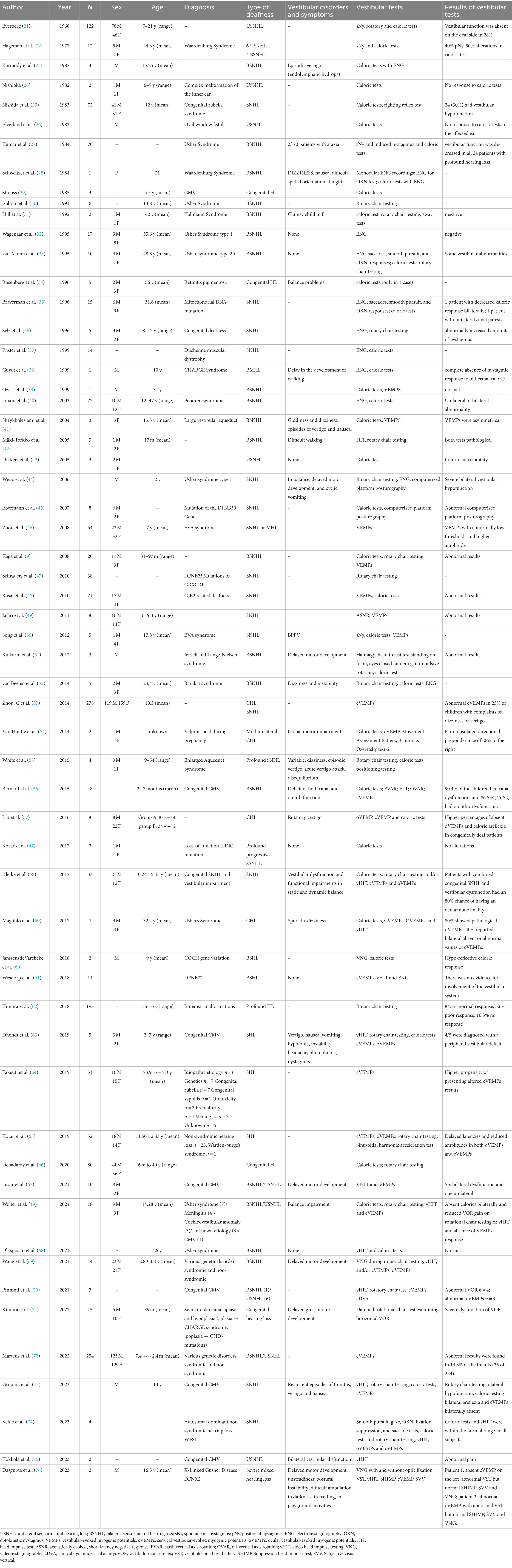
- 1Unit of Audiology, Department of Diagnostic, Clinical, and Public Health, University of Modena and Reggio Emilia, Modena, Italy
- 2Unit of Otorhinolaryngology, Department of Head and Neck, University of Verona, Verona, Italy
- 3Unit of Audiology, Department of Specialist Surgical Sciences, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 4Unit of Audiology, Primary Care Unit, Modena, Italy
Introduction: Congenital deafness is a pathological entity that represents an economical and social burden, affecting up to 0.2% of newborns in Europe. Sensorineural hearing loss (SHL) is caused by a variety of factors, including congenital abnormalities, perinatal infectious diseases and genetic syndromes. The inner ear’s vestibular system, nestled alongside the auditory organs, is crucial for balance maintenance. Its close connection with the auditory system means that disturbances in one often coincide with disturbances in the other, highlighting their intertwined functions. With this review we aim to describe objective vestibular tests found in literature and to study their use for diagnosis of vestibular disturbances in patients affected by congenital deafness.
Methods: The review is conducted with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 guidelines. The search string used was: [(congenital deafness) OR (congenital hearing loss) OR (congenital hypoacusia)] AND [(vestibular disorders) OR (vertigo)]. An initial abstract reading selection was made, and a subsequent comprehensive full-text reading. For each article, we identified the type of vestibular test utilized and its corresponding outcome.
Results: Out of the initial—papers identified through the search string—articles met the eligibility criteria for further analysis through abstract and full-text reading. After further selection—articles were chosen for detailed examination, focusing on the data of patients.
Conclusion: Congenital hearing loss profoundly affects a child’s development, especially in language and communication skills, and it is frequently associated with a pathological vestibular system. Early identification allows timely intervention with personalized therapies. In current literature, there is still no gold standard test to identify balance disorders in patients with congenital hearing loss. There is considerable variability on the subject due to the inclusion of diverse patients with various diagnoses, alongside a wide range of available technologies. Managing such conditions necessitates collaboration among healthcare providers, ensuring comprehensive care through prompt diagnosis and personalized treatment plans. Ongoing research aims to further improve screening methods and develop precision medicine approaches tailored to individual needs.
Introduction
Congenital hearing loss is one of the most common forms of congenital disability in newborns worldwide and can affect one ear (unilateral hearing loss) or both ears (bilateral hearing loss). Despite the progress of neonatology, its incidence has remained constant in time. Hearing loss affects between 1 and 2 per 1,000 infants in the United States and in some categories of infants, such as those with familiarity for congenital deafness, the prevalence can be 10–20 times higher (1).
Screening programs have been implemented to allow timely treatment and avoid adverse consequences on the development of the child’s language and cognitive skills. About 50% of cases of congenital deafness are genetic, inherited by one or both parents. Genetic hearing loss can have both syndromic and non-syndromic causes. The other 50% are non-genetic (or environmental).
According to the site of lesion, hearing loss can be categorized in conductive (outer or middle ear) and sensorineural (inner ear or auditory nerve or central auditory pathway). Mixed hearing loss is defined as the cohexistence of the two (2).
Examples of congenital conductive hearing loss are external ear malformations, for example microtia, stenosis or atresia of the external auditory canal, that can occur both as a part of a clinical syndrome (i.e., CHARGE syndrome) or isolatedly, but also malformations of the ossicles, as ossicular chain fixation, usually part of a syndrome (e.g., Treacher-Collins syndrome, branchio-oto-renal syndrome, DiGeorge syndrome, Beckwith-Wiedemann syndrome).
Sensorineural congenital genetic hearing loss can be associated with syndromes as Waardenburg syndrome, Usher syndrome, Pendred syndrome, Alport syndrome, and Jervell and Lange-Nielsen syndrome or can be linked to isolated gene mutations, commonly GJB2 and STRC genes.
Non-genetic causes of sensorineural congenital hearing loss are (3).
• in-utero infections by TORCH complex pathogens. The most common cause is congenital CMV, which can determine delayed or progressive sensorineural hearing loss (SNHL), both monolaterally and bilaterally. Also toxoplasma, rubella, Zika virus and syphilis can be responsible.
• Inner ear malformations, for example Enlarged Vestibular Aqueduct (EVA) Syndrome, manifesting with varying degrees of progressive deafness, sometimes associated with vestibular disorders
• Perilymph fistula, very uncommon, determining fluctuating severe SNHL, disequilibrium, and aural fullness
The vestibular system is constituted by peripheral structures linked to a complex central neural network, and contributes to give to the brain inputs regarding movement and orientation of the body in space.
Specifically, informations from the peripheral vestibular apparatus, located in the inner ear, are carried to the brainstem through the inferior and superior vestibular nerves (cranial nerve VIII). Those informations are represented by angular acceleration of the head in the space, detected by the sensory hair cells present in the ampullae of the semicircular canals, and linear acceleration, to which the maculae of the otolith organs, utricle and saccule, are dedicated. In the brainstem the vestibular nuclei receive peripheral vestibular inputs and integrate them with sensory afferences from visual and proprioceptive systems. Ultimately, motor effereces are conveyed to the brain and spinal cord, for the control of eye movement (vestibulo-ocular reflexes), balance maintenance and postural adjustments (vestibulospinal reflexes).
A dysfunction of this innately complex system can be peripheral or central and May present acutely or chronically.
A peripheral vestibular dysfunction is a pathology of the vestibular system itself: the membranous labyrinth and the superior and inferior vestibular nerves. On the other hand, a central vestibular disorder affects the central nervous system itself, for instance in trauma, stroke and demyelinating conditions (4, 5).
Similarly to congenital hearing loss, congenital vestibular disorders May be determined by genetic disorders, syndromic or isolated, but also be associated with structural anomalies, malformations and exposure to prenatal insults, either infectious noxae or ototoxic drugs.
The diagnosis of vestibular disorders May involve imaging, such as magnetic resonance imaging (MRI) when a central lesion is suspected from history of physical examination, or computed tomography (CT), but also clinical tests, with various degrees of complexity and availability, depending on the specific setting (4).
For example, in an emergency room setting, the HINTS examination (head impulse, nystagmus, test of skew) is a valid tool to differentiate central or peripheral causes of acute vertigo (6).
On the other hand, the diagnosis of vestibular dysfunctions May often be a challenge, because of symptom variability and patients’ comorbidities, requiring objective vestibular tests in order to reach an accurate diagnosis. These tests can identify the site of lesion (central vs. peripheral, side but also the specific structure involved, e.g., superior vs. inferior vestibular nerve), quantify vestibular function, assess compensation status and monitor disease’s progression (7).
Even though vestibular tests can give pathophysiological information about vestibular function, they are unable to determine a specific diagnosis, or the impact of a certain deficit on level of disability or quality of life.
Analysis and interpretation of eye movements represents one of the main tools to verify vestibular system function through its interaction with the visual system.
Pathologic nystagmus (spontaneous, gaze, positional, and positioning) can represent a sign of lesion of peripheral vestibular system as well as lesions of cerebellum or any site involved in the central vestibular pathway.
Videonystagmography (VNG) or electronystagmography (ENG) can be used to record nystagmus (whether spontaneous or induced by various stimuli, such as rotational, caloric, optokinetic, and gaze testing) and other eye movements, including saccades or pursuit movements during oculomotor assessments.
Vestibular evoked myogenic potentials (VEMPs) represent a neurophysiologic test to assess the function of utricule and saccule, the otholitic organs. In particular, the oVEMPs (ocular VEMPs) determine the function of the utricule and superior vestibular nerve while the cVEMPs (cervical VEMPs) evaluate the saccule and the inferior vestibular nerve.
Auditory pathway function (from CN VIII to the mesencephalon) can be evaluated through Auditory brainstem response (ABR), or Brainstem auditory evoked potentials (BAEP) (4, 7).
The association between SNHL and vestibular disorders is described in literature, however frequently underestimated by most professionals. Vestibular impairment has repercussions on gross motor functions, with delayed motor development and postural control, hindering the achievement of motor milestones like head control, independent sitting and walking (8). Fine motor functions are however usually preserved, in the absence of CNS involvement (9).
The prevalence of vestibular and balance disorders in children with SNHL is elevated, in fact some estimates indicate that almost 70% of children with SNHL have vestibular system impairment, with 20–40% having severe bilateral vestibular loss (8). Eventually, the integration between pyramidal and extrapiramidal motor systems and visual and somatosensory systems, associated with intellectual development, compensate for vestibular failure, and these children catch up with their peers in terms of development and motor function (9).
The aim of this review is to analyze the role of objective vestibular tests in studying vestibular function in patients with congenital deafness.
Materials and methods
This review was conducted with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) 2020 guidelines (See Figure 1). The research was carried out using the Pubmed, Scopus and Cochrane database with the following research string: [(congenital deafness) OR (congenital hearing loss) OR (congenital hypoacusia)] AND [(vestibular disorders) OR (vertigo)]. Three reviewers (ES, CL, and EZ) performed the literature search and the abstract and full text reading. All the articles found were included, without any period restriction. Last research on the database was performed in January 2024. The inclusion criteria for the initial abstract reading selection were as follows: articles focusing on congenital hearing loss, inclusion of objective vestibular tests, relevance to the inherent topic, and written in English. The exclusion criteria for this phase included the absence of evidence for congenital hearing loss, lack of objective vestibular tests, unrelated topic, and non-English papers. Following the initial abstract-based selection, a comprehensive full-text reading was conducted, incorporating additional criteria.
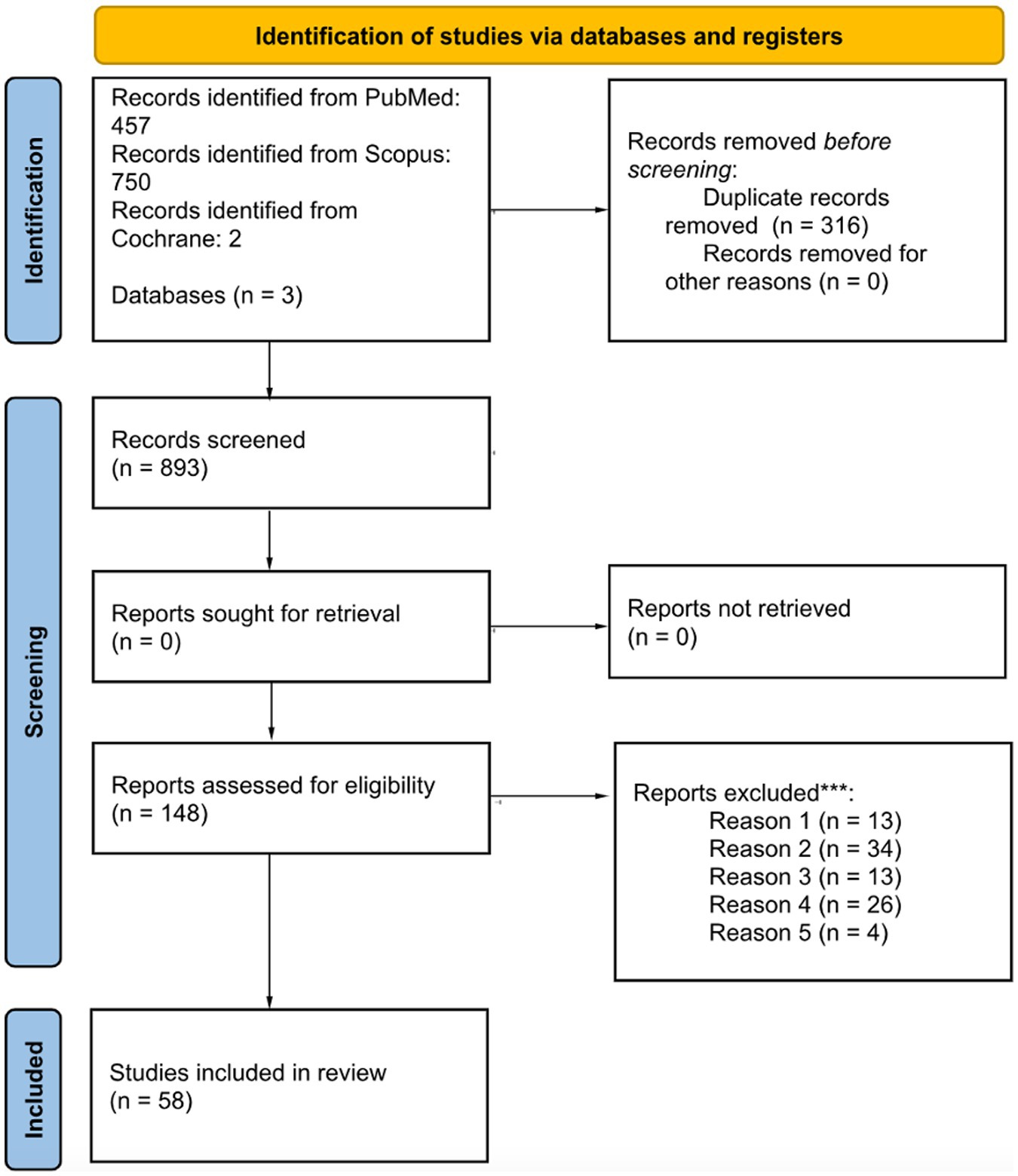
Figure 1. Literature selection following the PRISMA statement guidelines. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. ***Reason 1 = absence of a diagnosis of congenital deafness; reason 2 = lack of objective vestibular tests; reason 3 = unavailability of full-text articles; reason 4 = insufficient data; reason 5 = cochlear implant.
The inclusion criteria for the second selection phase were: individuals with congenital deafness who had undergone objective vestibular tests, the availability of full-text articles, and complete data. Exclusion criteria included the absence of a diagnosis of congenital deafness, lack of objective vestibular tests, unavailability of full-text articles, insufficient data (i.e., lack of specific data regarding the number of patients and the type of deafness) and the presence of cochlear implant. The selected articles were then analyzed in detail, extracting data about: type of study design, number of patients, sex and age of patients, diagnosis and type of deafness, types of vestibular disorders and their related symptoms, audiometry, and vestibular tests.
Results
A total of 1,209 papers were selected using the aforementioned research string. A total of 316 duplicated articles were eliminated resulting in 893 articles eligible for further analysis. First, an abstract reading selection was made according to the inclusion criteria and 148 abstracts were selected. After this, a further exclusion reading full-length papers was made, with 59 articles selected, following the pre-established criteria. The process of literature selection following the PRISMA statement guidelines is reported in Figure 1.
A total of 1,700 patients (sex was not always available) were reported in the selected papers. The articles analyzed cover a time span from 1960 to 2023. They examine patients with congenital sensorissneural hearing loss, both unilateral and bilateral. The main vestibular symptoms considered by each article were analyzed, including vertigo, ataxia, dizziness, nausea, balance problems, delayed walking, and paroxysmal positional vertigo. Furthermore, the various types of vestibular tests used and their results were analyzed in detail. The details of patients’ results are reported in Table 1.
Discussion
Hearing loss is the most common sensory disorder in newborns, affecting approximately 0.1–0.2% of infants who are born deaf or hard-of-hearing (10). This incidence surpasses that of other congenital disorders detected through newborn screening, such as 13 out of 100,000 for haemoglobinopathies, 10 out of 100,000 for phenylketonuria, and 25 out of 100,000 for congenital hypothyroidism (11). The American Speech-Language-Hearing Association reports that the percentage of newborns with permanent hearing loss at birth is about 3% and that the number increases to 6% by the time children get old. Among the myriad of factors contributing to neonatal hearing loss, genetic causes, environmental exposures, and congenital infections—particularly cytomegalovirus (CMV)—play significant roles. Unlike postlingual hearing loss, prelingual deafness delays the development of auditory neural pathways, hindering normal speech and language acquisition. Early identification and timely intervention are crucial for preventing delays in language development. Treatment options include early intervention services, hearing aid amplification, and cochlear implantation. In addition to hearing loss, there is growing interest in understanding the impact of vestibular dysfunction on children with congenital hearing impairment. The vestibular system, located in the inner ears alongside the hearing organs, plays a vital role in maintaining balance, given the interconnectedness of the auditory and balance systems, disruptions in one system can often correlate with disruptions in the other (8).
It has been demonstrated that spatial hearing and balance are connected, in that a single fixed sound source can provide sufficient spatial cues for the central nervous system to better control postural stability (12). The vestibular system enables gaze stability during head movements through the vestibulo-ocular reflex (VOR) originating from the semicircular canals. It also participates in the perception of verticality and is involved in producing muscular synergies necessary for postural control and orientation via vestibulospinal reflexes. Thus, the vestibular system plays a crucial role in postural orientation and elaborates various postural adjustments essential for acquiring the different stages of postural−motor development (13). Almost 70% of children presenting with sensorineural hearing loss (SNHL) have vestibular system disturbances, with 20–40% having severe bilateral vestibular loss. Numerous causes of hearing loss can be linked to vestibular impairment. These include conditions such as complete partition (types 1–3), enlarged vestibular aqueduct syndrome, cochlear nerve deficiency, congenital CMV infection, meningitis, and exposure to ototoxic medications. In affected children, vestibular impairment May manifest progressively, either in conjunction with a similar Decline in hearing levels or independently of it. This population must be rehabilitated early in order to avoid motor and cognitional delayed development (14). However, research on the effects of vestibular dysfunction in this population remains limited.
This article reviews the current literature on the work-up of vestibular disorders with objective vestibular tests for diagnosing balance disorders in patients affected by congenital sensorineural hearing loss. Early diagnosis and subsequent rehabilitation plays a pivotal role in optimizing outcomes for patients with congenital hearing loss and vestibular disorders. By identifying and addressing these conditions promptly, we empower children to thrive and communicate effectively.
In our review, we found significant heterogeneity among the cases presented, both in terms of the etiology of hearing loss and regarding the vestibular tests used, the variability can be attributed to the fact that the various articles were written in different years and countries, thus reflecting diverse technological advancements and availability of materials. The wide time span during which the articles analyzed in this review were published (1960–2023) has led to a significant change in the use of vestibular tests for patient evaluation. In fact, overall, the most commonly used test was the caloric test, at least until 1990, when VEMPs (Vestibular Evoked Myogenic Potentials) were introduced and became widespread. Therefore, there is an inherent inconsistency in the types of tests used for the evaluation of vestibular disorders.
We excluded patients with cochlear implants (IC) from the review to concentrate on individuals who had not undergone surgical procedures that might impact the neurological organization of the inner ear. Several studies have demonstrated that children with cochlear implants exhibit altered sacculi, characterized by the absence of vestibular-evoked myogenic potentials (VEMPs) in response to click stimuli (15). Vestibular dysfunction can be found in almost 60% of post-IC patients (16).
Moreover, the results vary greatly because we decided to include both patients with non-syndromic hearing loss and those with more severe syndromes that also cause extreme anatomical alterations of the inner ear structures. This variation is reflected in the results, ranging from patients who are essentially asymptomatic without any motor balance disturbance to others experiencing symptoms such as vertigo attacks, nystagmus, postural instability, and complete motor disability. In some cases, despite evidence of vestibular test abnormalities, patients did not exhibit balance disturbances, indicating the remarkable adaptability of physiological systems (17). Additionally, including both unilateral and bilateral congenital deafness makes the results even more heterogeneous. Vestibular tests, in fact, have different interpretations depending on the case, as with the caloric test, which is not always appropriate in cases of bilateral congenital deafness. The wide age range of the patients included in the study is also a factor that contributes to the heterogeneity of the results. The outcomes of the tests can indeed be influenced by the patient’s cooperation, which clearly varies based on age. Hearing evaluation has been conducted using age appropriate tools. Hearing tests included pure tone audiometry, vocal audiometry, tympanometry, auditory brainstem response (ABR), and otoacoustic emissions. In addition to audiological and vestibular tests, patients underwent computed tomography (CT) and magnetic resonance imaging (MRI) to identify congenital anomalies of the vestibular apparatus such as enlarged vestibular aqueduct syndrome (EVA) or CHARGE syndrome.
Genetic tests were also conducted to assess potential genetic mutations in syndromes. Perinatal tests were performed to screen for CMV or other pathogens infection such as urine/blood PCR, maternal seroconversion, amniotic fluid sampling, and ophthalmologic evaluations were conducted in cases where alterations of vision were suspected (18). In the analyzed articles, assessments addressed the labyrinthine (semicircular canal) function, otolith function and integrated balance.
We found that the majority of studies after 1990 included for vestibular testing used VEMPS, cVEMPs or oVEMPS. The greatest advantage of the cVEMP and oVEMP tests is their ability to measure a different part of the vestibular system (i.e., the otolithic end organs), whereas videonystagmography (VNG) is typically used for performing caloric and rotational tests that assess the function of the horizontal semicircular canal and its connections with the superior vestibular nerve. VEMP examinations evaluate the right and left labyrinths separately, making VEMPs further useful in localizing the side of the lesion. Another advantage is that both of these tests are relatively quick and well-tolerated by patients.
Other tests utilized were rotatory test, caloric test with or without ENG, posturography, acoustically evoked, short latency negative response (ASNR), Dynamic visual acuity (DVA). Some authors have also tested dynamic balance in simulated conditions, using a virtual reality simulator.
Together with these tests, some authors have chosen to further investigate the balance of the patients using the Bruininks-Oseretsky Test of Motor proficiency-2 (BOT-2) and the challenging environmental assessment lab (CEAL) (19). The BOT-2 is an assessment tool for motor skills used to evaluate an individual’s ability to perform basic motor tasks, so it does not rely on objective response from specific gear but rely on the competence of qualified professionals. The BOT-2 consists of eight subtests that assess eye-hand coordination, manual dexterity, movement speed, static and dynamic balance, and muscle strength. The subtests are divided into two main areas: fine motor and gross motor skills.
Gerdsen et al. (20) have recently proposed a clinical vestibular testing algorithm in children to detect vestibular hypofunction. The first test which is used is vHIT since this test is quick, has low burden and high specificity, followed by caloric test o cVEMP depending on the age of the patient. In HIT, patients focus on a stationary target while experiencing sudden, small head rotations in the plane of each semicircular canal. This test evaluates the effectiveness of compensatory eye movements, assessing semicircular canal function studying the vestibulo-ocular reflex (VOR). vHIT test is valuable because it evaluates the function of both the horizontal and vertical semicircular canals, providing a comprehensive assessment of the vestibular system. By measuring the eye movements in response to rapid, small head impulses, the vHIT detects deficits in VOR, which is critical for maintaining stable vision during head movements. Combining vHIT with cervical and cVEMP and oVEMP tests enables a complete evaluation of all vestibular receptors, including both the semicircular canals (horizontal and vertical) and the otolithic organs (saccule and utricle). This integrated approach enhances the diagnostic accuracy for a wide range of vestibular disorders by assessing the entire vestibular apparatus.
This review highlights that the available data are limited due to significant heterogeneity, and there is no exact consensus on the methodology for conducting vestibular studies. This is allegedly mostly due to the deverseness of the conditions causing the hearing loss, the age of the patients and advance and availability of the technologies. In our study articles have been ruled out due to the absence of objective reproducible vestibular tests, so in the future we hope we’ll find a useful diagnostic algorithm in order to get early diagnosis for vestibular impairment in congenital deaf patients. In future studies, it will be crucial to ensure comprehensive reporting of all vestibular test results and to provide detailed specifications regarding which patients participated in specific tests within case series.
Conclusion
Congenital hearing loss significantly impacts a child’s development, particularly language acquisition and communication skills. Early identification of hearing impairment allows for timely intervention, which can mitigate the adverse effects on language development. Individualized therapies, including hearing aids and cochlear implants, are most effective when initiated early.
In light of the frequent association between unilateral or bilateral congenital hearing loss and vestibular disorders, it is crucial to develop a diagnostic algorithm for the early detection of any associated vestibular issues. To date, there is still no consensus on which vestibular tests should be the first choice, largely due to the significant heterogeneity of studies in the literature and the diversity of patients. In the future, studies are needed to determine which vestibular test is most appropriate for assessing vestibular symptoms in patients with congenital deafness, taking into account the patient’s age and level of cooperation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
EG: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Visualization, Writing – review & editing. ES: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CL: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Investigation. EZ: Investigation, Methodology, Writing – original draft, Writing – review & editing, Formal analysis. DM: Conceptualization, Data curation, Project administration, Supervision, Validation, Visualization, Writing – review & editing. EA: Supervision, Validation, Visualization, Writing – review & editing. SP: Supervision, Validation, Visualization, Writing – review & editing. RN: Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hardani, AK, Goodarzi, E, Delphi, M, and Badfar, G. Prevalence and risk factors for hearing loss in neonates admitted to the neonatal intensive care unit: a hospital study. Cureus. (2020) 12:e11207. doi: 10.7759/cureus.11207
2. Renauld, JM, and Basch, ML. Congenital deafness and recent advances towards restoring hearing loss. Curr Protoc. (2021) 1:e76. doi: 10.1002/cpz1.76
3. Korver, AMH, Smith, RJH, Van Camp, G, Schleiss, MR, Bitner-Glindzicz, MAK, Lustig, LR, et al. Congenital hearing loss. Nat Rev Dis Primer. (2017) 3:16094. doi: 10.1038/nrdp.2016.94
4. Dougherty, JM, Carney, M, Hohman, MH, and Emmady, PD. Vestibular dysfunction In: WB Thomas, editor. StatPearls. Treasure Island, FL: StatPearls Publishing (2024)
5. Strupp, M, Dlugaiczyk, J, Bettina Ertl-Wagner, B, Rujescu, D, Westhofen, M, and Dieterich, M. Vestibular disorders. Dtsch Ärztebl Int. (2020) 117:300–10. doi: 10.3238/arztebl.2020.0300
6. Gottlieb, M, Peksa, GD, and Carlson, JN. Head impulse, nystagmus, and test of skew examination for diagnosing central causes of acute vestibular syndrome. Cochrane Database Syst Rev. (2023) 2023:11. doi: 10.1002/14651858.CD015089.pub2
7. Narayana Swamy, S, Yuvaraj, P, Pruthi, N, Thennarasu, K, and Rajasekaran, AK. Comprehensive normative data for objective vestibular tests. Cureus. (2023) 15:e40080. doi: 10.7759/cureus.40080
8. Santos, TGT, Venosa, AR, and Sampaio, ALL. Association between hearing loss and vestibular disorders: a review of the interference of hearing in the balance. Int J Otolaryngol Head Amp Neck Surg. (2015) 4:173–9. doi: 10.4236/ijohns.2015.43030
9. Kaga, K, Shinjo, Y, Jin, Y, and Takegoshi, H. Vestibular failure in children with congenital deafness. Int J Audiol. (2008) 47:590–9. doi: 10.1080/14992020802331222
10. Chari, DA, and Chan, DK. Diagnosis and treatment of congenital sensorineural hearing loss. Curr Otorhinolaryngol Rep. (2017) 5:251–8. doi: 10.1007/s40136-017-0163-3
11. Mehl, AL, and Thomson, V. Newborn hearing screening: the great omission. Pediatrics. (1998) 101:E4. doi: 10.1542/peds.101.1.e4
12. Zhong, X, and Yost, WA. Relationship between postural stability and spatial hearing. J Am Acad Audiol. (2013) 24:782–8. doi: 10.3766/jaaa.24.9.3
13. Reynard, P, Ortega-Solís, J, Tronche, S, Darrouzet, V, and Thai-Van, H. Guidelines of the French society of otorhinolaryngology and head and neck surgery (SFORL) for vestibular rehabilitation in children with vestibular dysfunction. A systematic review. Arch Pediatr Organe Off Soc Francaise Pediatr. (2024) 31:217–23. doi: 10.1016/j.arcped.2024.02.006
14. Beck, DL, Petrak, M, Madell, JR, and Cushing, SL. Update 2015: pediatric vestibular, balance, and hearing disorders. Hear Rev. (2015) 22:14
15. Jin, Y, Nakamura, M, Shinjo, Y, and Kaga, K. Vestibular-evoked myogenic potentials in cochlear implant children. Acta Otolaryngol. (2006) 126:164–9. doi: 10.1080/00016480500312562
16. Licameli, G, Zhou, G, and Kenna, MA. Disturbance of vestibular function attributable to cochlear implantation in children. Laryngoscope. (2009) 119:740–5. doi: 10.1002/lary.20121
17. Kovač, J, Klančar, G, and Battelino, S. Discovering the unexpected with the utilization of NGS in diagnostics of non-syndromic hearing loss disorders: the family case of ILDR1-dependent hearing loss disorder. Front Genet. (2017) 8:95. doi: 10.3389/fgene.2017.00095
18. Chebib, E, Maudoux, A, Benoit, C, Bernard, S, Belarbi, N, Parodi, M, et al. Predictors of cochleovestibular dysfunction in children with congenital cytomegalovirus infection. Eur J Pediatr. (2022) 181:2909–18. doi: 10.1007/s00431-022-04495-8
19. Wolter, NE, Gordon, KA, Campos, J, Vilchez Madrigal, LD, Papsin, BC, and Cushing, SL. Impact of the sensory environment on balance in children with bilateral cochleovestibular loss. Hear Res. (2021) 400:108134. doi: 10.1016/j.heares.2020.108134
20. Gerdsen, M, Hundscheid, TM, Boudewyns, A, Van Rompaey, V, Van De Berg, R, and Widdershoven, JCC. Vestibular assessment in children with sensorineural hearing loss: diagnostic accuracy and proposal for a diagnostic algorithm. Front Neurol. (2024) 15:1349554. doi: 10.3389/fneur.2024.1349554
21. Everberg, G. Unilateral total deafness in children clinical problems with a special view to vestibular function. Acta Otolaryngol. (1960) 52:253–69. doi: 10.3109/00016486009123146
22. Hageman, MJ, and Oosterveld, WJ. Vestibular findings in 25 patients with Waardenburg’s syndrome. Arch Otolaryngol. (1977) 103:648–52. doi: 10.1001/archotol.1977.00780280048006
23. Karmody, CS. Congenital deafness and episodic Vertigo. Otolaryngol Neck Surg. (1982) 90:602–5. doi: 10.1177/019459988209000517
25. Nishida, Y, Ueda, K, and Fung, KC. Congenital rubella syndrome: function of equilibrium of 80 cases with deafness. Laryngoscope. (1983) 93:938–40. doi: 10.1288/00005537-198307000-00018
26. Elverland, HH, and Mair, IW. Recurrent meningitis, congenital anacusis and Mondini anomaly. Acta Otolaryngol. (1983) 95:147–51. doi: 10.3109/00016488309130928
27. Kumar, A, Fishman, G, and Torok, N. Vestibular and auditory function in Usher’s syndrome. Ann Otol Rhinol Laryngol. (1984) 93:600–8. doi: 10.1177/000348948409300613
28. Schweitzer, VG, and Clack, TD. Waardenburg’s syndrome: a case report with CT scanning and cochleovestibular evaluation. Int J Pediatr Otorhinolaryngol. (1984) 7:311–22. doi: 10.1016/S0165-5876(84)80014-2
29. Strauss, M. A clinical pathologic study of hearing loss in congenital cytomegalovirus infection. Laryngoscope. (1985) 95:951–62. doi: 10.1288/00005537-198508000-00014
30. Enbom, H, Magnusson, M, and Pyykkö, I. Postural compensation in children with congenital or early acquired bilateral vestibular loss. Ann Otol Rhinol Laryngol. (1991) 100:472–8. doi: 10.1177/000348949110000609
31. Hill, J, Elliott, C, and Colquhoun, I. Audiological, vestibular and radiological abnormalities in Kallman’s syndrome. J Laryngol Otol. (1992) 106:530–4. doi: 10.1017/S0022215100120067
32. Wagenaar, M, ter Rahe, B, van Aarem, A, Huygen, P, Admiraal, R, Bleeker-Wagemakers, E, et al. Clinical findings in obligate carriers of type I usher syndrome. Am J Med Genet. (1995) 59:375–9. doi: 10.1002/ajmg.1320590319
33. Van Aarem, A, Cremers, CWRJ, Pinckers, AJLG, Huygen, PLM, Hombergen, GCJH, and Kimberling, BJ. The usher syndrome type 2A: clinical findings in obligate carriers. Int J Pediatr Otorhinolaryngol. (1995) 31:159–74. doi: 10.1016/0165-5876(94)01081-8
34. Rosenberg, T, and Parving, A. A syndrome with retinitis pigmentosa, progressive hearing impairment, vestibular dysfunction, and congenital cataract. Acta Ophthalmol Scand Suppl. (1996) 74:50–3. doi: 10.1111/j.1600-0420.1996.tb00387.x
35. Braverman, I, Jaber, L, Levi, H, Adelman, C, Arons, KS, Fischel-Ghodsian, N, et al. Audiovestibular findings in patients with deafness caused by a mitochondrial susceptibility mutation and precipitated by an inherited nuclear mutation or aminoglycosides. Arch Otolaryngol Head Neck Surg. (1996) 122:1001–4. doi: 10.1001/archotol.1996.01890210073016
36. Selz, PA, Girardi, M, Konrad, HR, and Hughes, LF. Vestibular deficits in deaf children. Otolaryngol Neck Surg. (1996) 115:70–7. doi: 10.1016/S0194-5998(96)70139-0
37. Pfister, MHF, Apaydin, F, Turan, O, Bereketoglu, M, Bilgen, V, Braendle, U, et al. Clinical evidence for dystrophin dysfunction as a cause of hearing loss in locus DFN4. Laryngoscope. (1999) 109:730–5. doi: 10.1097/00005537-199905000-00010
38. Guyot, JP, and Vibert, D. Patients with CHARGE association: a model to study saccular function in the human. Ann Otol Rhinol Laryngol. (1999) 108:151–5. doi: 10.1177/000348949910800209
39. Ozeki, H, Matsuzaki, M, and Murofushi, T. Vestibular evoked myogenic potentials in patients with bilateral profound hearing loss. ORL J Oto-Rhino-Laryngol Its Relat Spec. (1999) 61:80–3. doi: 10.1159/000027646
40. Luxon, LM, Cohen, M, Coffey, RA, Phelps, PD, Britton, KE, Jan, H, et al. Neuro-otological findings in Pendred syndrome. Int J Audiol. (2003) 42:82–8. doi: 10.3109/14992020309078339
41. Sheykholeslami, K, Schmerber, S, Habiby Kermany, M, and Kaga, K. Vestibular-evoked myogenic potentials in three patients with large vestibular aqueduct. Hear Res. (2004) 190:161–8. doi: 10.1016/S0378-5955(04)00018-8
42. Mäki-Torkko, E, and Magnusson, M. An office procedure to detect vestibular loss in children with hearing impairment. Eur Arch Otorrinolaringol. (2005) 262:328–30. doi: 10.1007/s00405-004-0807-z
43. Dikkers, FG, Verheij, JBGM, and Van Mechelen, M. Hereditary congenital unilateral deafness: a new disorder? Ann Otol Rhinol Laryngol. (2005) 114:332–7. doi: 10.1177/000348940511400414
44. Weiss, AH, and Phillips, JO. Congenital and compensated vestibular dysfunction in childhood: an overlooked entity. J Child Neurol. (2006) 21:572–9. doi: 10.1177/08830738060210071501
45. Ebermann, I, Walger, M, Scholl, HPN, Charbel Issa, P, Lüke, C, Nürnberg, G, et al. Truncating mutation of the DFNB59 gene causes cochlear hearing impairment and central vestibular dysfunction. Hum Mutat. (2007) 28:571–7. doi: 10.1002/humu.20478
46. Zhou, G, Gopen, Q, and Kenna, MA. Delineating the hearing loss in children with enlarged vestibular aqueduct. Laryngoscope. (2008) 118:2062–6. doi: 10.1097/MLG.0b013e31818208ad
47. Schraders, M, Lee, K, Oostrik, J, Huygen, PLM, Ali, G, Hoefsloot, LH, et al. Homozygosity mapping reveals mutations of GRXCR1 as a cause of autosomal-recessive nonsyndromic hearing impairment. Am J Hum Genet. (2010) 86:138–47. doi: 10.1016/j.ajhg.2009.12.017
48. Kasai, M, Hayashi, C, Iizuka, T, Inoshita, A, Kamiya, K, Okada, H, et al. Vestibular function of patients with profound deafness related to GJB2 mutation. Acta Otolaryngol. (2010) 130:990–5. doi: 10.3109/00016481003596508
49. Jafari, Z, and Asad, MS. The effect of saccular function on static balance ability of profound hearing-impaired children. Int J Pediatr Otorhinolaryngol. (2011) 75:919–24. doi: 10.1016/j.ijporl.2011.04.006
50. Song, JJ, Hong, SK, Kim, JS, and Koo, JW. Enlarged vestibular aqueduct may precipitate benign paroxysmal positional vertigo in children. Acta Otolaryngol. (2012) 132:S109–17. doi: 10.3109/00016489.2012.662714
51. Kulkarni, AM, Rajput, K, Raglan, E, Abrams, D, and Bitner-Glindzicz, M. Is gross motor delay secondary to bilateral vestibular hypofunction in Jervell and Lange-Nielsen syndrome? Audiol Med. (2012) 10:93–8. doi: 10.3109/1651386X.2012.686165
52. van Beelen, E, Leijendeckers, JM, Admiraal, RJC, Huygen, PLM, Hoefsloot, LH, Pennings, RJE, et al. Audiometric characteristics of a Dutch family with a new mutation in GATA3 causing HDR syndrome. Audiol Neurootol. (2014) 19:106–14. doi: 10.1159/000356303
53. Zhou, G, Dargie, J, Dornan, B, and Whittemore, K. Clinical uses of cervical vestibular-evoked myogenic potential testing in pediatric patients. Medicine. (2014) 93:e37. doi: 10.1097/MD.0000000000000037
54. Van Houtte, E, Casselman, J, Janssens, S, De Kegel, A, Maes, L, and Dhooge, I. Middle and inner ear malformations in two siblings exposed to valproic acid during pregnancy: a case report. Int J Pediatr Otorhinolaryngol. (2014) 78:2007–10. doi: 10.1016/j.ijporl.2014.08.030
55. White, J, and Krakovitz, P. Nystagmus in enlarged vestibular aqueduct: a case series. Audiol Res. (2015) 5:120. doi: 10.4081/audiores.2015.120
56. Bernard, S, Wiener-Vacher, S, Van Den Abbeele, T, and Teissier, N. Vestibular disorders in children with congenital cytomegalovirus infection. Pediatrics. (2015) 136:e887–95. doi: 10.1542/peds.2015-0908
57. Lin, BY, and Young, YH. Assessing residual vestibular function in adults with congenital hearing loss. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol Head Neck Surg. (2016) 273:4209–14. doi: 10.1007/s00405-016-4137-8
58. Kletke, S, Batmanabane, V, Dai, T, Vincent, A, Li, S, Gordon, KA, et al. The combination of vestibular impairment and congenital sensorineural hearing loss predisposes patients to ocular anomalies, including usher syndrome. Clin Genet. (2017) 92:26–33. doi: 10.1111/cge.12895
59. Magliulo, G, Iannella, G, Gagliardi, S, Iozzo, N, Plateroti, R, Mariottini, A, et al. Usher’s syndrome type II: a comparative study of genetic mutations and vestibular system evaluation. Otolaryngol Neck Surg. (2017) 157:853–60. doi: 10.1177/0194599817715235
60. JanssensdeVarebeke, SPF, Van Camp, G, Peeters, N, Elinck, E, Widdershoven, J, Cox, T, et al. Bi-allelic inactivating variants in the COCH gene cause autosomal recessive prelingual hearing impairment. Eur J Hum Genet EJHG. (2018) 26:587–91. doi: 10.1038/s41431-017-0066-2
61. Wesdorp, M, Schreur, V, Beynon, AJ, Oostrik, J, van de Kamp, JM, Elting, MW, et al. Further audiovestibular characterization of DFNB77, caused by deleterious variants in LOXHD1, and investigation into the involvement of Fuchs corneal dystrophy. Clin Genet. (2018) 94:221–31. doi: 10.1111/cge.13368
62. Kimura, Y, Masuda, T, and Kaga, K. Vestibular function and gross motor development in 195 children with congenital hearing loss - assessment of inner ear malformations. Otol Neurotol. (2018) 39:196–205. doi: 10.1097/MAO.0000000000001685
63. Dhondt, C, Maes, L, Oostra, A, and Dhooge, I. Episodic vestibular symptoms in children with a congenital cytomegalovirus infection: a case series. Otol Neurotol Off Publ Am Otol Soc Am Neurotol Soc Eur Acad Otol Neurotol. (2019) 40:e636–42. doi: 10.1097/MAO.0000000000002244
64. Takeuti, AA, Correa, APS, Leao, EM, and Favero, ML. The relationship between the etiology of profound Prelingual sensorineural hearing loss and the results of vestibular-evoked myogenic potentials. Int Arch Otorhinolaryngol. (2019) 23:1–6. doi: 10.1055/s-0038-1649491
65. Kotait, MA, Moaty, AS, and Gabr, TA. Vestibular testing in children with severe-to-profound hearing loss. Int J Pediatr Otorhinolaryngol. (2019) 125:201–5. doi: 10.1016/j.ijporl.2019.07.015
66. Dehadaray, A, Gaikwad, V, Kaushik, M, Mishra, P, and Belsare, S. Vestibular evaluation in patients with congenital profound hearing loss using ice cold water caloric test and rotational chair test. Indian J Otol. (2020) 26:132–4. doi: 10.4103/indianjotol.INDIANJOTOL_11_19
67. Lazar, A, Löfkvist, U, Verrecchia, L, and Karltorp, E. Identical twins affected by congenital cytomegalovirus infections showed different audio-vestibular profiles. Acta Paediatr Oslo Nor. (2021) 110:30–5. doi: 10.1111/apa.15561
68. D’Esposito, F, Randazzo, V, Cennamo, G, Centore, N, Maltese, PE, Malesci, R, et al. Novel USH1G homozygous variant underlying USH2-like phenotype of usher syndrome. Eur J Ophthalmol. (2021) 31:NP18–22. doi: 10.1177/1120672119879392
69. Wang, A, Shearer, AE, Zhou, GW, Kenna, M, Poe, D, Licameli, GR, et al. Peripheral vestibular dysfunction is a common occurrence in children with non-syndromic and syndromic genetic hearing loss. Front Neurol. (2021) 12:714543. doi: 10.3389/fneur.2021.714543
70. Pinninti, S, Christy, J, Almutairi, A, Cochrane, G, Fowler, KB, and Boppana, S. Vestibular, gaze, and balance disorders in asymptomatic congenital cytomegalovirus infection. Pediatrics. (2021) 147:e20193945. doi: 10.1542/peds.2019-3945
71. Kimura, Y, and Kaga, K. Comparison of vestibular ocular reflex and gross motor development in children with semicircular canal aplasia and hypoplasia. Int J Pediatr Otorhinolaryngol. (2022) 162:111303. doi: 10.1016/j.ijporl.2022.111303
72. Martens, S, Dhooge, I, Dhondt, C, Vanaudenaerde, S, Sucaet, M, Van Hoecke, H, et al. Three years of vestibular infant screening in infants with sensorineural hearing loss. Pediatrics. (2022) 150:e2021055340. doi: 10.1542/peds.2021-055340
73. Grijpink, LCM, Vossen, ACTM, Bruintjes, TD, Verbist, BM, Locher, H, and Rotteveel, LJC. Endolymphatic hydrops and fluctuating hearing loss in a patient with congenital cytomegalovirus infection. Otolaryngol Case Rep. (2023) 28:100552. doi: 10.1016/j.xocr.2023.100552
74. Velde, HM, Huizenga, XJJ, Yntema, HG, Haer-Wigman, L, Beynon, AJ, Oostrik, J, et al. Genotype and phenotype analyses of a novel WFS1 variant (c.2512C>T p.(Pro838Ser)) associated with DFNA6/14/38. Genes. (2023) 14:457. doi: 10.3390/genes14020457
75. Kokkola, E, Niemensivu, R, Lappalainen, M, Palomäki, M, Nieminen, T, Boppana, S, et al. Long-term outcome of vestibular function and hearing in children with congenital cytomegalovirus infection: a prospective cohort study. Eur Arch Otorrinolaringol. (2023) 280:3141–7. doi: 10.1007/s00405-022-07816-7
Keywords: congenital deafness, vestibular tests, deafness, vertigo, hypoacusia
Citation: Genovese E, Segato E, Liberale C, Zampieri E, Monzani D, Apa E, Palma S and Nocini R (2024) Congenital deafness and vestibular disorders: a systematic literature review. Front. Neurol. 15:1463234. doi: 10.3389/fneur.2024.1463234
Edited by:
Widdershoven Josine, Maastricht University Medical Centre, NetherlandsReviewed by:
Eyyup Kara, Istanbul University-Cerrahpasa, TürkiyePaz Pérez-Vázquez, Central University Hospital of Asturias, Spain
Copyright © 2024 Genovese, Segato, Liberale, Zampieri, Monzani, Apa, Palma and Nocini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlotta Liberale, Y2FybG90dGEubGliZXJhbGVAZ21haWwuY29t
 Elisabetta Genovese
Elisabetta Genovese Erika Segato2
Erika Segato2 Carlotta Liberale
Carlotta Liberale Silvia Palma
Silvia Palma Riccardo Nocini
Riccardo Nocini