- 1Emergency Department, Shaoxing People’s Hospital, Shaoxing, Zhejiang, China
- 2Department of Neurology, Shanghai Fifth People’s Hospital, Fudan University, Shanghai, China
- 3Suzhou Industrial Park Monash Research Institute of Science and Technology, Suzhou, Jiangsu Province, China
- 4Southeast University-Monash University Joint Graduate School, Suzhou, Jiangsu Province, China
- 5Monash University-Southeast University Joint Research Institute, Suzhou, Jiangsu Province, China
- 6Department of Neurology, Shanghai Yangpu District Shidong Hospital, Shanghai, China
- 7Department of Military Health Statistics, School of Health Service, People's Liberation Army, Naval Medical University, Shanghai, China
Background: The present study aimed to develop a reliable and straightforward Nomogram by integrating various parameters to accurately predict the likelihood of early neurological deterioration (END) in patients with acute ischemic stroke (AIS).
Methods: Acute ischemic stroke patients from Shaoxing People’s Hospital, Shanghai Yangpu District Shidong Hospital, and Shanghai Fifth People’s Hospital were recruited based on specific inclusion and exclusion criteria. The primary outcome was END. Using the LASSO logistic model, a predictive Nomogram was generated. The performance of the Nomogram was evaluated using the ROC curve, the Hosmer-Lemeshow test, and a calibration plot. Additionally, the decision curve analysis was conducted to assess the effectiveness of the Nomogram.
Results: It was found that the Nomogram generated in the present study showed strong discriminatory performance in both the training and the internal validation cohorts when their ROC-AUC values were 0.715 (95% CI 0.648–0.782) and 0.725 (95% CI 0.631–0.820), respectively. Similar results were observed in two external validation cohorts when their ROC-AUC values were 0.685 (95% CI 0.541–0.829) and 0.673 (95% CI 0.545–0.800), respectively. In addition, CAD, SBP, neutrophils, TBil, and LDL were found to be positively correlated with the occurrence of END post-stroke, while lymphocytes and UA were negatively correlated.
Conclusion: Our study developed a novel Nomogram that includes CAD, SBP, neutrophils, lymphocytes, TBil, UA, and LDL and it demonstrated strong discriminatory performance in identifying AIS patients who are likely to develop END.
Introduction
Ischemic stroke (IS) represents the most common subtype of stroke and is a leading cause of mortality worldwide (1–3). Early neurological deterioration (END) refers to decline in neurological functions occurring within hours or days after acute ischemic stroke (AIS) onset. Previous studies have shown that the incidence of END varies from 5 to 40%, influenced by different inclusion criteria and assessment methods (4–6). Research has shown that END increases the risk of mortality and disability in AIS patients (7, 8). Due to the complex etiology and pathogenesis of END in AIS, there is still a lack of accurate and reliable early predictive markers, as well as effective prevention and treatment strategies. Consequently, it is imperative to investigate risk factors associated with END in AIS patients with an aim to minimize the occurrence of END.
Previous studies have tentatively used a number of predictors to identify END in AIS patients, such as hyperglycemia, baseline NIHSS score, and proximal artery occlusion (9, 10). Duan et al. found that elevated levels of HsCRP were independently correlated with END in AIS patients with atrial fibrillation (11). Furthermore, our previous study suggested that serum total bilirubin may be a potential biomarker for END in ischemic stroke patients (12). Despite these findings, an all-encompassing prognostic score that accurately predicts outcomes of AIS patient is still lacking.
Therefore, this study aimed to develop a Nomogram that integrated various parameters to accurately predict the likelihood of END in AIS patients.
Materials and methods
Subjects of the study
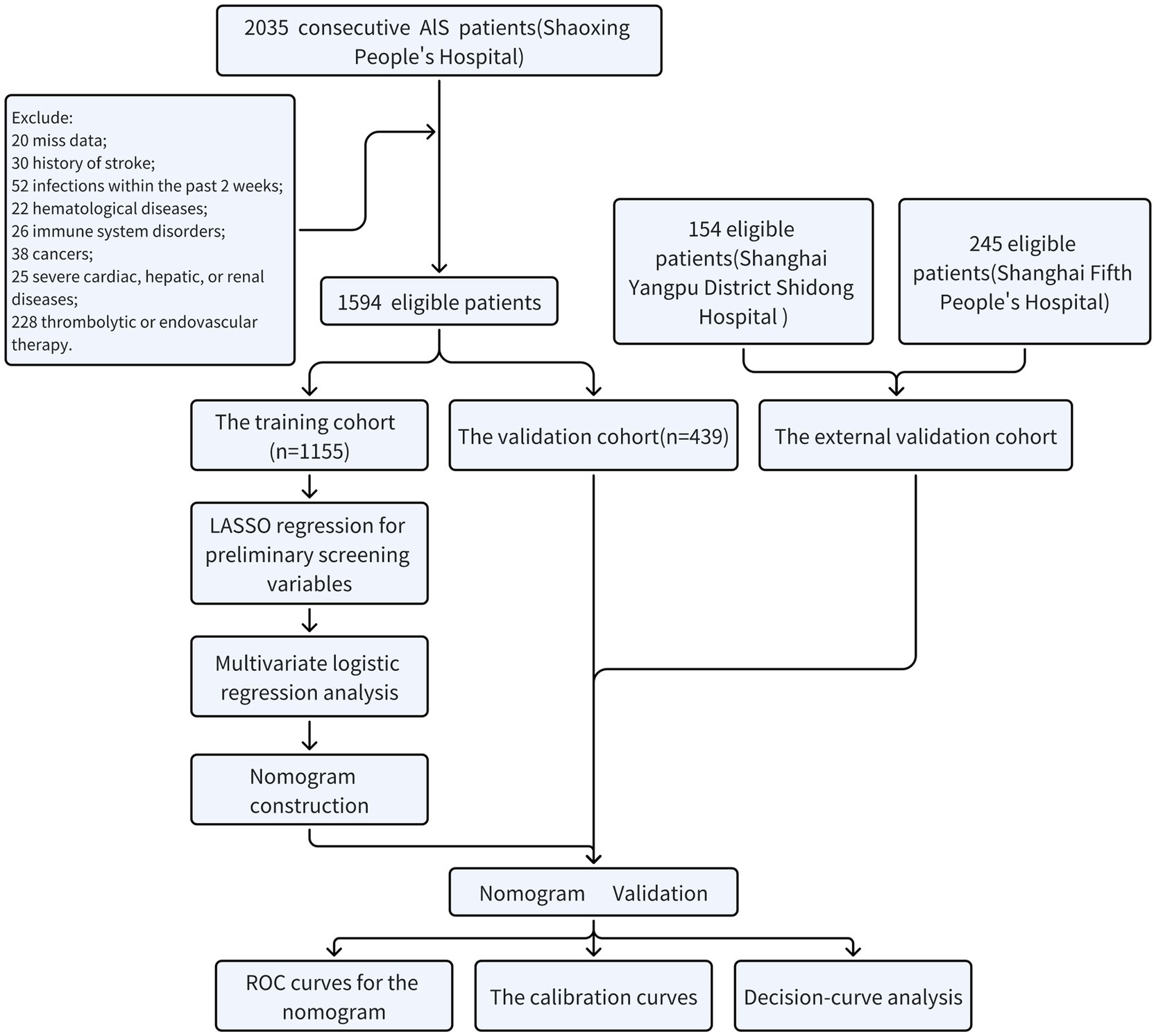
Acute ischemic stroke patients admitted to Shaoxing People’s Hospital from January 2018 to October 2023, Shanghai Yangpu District Shidong Hospital from January 2021 to October 2023, and Shanghai Fifth People’s Hospital from January 2018 to October 2020 were included in the present retrospective study. Inclusion criteria were: (1) aged ≥18 years; (2) completed brain computed tomography or magnetic resonance imaging scans during their hospitalization; (3) symptoms deteriorated within 48 h. Exclusion criteria were: (1) did not complete routine blood tests or were unable to obtain relevant parameters in the emergency department or on the day of admission; (2) history of stroke; (3) infections within the past 2 weeks; (4) patients with hematological diseases; (5) patients with immune system disorders; (6) patients with cancers; (7) patients with severe cardiac, hepatic, or renal diseases; (8) patients who received thrombolytic or endovascular therapy. Figure 1 showed the process of screening eligible participants. Participants in Shaoxing People’s Hospital were randomly allocated to the training and the internal validation cohorts in a 7:3 ratio (13, 14). Participants from Shanghai Yangpu District Shidong Hospital and Shanghai Fifth People’s Hospital were treated as external validation cohorts. They were referred to as the Shidong Hospital cohort and the Fifth Hospital cohort, respectively. This study was approved by the Ethics Committee of Shaoxing People’s Hospital (2021-KY-330-01), Shanghai Fifth People’s Hospital(2018 Ethics Approval NO.001), and Shanghai Yangpu District Shidong Hospital (2021-041-02).
Data collection
Demographic information, physical examination, and laboratory findings were documented upon admission. Demographic information included age, gender, and smoking and drinking habits. Physical examination data included measurements of systolic blood pressure (SBP) and diastolic blood pressure (DBP). Laboratory findings included counts of white blood cells (WBC), neutrophils, lymphocytes, monocytes, platelets, levels of the C-reactive protein (CRP), total bilirubin (TBil), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), apolipoprotein A (apoA), apolipoprotein B (apoB), uric acid (UA), creatinine, urea nitrogen (UN), fasting blood glucose (FBG), glycosylated hemoglobin (GHb), alanine aminotransferase (ALT), and the aspartate aminotransferase (AST). Additionally, information on the medication history (including anticoagulants, antihypertensives, antidiabetics, and statins) as well as comorbidities [such as history of coronary heart disease (CAD), hypertension, diabetes mellitus (DM), and atrial fibrillation (AF)] was collected. Neurological functions were assessed using the National Institutes of Health Stroke Scale (NIHSS) on the admission day and subsequently within the first 7 days by experienced clinicians. Ischemic stroke was classified into subtypes based on the criteria outlined in the Org 10,172 Acute Stroke Treatment Trial (15).
Outcome assessment
The severity of stroke was assessed upon admission and subsequently 2–3 times daily for 7 days using the NIHSS score. All neurologists at each center received standardized training in NIHSS scoring and were blinded to the present study. Each participant underwent assessment by two certified neurologists from their assigned medical team. In cases of disagreement in NIHSS scoring, a third neurologist from each center was consulted to reach a final decision. Early neurological deterioration (END) was defined as an increase of ≥2 points in the NIHSS total score within 7 days of admission.
Statistical analysis
The statistical analysis was performed using the R software version 3.6.2.1 Categorical variables were presented as counts and percentages [n (%)], whereas continuous variables were presented as medians with interquartile ranges (IQR). The independent sample t-test or Mann–Whitney U test was applied to compare continuous variables, and the Chi-square test or Fisher’s exact test was used to compare categorical variables. The LASSO regression analysis was conducted using the “Glmnet” software package to select the optimal subset of features. Based on the results of the LASSO regression analysis, a Nomogram was developed utilizing seven characteristic variables (CAD, SBP, neutrophils, lymphocytes, TBil, UA, and LDL). The performance of the Nomogram was then tested using data from the training cohort, internal validation cohort, and the external cohort. The discriminative performance of the Nomogram was evaluated by calculating the area under the receiver operating characteristic curve (AUC-ROC). Calibration was conducted by generating a calibration plot with 1,000 bootstrap resamples to assess the agreement between the actual and predicted outcomes. The Decision curve analysis was utilized to evaluate the clinical utility of the Nomogram. Statistically significant differences were indicated when p < 0.05.
Results
Baseline characteristics of subjects
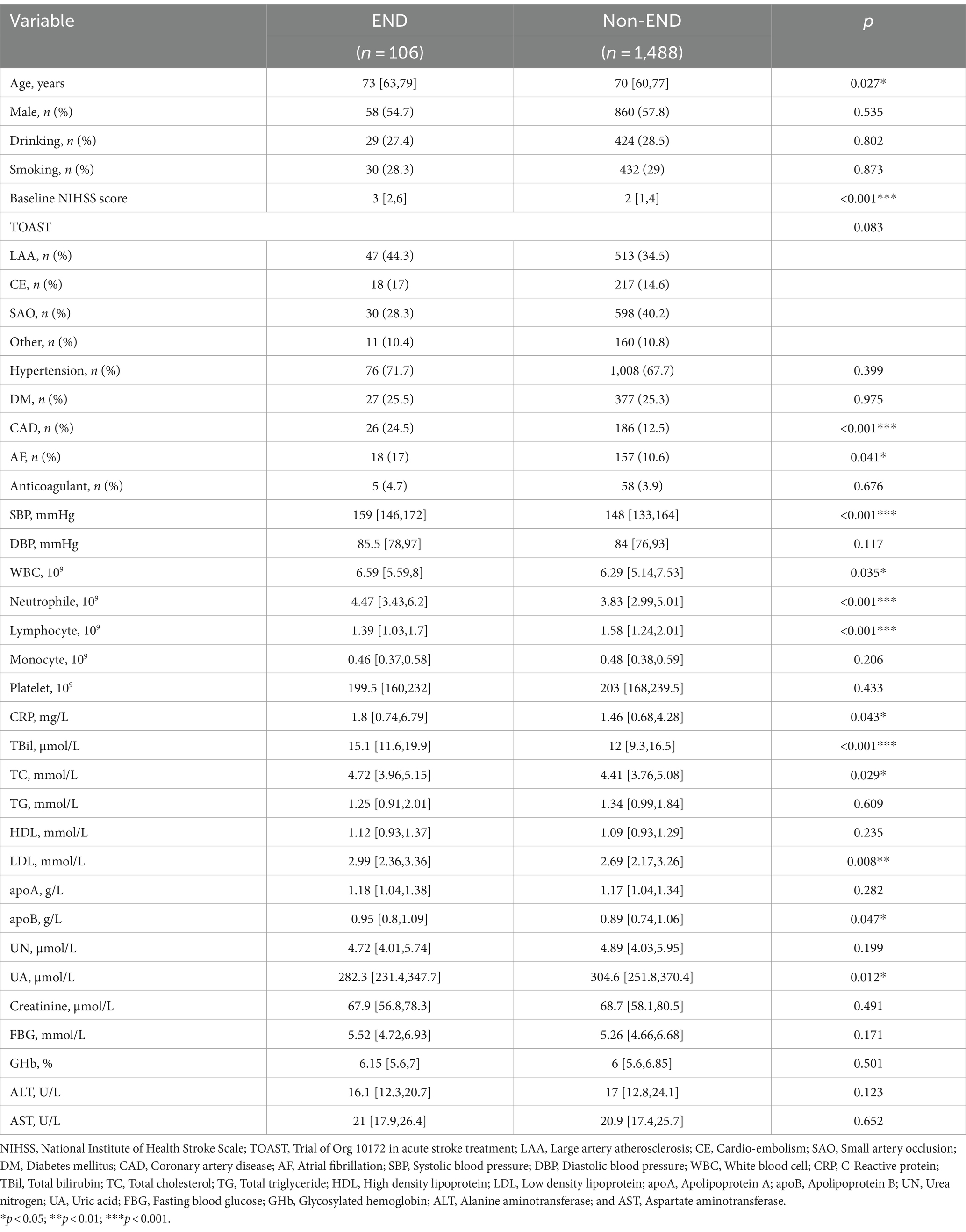
Patients in Shaoxing People’s Hospital were categorized into END and non-END groups. The Univariate analysis revealed that age, baseline NIHSS score, CAD, AF, SBP, WBC, neutrophil, lymphocyte, CRP, TBil, TC, LDL, apoA, and UA were potentially associated with END (p < 0.05). Compared to patients in the non-END group, patients in the END group were older and had higher levels of the baseline NIHSS score, SBP, WBC, neutrophil, CRP, TBil, TC, LDL, apoA, CAD, and AF. In contrast, patients in the END group exhibited a lower count of lymphocytes and a lower level of UA (Table 1).
Patients in Shanghai Yangpu District Shidong Hospital were also divided into the END group and the non-END group. The Univariate analysis demonstrated that age, smoking, and hypertension were statistically significant factors associated with the occurrence of END (p < 0.05). Furthermore, compared to the non-END group, the END group exhibited an older age and a greater proportion of hypertension (Supplementary Table 1).
Patients in Shanghai Fifth People’s Hospital were divided into the END group and the non-END group as well. The Univariate analysis demonstrated that hypertension and the use of anticoagulant drugs were statistically significant factors associated with the occurrence of END (p < 0.05). The END group had a higher proportion of patients with hypertension and anticoagulant drug use compared to the non-END group (Supplementary Table 2).
Baseline characteristics of the training and the validation cohorts
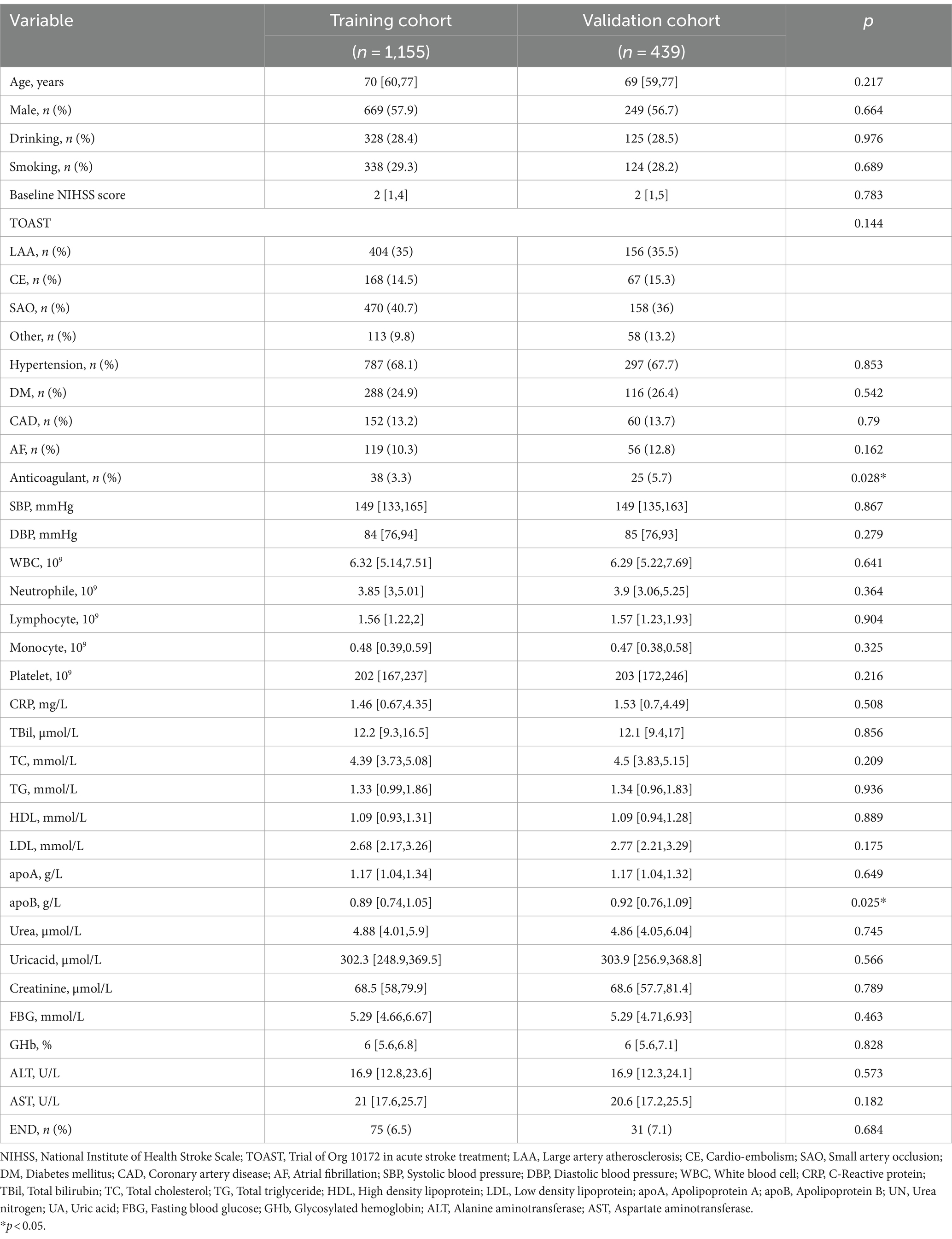
Patients in Shaoxing People’s Hospital were randomly assigned to the training and the internal validation cohorts in a ratio of 7:3. The training cohort consisted of 1,155 patients (median age: 70 years; 57.9% male), whereas the internal validation cohort consisted of 439 patients (median age: 69 years; 56.7% male). Table 2 displayed the demographic and clinical characteristics of the two cohorts. No significant difference in END was observed between the two cohorts (6.5 vs. 7.1%, p = 0.684). However, significant difference was found in the level of ApoB (p = 0.025) and the use of anticoagulant drugs (p = 0.028) between the cohorts, while no significant difference was observed in other variables.
Additionally, detailed baseline characteristics of patients from Shanghai Yangpu District Shidong Hospital and Shanghai Fifth People’s Hospital can be found in Supplementary Table 3. The median age of patients enrolled in the study at Shanghai Yangpu District Shidong Hospital was 62 years, and male patients accounted for 73.4%. The most common vascular risk factor observed was hypertension (42.9%), followed by DM (27.3%). A total of 16 patients with END were documented, representing a prevalence of 10.4%. In Shanghai Fifth People’s Hospital, the median age of enrolled patients was 68 years, and 68.2% were males. The predominant vascular risk factor observed was hypertension (65.7%), followed by DM (36.3%). A total of 18 patients of END were recorded, indicating a prevalence of 7.3% (Supplementary Table 3).
The Univariate analysis of risk factors associated with END in the training cohort
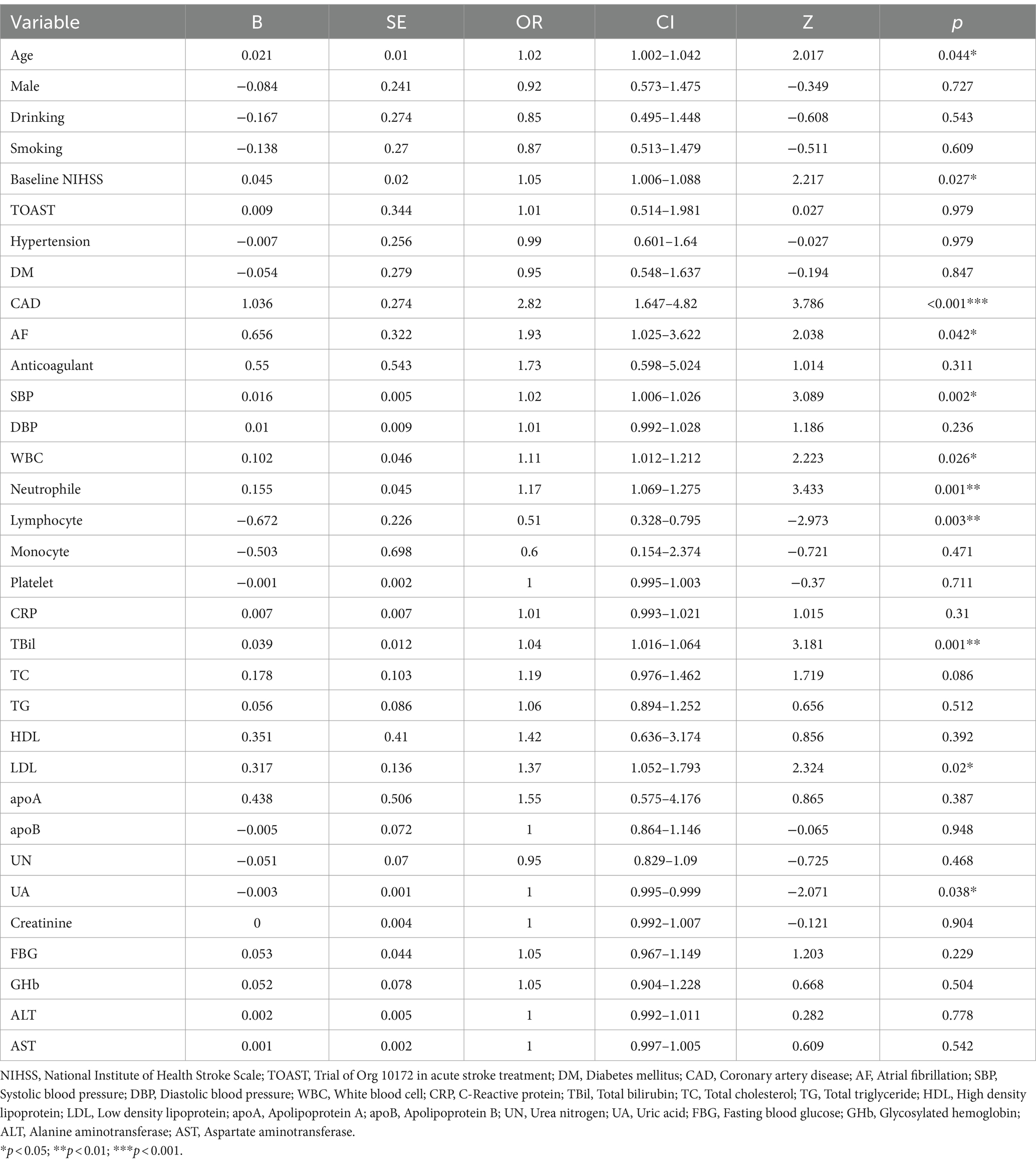
The single-factor logistic regression analysis was conducted on risk factors associated with END within the training cohort. It was found that age (OR 1.02, 95% CI 1.002–1.042, p = 0.044), baseline NIHSS (OR 1.05, 95% CI 1.006–1.088, p = 0.027), CAD (OR 2.82, 95% CI 1.647–4.82, p < 0.001), AF (OR 1.93, 95% CI 1.025–3.622, p = 0.042), SBP (OR 1.02, 95% CI 1.006–1.026, p = 0.002), WBC (OR 1.11, 95% CI 1.012–1.212, p = 0.026), neutrophils (OR 1.17, 95% CI 1.069–1.275, p = 0.001), lymphocytes (OR 0.51, 95% CI 0.328–0.795, p = 0.003), TBil (OR 1.04, 95% CI 1.016–1.064, p = 0.001), LDL (OR 1.37, 95% CI 1.052–1.793, p = 0.02), and UA (OR 1, 95% CI 0.995–0.999, p = 0.038) were significantly associated with END (Table 3).
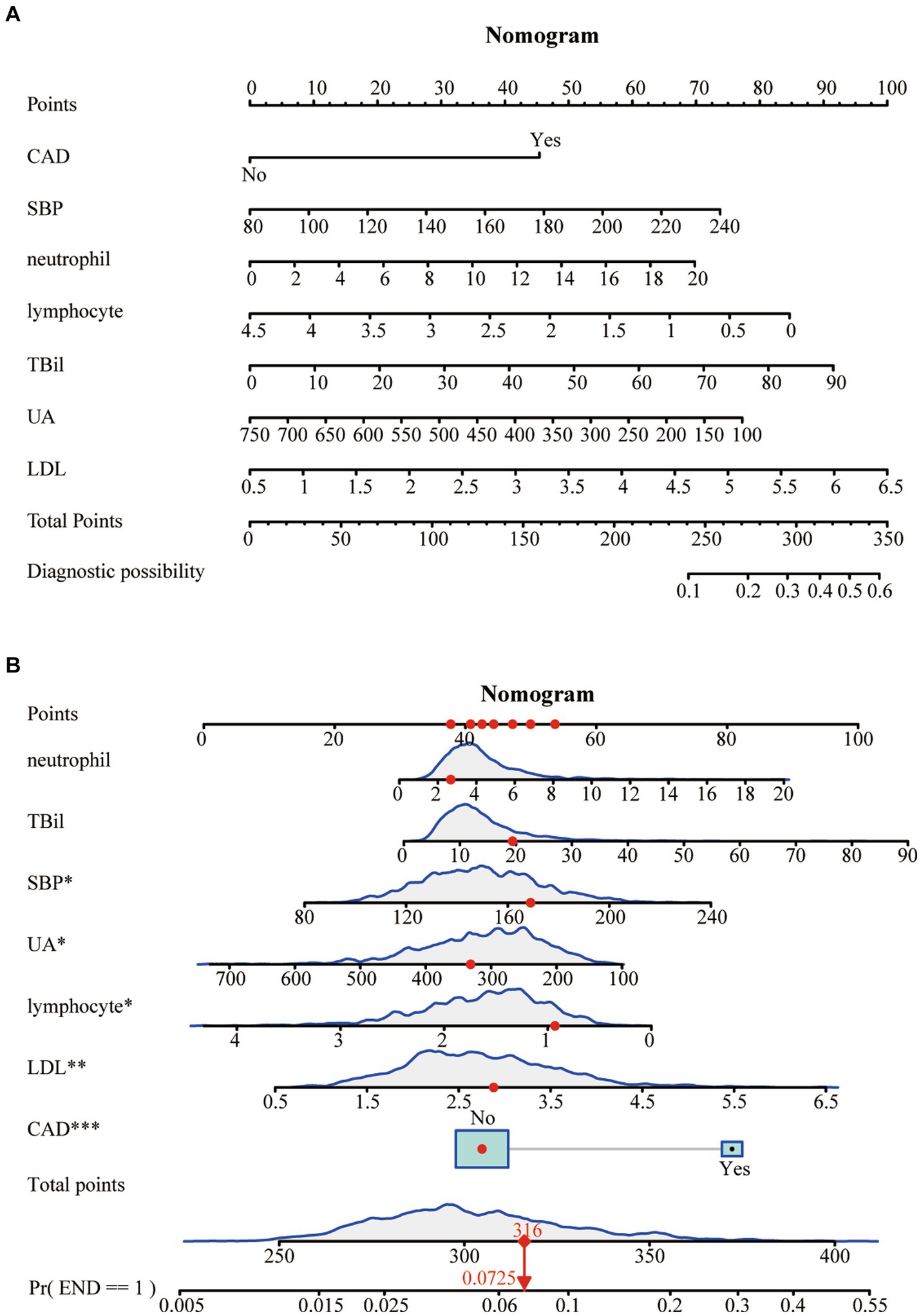
The LASSO regression analysis
To mitigate potential multicollinearity among variables, the LASSO regression analysis was utilized to identify key variables (Figure 2A). This employed 10-fold cross-validation to select the Lambda parameter that minimized the mean square error, which yielded the optimal model (Figure 2B). Seven significant variables were identified: CAD, SBP, neutrophils, lymphocytes, TBil, UA, and LDL, with a Lambda value of 0.01233904.
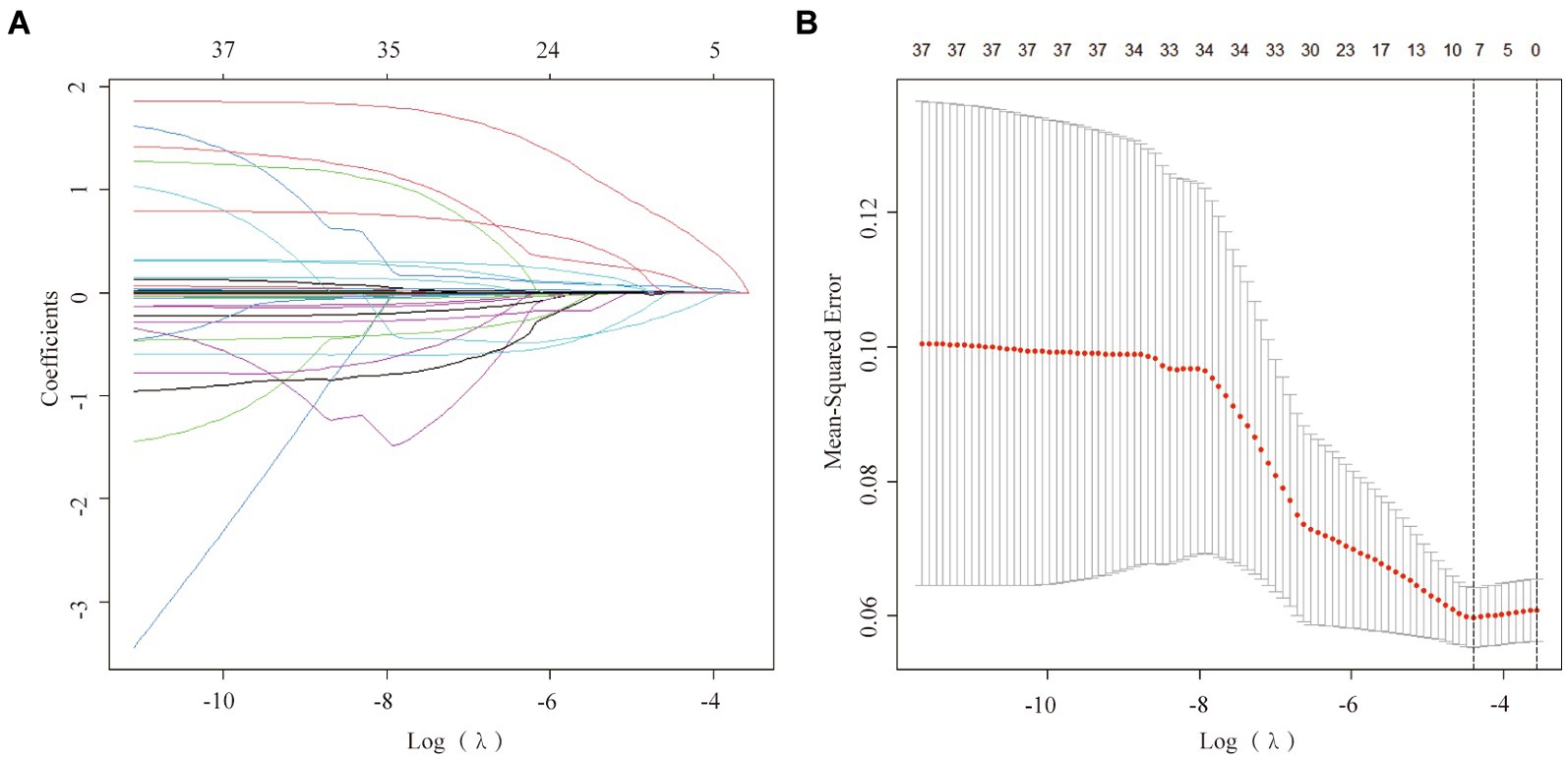
Figure 2. Factor selection using the least absolute shrinkage and selection operator (LASSO) logistic regression. (A) The LASSO coefficient profiles of the candidate variables. The binomial deviance is plotted versus log (λ). (B) Tuning parameter (λ) selection in the LASSO logistic regression performed using 10-fold cross-validation via the minimum criteria.
The Multivariable logistic regression analysis was performed on these seven characteristic variables obtained from the LASSO regression analysis (Table 4). After adjusting all potential confounders, CAD (OR 3.09, 95% CI 1.732–5.505, p < 0.001), SBP (OR 1.01, 95% CI 1–1.023, p = 0.038), lymphocytes (OR 0.63, 95% CI 0.402–0.976, p = 0.039), UA (OR 1, 95% CI 0.995–0.999, p = 0.034), and LDL (OR 1.51, 95% CI 1.132–2.022, p = 0.005) were identified as independent predictors of END in this model (Table 4).
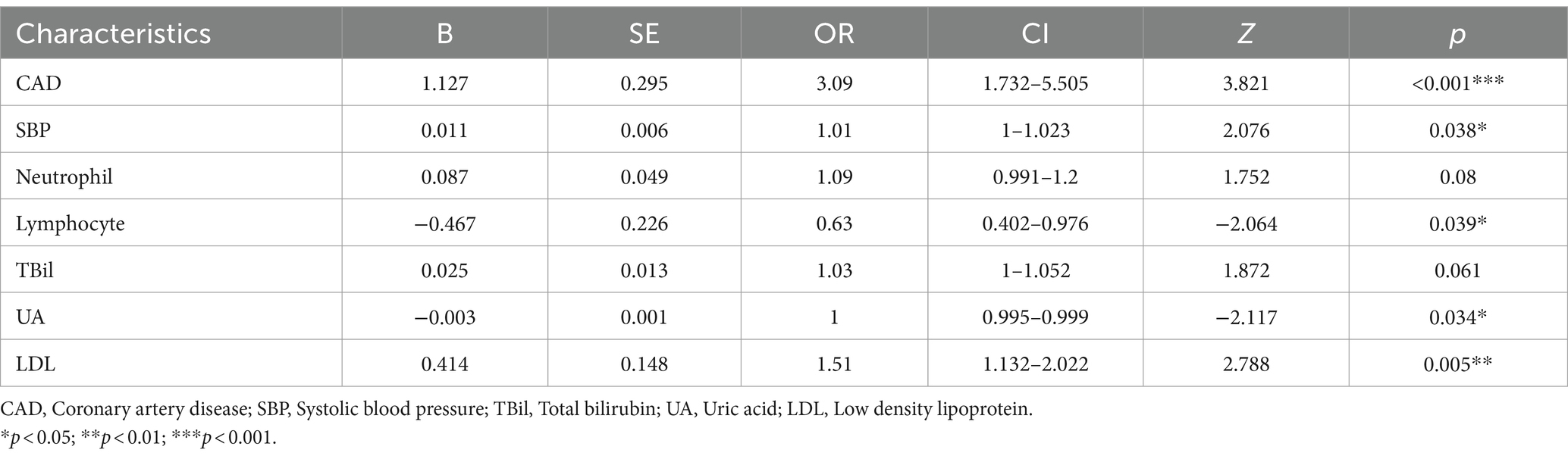
Construction of the nomogram
A Nomogram was constructed based on the seven characteristic variables (CAD, SBP, neutrophils, lymphocytes, TBil, UA, and LDL) identified by the LASSO regression analysis. It predicted the probability of END by assigning scores to each independent predictor on a scale from 0 to 100. Higher cumulative scores on the Nomogram indicated an elevated risk of END, whereas lower scores indicated a reduced probability (Figure 3A). For example, when the values for neutrophils = 2.7*109, total bilirubin = 20 μmoL/L, SBP = 170 mmHg, uric acid = 320 μmoL/L, lymphocytes = 0.9*109, LDL = 2.9 mmoL/L, and no history of CAD were entered, the estimated risk of END was 7.25% (Figure 3B).
Validation of the nomogram in the training and the validation cohorts
The training cohort demonstrated a Hosmer-Lemeshow χ2 of 8.14 (p = 0.519), with an area under the ROC curve of 0.715 (95% CI 0.648–0.782) (Figure 4A). In the internal validation cohort, the Hosmer-Lemeshow χ2 was 4.457 (p = 0.879), and the area under the ROC curve was 0.725 (95% CI 0.631–0.820) (Figure 4B). Furthermore, in the patient cohorts enrolled at Shanghai Yangpu District Shidong Hospital, the Hosmer-Lemeshow χ2 was 12.02 (p = 0.212), with an area under the ROC curve of 0.685 (95% CI 0.541–0.829) (Figure 4C). In the patient cohort enrolled in Shanghai Fifth People’s Hospital, the Hosmer-Lemeshow χ2 was 6.86 (p = 0.652), with an area under the ROC curve of 0.673 (95% CI 0.545–0.800) (Figure 4D). These findings further demonstrated strong calibration and discrimination. Calibration plots, comparing the predicted probability of END using the Nomogram to the observed probability of END, revealed a significant prediction accuracy in all the cohorts (Figure 5).
Figure 4. ROC curves were generated for the Nomogram in the training cohort (A), the internal validation cohort (B), the Shidong Hospital validation cohort (C), and the Shanghai Fifth Hospital validation cohort (D).
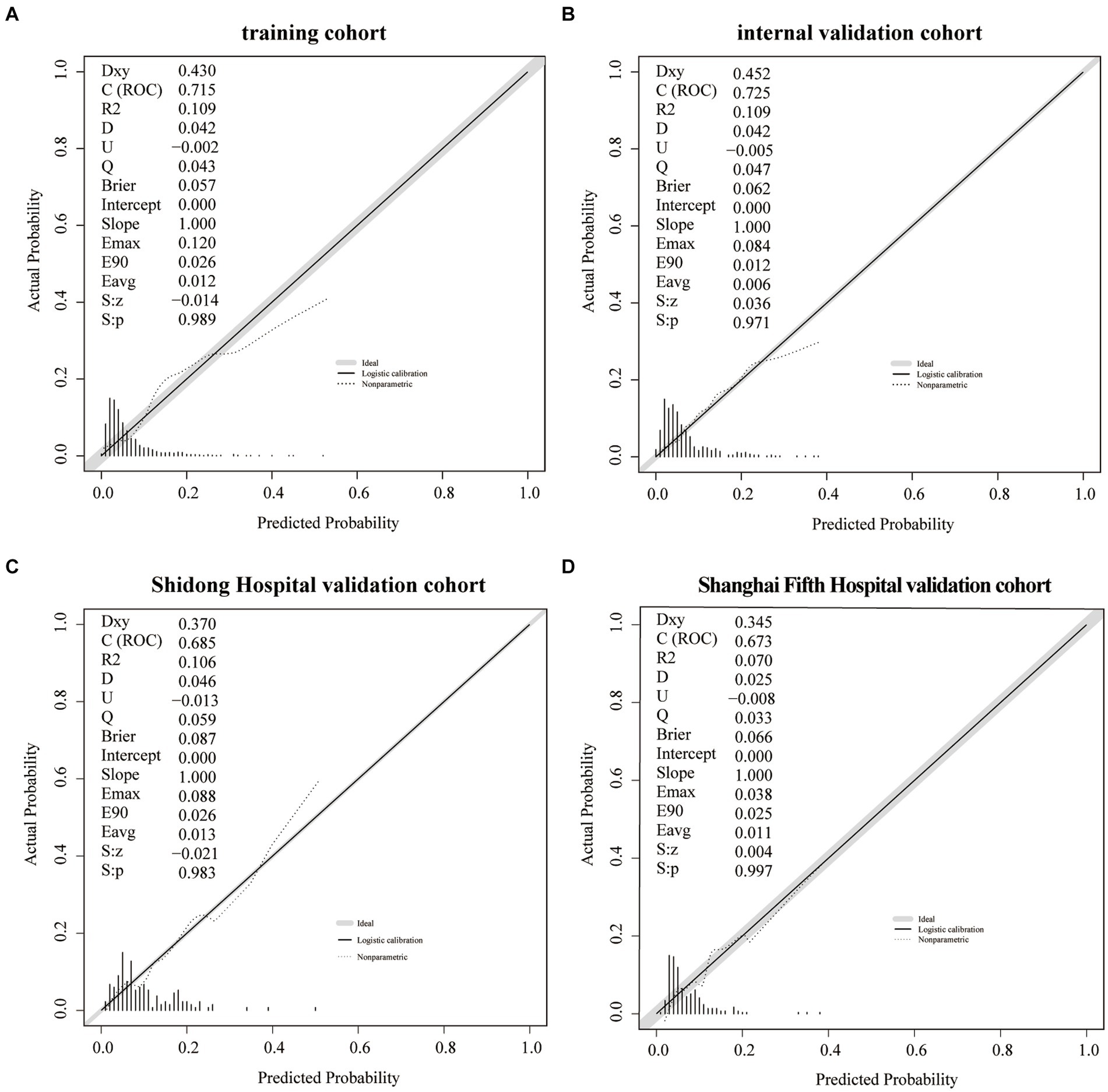
Figure 5. A calibration plot of the Nomogram in the training cohort (A), the internal validation cohort (B), the Shidong Hospital validation cohort (C), and the Shanghai Fifth Hospital validation cohort (D).
The Decision curve analysis was performed on the Nomogram to evaluate its predicability for END and to determine whether the model provides a greater net benefit. The Nomogram showed that when the risk thresholds ranged from 0 to 0.2 in the training cohort (Figure 6A), from 0 to 0.25 in the internal validation cohort (Figure 6B), from 0 to 0.55 in the Shanghai Yangpu District Shidong Hospital validation cohort (Figure 6C) and from 0 to 0.4 in the Shanghai Fifth People’s Hospital validation cohort (Figure 6D), adopting the strategy with the greatest net benefit ensured that all AIS patients would choose this strategy, thus ensuring that no AIS patient would miss out on it. At a risk threshold of 0.1 for AIS patients, net benefits of 2, 1.6, 2.5, and 1% in the training and validation cohorts, respectively, were yielded (Figure 6).
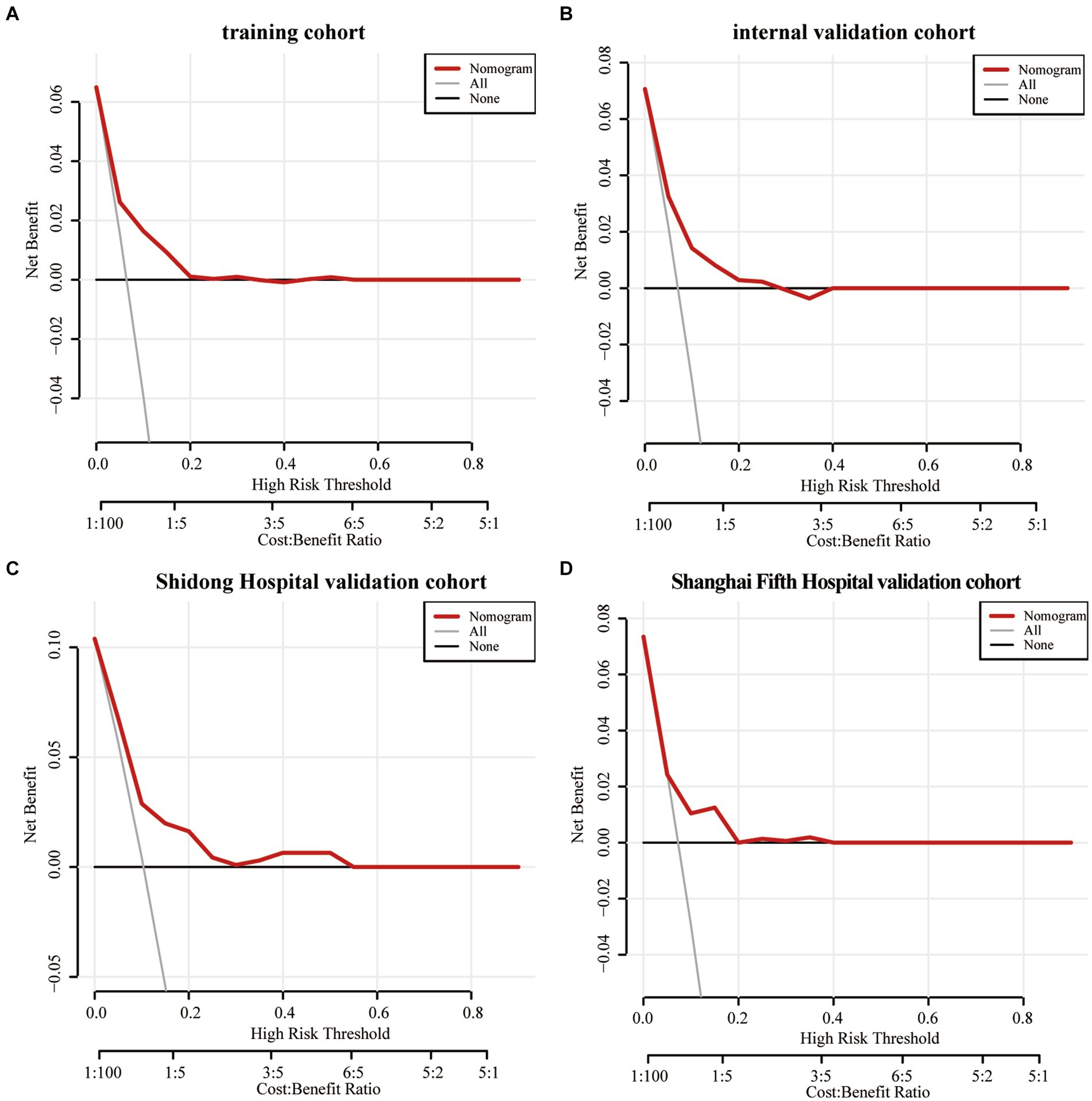
Figure 6. The decision curve analysis of the Nomogram of the training cohort (A), the internal validation cohort (B), the Shidong Hospital validation cohort (C), and the Shanghai Fifth Hospital validation cohort (D).
Comparison between different indicators in the nomogram
The ROC analysis was performed for each indicator in the Nomogram. The results demonstrated that the AUC of neutrophils was larger than that of other indicators in the internal validation cohort (AUC = 0.653, 95%CI 0.556–0.750) and Shanghai Yangpu District Shidong Hospital validation cohort (AUC = 0.658, 95%CI 0.561–0.754). Conversely, in the Shanghai Fifth People’s Hospital validation cohort, the total bilirubin AUC surpassed other indicators (AUC = 0.628, 95%CI 0.500–0.756) (Supplementary Figure 1).
Discussion
The present study introduced a novel Nomogram that included CAD, SBP, neutrophils, lymphocytes, TBil, UA, and LDL as predictors for END in AIS patients. These variables are routinely collected in the clinical setting; therefore, the Nomogram is practical for risk stratification. ROC curves and calibration plots were used in both the training and validation cohorts to evaluate the discrimination and calibration performance of the Nomogram. The results demonstrated ROC-AUC values of 0.715 (95% CI 0.648–0.782) and 0.725 (95% CI 0.631–0.820) in the training and the internal validation cohorts, respectively, signifying strong discriminatory performance. The external validation cohorts also exhibited ROC-AUC values of 0.685 (95% CI 0.541–0.829) and 0.673 (95% CI 0.545–0.800), indicating good discriminatory performance as well. Additionally, calibration curves provided further evidence on the model’s reliable calibration capacity. Importantly, CAD, SBP, neutrophil, TBil, and LDL were positively correlated with END occurrence post-stroke, while lymphocytes and UA were negatively correlated.
Previous studies have constructed other models to predict the risk of developing END in AIS patients. However, their scoring systems were limited in the ability to predict END using a small number of parameters. Wang et al. (16) developed a scoring system to predict END based on MRI-derived radiomics and clinical metrics. However, MRI examination is expensive and time-consuming, which may negatively impact the timing of diagnosis and therapy and increase the probability of END. Xie et al. (17) developed a prediction model consisting of the initial NIHSS score, middle cerebral artery stenosis, and carotid stenosis ≥50% to predict END in AIS patients. However, this study was conducted in a single center and included a relatively small number of patients. In contrast, our multi-center study effectively addressed these concerns by analyzing a larger patient cohort, which enhanced the reliability of the findings.
Coronary heart diseases, hypertension, and ischemic stroke often simultaneously occur and share common risk factors. Previous studies have shown that patients with ischemic stroke may experience clinical symptoms of coronary artery diseases, and effective management of coronary heart diseases might potentially reduce the risk of ischemic stroke (18). A substantial 10-year study conducted in China further substantiated the notion that coronary heart diseases may independently predict the recurrence of stroke (19). Our study further reinforces this concept by highlighting that coronary heart diseases independently predict END in AIS patients. Hypertension is a significant risk factor of AIS and can lead to more severe neurological deficits, worsening functional outcomes of AIS patients. This is likely attributed to the complex interplay between cerebrovascular autoregulation, neuronal activity, and brain bioenergetics (20). Turana et al. (21) reported a positive correlation between systolic blood pressure (SBP) and the incidence of stroke. In many Asian countries, adherence to hypertension treatment is crucial for stroke prevention. A cohort study by Zhou et al. (22) identified SBP as a risk factor for predicting the recurrence of ischemic stroke, which is consistent with our data demonstrating a strong correlation between SBP and END in patients with AIS. Therefore, effective management of coronary heart diseases and blood pressure has the potential to mitigate adverse outcomes in patients with AIS.
Oxidative stress, inflammatory response, and cholesterol accumulation collectively contribute to the progression of stroke (23). Studies have shown that ischemia–reperfusion injury generates a significant amount of oxygen free radicals, which result in neuronal oxidative stress and lead to compromised functions of cellular components including proteins, phospholipids, and nucleic acids. Such damage also impacts the structure of mitochondria and triggers the release of excitatory amino acids, ultimately accelerating neuronal necrosis (24). Post-ischemic brain injury activates the nuclear factor κB, which upregulates the expression of inflammatory factors, chemokines, and cell adhesion molecules. This cascade of events recruits inflammatory cells to the site of injury, resulting in microvascular occlusion, generation of oxygen free radicals, release of cytotoxic enzymes, and vasomotor changes that exacerbate post-ischemic brain injury (25, 26).
Uric acid, an essential endogenous antioxidant, plays a crucial role in protecting the brain from oxidative injury by inhibiting the accumulation of reactive oxygen species and the formation of nitrotyrosine (27). Animal studies have shown that high concentrations of uric acid can ameliorate brain tissue injury and the production of reactive oxygen species in a rat model of middle cerebral artery occlusion (28). Furthermore, a prospective study involving 881 AIS patients revealed an inverse relationship between neurological impairment, lesion volume on admission, and the level of uric acid (29). Our study supports these findings by demonstrating a negative association between the concentration of uric acid and the occurrence of END in AIS patients. Some researchers proposed that neutrophils may enhance the immune response in patients with ischemic stroke by producing pro-inflammatory cytokines (30) and triggering the expression of matrix metalloproteinase-9 (MMP-9), thereby contributing to the disruption of the blood–brain barrier and exacerbating brain damage (31). Conversely, lymphocytes are thought to have a protective role in inflammatory response to ischemic injury (32). A decrease in lymphocytes may indicate a stress response associated with cortisol, potentially increasing the production of pro-inflammatory cytokines (33). Our study demonstrated that neutrophils and lymphocytes were independent risk factors for END after AIS, with neutrophils positively correlated and lymphocytes negatively correlated with END occurrence. These findings are consistent with those of a previous study (34). Additionally, bilirubin can activate microglia and induce the release of inflammatory factors, such as tumor necrosis factor alpha (TNF-α), interleukin 1β, and interleukin 6, thus promoting neurotoxicity (35, 36). A study showed a positive correlation between the level of the serum bilirubin and the NIHSS, suggesting that higher concentrations of the serum bilirubin were associated with more severe stroke, which may be served as useful markers to reflect the severity of illness (37). Our findings also support this, showing a positive correlation between total bilirubin and the occurrence of END in AIS patients. Higher plasma levels of LDL result in increased adhesion of circulating monocytes to arterial endothelial cells, promoting LDL entry into the vascular intima (38). This process can cause damage to endothelial cells, formation of foam cells, activation of cell surface receptors on monocytes and vascular smooth muscle cells, initiation of inflammation, and promotion of AIS development (38, 39). A meta-analysis showed that for each 1 mmol/L decrease in LDL-C, there was a 17% decrease in the incidence of stroke (40, 41). A study by Amarenco et al. investigated the target level of LDL cholesterol for secondary prevention of cerebrovascular events. They found that maintaining the level of LDL below 1.8 mmol/L in patients with ischemic stroke or transient ischemic attack and evidence of atherosclerosis was associated with a lower risk of vascular events compared to those with their LDL between 2.3 and 2.8 mmol/L (42). Our study further confirmed that LDL is an independent predictor of END in AIS patients.
The Decision curve analysis (DCA) is a novel approach for evaluating the performance of predictive models, such as nomograms (43, 44). The results of the DCA in this study demonstrated the efficacy of nomograms in predicting END and illustrating the overall benefit of clinical outcomes at various threshold probabilities. A net benefit of zero in DCA indicates that no treatment would be required for AIS patients. AIS patients at a higher risk of END may benefit from additional interventions, including endovascular therapy, hematoma resection, cranial decompression, or specific medications. Results of the DCA supported the idea that the Nomogram can effectively identify high-risk patients who may develop END.
To the best of our knowledge, there is limited research conducted in a multicenter format exploring the predictive role of the Nomogram in assessing END in AIS patients. In this study, we developed a new Nomogram model specifically designed to predict END in AIS patients. The Nomogram model exhibited excellent predictive performance and accuracy. Furthermore, the Nomogram prediction model identified key clinical factors that can be utilized for early prediction of END in AIS patients, thereby maximizing clinical benefits.
However, this study has a number of limitations that should be acknowledged. Firstly, being a retrospective study, it is susceptible to selection and recall bias. Secondly, certain inflammatory biomarkers, such as interleukin 6 and tumor necrosis factor α, were not measured in this study and will be evaluated in future research. Furthermore, the participants were exclusively from East China, it is challenging to extrapolate our findings to other populations. Therefore, it is essential to test the Nomogram in other populations to ensure its generalizability beyond China.
Conclusion
Our study developed a novel Nomogram that included CAD, SBP, neutrophils, lymphocytes, TBil, UA, and LDL to predict the risk of developing END in AIS patients. The number of neutrophils and the level of total bilirubin were strong predictors of END. Future large-scale studies are needed to improve the accuracy of the Nomogram model in predicting END in AIS patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
This study was approved by the Ethics Committee of Shaoxing People’s Hospital (2021-KY-330-01), Shanghai Fifth People’s Hospital (2018 Ethics Approval NO.001), and Shanghai Yangpu District Shidong Hospital (2021-041-02). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YZ: Data curation, Formal analysis, Writing – original draft. YL: Data curation, Formal analysis, Writing – review & editing. HL: Data curation, Formal analysis, Writing – review & editing. ZW: Data curation, Formal analysis, Writing – review & editing. XY: Formal analysis, Writing – review & editing. PZ: Writing – review & editing. DW: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Shanghai Committee of Science and Technology (Grant No. 23JC1401803), Enbipu Co., Ltd. of Shiyao Group (Grant No. YXSY-2022-24), the Zhejiang Medical Science and Technology Project (Grant No. 2023RC287), and Shaoxing Basic Public Welfare Program (Grant No. 2022A14018).
Acknowledgments
The authors appreciate the valuable suggestions from other members of their teams.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1433010/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | ROC curves were generated to evaluate the performance of different indicators within the Nomogram using the internal validation cohort (A), the Shidong Hospital validation cohort (B), and the Shanghai Fifth Hospital validation cohort (C).
Footnotes
References
1. Liu, L, Villavicencio, F, Yeung, D, Perin, J, Lopez, G, Strong, KL, et al. National, regional, and global causes of mortality in 5-19-year-olds from 2000 to 2019: a systematic analysis. Lancet Glob Health. (2022) 10:e337–47. doi: 10.1016/S2214-109X(21)00566-0
2. Shim, R, and Wong, CH. Ischemia, immunosuppression and infection—tackling the predicaments of post-stroke complications. Int J Mol Sci. (2016) 17:64. doi: 10.3390/ijms17010064
3. Eren, F, and Yilmaz, SE. Neuroprotective approach in acute ischemic stroke: a systematic review of clinical and experimental studies. Brain Circ. (2022) 8:172–9. doi: 10.4103/bc.bc_52_22
4. Hou, L, Zhang, Y, Zheng, D, Shi, H, Zou, C, Zhang, H, et al. Increasing trimethylamine N-oxide levels as a predictor of early neurological deterioration in patients with acute ischemic stroke. Neurol Res. (2020) 42:153–8. doi: 10.1080/01616412.2019.1710416
5. Kwon, HM, Lee, YS, Bae, HJ, and Kang, DW. Homocysteine as a predictor of early neurological deterioration in acute ischemic stroke. Stroke. (2014) 45:871–3. doi: 10.1161/STROKEAHA.113.004099
6. Kanamaru, T, Suda, S, Muraga, K, Okubo, S, Watanabe, Y, Tsuruoka, S, et al. Albuminuria predicts early neurological deterioration in patients with acute ischemic stroke. J Neurol Sci. (2017) 372:417–20. doi: 10.1016/j.jns.2016.11.007
7. Liu, P, Liu, S, Feng, N, Wang, Y, Gao, Y, and Wu, J. Association between neurological deterioration and outcomes in patients with stroke. Ann Transl Med. (2020) 8:4. doi: 10.21037/atm.2019.12.36
8. Mori, M, Naganuma, M, Okada, Y, Hasegawa, Y, Shiokawa, Y, Nakagawara, J, et al. Early neurological deterioration within 24 hours after intravenous rt-PA therapy for stroke patients: the stroke acute management with urgent risk factor assessment and improvement rt-PA registry. Cerebrovasc Dis. (2012) 34:140–6. doi: 10.1159/000339759
9. Seners, P, Turc, G, Oppenheim, C, and Baron, JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. (2015) 86:87–94. doi: 10.1136/jnnp-2014-308327
10. Liu, H, Liu, K, Zhang, K, Zong, C, Yang, H, Li, Y, et al. Early neurological deterioration in patients with acute ischemic stroke: a prospective multicenter cohort study. Ther Adv Neurol Disord. (2023) 16:81538465. doi: 10.1177/17562864221147743
11. Duan, Z, Guo, W, Tang, T, Tao, L, Gong, K, and Zhang, X. Relationship between high-sensitivity C-reactive protein and early neurological deterioration in stroke patients with and without atrial fibrillation. Heart Lung. (2020) 49:193–7. doi: 10.1016/j.hrtlng.2019.10.009
12. Zhong, P, Wu, D, Ye, X, Wang, X, Zhou, Y, Zhu, X, et al. Association of circulating total bilirubin level with ischemic stroke: a systemic review and meta-analysis of observational evidence. Ann Transl Med. (2019) 7:335. doi: 10.21037/atm.2019.06.71
13. Ping, Z, Min, L, Qiuyun, L, Xu, C, and Qingke, B. Prognostic nomogram for the outcomes in acute stroke patients with intravenous thrombolysis. Front Neurosci. (2022) 16:1017883. doi: 10.3389/fnins.2022.1017883
14. Jin, G, Hu, W, Zeng, L, Diao, M, Chen, H, Chen, J, et al. Development and verification of a nomogram for predicting short-term mortality in elderly ischemic stroke populations. Sci Rep. (2023) 13:12580. doi: 10.1038/s41598-023-39781-4
15. Adams, HJ, Bendixen, BH, Kappelle, LJ, Biller, LJ, Love, LB, Gordon, DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
16. Wang, J, Fu, K, Wang, Z, Wang, N, Wang, X, Xu, T, et al. MRI-based clinical-radiomics nomogram to predict early neurological deterioration in isolated acute pontine infarction: a two-center study in Northeast China. BMC Neurol. (2024) 24:39. doi: 10.1186/s12883-024-03533-2
17. Xie, X, Xiao, J, Wang, Y, Pan, L, Ma, J, Deng, L, et al. Predictive model of early neurological deterioration in patients with acute ischemic stroke: a retrospective cohort study. J Stroke Cerebrovasc Dis. (2021) 30:105459. doi: 10.1016/j.jstrokecerebrovasdis.2020.105459
18. Zhu, H, Li, Z, Lv, J, and Zhao, R. Effects of cerebral small vessel disease on the outcome of patients with ischemic stroke caused by large artery atherosclerosis. Neurol Res. (2018) 40:381–90. doi: 10.1080/01616412.2018.1446283
19. Xu, J, Zhang, X, Jin, A, Pan, Y, Li, Z, Meng, X, et al. Trends and risk factors associated with stroke recurrence in China, 2007-2018. JAMA Netw Open. (2022) 5:e2216341. doi: 10.1001/jamanetworkopen.2022.16341
20. Ferrari, F, and Villa, RF. Brain bioenergetics in chronic hypertension: risk factor for acute ischemic stroke. Biochem Pharmacol. (2022) 205:115260. doi: 10.1016/j.bcp.2022.115260
21. Turana, Y, Tengkawan, J, Chia, YC, Nathaniel, M, Wang, JG, Sukonthasarn, A, et al. Hypertension and stroke in Asia: a comprehensive review from HOPE Asia. J Clin Hypertens. (2021) 23:513–21. doi: 10.1111/jch.14099
22. Zhuo, Y, Wu, J, Qu, Y, Yu, H, Huang, X, Zee, B, et al. Clinical risk factors associated with recurrence of ischemic stroke within two years: a cohort study. Medicine (Baltimore). (2020) 99:e20830. doi: 10.1097/MD.0000000000020830
23. Choi, SH, Sviridov, D, and Miller, YI. Oxidized cholesteryl esters and inflammation. Biochim Biophys Acta Mol Cell Biol Lipids. (2017) 1862:393–7. doi: 10.1016/j.bbalip.2016.06.020
24. Poh Loh, K, Hong Huang, S, de Silva, R, Tan, BH, and Zhun Zhu, Y. Oxidative stress: apoptosis in neuronal injury. Curr Alzheimer Res. (2006) 3:327–37. doi: 10.2174/156720506778249515
25. Chu, AJ . Tissue factor mediates inflammation. Arch Biochem Biophys. (2005) 440:123–32. doi: 10.1016/j.abb.2005.06.005
26. Macrez, R, Ali, C, Toutirais, O, le Mauff, B, Defer, G, Dirnagl, U, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. (2011) 10:471–80. doi: 10.1016/S1474-4422(11)70066-7
27. Amaro, S, Jiménez-Altayó, F, and Chamorro, Á. Uric acid therapy for vasculoprotection in acute ischemic stroke. Brain Circ. (2019) 5:55–61. doi: 10.4103/bc.bc_1_19
28. Zhang, B, Yang, N, Lin, SP, and Zhang, F. Suitable concentrations of uric acid can reduce cell death in models of OGD and cerebral ischemia-reperfusion injury. Cell Mol Neurobiol. (2017) 37:931–9. doi: 10.1007/s10571-016-0430-8
29. Chamorro, A, Obach, V, Cervera, A, Revilla, M, Deulofeu, R, Aponte, JH. Prognostic significance of uric acid serum concentration in patients with acute ischemic stroke. Stroke. (2002) 33:1048–52. doi: 10.1161/hs0402.105927
30. Neuberger, U, Möhlenbruch, MA, Herweh, C, Ulfert, C, Bendszus, M, and Pfaff, J. Classification of bleeding events: comparison of ECASS III (European cooperative acute stroke study) and the new Heidelberg bleeding classification. Stroke. (2017) 48:1983–5. doi: 10.1161/STROKEAHA.117.016735
31. Jin, P, Li, X, Chen, J, Zhang, ZR, Hu, WW, Chen, LY, et al. Platelet-to-neutrophil ratio is a prognostic marker for 90-days outcome in acute ischemic stroke. J Clin Neurosci. (2019) 63:110–5. doi: 10.1016/j.jocn.2019.01.028
32. Gusdon, AM, Gialdini, G, Kone, G, Baradaran, H, Merkler, AE, Mangat, HS, et al. Neutrophil-lymphocyte ratio and perihematomal edema growth in intracerebral hemorrhage. Stroke. (2017) 48:2589–92. doi: 10.1161/STROKEAHA.117.018120
33. Park, BJ, Shim, JY, Lee, HR, Lee, JH, Jung, DH, Kim, HB, et al. Relationship of neutrophil-lymphocyte ratio with arterial stiffness and coronary calcium score. Clin Chim Acta. (2011) 412:925–9. doi: 10.1016/j.cca.2011.01.021
34. Petrone, AB, Eisenman, RD, Steele, KN, Mosmiller, LT, Urhie, O, and Zdilla, MJ. Temporal dynamics of peripheral neutrophil and lymphocytes following acute ischemic stroke. Neurol Sci. (2019) 40:1877–85. doi: 10.1007/s10072-019-03919-y
35. Watchko, JF, and Maisels, MJ. The enigma of low bilirubin kernicterus in premature infants: why does it still occur, and is it preventable? Semin Perinatol. (2014) 38:397–406. doi: 10.1053/j.semperi.2014.08.002
36. Watchko, JF, and Tiribelli, C. Bilirubin-induced neurologic damage—mechanisms and management approaches. N Engl J Med. (2013) 369:2021–30. doi: 10.1056/NEJMra1308124
37. Luo, Y, Li, JW, Lu, ZJ, Wang, C, Guan, DN, and Xu, Y. Serum bilirubin after acute ischemic stroke is associated with stroke severity. Curr Neurovasc Res. (2012) 9:128–32. doi: 10.2174/156720212800410876
38. Steinberg, D . Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. (1997) 272:20963–6. doi: 10.1074/jbc.272.34.20963
39. BOULLIER, A, BIRD, DA, CHANG, MK, DENNIS, EA, FRIEDMAN, P, GILLOTTE-TAYLOR, K, et al. Scavenger receptors, oxidized LDL, and atherosclerosis. Ann N Y Acad Sci. (2001) 947:214–23. doi: 10.1111/j.1749-6632.2001.tb03943.x
40. Moonis, M, Kane, K, Schwiderski, U, Sandage, BW, and Fisher, M. HMG-CoA reductase inhibitors improve acute ischemic stroke outcome. Stroke. (2005) 36:1298–300. doi: 10.1161/01.STR.0000165920.67784.58
41. Bellosta, S, Fed, N, Bernini, F, Paoletti, R, and Corsini, A. Non-lipid-related effects of statins. Ann Med. (2000) 32:164–76. doi: 10.3109/07853890008998823
42. Amarenco, P, Kim, JS, Labreuche, J, Charles, H, Abtan, J, Béjot, Y, et al. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. (2020) 382:9–19. doi: 10.1056/NEJMoa1910355
43. Zhang, X, Yuan, K, Wang, H, Gong, P, Jiang, T, Xie, Y, et al. Nomogram to predict mortality of endovascular thrombectomy for ischemic stroke despite successful recanalization. J Am Heart Assoc. (2020) 9:e14899. doi: 10.1161/JAHA.119.014899
Keywords: outcome, early neurological deterioration, nomogram, ischemic stroke, predict
Citation: Zhou Y, Luo Y, Liang H, Wei Z, Ye X, Zhong P and Wu D (2024) Predictors of early neurological deterioration in patients with acute ischemic stroke. Front. Neurol. 15:1433010. doi: 10.3389/fneur.2024.1433010
Edited by:
Giovanni Merlino, Udine University Hospital, ItalyReviewed by:
Albert Akpalu, University of Ghana, GhanaBin Yu, Nanjing University of Chinese Medicine, China
Jinhua Xue, Gannan Medical University, China
Copyright © 2024 Zhou, Luo, Liang, Wei, Ye, Zhong and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danhong Wu, RGFuaG9uZ3d1QGZ1ZGFuLmVkdS5jbg==; Ping Zhong, emhwengyMDE3QDE2My5jb20=
 Yang Zhou
Yang Zhou Yufan Luo
Yufan Luo Huazheng Liang3,4,5
Huazheng Liang3,4,5 Zhenyu Wei
Zhenyu Wei Danhong Wu
Danhong Wu