
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 18 July 2024
Sec. Neuroinfectious Diseases
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1429354
 Yutaka Fuchinoue1*
Yutaka Fuchinoue1* Kosuke Kondo1
Kosuke Kondo1 Yuki Sakaeyama1
Yuki Sakaeyama1 Chie Nakada1
Chie Nakada1 Sayaka Terazono1
Sayaka Terazono1 Syuhei Kubota1
Syuhei Kubota1 Masataka Mikai1
Masataka Mikai1 Mituyoshi Abe1
Mituyoshi Abe1 Shinji Ujiie2
Shinji Ujiie2 Toshisuke Morita2
Toshisuke Morita2 Nobuo Sugo1
Nobuo Sugo1Objective: To determine the usefulness of cerebrospinal fluid (CSF) presepsin in the diagnosis of neurosurgical postoperative meningitis (POM).
Methods: The study included patients admitted to the Department of Neurosurgery, Toho University Medical Center Omori Hospital from May 1, 2020 to March 31, 2022 with suspected meningitis after neurosurgery who clinically required CSF sampling and patients who underwent CSF sampling for examination of idiopathic normal pressure hydrocephalus (iNPH). Participants were divided into a POM and a postoperative non meningitis (PONM) group based on the POM diagnostic criteria established for this study. The control group included patients from whom a CSF sample for iNPH was collected by tap test.
Results: A total of 238 CSF samples were collected from 90 patients. There were 39 samples in the POM, 180 samples in the PONM, and 19 samples in the control group. CSF presepsin levels in the POM were significantly higher than in the PONM group (1764.5 and 440.9 pg./mL, respectively; p < 0.0001). The control group had CSF presepsin levels of 95.5 pg./mL. A cutoff value of 669 pg./mL for CSF presepsin in POM and PONM groups had 76.9% sensitivity and 78.3% specificity for the diagnosis of POM. In analyzes including only subarachnoid hemorrhage (SAH) cases (123 samples), CSF presepsin (1251.2 pg./mL) in the POM was significantly higher than in the PONM subgroup (453.9 pg./mL; p < 0.0001). The cutoff value for presepsin in CSF among patients with SAH (669 pg./mL) had 87.5% sensitivity and 76.6% specificity, similar to that of all patients.
Conclusion: CSF presepsin is a useful marker in the diagnosis of neurosurgical POM even in patients with blood components, such as SAH. When POM is suspected, measurement of CSF presepsin may be recommended in addition to a general CSF examination.
The incidence of bacterial meningitis after neurosurgery is 0.3–25% (1–4). Particularly, patients with intraventricular and lumbar catheters are at risk of postoperative meningitis (POM) (5, 6) and nearly one third (10–27%) of patients with an external ventricular drainage have meningitis (7–11). In a previous study including 6,243 neurosurgical patients, patients with/without POM had a 3-month mortality rate of 13.7 and 4.7%, respectively (1). Moreover, a previous review of 334 craniotomies found a 29% mortality rate from POM (12). Thus, a prompt POM diagnosis is essential to improve patient outcomes (5).
POM symptoms include headache, nausea, malaise, altered consciousness, convulsions, neck stiffness, and other meningeal signs, as well as skin redness and tenderness associated with subcutaneous shunting and fever without other obvious foci of infection (13). However, it is difficult to diagnose POM based only on clinical symptoms; cerebrospinal fluid (CSF) analysis is a cornerstone in diagnosing meningitis. In fact, the diagnosis of bacterial meningitis caused by paranasal sinusitis or otitis media is routinely based on CSF cell counts, glucose, and protein concentration (14). However, the CSF cell counts and protein levels increase after neurosurgical operations for subarachnoid hemorrhage (SAH) or intraventricular hemorrhage due to bloody CSF (14). CSF cultures have a higher diagnostic performance but are limited by its low positivity rates, the presence of contaminating bacteria, and the long time required to obtain culture results (15).
Presepsin is a soluble CD14 protein composed of 64 amino acids (16). CD14 is a glycolipid-anchored membrane glycoprotein expressed on cells of the myelomonocyte lineage (17). CD14 binds to bacteria and is phagocytosed by granulocytes together with the bacteria. CD14 is then cleaved by intracellular digestive enzymes into presepsin, which is released into the blood (16). From an early stage of infection, presepsin is found at high concentrations in the blood of patients with sepsis, reflecting disease severity (18). Notably, presepsin is less susceptible to noninfectious inflammatory conditions such as trauma, burns, and surgery than other conventional sepsis markers such as procalcitonin and C-reactive protein (CRP) (19, 20). So far, few studies have used CSF presepsin for the diagnosis of POM in the field of neurosurgery (14, 21, 22); thus, we aimed to investigate the utility of CSF presepsin for diagnosing meningitis after neurosurgery.
The study subjects were patients who were suspected post meningitis after neurosurgical operations at Toho University Medical Center Omori Hospital between May 1, 2020, and March 31, 2022, and clinically required CSF sampling. Cases for whom CSF was collected for the diagnosis of idiopathic normal pressure hydrocephalus (iNPH) were also eligible for inclusion in our final analysis. CSF was collected through a lumbar puncture or a lumbar or ventricular drain. Routine CSF tests, CSF culture, and CSF presepsin levels were evaluated in this study.
Based on the United States guidelines for bacterial meningitis and Ortiz’s report (6, 23), this study adopted the following diagnostic criteria for POM: A body temperature ≥ 38°C, serum CRP ≥ 6 mg/dL, CSF cell count ≥1,000/μL, CSF polymorphonuclear cell ratio ≥ 80%, CSF protein ≥100 mg/dL, CSF glucose ≤40 mg/dL, and a CSF glucose: serum ratio < 0.4. POM Diagnosis was based on meeting ≥4 of the above criteria, and comprehensive evaluation by ≥2 Japan neurosurgical society board certified neurosurgeons.
In this study, patients who developed postoperative meningitis were included in the postoperative meningitis case group (POM-C), while those who did not were the postoperative non meningitis case group (PONM-C). Patient CSF specimens satisfying postoperative meningitis diagnostic criteria were categorized as postoperative meningitis specimens (POM-S), and specimens not doing so as PONM-S. A patient with multiple CSF tests had each specimen categorized into either a POM-S or a PONM-S. We also evaluated general CSF data, blood examination data, and clinical findings on the same day as CSF presepsin levels were measured. CSF collected by the tap test by lumbar puncture for iNPH was used as a control group.
CSF samples were first centrifuged (3,000 rpm × 10 min at 4°C) and the supernatant placed into a container for measurement (measurement time: 19 min) with STACIA (manufactured by LSI Medience, Tokyo, Japan).
The measurement protocol was as follows: (1) 25 μL of sample diluent was added to a 25 μL of CSF and heated to 37°C for 3.5 min, (2) 50 μL of enzyme-labeled antibody reagent was then added to the mixture and heated to 37°C for 4.2 min, (3) subsequently, 50 μL of magnetic latex reagent was added to the mixture and heated to 37°C for 4.4 min, (4) the mixture was washed out and 100 μL of organic solution was added to the extract and warmed to 37°C for 2.7 min before measuring luminescence measured.
The Kruskal–Wallis test and Steel–Dwass method were used to compare the three patient groups (POM-C, PONM-C, and iNPH). The chi-squared test or Mann–Whitney U test were used to compare categorical and continuous variables between sample groups (POM-S and PONM-S). Univariate analysis using logistic regression was performed using POM as dependent variable and age, sex, serum glucose, CSF presepsin, CSF mononuclear cell ratio and POM diagnostic criteria CRP, CSF cell count, CSF polymorphonuclear cell ratio, CSF protein, CSF glucose, and CSF glucose: serum ratio as independent variables. In addition, receiver operating curve (ROC) analysis was performed to calculate the cutoff values for POM-S and the PONM-S. A subgroup analysis was performed for patients with SAH to illustrate the diagnostic utility of Presepsin in the presence of blood in CSF. A linear mixed analysis was performed on postoperative changes in presepsin, CRP, and white blood cell counts (WBC) over time from postoperative day 1 to 9 for patients with SAH. Statistical significance was set at p < 0.05. IBM SPSS Statistics 26 (IBM Corp., Armonk, NY, United States) was used for data analysis. This study was approved by the Ethics Committee of Toho University Omori Medical Center (approval number: M19212).
A total of 253 samples from 90 patients including 234 samples from neurosurgical cases and 19 from the control group. In neurosurgical cases, after excluding 15 samples from patients that had not undergone operation, 39 and 180 samples were obtained in POM-S and PONM-S, respectively (Figure 1).
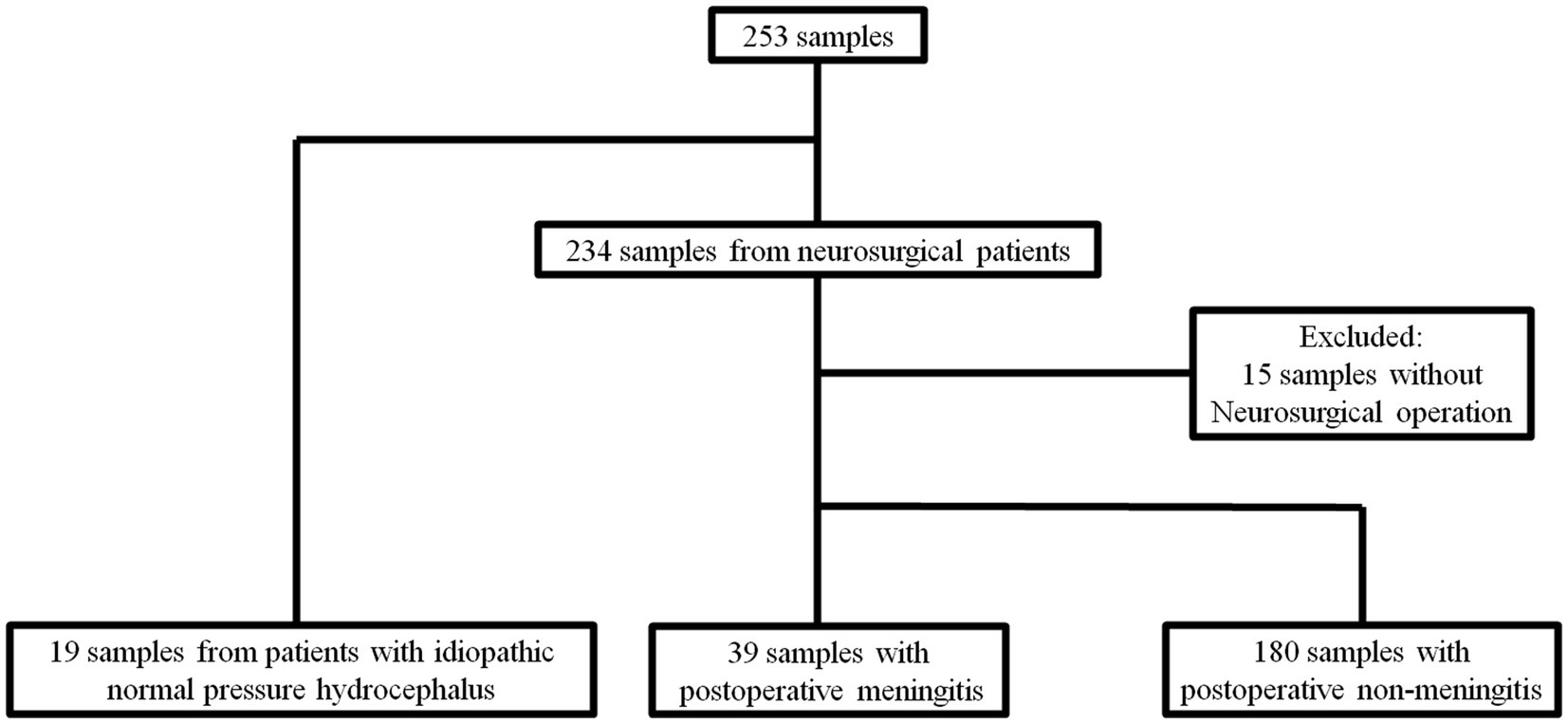
Figure 1. Flow diagram of the sample algorithm. A total of 253 samples from 90 patients including 234 samples from neurosurgical cases and 19 from the control group. In neurosurgical cases, after excluding 15 samples from patients that had not undergone operation, 39 and 180 samples were obtained in POM-S and PONM-S, respectively. iNPH, idiopathic normal pressure hydrocephalus; POM, Postoperative meningitis; POMN, Postoperative nonmeningitis.
The clinical characteristics of the POM-C, PONM-C, and iNPH control groups are shown in Table 1. The average age of the patients was 56.8 years in the POM, 60.4 years in the PONM, and 74.8 years in the control group. There was a significant difference (p < 0.01) between the three groups, but there no differences between the POM-C and PONM-C groups (p = 0.75). There was no significant difference in gender between the POM-C, PONM-C, and the control group (p = 0.68). The causative diseases in the POM-C were 12 cases of SAH, 6 cases of cerebral hemorrhage, 3 cases of brain tumor, and 1 case of others. In the PONM-C, there were 20 cases of SAH, 6 cases of cerebral hemorrhage, 15 cases of brain tumor, one case of hydrocephalus, seven other cases, and there was no significant difference in the causative disease between the POM-C and the PONM-C (p = 0.98). Treatment details for the POM-C, PONM-C included coiling, ventricular drainage, endoscopic surgery, clipping, shunt, tumor resection, others, and there was no significant difference between the 2 groups (p = 0.94) (Table 1). Next, the three groups were compared based on CSF samples. There were no differences among the sample groups according to disease procedure.
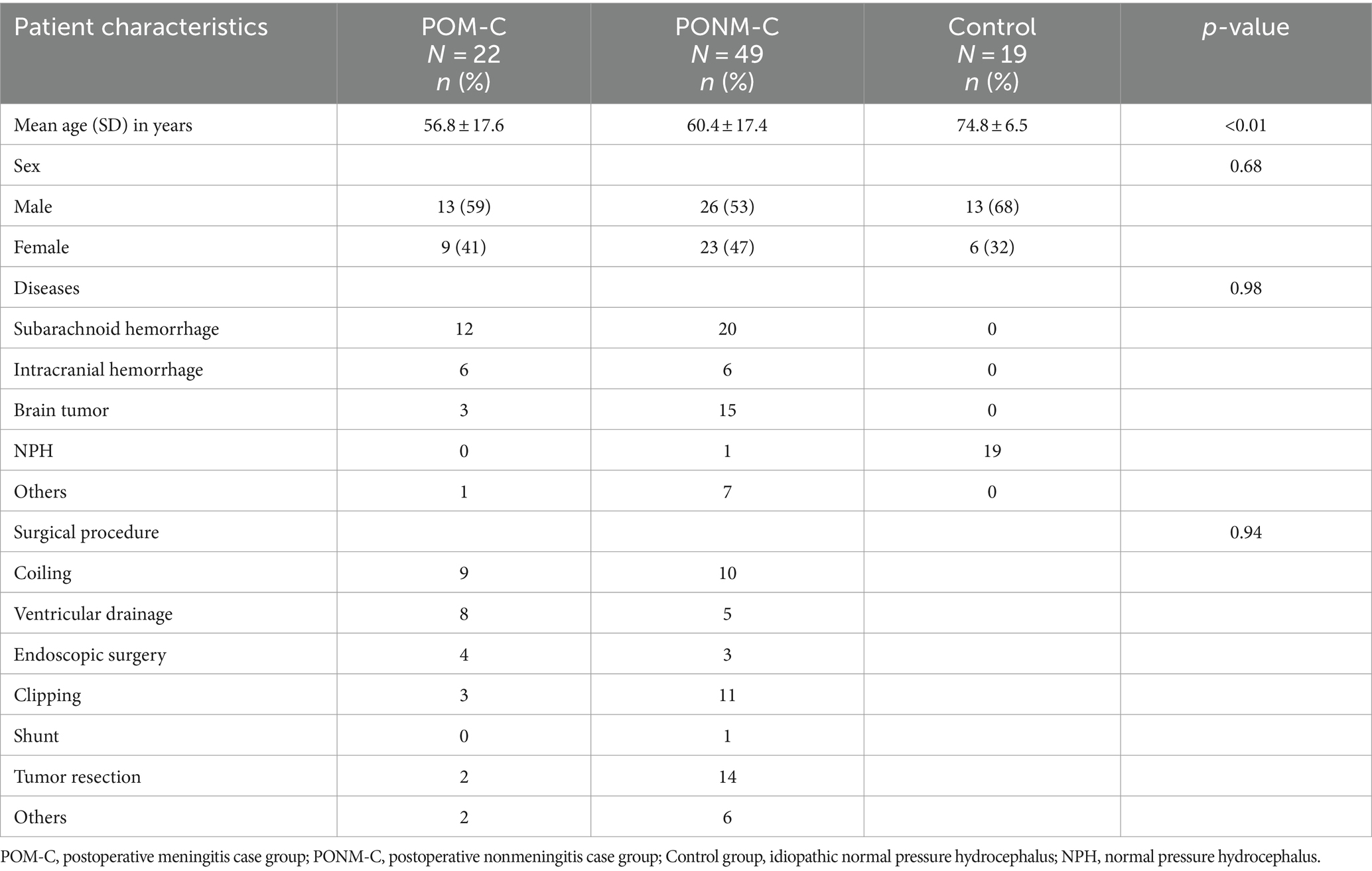
Table 1. Characteristics of patients who underwent neurosurgery at Toho University Medical Center Omori Hospital, Japan, 2020–2022 according to diagnostic criteria.
Patients’ sample characteristics are shown in Table 2. Patients’ body temperatures were significantly higher in the POM-S group than in the PONM-S group (p < 0.0001). CSF presepsin levels were significantly higher in the POM-S group than in the PONM-S group (1764.5 and 440.9 pg./mL, respectively; p < 0.0001). The CRP level (p < 0.0001), CSF cell count (p < 0.0001), CSF polymorphonuclear cell ratio (p < 0.0001), and CSF protein (p < 0.0001) level were significantly higher in the POM-S group than in the PONM-S. However, CSF mononuclear cell ratio (p < 0.0001), CSF glucose level (p < 0.0001), and CSF glucose: serum ratio (p < 0.0001) were significantly lower in the POM-S group than in the PONM-S. The mean values of CSF presepsin of patients with and without renal dysfunction (eGFR<60) were 475.0 and 703.8, respectively. These were not statistically significantly different (p = 0.58). There were 10 culture-positive cases. The isolates were Enterococcus faecalis, Staphylococcus epidermidis, Staphylococcus capitis, Staphylococcus warneri, Staphylococcus aureus, Enterobacter aerogenes were detected. The mean values of presepsin in culture-positive and culture-negative cases were 1,096 pg./mL (±793.0) and 603.6 pg./mL (± 1044.4), respectively, indicating that presepsin levels were significantly higher in culture-positive cases than in culture-negative cases (p = 0.02).
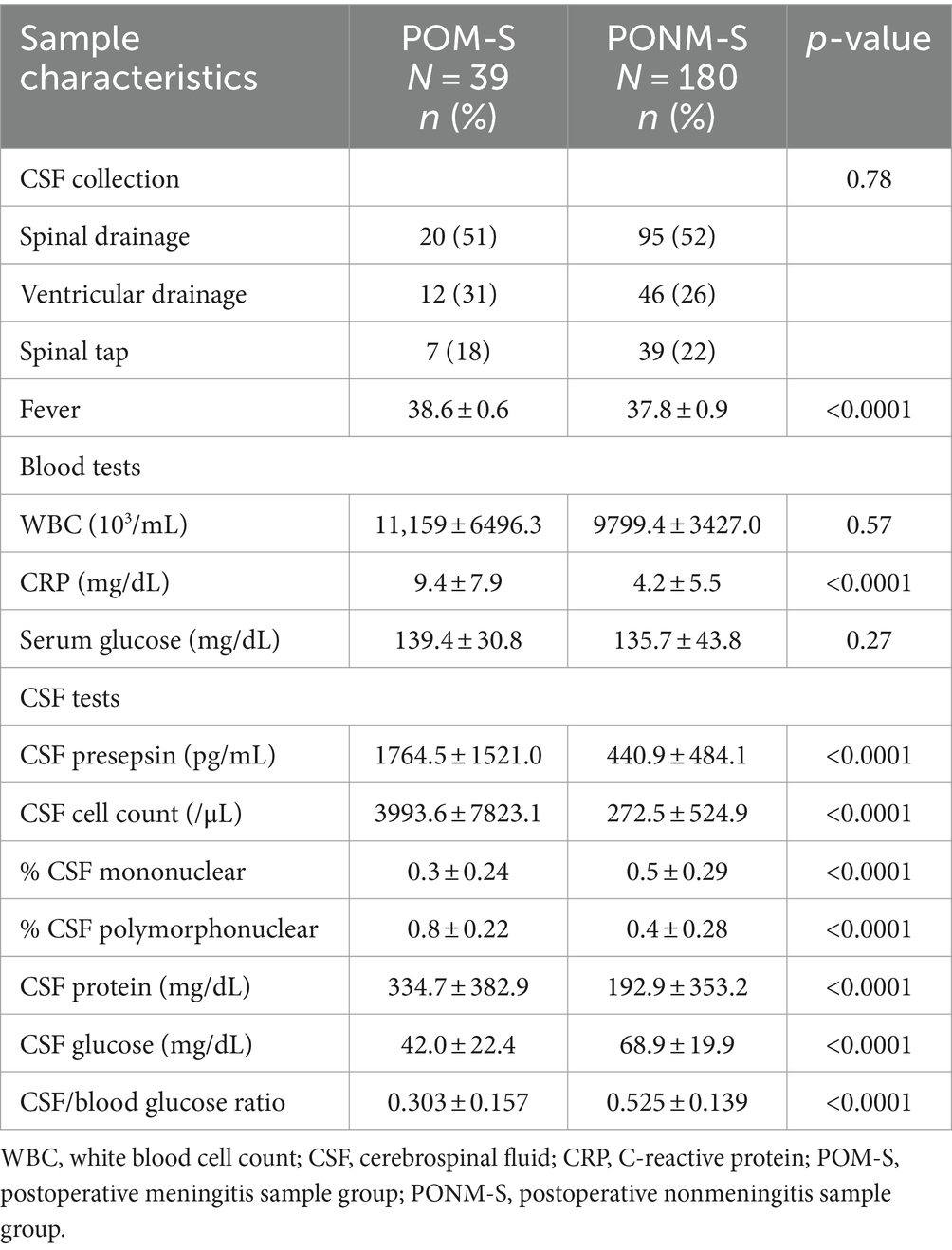
Table 2. CSF sample characteristics of patients who underwent neurosurgery at Toho University Medical Center Omori Hospital, Japan, 2020–2022.
Univariate analysis with the presence or absence of POM as the objective variable showed significant differences in fever, CSF presepsin, CRP, CSF cell count, CSF mononuclear cell ratio, CSF polymorphonuclear cell ratio, CSF glucose, and CSF glucose: serum ratio (Table 3). The univariate analysis of this study included fever, CRP, CSF cell count, CSF polymorphonuclear cell ratio, CSF glucose, CSF protein, and CSF glucose: serum ratio, which were the diagnostic criteria for the POM. When multivariate analysis was performed without POM diagnostic criteria, CSF presepsin was the only predictive factor of POM (Table 4).
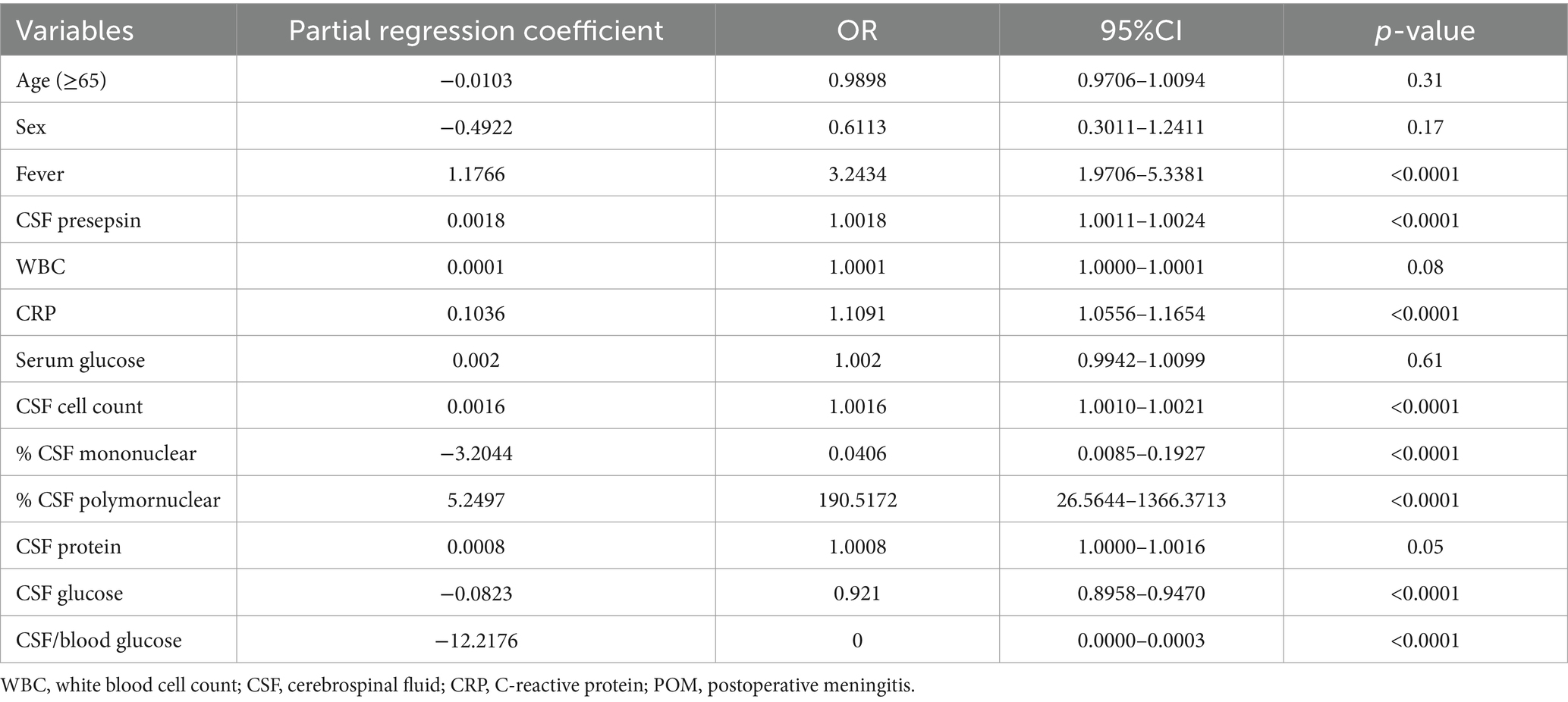
Table 3. Univariate logistic regression illustrating factors predictive of POM among CSF samples from patients who underwent neurosurgery at Toho University Medical Center Omori Hospital, Japan, 2020–2022 using diagnostic criteria as independent variables.
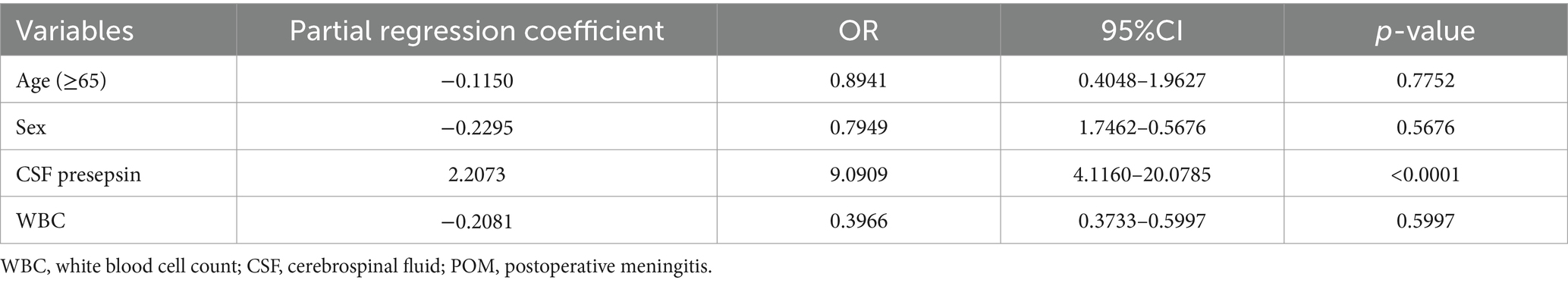
Table 4. Multivariate logistic regression illustrating factors predictive of POM among CSF samples from patients who underwent neurosurgery at Toho University Medical Center Omori Hospital, Japan, 2020–2022 excluding diagnostic criteria independent variables.
ROC analysis showed a sensitivity of 76.9% and specificity of 78.3% for a cutoff value of 669 pg./mL for CSF presepsin levels in the POM-S and PONM-S groups. In addition, the area under the curve (AUC) for CSF presepsin was 0.86 (95%CI 0.79–0.92). This was the second highest value compared to the AUC of other parameters (fever, WBC, CRP, CSF cell count, CSF mononuclear cell ratio, CSF polymorphonuclear cell ratio, CSF protein, CSF glucose, and CSF glucose; serum ratio) (Table 5).
We compared the clinical characteristics of patients with SAH with ≥1 sample confirming POM to those who did not. There were no significant differences in age, sex, and surgical procedure between the two patient groups (Table 6).
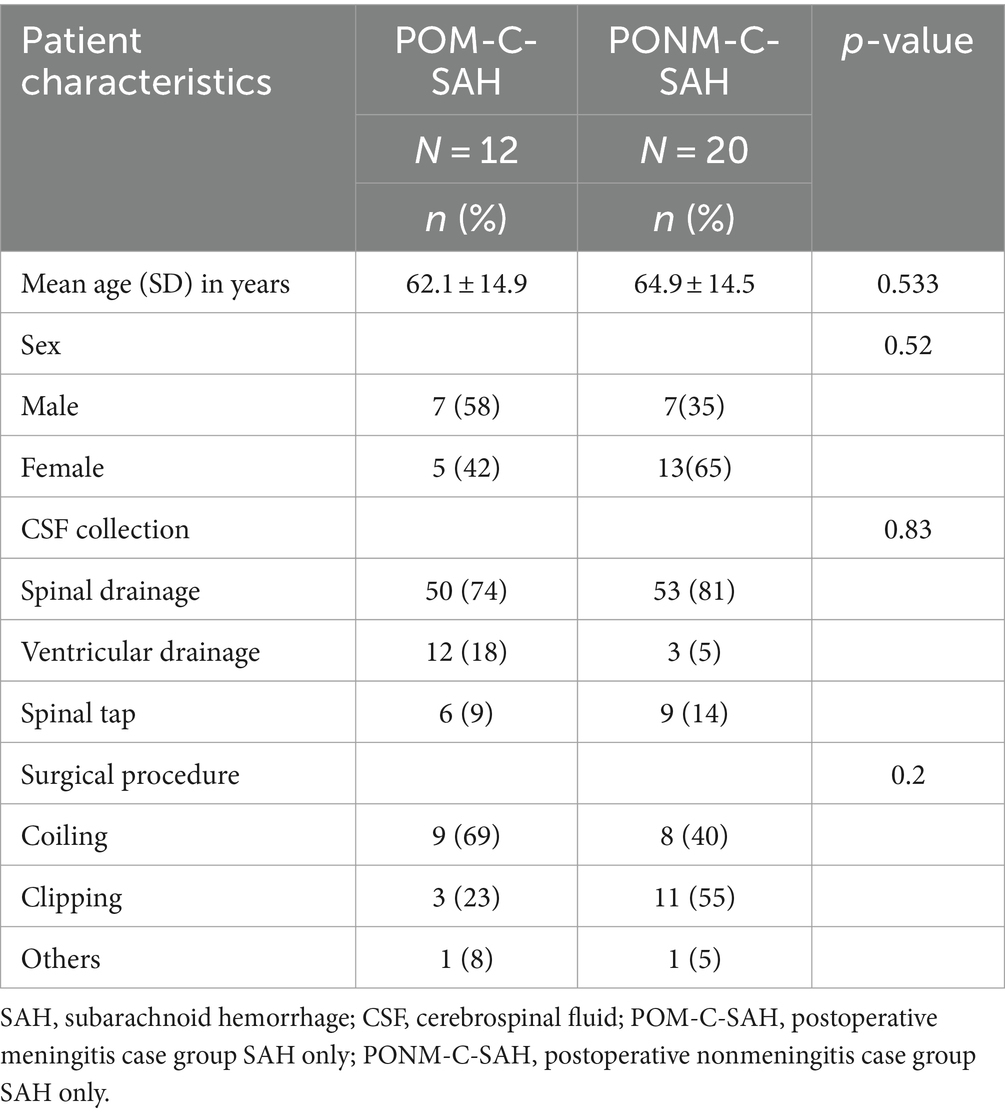
Table 6. Characteristics of patients who underwent neurosurgery who had SAH at Toho University Medical Center Omori Hospital, Japan, 2020–2022 (n = 32).
CSF samples were also examined for patients with SAH. Among patients with SAH, there were a total of 123 samples, of which 16 met the criteria for POM-S and 107 did not. Body temperature was significantly higher in the POM-S than in the PONM-S (p < 0.001). CSF presepsin levels in the POM-S and PONM-S averaged 1251.2 and 453.9 pg./mL, respectively (p < 0.0001). CRP level (p = 0.0014), CSF cell count (p < 0.0001), CSF polynuclear cell ratio (p = 0.02), and CSF protein level (p = 0.0069) were significantly higher in the POM-S than in the PONM-S. Conversely, CSF glucose level (p = 0.0002) and CSF glucose: serum ratio (p < 0.0001) were significantly lower in the POM-S than in the PONM-S (Table 7).
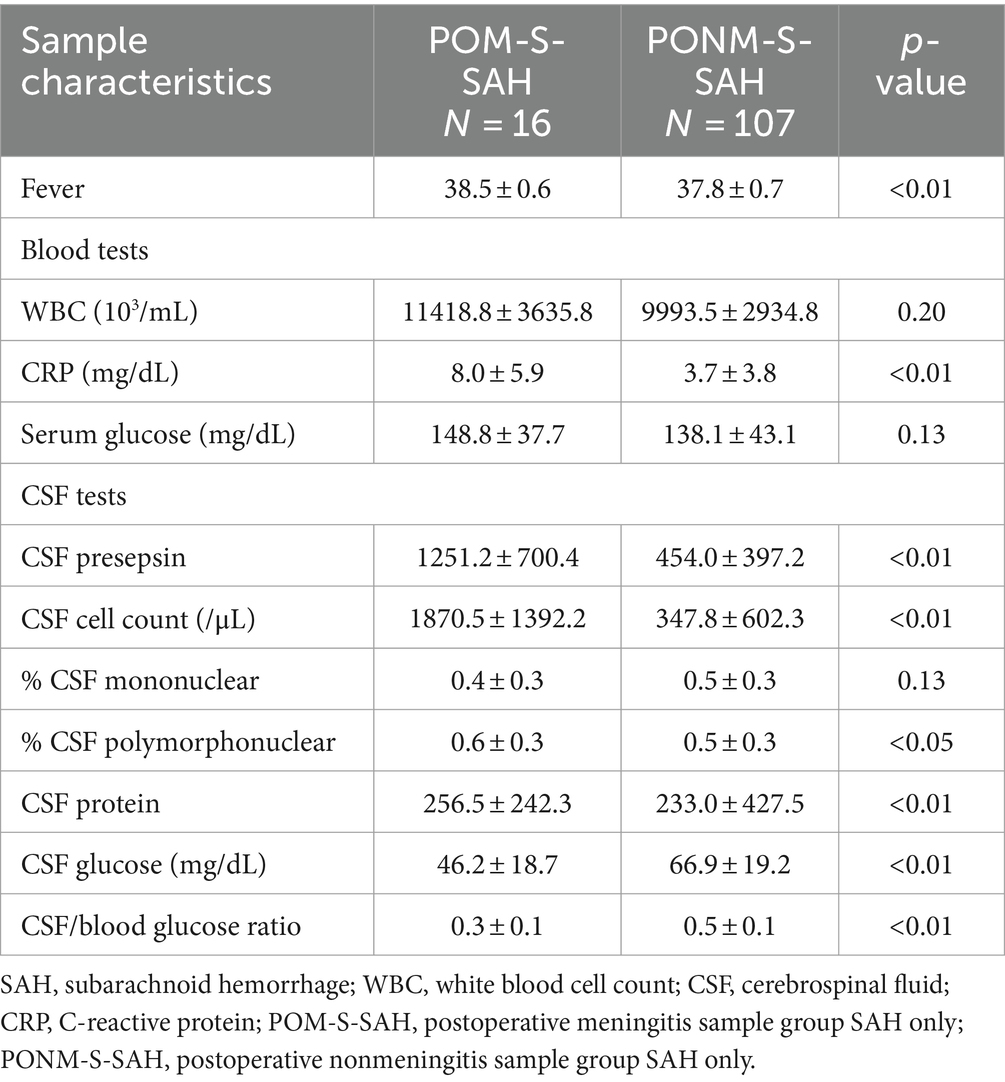
Table 7. CSF sample characteristics of patients who underwent neurosurgery with SAH at Toho University Medical Center Omori Hospital, Japan, 2020–2022.
ROC analysis indicated a similar CSF presepsin cutoff value of 669 pg./mL for POM-S-SAH and PONM-S-SAH groups to that of all samples (sensitivity: 87.5%, specificity: 76.6%, area under the curve: 0.86, 95%CI 0.72–0.97).
Finally, we evaluated changes in CSF presepsin, CRP, and WBC levels over time among SAH cases. The median diagnosis of postoperative meningitis was 5 days postoperatively. There were significant differences in CSF presepsin levels over time between the POM-S and the PONM-S (p = 0.03); but no differences between groups in CRP levels and WBC (Figure 2).
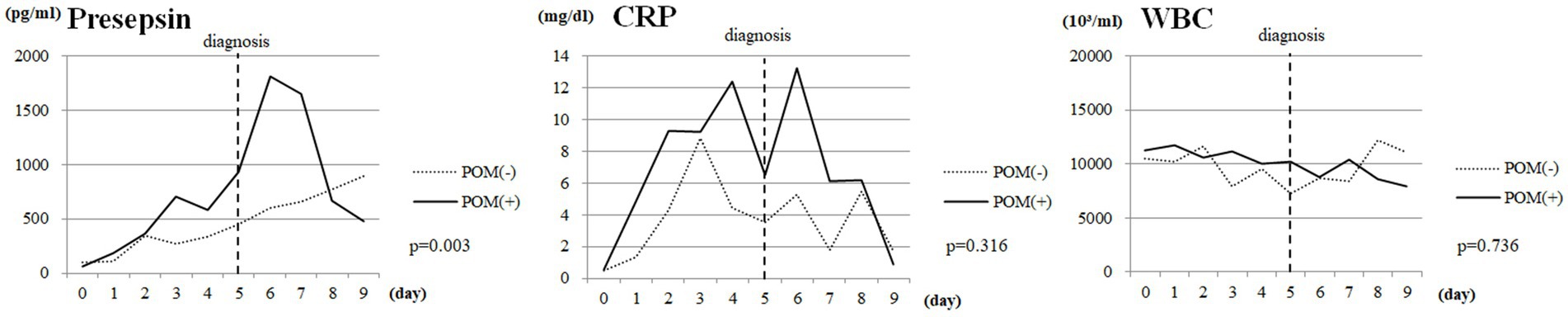
Figure 2. Changes over time in CSF presepsin, CRP, and WBC levels in SAH cases. Vertical dotted lines indicate the date of diagnosis of POM. CSF, cerebrospinal fluid; CRP, C-reactive protein; WBC, white blood cell count; SAH, Subarachnoid hemorrhage; POM, Postoperative meningitis; POMN, Postoperative nonmeningitis.
To the best of our knowledge, only four articles measured CSF presepsin levels for meningitis, one involving predictions (14, 21, 22, 24). Among those reports, the presepsin level in normal CSF was 50–100 pg./mL, increasing to 640.8 pg./mL in cases with bacterial meningitis demonstrating the usefulness of CSF presepsin level measurement for the diagnosis of bacterial meningitis (21). Further, in the that report, the CSF presepsin cutoff of 321 pg./mL did not vary with the presence or absence of blood in the CSF (21). However, these reports focused on community-acquired bacterial meningitis. The only report that measured CSF presepsin of POM is that of Zheng et al. They reported a CSF presepsin cutoff value of 1257.4–1276.2 pg./mL for POM and ventriculitis in neurosurgery, although they excluded patients with SAH (22). SAH is considered a disease with a high risk of POM because ventricular, cisternal, and spinal drains are often inserted to prevent spasm and to control intracranial pressure. Blood contamination in the CSF makes the diagnosis of bacterial meningitis difficult because it increases cell numbers in the CSF, and blood cells consume glucose producing lactic acid (25). In addition, the diagnosis of meningitis is difficult in cases with bloody CSF, such as SAH. Our study is the first report to demonstrate the utility of CSF presepsin in the diagnosis of neurosurgical POM in all patients, including those with SAH.
CD14 is found on the plasma membrane of granulocytes, macrophages, and monocytes and plays an important role in regulating the immune response to endotoxins (26). The CD14 on the cell membrane is internalized by phagocytic cells together with bacteria, digested, and cleaved by intracellular digestive enzymes, and converted into presepsin. Thereafter, it is released extracellularly and measured (27). Presepsin has been used as a novel marker to diagnose sepsis since its discovery in 2004 (3). In 2008, “PATHFAST Presepsin” (LSI Medience Corporation, Tokyo, Japan) was developed for fully automated measurement of presepsin with a chemiluminescent enzyme immunoassay, and numerous clinical evaluations have been conducted (28, 29). A multicenter clinical trial was conducted by Endo et al. (30) in 2012, which showed comparable or better results against the existing markers procalcitonin, IL-6, and CRP in the diagnosis of sepsis. The presepsin levels quantified during Gram-positive bacterial infection do not vary from those in Gram-negative bacterial infections (14, 30). Other common sepsis markers are CRP, procalcitonin, and interleukin-6 (IL-6). Procalcitonin is produced by cells stimulated by IL-6, but as protein synthesis is required for IL-6 production, this delays procalcitonin detection in the blood. Conversely, presepsin blood levels increase 2 h after infection peak in 3 h (19, 20, 31).
In this study, comparing the AUC of CSF presepsin to other parameters, CSF presepsin had the second highest value. This suggests that CSF presepsin is superior to routine parameters in detecting POM. It also had the third highest specificity. This suggests that the addition of CSF presepsin to existing parameters may provide a more accurate diagnosis of POM. In addition, markers other than CSF presepsin may be difficult to prove that the patient has POM on their own, while CSF presepsin, which is not affected by inflammation or bleeding associated with surgical invasion, may be able to diagnose POM on its own. During the postoperative course of cases with SAH, CSF presepsin levels peaked on the seventh postoperative day and were significantly higher in cases with POM than in cases with PONM. By contrast, CRP values showed a bimodal peak on postoperative days 3–4 and 6–7 but did not differ between POM and PONM cases. WBC counts did not change over time in both groups. In Figure 2, presepsin showed an upward trend from the date of diagnosis, whereas CRP reached its first peak before the date of diagnosis of POM. This suggests that CRP is rising due to non-infectious influences such as surgical invasion. In contrast, presepsin unimodal peak possibly reflects postoperative infection only as it is not affected by the surgical invasion. These results suggest that CSF presepsin may be a more accurate diagnostic marker for POM than other inflammatory markers. In our study, the CSF presepsin level in the control group (95.5 pg./mL) was comparable to the normal CSF presepsin (50–100 pg./mL) reported by Abudeev et al. (21). The cutoff of 669 pg./mL obtained in the present study lies within the range of the CSF presepsin levels described for POM in previous studies (625–1276.2 pg./mL) (14, 22).
Our study has some limitations. First, it was conducted at a single center, which limits its generalizability. Second, our sample size may limit group comparisons. However, a significant difference was obtained between the POM-S and the PONM-S groups. Nevertheless, future research should include more patients in a multicenter study. Third, blood presepsin and CSF lactate levels were not measured. However, CSF lactate levels are considered Grade D by the Infectious Diseases Society of America as they may be elevated by anaerobic metabolism, and metabolism by leukocytes in the CSF (23).
CSF presepsin is a useful marker in the diagnosis of neurosurgical POM and in cases of SAH or when there is a blood component in the CSF. When POM is suspected, measurement of CSF presepsin may be recommended in addition to a general CSF examination.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Toho University Omori Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. KK: Data curation, Investigation, Methodology, Validation, Writing – review & editing. YS: Data curation, Investigation, Methodology, Validation, Writing – review & editing. CN: Data curation, Investigation, Methodology, Validation, Writing – review & editing. ST: Data curation, Investigation, Methodology, Validation, Writing – review & editing. SK: Data curation, Investigation, Methodology, Validation, Writing – review & editing. MM: Data curation, Investigation, Methodology, Validation, Writing – review & editing. MA: Data curation, Investigation, Methodology, Validation, Writing – review & editing. SU: Data curation, Investigation, Methodology, Validation, Writing – review & editing. TM: Writing – review & editing, Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation. NS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
We are grateful to Chiaki Nishimura, Professor Emeritus of Toho University, for helping us with the statistical analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Korinek, AM, Baugnon, T, Golmard, JL, van Effenterre, R, Coriat, P, and Puybasset, L. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. (2008) 10:626–36. doi: 10.1016/S1474-4422(11)70109-0
2. McClelland, S 3rd, and Hall, WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. (2007) 45:55–9. doi: 10.1086/518580
3. Reichert, MC, Medeiros, EA, and Ferraz, FA. Hospital-acquired meningitis in patients undergoing craniotomy: incidence, evolution, and risk factors. Am J Infect Control. (2002) 30:158–64. doi: 10.1067/mic.2002.119925
4. Tavares, WM, Machado, AG, Matushita, H, and Plese, JP. CSF markers for diagnosis of bacterial meningitis in neurosurgical postoperative patients. Arq Neuro Psiquiatr. (2006) 64:592–5. doi: 10.1590/s0004-282x2006000400012
5. Federico, G, Tumbarello, M, Spanu, T, Roselli, R, Iacoangeli, M, Scerrati, M, et al. Risk factors and prognostic indicators of bacterial meningitis in a cohort of 3580 postneurosurgical patients. Scand J Infect Dis. (2001) 33:533–7. doi: 10.1080/00365540110026557
6. Hernández Ortiz, OH, García García, HI, Muñoz Ramírez, F, Cardona Flórez, JS, Gil Valencia, BA, Medina Mantilla, SE, et al. Development of a prediction rule for diagnosing postoperative meningitis: a cross-sectional study. J Neurosurg. (2018) 128:262–71. doi: 10.3171/2016.10.JNS16379
7. Maniker, AH, Vaynman, AY, Karimi, RJ, Sabit, AO, and Holland, B. Hemorrhagic complications of external ventricular drainage. Neurosurgery. (2006) 59:ONS419–25. doi: 10.1227/01.NEU.0000222817.99752.E6
8. Lozier, AP, Sciacca, RR, Romagnoli, MF, and Connolly, ES Jr. Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. (2002) 51:170–82. doi: 10.1097/00006123-200207000-00024
9. Lyke, KE, Obasanjo, OO, Williams, MA, O’Brien, M, Chotani, R, and Perl, TM. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis. (2001) 33:2028–33. doi: 10.1086/324492
10. Park, P, Garton, HJ, Kocan, MJ, and Thompson, BG. Risk of infection with prolonged ventricular catheterization. Neurosurgery. (2004) 55:594–601. doi: 10.1227/01.NEU.0000134289.04500.EE
11. Hill, M, Baker, G, Carter, D, Henman, LJ, Marshall, K, Mohn, K, et al. A multidisciplinary approach to end external ventricular drain infections in the neurocritical care unit. J Neurosci Nurs. (2012) 44:188–93. doi: 10.1097/JNN.0b013e3182527672
12. Kourbeti, IS, Vakis, AF, Ziakas, P, Karabetsos, D, Potolidis, E, Christou, S, et al. Infections in patients undergoing craniotomy: risk factors associated with post-craniotomy meningitis. J Neurosurg. (2015) 122:1113–9. doi: 10.3171/2014.8.JNS132557
13. Carmel, PW, and Greif, LK. The aseptic meningitis syndrome: a complication of posterior fossa surgery. Pediatr Neurosurg. (1993) 19:276–80. doi: 10.1159/000120744
14. Stubljar, D, Kopitar, AN, Groselj-Grenc, M, Suhadolc, K, Fabjan, T, and Skvarc, M. Diagnostic accuracy of presepsin (sCD14-ST) for prediction of bacterial infection in cerebrospinal fluid samples from children with suspected bacterial meningitis or ventriculitis. J Clin Microbiol. (2015) 53:1239–44. doi: 10.1128/JCM.03052-14
15. Blomstedt, GC . Infections in neurosurgery: a retrospective study of 1143 patients and 1517 operations. Acta Neurochir. (1985) 78:81–90. doi: 10.1007/BF01808684
16. Yaegashi, Y, Shirakawa, K, Sato, N, Suzuki, Y, Kojika, M, Imai, S, et al. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J Infect Chemother. (2005) 11:234–8. doi: 10.1007/s10156-005-0400-4
17. Antony, JC, and Kevin, AD. “Antigen Clearance”. In: P. J. Delves, T. M. Roitt, editors. Encyclopedia of Immunology (Second Edition). Cambridge, Massachusetts: Elsevier Science (1998). p.182–188.
18. Kojika, M, Takahashi, G, and Matsumoto, N. Serum levels of soluble CD14 subtype reflect the APACHEII and SOFA scores. Med Postgraduates. (2010) 48:46–50.
19. Dupuy, AM, Philippart, F, Péan, Y, Lasocki, S, Charles, PE, Chalumeau, M, et al. Role of biomarkers in the management of antibiotic therapy: an expert panel review: I – currently available biomarkers for clinical use in acute infections. Ann Int Care. (2013) 3:22. doi: 10.1186/2110-5820-3-22
20. Quenot, JP, Luyt, CE, Roche, N, Chalumeau, M, Charles, PE, Claessens, YE, et al. Role of biomarkers in the management of antibiotic therapy: an expert panel review II: clinical use of biomarkers for initiation or discontinuation of antibiotic therapy. Ann Int Care. (2013) 3:21. doi: 10.1186/2110-5820-3-21
21. Abudeev, SA, Kiselev, KV, Kruglyakov, NM, Belousova, KA, Lobanova, IN, Parinov, OV, et al. Cerebrospinal fluid presepsin as a marker of nosocomial infections of the central nervous system: a prospective observational study. Front Neurol. (2018) 9:58. doi: 10.3389/fneur.2018.00058
22. Zheng, G, Zhang, C, Zhang, G, and Shao, C. Evaluation of the diagnostic and prognostic value of CSF presepsin levels in patients with postneurosurgical ventriculitis/meningitis. Infect Drug Resist. (2021) 14:2901–9. doi: 10.2147/IDR.S325635
23. Tunkel, AR, Hartman, BJ, Kaplan, SL, Kaufman, BA, Roos, KL, Scheld, WM, et al. Practice guide-lines for the management of bacterial meningitis. Clin Fect dis. (2004) 39:1267–84. doi: 10.1086/425368
24. Wright, SD, Ramos, RA, Tobias, PS, Ulevitch, RJ, and Mathison, JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. (1990) 249:1431–3. doi: 10.1126/science.1698311
25. Zhang, X, Liu, D, Liu, YN, Wang, R, and Xie, LX. The accuracy of presepsin (sCD14-ST) for the diagnosis of sepsis in adults: a meta-analysis. Crit Care. (2015) 19:323. doi: 10.1186/s13054-015-1032-4
26. Shirakawa, K, Naitou, K, Hirose, J, Takahashi, T, and Furusako, S. Presepsin (sCD14-ST): development and evaluation of one-step ELISA with a new standard that is similar to the form of presepsin in septic patients. Clin Chem Lab Med. (2011) 49:937–9. doi: 10.1515/CCLM.2011.145
27. Shozushima, T, Takahashi, G, Matsumoto, N, Kojika, M, Okamura, Y, and Endo, S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satified diagnostic criteria of systemic inflammatory response syndrome. J Inf Chemother. (2011) 17:764–9. doi: 10.1007/s10156-011-0254-x
28. Endo, S, Suzuki, Y, Takahashi, G, Shozushima, T, Ishikura, H, Murai, A, et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J Infect Chemother. (2012) 18:891–7. doi: 10.1007/s10156-012-0435-2
29. Okamura, Y, and Yokoi, H. Development of a point-of-care assay system for measurement of presepsin (sCD14-ST). Clin Chim Acta. (2011) 412:2157–61. doi: 10.1016/j.cca.2011.07.024
30. Nockher, WA, Wick, M, and Pfister, HW. Cerebrospinal fluid levels of soluble CD14 in inflammatory and non-inflammatory diseases of the CNS: upregulation during bacterial infections and viral meningitis. J Neuroimmunol. (1999) 101:161–9. doi: 10.1016/s0165-5728(99)00141-1
Keywords: presepsin, CSF presepsin, soluble CD14 subtype, meningitis, postoperative meningitis, neurosurgical postoperative meningitis
Citation: Fuchinoue Y, Kondo K, Sakaeyama Y, Nakada C, Terazono S, Kubota S, Mikai M, Abe M, Ujiie S, Morita T and Sugo N (2024) Usefulness of cerebrospinal fluid presepsin (soluble CD14 subtype) as a new marker in the diagnosis of neurosurgical postoperative meningitis. Front. Neurol. 15:1429354. doi: 10.3389/fneur.2024.1429354
Received: 08 May 2024; Accepted: 09 July 2024;
Published: 18 July 2024.
Edited by:
Peter R. Williamson, National Institutes of Health (NIH), United StatesReviewed by:
Jian-Xin Zhou, Capital Medical University, ChinaCopyright © 2024 Fuchinoue, Kondo, Sakaeyama, Nakada, Terazono, Kubota, Mikai, Abe, Ujiie, Morita and Sugo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yutaka Fuchinoue, eXV0YWthLmZ1Y2hpbm91ZUBtZWQudG9oby11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.