- 1Department of Neurology and Institute of Neurology of First Affiliated Hospital, Institute of Neuroscience, Fujian Key Laboratory of Molecular Neurology, Fujian Medical University, Fuzhou, China
- 2Department of Neurology, National Regional Medical Center, Binhai Campus of the First Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 3Department of Radiology, First Affiliated Hospital of Fujian Medical University, Fuzhou, China
Introduction: Hemorrhagic transformation (HT) is a severe complication in patients with acute ischemic stroke due to large vessel occlusion (AIS-LVO) after endovascular treatment (EVT). We hypothesize that asymmetry of the internal cerebral veins (ICVs) on baseline CT angiogram (CTA) may serve as an adjunctive predictor of HT.
Methods: We conducted a study on consecutive AIS-LVO patients from November 2020 to April 2022. These patients had anterior circulation occlusions and were treated with EVT. Asymmetrical ICVs were assessed using CTA and defined as hypodensity (reduced opacification) on the ipsilateral side of occlusion compared to the contralateral side. The primary outcome was HT, defined as hemorrhage within the ischemic territory. This was evaluated using follow-up imaging (CT scan or magnetic resonance imaging) performed 48 h post-EVT. HT was classified into four subtypes based on the European Cooperative Acute Stroke Study-II criteria.
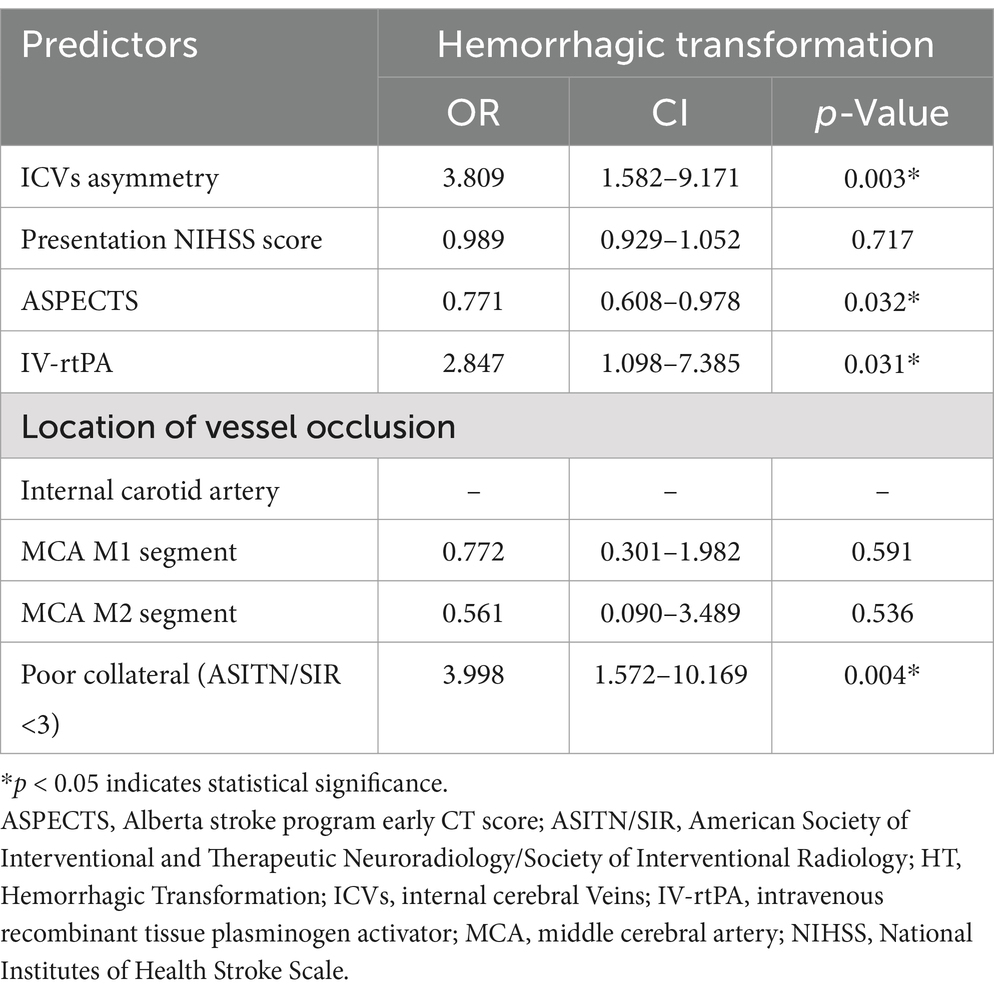
Results: A total of 126 patients were included, with an HT rate of 49.2% (62/126). ICV asymmetry was observed in 54.0% (68/126) of patients. The ICV asymmetry group exhibited a significantly higher risk of parenchymatous hematoma-type HT (33.8% vs. 15.5%, p = 0.019) and symptomatic intracerebral hemorrhage (sICH) (23.5% vs. 5.2%, p = 0.004). In multivariate logistic regression, ICV asymmetry (OR 3.809, 95% CI 1.582–9.171), baseline Alberta Stroke Program Early CT Score (OR 0.771, 95% CI 0.608–0.978), intravenous recombinant tissue plasminogen activator (OR 2.847, 95% CI 1.098–2.7.385), and poor collateral circulation (OR 3.998, 95% CI 1.572–10.169) were identified as independent risk factors of HT.
Conclusion: ICV asymmetry, likely resulting from impaired autoregulation or tissue micro-perfusion hampering cerebral blood flow (CBF), is a novel radiological sign that independently predicts HT. It is associated with a higher risk of sICH in AIS-LVO patients after EVT. Further research is warranted to validate these findings.
1 Introduction
Endovascular thrombectomy (EVT) has become the standard of care for patients with acute ischemic stroke caused by large vessel occlusion (AIS-LVO) in recent years (1). The time window for EVT was extended to 24 h based on findings from the DAWN and DIFFUSE-3 trials, which utilized diffusion-weighted imaging to better understand stroke evolution. As a result, more AIS-LVO patients could be treated. However, severe complications, particularly hemorrhagic transformation (HT), pose significant challenges to its widespread application. Intracranial HT is a common and serious complication often associated with poor outcomes (2). Reported incidence rates in clinical trials are as high as 49.5% (3). Given its clinical impact, identifying risk factors associated with HT is critically important to help clinicians make informed treatment decisions and improve patient prognosis.
Arterial collaterals only reflect the arterial portion of the microcirculation and are not a reliable indicator of cerebral perfusion (4). Conversely, venous drainage is generally a better reflection of cerebral microcirculation and perfusion. Unobstructed venous drainage indicates adequate cerebral blood flow through brain tissue (5).
The internal cerebral veins (ICVs), located near the interventricular foramen, are responsible for draining venous drainage from the deep structures of the cerebral hemisphere. These veins converge to form the great vein of Galen, which subsequently drains into the straight sinus (6). In cases of anterior large vessel occlusion, patients may experience reduced blood flow in deep brain tissues, potentially leading to hypo-opacification of the ipsilateral ICV, a phenomenon referred to as ICV asymmetry.
Recent studies have shown that ICV asymmetry is associated with poor functional outcomes (7, 8). Favorable venous outflow profiles, on the other hand, are associated with reduced early edema progression and improved functional outcomes in AIS-LVO patients undergoing EVT (9). However, limited research has specifically explored the relationship between ICV asymmetry and HT after EVT.
We hypothesized that ICV asymmetry is associated with HT in AIS-LVO patients who underwent EVT. We verified this hypothesis by determining ICV symmetry using baseline CTA images and evaluated HT through 48-h follow-up head non-contrast CT (NCCT) or magnetic resonance imaging (MRI) after EVT.
2 Materials and methods
2.1 Study population
In an ongoing study (ClinicalTrials.gov Identifier NCT 04637074), we prospectively collected demographic characteristics and clinical and imaging data of patients with AIS-LVO who underwent EVT between November 2020 and April 2022. Following this data collection, a retrospective analysis of all patients was performed to analyze the relationship between ICV asymmetry and HT. The data collection process was approved by the ethics committee, and written informed consent was obtained from all patients.
The inclusion criteria were as follows: (1) age ≥ 18 years old; (2) EVT < 24 h after stroke onset; and (3) large vessel occlusion of the anterior circulation (internal carotid artery or first (M1) or second (M2) segment of the middle cerebral artery) determined by CT angiography (CTA) examination. The exclusion criteria were as follows: (1) subarachnoid hemorrhage or intracranial hemorrhage determined by a baseline NCCT scan; (2) poor CTA image quality resulting from motion artifacts and inability to assess ICVs asymmetry; (3) absence of follow-up head NCCT/MRI after EVT; and (4) history of iodine allergy.
2.2 Imaging acquisition and treatment
All patients underwent head NCCT or CTA performed on a 64-section CT scanner (Toshiba Medical Systems, Nasu, Japan). NCCT was performed (120 kV; 300 mA; section thickness, 5 mm) from the foramen magnum to the vertex. In our study, helical CTA was performed using a bolus tracking method to determine the optimal timing for image acquisition. After administering 40 mL of an iodinated contrast medium (iopamidol 370, Braccosine, Shanghai, China) at a flow rate of 5.0 mL/s, followed by a 35-mL saline flush using a two-channel high-pressure injector, helical CTA was conducted from the foramen magnum to the vertex with a section thickness of 0.625 mm.
All patients underwent follow-up MRI using a 1.5-T scanner (Signa Excite; GE Medical Systems, Milwaukee, WI, United States) with an 8-channel head coil. The MR protocol included a routine head scan including T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and T2-weighted fluid-attenuated inversion recovery (FLAIR).
All patients who presented to our hospital underwent an urgent NCCT of the brain to exclude intracranial hemorrhage and were treated with intravenous recombinant tissue plasminogen activator (IV-rtPA) if they met the criteria. Consequently, EVT was usually performed if a large vessel occlusion was confirmed by CTA.
2.3 Imaging analysis
Imaging data were independently reviewed by two neuroradiologists, each with over 5 years of experience in stroke imaging, who were blinded to all patients’ clinical and outcome data. Any disagreements between the two observers were resolved by a senior medical radiologist. All patients underwent non-contrast head CT (NCCT) and CT angiography (CTA), followed by NCCT or MRI 48 h post-EVT.
The Alberta Stroke Program Early CT Score (ASPECTS) (10) was used to assess the early ischemic burden on baseline NCCT imaging. Baseline CTA source images were used to assess ICV opacification. We assessed the degree of ICV opacification using maximum intensity projection (MIP) sequences on axial sections. ICV asymmetry was defined as lesser opacification (hypodensity) of the ICV on the ipsilateral ischemic hemisphere compared to the contralateral ICV. Conversely, if the degree of opacification was equal or more than the contralateral ICV, the ICVs are considered symmetric.
Collateral circulation was graded on pre-recanalization angiograms using the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) guidelines, with grades 3–4 classified as good collaterals. Successful recanalization of the occluded artery after EVT was defined as a modified Thrombolysis in Cerebral Infarction (mTICI) grade 2b/3 on final angiography. Any disagreement was resolved through consensus coordination.
2.4 Clinical data collection
We prospectively collected all the variables, such as demographic characteristics, vascular risk factors (hypertension, hyperlipidemia, diabetes mellitus, smoking, and atrial fibrillation), previous medication history (antiplatelets and anticoagulants), blood pressure, baseline stroke scale (e.g., National Institutes of Health Stroke Scale [NIHSS]), imaging data, procedural details, time-metric data, and prognosis outcome (e.g., modified Rankin Scale [mRS] at 90 days). The probable stroke etiology was categorized according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria (11).
2.5 Outcomes
HT refers to hemorrhagic transformation within the ischemic region, evaluated on follow-up imaging after EVT and classified into four subtypes based on the European Cooperative Acute Stroke Study-II (ECASS II) (12). In cases of inconsistency between the two ratings, the severity rating takes precedence. Symptomatic intracranial hemorrhage (sICH) is defined as neurological deterioration (resulting in an increase of ≥4 points on the NIHSS within 48 h) accompanied by evidence of intracranial hemorrhage on brain CT or MRI performed 48 h after treatment. The modified Rankin scale (mRS) score after 3 months was used to evaluate the patient’s clinical outcomes, and patients with an mRS score of 3 to 6 were classified as having a poor prognosis.
2.6 Statistical analysis
Normally distributed continuous variables were expressed as mean ± standard deviation (SD), and non-normally distributed variables were described as median (interquartile range, IQR). Categorical variables were expressed as frequency (percentage). The chi-squared test was used to compare categorical variables between patients with HT and those with non-HT. The Mann–Whitney U test was employed to analyze non-normally distributed variables between the two groups. A Student unpaired t-test was performed for normally distributed variables between the two groups. Multivariable binary logistic regression was performed with factors associated with HT with p < 0.1. Collinearity among predictors was evaluated using the Spearman correlation coefficient and the variance inflation factor (VIF). Interrater reliability of ICV status was tested by κ statistics. Statistical significance was set as a probability value of <0.05, and all reported results were two-sided. Statistical analyses were conducted using SPSS Version 25.0 (SPSS Inc., Chicago, IL, United States).
3 Results
3.1 Baseline characteristics of patients
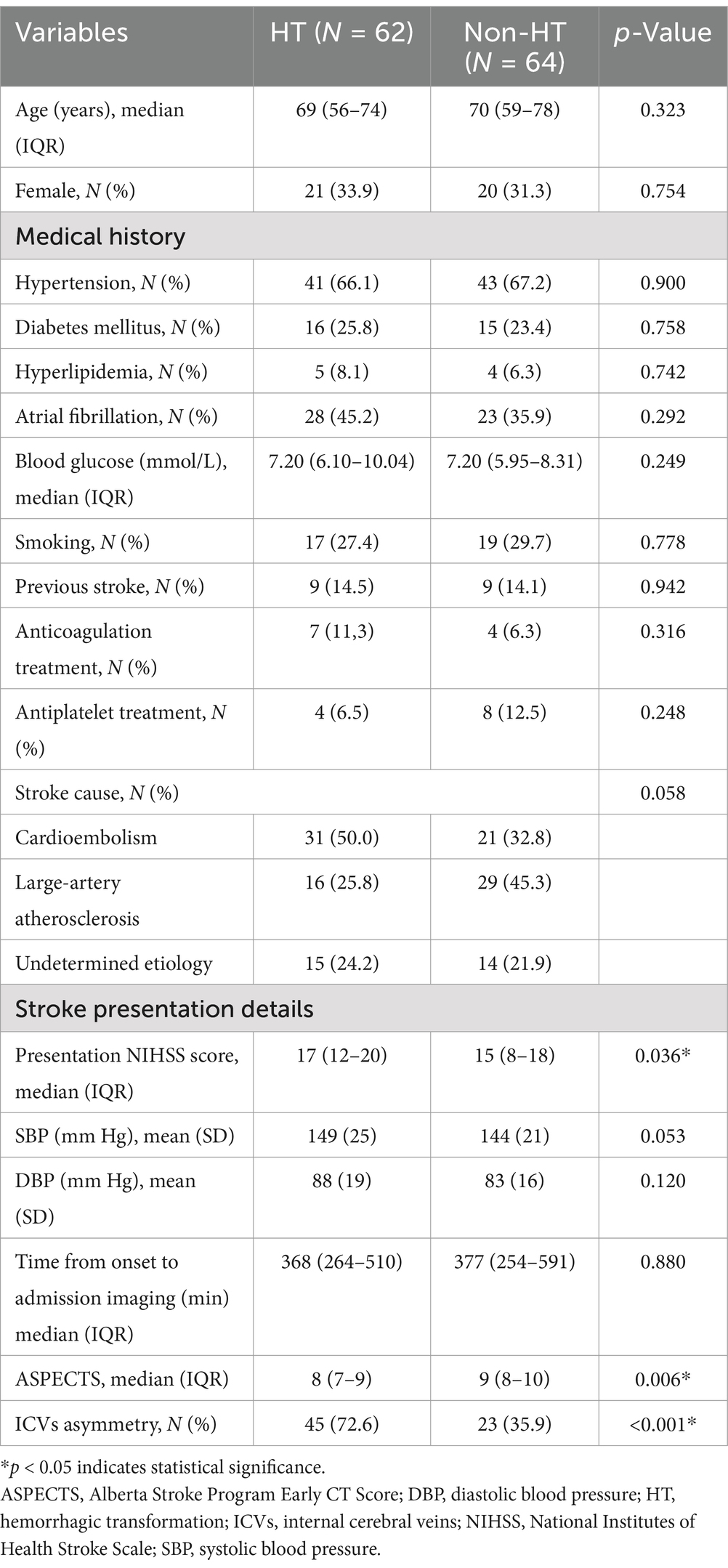
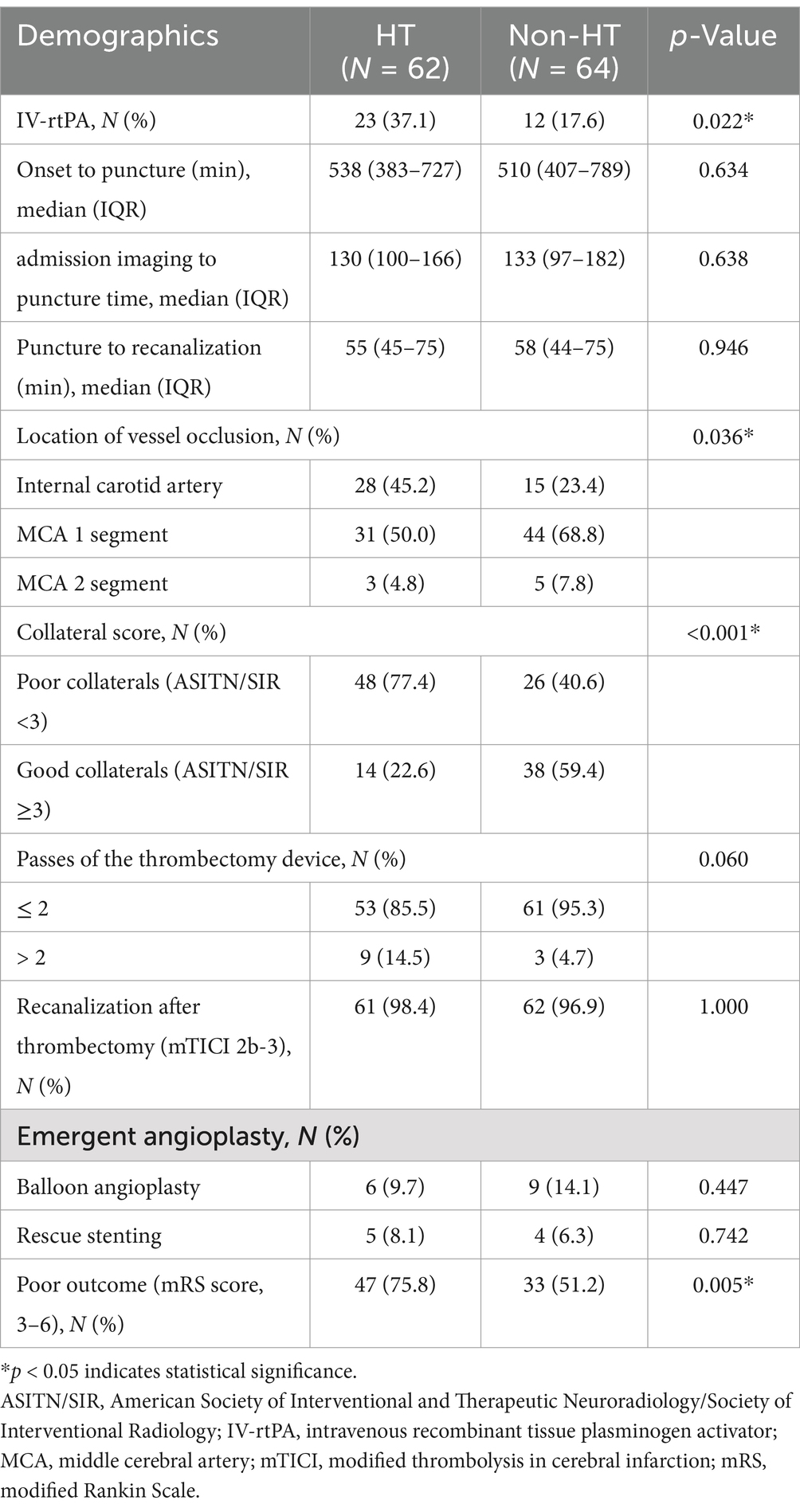
A total number of 126 patients met the inclusion criteria (Figure 1). Overall, the HT rate was 49.2% (62/126). Of the 126 patients, 84 (66.7%) had hypertension, 31 (24.6%) had diabetes mellitus, 51 (40.5%) had atrial fibrillation, and only 9 (7.1%) had hyperlipidemia. Based on the TOAST classification, the etiology of stroke was large-artery atherosclerosis in 45 (35.7%) patients and cardiogenic embolism in 51 (40.5%) patients. A total of 29 (23.0%) patients had an undetermined reason for stroke. All baseline demographic and clinical characteristics are presented in Table 1. The median time from symptom onset to puncture was 511.50 min (range 403.5–758.00 min), while the median time from puncture to recanalization was 56.0 min (range 44.75–74.5 min). More details are shown in Table 2.
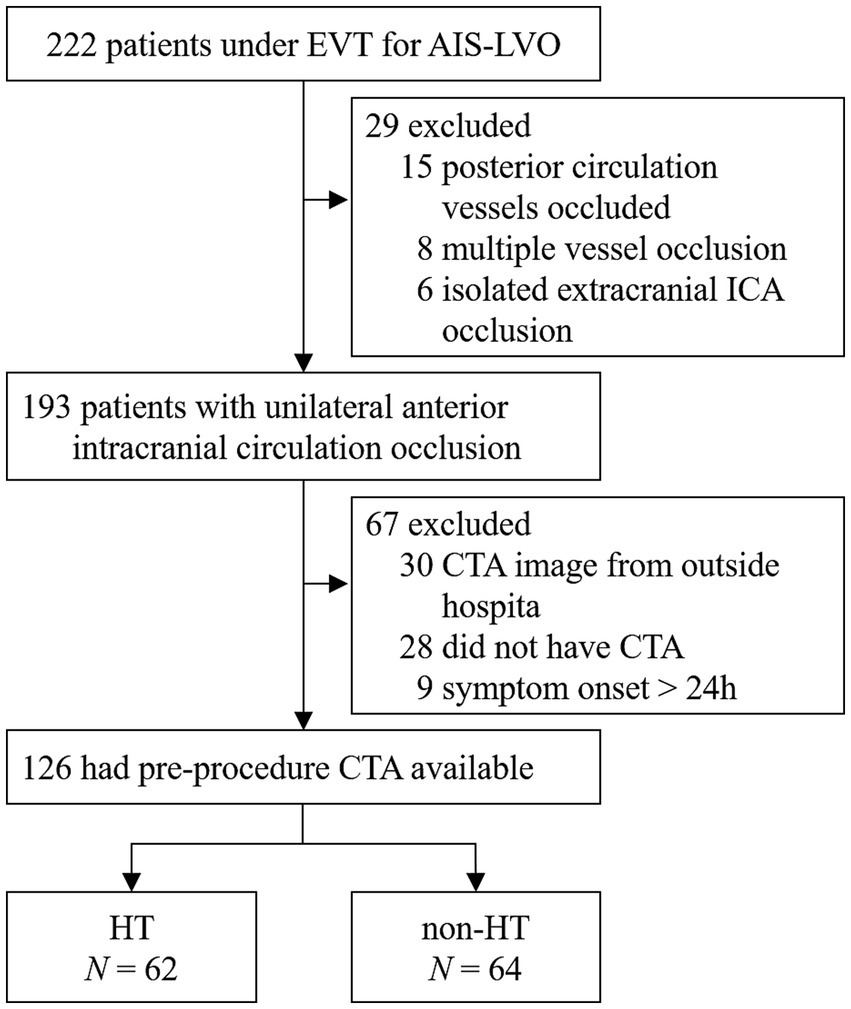
Figure 1. A flow chart of patient selection. EVT, endovascular treatment; AIS-LVO, acute ischemic stroke due to large vessel occlusion; CTA, computed tomography angiography; MCA, middle cerebral artery; ICA, internal carotid artery; HT, hemorrhagic transformation.
3.2 Association between ICV asymmetry and the risk of HT
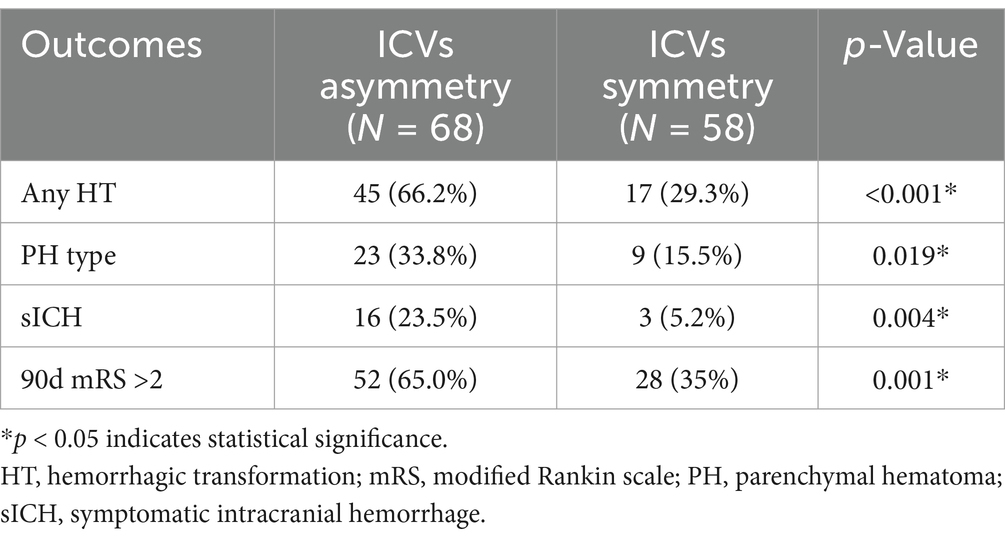
In the univariate analysis, the factors associated with HT compared with the non-HT group were ICV asymmetry (45% versus 23%, respectively, p < 0.001), baseline NIHSS score (median, 17 vs. 15, respectively, p = 0,036), baseline ASPECTS (mean, 8 versus 9, respectively; p = 0.006), IV-rtPA (23% vs. 12%, respectively; p = 0.022), and poor collateral circulation (48% vs. 23%, respectively, p < 0.001). These findings are summarized in Tables 1, 2. Compared with the ICV symmetry group, the ICV asymmetry group exhibited a higher risk of any HT (66.2% vs. 29.3%, p < 0.001), parenchymatous hematoma (PH) type HT (33.8% vs. 15.5%, p = 0.019), and sICH (23.5% vs. 5.2%, p = 0.004) (Table 3).
The multivariate logistic regression model adjusted for the following factors: ICV asymmetry, presentation NIHSS score, ASPECTS, IV-rtPA, location of vessel occlusion, and poor collateral (ASITN/SIR <3). The analysis revealed that ICV asymmetry was associated with a higher risk of HT (OR 3.809, 95% CI 1.582–9.171), as illustrated in Figure 2. Baseline ASPECTS (OR 0.771, 95% CI 0.608–0.978) was also linked with a lower risk of HT. IV-rtPA (OR 2.847, 95% CI 1.098–2.7.385) and poor collateral circulation (OR 3.998, 95% CI 1.572–10.169) are also independent risk factors of HT. Detailed results are organized in Table 4 and Figure 3. The Spearman correlation coefficient between ICV asymmetry and collateral circulation was −0.224 (p = 0.012). Collinearity diagnostics showed a VIF of 1.052 and a tolerance of 0.950, indicating no significant collinearity. Interrater reliability for ICV asymmetry on baseline CTA source images was substantial (κ = 0.86, 95% CI 0.74 to 0.97). For HT determination, the interrater reliability was excellent (κ = 0.92, 95% CI 0.85 to 0.99), while for HT classification, it was also high (κ = 0.90, 95% CI 0.84 to 0.97). These results indicate strong and consistent agreement across different assessments.
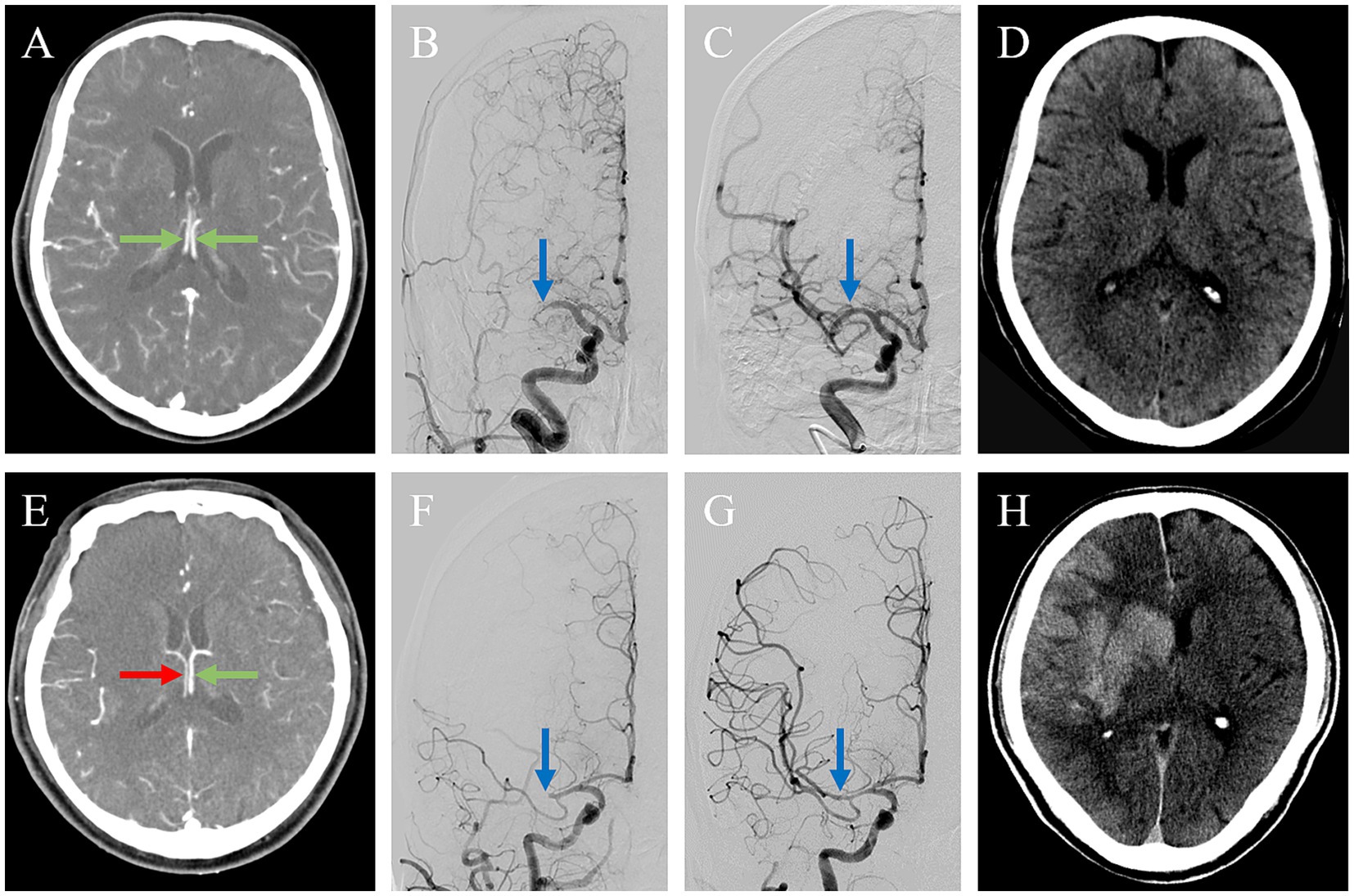
Figure 2. Association between ICV asymmetry and HT. Two patients with acute ischemic stroke due to occlusion of the M1 segment of the right middle cerebral artery (MCA), (A–D) 1 with internal cerebral veins (ICVs) symmetry and (E-H) 1 with ICVs asymmetry. In the first patient, axial maximum intensity projection imaging shows the ICVs running parallel to each other and symmetrical opacification (green arrows) (A). After endovascular thrombectomy, the initial proximal MCA occlusion (blue arrow) (B) was successfully reperfused with a modified Thrombolysis in Cerebral Infarction (mTICI) of 3(blue arrow) (C). The non-contrast CT on 24 h showed a small acute infarct without hemorrhagic transformation. The second patient exhibited a poor ICV profile in the right hemisphere [(E), red arrow] and good opacification in the left (green arrow). The thrombus on the right proximal MCA (blue arrow) (F) was successfully reperfused with an mTICI of 3 (blue arrow) (G). The non-contrast CT on 24 h showed hemorrhagic transformation with a cerebral hernia (H).

Figure 3. Forest plot visualizing the results of the multivariate regression analysis. ICVs, internal cerebral veins; NIHSS, National Institutes of Health stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; IV-rtPA, intravenous recombinant tissue plasminogen activator; MCA, middle cerebral artery.
4 Discussion
HT is a main side effect of EVT, making the identification of predictors crucial for improving patient outcomes. According to the results of our study, ICV asymmetry on baseline CTA can independently predict HT in patients with anterior circulation AIS-LVO after EVT. Moreover, ICV asymmetry is associated with sICH, which is a severe and life-altering complication.
HT after recanalization therapy in acute ischemic stroke is a severe complication associated with high rates of disability and mortality, especially due to parenchymal hematoma (PH) or sICH (13). Clinical features accompanied by an increased risk of HT in AIS-LVO patients include treatment with IV-rtPA, baseline NIHSS scores, glucose levels, atrial fibrillation, and poor collateral circulation (14). Our study is a noteworthy finding that adds to previous studies, which showed that ICV asymmetry is an independent predictor of poor functional outcomes after intravenous thrombolysis (7) and EVT (8). Interestingly, asymmetrical prominent cortical vein signs can also predict early neurological deterioration in acute ischemic stroke patients (15). This highlights the significance of retrograde venous flow in predicting clinical outcomes in AIS.
The ICVs drain the deep cerebral white matter, including regions such as the thalamus, basal ganglia, hippocampus, and medial temporal lobe, through the thalamostriate and anterior septal veins. These veins receive their arterial supply from the lateral lenticulostriate artery, medial lenticulostriate artery, and anterior choroidal artery. This extensive drainage network makes the ICVs an effective surrogate imaging biomarker for assessing cerebral perfusion in brain tissue. Although quantitative metrics, such as HU values, could enhance the precision of ICV asymmetry assessment, this study utilized qualitative visual assessments due to the limitations of imaging protocols. This method aligns with real-world clinical practice and demonstrates substantial interrater reliability. Hypo-opacification in the ipsilateral ICV reflects hypoperfusion in the cerebral hemisphere and is associated with an increased risk of HT. This finding aligns with prior studies showing that hypoperfusion detected by MRI predicts HT in acute ischemic stroke (16).
Several other studies (17, 18) used the cortical vein opacification score (COVES) to predict prognosis in AIS-LVO patients who underwent EVT. The COVES quantifies venous opacification on single-phase CTA of the vein of Labbé, sphenoparietal sinus, and superficial middle cerebral was used to determine venous outflow (VO) profiles. However, those superficial venous systems are more prone to variation than the deep venous system and are more difficult to identify. Conversely, the ICVs are located close to each other for effortless parallel comparison. Our study’s high degree of consistency for ICV asymmetry (κ = 0.86) shows that this is an easy assessment method. Therefore, using this robust technique, our study showed that ICV asymmetry independently predicts HT in patients with AIS-LVO. However, the reasons for the association between ICV asymmetry and HT were not totally demonstrated. We hypothesized that the poor ICV flow is a hallmark of hypoperfusion in the microcirculation, leading to the transformation of the ischemic penumbra to a huge cerebral infarction. When vessel occlusion occurs, the integrity of the blood–brain barrier (BBB) will be disrupted with the extension of ischemic time, leading to the extravasation of red blood cells from the blood vessels, resulting in HT. However, EVT was performed within the recommended time window.
According to the results of our study, other predictors for HT were poor collateral circulation, baseline ASPECTS, and IV-tPA. Previous studies have stated that good collateral circulation could diminish the severity of neurological symptoms and improve clinical outcomes (19, 20). Our study found that patients with poor collateral circulation had a higher risk of HT after receiving EVT. Interestingly, we observed that ICV asymmetry was often associated with poor collateral circulation on DSA (digital subtraction angiography), although these data were not explicitly shown. However, it is remarkable that a significant number of AIS-LVO patients developed ICV asymmetry despite good collateral circulation (18), possibly owing to damaged autoregulation or tissue micro-perfusion that hampers cerebral blood flow (CBF) on a tissue level (21).
Additionally, individual anatomical variations in venous drainage pathways may also contribute to ICV asymmetry. For instance, asymmetry in the size, structure, or dominance of internal cerebral veins (ICVs) could result in differential venous drainage patterns even in the presence of adequate collateral circulation. Variations in venous collateral pathways or compensatory shifts in drainage dynamics due to ischemia might further exacerbate this asymmetry. This may explain the missing connection between ICV asymmetry, arterial collateral status, and HT in this study. In addition, an increasing number of research studies are needed to elucidate the underlying pathophysiological mechanisms.
Lower ASPECTS, indicating a larger infarct core, has been consistently associated with HT risk due to more severe ischemic damage and loss of vascular integrity (3, 22). The robust association between baseline ASPECTS and HT in this study reconfirmed the previous research and further identified the infarct volume as one of the most significant factors influencing HT after EVT. IV-rtPA is another variable that is certain and has been established to be associated with HT. The coagulopathy and immune invasion of the neurovascular unit induced by the tPA exacerbate HT risk after reperfusion therapy (23, 24). Unlike IV-rtPA and ASPECTS, which predominantly reflect arterial mechanisms or the extent of ischemic burden, ICV asymmetry provides unique insights into delayed venous outflow and microvascular hemodynamics, both of which may contribute to an increased risk of HT. Our findings build upon previous research by highlighting ICV asymmetry as an independent risk factor for HT, offering a novel perspective on venous involvement in HT pathogenesis.
We recognized that our study has several limitations. First, this study had a small sample size and was a single-center retrospective cohort study. Second, we used visual inspection to assess ICV asymmetry, and any anatomical variation in the ICVs could potentially bias the results. Additionally, the number of variables included in the multivariate analysis may have been excessive for the small cohort size, possibly leading to issues such as overfitting. This discrepancy should be mentioned in the limitations of our study to ensure readers understand the potential impact on our findings.
5 Conclusion
Our study indicates that ICV asymmetry could independently predict HT and is associated with sICH in patients with anterior circulation AIS-LVO after EVT. Due to the relatively small size of our cohort, our study results should be cautiously interpreted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving human participants were approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University. The studies were conducted in accordance with local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
KL: Conceptualization, Data curation, Writing – original draft, Formal analysis. WZ: Data curation, Funding acquisition, Investigation, Writing – original draft. QW: Data curation, Methodology, Project administration, Writing – original draft. YZ: Formal analysis, Investigation, Writing – original draft. BY: Data curation, Funding acquisition, Methodology, Writing – original draft. YF: Supervision, Validation, Writing – review & editing. NW: Conceptualization, Supervision, Validation, Writing – review & editing. LF: Conceptualization, Formal analysis, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Young and Middle-aged Talent Training Project in Health System of Fujian Province, grant number 2015-ZQN-JC-21; the Major Scientific Research Program for Young and Middle-aged Health Professionals of Fujian Province, grant number 2022ZQNZD005; the Provincial Health and Youth Research Project, grant number 2020QNB027; the Joint Funds for the Innovation of Science and Technology of Fujian Province, grant number 2023Y9029.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1465481/full#supplementary-material
References
1. Goyal, M, Menon, BK, van Zwam, WH, Dippel, DWJ, Mitchell, PJ, Demchuk, AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomized trials. Lancet Lond Engl. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
2. Hong, JM, Kim, DS, and Kim, M. Hemorrhagic transformation after ischemic stroke: mechanisms and management. Front Neurol. (2021) 12:703258. doi: 10.3389/fneur.2021.703258
3. Hao, Y, Yang, D, Wang, H, Zi, W, Zhang, M, Geng, Y, et al. Predictors for symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke. Stroke. (2017) 48:1203–9. doi: 10.1161/STROKEAHA.116.016368
4. Mangiardi, M, Bonura, A, Iaccarino, G, Alessiani, M, Bravi, MC, Crupi, D, et al. The pathophysiology of collateral circulation in acute ischemic stroke. Diagnostics. (2023) 13:2425. doi: 10.3390/diagnostics13142425
5. Jansen, IGH, van Vuuren, AB, van Zwam, WH, van den Wijngaard, IR, Berkhemer, OA, Lingsma, HF, et al. Absence of cortical vein opacification is associated with lack of intra-arterial therapy benefit in stroke. Radiology. (2018) 286:643–50. doi: 10.1148/radiol.2017162445
6. Kubo, M, Kuwayama, N, Massoud, TF, and Hacein-Bey, L. Anatomy of intracranial veins. Neuroimaging Clin N Am. (2022) 32:637–61. doi: 10.1016/j.nic.2022.05.002
7. Sharma, VK, Yeo, LLL, Teoh, HL, Shen, L, Chan, BPL, Seet, RC, et al. Venous outflow profiles are linked to cerebral edema formation at noncontrast head CT after treatment in acute ischemic stroke regardless of collateral vessel status at CT angiography. J Stroke Cerebrovasc Dis. (2014) 23:e39–45. doi: 10.1016/j.jstrokecerebrovasdis.2013.08.007
8. Myint, MZ, Yeo, LL, Tan, BYQ, The, EZ, Lim, MC, Sia, C-H, et al. Internal cerebral vein asymmetry is an independent predictor of poor functional outcome in endovascular thrombectomy. J Neuro Interventional Surg. (2022) 14:683–7. doi: 10.1136/neurintsurg-2021-017684
9. Faizy, TD, Kabiri, R, Christensen, S, Mlynash, M, Kuraitis, G, Meyer, L, et al. Venous outflow profiles are linked to cerebral edema formation at noncontrast head CT after treatment in acute ischemic stroke regardless of collateral vessel status at CT angiography. Radiology. (2021) 299:682–90. doi: 10.1148/radiol.2021203651
10. Barber, PA, Demchuk, AM, Zhang, J, and Buchan, AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS study group. Alberta Stroke Programme Early CT Score. Lancet Lond Engl. (2000) 355:1670–4. doi: 10.1016/s0140-6736(00)02237-6
11. Zhang, H, Li, Z, Dai, Y, Guo, E, Zhang, C, and Wang, Y. Ischaemic stroke etiological classification system: the agreement analysis of CISS, SPARKLE and TOAST. Stroke Vasc Neurol. (2019) 4:123–8. doi: 10.1136/svn-2018-000226
12. Hacke, W, Kaste, M, Fieschi, C, von Kummer, R, Davalos, A, Meier, D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet Lond Engl. (1998) 352:1245–51. doi: 10.1016/s0140-6736(98)08020-9
13. Yedavalli, VS, Salim, HA, Musmar, B, Adeeb, N, Essibayi, MA, ElNaamani, K, et al. Symptomatic intracerebral hemorrhage in proximal and distal medium middle cerebral artery occlusion patients treated with mechanical thrombectomy. J Neuro Interventional Surg. (2024):jnis-2024-021879. doi: 10.1136/jnis-2024-021879
14. Jensen, M, Schlemm, E, Cheng, B, Lettow, I, Quandt, F, Boutitie, F, et al. Clinical characteristics and outcome of patients with hemorrhagic transformation after intravenous thrombolysis in the WAKE-UP trial. Front Neurol. (2020) 11:957. doi: 10.3389/fneur.2020.00957
15. Li, W, Xiao, W-M, Luo, G-P, Liu, Y-L, Qu, J-F, Fang, X-W, et al. Asymmetrical cortical vein sign predicts early neurological deterioration in acute ischemic stroke patients with severe intracranial arterial stenosis or occlusion. BMC Neurol. (2020) 20:331. doi: 10.1186/s12883-020-01907-w
16. Suh, CH, Jung, SC, Cho, SJ, Woo, D-C, Oh, WY, Lee, JG, et al. MRI for prediction of hemorrhagic transformation in acute ischemic stroke: a systematic review and meta-analysis. Acta Radiol Stockh Swed. (2020) 61:964–72. doi: 10.1177/0284185119887593
17. Faizy, TD, Mlynash, M, Kabiri, R, Christensen, S, Kuraitis, G, Meyer, L, et al. Favourable arterial, tissue-level and venous collaterals correlate with early neurological improvement after successful thrombectomy treatment of acute ischaemic stroke. J Neurol Neurosurg Psychiatry. (2022) 93:701–6. doi: 10.1136/jnnp-2021-328041
18. Faizy, TD, Kabiri, R, Christensen, S, Mlynash, M, Kuraitis, GM, Broocks, G, et al. Favorable venous outflow profiles correlate with favorable tissue-level collaterals and clinical outcome. Stroke. (2021) 52:1761–7. doi: 10.1161/STROKEAHA.120.032242
19. Liebeskind, DS, Luff, MK, Bracard, S, Guillemin, F, Jahan, R, Jovin, TG, et al. Collaterals at angiography guide clinical outcomes after endovascular stroke therapy in HERMES. J Neuro Interventional Surg. (2024):jnis-2024-021808. doi: 10.1136/jnis-2024-021808
20. Olthuis, SGH, Pirson, FAV, Pinckaers, FME, Hinsenveld, WH, Nieboer, D, Ceulemans, A, et al. Endovascular treatment versus no endovascular treatment after 6–24 h in patients with ischaemic stroke and collateral flow on CT angiography (MR CLEAN-LATE) in the Netherlands: a multicentre, open-label, blinded-endpoint, randomised, controlled, phase 3 trial. Lancet. (2023) 401:1371–80. doi: 10.1016/S0140-6736(23)00575-5
21. McKetton, L, Venkatraghavan, L, Poublanc, J, Sobczyk, O, Crawley, AP, Rosen, C, et al. Importance of collateralization in patients with large artery intracranial occlusive disease: long-term longitudinal assessment of cerebral hemodynamic function. Front Neurol. (2018) 9:226. doi: 10.3389/fneur.2018.00226
22. Kuang, Y, Zhang, L, Ye, K, Jiang, Z, Shi, C, and Luo, L. Clinical and imaging predictors for hemorrhagic transformation of acute ischemic stroke after endovascular thrombectomy. J Neuroimaging. (2024) 34:339–47. doi: 10.1111/jon.13191
23. Shi, K, Zou, M, Jia, D-M, Shi, S, Yang, X, Liu, Q, et al. tPA mobilizes immune cells that exacerbate hemorrhagic transformation in stroke. Circ Res. (2021) 128:62–75. doi: 10.1161/CIRCRESAHA.120.317596
Keywords: internal cerebral veins, hemorrhagic transformation, acute ischemic stroke, endovascular thrombectomy, large vessel occlusion
Citation: Lin K, Zhao W, Wu Q, Zheng Y, Yang B, Fu Y, Wang N and Fang L (2025) A cross-sectional study on the correlation between internal cerebral vein asymmetry and hemorrhagic transformation following endovascular thrombectomy. Front. Neurol. 15:1465481. doi: 10.3389/fneur.2024.1465481
Edited by:
Lucio D’Anna, Imperial College London, United KingdomReviewed by:
Yao Yu, The Affiliated Hospital of Qingdao University, ChinaZhongming Qiu, Xinqiao Hospital, China
(Brig) Sankar Gorthi, Bharati Hospital, India
Copyright © 2025 Lin, Zhao, Wu, Zheng, Yang, Fu, Wang and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Wang, bmluZ3dhbmdAZmptdS5lZHUuY24=; Ling Fang, cGF1bGluZWZ6QDEyNi5jb20=
 Kunxin Lin
Kunxin Lin Wenlong Zhao
Wenlong Zhao Quanhong Wu1,2
Quanhong Wu1,2 Ying Fu
Ying Fu Ning Wang
Ning Wang