
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 05 November 2024
Sec. Neurocritical and Neurohospitalist Care
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1433025
This article is part of the Research Topic The Role of Environmental Stressors in Neurocritical Patient Outcomes View all 10 articles
 Liren Hu1,2†
Liren Hu1,2† Sirui Geli1,2
Sirui Geli1,2 Feiyu Long1,2
Feiyu Long1,2 Liang Nie3
Liang Nie3 Jiali Wu1,2
Jiali Wu1,2 Jun Zhou1,2
Jun Zhou1,2 Maohua Wang1,2*
Maohua Wang1,2* Yingxu Chen1,2*
Yingxu Chen1,2*Introduction: A bibliometric analysis is used to assess the impact of research in a particular field. However, a specialized bibliometric analysis focused on hypothermic brain protection has not yet been conducted. This study aimed to identify the 100 most-cited articles published in the field of hypothermic brain protection and analyze their bibliometric characteristics.
Methods: After screening articles from the Web of Science citation database, complete bibliographic records were imported into Python for data extraction. The following parameters were analyzed: title, author’s name and affiliation, country, publication year, publication date, first author, corresponding author, study design, language, number of citations, journal impact factors, keywords, Keywords Plus®, and research topic.
Results: The 100 articles were published between 1990 and 2016. The citation frequency for each publication ranged from 86 to 470. Among the 100 articles, 73 were original articles, 18 were review articles, 8 were clinical articles, and 1 was editorial material. These papers were published in 37 journals, with the Journal of Cerebral Blood Flow and Metabolism being the most prolific with 15 papers. Eighteen countries contributed to the 100 publications, 51 of which were from United States institutions. In addition, the keywords in the Sankey plot indicated that research in the field of hypothermic brain protection is growing deeper and overlapping with other disciplines.
Discussion: The results provide an overview of research on hypothermic brain protection, which may help researchers better understand classical research, historical developments, and new discoveries, as well as providing ideas for future research.
• The unique advantage of this study is that it is the first bibliometric study to identify and characterize articles in the field of hypothermic brain protection in all journals of the Science Network (SCIE).
• Most bibliometric studies exclude nonprofessional journals.
• We generated a more comprehensive list of the 100 most-cited articles in the field of hypothermic brain protection by including all journals in the analysis.
Hypothermic brain protection is an important technique used in neurosurgery to mitigate ischemic and hypoxic injuries. Moreover, lowering the temperature of the brain tissue can protect neurological function (1). Recent research has focused on understanding the mechanisms of hypothermic brain protection and its ability to protect brain functions (2). Hypothermia inhibits the generation of free radicals, reduces apoptosis, and stabilizes cell membrane integrity (3). Clinical methods for hypothermic brain protection include surface, nasopharyngeal, and intravenous cooling (4). However, hypothermia can also lead to complications, such as immunosuppression and renal failure, highlighting the need for further investigation to determine the optimal hypothermic brain protection protocol (5).
Several related studies in the field of hypothermic brain protection have been published in various journals. Analysis of these articles is crucial to evaluate their impact on basic research, clinical practice, and the surgical profession. Bibliometric analysis is an excellent method to assess this impact (6).
Bibliometrics is a discipline that uses quantitative and statistical methods to analyze scientific literature (7). It provides valuable information and reveals the laws and trends in the development of a particular scientific discipline (8). In neurological surgery, bibliometric analysis has been widely applied to analyze research hotspots and developmental trajectories of various diseases, including cerebral aneurysms, stroke, and traumatic brain injury (9–11). This analysis provides guidance for future research directions and resource allocation in the field of neurosurgery. However, specialized bibliometric analyses have not yet been performed in the field of hypothermic brain protection.
Therefore, this study aimed to identify the 100 most-cited articles published in the field of hypothermic brain protection using bibliometric methods. We analyzed their bibliometric characteristics to provide insights into the developments in this field.
This study analyzed and described previously published articles; therefore, no ethical approval was required.
The Clarivate Analytics’ Web of Science (WOS) (1980–present) citation database was used as the data source to identify articles in the field of hypothermic brain protection and track their citations. Considering the broad range of topics covered in the articles on hypothermia-related brain protection, we conducted a pre-search to determine the best search formula. The last search was conducted on August 21, 2023, using the expressions detailed in Supplementary Table S1. All obtained references, including bibliographic and citation data, were exported from the database and subsequently imported into document management software (Zotero, 6.0.30; https://www.zotero.org/) to remove duplications and screen them. The search strategy produced 1,847 records, listed in descending order, based on the number of citations retrieved from the source database.
Two independent researchers (Geli and Huang) screened the literature in the database in descending order based on WOS citations. Literature in the field of non-hypothermic brain protection was excluded based on the title and abstract. For uncertain articles, the full text was obtained for accurate inclusion or exclusion determination. During the study selection process, discrepancies between investigators were resolved by a third investigator (Hu). The evaluation was terminated at the 100th paper with the highest number of citations. Finally, the 100 most-cited articles in the field of hypothermic brain protection were listed for further analysis. Complete bibliographic records were exported from the WOS in plain text or Excel (Microsoft Corporation, Redmond, WA, United States) format and imported into Python (version 3.11.5; https://www.python.org/downloads/release/python-3115/) for data extraction.
The following information was extracted using Python and stored in Microsoft Excel (Microsoft Corporation): article title, author, abstract, keywords, year of publication, published journal, cited references, PMID, DOI, total citations, and annual average citations. We also determined the impact factor (IF) of the articles based on the currently published journal citation report (JCR® IF 2023).
Descriptive statistics of the selected articles were analyzed using Microsoft Excel 2023 (Microsoft Corporation), Python (version 3.11.5), and VOSviewer [version 1.6.20; developed by van Eck and Waltman (12)], a literature knowledge visualization software for constructing a bibliometric network. Before data analysis, the obtained data were standardized. All authors were checked through their institutions or email addresses to eliminate potential confusion from authors sharing the same names and initials, ensuring that they were specific individuals. The names of all institutions, such as universities and research centers, were reviewed and included at the same level, whereas the individual departments or research units under them were removed. Articles from Northern Ireland and Britain were considered to be from the United Kingdom. Different keywords with the same meaning were merged into one term (e.g., head injury and brain injury were collectively classified as brain injury). Some authors in the top 20 articles did not provide keywords (n = 7), and three independent researchers discussed and determined the keywords for these articles based on Keywords Plus®. All references were standardized to create a unified list. Excel (Microsoft Corporation) was used to list the basic characteristics of the selected documents in a tabular form. The bibliometric network was graphically generated using the VOSviewer software (12). In a network visualization map, different nodes represent different elements such as countries, institutions, authors, or terms. The links between nodes represent relationships such as coauthors, co-citations, or co-occurrences, and are weighted by the total link strength (13). Python (version 3.11.5) and R (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria) were used to construct radar charts describing article types and publication years and a Sankey plot was created to describe the relationships among authors, countries, and keywords. The packages used in Python and R are listed in Supplementary Table S2. The research process is shown in Figure 1.
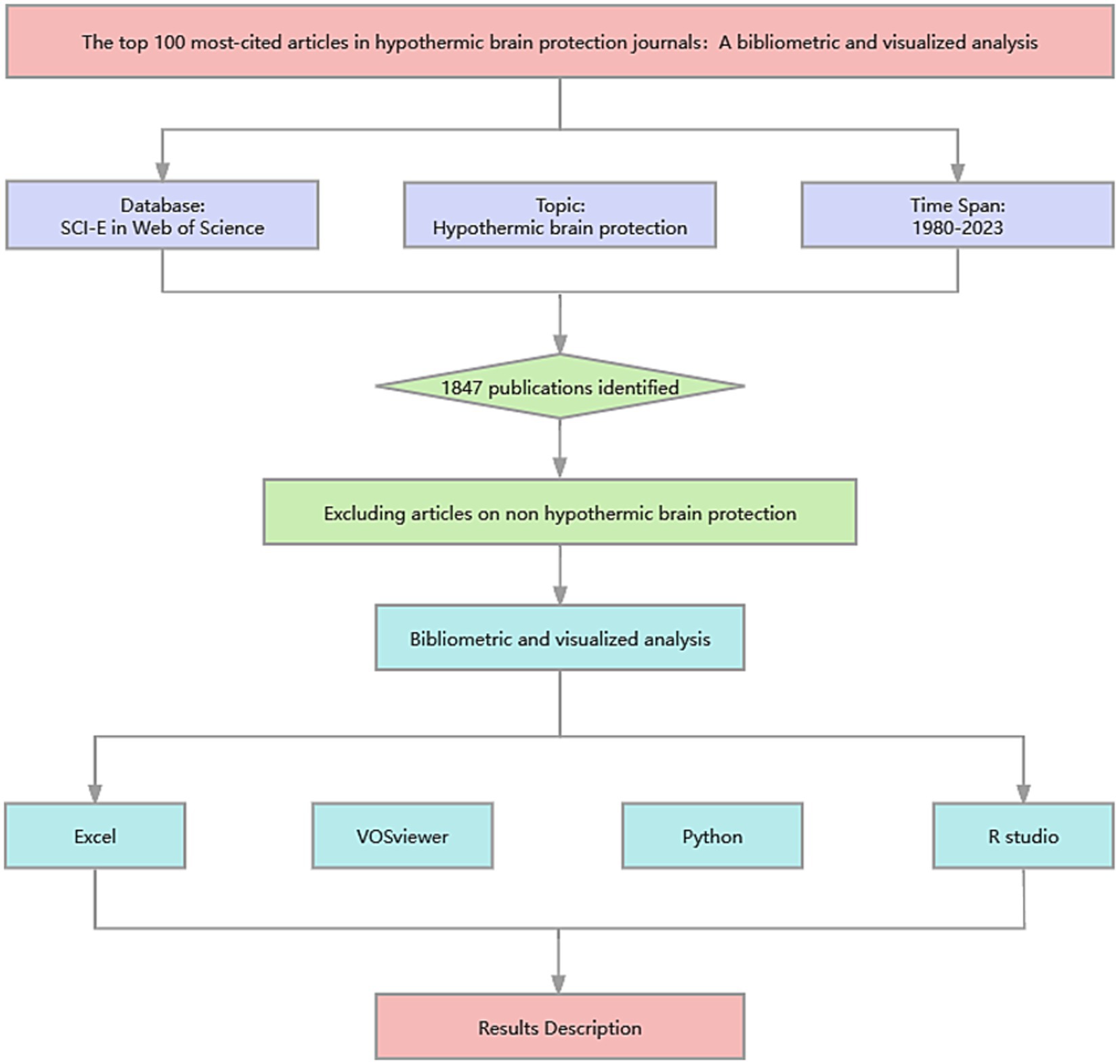
Figure 1. Study flow diagram created using ProcessOn.com. The Web of Science (WOS) (1980–present) citation database was used to identify articles in the field of hypothermic brain protection. The search strategy yielded 1,847 records, with the final selection of 100 articles made by the researchers. Data were then standardized and imported into Excel, VOSviewer, Python, and R Studio for bibliometric and visual analyses.
The 100 most-cited articles from all journals were published between 1990 and 2016, as shown in Table 1, and are listed in descending order based on the total WOS citation count on the search day (1, 14–112). Additionally, the table includes the year of publication, journal name, IF, first author, corresponding author, and average citations per year count based on WOS data.
The citation frequency of these 100 studies ranged from 86 to 470 times (mean = 156), with the average annual citation volume ranging from 2.9 to 19.4 times (mean = 7.3). The median publication year for these articles is 1999. Approximately one-fifth of the articles (n = 18) were cited more than 200 times, and only six articles were cited more than 400 times. The most-cited article, titled “Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia” was published by Globus et al. (14) in the Journal of Neurochemistry in 1995 and has 470 citations to date.
We also created a network visualization based on the number of citations per article (Figure 2). Globus et al. (14), Clifton et al. (15), Kuboyama et al. (16), Ginsberg et al. (17), Dietrich et al. (18), and Colbourne et al. (19) all received more than 400 citations, making them the largest nodes in the graph. Todd et al. (20) and Erecinska et al. (21) also received a high number of citations.
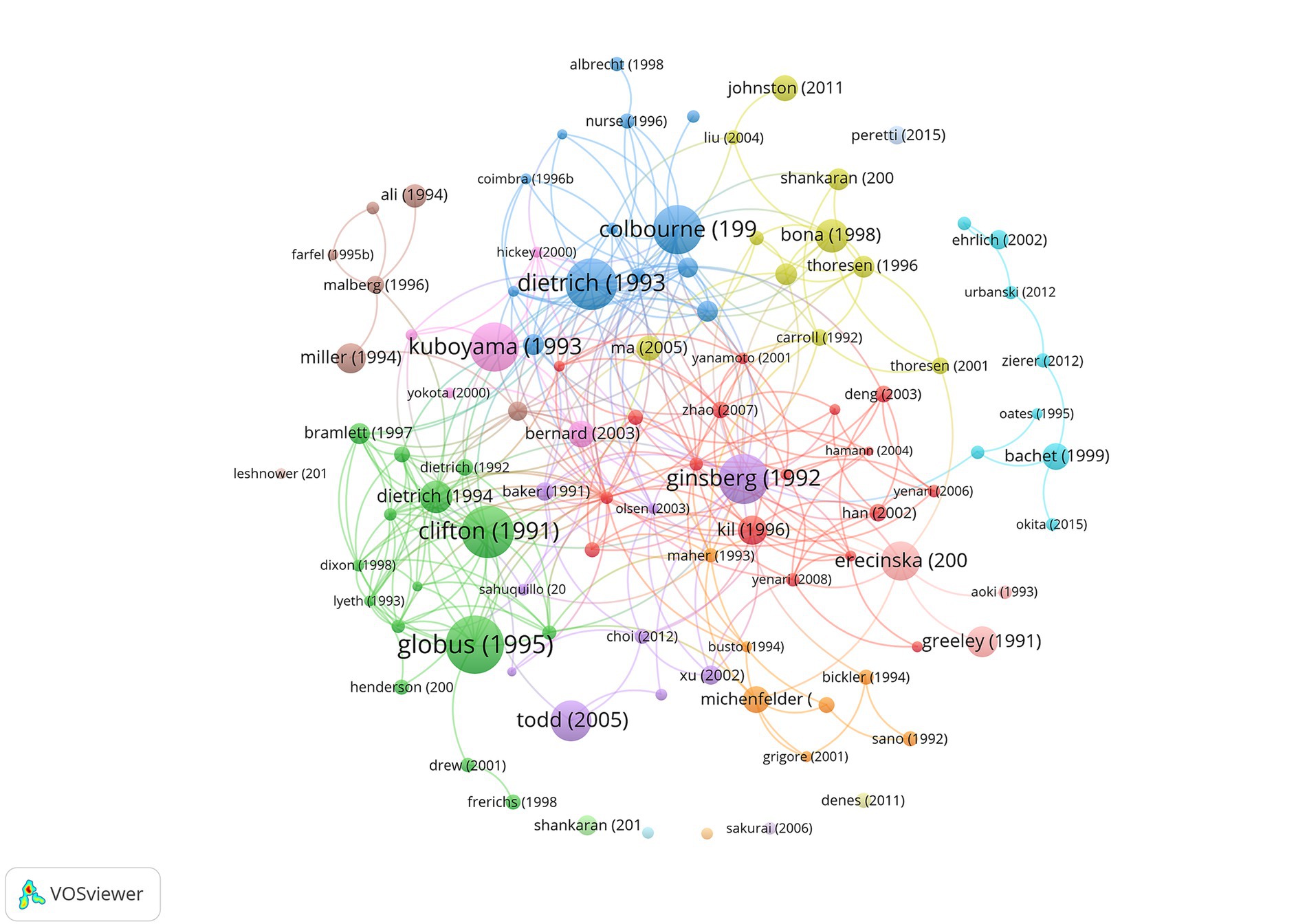
Figure 2. Citation of documents in hypothermic brain protection journals created using VOSviewer. Labels and circles were used to represent articles, representing authors’ name and year of publication indicated. The size of each label and circle was based on the weight of the article.
Among the 100 most-cited articles, 1991–1996 had the most publications (n = 42) (Figure 3), with the highest number of publications occurring in 1996 (n = 9) and a smaller peak occurring in 2007 (n = 6). The lowest numbers of publications occurred in 1990, 1997, 2010, and 2016 (n = 1). Among the types of published articles, randomized controlled trials were the most common (n = 73), followed by reviews (n = 18), whereas case reports, clinical studies, and other types of articles were the least common (n = 9) (Table 2).
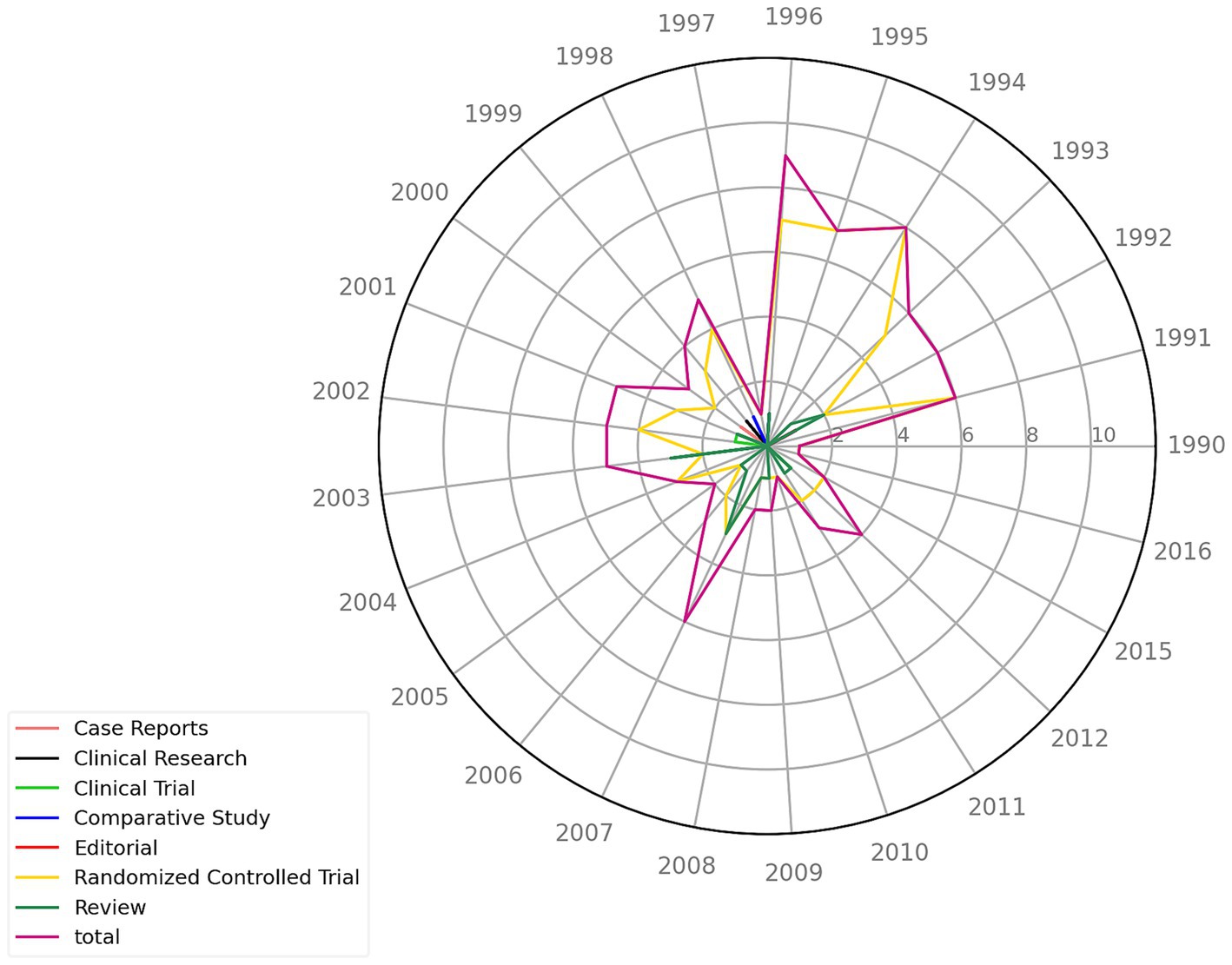
Figure 3. The 100 most-cited articles by publication year. The radar chart depicts the publication years of articles, spanning from 1990 to 2016. Each colored line represents a different type of article, with the outermost purple line representing the total number of publications per year.
We established a collaborative network based on the authors who published two or more papers (Figure 4). Dietrich, Ginsberg, and Busto published the most relevant articles; therefore, their nodes were the largest. In addition, we observed close collaboration among multiple authors. For example, Dietrich closely cooperated with Busto, Alonso, and others (Figure 4A). More detailed and specific collaborations between the authors can be found in the author coupling diagram (Figure 4B). There are five main color classifications, with yellow representing authors such as Dietrich, Busto, and Ginsberg, who have the highest collaborations. There are many collaborations among the authors, represented in green, such as Markgraf, Clifton, and Marion. The authors represented in red, such as Yenari, Steinberg, Chan, and Graham, strongly cooperated with each other. The authors represented in blue, such as Colbourne, Wieloch, Corbett, and Yanamoto, strongly cooperated with each other. Purple represents authors such as Thoresen, Loberg, and Chakkarapani, who participated in many collaborations.
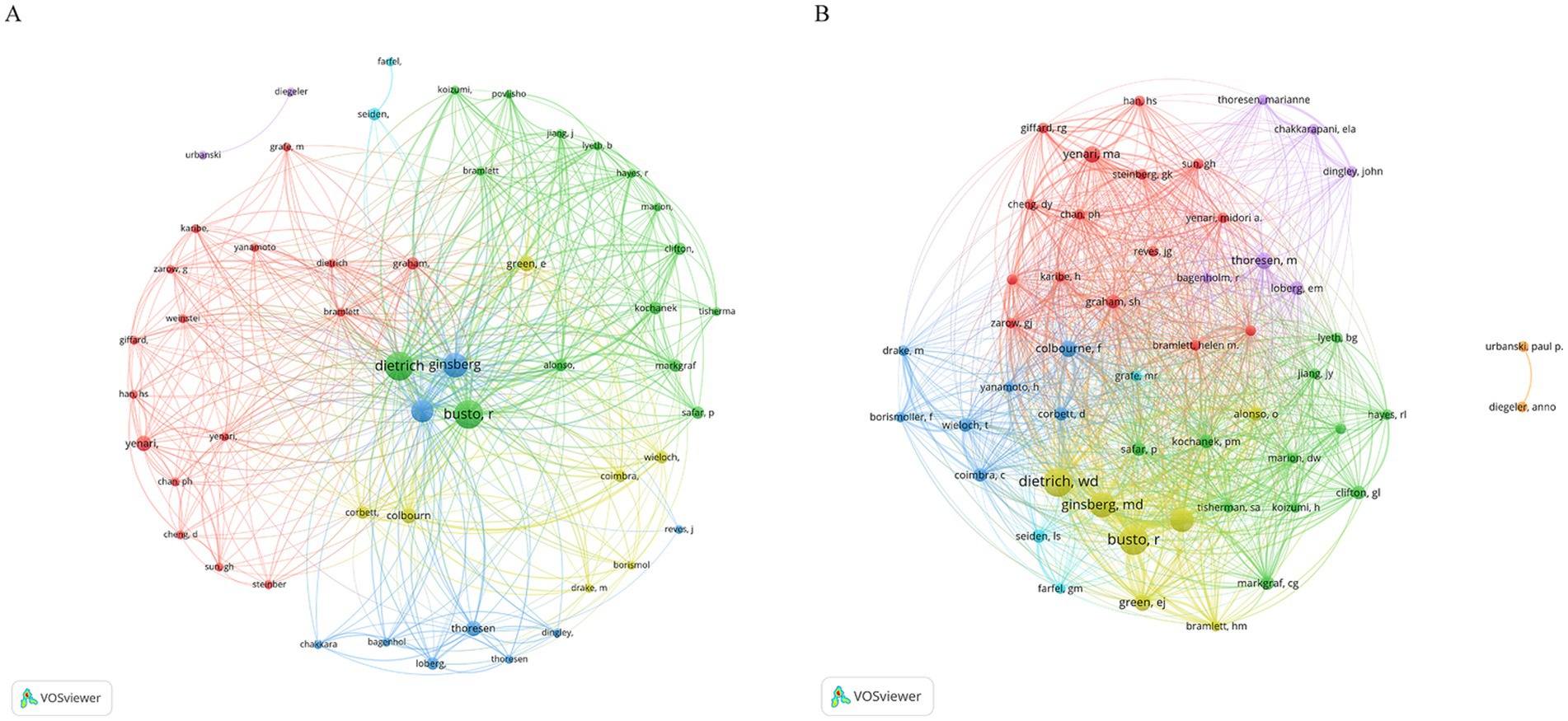
Figure 4. The author network visualized using VOSviewer software. The minimum number of documents by any one author is ≥2. Author citation (A) and the bibliometric coupling (B) are shown using labels and circles, showcasing each author’s name. The size of the label and circle varied according to the author’s weight.
Visual networks and statistical charts were developed for both countries and their institutions. The 100 most-cited articles included 136 countries/regions and 258 institutions. The maps clearly indicated the presence of clusters. It is evident from the number of publications and international cooperation that the United States has the greatest influence among many countries. In addition, Japan has established strong scientific relationships with countries such as Germany, Canada, and Sweden (Figure 5). Among the countries that published articles, the United States (n = 51), the United Kingdom (n = 8), and Japan (n = 8) had the highest number of published articles, whereas the remaining articles were scattered among other countries (Table 3).
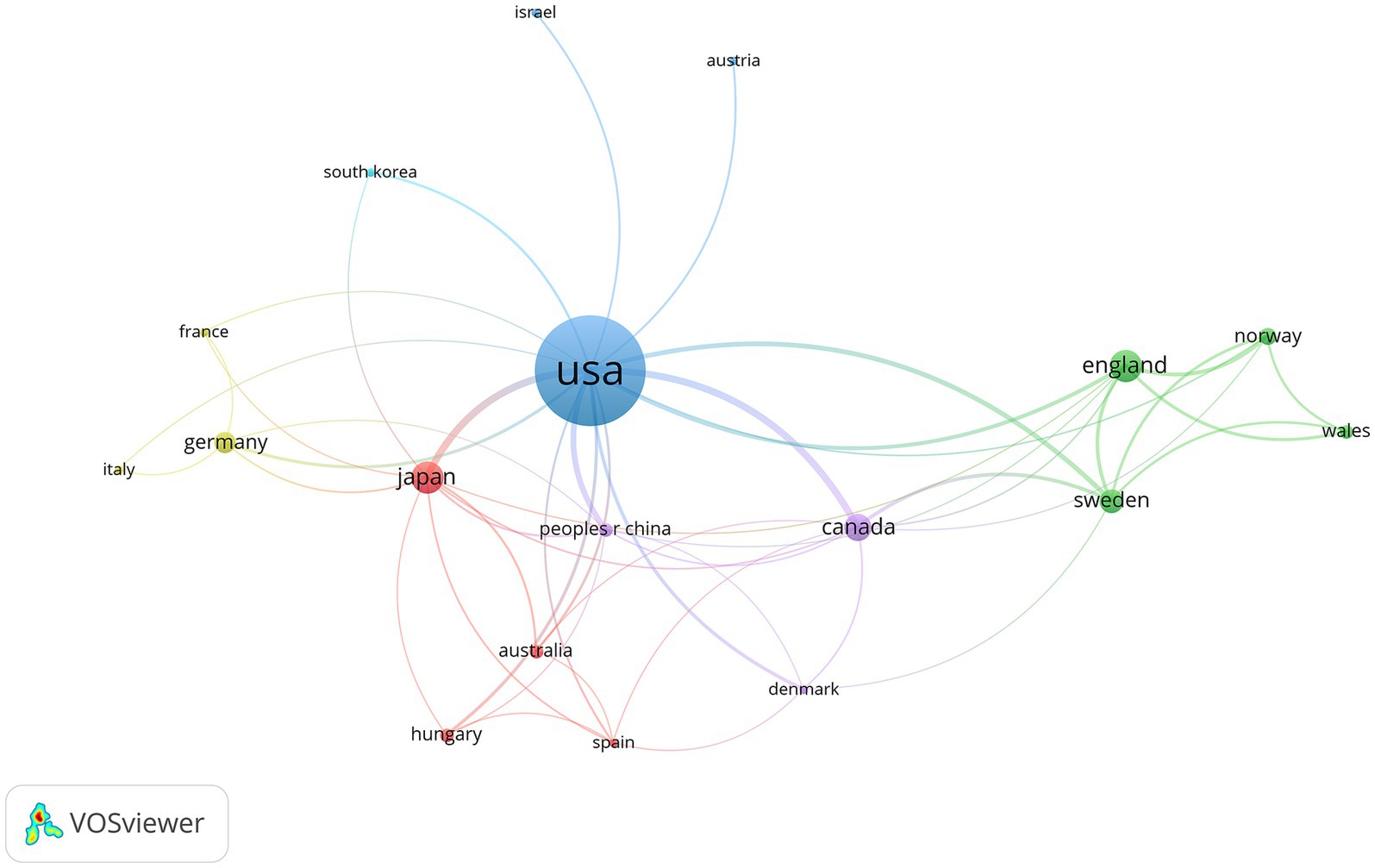
Figure 5. Countries cited in the 100 most-cited articles on hypothermic brain protection created by VOSviewer. The minimum number of documents from any one country is ≥1. Countries are represented by labels and circles, with the size of each country’s label and circle determined by its weight.
Multiple institutions also formed regional cooperative networks. The University of Miami has close cooperation with the University of Texas, the University of Pittsburgh, and others. Stanford University has close cooperation with Duke University, the University of California, San Francisco University, and others. The Memorial University of Newfoundland cooperates closely with Emory University, Yale University, and others (Figure 6).
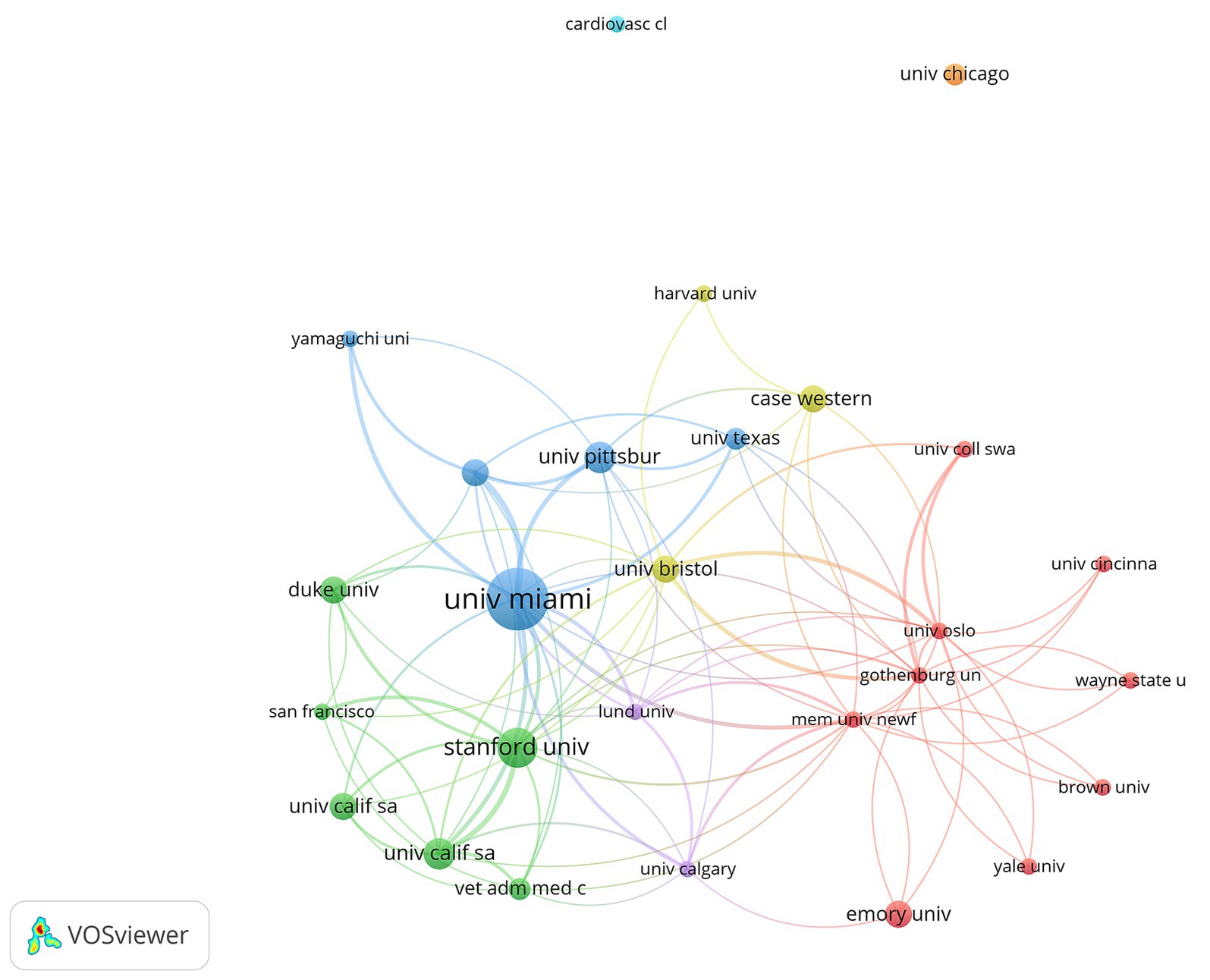
Figure 6. Citation of institutions in the 100 most-cited articles in hypothermic brain protection journals created by VOSviewer. The minimum number of documents from any one country is ≥2. Institutions are represented by labels and circles, with the size of each institution’s label and circle determined by its weight.
Journals with at least two publications and their main characteristics are listed in Table 4. There are 19 journals on the list, and the two journals with the highest publication volumes in the field of hypothermic brain protection are the Journal of Cerebral Blood Flow and Metabolism and Stroke, with 15 and 10 articles published, respectively. Additionally, five articles were published in Anesthesiology, the Journal of Neurotrauma, and the Journal of Thoracic and Cardiovascular Surgery.
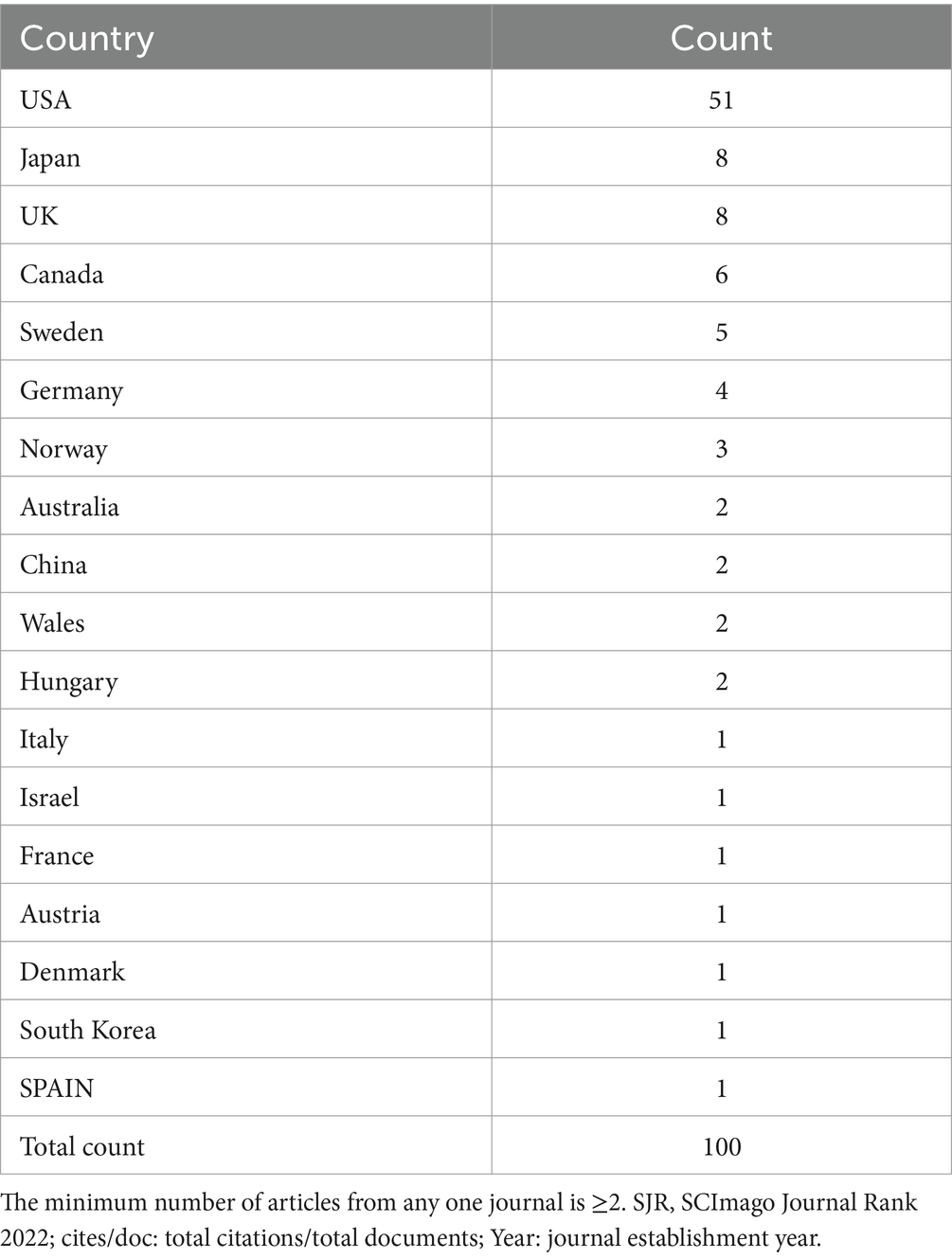
Table 4. Journal distribution and main characteristics of the 100 most-cited articles on hypothermic brain protection.
The relationships among the authors, countries, and research keywords of the 20 most-cited articles in hypothermic brain protection journals are shown in Figure 7. Other keywords related to hypothermic brain protection with a high frequency of occurrence included hyperthermia, dopamine, ischemia, metabolism, surgery, and trauma.
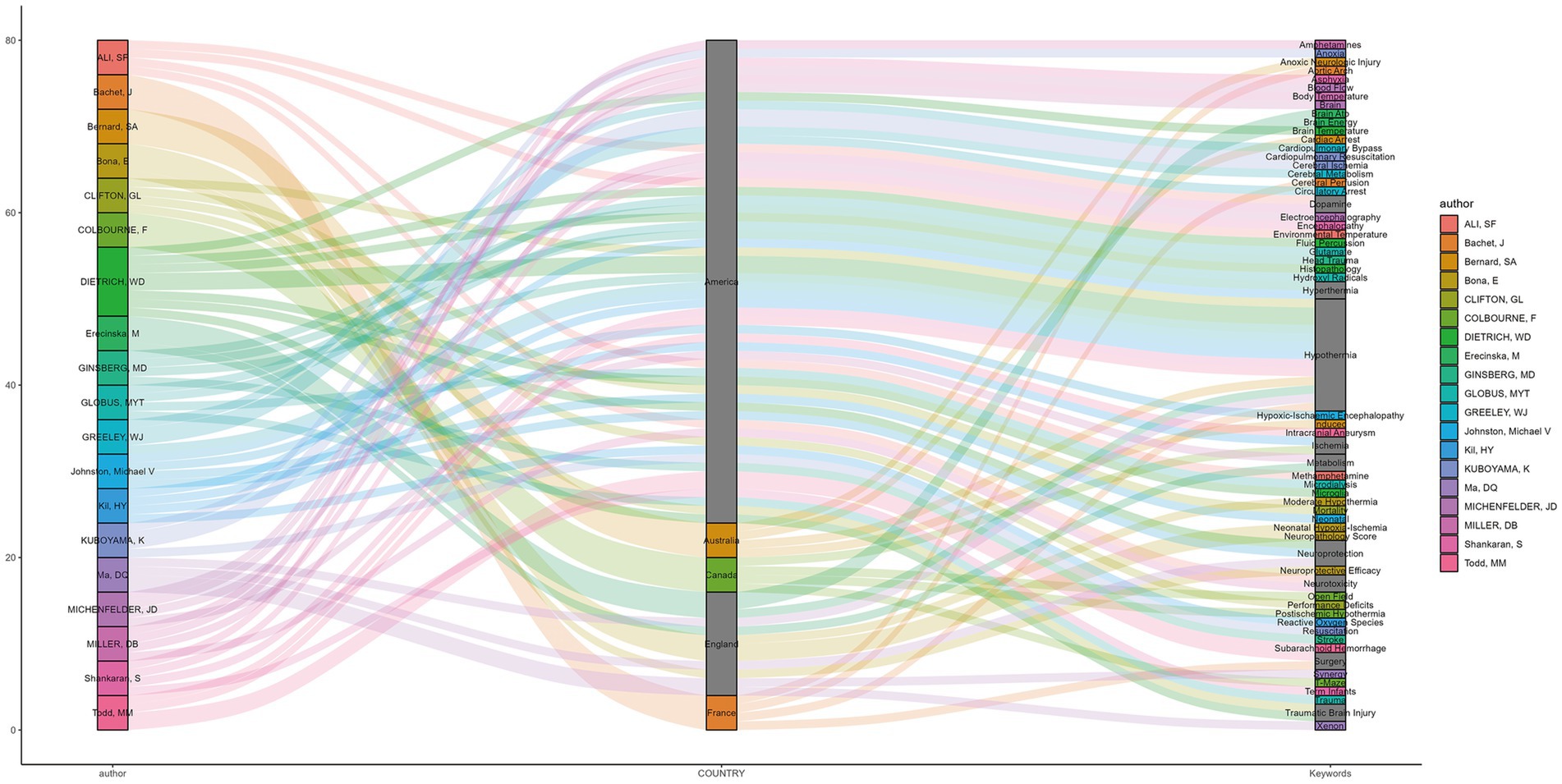
Figure 7. Sankey plot showing the relationships among authors, countries, and research keywords of the 20 most-cited articles. The left column represents the authors’ name, the middle column represents the authors’ country, and the right column represents the articles’ keywords. These columns are connected with corresponding lines on a one-to-one basis.
With continuous advances in science and technology, research in the biomedical field is expanding in terms of breadth and depth, leading to an exponential increase in the number of related studies (113). Effectively screening useful information from this vast body of literature poses a major challenge for researchers. Bibliometric analysis, a key tool in modern medical research, allows researchers to quantitatively analyze scientific literature to reveal development dynamics and trends in the research field. Using bibliometric analysis, researchers can better understand the development of a discipline, identify cutting-edge fields and research hotspots, evaluate the impact and quality of academic achievements, and provide valuable references and guidance for future medical research and strategic planning (10).
Hypothermic brain protection is a key area of medical research aimed at reducing brain injury and improving the tolerance to cerebral ischemia, trauma, and other conditions. Hypothermic brain protection techniques have shown potential neuroprotective effects in patients with neurological ischemia or injury (114–116). Although there are challenges to its clinical application, research on hypothermic brain protection is advancing, providing new ideas and methods for improving the treatment of brain injuries and neurological diseases.
The research that is most cited in a specific field is often considered a milestone and can be referred to as “classic (117, 118).” The frequency of citations in a paper generally reflects its importance, indicating that it has gained recognition from researchers in the relevant field as well as sparking discussions and guiding new research directions (119, 120). Owing to its pioneering contributions, this study provides important reference values for further analysis. This study identified and analyzed the 100 most-cited articles in the field of hypothermic brain protection, providing an historical overview of the development of the research field over time, defining interesting trends, and potentially offering clues for the future development of basic research and clinical practice in hypothermic brain protection.
Since Busto et al. (121, 122) first proposed the use of mild hypothermia (33–35°C) to treat brain injuries in the 1980s, experts have recognized its protective effects on the body, particularly on the brain. The question of whether lowering body temperature to induce hypothermia can effectively shield the brain has sparked interest in clinical and basic research. The 100 most cited articles listed in this study have shown that hypothermia can improve brain function and provide significant protection (1, 14–112). The current mechanisms of hypothermic brain protection include reducing brain energy metabolism, protecting the blood brain barrier, decreasing brain swelling and pressure, preventing lactic acid accumulation, limiting the release of harmful amino acids, blocking the detrimental effects of calcium, inhibiting nitric oxide production, reducing the generation of oxygen radicals, enhancing the elimination of oxygen radicals, suppressing the expression of genes associated with cellular damage, and reducing inflammation and neuronal cell death (2, 123).
Hypothermic brain protection is used to lower a patient’s body or brain temperature to decrease brain oxygen consumption and facilitate recovery (124). Mild hypothermia (33–35°C) and moderate hypothermia (28–32°C) are commonly used, and studies have indicated that 33°C is the optimal temperature for treatment (125). Deep hypothermia (17–27°C) is reserved for specific patients (for example, those with aortic stenosis or aortic dissection) due to more severe complications (126).
However, some recent large-scale clinical studies have refuted this view. In a study conducted in 2005 to determine whether hypothermia during craniotomy can improve the prognosis of patients with acute aneurysmal subarachnoid hemorrhage, there was no significant difference in hospitalization time, total hospitalization time, or follow-up mortality in the intensive care unit between the hypothermia group and the normal temperature group during craniotomy (20). Another randomized controlled trial published in 2010 showed no correlation between intraoperative hypothermia or supplementation of protective drugs and neurological prognosis in patients undergoing temporary clipping during cerebral aneurysm surgery (127). At the same time, some systematic reviews also expound similar viewpoints (128–130).
There is a guideline stating that the duration of brain hypothermia should be sufficient to provide brain protection (131). For patients with craniocerebral injury, it is challenging to achieve favorable clinical outcomes with short-term (24–48 h) mild hypothermia treatment. It is recommended that the duration of mild hypothermia treatment for such patients be maintained for at least 3–5 days. Therefore, additional research is required to determine the appropriateness of utilizing low temperatures in varying circumstances, along with the corresponding low-temperature strategy and duration of maintenance.
The most-cited studies in the field of hypothermic brain protection generally describe the effects of posttraumatic hypothermia on neuronal damage in rats with traumatic brain injury (TBI) (14). Research has shown that TBI leads to a significant increase in the glutamate and hydroxyl radical levels in the brain, with a positive correlation between these two factors. Post-traumatic hypothermia effectively suppresses these elevations, indicating a potential link between glutamate release and hydroxyl radical production in the brain after TBI. This groundbreaking discovery has had a significant guiding influence on clinical treatment, making it the most influential article. Six articles, with a total citation count of over 400 in this field, can be referred to as “classics.” The six authors and their teams—Globus et al. (14), Clifton et al. (15), Kuboyama et al. (16), Ginsberg et al. (17), Dietrich et al. (18), and Colbourne et al. (19)—have all made significant contributions to further research in the field of hypothermic brain protection.
The temporal distribution of these 100 articles revealed that they were published between 1990 and 2016, whereas the years that produced a relatively large number of highly influential articles were 1991–1996 and 2007. Furthermore, it must be emphasized that the total citation count of publications over the past 3 years may have been underestimated, considering that recently-published articles will take time to attract citations. Over time, an increasing number of recently-published studies have become highly cited (132). Among the 100 articles, the proportion of basic research was the highest (n = 73), followed by reviews (n = 18), whereas the proportions of clinical research and case reports were low (n = 8). These findings indicate that although we have observed the protective effect of hypothermia, we still have only a partial understanding of its mechanism, and the study of the specific mechanism of hypothermic brain protection remains a hot topic (123).
Regarding countries and institutions, most of the 100 most-cited articles in the field of hypothermic brain protection were from the United States (n = 51), which also had an overwhelming number of citations, indicating that the United States is the most influential country in this field. The United States has always led the world in the field of hypothermic brain protection research, and its continuously innovative medical technology and cutting-edge research results have inspired tremendous progress. The most-cited authors were from the United States. The University of Miami and Stanford University the institutions with the most cited articles, reflecting their authority in the field of hypothermic brain protection (35, 39). In terms of international cooperation, the United States cooperates closely with multiple countries, whereas Japan cooperates closely with Germany, the United Kingdom, and Sweden. At the institutional level, Stanford University cooperates closely with the University of California, Emory University, and Duke University (42).
The most-cited research in this field is more likely to be published in highly-influential neurosurgical journals such as the Journal of Cerebral Blood Flow and Metabolism and Stroke (15, 36). In addition, several studies published in these journals, apart from those in the field of neurosurgery, involve the intersection of anesthesia and critical care, as well as pediatrics and neurosurgery, such as anesthesiology, critical care medicine, and pediatric research (16, 22, 30). Interestingly, these results suggest that hypothermic brain protection has aroused great interest not only among neurologists but also among anesthesiologists and pediatricians due to its close connection to their clinical work.
The Sankey plot illustrated the relationships among authors, countries, and agreed-upon keywords. The keywords used were further extended to include “hypothermia,” “brain,” “neuroprotection,” “surgery,” “trauma,” “ischemia,” “metabolism,” and others. A relatively novel keyword, “xenon,” was used to explore the treatment of neonatal hypoxic-ischemic brain injury (31, 38, 73). The experimental data from these studies showed that low-concentration xenon combined with mild hypothermia may be a safe and effective treatment for perinatal asphyxia. Most articles mentioned keywords such as “hypothermia” and “neuroprotection” in their titles; however, a few articles do not mention them. We recommend that the terms “hypothermia” and “neuroprotection” are included in the title so readers can easily identify the nature of the article and index it in search databases.
Keywords Plus® was used with the Clarivate Analytics1 algorithm, which is based on repeated words or phrases appearing in the reference lists of indexed articles (133). In the absence of author keywords, Keywords Plus® is considered to have special value. However, compared with keywords, the number of Keywords Plus® was greater, and the concentration of keywords was not strong. The use of Keyword Plus® to construct Sankey plots may have caused data distortion. Therefore, we constructed the Sankey plot using keywords. Seven of the 20 most-cited articles in this field did not provide keywords, and three researchers summarized the keywords after their discussion according to Keywords Plus®.
This study has several limitations. The first limitation is inherent in citation analysis which is based on the absolute number of citations of an article. The number of citations is a substitute for this influence; however many factors can affect the citation rate. Generally the number of citations an article receives reflects its impact and level of recognition by academic and clinical communities. However the number of citations depends on many factors such as factors related to the paper including quality length publication year and literature type; factors related to journals such as journal influence language and publication format; and factors related to the author such as reputation academic ranking and productivity. Second only English versions of the articles were included in the study; however English is the most widely-used language worldwide making it possible for articles published in English to be widely read and cited. Third the search was conducted only on the WOS database which may have resulted in missing several relevant publications from other databases such as Google Scholar Scopus and PubMed. Searching databases from multiple sources can yield more complete citation results because the number of citations varies among databases from different sources (134). However different databases have different reference-counting methods which may be unsuitable for merging data from different databases. Among these databases WOS is one of the most commonly used databases for analyzing highly-cited articles in a particular field (135–137). The fourth limitation was the time factor because the WOS currently covers only articles published after 1980. We may have missed articles published before 1980 with higher citation frequencies than those included in this study. Additionally recently published articles require time to attract citations. Therefore the citation frequency in early studies should be higher than that in recently published studies and some recently-published representative works may not be included. Quotations may not fully represent the true academic value and the impact of a study should be evaluated from all aspects. Future work can start by updating the time periods searching in and merging multiple databases and establishing algorithms for the influence of new and old articles. Nevertheless the results of this study provide valuable insights for researchers.
This bibliometric study offers a comprehensive overview of the advancements, trends, and current trajectories in both basic research and clinical applications within the domain of hypothermic brain protection. By conducting a bibliometric analysis of the WOS database, this study identified and thoroughly analyzed the 100 most impactful articles that have significantly contributed to advancing this field. Rapid growth has been observed in the field of hypothermic brain protection. The United States emerged as a dominant force in terms of the number of highly influential articles, prominent academic institutions, and leading scientists in this area of research. Predominantly, articles in this field focus on basic research and delve into the underlying mechanisms. Keywords, such as “hypothermia” and “neuroprotection” intersect with other fields, enriching the comprehension of mechanisms and broadening their applicability. Continuous and profound research endeavors in this sphere promise a deeper understanding of the molecular pathophysiological mechanisms underlying various diseases, thereby unveiling potential therapeutic targets.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
LH: Writing – original draft, Visualization, Software, Methodology, Investigation, Data curation, Conceptualization. SG: Writing – original draft, Visualization, Software, Methodology, Data curation. FL: Writing – original draft, Resources, Methodology, Formal analysis, Data curation. LN: Writing – review & editing, Visualization, Resources, Methodology, Funding acquisition, Formal analysis. JW: Writing – review & editing, Validation, Supervision, Resources, Funding acquisition. JZ: Funding acquisition, Writing – review & editing, Supervision. MW: Writing – review & editing, Validation, Supervision, Resources, Project administration, Funding acquisition. YC: Project administration, Methodology, Writing – review & editing, Validation, Funding acquisition.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was supported by the Sichuan Science and Technology Program (2022YFS0632), the Joint Foundation of the Luzhou Government and Southwest Medical University (2021LZXNYD-J28), Southwest Medical University Technology Program (2023QN013), and 2021-year Zigong City Health Commission Project (2021yb077).
The authors would like to thank Natalie from Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1433025/full#supplementary-material
1. Henderson, WR, Dhingra, VK, Chittock, DR, Fenwick, JC, and Ronco, JJ. Hypothermia in the management of traumatic brain injury. A systematic review and meta-analysis. Intensive Care Med. (2003) 29:1637–44. doi: 10.1007/s00134-003-1848-2
2. Yenari, MA, and Han, HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. (2012) 13:267–78. doi: 10.1038/nrn3174
3. Polderman, KH . Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. (2009) 37:S186–202. doi: 10.1097/CCM.0b013e3181aa5241
4. van der Worp, HB, Macleod, MR, and Kollmar, REuropean Stroke Research Network for Hypothermia (EuroHYP). Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials? J Cereb Blood Flow Metab. (2010) 30:1079–93. doi: 10.1038/jcbfm.2010.44
5. Polderman, KH . Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. (2008) 371:1955–69. doi: 10.1016/S0140-6736(08)60837-5
6. Ponce, FA, and Lozano, AM. Highly cited works in neurosurgery. Part I: the 100 top-cited papers in neurosurgical journals. J Neurosurg. (2010) 112:223–32. doi: 10.3171/2009.12.JNS091599
7. Ninkov, A, Frank, JR, and Maggio, LA. Bibliometrics: methods for studying academic publishing. Perspect Med Educ. (2022) 11:173–6. doi: 10.1007/s40037-021-00695-4
8. Mingers, J, and Leydesdorff, L. A review of theory and practice in scientometrics. Eur J Oper Res. (2015) 246:1–19. doi: 10.1016/j.ejor.2015.04.002
9. Vinkler, P . An attempt at a bibliometric analysis of the most cited works on aneurysms. Neurosurg Rev. (2007) 30:137–47.
10. Xie, L, Chen, Z, Wang, H, Zheng, C, and Jiang, J. Bibliometric and visualized analysis of scientific publications on atlantoaxial spine surgery based on Web of Science and VOSviewer. World Neurosurg. (2020) 137:435–442.e4. doi: 10.1016/j.wneu.2020.01.171
11. Ponce, FA, and Lozano, AM. Highly cited works in neurosurgery. Part II: the citation classics. J Neurosurg. (2010) 112:233–46. doi: 10.3171/2009.12.JNS091600
12. van Eck, NJ, and Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
13. Wu, H, Sun, Z, Tong, L, Wang, Y, Yan, H, and Sun, Z. Bibliometric analysis of global research trends on male osteoporosis: a neglected field deserves more attention. Arch Osteoporos. (2021) 16:154. doi: 10.1007/s11657-021-01016-2
14. Globus, MY, Alonso, O, Dietrich, WD, Busto, R, and Ginsberg, MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem. (1995) 65:1704–11. doi: 10.1046/j.1471-4159.1995.65041704.x
15. Clifton, GL, Jiang, JY, Lyeth, BG, Jenkins, LW, Hamm, RJ, and Hayes, RL. Marked protection by moderate hypothermia after experimental traumatic brain injury. J Cereb Blood Flow Metab. (1991) 11:114–21. doi: 10.1038/jcbfm.1991.13
16. Kuboyama, K, Safar, P, Radovsky, A, Tisherman, SA, Stezoski, SW, and Alexander, H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. (1993) 21:1348–58. doi: 10.1097/00003246-199309000-00019
17. Ginsberg, MD, Sternau, LL, Globus, MY, Dietrich, WD, and Busto, R. Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovasc Brain Metab Rev. (1992) 4:189–225.
18. Dietrich, WD, Busto, R, Alonso, O, Globus, MY, and Ginsberg, MD. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. (1993) 13:541–9. doi: 10.1038/jcbfm.1993.71
19. Colbourne, F, and Corbett, D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. (1995) 15:7250–60. doi: 10.1523/JNEUROSCI.15-11-07250.1995
20. Todd, MM, Hindman, BJ, Clarke, WR, and Torner, JCIntraoperative Hypothermia for Aneurysm Surgery Trial (IHAST) Investigators. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. (2005) 352:135–45. doi: 10.1056/NEJMoa040975
21. Erecinska, M, Thoresen, M, and Silver, IA. Effects of hypothermia on energy metabolism in mammalian central nervous system. J Cereb Blood Flow Metab. (2003) 23:513–30. doi: 10.1097/01.WCB.0000066287.21705.21
22. Bona, E, Hagberg, H, Løberg, EM, Bågenholm, R, and Thoresen, M. Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short-and long-term outcome. Pediatr Res. (1998) 43:738–45. doi: 10.1203/00006450-199806000-00005
23. Dietrich, WD, Alonso, O, Busto, R, Globus, MY, and Ginsberg, MD. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol. (1994) 87:250–8. doi: 10.1007/BF00296740
24. Greeley, WJ, Kern, FH, Ungerleider, RM, Boyd, JL III, Quill, T, Smith, LR, et al. The effect of hypothermic cardiopulmonary bypass and total circulatory arrest on cerebral metabolism in neonates, infants, and children. J Thorac Cardiovasc Surg. (1991) 101:783–94. doi: 10.1016/S0022-5223(19)36647-4
25. Kil, HY, Zhang, J, and Piantadosi, CA. Brain temperature alters hydroxyl radical production during cerebral ischemia/reperfusion in rats. J Cereb Blood Flow Metab. (1996) 16:100–6. doi: 10.1097/00004647-199601000-00012
26. Miller, DB, and O’Callaghan, JP. Environment-, drug-and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. (1994) 270:752–60.
27. Bernard, SA, and Buist, M. Induced hypothermia in critical care medicine: a review. Crit Care Med. (2003) 31:2041–51. doi: 10.1097/01.CCM.0000069731.18472.61
28. Bachet, J, Guilmet, D, Goudot, B, Dreyfus, GD, Delentdecker, P, Brodaty, D, et al. Antegrade cerebral perfusion with cold blood: a 13-year experience. Ann Thorac Surg. (1999) 67:1874–8. doi: 10.1016/s0003-4975(99)00411-7
29. Johnston, MV, Fatemi, A, Wilson, MA, and Northington, F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. (2011) 10:372–82. doi: 10.1016/S1474-4422(11)70016-3
30. Michenfelder, JD, and Milde, JH. The relationship among canine brain temperature, metabolism, and function during hypothermia. Anesthesiology. (1991) 75:130–6. doi: 10.1097/00000542-199107000-00021
31. Ma, D, Hossain, M, Chow, A, Arshad, M, Battson, RM, Sanders, RD, et al. Xenon and hypothermia combine to provide neuroprotection from neonatal asphyxia. Ann Neurol. (2005) 58:182–93. doi: 10.1002/ana.20547
32. Ali, SF, Newport, GD, Holson, RR, Slikker, W Jr, and Bowyer, JF. Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res. (1994) 658:33–8. doi: 10.1016/s0006-8993(09)90007-5
33. Shankaran, S, Laptook, A, Wright, LL, Ehrenkranz, RA, Donovan, EF, Fanaroff, AA, et al. Whole-body hypothermia for neonatal encephalopathy: animal observations as a basis for a randomized, controlled pilot study in term infants. Pediatrics. (2002) 110:377–85. doi: 10.1542/peds.110.2.377
34. Coimbra, C, and Wieloch, T. Moderate hypothermia mitigates neuronal damage in the rat brain when initiated several hours following transient cerebral ischemia. Acta Neuropathol. (1994) 87:325–31. doi: 10.1007/BF00313599
35. Xu, L, Yenari, MA, Steinberg, GK, and Giffard, RG. Mild hypothermia reduces apoptosis of mouse neurons in vitro early in the cascade. J Cereb Blood Flow Metab. (2002) 22:21–8. doi: 10.1097/00004647-200201000-00003
36. Coimbra, C, Drake, M, Boris-Möller, F, and Wieloch, T. Long-lasting neuroprotective effect of postischemic hypothermia and treatment with an anti-inflammatory/antipyretic drug. Evidence for chronic encephalopathic processes following ischemia. Stroke. (1996) 27:1578–85. doi: 10.1161/01.str.27.9.1578
37. Thoresen, M, Bågenholm, R, Løberg, EM, Apricena, F, and Kjellmer, I. Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child Fetal Neonatal Ed. (1996) 74:F3–9. doi: 10.1136/fn.74.1.f3
38. Hobbs, C, Thoresen, M, Tucker, A, Aquilina, K, Chakkarapani, E, and Dingley, J. Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke. (2008) 39:1307–13. doi: 10.1161/STROKEAHA.107.499822
39. Bramlett, HM, Dietrich, WD, Green, EJ, and Busto, R. Chronic histopathological consequences of fluid-percussion brain injury in rats: effects of post-traumatic hypothermia. Acta Neuropathol. (1997) 93:190–9. doi: 10.1007/s004010050602
40. Ehrlich, MP, JN, MC, Zhang, N, Weisz, DJ, Juvonen, T, Bodian, CA, et al. Effect of hypothermia on cerebral blood flow and metabolism in the pig. Ann Thorac Surg. (2002) 73:191–7. doi: 10.1016/s0003-4975(01)03273-8
41. Colbourne, F, Li, H, and Buchan, AM. Indefatigable CA1 sector neuroprotection with mild hypothermia induced 6 hours after severe forebrain ischemia in rats. J Cereb Blood Flow Metab. (1999) 19:742–9. doi: 10.1097/00004647-199907000-00003
42. Shankaran, S, Barnes, PD, Hintz, SR, Laptook, AR, Zaterka-Baxter, KM, McDonald, S, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. (2012) 97:F398–404. doi: 10.1136/archdischild-2011-301524
43. Green, EJ, Dietrich, WD, van Dijk, F, Busto, R, Markgraf, CG, McCabe, PM, et al. Protective effects of brain hypothermia on behavior and histopathology following global cerebral ischemia in rats. Brain Res. (1992) 580:197–204. doi: 10.1016/0006-8993(92)90945-6
44. Peretti, D, Bastide, A, Radford, H, Verity, N, Molloy, C, Martin, MG, et al. RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature. (2015) 518:236–9. doi: 10.1038/nature14142
45. Baker, AJ, Zornow, MH, Grafe, MR, Scheller, MS, Skilling, SR, Smullin, DH, et al. Hypothermia prevents ischemia-induced increases in hippocampal glycine concentrations in rabbits. Stroke. (1991) 22:666–73. doi: 10.1161/01.str.22.5.666
46. Han, HS, Qiao, Y, Karabiyikoglu, M, Giffard, RG, and Yenari, MA. Influence of mild hypothermia on inducible nitric oxide synthase expression and reactive nitrogen production in experimental stroke and inflammation. J Neurosci. (2002) 22:3921–8. doi: 10.1523/JNEUROSCI.22-10-03921.2002
47. Deng, H, Han, HS, Cheng, D, Sun, GH, and Yenari, MA. Mild hypothermia inhibits inflammation after experimental stroke and brain inflammation. Stroke. (2003) 34:2495–501. doi: 10.1161/01.STR.0000091269.67384.E7
48. Zhao, H, Steinberg, GK, and Sapolsky, RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab. (2007) 27:1879–94. doi: 10.1038/sj.jcbfm.9600540
49. Dietrich, WD . The importance of brain temperature in cerebral injury. J Neurotrauma. (1992) 9:S475–85.
50. Carroll, M, and Beek, O. Protection against hippocampal CA1 cell loss by post-ischemic hypothermia is dependent on delay of initiation and duration. Metab Brain Dis. (1992) 7:45–50. doi: 10.1007/BF01000440
51. Malberg, JE, Sabol, KE, and Seiden, LS. Co-administration of MDMA with drugs that protect against MDMA neurotoxicity produces different effects on body temperature in the rat. J Pharmacol Exp Ther. (1996) 278:258–67.
52. Thoresen, M, Satas, S, Løberg, EM, Whitelaw, A, Acolet, D, Lindgren, C, et al. Twenty-four hours of mild hypothermia in unsedated newborn pigs starting after a severe global hypoxic-ischemic insult is not neuroprotective. Pediatr Res. (2001) 50:405–11. doi: 10.1203/00006450-200109000-00017
53. Bramlett, HM, Green, EJ, Dietrich, WD, Busto, R, Globus, MY, and Ginsberg, MD. Posttraumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits. J Neurotrauma. (1995) 12:289–98. doi: 10.1089/neu.1995.12.289
54. Choi, HA, Badjatia, N, and Mayer, SA. Hypothermia for acute brain injury—mechanisms and practical aspects. Nat Rev Neurol. (2012) 8:214–22. doi: 10.1038/nrneurol.2012.21
55. Maher, J, and Hachinski, V. Hypothermia as a potential treatment for cerebral ischemia. Cerebrovasc Brain Metab Rev. (1993) 5:277–300.
56. Dénes, A, Ferenczi, S, and Kovács, KJ. Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood-brain barrier damage and brain oedema independently of infarct size. J Neuroinflammation. (2011) 8:164. doi: 10.1186/1742-2094-8-164
57. Frerichs, KU, and Hallenbeck, JM. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab. (1998) 18:168–75. doi: 10.1097/00004647-199802000-00007
58. Dietrich, WD, Atkins, CM, and Bramlett, HM. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J Neurotrauma. (2009) 26:301–12. doi: 10.1089/neu.2008.0806
59. Todd, MM, and Warner, DS. A comfortable hypothesis reevaluated. Cerebral metabolic depression and brain protection during ischemia. Anesthesiology. (1992) 76:161–4. doi: 10.1097/00000542-199202000-00002
60. Karibe, H, Chen, J, Zarow, GJ, Graham, SH, and Weinstein, PR. Delayed induction of mild hypothermia to reduce infarct volume after temporary middle cerebral artery occlusion in rats. J Neurosurg. (1994) 80:112–9. doi: 10.3171/jns.1994.80.1.0112
61. Nurse, S, and Corbett, D. Neuroprotection after several days of mild, drug-induced hypothermia. J Cereb Blood Flow Metab. (1996) 16:474–80. doi: 10.1097/00004647-199605000-00014
62. Zierer, A, El-Sayed Ahmad, A, Papadopoulos, N, Moritz, A, and Diegeler, A. Selective antegrade cerebral perfusion and mild (28°C–30°C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J Thorac Cardiovasc Surg. (2012) 144:1042–50. doi: 10.1016/j.jtcvs.2012.07.063
63. Bickler, PE, Buck, LT, and Hansen, BM. Effects of isoflurane and hypothermia on glutamate receptor-mediated calcium influx in brain slices. Anesthesiology. (1994) 81:1461–9. doi: 10.1097/00000542-199412000-00022
64. Sakurai, T, Itoh, K, Higashitsuji, H, Nonoguchi, K, Liu, Y, Watanabe, H, et al. Cirp protects against tumor necrosis factor-alpha-induced apoptosis via activation of extracellular signal-regulated kinase. Biochim Biophys Acta. (2006) 1763:290–5. doi: 10.1016/j.bbamcr.2006.02.007
65. Drew, KL, Rice, ME, Kuhn, TB, and Smith, MA. Neuroprotective adaptations in hibernation: therapeutic implications for ischemia-reperfusion, traumatic brain injury and neurodegenerative diseases. Free Radic Biol Med. (2001) 31:563–73. doi: 10.1016/s0891-5849(01)00628-1
66. Sano, T, Drummond, JC, Patel, PM, Grafe, MR, Watson, JC, and Cole, DJ. A comparison of the cerebral protective effects of isoflurane and mild hypothermia in a model of incomplete forebrain ischemia in the rat. Anesthesiology. (1992) 76:221–8. doi: 10.1097/00000542-199202000-00011
67. Liu, L, and Yenari, MA. Therapeutic hypothermia: neuroprotective mechanisms. Front Biosci. (2007) 12:816–25. doi: 10.2741/2104
68. Churn, SB, Taft, WC, Billingsley, MS, Blair, RE, and DeLorenzo, RJ. Temperature modulation of ischemic neuronal death and inhibition of calcium/calmodulin-dependent protein kinase II in gerbils. Stroke. (1990) 21:1715–21. doi: 10.1161/01.str.21.12.1715
69. Marion, DW, Leonov, Y, Ginsberg, M, Katz, LM, Kochanek, PM, Lechleuthner, A, et al. Resuscitative hypothermia. Crit Care Med. (1996) 24:81S–9S. doi: 10.1097/00003246-199602001-00008
70. Urbanski, PP, Lenos, A, Bougioukakis, P, Neophytou, I, Zacher, M, and Diegeler, A. Mild-to-moderate hypothermia in aortic arch surgery using circulatory arrest: a change of paradigm? Eur J Cardiothorac Surg. (2012) 41:185–91. doi: 10.1016/j.ejcts.2011.03.060
71. Corbett, D, Hamilton, M, and Colbourne, F. Persistent neuroprotection with prolonged postischemic hypothermia in adult rats subjected to transient middle cerebral artery occlusion. Exp Neurol. (2000) 163:200–6. doi: 10.1006/exnr.2000.7369
72. Yenari, M, Kitagawa, K, Lyden, P, and Perez-Pinzon, M. Metabolic downregulation: a key to successful neuroprotection? Stroke. (2008) 39:2910–7. doi: 10.1161/STROKEAHA.108.514471
73. Thoresen, M, Hobbs, CE, Wood, T, Chakkarapani, E, and Dingley, J. Cooling combined with immediate or delayed xenon inhalation provides equivalent long-term neuroprotection after neonatal hypoxia-ischemia. J Cereb Blood Flow Metab. (2009) 29:707–14. doi: 10.1038/jcbfm.2008.163
74. Pacini, D, Leone, A, Di Marco, L, Marsilli, D, Sobaih, F, Turci, S, et al. Antegrade selective cerebral perfusion in thoracic aorta surgery: safety of moderate hypothermia. Eur J Cardiothorac Surg. (2007) 31:618–22. doi: 10.1016/j.ejcts.2006.12.032
75. Albrecht, RF 2nd, Wass, CT, and Lanier, WL. Occurrence of potentially detrimental temperature alterations in hospitalized patients at risk for brain injury. Mayo Clin Proc. (1998) 73:629–35. doi: 10.1016/S0025-6196(11)64885-4
76. Yenari, MA, and Han, HS. Influence of hypothermia on post-ischemic inflammation: role of nuclear factor kappa B (NFkappaB). Neurochem Int. (2006) 49:164–9. doi: 10.1016/j.neuint.2006.03.016
77. Clark, RS, Kochanek, PM, Marion, DW, Schiding, JK, White, M, Palmer, AM, et al. Mild posttraumatic hypothermia reduces mortality after severe controlled cortical impact in rats. J Cereb Blood Flow Metab. (1996) 16:253–61. doi: 10.1097/00004647-199603000-00010
78. Liu, Y, Barks, JD, Xu, G, and Silverstein, FS. Topiramate extends the therapeutic window for hypothermia-mediated neuroprotection after stroke in neonatal rats. Stroke. (2004) 35:1460–5. doi: 10.1161/01.STR.0000128029.50221.fa
79. Swain, JA, McDonald, TJ Jr, Griffith, PK, Balaban, RS, Clark, RE, and Ceckler, T. Low-flow hypothermic cardiopulmonary bypass protects the brain. J Thorac Cardiovasc Surg. (1991) 102:76–84. doi: 10.1016/S0022-5223(19)36586-9
80. Koizumi, H, and Povlishock, JT. Posttraumatic hypothermia in the treatment of axonal damage in an animal model of traumatic axonal injury. J Neurosurg. (1998) 89:303–9. doi: 10.3171/jns.1998.89.2.0303
81. Lee, JH, Wei, ZZ, Cao, W, Won, S, Gu, X, Winter, M, et al. Regulation of therapeutic hypothermia on inflammatory cytokines, microglia polarization, migration and functional recovery after ischemic stroke in mice. Neurobiol Dis. (2016) 96:248–60. doi: 10.1016/j.nbd.2016.09.013
82. Sahuquillo, J, and Vilalta, A. Cooling the injured brain: how does moderate hypothermia influence the pathophysiology of traumatic brain injury. Curr Pharm Des. (2007) 13:2310–22. doi: 10.2174/138161207781368756
83. Okita, Y, Miyata, H, Motomura, N, and Takamoto, SJapan Cardiovascular Surgery Database Organization. A study of brain protection during total arch replacement comparing antegrade cerebral perfusion versus hypothermic circulatory arrest, with or without retrograde cerebral perfusion: analysis based on the Japan Adult Cardiovascular Surgery Database. J Thorac Cardiovasc Surg. (2015) 149:S65–73. doi: 10.1016/j.jtcvs.2014.08.070
84. Aoki, M, Nomura, F, Stromski, ME, Tsuji, MK, Fackler, JC, Hickey, PR, et al. Effects of pH on brain energetics after hypothermic circulatory arrest. Ann Thorac Surg. (1993) 55:1093–103. doi: 10.1016/0003-4975(93)90014-9
85. Dietrich, WD, Busto, R, and Bethea, JR. Postischemic hypothermia and IL-10 treatment provide long-lasting neuroprotection of CA1 hippocampus following transient global ischemia in rats. Exp Neurol. (1999) 158:444–50. doi: 10.1006/exnr.1999.7115
86. Büki, A, Koizumi, H, and Povlishock, JT. Moderate posttraumatic hypothermia decreases early calpain-mediated proteolysis and concomitant cytoskeletal compromise in traumatic axonal injury. Exp Neurol. (1999) 159:319–28. doi: 10.1006/exnr.1999.7139
87. Dixon, CE, Markgraf, CG, Angileri, F, Pike, BR, Wolfson, B, Newcomb, JK, et al. Protective effects of moderate hypothermia on behavioral deficits but not necrotic cavitation following cortical impact injury in the rat. J Neurotrauma. (1998) 15:95–103. doi: 10.1089/neu.1998.15.95
88. Yenari, MA, Iwayama, S, Cheng, D, Sun, GH, Fujimura, M, Morita-Fujimura, Y, et al. Mild hypothermia attenuates cytochrome c release but does not alter Bcl-2 expression or caspase activation after experimental stroke. J Cereb Blood Flow Metab. (2002) 22:29–38. doi: 10.1097/00004647-200201000-00004
89. Leshnower, BG, Myung, RJ, Kilgo, PD, Vassiliades, TA, Vega, JD, Thourani, VH, et al. Moderate hypothermia and unilateral selective antegrade cerebral perfusion: a contemporary cerebral protection strategy for aortic arch surgery. Ann Thorac Surg. (2010) 90:547–54. doi: 10.1016/j.athoracsur.2010.03.118
90. Yanamoto, H, Nagata, I, Niitsu, Y, Zhang, Z, Xue, JH, Sakai, N, et al. Prolonged mild hypothermia therapy protects the brain against permanent focal ischemia. Stroke. (2001) 32:232–9. doi: 10.1161/01.str.32.1.232
91. Farfel, GM, and Seiden, LS. Role of hypothermia in the mechanism of protection against serotonergic toxicity. II. Experiments with methamphetamine, p-chloroamphetamine, fenfluramine, dizocilpine and dextromethorphan. J Pharmacol Exp Ther. (1995) 272:868–75.
92. Mellergård, P, and Nordström, CH. Intracerebral temperature in neurosurgical patients. Neurosurgery. (1991) 28:709–13. doi: 10.1227/00006123-199105000-00012
93. Olsen, TS, Weber, UJ, and Kammersgaard, LP. Therapeutic hypothermia for acute stroke. Lancet Neurol. (2003) 2:410–6. doi: 10.1016/s1474-4422(03)00436-8
94. Ausman, JI, McCormick, PW, Stewart, M, Lewis, G, Dujovny, M, Balakrishnan, G, et al. Cerebral oxygen metabolism during hypothermic circulatory arrest in humans. J Neurosurg. (1993) 79:810–5. doi: 10.3171/jns.1993.79.6.0810
95. Grigore, AM, Mathew, J, Grocott, HP, Reves, JG, Blumenthal, JA, White, WD, et al. Prospective randomized trial of normothermic versus hypothermic cardiopulmonary bypass on cognitive function after coronary artery bypass graft surgery. Anesthesiology. (2001) 95:1110–9. doi: 10.1097/00000542-200111000-00014
96. Hickey, RW, Ferimer, H, Alexander, HL, Garman, RH, Callaway, CW, Hicks, S, et al. Delayed, spontaneous hypothermia reduces neuronal damage after asphyxial cardiac arrest in rats. Crit Care Med. (2000) 28:3511–6. doi: 10.1097/00003246-200010000-00027
97. Jatana, M, Singh, I, Singh, AK, and Jenkins, D. Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. (2006) 59:684–9. doi: 10.1203/01.pdr.0000215045.91122.44
98. Karibe, H, Chen, SF, Zarow, GJ, Gafni, J, Graham, SH, Chan, PH, et al. Mild intraischemic hypothermia suppresses consumption of endogenous antioxidants after temporary focal ischemia in rats. Brain Res. (1994) 649:12–8. doi: 10.1016/0006-8993(94)91043-x
99. Ohta, H, Terao, Y, Shintani, Y, and Kiyota, Y. Therapeutic time window of post-ischemic mild hypothermia and the gene expression associated with the neuroprotection in rat focal cerebral ischemia. Neurosci Res. (2007) 57:424–33. doi: 10.1016/j.neures.2006.12.002
100. Yokota, S, Imagawa, T, Miyamae, T, Ito, SI, Nakajima, S, Nezu, A, et al. Hypothetical pathophysiology of acute encephalopathy and encephalitis related to influenza virus infection and hypothermia therapy. Pediatr Int. (2000) 42:197–203. doi: 10.1046/j.1442-200x.2000.01204.x
101. Dietrich, WD, Lin, B, Globus, MY, Green, EJ, Ginsberg, MD, and Busto, R. Effect of delayed MK-801 (dizocilpine) treatment with or without immediate postischemic hypothermia on chronic neuronal survival after global forebrain ischemia in rats. J Cereb Blood Flow Metab. (1995) 15:960–8. doi: 10.1038/jcbfm.1995.122
102. Yanamoto, H, Hong, SC, Soleau, S, Kassell, NF, and Lee, KS. Mild postischemic hypothermia limits cerebral injury following transient focal ischemia in rat neocortex. Brain Res. (1996) 718:207–11. doi: 10.1016/0006-8993(96)00122-9
103. Busto, R, Globus, MY, Neary, JT, and Ginsberg, MD. Regional alterations of protein kinase C activity following transient cerebral ischemia: effects of intraischemic brain temperature modulation. J Neurochem. (1994) 63:1095–103. doi: 10.1046/j.1471-4159.1994.63031095.x
104. Markgraf, CG, Clifton, GL, and Moody, MR. Treatment window for hypothermia in brain injury. J Neurosurg. (2001) 95:979–83. doi: 10.3171/jns.2001.95.6.0979
105. Lyeth, BG, Jiang, JY, and Liu, S. Behavioral protection by moderate hypothermia initiated after experimental traumatic brain injury. J Neurotrauma. (1993) 10:57–64. doi: 10.1089/neu.1993.10.57
106. Doyle, KP, Suchland, KL, Ciesielski, TM, Lessov, NS, Grandy, DK, Scanlan, TS, et al. Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke. (2007) 38:2569–76. doi: 10.1161/STROKEAHA.106.480277
107. Hamann, GF, Burggraf, D, Martens, HK, Liebetrau, M, Jäger, G, Wunderlich, N, et al. Mild to moderate hypothermia prevents microvascular basal lamina antigen loss in experimental focal cerebral ischemia. Stroke. (2004) 35:764–9. doi: 10.1161/01.STR.0000116866.60794.21
108. Coimbra, C, Boris-Möller, F, Drake, M, and Wieloch, T. Diminished neuronal damage in the rat brain by late treatment with the antipyretic drug dipyrone or cooling following cerebral ischemia. Acta Neuropathol. (1996) 92:447–53. doi: 10.1007/s004010050545
109. Truettner, JS, Alonso, OF, Bramlett, HM, and Dietrich, WD. Therapeutic hypothermia alters microRNA responses to traumatic brain injury in rats. J Cereb Blood Flow Metab. (2011) 31:1897–907. doi: 10.1038/jcbfm.2011.33
110. Farfel, GM, and Seiden, LS. Role of hypothermia in the mechanism of protection against serotonergic toxicity. I. Experiments using 3,4-methylenedioxymethamphetamine, dizocilpine, CGS 19755 and NBQX. J Pharmacol Exp Ther. (1995) 272:860–7.
111. Oates, RK, Simpson, JM, Turnbull, JA, and Cartmill, TB. The relationship between intelligence and duration of circulatory arrest with deep hypothermia. J Thorac Cardiovasc Surg. (1995) 110:786–92. doi: 10.1016/S0022-5223(95)70112-5
112. MacLellan, CL, Girgis, J, and Colbourne, F. Delayed onset of prolonged hypothermia improves outcome after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. (2004) 24:432–40. doi: 10.1097/00004647-200404000-00008
113. Zhu, S, Liu, Y, Gu, Z, and Zhao, Y. Research trends in biomedical applications of two-dimensional nanomaterials over the last decade—a bibliometric analysis. Adv Drug Deliv Rev. (2022) 188:114420. doi: 10.1016/j.addr.2022.114420
114. Centola, L, Kanamitsu, H, Kinouchi, K, Fuji, Y, Ito, H, Maeda, K, et al. Deep hypothermic circulatory arrest activates neural precursor cells in the neonatal brain. Ann Thorac Surg. (2020) 110:2076–81. doi: 10.1016/j.athoracsur.2020.02.058
115. Broad, KD, Fierens, I, Fleiss, B, Rocha-Ferreira, E, Ezzati, M, Hassell, J, et al. Inhaled 45-50% argon augments hypothermic brain protection in a piglet model of perinatal asphyxia. Neurobiol Dis. (2016) 87:29–38. doi: 10.1016/j.nbd.2015.12.001
116. Jiang, X, Gu, T, Liu, Y, Wang, C, Shi, E, Zhang, G, et al. Protection of the rat brain from hypothermic circulatory arrest injury by a chipmunk protein. J Thorac Cardiovasc Surg. (2018) 156:525–36. doi: 10.1016/j.jtcvs.2018.02.048
117. Van Noorden, R, Maher, B, and Nuzzo, R. The top 100 papers. Nature. (2014) 514:550–3. doi: 10.1038/514550a
118. Godin, B . On the origins of bibliometrics. Scientometrics. (2006) 68:109–33. doi: 10.1007/s11192-006-0086-0
119. Fardi, A, Kodonas, K, Lillis, T, and Veis, A. Top-cited articles in implant dentistry. Int J Oral Maxillofac Implants. (2017) 32:555–64. doi: 10.11607/jomi.5331
120. Tarazona, B, Lucas-Dominguez, R, Paredes-Gallardo, V, Alonso-Arroyo, A, and Vidal-Infer, A. The 100 most-cited articles in orthodontics: a bibliometric study. Angle Orthod. (2018) 88:785–96. doi: 10.2319/012418-65.1
121. Busto, R, Dietrich, WD, Globus, MY, and Ginsberg, MD. Postischemic moderate hypothermia inhibits CA1 hippocampal ischemic neuronal injury. Neurosci Lett. (1989) 101:299–304. doi: 10.1016/0304-3940(89)90549-1
122. Busto, R, Globus, MY, Dietrich, WD, Martinez, E, Valdés, I, and Ginsberg, MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke. (1989) 20:904–10. doi: 10.1161/01.str.20.7.904
123. Tveita, T, and Sieck, GC. Physiological impact of hypothermia: the good, the bad, and the ugly. Physiology. (2022) 37:69–87. doi: 10.1152/physiol.00025.2021
124. Nielsen, N, and Friberg, H. Changes in practice of controlled hypothermia after cardiac arrest in the past 20 years: a critical care perspective. Am J Respir Crit Care Med. (2023) 207:1558–64. doi: 10.1164/rccm.202211-2142CP
125. Hsieh, YC, Lin, SF, Huang, JL, Hung, CY, Lin, JC, Liao, YC, et al. Moderate hypothermia (33°C) decreases the susceptibility to pacing-induced ventricular fibrillation compared with severe hypothermia (30°C) by attenuating spatially discordant alternans in isolated rabbit hearts. Acta Cardiol Sin. (2014) 30:455–65.
126. Lin, Y, Chen, MF, Zhang, H, Li, RM, and Chen, LW. The risk factors for postoperative cerebral complications in patients with Stanford type A aortic dissection. J Cardiothorac Surg. (2019) 14:178. doi: 10.1186/s13019-019-1009-5
127. Hindman, BJ, Bayman, EO, Pfisterer, WK, Torner, JC, and Todd, MMIHAST Investigators. No association between intraoperative hypothermia or supplemental protective drug and neurologic outcomes in patients undergoing temporary clipping during cerebral aneurysm surgery: findings from the intraoperative hypothermia for aneurysm surgery trial. Anesthesiology. (2010) 112:86–101. doi: 10.1097/ALN.0b013e3181c5e28f
128. Zhao, ZX, Wu, C, and He, M. A systematic review of clinical outcomes, perioperative data and selective adverse events related to mild hypothermia in intracranial aneurysm surgery. Clin Neurol Neurosurg. (2012) 114:827–32. doi: 10.1016/j.clineuro.2012.05.008
129. Li, LR, You, C, and Chaudhary, B. Intraoperative mild hypothermia for postoperative neurological deficits in people with intracranial aneurysm. Cochrane Database Syst Rev. (2016) 2016:CD008445. doi: 10.1002/14651858.CD008445.pub3
130. Galvin, IM, Levy, R, Boyd, JG, Day, AG, and Wallace, MC. Cooling for cerebral protection during brain surgery. Cochrane Database Syst Rev. (2015) 1:CD006638. doi: 10.1002/14651858.CD006638.pub3
131. Bratton, SL, Chestnut, RM, Ghajar, J, McConnell Hammond, FF, Harris, OA, Hartl, R, et al. J Neurotrauma. (2007) 24:S21–5. doi: 10.1089/neu.2007.9993
132. Jiang, Y, Hu, R, and Zhu, G. Top 100 cited articles on infection in orthopaedics: a bibliometric analysis. Medicine. (2019) 98:e14067. doi: 10.1097/MD.0000000000014067
133. Garfield, E, and Sher, IH. KeyWords Plus™—algorithmic derivative indexing. J Am Soc Inform Sci. (1993) 44:298–9. doi: 10.1002/(SICI)1097-4571(199306)44:5<298:AID-ASI5>3.0.CO;2-A
134. Bakkalbasi, N, Bauer, K, Glover, J, and Wang, L. Three options for citation tracking: Google Scholar, Scopus and Web of Science. Biomed Digit Libr. (2006) 3:7. doi: 10.1186/1742-5581-3-7
135. Trapp, J . Web of Science, Scopus, and Google Scholar citation rates: a case study of medical physics and biomedical engineering: what gets cited and what doesn’t? Australas Phys Eng Sci Med. (2016) 39:817–23. doi: 10.1007/s13246-016-0478-2
136. Anker, MS, Hadzibegovic, S, Lena, A, and Haverkamp, W. The difference in referencing in Web of Science, Scopus, and Google Scholar. ESC Heart Fail. (2019) 6:1291–312. doi: 10.1002/ehf2.12583
Keywords: hypothermia, brain protection, surgery, bibliometric analysis, citations highlights
Citation: Hu L, Geli S, Long F, Nie L, Wu J, Zhou J, Wang M and Chen Y (2024) The 100 most-cited articles in hypothermic brain protection journals: a bibliometric and visualized analysis. Front. Neurol. 15:1433025. doi: 10.3389/fneur.2024.1433025
Received: 15 May 2024; Accepted: 15 October 2024;
Published: 05 November 2024.
Edited by:
Luis Rafael Moscote-Salazar, Colombian Clinical Research Group in Neurocritical Care, ColombiaReviewed by:
Luis Alberto Camputaro, Specialized Institute “Hospital El Salvador”, El SalvadorCopyright © 2024 Hu, Geli, Long, Nie, Wu, Zhou, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maohua Wang, d2FuZ21hb2h1YUBzd211LmVkdS5jbg==; Yingxu Chen, MTE0OTc1MTY0OEBxcS5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.