- 1Evidence-Based Nursing Center, West China Hospital, West China School of Nursing, Sichuan University, Chengdu, China
- 2Department of Neurosurgery, West China Hospital, West China School of Nursing, Sichuan University, Chengdu, China
- 3Department of Nursing, West China Hospital, West China School of Nursing, Sichuan University, Chengdu, China
Background: Recent studies have investigated the epidemiological burden of sleep-disordered breathing (SDB) in patients with stroke; however, the results have been inconsistent, and the temporal trends of SDB after stroke remain unclear.
Objective: To perform a systematic review and meta-analysis of the prevalence and incidence of post-stroke SDB, evaluate demographic and clinical characteristic predictors of post-stroke SDB, and examine temporal trends in the overall burden of post-stroke SDB.
Methods: We searched PubMed, MEDLINE, Embase, Web of Science, CINAHL, and the Cochrane Library for studies reporting the burden of SDB in stroke patients published between 1 January 2010 and 30 December 2023. Two researchers independently screened the records for eligibility, extracted the data, and assessed the quality of the studies. Data were analyzed using random effects meta-analyses, and sources of heterogeneity were explored using subgroup analyses and meta-regression analyses.
Results: Out of the 8,799 references retrieved, none examined the incidence of SDB after stroke. However, 85 studies from 26 countries examined the prevalence of SDB and were included. The overall prevalence of SDB, mild SDB, and moderate to severe SDB were 60.0% (95% CI, 60.0–70.0%), 30.0% (95% CI, 23.0–37.0%), and 45.0% (95% CI, 33.0–57.0%), respectively. Meta-regression revealed that sex (p < 0.0001) and sample size (p < 0.01) were sources of heterogeneity among the studies. The pooled overall prevalence of SDB remained stable over time.
Conclusion: SDB is common in patients with stroke, and no reduction in the high prevalence of SDB has been observed over time, suggesting that early screening and prevention of post-stroke SDB still have not received sufficient attention. Moreover, additional studies investigating the incidence of this disease are needed to inform clinical practice.
1 Background
Stroke is a leading cause of death and disability worldwide (1). After a stroke, patients may experience various physical dysfunctions, including motor, speech, and swallowing dysfunctions. Although the long-term functional recovery of stroke survivors is crucial for their rehabilitation and return to normal activities (2, 3), the neuropsychiatric symptoms and complications of stroke patients have received increasing attention in recent years. Post-stroke neuropsychiatric symptoms, such as sleep-disordered breathing (SDB), chronic fatigue, and delirium, have been shown to be associated with reduced quality of life and hindered rehabilitation progress. In addition, these symptoms contribute to an increased medical burden, resulting in increased costs and an increased risk of all-cause mortality (4, 5). Among the multiple neuropsychiatric symptoms in stroke patients, SDB is one of the most common symptoms and is potentially fatal. The updated American Heart Association/American Stroke Association guidelines recommend screening for SDB among stroke patients (6). A recent study demonstrated that SDB was a significant risk factor for stroke (7). Nearly half of stroke patients experience SDB (8, 9). Poor sleep quality caused by SDB can worsen the risk of stroke onset, stroke recurrence, and poor functional recovery as well as increase the incidence of stroke-related risk factors (e.g., hypertension, atrial fibrillation, and cardiovascular disease) (10). Furthermore, SDB can adversely affect an individual’s quality of life; impair cognitive, social–emotional, or occupational functioning; and increase mortality rates, which creates a vicious cycle (11, 12).
In fact, the results of several clinical studies indicate that stroke patients have a low tolerance to hypoxemia and unstable hemodynamic changes due to concurrent SDB during hospitalization, especially in the acute phase. Continuous positive airway pressure therapy, which is the gold standard treatment for SDB, should be initiated as soon as possible (13, 14). However, assessing the burden of SDB in individuals with stroke is challenging. For example, stroke patients with impaired consciousness may struggle to cooperate, whereas those with severe physical dysfunction may have limitations during polysomnography (PSG) monitoring, such as the inability to move both upper limbs, which affects pulse oxygen monitoring. Moreover, a controlled environment for sleep monitoring is required and variations in individual tolerance may affect the assessment of results (15). Katzan et al. found that stroke patients often reported physical dysfunction and limited social engagement but rarely reported SDB-related symptoms, suggesting that the true impact of SDB in stroke patients may be masked, posing a challenge to timely risk assessment and the implementation of tailored interventions (16).
Many studies have reported the impact of SDB on stroke or the role of regular sleep in stroke recovery (17–19). A previous meta-analysis published in 2019 searched databases from inception to April 7, 2017, and included 89 studies investigating the prevalence of SDB after stroke and transient ischemic attack (20). However, our initial literature search revealed that more than 40 relevant articles were published afterward, and the reported burden of SDB varied considerably across studies. Furthermore, no systematic reviews have been published to assess the time trends of SDB burden in stroke patients. Therefore, the primary objective of this systematic review and meta-analysis was to examine the overall prevalence and incidence of SDB in stroke patients. The secondary objectives were to examine the prevalence and incidence of different severities of SDB, changes in overall SDB prevalence and incidence over time, and differences in the prevalence and incidence of SDB by gender, region, publication year, phase of stroke, study design, and SDB assessment methods.
2 Methods
2.1 Study design and registration
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (21) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines (22). The protocol was registered in the international prospective register of systematic reviews (PROSPERO registration number CRD42023443328).
2.2 Search strategy and selection criteria
Two authors independently and systematically searched the PubMed, MEDLINE, Embase, Web of Science, CINAHL, and Cochrane Library databases to identify cross-sectional, longitudinal studies that reported the prevalence of SDB or different severities of SDB in individuals who experienced hemorrhagic stroke, ischemic stroke, or transient ischemic attack. Furthermore, we searched the reference lists of key reviews and meta-analyses. An initial scoping search was performed using PubMed to collate relevant keywords and medical subject headings. The initial combination of search terms was as follows: (“stroke” OR “cerebrovascular disorders” OR “brain infarction” OR “brain ischemia” OR “cerebrovascular accident*” OR “ischemic stroke” OR “hemorrhagic stroke” OR “ischemic attack, transient”) AND (“sleep disordered breathing” OR “sleep apnea syndromes” OR “sleep apnea, obstructive” OR “sleep apnea, central”) AND (“prevalence” OR “epidemiology” OR “incidence”). Next, we modified the search strategy to suit each database. Only human studies published from 1 January 2010 to 30 December 2023 were included. Our complete search strategy is available in Supplementary Table 1.
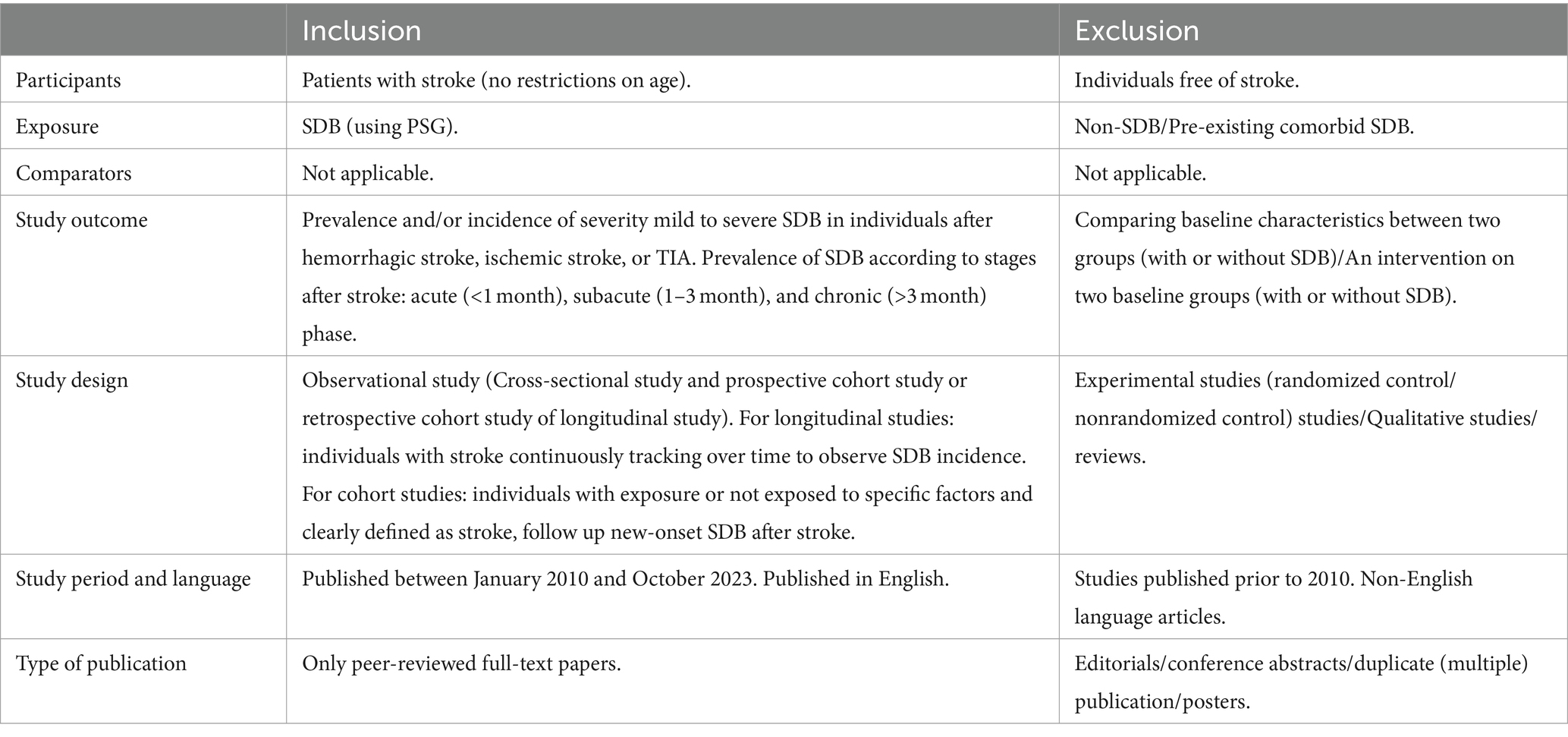
The inclusion and exclusion criteria were established according to the Population, Intervention, Comparators, Outcomes, and Study (PICOS) framework, as shown in Table 1. In addition, if multiple studies used overlapping data, we selected the study with the largest sample size. SDB was diagnosed based on the International Classification of Sleep Disorders, Diagnostic and Statistical Manual of Mental Disorders, or International Classification of Diseases (23) and assessed by PSG or questionnaires.
2.3 Study selection
We imported the results from each database into the reference management software package EndNote and removed duplicates. When the results of the same study were reported in multiple publications, we included only the article reporting the largest sample size in the data synthesis. Next, two authors reviewed the titles and abstracts of the retrieved studies and excluded studies that were clearly not relevant (e.g., studies focusing on patients with cardiovascular disease, animal studies, or studies that examined periodic limb movements during sleep as the outcome) by using the Rayyan systematic review application (24) and screened independently the remaining references for relevance based on the full eligibility criteria. Any disagreements were resolved by discussion or by consulting a third reviewer (YJ).
2.4 Data extraction
The data were extracted by two independent investigators (XFS and SSL) using a standardized data extraction sheet. The data were then cross-checked for agreement, and any disagreements were resolved by consulting a third investigator (YJ). The following data were extracted: first author, publication year, country, study design, different stroke subtypes, phase of stroke, mean/median age, percentage of males, source of patients, tools used for SDB assessment, sample size, etc. If any data were unclear or unavailable, attempts were made to contact the study authors.
2.5 Risk of bias assessment
Two reviewers independently assessed the quality of each survey by using the Joanna Briggs Institute’s Critical Appraisal Checklist for Prevalence Studies (25), and any discrepancies were resolved by consensus. The Joanna Briggs Institute’s Critical Appraisal Checklist for Observational Studies consists of 9 items across 3 domains, namely, participants (questions 1, 2, 4, and 9), outcome measurements (questions 6 and 7), and statistics (questions 3, 5, and 8). This tool is used to evaluate the overall quality of prevalence studies. Two authors independently conducted the appraisal (XFS and SSL), and disagreements were resolved through discussion or by a third reviewer (YJ) where required.
2.6 Data synthesis
All the statistical analyses were conducted with Stata (version 16.0) software. The primary outcome in this systematic review and meta-analysis was the prevalence of SDB in stroke patients. The 95% confidence intervals (95% CIs) were calculated. Prevalence estimates were calculated by pooling study-specific estimates using the DerSimonian and Laird random effects model (26). The heterogeneity among the studies was assessed using Cochran’s Q statistic. The magnitude of heterogeneity was measured using the I-square (I2) statistic. The I2 statistic ranged from 0 to 100% (an I2 of 0 to 25% indicated no or mild heterogeneity, 25–50% indicated moderate heterogeneity, and I2 > 50% indicated substantial heterogeneity) (27). If significant heterogeneity was not observed, the fixed effects model was used to calculate the pooled prevalence and 95% CI of SDB; otherwise, a random effects model was used.
Subgroup analyses and meta-regressions were performed to explore potential sources of heterogeneity. Subgroup analyses were performed based on the following study characteristics: study country, proportion of male patients, study design, phase of stroke, tools used for SDB assessment, and study design. We investigated publication bias by constructing funnel plots and assessing the significance of Egger’s weighted regression test. The trim-and-fill method, which was developed by Duval and Tweedie (28), was used to adjust the pooled effect sizes for publication bias. This method estimates the number of missing studies. Meta-analysis was only performed when an outcome was reported by at least three original studies. The threshold for statistical significance was set at p < 0.05, and all tests were two-sided.
3 Results
3.1 Search results and study selection
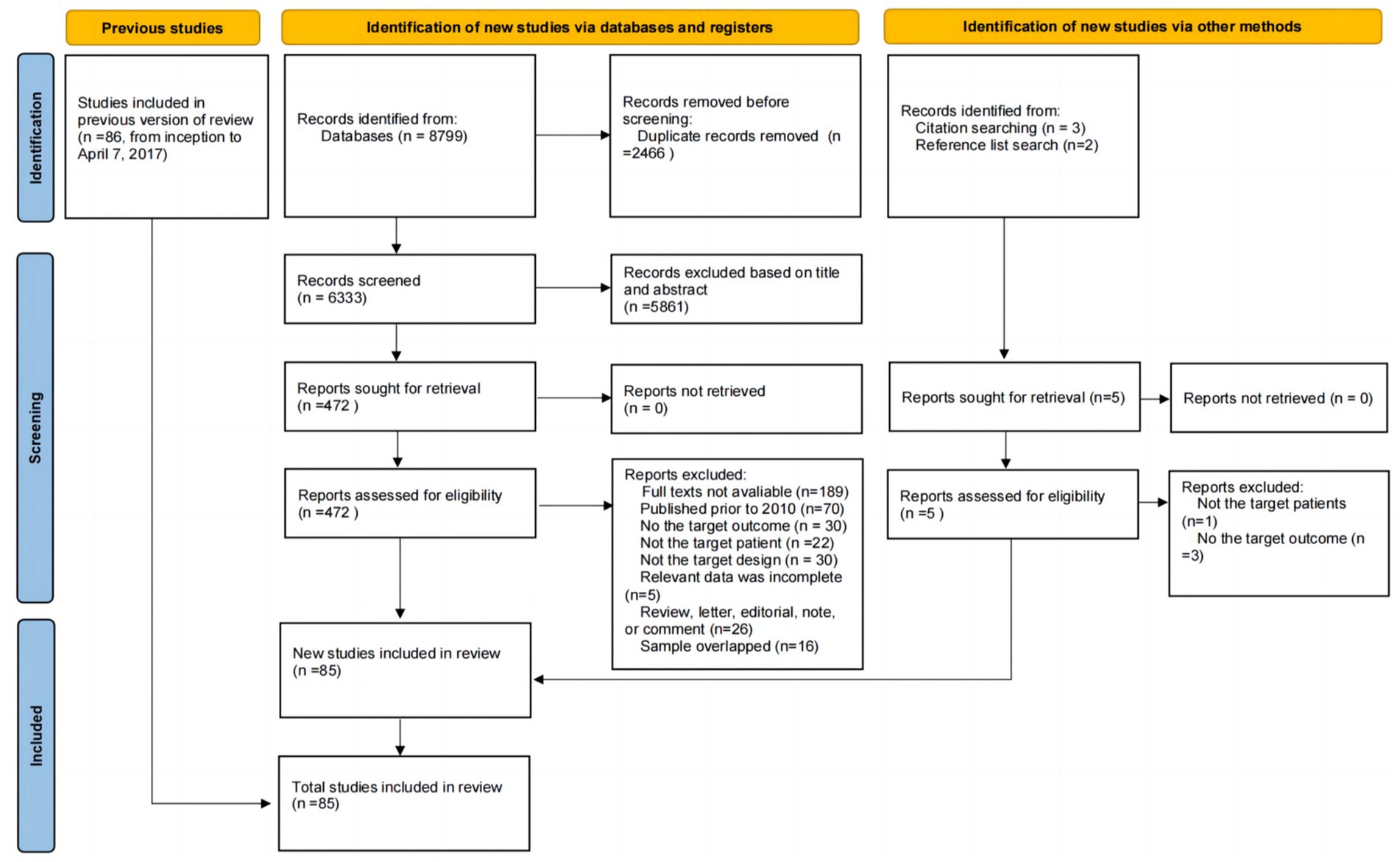
The search strategy yielded a total of 8,799 unique citations, and duplicates were removed (n = 2,466). After screening the titles, 5,429 studies were excluded. After screening the abstracts, 432 studies were excluded. Thus, 472 articles remained for full-text evaluation. Of those studies, the number and reason for exclusion were as follows: Thirty studies did not report the target outcome, the full text of 189 studies was unavailable, 22 studies did not include the target patient group, 30 studies had an inappropriate study design, 70 studies were published prior to 2010, 16 studies had overlapping samples, and 5 studies had incomplete data. Furthermore, five potentially eligible studies were identified by searching the reference lists of relevant studies; however, only one of these studies fully met the eligibility criteria, while the other four studies were excluded because they did not report prevalence data or did not examine the target patient group. Ultimately, a total of 85 studies were included in this updated systematic review and meta-analysis, consisting of 61 new studies (29–89) and 24 older studies (11, 90–112). The details of the selection process are shown in Figure 1.
3.2 Study characteristics
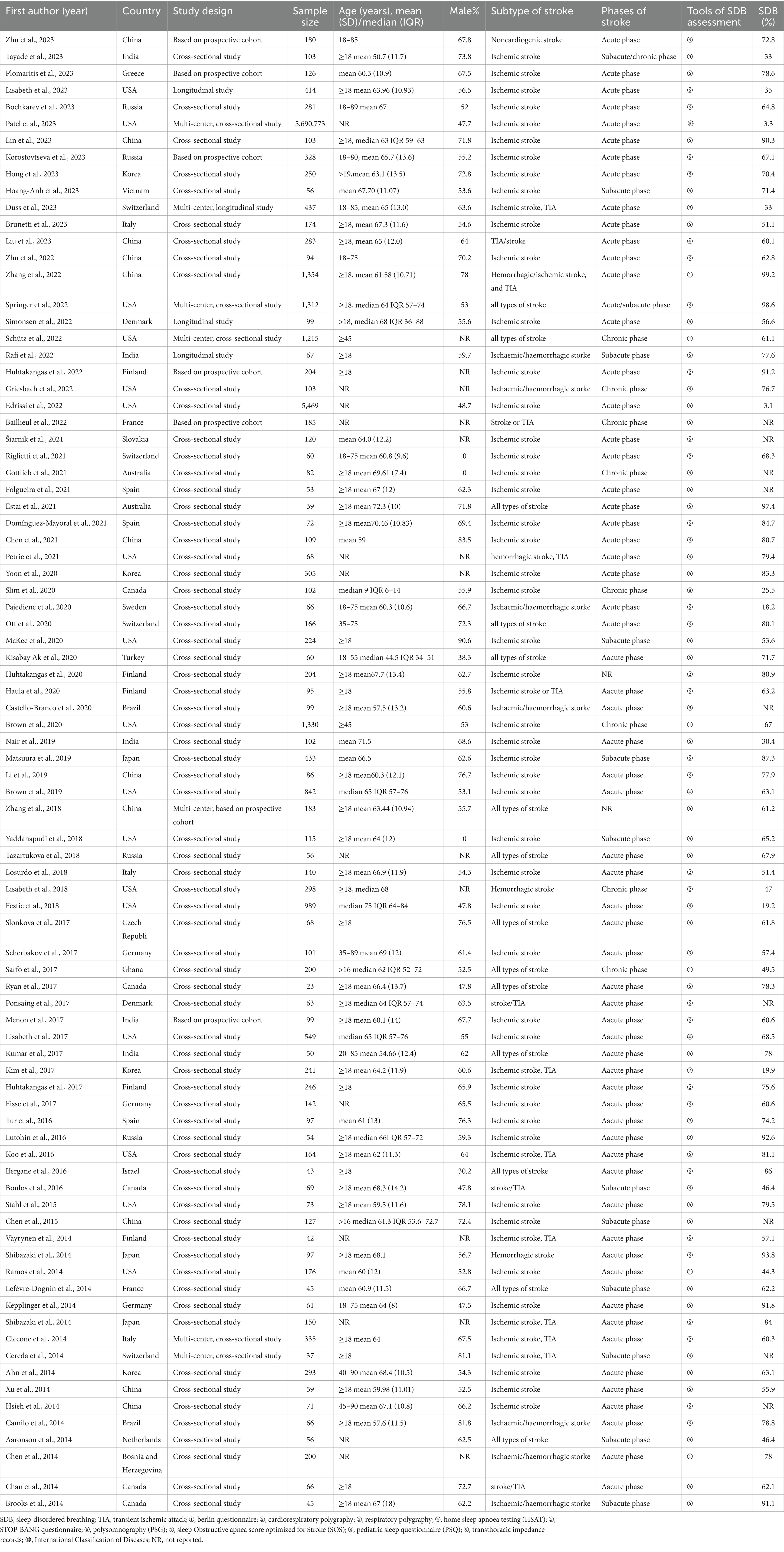
The characteristics of the 85 included studies are shown in Table 2. The included studies were published between 2010 and 2023, with the largest number (n = 12) published in 2023. More than half of the included studies were published in 2017 or later. Among the 88 included observational studies, 74 were cross-sectional studies (including 69 single-center and five multicenter studies), and 11 were baseline data or at a certain time point based on longitudinal studies (including nine single-center and two multi-center study). Altogether, these studies included 5,714,316 patients with stroke, including those with ischemic stroke, transient ischemic attack, and hemorrhagic stroke. The sample sizes across the 85 reviewed studies ranged from 23 to 5,690,773.
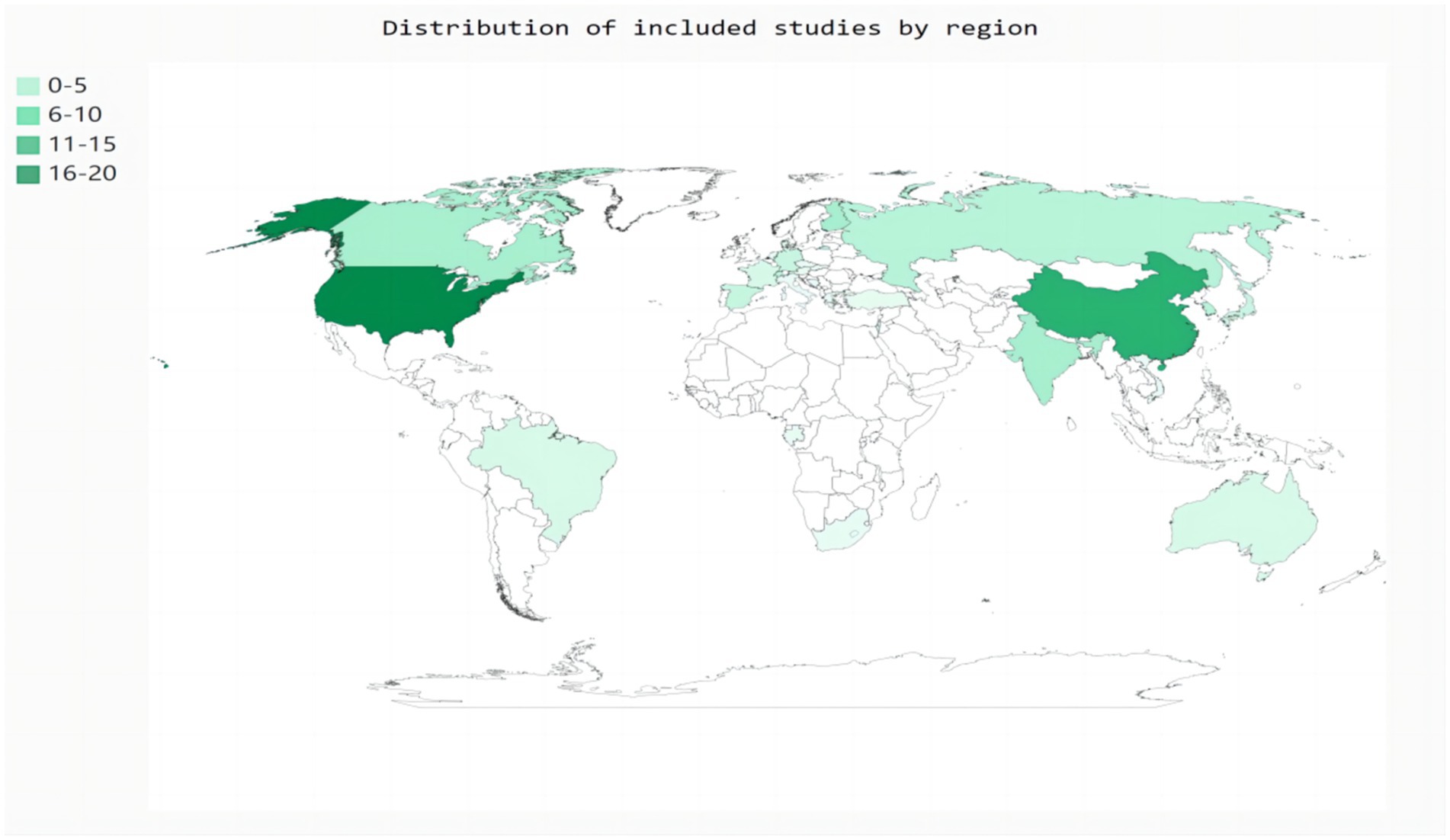
Eighty-two studies included hospital-based inpatients and outpatients (including stroke units, neural psychiatric disorders and mental health centers, and tertiary care centers). Five studies included population-based samples: three in the United States, one in Canada, and one in China. However, four studies did not report the study setting. The studies were conducted across 26 countries (Figure 2). The diagnostic tools used for SDB varied among studies. Most studies (n = 59) used PSG to assess SDB; eight studies used cardiorespiratory polygraphy; five studies used home sleep apnea testing; four studies used the Berlin questionnaire; three studies used the STOP-Bang score; two studies used respiratory polygraphy; one study used the Sleep Obstructive Apnea Score optimized for Stroke; one study used transthoracic impedance records; one study used the Pediatric Sleep Questionnaire; and one study used the International Classification of Diseases, Ninth Revision, Clinical Modification. Seventy-six of the 85 studies reported the total prevalence of SDB, twenty-three studies reported the severity of SDB, and no studies reported the incidence of SDB. Moreover, more than half of the studies (n = 48) were designed to explore the prevalence of SDB in stroke patients with a range of chronic diseases rather than in patients with stroke alone. The stroke phase was reported in 83 out of the 85 studies. Strokes were identified using MRI (n = 9), CT (n = 5), or MRI or CT (n = 64); some studies lacked a detailed account of the stroke diagnosis method (n = 7). Forty-four studies focused on ischemic stroke only; forty studies included patients with ischemic stroke, transient ischemic attack, or hemorrhagic stroke; and one study used another stroke classification. Fifteen studies did not report the sex of the patients. Twelve studies did not report the ages of the patients. Twenty-five studies reported the median age, age range(s), or minimum age of stroke patients. The mean age across the remaining 48 studies ranged from 50.7 to 71.5 years.
3.3 Methodological quality of the included studies
The quality rating assessment results are presented in the Supplementary Table 2. Across each domain in all studies, more than 76.0% of the studies had a low risk of bias, more than 7.0% exhibited a moderate risk of bias, and more than 15% showed a high risk of bias. Specifically, only <30% of the studies met the criterion of having sufficient coverage in the data analysis. The other domains, including response rate adequacy, appropriate statistical analysis, standard measurements, detailed description of the study subjects and setting, appropriate sampling of study participants, and valid methods for identifying conditions, were found to be adequate for assessing the prevalence of SDB in more than 80% of the studies. Additionally, the remaining items, such as appropriate sample size and sample frame, were adequate for more than 60% of the studies for addressing the target population (Figure 3).
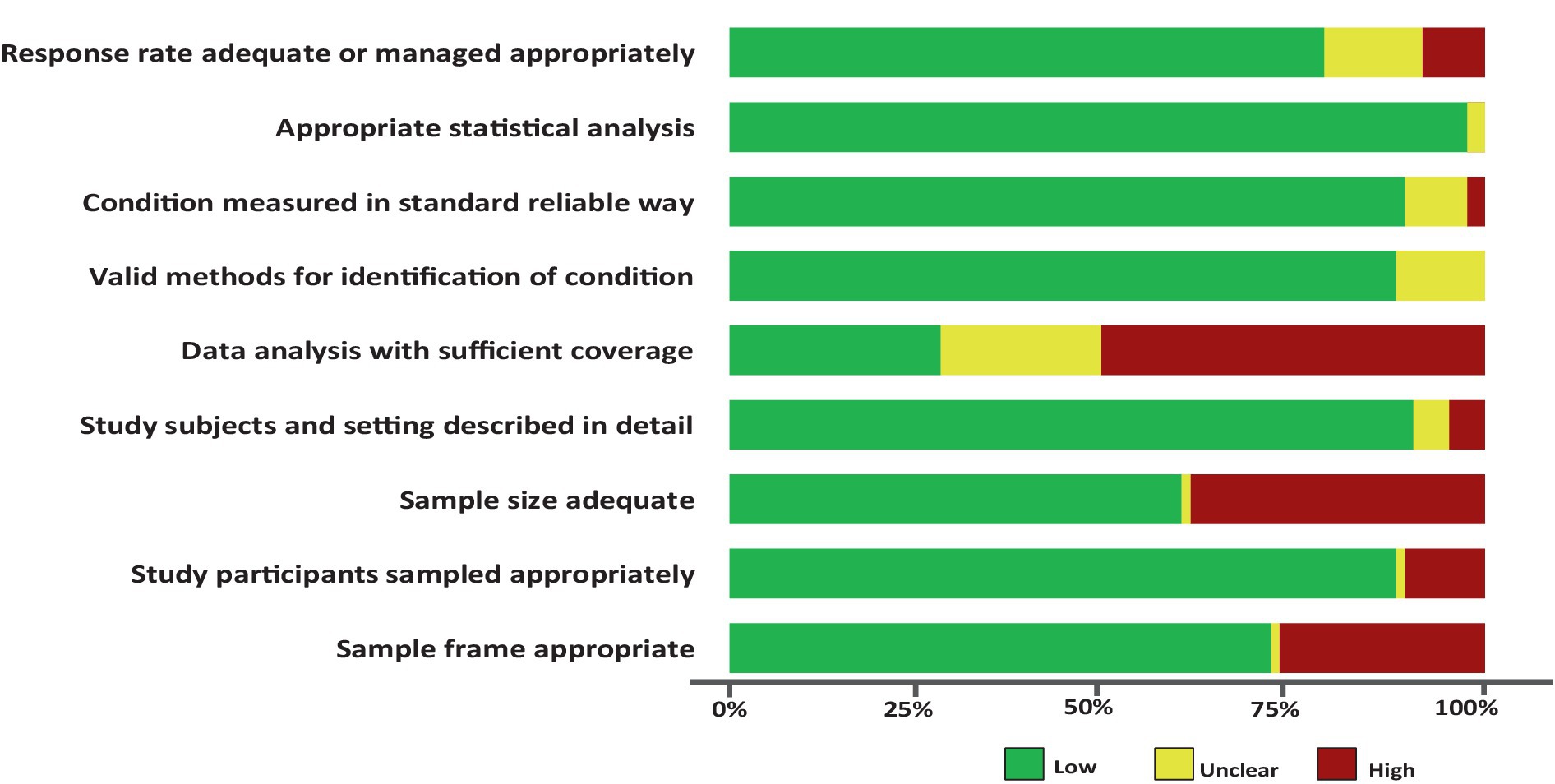
Figure 3. Risk of bias assessment summary table across all studies. *No weights were applied for different studies.
3.4 Prevalence of SDB
Seventy-six studies (5,713,479 patients) assessed the prevalence of SDB, and studies reporting the prevalence of only the subtypes of SDB were excluded from this pooled analysis. According to the random effects model, the overall pooled prevalence of SDB in patients with stroke was 65.0% (95% CI, 60.0–70.0%; I2 = 99.85) (Figure 4a). The pooled prevalence of mild and moderate-to-severe SDB were 30.0% (95% CI, 23.0–37.0%; I2 = 94.87) and 45.0% (95% CI, 33.0–57.0%; I2 = 94.86), respectively (Supplementary Figure 1).
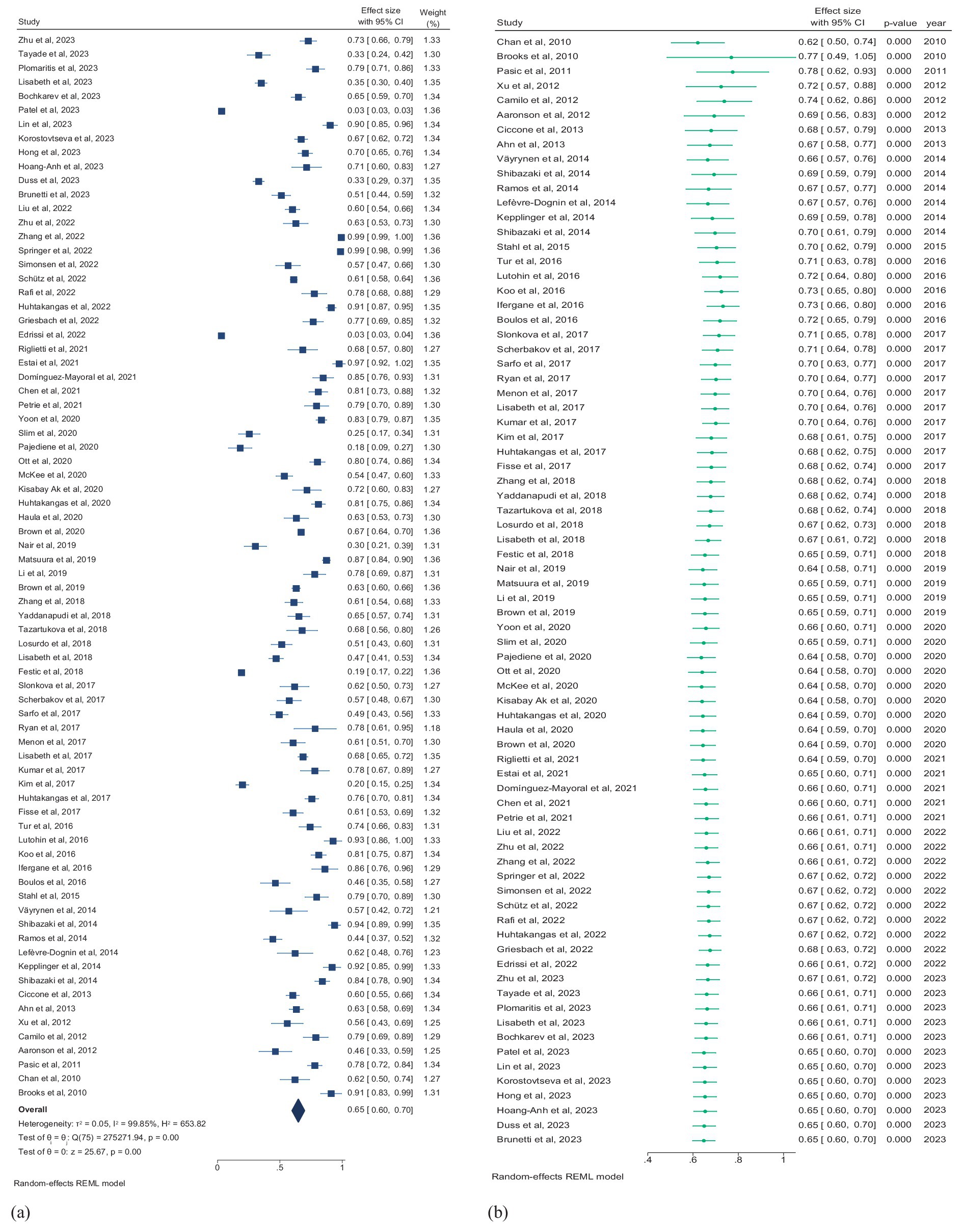
Figure 4. (a) Forest plot of overall prevalence of SDB in patients with stroke. (b) Forest plot of cumulative prevalence of SDB in stroke patients by year. CI, confidence interval.
3.5 Temporal trends in SDB prevalence
Multiple mergers were conducted in chronological order to dynamically assess the cumulative impact of publication year on the primary result. A cumulative time trend analysis of publication years revealed that the overall prevalence of SDB in stroke patients remained relatively stable from 2010 to 2023, fluctuating between 64 and 78%. The effect size also ranged from 0.49 to 1.05 over this period (Pfor trend < 0.01; Figure 4b).
3.6 Subgroup analyses
We conducted subgroup analyses to explore the discrepancy of the primary outcome regardless of the observed heterogeneity (Table 3). Our analysis across regions, study years, phases of stroke, gender, study designs, and assessment methods. Overall, studies in North America, Asia, and Europe exhibit geographical variances in prevalence. Among 22 studies conducted in North America (5,704,419 patients), the prevalence of SDB is the lowest (57.0%; 95% CI, 46.0–68.0%). Conversely, among 24 studies in Asia (4,563 patients), the prevalence is the highest (70.0%; 95% CI, 62.0–78.0%). The prevalence in 26 studies in Europe (3,955 patients) lies between North America and Asia. From a temporal perspective, the prevalence of SDB from 2010 to 2015 was 70.0% (95% CI, 62.0–79.0), from 2016 to 2020 was 62.0% (95% CI, 55.0–69.0), and from 2021 to 2023 was 65.0% (95%CI, 56.0–75.0). This indicates that the prevalence of SDB post-stroke fluctuates across different time periods, yet there is no evident reversal in the prevalence risk. In various phases of stroke, the prevalence of SDB also demonstrates differences, although the heterogeneity after stratification is not significantly reduced. In the chronic stage, the prevalence of SDB was the lowest. In contrast, the prevalence of SDB was highest in the subacute stage (Figures 5a–c), suggesting the alterations in SDB risk during different recovery periods post-stroke. Additionally, we performed stratified analyses according to gender (male ≤50% and male >50%), study design (cross-sectional study, cross-sectional analysis based on longitudinal study, and cross-sectional analysis based on prospective cohort), and SDB assessment method (PSG and other criteria). While some differences are present, these factors do not significantly affect the overall prevalence of SDB post-stroke (all p > 0.05; Figures 5d–f). This implies that in addition to the aforementioned possible interfering factors, the potential influencing factors of the prevalence of SDB after stroke remain a crucial issue worthy of attention.
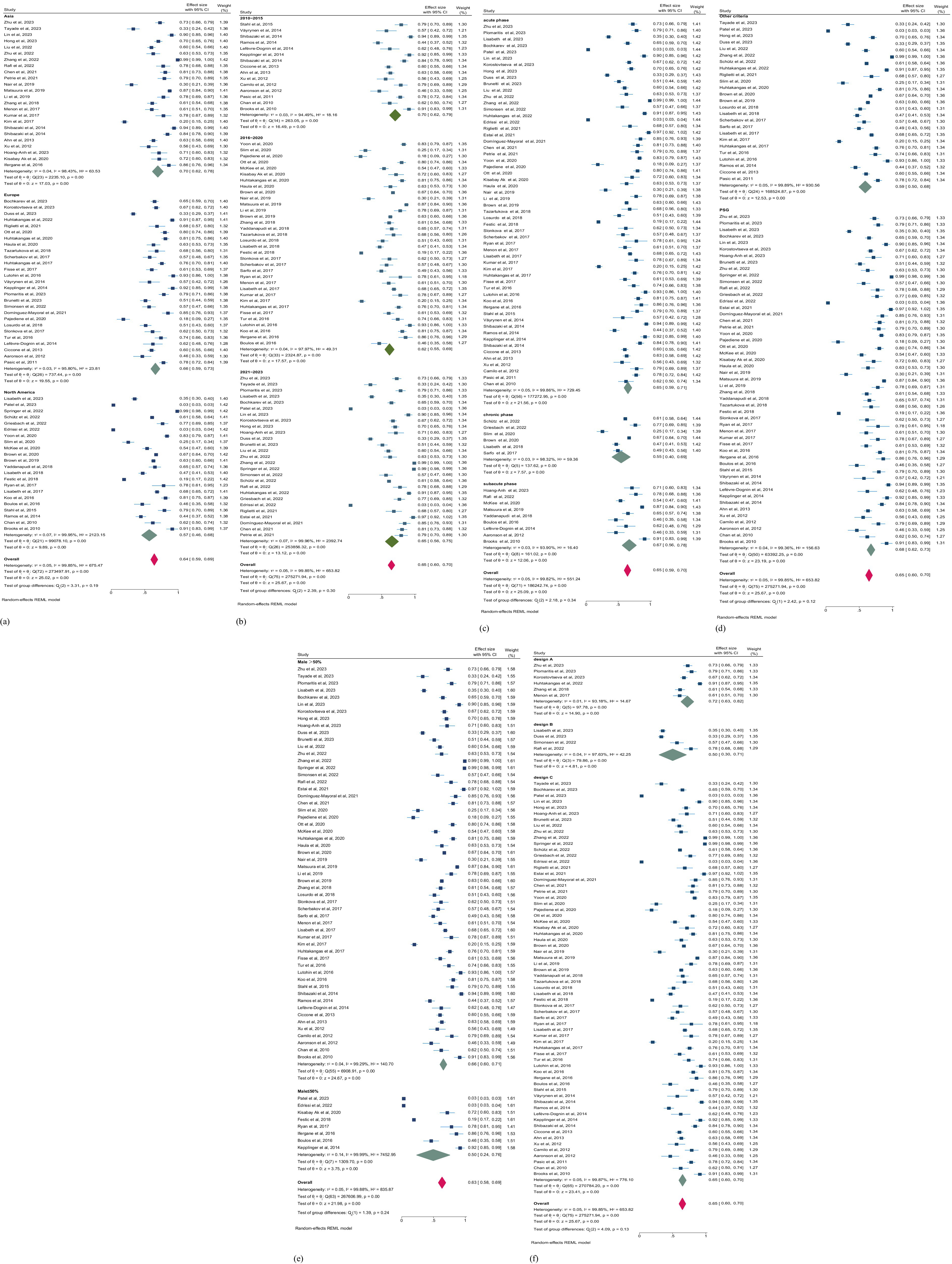
Figure 5. (a) Forest plot of prevalence of SDB in patients with stroke by region. (b) Forest plot of prevalence of SDB in patients with stroke by publication year. CI, confidence interval. (c) Forest plot of prevalence of SDB in patients with stroke by phases of stroke. (d) Forest plot of prevalence of SDB in patients with stroke by the tools of SDB assessment. CI, confidence interval. (e) Forest plot of prevalence of SDB in patients with stroke by gender. (f) Forest plot of prevalence of SDB in patients with stroke by study design. CI, confidence interval. Design A: Based on prospective cohort. Design B: longitudinal study. Design C: Cross-sectional study.
3.7 Meta-regression
According to the multivariate meta-regression model, the proportion of males (aOR = 1.54, 95% CI = 1.43–1.67; p < 0.0001) and the sample size (aOR = 0.99, 95% CI = 0.99–1.01; p < 0.01) were related to the prevalence of SDB, accounting for 64.31 and 10.24% of the variance in SDB prevalence, respectively (Supplementary Table 3). The study region, phase of stroke, region, and SDB assessment methods were not significantly associated with the prevalence of SDB (all p > 0.05).
3.8 Publication bias
We assessed publication bias using Egger test. The results indicated evidence of bias regarding the prevalence of SDB (t = 7.72; 95% CI = 0.023–0.040; p < 0.05). Nevertheless, based on our trim and fill analysis, we contend that this bias was minimal. Consequently, the pooled prevalence of SDB ranged from 64.7% (95% CI 59.8–69.7%) to 58.0% (95% CI 52.9–63.1%, Figure 6).
4 Discussion
4.1 Principal findings
Our meta-analysis of 85 studies published between 2010 and 2023 across 26 countries revealed that SDB burden was common among stroke patients. The overall prevalence of SDB, the prevalence of mild SDB, and the prevalence of moderate-to-severe SDB were 65, 30, and 45%, respectively. SDB most commonly occurs in the acute phase of stroke, and the overall prevalence of SDB is highest in Asia. The temporal trends of overall SDB prevalence were stable.
According to the time trend analysis conducted herein, the prevalence of SDB in stroke patients fluctuated between 62 and 77% from 2010 to 2023. Overall, the trend of the prevalence of SDB in stroke patients was stable. Nevertheless, enhancing screening for SDB in stroke patients and improving health education related to SDB in both stroke patients and their caregivers are particularly important. This includes enhancing sleep health literacy and health management methods among stroke patients and their caregivers, in turn enhancing the comprehensive health management of nonfunctional disorders in stroke to mitigate the impact of negative feedback regulation (i.e., SDB-stroke-SDB) on the risk of stroke recurrence. Moreover, the stable prevalence of SDB over time might suggest that existing interventions are to some extent effective. However, it also implies the need for new or improved methods to further reduce its prevalence. For instance, continuous positive airway pressure (CPAP), the gold standard intervention for SDB, has not decreased the prevalence of SDB after stroke. This indicates that potential factors such as the coverage, continuity, or patient compliance of CPAP intervention still need to be further considered. Additionally, the stable trend of the prevalence of SDB after stroke over time highlights the necessity to strengthen public education and the training of medical personnel to enhance the recognition of SDB after stroke. From the specific time period of the trend change in the prevalence of SDB, it can be observed that special public health events or economic fluctuations caused by events might be potential factors influencing the screening and intervention effects of SDB.
Several recent observational studies, including both prospective and retrospective cohort studies, have assessed the prevalence of SDB in stroke patients, but the results of these studies have been inconsistent (67, 73, 80, 82, 85, 88). Our systematic review and meta-analysis provided the latest pooled estimates of the prevalence of SDB in patients with stroke, and the findings were generally consistent with those of prior reports (20). The slight difference in prevalence findings may be attributed to disparities in sample sources and sizes, regional variations, cultural contexts, SDB assessment methods, and the timing of evaluations across studies. Moreover, SDB is a common disease, and its diagnosis relies primarily on PSG. Frequent awakenings during sleep is not only a symptom reported by stroke patients but also a characteristic manifestation of SDB. With respect to the pathological changes associated with stroke, worsening cerebral ischemia and hypoxia ultimately cause irreversible damage to neurons and the release of excessive excitatory amino acids and other toxic substances to disrupt the sleep–wake mechanism, which further increase the risk of SDB (9). In addition, physical dysfunction after stroke can lead to changes in nocturnal sleep posture, and an increase in supine sleep is one of the main causes of SDB (15). Thus, physicians at stroke centers recommend performing polysomnography for stroke patients to detect early signs of SDB. The potential risk of SDB in stroke patients without typical clinical manifestations is often overlooked in the stroke unit. In our meta-analysis, more than 50% of the studies included participants who had experienced a stroke and had an average age exceeding 60 years, which revealed atypical clinical manifestations of SDB in elderly individuals.
Taken together, our pooled results suggest that SDB burden is commonly observed in stroke patients, which serves as a reminder for medical professionals to carefully identify the underlying signs of SDB in stroke patients, particularly at night. Moreover, early clinical screening is necessary for stroke risk diagnosis and generalized treatment during the acute phase and healthcare during the recovery phase, as well as for preventing the exacerbation of SDB caused by conventional sleep aid medications.
4.2 Subgroup analysis and meta-regression findings
The analyses revealed that the prevalence of SDB after stroke was highest in Asia, followed by Europe and North America. Regional differences in the prevalence of SDB may be attributed to the limited ability to evaluate SDB diagnoses, which rely on a single sleep study or other diagnostic criteria. This approach may not adequately capture the actual sleep status of patients. Additionally, discrepancies in the sleep environment (e.g., hospital sleep centers or home monitoring) could introduce potential bias into the results. These findings indirectly indicate varying levels of emphasis on managing SDB in patients with stroke across different regions. Moreover, it is noteworthy that the majority of the research discussed in this meta-analysis originates from Asia, followed by Europe and North America. Limited research has been conducted in Oceania and North America, and related research in Africa is lacking. It is not feasible to perform subgroup analysis based on race and continent. Available epidemiological research on the prevalence of SDB in stroke patients is limited on some continents. Insufficient funding, limited regional focus, and a shortage of well-trained professionals are significant factors that impact the advancement and continuity of research. Specifically, some regions in Asia may have relatively insufficient investment in routine screening, diagnosis, and treatment of SDB. For example, the treatment costs related to SDB have not been fully included in medical insurance reimbursement, which may affect that some potential cases are not detected at early stage or patients’ initiative for diagnosis is not high due to costs, leading the aggravation of post-stroke SDB and thus being detected in large numbers. Secondly, the gold standard for post-stroke SDB is PSG, but the judgment of abnormal patterns often combines instrument types and manual analyze of professional sleep technician. In addition, some lifestyle and genetic factors in Asia may also be related to the relatively high prevalence of post-stroke SDB. Finally, cultural and socioeconomic factors, such as work pressure, the availability of social support systems, and differences in patients’ awareness of health issues may also cause differences in the prevalence of post-stroke SDB between different regions.
Subgroup analysis based on sex revealed that the prevalence of SDB was higher in studies with a proportion of males >50% than in studies with a proportion of males <50%. This result was similar to those of previous findings (68). The influence of gender on the prevalence of SDB after stroke may include physiological structure differences, hormone levels and sleep patterns. In addition, different living habits related to gender differences may interfere with the heterogeneity between studies (68). There is greater deposition of fat in the upper respiratory tract and abdomen of males, and males have longer airways than females; these differences may contribute to the increased vulnerability to airway collapse among males (113). Moreover, the clinical manifestations of SDB in females differ from those in males and are impacted by age-related and physiological states, such as menopause and pregnancy. Furthermore, compared with males, females commonly report atypical symptoms, including daytime fatigue, low energy intake, insomnia, morning headaches, mood disturbances, and nightmares (68, 114). Due to these differences in “atypical” clinical presentation, females with SDB are often underdiagnosed and undertreated compared with males. Therefore, healthcare professionals should focus on the potential signaling symptoms of SDB in female stroke patients and conduct timely screening and assessment.
In addition, the prevalence of SDB was highest between 2010 and 2015, followed by a slow decrease from 2016 to 2020. However, a slight increase occurred from 2021 to 2023. Research has indicated that SDB associated with stroke is connected to neurobiology, social psychology, and other contributing factors (115). Thus, additional research is needed to determine whether this increase is related to SDB resulting from anxiety and depression induced during the COVID-19 pandemic. For other outcomes, the heterogeneity between the subgroup analyses did not decrease, suggesting that the overall prevalence of SDB was not associated with the study design (116). Based on the use of SDB assessment tools, we found that PSG was more effective than other assessment methods for screening for the prevalence of SDB in stroke patients. In addition, the prevalence of SDB was greater among individuals with acute stroke than among individuals with stroke in the subacute and chronic phases. This difference could be attributed to the acute manifestations of stroke, including oropharyngeal dysfunction and hypoglossal nerve dysfunction, which contribute to retroglottic collapse and increase the risk of airway collapse.
The overall heterogeneity among studies was high, and the intergroup heterogeneity did not decrease following subgroup analysis. The I2 values exceeded 90% across groups. Previous systematic reviews of prevalence studies reported similar findings (20). However, our meta-regression analysis further revealed that sex and sample size accounted for the high heterogeneity in the overall prevalence estimates. Different sample sizes may become potential sources of heterogeneity by affecting statistical power and so on. Boulos et al. also reviewed on the possible influence of sample size in SDB (116).
4.3 Strengths and limitations
Our study had some limitations. First, after searching multiple databases, no direct studies on the incidence of SDB after stroke could still be found. Therefore, a comprehensive analysis of its incidence could not be conducted. However, our study points out the direction for future research. Second, there was still substantial heterogeneity among the included studies. However, heterogeneity is a inevitable problem for meta-analyses of observational studies (117, 118). Moreover, we conducted a meta-analysis of studies that met the inclusion criteria to examine the prevalence of SDB in stroke patients. We analyzed the sources and extent of study heterogeneity through subgroup analyses and multivariable random effects meta-regression analyses. Nevertheless, subgroup analysis revealed significant heterogeneity in terms of prevalence. Although we used a random effects model to deal with heterogeneity, potential factors such as methodological differences among different studies, sample size, population characteristics, model of measurement tools, measurement methods, environment, region, and uncontrollable factors (such as differences in hypoxia tolerance) still have an impact on the results. For example, there may be biases in the diagnostic criteria or patient selection in the included studies, which may overestimate or underestimate the true prevalence of SDB. Future studies can adopt more unified diagnostic criteria, increase sample diversity, and implement more stringent research designs to reduce heterogeneity and improve the reliability of research results. The meta-regression findings indicated that sex plays a significant role in the heterogeneity. However, due to the lack of data on the pooled number of SDB cases stratified by sex in most studies, further analyses could not be conducted. Third, due to certain studies provide information only on the median age or the minimum age threshold of the overall population included, thereby precluding the possibility of conducting meta-regression models to elucidate the impact of mean age on the prevalence of SDB among stroke patients. Finally, there is a lack of available data regarding the prevalence of SDB based on the location of the stroke. The thalamus, hypothalamus, basal ganglia, brainstem reticular structure, and base of the frontal lobe are implicated in the regulation of sleep, and stroke that occurs in these anatomical regions may lead to the development of SDB (115). Comparing variations in prevalence across different locations of stroke is important for the development of risk management policies. Hence, future research should also investigate the incidence of SDB in various stroke locations.
4.4 Policy, clinical and research implications
Our review identified several aspects that could be addressed in future research. First, our updated pooled estimates indicate a high prevalence of SDB in stroke patients, underscoring the importance of early assessment for warning signs of SDB in this population. There is currently a gold standard method for diagnosing SDB. However, the prevalence of SDB remains high, which means that it has failed to garner sufficient clinical attention. A small proportion of stroke patients undergo regular sleep monitoring, while the majority of these patients only seek treatment at sleep centers when they experience severe SDB. Hence, it is often difficult to determine the exact timing at which patients experience SDB based on observational studies. Second, in the routine clinical diagnosis and treatment of stroke and nursing care, complications related to SDB (i.e., daytime sleepiness, intermittent irregular snoring, and frequent nocturnal awakenings) are often overlooked in favor of focusing on long-term functional recovery (i.e., motor dysfunction or cognitive impairment) in stroke survivors. The aforementioned scenario complicates the assessment of the influence of concurrent SDB on long-term prognosis and functional recovery after stroke. Routine sleep screening of stroke patients is crucial for comprehensive health management and accurate care of physical and mental symptoms. In addition, the findings emphasize the need for healthcare professionals to establish routine screening programs to proactively evaluate the prevalence of SDB in stroke patients and to promptly implement early interventions and accurate care. Optimize screening and intervention measures for SDB after stroke, including but not limited to: integrating the SDB screening process in stroke units, using portable PSG monitoring equipment for early diagnosis, and conducting multidisciplinary team cooperation to ensure that patients receive timely and appropriate treatment. Moreover, the inclusion of sleep monitoring could be considered in routine health screenings for stroke survivors in primary care within the community or as an integral component of the care offered to patients experiencing acute or subacute stroke in inpatient care facilities. Given that not all stroke patients experience overt SDB, it is imperative for future research to investigate the protective factors that may mitigate SDB after stroke. At the same time, on the basis of strengthening the training of medical staff and raising public awareness of SDB, rationally optimize the allocation of resources to ensure that necessary SDB screening and management services can also be obtained in regions with limited resources. Furthermore, in different regions, especially in regions with a high prevalence of SDB, screening and management strategies should be formulated according to local medical resources, cultural and economic conditions. Finally, the disparities in the prevalence of SDB based on sex, region, and phase of stroke require attention, as they may affect the accurate prediction of the burden of SDB after stroke. Overall, this study offers valuable insights for the formulation and implementation of preventive measures and healthcare strategies.
5 Conclusion
SDB burden is common among stroke patients. Our latest pooled estimate of the overall prevalence of SDB in stroke patients helps to raise awareness among healthcare personnel regarding the evaluation of early warning signs or “atypical” symptoms of SDB after stroke, especially those who have the closest contact with patients in clinical care. According to our analysis, it is necessary to improve the management of SDB after stroke. Individuals in the acute stage of stroke and male stroke patients are considered high-risk populations, whereas it is challenging to assess SDB among female stroke patients.
Author contributions
XS: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. SL: Data curation, Methodology, Writing – review & editing. CW: Data curation, Methodology, Writing – review & editing. YC: Data curation, Methodology, Writing – review & editing. YL: Data curation, Methodology, Writing – review & editing. DW: Data curation, Software, Writing – review & editing. ZF: Conceptualization, Supervision, Writing – review & editing. YJ: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1432085/full#supplementary-material
References
1. Peng, S, Liu, X, Cao, W, Liu, Y, Liu, Y, Wang, W, et al. Global, regional, and national time trends in mortality for stroke, 1990-2019: an age-period-cohort analysis for the global burden of disease 2019 study and implications for stroke prevention. Int J Cardiol. (2023) 383:117–31. doi: 10.1016/j.ijcard.2023.05.001
2. Hazelton, C, Thomson, K, Todhunter-Brown, A, Campbell, P, Chung, CS, Dorris, L, et al. Interventions for perceptual disorders following stroke. Cochrane Database Syst Rev. (2022) 11:CD007039. doi: 10.1002/14651858.CD007039.pub3
3. Barclay, RE, Stevenson, TJ, Poluha, W, Semenko, B, and Schubert, J. Mental practice for treating upper extremity deficits in individuals with hemiparesis after stroke. Cochrane Database Syst Rev. (2020) 5:CD005950. doi: 10.1002/14651858.CD005950.pub5
4. Cai, W, Mueller, C, Li, YJ, Shen, WD, and Stewart, R. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. (2019) 50:102–9. doi: 10.1016/j.arr.2019.01.013
5. Tanayapong, P, and Kuna, ST. Sleep disordered breathing as a cause and consequence of stroke: a review of pathophysiological and clinical relationships. Sleep Med Rev. (2021) 59:101499. doi: 10.1016/j.smrv.2021.101499
6. Kleindorfer, DO, Towfighi, A, Chaturvedi, S, Cockroft, KM, Gutierrez, J, Lombardi-Hill, D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. (2021) 52:e364–467. doi: 10.1161/STR.0000000000000375
7. Kittner, SJ, Sekar, P, Comeau, ME, Anderson, CD, Parikh, GY, Tavarez, T, et al. Ethnic and racial variation in intracerebral hemorrhage risk factors and risk factor burden. JAMA Netw Open. (2021) 4:e2121921. doi: 10.1001/jamanetworkopen.2021.21921
8. Brunetti, V, Rollo, E, Broccolini, A, Frisullo, G, Scala, I, and Della, MG. Sleep and stroke: opening our eyes to current knowledge of a key relationship. Curr Neurol Neurosci Rep. (2022) 22:767–79. doi: 10.1007/s11910-022-01234-2
9. Mc Carthy, CE, Yusuf, S, Judge, C, Alvarez-Iglesias, A, Hankey, GJ, Oveisgharan, S, et al. Sleep patterns and the risk of acute stroke: results from the INTERSTROKE international Case-control study. Neurology. (2023) 100:e2191–203. doi: 10.1212/WNL.0000000000207249
10. Stone, KL, Blackwell, TL, Ancoli-Israel, S, Barrett-Connor, E, Bauer, DC, Cauley, JA, et al. Sleep disordered breathing and risk of stroke in older community-dwelling men. Sleep. (2016) 39:531–40. doi: 10.5665/sleep.5520
11. Ifergane, G, Ovanyan, A, Toledano, R, Goldbart, A, Abu-Salame, I, Tal, A, et al. Obstructive sleep apnea in acute stroke: a role for systemic inflammation. Stroke. (2016) 47:1207–12. doi: 10.1161/STROKEAHA.115.011749
12. Gottlieb, E, Landau, E, Baxter, H, Werden, E, Howard, ME, and Brodtmann, A. The bidirectional impact of sleep and circadian rhythm dysfunction in human ischaemic stroke: a systematic review. Sleep Med Rev. (2019) 45:54–69. doi: 10.1016/j.smrv.2019.03.003
13. Fu, S, Peng, X, Li, Y, Yang, L, and Yu, H. Effectiveness and feasibility of continuous positive airway pressure in patients with stroke and sleep apnea: a meta-analysis of randomized trials. J Clin Sleep Med. (2023) 19:1685–96. doi: 10.5664/jcsm.10680
14. Bravata, DM, Sico, J, Vaz Fragoso, CA, Miech, EJ, Matthias, MS, Lampert, R, et al. Diagnosing and treating sleep apnea in patients with acute cerebrovascular disease. J Am Heart Assoc. (2018) 7:e008841. doi: 10.1161/JAHA.118.008841
15. Baillieul, S, Dekkers, M, Brill, AK, Schmidt, MH, Detante, O, Pépin, JL, et al. Sleep apnoea and ischaemic stroke: current knowledge and future directions. Lancet Neurol. (2022) 21:78–88. doi: 10.1016/S1474-4422(21)00321-5
16. Katzan, IL, Thompson, NR, Uchino, K, and Lapin, B. The most affected health domains after ischemic stroke. Neurology. (2018) 90:e1364–71. doi: 10.1212/WNL.0000000000005327
17. Hermann, DM, and Bassetti, CL. Role of sleep-disordered breathing and sleep-wake disturbances for stroke and stroke recovery. Neurology. (2016) 87:1407–16. doi: 10.1212/WNL.0000000000003037
18. Baillieul, S, Tamisier, R, Camilo, MR, and Pontes-Neto, OM. Sleep apnea and ischemic stroke: more insights on a timeless association. Stroke. (2023) 54:2366–8. doi: 10.1161/STROKEAHA.123.043483
19. Iwuozo, EU, Enyikwola, JO, Asor, PM, Onyia, UI, Nwazor, EO, and Obiako, RO. Sleep disturbances and associated factors amongst stroke survivors in north central, Nigeria. Nigerian Postgraduate Med J. (2023) 30:193–9. doi: 10.4103/npmj.npmj_56_23
20. Seiler, A, Camilo, M, Korostovtseva, L, Haynes, AG, Brill, AK, Horvath, T, et al. Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis. Neurology. (2019) 92:e648–54. doi: 10.1212/WNL.0000000000006904
21. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. (2021) 10:89. doi: 10.1186/s13643-021-01626-4
22. Stroup, DF, Berlin, JA, Morton, SC, Olkin, I, Williamson, GD, Rennie, D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
23. Bhattacharyya, N, Zorrilla-Vaca, A, Schmitt, M, and Lozada, G. Screening for obstructive sleep apnoea in the USA: a representative cross-sectional study. Br J Anaesth. (2023) 130:e427–8. doi: 10.1016/j.bja.2022.12.016
24. Ouzzani, M, Hammady, H, Fedorowicz, Z, and Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
25. Munn, Z, Moola, S, Lisy, K, Riitano, D, and Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. (2015) 13:147–53. doi: 10.1097/XEB.0000000000000054
26. DerSimonian, R, and Laird, N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
27. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
28. Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
29. Huhtakangas, JK, Huhtakangas, J, Bloigu, R, and Saaresranta, T. Prevalence of sleep apnea at the acute phase of ischemic stroke with or without thrombolysis. Sleep Med. (2017) 40:40–6. doi: 10.1016/j.sleep.2017.08.018
30. Kim, KT, Moon, HJ, Yang, JG, Sohn, SI, Hong, JH, and Cho, YW. The prevalence and clinical significance of sleep disorders in acute ischemic stroke patients-a questionnaire study. Sleep Breathing. (2017) 21:759–65. doi: 10.1007/s11325-016-1454-5
31. Kumar, R, Suri, JC, and Manocha, R. Study of association of severity of sleep disordered breathing and functional outcome in stroke patients. Sleep Med. (2017) 34:50–6. doi: 10.1016/j.sleep.2017.02.025
32. Lisabeth, LD, Sanchez, BN, Chervin, RD, Morgenstern, LB, Zahuranec, DB, Tower, SD, et al. High prevalence of poststroke sleep-disordered breathing in Mexican Americans. Sleep Med. (2017) 33:97–102. doi: 10.1016/j.sleep.2016.01.010
33. Menon, D, Sukumaran, S, Varma, R, and Radhakrishnan, A. Impact of obstructive sleep apnea on neurological recovery after ischemic stroke: a prospective study. Acta Neurol Scand. (2017) 136:419–26. doi: 10.1111/ane.12740
34. Ponsaing, LB, Iversen, HK, and Jennum, P. Polysomnographic indicators of mortality in stroke patients. Sleep Breathing. (2017) 21:235–42. doi: 10.1007/s11325-016-1387-z
35. Ryan, CM, Wilton, K, Bradley, TD, and Alshaer, H. In-hospital diagnosis of sleep apnea in stroke patients using a portable acoustic device. Sleep Breathing. (2017) 21:453–60. doi: 10.1007/s11325-016-1438-5
36. Sarfo, FS, Jenkins, C, Mensah, NA, Saulson, R, Sarfo-Kantanka, O, Singh, A, et al. Prevalence and predictors of sleep apnea risk among Ghanaian stroke survivors. J Stroke Cerebrovasc Dis. (2017) 26:1602–8. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.027
37. Scherbakov, N, Sandek, A, Ebner, N, Valentova, M, Nave, AH, Jankowska, EA, et al. Sleep-disordered breathing in acute ischemic stroke: a mechanistic link to peripheral endothelial dysfunction. J Am Heart Assoc. (2017) 6:e006010. doi: 10.1161/JAHA.117.006010
38. Slonkova, J, Bar, M, Nilius, P, Berankova, D, Salounova, D, and Sonka, K. Spontaneous improvement in both obstructive sleep apnea and cognitive impairment after stroke. Sleep Med. (2017) 32:137–42. doi: 10.1016/j.sleep.2016.11.024
39. Festic, N, Alejos, D, Bansal, V, Mooney, L, Fredrickson, PA, Castillo, PR, et al. Sleep apnea in patients hospitalized with acute ischemic stroke: underrecognition and associated clinical outcomes. J Clin Sleep Med. (2018) 14:75–80. doi: 10.5664/jcsm.6884
40. Lisabeth, LD, Scheer, RV, Li, C, Case, E, Chervin, RD, Zahuranec, DB, et al. Intracerebral hemorrhage and sleep-disordered breathing. Sleep Med. (2018) 46:114–6. doi: 10.1016/j.sleep.2018.03.005
41. Losurdo, A, Brunetti, V, Broccolini, A, Caliandro, P, Frisullo, G, Morosetti, R, et al. Dysphagia and obstructive sleep apnea in acute, first-ever, ischemic stroke. J Stroke Cerebrovasc Dis. (2018) 27:539–46. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.051
42. Tazartukova, AD . Sleep-disordered breathing in patients with acute cerebral stroke. Pulmonologiya. (2018) 28:69–74. doi: 10.18093/0869-0189-2018-28-1-69-74
43. Yaddanapudi, SS, Pineda, MC, Boorman, DW, Bryne, RE, Hing, KL, and Sharma, S. High-resolution pulse oximetry (HRPO): a cost-effective tool in screening for obstructive sleep apnea (OSA) in acute stroke and predicting outcome. J Stroke Cerebrovasc Dis. (2018) 27:2986–92. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.030
44. Zhang, J, Gong, Z, Li, R, Gao, Y, Li, Y, Li, J, et al. Influence of lung function and sleep-disordered breathing on stroke: a community-based study. Eur J Neurol. (2018) 25:1307-e112. doi: 10.1111/ene.13722
45. Brown, DL, Shafie-Khorassani, F, Kim, S, Chervin, RD, Case, E, Morgenstern, LB, et al. Sleep-disordered breathing is associated with recurrent ischemic stroke. Stroke. (2019) 50:571–6. doi: 10.1161/STROKEAHA.118.023807
46. Li, J, You, SJ, Xu, YN, Yuan, W, Shen, Y, Huang, JY, et al. Cognitive impairment and sleep disturbances after minor ischemic stroke. Sleep Breathing. (2019) 23:455–62. doi: 10.1007/s11325-018-1709-4
47. Matsuura, D, Otaka, Y, Kamigaichi, R, Honaga, K, Kondo, K, and Liu, M. Prevalence, effect on functional outcome, and treatment of sleep-disordered breathing in patients with subacute stroke. J Clin Sleep Med. (2019) 15:891–7. doi: 10.5664/jcsm.7844
48. Nair, R, Radhakrishnan, K, Chatterjee, A, Gorthi, SP, and Prabhu, VA. Sleep apnea-predictor of functional outcome in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2019) 28:807–14. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.030
49. Brown, DL, He, K, Kim, S, Hsu, CW, Case, E, Chervin, RD, et al. Prediction of sleep-disordered breathing after stroke. Sleep Med. (2020) 75:1–6. doi: 10.1016/j.sleep.2020.05.004
50. Castello-Branco, RC, Cerqueira-Silva, T, Andrade, AL, Gonçalves, BMM, Pereira, CB, Felix, IF, et al. Association between risk of obstructive sleep apnea and cerebrovascular reactivity in stroke patients. J Am Heart Assoc. (2020) 9:e015313. doi: 10.1161/JAHA.119.015313
51. Haula, TM, Puustinen, J, Takala, M, and Holm, A. Relationship between SDB and short-term outcome in Finnish ischemic stroke patients. Brain Behav. (2020) 10:e01762. doi: 10.1002/brb3.1762
52. Huhtakangas, JK, Huhtakangas, J, Bloigu, R, and Saaresranta, T. Unattended sleep study in screening for sleep apnea in the acute phase of ischemic stroke. Sleep Med. (2020) 65:121–6. doi: 10.1016/j.sleep.2019.08.002
53. Kisabay, AAK, Saritas, AS, Batum, M, Göktalay, APDT, Horasan, PDGD, Selcuki, PDD, et al. Investigation of sleep breathing disorders in Young patients (under 55 years) with mild stroke. J Stroke Cerebrovasc Dis. (2020) 29:105263. doi: 10.1016/j.jstrokecerebrovasdis.2020.105263
54. McKee, Z, Wilson, RD, and Auckley, DH. Evaluation of an OSA risk stratifying and treatment protocol during inpatient rehabilitation of post-stroke patients. Sleep Breathing. (2020) 24:513–21. doi: 10.1007/s11325-019-01887-3
55. Ott, SR, Fanfulla, F, Miano, S, Horvath, T, Seiler, A, Bernasconi, C, et al. SAS care 1: sleep-disordered breathing in acute stroke and transient ischaemic attack - prevalence, evolution and association with functional outcome at 3 months, a prospective observational polysomnography study. ERJ Open Res. (2020) 6:00334–2019. doi: 10.1183/23120541.00334-2019
56. Pajediene, E, Pajeda, A, Urnieziute, G, Paulekas, E, Liesiene, V, Bileviciute-Ljungar, I, et al. Subjective and objective features of sleep disorders in patients with acute ischemic or haemorrhagic stroke: it is not only sleep apnoea which is important. Med Hypotheses. (2020) 136:109512. doi: 10.1016/j.mehy.2019.109512
57. Slim, M, Westmacott, R, Toutounji, S, Singh, J, Narang, I, Weiss, S, et al. Obstructive sleep apnea syndrome and neuropsychological function in pediatric stroke. Eur J Paediatr Neurol. (2020) 25:82–9. doi: 10.1016/j.ejpn.2019.11.006
58. Yoon, CW, Park, HK, Bae, EK, and Rha, JH. Sleep apnea and early neurological deterioration in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2020) 29:104510. doi: 10.1016/j.jstrokecerebrovasdis.2019.104510
59. Chen, Q, Fu, X, Wang, XH, Chen, J, Huang, JY, Mao, CJ, et al. Effect of sleep-disordered breathing during rapid eye movement sleep and non-rapid eye movement sleep on acute ischemic stroke. J Stroke Cerebrovasc Dis. (2021) 30:105913. doi: 10.1016/j.jstrokecerebrovasdis.2021.105913
60. Domínguez-Mayoral, A, Sánchez-Gómez, J, Guerrero, P, Ferrer, M, Gutiérrez, C, Aguilar, M, et al. High prevalence of obstructive sleep apnea syndrome in Spain's Stroke Belt. J Int Med Res. (2021) 49:3000605211053090. doi: 10.1177/03000605211053090
61. Estai, M, Walsh, J, Maddison, K, Shepherd, K, Hillman, D, McArdle, N, et al. Sleep-disordered breathing in patients with stroke-induced dysphagia. J Sleep Res. (2021) 30:e13179. doi: 10.1111/jsr.13179
62. Folgueira, AL, Valiensi, SM, De Francesco, L, Berrozpe, EC, Quiroga Narvaez, J, Martínez, OA, et al. Respiratory disorders during sleep in patients with acute ischemic stroke. Revista de la Facultad de Ciencias Medicas. (2021) 78:264–9. doi: 10.31053/1853.0605.v78.n3.28102
63. Gottlieb, E, Khlif, MS, Bird, L, Werden, E, Churchward, T, Pase, MP, et al. Sleep architectural dysfunction and undiagnosed obstructive sleep apnea after chronic ischemic stroke. Sleep Med. (2021) 83:45–53. doi: 10.1016/j.sleep.2021.04.011
64. Petrie, BK, Sturzoiu, T, Shulman, J, Abbas, S, Masoud, H, Romero, JR, et al. Questionnaire and portable sleep test screening of sleep disordered breathing in acute stroke and TIA. J Clin Med. (2021) 10:3568. doi: 10.3390/jcm10163568
65. Riglietti, A, Fanfulla, F, Pagani, M, Lucini, D, Malacarne, M, Manconi, M, et al. Obstructive and central sleep apnea in first ever ischemic stroke are associated with different time course and autonomic activation. Nat Sci Sleep. (2021) 13:1167–78. doi: 10.2147/NSS.S305850
66. Siarnik, P, Jurik, M, Klobucnikova, K, Kollár, B, Pirošová, M, Malík, M, et al. Sleep apnea prediction in acute ischemic stroke (SLAPS score): a derivation study. Sleep Med. (2021) 77:23–8. doi: 10.1016/j.sleep.2020.11.022
67. Baillieul, S, Bailly, S, Detante, O, Alexandre, S, Destors, M, Clin, R, et al. Sleep-disordered breathing and ventilatory chemosensitivity in first ischaemic stroke patients: a prospective cohort study. Thorax. (2022) 77:1006–14. doi: 10.1136/thoraxjnl-2021-218003
68. Edrissi, C, Rathfoot, C, Knisely, K, Sanders, CB, Poupore, N, and Nathaniel, T. Gender disparity in a cohort of stroke patients with incidence of obstructive sleep apnea. J Vasc Nurs. (2022) 40:17–27. doi: 10.1016/j.jvn.2021.10.002
69. Griesbach, GS, Howell, SN, and Masel, BE. Obstructive sleep apnea during the chronic stroke recovery period: comparison between primary haemorrhagic and ischaemic events. J Sleep Res. (2022) 31:e13460. doi: 10.1111/jsr.13460
70. Huhtakangas, JK, Saaresranta, T, Vähänikkilä, H, and Huhtakangas, J. Nocturnal hypoxemia and central apneas increase mortality, but not recurrent ischemic events after ischemic stroke: nocturnal hypoxemia and central apneas increase mortality after stroke. Sleep Med. (2022) 97:1–9. doi: 10.1016/j.sleep.2022.05.014
71. Liu, X, Lam, DCL, Mak, HKF, Ip, MSM, and Lau, KK. Associations of sleep apnea risk and oxygen desaturation indices with cerebral small vessel disease burden in patients with stroke. Front Neurol. (2022) 13:956208. doi: 10.3389/fneur.2022.956208
72. Rafi, A, Maqbool, M, Malik, SS, Shah, PA, and Kaul, R. Prevalence of obstructive sleep apnea in neurological disorders. JK Pract. (2022) 27:16–23. doi: 10.1002/mds.29223
73. Schutz, SG, Lisabeth, LD, Gibbs, R, Shi, X, Chervin, RD, Kwicklis, M, et al. Ten-year trends in sleep-disordered breathing after ischemic stroke: 2010 to 2019 data from the BASIC project. J Am Heart Assoc. (2022) 11:e024169. doi: 10.1161/JAHA.121.024169
74. Simonsen, SA, Andersen, AV, West, AS, Wolfram, F, Jennum, P, and Iversen, HK. Sleep-disordered breathing and cerebral small vessel disease—acute and 6 months after ischemic stroke. Sleep Breathing. (2022) 26:1107–13. doi: 10.1007/s11325-021-02482-1
75. Springer, MV, Lisabeth, LD, Gibbs, R, Shi, X, Case, E, Chervin, RD, et al. Racial and ethnic differences in sleep-disordered breathing and sleep duration among stroke patients. J Stroke Cerebrovasc Dis. (2022) 31:106822. doi: 10.1016/j.jstrokecerebrovasdis.2022.106822
76. Zhang, Y, Xia, X, Zhang, T, Zhang, C, Liu, R, Yang, Y, et al. Relationship between sleep disorders and the prognosis of neurological function after stroke. Front Neurol. (2022) 13:1036980. doi: 10.3389/fneur.2022.1036980
77. Zhu, R-l, Ouyang, C, Ma, R-l, and Wang, K. Obstructive sleep apnea is associated with cognitive impairment in minor ischemic stroke. Sleep Breathing. (2022) 26:1907–14. doi: 10.1007/s11325-022-02575-5
78. Bochkarev, MV, Korostovtseva, LS, Golovkova-Kucheryavaya, MS, Zheleznyakov, VE, Sviryaev, YV, and Yanishevsky, SN. Sleep-disordered breathing in the acute phase of ischemic stroke. Arterial Hypertension. (2023) 29:194–200. doi: 10.18705/1607-419X-2023-29-2-194-200
79. Brunetti, V, Testani, E, Losurdo, A, Vollono, C, Broccolini, A, di Iorio, R, et al. Association of Obstructive Sleep Apnea and Atrial Fibrillation in acute ischemic stroke: a cross-sectional study. J Pers Med. (2023) 13:527. doi: 10.3390/jpm13030527
80. Duss, SB, Bauer-Gambelli, SA, Bernasconi, C, Dekkers, MPJ, Gorban-Peric, C, Kuen, D, et al. Frequency and evolution of sleep-wake disturbances after ischemic stroke: a 2-year prospective study of 437 patients. Sleep Med. (2023) 101:244–51. doi: 10.1016/j.sleep.2022.10.007
81. Hoang-Anh, T, Duong-Minh, Q, Nguyen-Thi-Y, N, and Duong-Quy, S. Study of the obstructive sleep apnea syndrome in cerebral infarction patients. Front Neurol. (2023) 14:1132014. doi: 10.3389/fneur.2023.1132014
82. Hong, Y, Mo, H, Cho, SJ, and Im, HJ. Wake-up ischemic stroke associated with short sleep duration and sleep behavior: a stratified analysis according to risk of obstructive sleep apnea. Sleep Med. (2023) 101:497–504. doi: 10.1016/j.sleep.2022.11.038
83. Korostovtseva, L, Bochkarev, M, Amelina, V, Nikishkina, U, Osipenko, S, Vasilieva, A, et al. Sleep-disordered breathing and prognosis after ischemic stroke: it is not apnea-hypopnea index that matters. Diagnostics. (2023) 13:2246. doi: 10.3390/diagnostics13132246
84. Lin, HJ, Chen, PC, Liu, YH, and Hsu, CY. Increasing and high prevalence of moderate to severe obstructive sleep apnea in acute ischemic stroke in Taiwan. J Formos Med Assoc. (2023) 123:408–14. doi: 10.1016/j.jfma.2023.09.005
85. Lisabeth, LD, Zhang, G, Chervin, RD, Shi, X, Morgenstern, LB, Campbell, M, et al. Longitudinal assessment of sleep apnea in the year after stroke in a population-based study. Stroke. (2023) 54:2356–65. doi: 10.1161/STROKEAHA.123.042325
86. Patel, UK, Rao, A, Manihani, GSD, Patel, N, George, C, Vijayakumar, JS, et al. Prevalence and outcomes of depression, obstructive sleep apnea, and concurrent anxiety (DOCA) in stroke survivors: insights from a Nationwide study. Cureus J Med Sci. (2023) 15:e41968. doi: 10.7759/cureus.41968
87. Plomaritis, P, Theodorou, A, Michalaki, V, Stefanou, MI, Palaiodimou, L, Papagiannopoulou, G, et al. Periodic limb movements during sleep in acute stroke: prevalence, severity and impact on post-stroke recovery. J Clin Med. (2023) 12:5881. doi: 10.3390/jcm12185881
88. Tayade, K, Vibha, D, Tripathi, M, Singh, RK, and Elavarasi, A. Association between self and caregiver reported sleep problems and polysomnographic findings in post-stroke patients: a cross sectional study. Neurology. (2023) 100:2603. doi: 10.1212/WNL.0000000000202666
89. Zhu, Q, Chen, L, Xu, Q, Xu, J, Zhang, L, and Wang, J. Association between obstructive sleep apnea and risk for post-stroke anxiety: a Chinese hospital-based study in noncardiogenic ischemic stroke patients. Sleep Med. (2023) 107:55–63. doi: 10.1016/j.sleep.2023.04.016
90. Brooks, D, Davis, L, Vujovic-Zotovic, N, Boulias, C, Ismail, F, Richardson, D, et al. Sleep-disordered breathing in patients enrolled in an inpatient stroke rehabilitation program. Arch Phys Med Rehabil. (2010) 91:659–62. doi: 10.1016/j.apmr.2009.12.019
91. Chan, W, Coutts, SB, and Hanly, P. Sleep apnea in patients with transient ischemic attack and minor stroke opportunity for risk reduction of recurrent stroke? Stroke. (2010) 41:2973–5. doi: 10.1161/STROKEAHA.110.596759
92. Pasic, Z, Smajlovic, D, Dostovic, Z, Kojic, B, and Selmanovic, S. Incidence and types of sleep disorders in patients with stroke. Med Arh. (2011) 65:225–7. doi: 10.5455/medarh.2011.65.225-227
93. Aaronson, JA, van Bezeij, T, van den Aardweg, JG, van Bennekom, CA, and Hofman, WF. Diagnostic accuracy of nocturnal oximetry for detection of sleep apnea syndrome in stroke rehabilitation. Stroke. (2012) 43:2491–3. doi: 10.1161/STROKEAHA.112.665414
94. Camilo, MR, Fernandes, RM, Sander, HH, Nobre, F, Santos-Pontelli, T, Santos, AC, et al. Supine sleep and positional sleep apnea after acute ischemic stroke and intracerebral hemorrhage. Clinics. (2012) 67:1357–60. doi: 10.6061/clinics/2012(12)02
95. Hsieh, SW, Lai, CL, Liu, CK, Hsieh, CF, and Hsu, CY. Obstructive sleep apnea linked to wake-up strokes. J Neurol. (2012) 259:1433–9. doi: 10.1007/s00415-011-6370-9
96. Xu, J, Deng, L, Zou, X, Liu, H, Yu, Y, and Ding, Y. [Impact of obstructive sleep apnea-hypopnea syndrome on cerebral microbleeds in patients with cerebral infarction]. J Southern Med Univ. (2012) 32:1362–5. doi: 10.3969/j.issn.1673-4254.2012.09.034
97. Ahn, SH, Kim, JH, Kim, DU, Choo, IS, Lee, HJ, and Kim, HW. Interaction between sleep-disordered breathing and acute ischemic stroke. J Clin Neurol. (2013) 9:9–13. doi: 10.3988/jcn.2013.9.1.9
98. Cereda, CW, Tamisier, R, Manconi, M, Andreotti, J, Frangi, J, Pifferini, V, et al. Endothelial dysfunction and arterial stiffness in ischemic stroke the role of sleep-disordered breathing. Stroke. (2013) 44:1175–8. doi: 10.1161/STROKEAHA.111.000112
99. Ciccone, A, Proserpio, P, Roccatagliata, DV, Nichelatti, M, Gigli, GL, Parati, G, et al. Wake-up stroke and TIA due to paradoxical embolism during long obstructive sleep apnoeas: a cross-sectional study. Thorax. (2013) 68:97–104. doi: 10.1136/thoraxjnl-2012-201643
100. Shibazaki, K, Kimura, K, Uemura, J, Sakai, K, Fujii, S, Sakamoto, Y, et al. Atrial fibrillation is associated with severe sleep-disordered breathing in patients with ischaemic stroke and transient ischaemic attack. Eur J Neurol. (2013) 20:266–70. doi: 10.1111/j.1468-1331.2012.03837.x
101. Kepplinger, J, Barlinn, K, Boehme, AK, Gerber, J, Puetz, V, Pallesen, LP, et al. Association of sleep apnea with clinically silent microvascular brain tissue changes in acute cerebral ischemia. J Neurol. (2014) 261:343–9. doi: 10.1007/s00415-013-7200-z
102. Lefèvre-Dognin, C, Stana, L, Jousse, M, Lucas, C, Sportouch, P, Bradai, N, et al. Lack of repercussions of sleep apnea syndrome on recovery and attention disorders at the subacute stage after stroke: a study of 45 patients. Ann Phys Rehabil Med. (2014) 57:618–28. doi: 10.1016/j.rehab.2014.09.008
103. Ramos, AR, Guilliam, D, Dib, SI, and Koch, S. Race/ethnic differences in obstructive sleep apnea risk in patients with acute ischemic strokes in South Florida. Sleep Breathing. (2014) 18:165–8. doi: 10.1007/s11325-013-0865-9
104. Shibazaki, K, Kimura, K, Aoki, J, Uemura, J, Fujii, S, and Sakai, K. Dysarthria plus dysphagia is associated with severe sleep-disordered breathing in patients with acute intracerebral hemorrhage. Eur J Neurol. (2014) 21:344–8. doi: 10.1111/ene.12323
105. Vayrynen, K, Kortelainen, K, Numminen, H, Miettinen, K, Keso, A, Tenhunen, M, et al. Screening sleep disordered breathing in stroke unit. Sleep Disord. (2014) 2014:317615:1–7. doi: 10.1155/2014/317615
106. Chen, CY, Chen, CL, and Yu, CC. Obstructive sleep apnea is independently associated with arterial stiffness in ischemic stroke patients. J Neurol. (2015) 262:1247–54. doi: 10.1007/s00415-015-7699-2
107. Stahl, SM, Yaggi, HK, Taylor, S, Qin, L, Ivan, CS, Austin, C, et al. Infarct location and sleep apnea: evaluating the potential association in acute ischemic stroke. Sleep Med. (2015) 16:1198–203. doi: 10.1016/j.sleep.2015.07.003
108. Boulos, MI, Wan, A, Im, J, Elias, S, Frankul, F, Atalla, M, et al. Identifying obstructive sleep apnea after stroke/TIA: evaluating four simple screening tools. Sleep Med. (2016) 21:133–9. doi: 10.1016/j.sleep.2015.12.013
109. Koo, BB, Bravata, DM, Tobias, LA, Mackey, JS, Miech, EJ, Matthias, MS, et al. Observational study of obstructive sleep apnea in wake-up stroke: the SLEEP TIGHT study. Cerebrovasc Dis. (2016) 41:233–41. doi: 10.1159/000440736
110. Lutohin, GM, Geraskina, LA, and Fonyakin, AV. Sleep-disordered breathing syndrome in acute ischemic stroke. Zh Nevrol Psikhiatr Im S S Korsakova. (2016) 116:14–20. doi: 10.17116/jnevro201611612214-20
111. Tur, S, de la Pena, M, Vives, B, Martínez, AB, Gorospe, A, Legarda, I, et al. Sleep apnea syndrome and patent foramen ovale: a dangerous association in ischemic stroke? Sleep Med. (2016) 25:29–33. doi: 10.1016/j.sleep.2016.07.014
112. Fisse, AL, Kemmling, A, Teuber, A, Wersching, H, Young, P, Dittrich, R, et al. The Association of Lesion Location and Sleep Related Breathing Disorder in patients with acute ischemic stroke. PLoS One. (2017) 12:e0171243. doi: 10.1371/journal.pone.0171243
113. Bonsignore, MR, Saaresranta, T, and Riha, RL. Sex differences in obstructive sleep apnoea. European respiratory review: an official journal of the European respiratory. Society. (2019) 28:190030. doi: 10.1183/16000617.0030-2019
114. André, S, Andreozzi, F, Van Overstraeten, C, Ben Youssef, S, Bold, I, Carlier, S, et al. Cardiometabolic comorbidities in obstructive sleep apnea patients are related to disease severity, nocturnal hypoxemia, and decreased sleep quality. Respir Res. (2020) 21:35. doi: 10.1186/s12931-020-1284-7
115. Krause, AJ, Simon, EB, Mander, BA, Greer, SM, Saletin, JM, Goldstein-Piekarski, AN, et al. The sleep-deprived human brain. Nat Rev Neurosci. (2017) 18:404–18. doi: 10.1038/nrn.2017.55
116. Boulos, MI, Dharmakulaseelan, L, Brown, DL, and Swartz, RH. Trials in sleep apnea and stroke: learning from the past to direct future approaches. Stroke. (2021) 52:366–72. doi: 10.1161/STROKEAHA.120.031709
117. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.ED000142
Keywords: sleep-disordered breathing, stroke, epidemiology, systematic review, meta-analysis, meta-regression
Citation: Su X, Liu S, Wang C, Cai Y, Li Y, Wang D, Fan Z and Jiang Y (2024) Prevalence, incidence, and the time trends of sleep-disordered breathing among patients with stroke: a systematic review and meta-analysis. Front. Neurol. 15:1432085. doi: 10.3389/fneur.2024.1432085
Edited by:
Valerio Brunetti, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Queran Lin, Imperial College London, United KingdomIrene Scala, Agostino Gemelli University Polyclinic (IRCCS), Italy
Copyright © 2024 Su, Liu, Wang, Cai, Li, Wang, Fan and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Jiang, aHhobGp5MjAxOEAxNjMuY29t; Zhaofeng Fan, OTE0NjA5MTQxQHFxLmNvbQ==
 Xiaofeng Su
Xiaofeng Su Shanshan Liu1
Shanshan Liu1 Zhaofeng Fan
Zhaofeng Fan Yan Jiang
Yan Jiang