherothrombosis, ischemic stroke, randomized controlled trial
Citation: Okazaki S, Yamamoto H, Asakura K, Omae K, Maeda H, Tanaka K, Yamamoto S, Hirano T, Iguchi Y, Sakaguchi M, Koga M, Ihara M, Toyoda K, Noguchi T, Sakai N and Yamagami H (2024) Optimal antithrombotic therapy in ischemic stroke patients with non-valvular atrial fibrillation and atherothrombosis: study protocol for a randomized controlled trial. Front. Neurol. 15:1468523. doi: 10.3389/fneur.2024.1468523
Edited by:
Giovanni Merlino, Udine University Hospital, ItalyReviewed by:
Masahiro Uemura, Niigata University, JapanAkash Batta, Dayanand Medical College & Hospital, India
Copyright © 2024 Okazaki, Yamamoto, Asakura, Omae, Maeda, Tanaka, Yamamoto, Hirano, Iguchi, Sakaguchi, Koga, Ihara, Toyoda, Noguchi, Sakai and Yamagami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hiroshi Yamagami, eWFtYWdhbWkuYnJhaW5Ab3V0bG9vay5jb20=
 Shuhei Okazaki
Shuhei Okazaki Haruko Yamamoto
Haruko Yamamoto Koko Asakura
Koko Asakura Katsuhiro Omae
Katsuhiro Omae Hirotada Maeda
Hirotada Maeda Kanta Tanaka
Kanta Tanaka Shiro Yamamoto
Shiro Yamamoto Teruyuki Hirano
Teruyuki Hirano Yasuyuki Iguchi
Yasuyuki Iguchi Manabu Sakaguchi
Manabu Sakaguchi Masatoshi Koga
Masatoshi Koga Masafumi Ihara
Masafumi Ihara Kazunori Toyoda
Kazunori Toyoda Teruo Noguchi9
Teruo Noguchi9 Nobuyuki Sakai
Nobuyuki Sakai Hiroshi Yamagami
Hiroshi Yamagami