
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 07 October 2024
Sec. Endovascular and Interventional Neurology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1455135
Introduction: Carotid artery stenting is an alternative interventional treatment to carotid endarterectomy. However, preprocedural considerations and anatomical risk factor analyses for carotid artery stenting are currently insufficient. Therefore, we investigated the high-risk anatomical appearance of carotid artery stenting from the neurointerventionist perspective to predict periprocedural complications.
Materials and methods: We retrospectively reviewed patients with carotid stenosis who underwent carotid artery stenting at a comprehensive stroke center between January 2012 and December 2021. We compared the demographic characteristics, medical history, and anatomical appearance of the stenotic segment in patients with and without complications.
Results: We analyzed a total of 148 patients (64 women [43.2%]; median age, 73.0 [interquartile range, 65.5–79.0]). Complications occurred in 39 of the 148 patients, primarily minor and transient. Of baseline or procedural characteristics, a high initial National Institutes of Health Stroke Scale score (p = 0.04), symptomatic stenosis (p = 0.01), and curve-centered plaque of the proximal ICA (p = 0.01) were significantly associated with carotid artery stenting complications in unadjusted analysis. Curve-centered plaque remained an independent risk factor for carotid artery stenting complications after adjustment (odds ratio 2.23[1.02–4.88], p = 0.04).
Conclusion: High-risk vascular anatomical features, such as curve-centered plaque, are associated with a high frequency of periprocedural complications of carotid artery stenting. Tailored patient selection for carotid stenosis is crucial to prevent complications. Patients with curve-centered plaque should consider alternative treatment options such as carotid endarterectomy to achieve optimal clinical results.
Carotid stenosis is a significant cause of ischemic stroke (1). Carotid endarterectomy (CEA) and carotid artery stenting (CAS) are widely performed to prevent ischemic stroke in patients with carotid stenosis (2). Many studies have reported the effectiveness and safety of CAS (3, 4). CAS offers the advantages of being less invasive, reduced potential for postoperative wound complications, reduced postoperative pain, and a short length of hospital stay. Therefore, the frequency of CAS has recently increased (5, 6).
Nevertheless, tortuosity around the targeted neck vessels, which is relatively common in the older population (7), could affect the clinical outcomes of CAS (8, 9). Most periprocedural complications occur during the delivery of the devices, such as microwires, embolic protection devices (EPDs), or carotid stent systems to the stenotic segment (10). Thus, risk evaluation of high-risk anatomical appearances before CAS is crucial for optimal clinical outcomes.
During carotid artery stenting, we experienced that the stent or balloon often got caught in the plaque when the plaque was located in the center if the curved proximal ICA. A curve-centered plaque (CCP) is a large plaque associated with stenosis located within the severely tortuous carotid artery, which is the lesion target of the stenting procedure. Severe stenosis with a large plaque in a severely curved vessel can pose a substantial challenge in CAS (9, 10). Few studies have investigated the impact of a high-risk anatomical appearance with extracranial ICA tortuosity on CAS (9, 10).
This study aimed to analyze the association between high-risk anatomical appearances of the carotid artery, such as CCP, and periprocedural complications of CAS. We evaluated only neurological and cardiovascular complications.
Consecutive carotid stenosis patients who underwent endovascular balloon angioplasty and stent placement at a comprehensive stroke center between January 1, 2012, and December 31, 2021, were retrospectively selected from a prospective neuro-intervention database and stroke registry. The local institutional review board approved this study, and the need for written informed consent was waived because of the study’s retrospective nature. According to our management protocol, CAS was considered in all patients presumed to require revascularization. However, CAS was not considered for patients who did not have procedural equipoise or vasculitis or those who underwent dissection and iatrogenic occlusion (e.g., surgery or endovascular treatment). CAS was also not performed when patients or their proxies did not provide consent or when a medical condition contraindicated CAS. All procedures were performed when severe (>70%) underlying proximal carotid artery atherosclerotic stenosis was observed according to the NASCET criteria on DSA. Additionally, duplex procedures confirmed the detection of a peak systolic velocity >230 cm/s before the duplex ultrasound (DUS). CAS for asymptomatic carotid stenosis was performed for progressive stenosis on follow-up DUS, severe stenosis with ulceration, and contralateral carotid occlusion. Patients with a modified Rankin Scale of 3 or less who underwent CAS for atherosclerotic stenosis of the proximal ICA were included in the analysis. Patients with tandem lesions, those who underwent stenting at locations other than the proximal ICA, and those with emergent CAS were excluded. Depending on periprocedural events and long-term follow-up, patients were classified into the complication or non-complication group (Figure 1).
Dual antiplatelet therapy (DAPT) with aspirin and clopidogrel was generally administered to treat CAS. The patients received 100 mg aspirin and 75 mg clopidogrel daily for at least 14 days before the procedure. DAPT was administered upon admission to patients who had undergone acute ischemic stroke. If bleeding complications were absent, DAPT was continued for at least 6 months postoperatively, and single antiplatelet therapy was continued as life-long treatment.
Patient data were retrospectively retrieved from a prospective database at our institute. The factors for comparison between the two groups were extracted from electronic medical records and included demographic characteristics (age, sex, and cerebrovascular risk factors), clinical characteristics (procedure-related information and modified Rankin scores at baseline and discharge), and radiographic and angiographic characteristics (complications, nature of stenosis, and devices).
All endovascular treatments were performed by three neurointerventionalists at our institution who treated all patients with carotid stenosis. Except on emergency cases, CAS was performed in two stages after confirming the stenotic lesion and its characteristics through digital subtraction angiography. All procedures were performed via the percutaneous transfemoral route under local anesthesia. After placement of the sheath introducer, heparin was administered intravenously to maintain an activated clotting time >2-fold above normal. EPDs were used in all the patients. Spider FX (Medtronic, Minneapolis, Minnesota, United States) or Emboshield Nav6 (Abbott Vascular, Abbott Park, Illinois, United States) was mainly used as EPDs. Rarely, the Mo.Ma Ultra Proximal Cerebral Protection Device (Medtronic, Minneapolis, Minnesota, United States) was used at the discretion of the neurointerventionists. Typically, a 6F Shuttle (Cook Medical Inc., Bloomington, Indiana, United States) or NeuroMax (Penumbra Inc., Alameda, California, United States) was employed for the procedure. Predilatation was generally performed with a 3.0–4.0 mm diameter angioplasty balloon, and postdilatation was performed with a 4.0–7.0 mm diameter angioplasty balloon. A neurointerventionist determined the diameters of the balloon catheters. The stent was deployed in all cases, and the type of stent was determined at the discretion of the neurointerventionists. After dilatation, conventional angiography was repeated to evaluate periprocedural complications, including in-stent thrombosis or flow limitations. Finally, intracranial angiography was performed to confirm distal thrombus embolization.
The final diagnosis of severe carotid artery stenosis was based on DUS, with peak systolic velocity and B-mode plaque images. All patients underwent conventional CT or MRI, including contrast-enhanced angiography of the carotid vessels. Two neuroradiologists who were blinded to the available clinical data independently assessed all images. Any discrepancies were resolved by consensus.
The anatomic characteristics of the lesions were analyzed individually via neuroimaging (MRA, CTA, DUS, and DSA) before the procedure. Neuroimaging was performed to assess the lesion shape, location, ulceration, calcification, degree of stenosis, lesion length, and contralateral carotid artery status. Using the DUS B-mode test, plaque characteristics were classified as hypoechoic, isoechoic, or hyperechoic. The ICA tortuosity index (TI) was calculated for all the available angiograms. The ICA TI was determined based on a 3-point system that measured the degree of deviation of the internal carotid from the axis between the common and distal internal carotid arteries. The TI was measured to determine the morphology of the segment between the initiation of the ICA and the sites of the EPDs. The TI was defined as the sum of all angles diverging from the ideal straight axis. Angle α was defined as the angle between the initial ICA’s axis and the ICA’s tangent. Angle β was defined as the angle between the axes of the first and second segments of the ICA. The sum of angle α and β was defined as TI (10). CCP was defined as a large plaque associated with stenosis located within the severely tortuous carotid artery and was the lesion target of the stenting procedure (Figures 2A–D). The ostial-centered lesion was defined as the location of maximal stenosis in the internal carotid ostium (11). Three neurointerventionists retrospectively evaluated all DSA images for the following anatomical higher-risk appearances: high ICA TI, CCP, degree of carotid stenosis, lesion location (carotid bulb or supra-bulb), ostial-centered lesion, length of stenosis >15 mm, and ulceration and calcification. Additionally, the echogenicity (hypoechoic, isoechoic, or hyperechoic) of the plaque on DUS was evaluated.
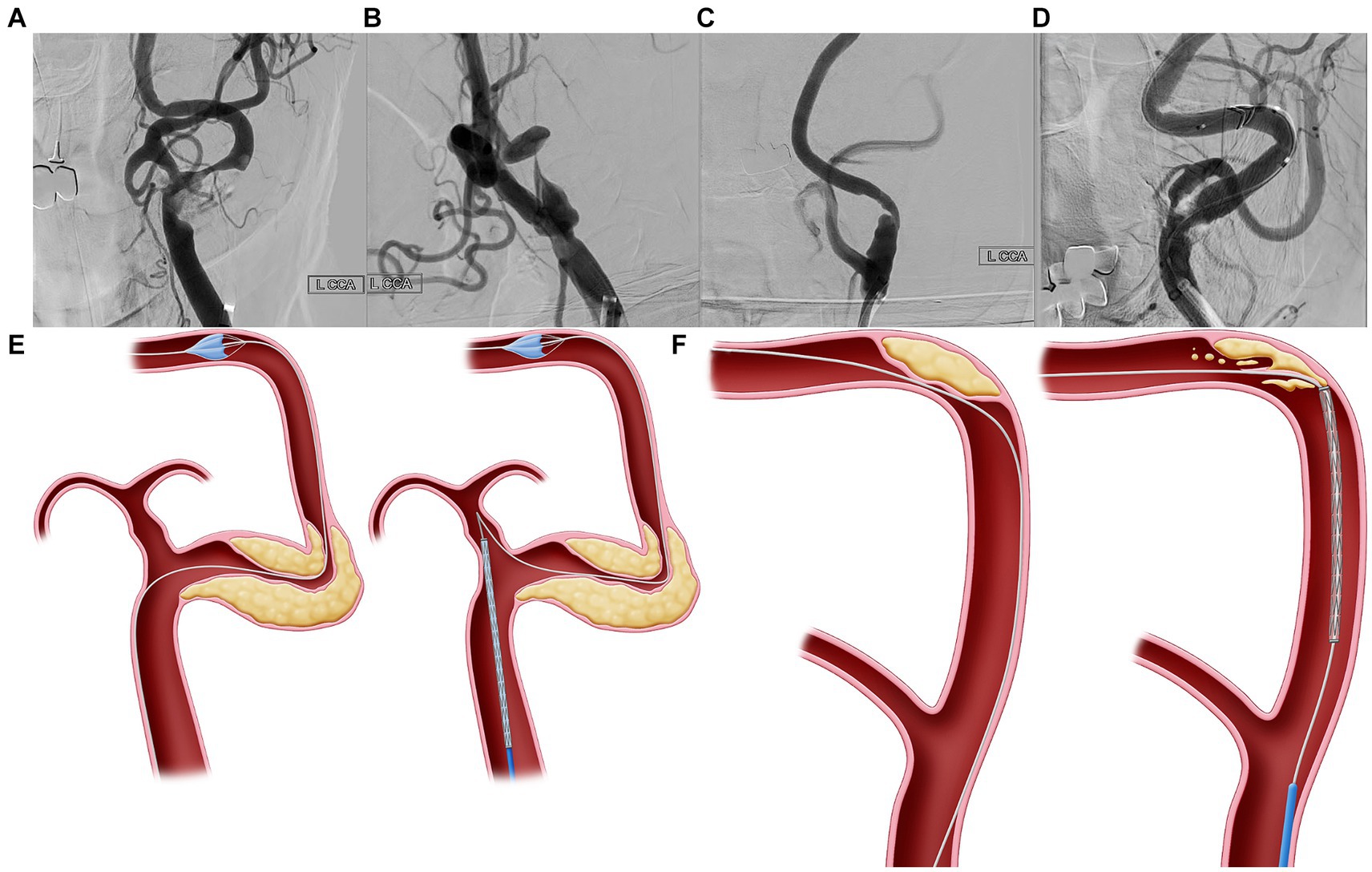
Figure 2. Curve-centered plaque. (A–D) Curve-centered plaque (CCP) is a plaque associated with stenosis located within the severe tortuous carotid artery, which is the lesion target of the stenting procedure. (E) In vascular structures with severe curves, when delivering the device through a wire which has advanced to the distal segment, the devices with wire may fall into the external carotid artery due to failure to overcome the angle. Thus, since the delivery of devices is impossible, there is no choice but to advance the guiding system to the stenotic area. In this case, more than necessary power is accumulated in the guiding catheter during device delivery, which can possibly lead to dangerous or uncontrolled events. (F) Second, because the plaque is located at the center of the bent carotid artery, the CAS device cannot be advanced along the wire and can possibly dig into the plaque. If the interventionist pushed more of the device to pass through the bent vessel, the wire would adhere more closely to the center of the curve where the plaque was located, potentially increasing the risk of periprocedural complications.
Stroke neurologists re-evaluated the neurological status after the procedure, and the patients were monitored in the neurointensive care unit for 1 day. All patients were evaluated with simple diffusion-weighted images immediately after the procedure. Patients with a >2-point increase in the National Institutes of Health Stroke Scale (NIHSS) were evaluated using non-contrast brain CT or MRI. DUS were performed to evaluate the restenosis at discharge. Clinical outcomes were assessed by measuring mRS scores at 90 days. Other clinical and imaging outcomes evaluated included early neurological deterioration (END), length of hospital stay, re-stenosis on follow-up imaging, and periprocedural complications. DUS was performed before the procedure and at 1, 6, and 18 months after the intervention. Procedure time was defined as the interval from the puncture to the final angiography.
Periprocedural complications were defined as newly developed neurological deterioration or abnormal neuroimaging features that developed within 1 week after CAS. We regarded any neurological symptom, including ischemic stroke, a malpositioned stent, vasospasm, dissection, myocardial infarction, distal occlusion, an asymptomatic DWI lesion, hyperperfusion/reperfusion events, postprocedural headache, ocular symptoms, instant thrombosis, pseudoaneurysm, and any cardiorespiratory arrest after the procedure as complications of CAS. Cardiorespiratory arrest was defined as a new condition requiring endotracheal intubation or chest compression within 1 day after CAS.
In addition to those mentioned above, complications associated with CAS include access site complications such as hematoma and infection, and contrast medium-induced nephropathy.
We compared baseline characteristics, clinical status, procedural characteristics, and clinical outcomes between the patients who experienced complications and those who did not. Differences in baseline categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test. In contrast, differences in continuous variables were compared using Student’s t-test or the Mann–Whitney U-test, as appropriate. Multivariate logistic regression analysis was performed to identify the independent variables contributing to complications. Variables with a p-value <0.2 in a univariate analysis were included as candidate variables in a multivariate analysis and removed by backward stepwise selection. Additional forward selection analyses confirmed the final model. Statistical significance was defined as a two-tailed p-value of <0.05. All statistical analyses were performed using the R statistical software (version 2.14.0; R Foundation for Statistical Computing, Vienna, Austria).
Over 10 years, 218 carotid stenting procedures were performed, of which 148 met the inclusion criteria (Figure 1). A total of 148 patients (64 women [43.2%]; median age, 73.0 [interquartile range, 65.5–79.0]) were analyzed (Table 1). There were no differences between the two groups regarding age, sex, the baseline mRS score, or underlying disease. However, the complications group had a higher initial NIHSS score than did the non-complications group, and the proportion of patients with symptomatic stenosis was higher in the complications group than in the non-complications group.
There was no difference between the two groups concerning the devices used, ICA TI, degree of stenosis, ulceration, echogenicity, or contralateral carotid artery occlusion; however, the frequency of CCP was significantly higher in the complication group than in the non-complication group (Table 2).
Complications occurred in 39 (26.3%) of 148 patients, most of which were minor and transient. Asymptomatic DWI high-signal lesions were the most common complication, occurring in 12 (30.8%) patients. Ischemic stroke occurred in six (15.4%) patients, but all had minor symptoms, and no patients had sequelae after 3 months.
When comparing the clinical outcomes between the two groups, there were no significant differences in the prevalence of re-stenosis, mRS at 90 days, or mortality at 90 days. However, the procedure and hospitalization periods were longer in the complications group than in the non-complications group (Table 3).
The univariate analysis revealed that symptomatic stenosis, CCP, and procedure time were associated with periprocedural complications. However, in the multivariate analysis, only CCP was an independent risk factor for periprocedural complications of CAS (Table 4).
A CCP is a large plaque associated with stenosis located within the severely tortuous carotid artery and is the lesion target of the stenting procedure. Delivering the CAS system through a CCP to avoid plaque irritation during the procedure is challenging. Our study showed that complications occurred more frequently in cases with CCP at the proximal ICA. Thus, we suggest a more specific concept of ICA tortuosity to predict periprocedural complications during CAS.
The CAS is currently performed as an alternative interventional treatment option for CEA (12). It is an effective and safe procedure for increasing blood flow through a previously blocked artery and reducing the risk of stroke without requiring general anesthesia or open surgery (13, 14). However, preprocedural considerations and anatomical risk factor analyses for CAS are currently insufficient. We investigated the risk factors that predict periprocedural complications from the standpoint of neurointerventionists. In our study, periprocedural complications occurred in 26.3% (39/148) of the CAS procedures performed over 10 years. The incidence of complications was relatively frequent, probably because the researchers included complications that were minor or could be ignored. Thus, it rarely lasted for 90 days, or it caused severe neurological deficits.
The CAS is non-inferior to CEA regarding the efficacy and safety of carotid revascularization (3, 4, 15). It offers the advantages of simpler and shorter procedure than that of CEA (13). Nevertheless, minor and non-disabling stroke incidence is more frequently reported in patients who undergo CAS than in those who undergo CEA, especially during the perioperative period (3, 4, 12, 16). Long-term procedure-related deaths or strokes are more frequent in the CAS group than in the CEA group, although the differences are minor (4). There have been several studies on the impact of extracranial ICA tortuosity on the clinical outcome of CAS (8, 10). However, screening patients for CAS according to ICA tortuosity does not provide clear guidelines or distinctive features in clinical practice.
Specifically, two major problems occur during CAS in the carotid artery with CCP. In vascular structures with severe curves, when delivering the device through a wire that has advanced to the distal segment, the devices with the wire may fall into the external carotid artery due to failure to overcome the angle (Figure 2E). Thus, because the delivery of devices is impossible, the guiding system must be advanced toward the stenotic area. In this case, more than necessary power accumulates in the guiding catheter during device delivery, leading to dangerous or uncontrolled events. Second, because the plaque is located at the center of the bent carotid artery, CAS devices cannot be advanced along the wire and can dig into the plaque (Figure 2F). If the interventionist pushed more of the device to pass through the bent vessel, the wire would adhere more closely to the center of the curve where the plaque was located, potentially increasing the risk of periprocedural complications.
The present study had several limitations. First, the inherent biases owing to the single-center retrospective design, with a relatively small number of patients in each group, affected the results. Second, the definition of CCP is still non-specific and vague because the precise angle of the carotid artery was not determined. Nevertheless, CCP is a more intuitive and practical tool for screening high-risk CAS groups. Third, we only assessed the tortuosity of the extracranial ICA; the tortuosities of other relevant vessels, including the aortic arch and common carotid artery, were not assessed. In addition, we assessed the vessel anatomy based on anteroposterior and lateral projection without three-dimensional imaging, which can provide the most precise angle measurements. However, our study was designed to pursue a practical situation without advanced neuroimaging. Above all, the 10-year study period may affected the results, associated with developing medications and devices. Nevertheless, no significant novel therapeutic advances have been made in the endovascular treatment of carotid artery stenosis for about 10 years.
Furthermore, clinical outcomes are affected by other factors, including the skill or experience level of the neurointerventionist and the type of device selected. Therefore, further studies with larger sample sizes are warranted. Nevertheless, in patients with CCP, it might be better to consider CEA rather than CAS, and developing devices and techniques to overcome CCP is necessary.
In conclusion, although minor and transient, CCP was independently associated with a high frequency of periprocedural complications during CAS. Careful patient screening is crucial for improving the outcomes of CAS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Jeju National University Hospital/JEJUNUH2023-01-018. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
C-HK: Conceptualization, Data curation, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing. J-KR: Investigation, Resources, Supervision, Writing – review & editing. HK: Data curation, Formal analysis, Methodology, Project administration, Writing – review & editing. JC: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing. J-GK: Conceptualization, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by a research grant from Jeju National University Hospital in 2020.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CEA, carotid endarterectomy; CAS, carotid artery stenting; EPD, embolic protection device; CCP, curve-centered plaque; DSA, digital subtraction angiography; DUS, duplex ultrasound; DAPT, dual antiplatelet therapy; NIHSS, National Institutes of Health Stroke Scale.
1. Petty, GW, Brown, RD Jr, Whisnant, JP, Sicks, JD, O'Fallon, WM, and Wiebers, DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. (1999) 30:2513–6. doi: 10.1161/01.STR.30.12.2513
2. Writing Group MembersMozaffarian, D, Benjamin, EJ, Go, AS, Arnett, DK, Blaha, MJ, et al. Heart disease and stroke Statistics-2016 update: a report from the American Heart Association. Circulation. (2016) 133:e38–e360. doi: 10.1161/CIR.0000000000000350
3. Bonati, LH, Dobson, J, Algra, A, Branchereau, A, Chatellier, G, Fraedrich, G, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet. (2010) 376:1062–73. doi: 10.1016/S0140-6736(10)61009-4
4. Halliday, A, Bulbulia, R, Bonati, LH, Chester, J, Cradduck-Bamford, A, Peto, R, et al. Second asymptomatic carotid surgery trial (ACST-2): a randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet. (2021) 398:1065–73. doi: 10.1016/S0140-6736(21)01910-3
5. Lichtman, JH, Jones, MR, Leifheit, EC, Sheffet, AJ, Howard, G, Lal, BK, et al. Carotid endarterectomy and carotid artery stenting in the US Medicare population, 1999-2014. JAMA. (2017) 318:1035–46. doi: 10.1001/jama.2017.12882
6. Suh, SH. The annual trends between Neurointerventional and neurosurgical procedures in Korea: analysis using HIRA data from 2010 to 2016. Neurointervention. (2017) 12:77–82. doi: 10.5469/neuroint.2017.12.2.77
7. Togay-Isikay, C, Kim, J, Betterman, K, Andrews, C, Meads, D, Tesh, P, et al. Carotid artery tortuosity, kinking, coiling: stroke risk factor, marker, or curiosity? Acta Neurol Belg. (2005) 105:68–72.
8. Vatan, MB, Acar, BA, Aksoy, M, Can, Y, Varim, C, Agac, MT, et al. Predictors of periprocedural complications of carotid artery stenting – a multivariate analysis of a single-Centre experience. Vasa. (2016) 45:387–93. doi: 10.1024/0301-1526/a000550
9. Faggioli, G, Ferri, M, Gargiulo, M, Freyrie, A, Fratesi, F, Manzoli, L, et al. Measurement and impact of proximal and distal tortuosity in carotid stenting procedures. J Vasc Surg. (2007) 46:1119–24. doi: 10.1016/j.jvs.2007.08.027
10. Wang, QZ, Liu, CL, Yan, B, Fan, XB, Zhang, M, Li, YK, et al. Correlation of extracranial internal carotid artery tortuosity index and Intraprocedural complications during carotid artery stenting. Eur Neurol. (2012) 68:65–72. doi: 10.1159/000337682
11. Sayeed, S, Stanziale, SF, Wholey, MH, and Makaroun, MS. Angiographic lesion characteristics can predict adverse outcomes after carotid artery stenting. J Vasc Surg. (2008) 47:81–7. doi: 10.1016/j.jvs.2007.09.047
12. Muller, MD, Lyrer, PA, Brown, MM, and Bonati, LH. Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis. Stroke. (2021) 52:E3–5. doi: 10.1161/STROKEAHA.120.030521
13. Brown, MM, Rogers, J, Bland, JM, and Investigators, C. Endovascular versus surgical treatment in patients with carotid stenosis in the carotid and vertebral artery transluminal angioplasty study (CAVATAS): a randomised trial. Lancet. (2001) 357:1729–37. doi: 10.1016/S0140-6736(00)04893-5
14. Nahser, HC, and Investi, ICSS. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (international carotid stenting study): an interim analysis of a randomised controlled trial. Lancet. (2010) 376:985. doi: 10.1016/S0140-6736(10)60239-5
15. Gurm, HS, Yadav, JS, Fayad, P, Katzen, BT, Mishkel, GJ, Bajwa, TK, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. (2008) 358:1572–9. doi: 10.1056/NEJMoa0708028
16. Bonati, LH, Dobson, J, Featherstone, RL, Ederle, J, van der Worp, HB, de Borst, GJ, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the international carotid stenting study (ICSS) randomised trial. Lancet. (2015) 385:529–38. doi: 10.1016/S0140-6736(14)61184-3
Keywords: carotid stenosis, carotid stenting, stroke, complication, carotid angiography
Citation: Kang C-H, Rhim J-K, Kim HJ, Choi JC and Kim J-G (2024) Curve-centered plaques raise the risk of peri-operative neurological and cardiovascular complications during angioplasty and stenting for severe carotid stenosis. Front. Neurol. 15:1455135. doi: 10.3389/fneur.2024.1455135
Received: 26 June 2024; Accepted: 05 September 2024;
Published: 07 October 2024.
Edited by:
Sherief Ghozy, Mayo Clinic, United StatesReviewed by:
Sandeep Nayak, Yale New Haven Health System, United StatesCopyright © 2024 Kang, Rhim, Kim, Choi and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joong-Goo Kim, bGlsaXMxMTE4QG5hdmVyLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.