- 1Department of Neurosurgery, Lithuanian University of Health Sciences, Kaunas, Lithuania
- 2Department of Immunology and Allergology, Lithuanian University of Health Sciences, Kaunas, Lithuania
- 3Laboratory of Immunology, Department of Immunology and Allergology, Lithuanian University of Health Sciences, Kaunas, Lithuania
Background: Accumulation of transthyretin amyloids (ATTR) is detected in ligamentum flavum in about 1/3 of patients underwent surgery for spinal stenosis. However, the significance of this finding is not known. The aim of this systematic review and meta-analysis is to analyze the incidence and importance of ATTR in patients with spinal stenosis who underwent spinal surgery.
Methods: The primary outcome measure was incidence of ATTR in patients with spinal stenosis. English language observational studies published within 10 years period were searched in Pubmed and Taylor and Francis databases.
Results: Nine articles were included in the systematic review. The incidence of positive ATTR among patients who underwent lumbar spinal surgery was 48% (95%CI 38–58%). ATTR deposits were found in the lumbar region the most frequently. Seven studies showed that patients with positive ATTR were older than those with negative. Five studies investigated and found a significant relationship between the ligamentum flavum thickness and positive ATTR. Five studies investigated cardiac involvement among patients with positive ATTR.
Conclusion: ATTR deposits are frequently found in older patients with spinal stenosis, especially in the lumbar region. The presence of ATTR deposits is related to ligamentum flavum thickness.
Introduction
Amyloidosis is a disorder of protein accumulation, during which the normal architecture and function of the tissue in which the protein accumulates is disturbed (1). More than 36 amyloid precursor proteins are known. Depending on the amount and localization of amyloid deposits, the spectrum and severity of diseases varies.
Transthyretin (TTR), a serum protein synthesized mainly in the liver, causes two types of systemic amyloidosis. Wild-type transthyretin amyloidosis (ATTR) (or senile systemic amyloidosis) is a non-hereditary form of amyloidosis caused by dissociation and misfolding of serum protein transthyretin (1). Wild-type ATTR is a normal genotype of TTR. Another type is a hereditary systemic amyloidosis, which is associated with abnormal genetic sequence (variant TTR). Wild-type ATTR primarily manifests as cardiomyopathy while ATTR due to a genetic variant manifests as cardiomyopathy and/or polyneuropathy (1). Moreover, they are also found in tendons, ligaments, and joints, particularly in older individuals (1, 2). For example, accumulations of wild-type ATTR are detected in ligamentum flavum in 33–45% of patients underwent surgery for spinal stenosis (2).
Spinal stenosis is defined as the narrowing of the spinal canal causing clinical symptoms such as numbness, fatigue and/or pain in the neck, buttocks and/or legs that increase with activities such as walking and standing (3–5). Spinal stenosis can involve the cervical, thoracic, or lumbar spine, being either monosegmental or multisegmental, and unilateral or bilateral (4). Lumbar spinal stenosis is the most frequent form and according to the systematic review and meta-analysis its incidence varies from 11 to 39% (3). Symptomatic lumbar spinal stenosis is characterized by low back and leg pain in the setting of compression of the central canal and/or exiting nerve roots by disk, osteophyte, ligamentum flavum, or other structures (5). Spinal stenosis usually develops due to many reasons such as degenerative diseases, iatrogenic factors, trauma, metabolic, infectious or rheumatological diseases (4). Treatment of spinal stenosis can be conservative (nonsteroidal anti-inflammatory medications, acetaminophen, and other medications, physical therapy, epidural steroid and local anesthetics injections) and surgical (spinal decompression surgery) (5). Direct surgical decompression, in which bone and/or disk are moved away from the affected nerve root(s), can be performed through an open or minimally invasive approach for lumbar spinal stenosis. In patients with concomitant degenerative spondylolisthesis and/or scoliosis, decompression for lumbar spinal stenosis is often performed in combination with lumbar arthrodesis (fusion), in which adjacent vertebrae are fused to prevent motion (5).
The clinical relevance of ATTR in ligamentum flavum is not fully understood. Scientists investigate whether ATTR depositions in spinal cord could be related to outcomes of spinal stenosis and prevalence of other disorders such as heart damage or systemic amyloidosis (6, 7).
The aim of this systemic review is to analyze the incidence and importance of ATTR in patients with spinal stenosis who underwent spinal surgery.
Methods and materials
Measured outcomes
Incidence of amyloidosis in patients with spinal stenosis was the exposure of interest. The primary outcome measure was incidence of ATTR in patients with spinal stenosis. Secondary outcomes of interest included: localization of amyloid deposits, relation between ligamentum flavum thickness and amyloid deposits, relation between amyloid deposits and systemic amyloidosis and/or another local amyloidosis (such as carpal syndrome or cardiac amyloidosis). Data about the number of participants and their age was also collected.
Eligibility criteria
English language observational studies (cross-sectional) were included if they reported incidence of ATTR in patients with spinal stenosis published within the period of 2012–2022 years.
Search strategy and statistical analysis
Studies were searched in Pubmed and Taylor and Francis databases. The Medical Subject Heading (MeSH) terms of “spinal stenosis” or “ligamentum flavum” and “amyloid” or “amyloidosis” were used. In addition, combined text words of (spinal stenosis AND amyloid) OR (spinal stenosis AND amyloidosis) OR (ligamentum flavum AND amyloid) OR (ligamentum flavum AND amyloidosis) were used to find relevant studies. Systematic review was performed according to the PRISMA guidelines (8). Statistical heterogeneity was assessed using the I2 statistic. We conducted a random-effects meta-analysis by the DerSimonian and Laird method. Before pooling, the Freeman-Tukey double arcsine transformed proportion was used to ensure admissible CIs given its improved estimation of CIs in the presence of 0 events and its more stable estimation of variances. The exact method was used for CI computation for pooled estimation of prevalence and incidence.
Results and discussion
Study characteristics
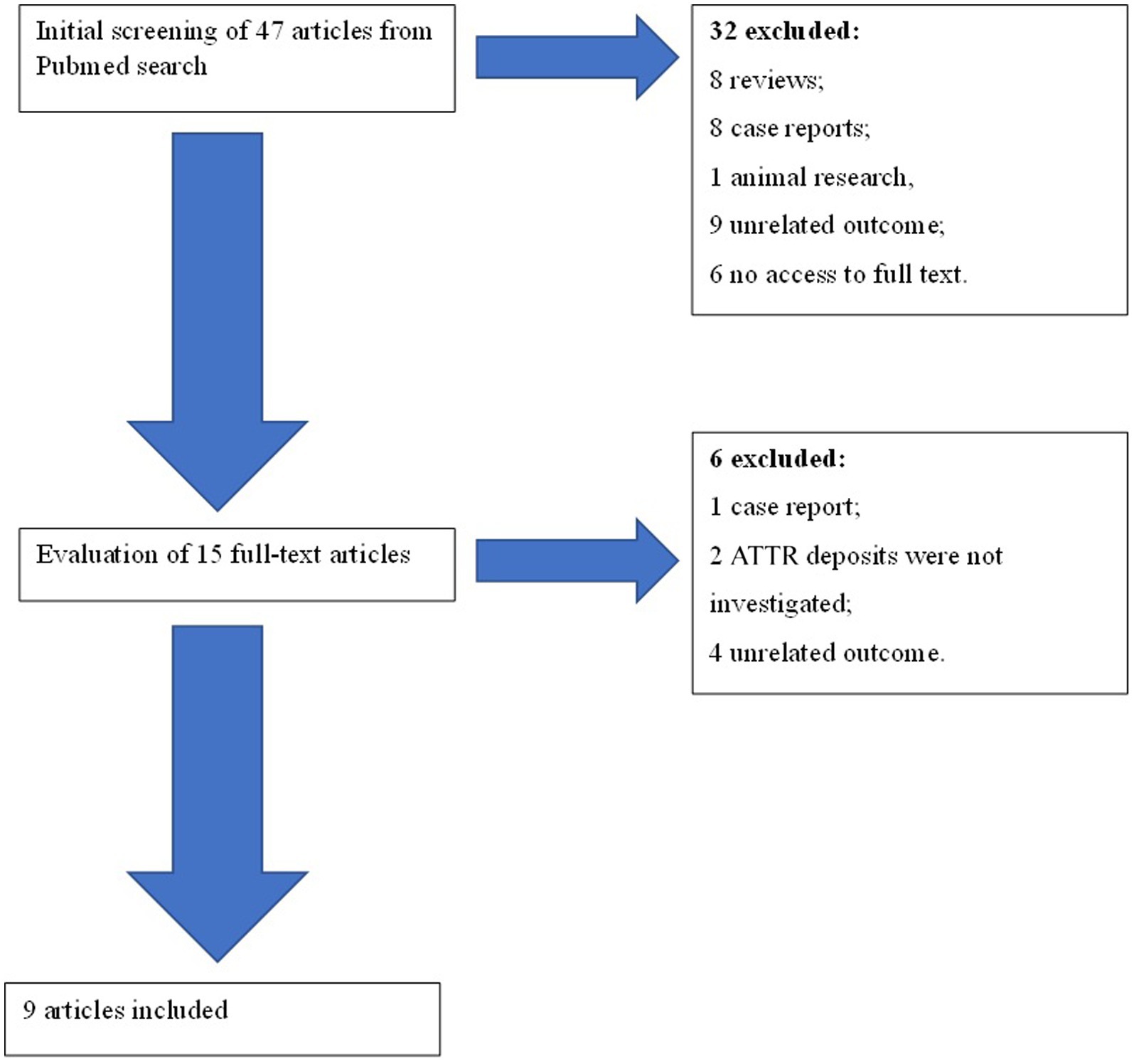
A total of 47 articles were found. Of these articles, 32 were eliminated after an initial screening of their titles and abstracts (Figure 1). The full texts of the 15 remaining articles were assessed in detail. Six articles were rejected. Finally, nine articles were included in the systematic review. Articles were included after evaluation of their quality according to Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies of National Heart, Lung and Blood Institute (Table 1) (9).
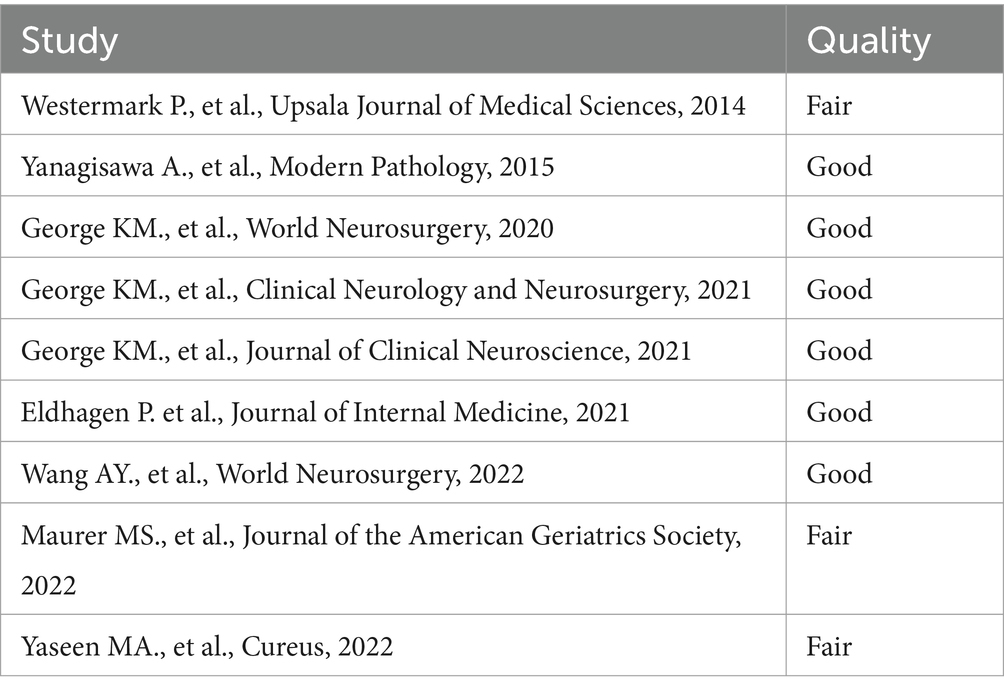
Table 1. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies of National Heart, Lung and Blood Institute.
Measurement of ATTR and ligamentum flavum thickness
Amyloidosis in pathologic specimens was detected with Congo red staining and demonstration of apple-green birefringence under polarized light and in all studies. Wild type ATTR was confirmed by the presence of wild type ATTR by typing and the absence of mutations in the ATTR gene sequence from genomic DNA in three studies (10–12). Wild type ATTR was detected using monoclonal antibodies to ATTR in four studies (13–16). Tandem mass spectrometry-based analysis was used in two studies (17, 18).
Ligamentum flavum thickness was measured using an axial T2-weighted magnetic resonance images (MRI) slice in three studies (11, 12, 18) and using an axial T1- weighted MRI in two studies (13, 15). Three studies indicated that bilateral measurements were made (11, 12, 18). The average of the three measurements of MRI images was taken as a final value in two studies (12, 15) and the arithmetic mean of the two values was recorded in one study (11). Ligamentum flavum thickness was measured in affected lumbar levels, but lumbar Ligamentum flavum burden was calculated as a mean Ligamentum flavum thickness from each lumbar level in two studies (12, 18).
Measurement of cardiac involvement
Five studies evaluated cardiac involvement. Three studies evaluated only clinical symptoms of cardiac failure, hypertension, arrhythmia and myocardial infarction usually based on history (10, 11, 15). Eldhagen et al. (14) performed cardiovascular investigation with electrocardiography, medical history, physical examination, N-terminal pro–B-type natriuretic peptide (NT-proBNP), echocardiography with strain analysis and cardiovascular MRI (CMRI; Siemens 1 5 T) with gadolinium contrast for patients with positive ATTR deposits. Yaseen et al. (13) also performed cardiac investigation which involved medical history, physical examination, NT-proBNP, electrocardiography, echocardiography and Tc-99 m pyrophosphate planar imaging with concomitant single photon emission computed tomography (SPECT).
Measurement of carpal tunnel syndrome
Four studies evaluated carpal tunnel syndrome. Information about carpal tunnel symptoms, surgery or muscle tears was obtained from chart review or history in all these studies (10–12, 14).
Studies populations
Patients who underwent lumbar spinal surgery were involved in all studies. Spinal stenosis as indication for surgical treatment was in all studies. Four studies additionally involved patients with disk herniation (10–12, 15) and one study—with disk degeneration (13). Overall, 1,339 patients underwent investigation for ATTR deposits in ligamentum flavum. The average patients’ age was about 70 years.
Incidence of ATTR in patients with spinal stenosis
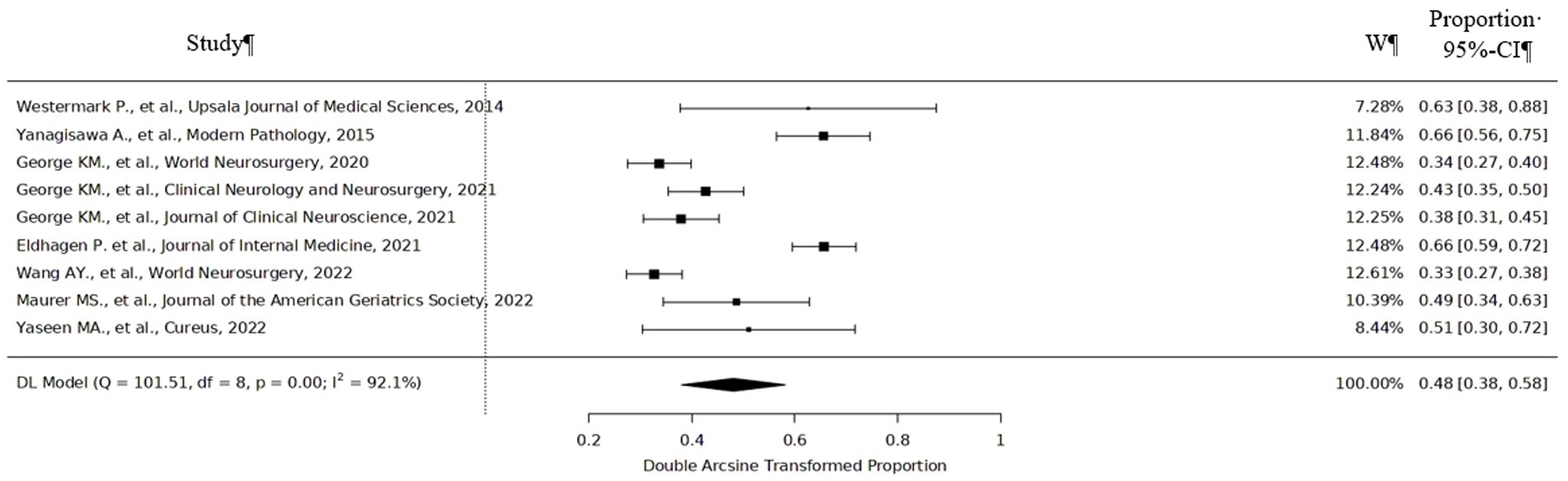
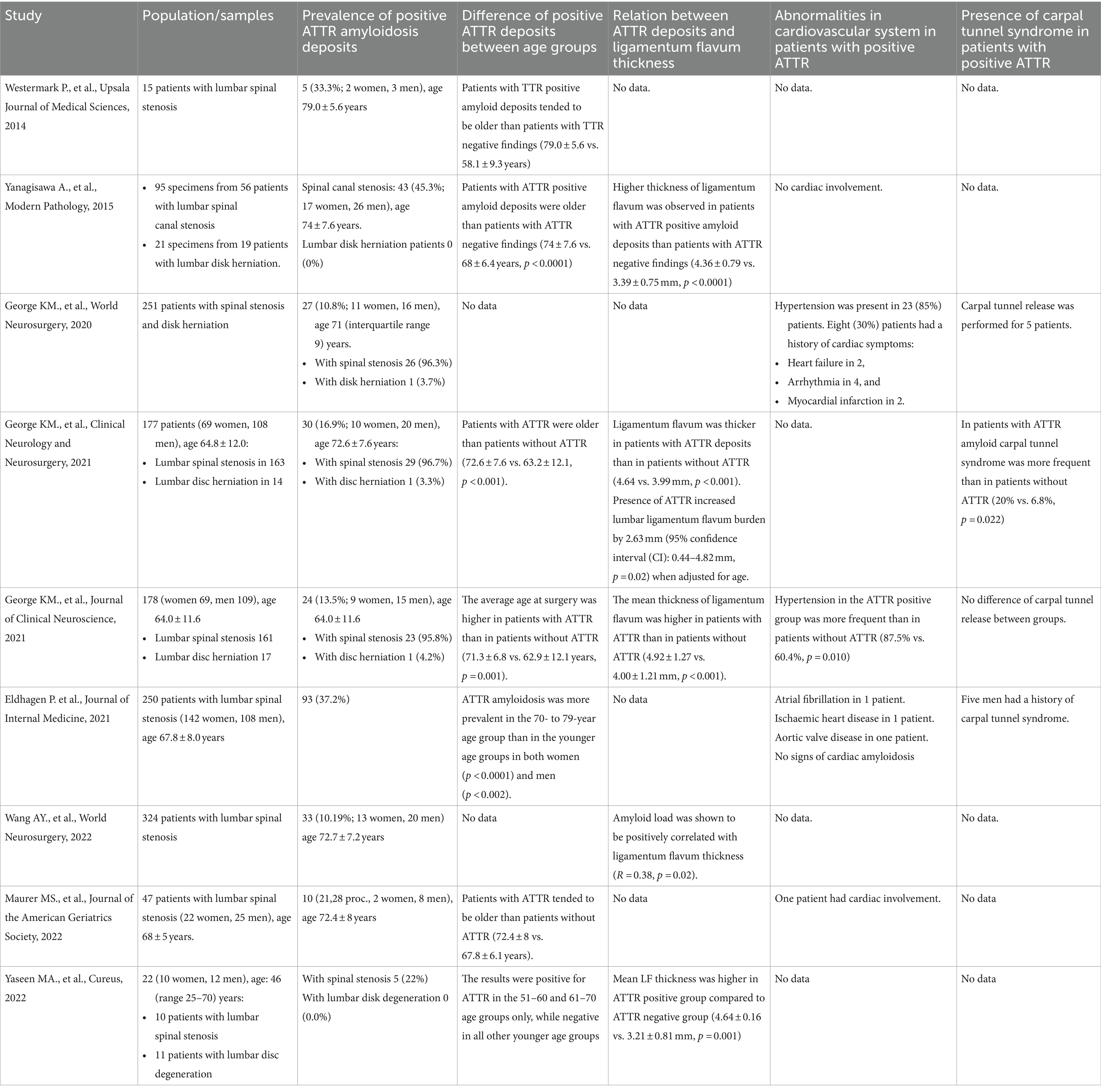
According to meta-analysis, the incidence of positive ATTR among patients who underwent lumbar spinal surgery was 48% (95%CI 38–58%) varying from 33% (95%CI 27–38%) to 66% (95%CI 56–75%) (Figure 2). ATTR deposits were found in the lumbar region the most frequently. 70% specimens that were ATTR positive were found to occur in the L3-L4 and L4-L5 levels (8). One study showed that 24 (89%) patients had ATTR deposits in the lumbar region, and 3 (11%) patients had ATTR deposits outside of the lumbar spine (2 had ATTR in the cervical level, while 1 had deposition in the thoracic level) (10). Only three positive ATTR cases were found among patients with disk herniation and no ATTR deposits among patients with lumbar disk degeneration.
There was no significant difference between females and males in all studies. Seven studies showed that patients with positive ATTR were older than those with negative. Westermark et al. (16) noticed a tendency that patients with ATTR positive amyloid deposits were older than patients with ATTR negative findings (79.0 ± 5.6 vs. 58.1 ± 9.3 years). Yanagisawa et al., George et al., George et al., and Maurer et al. provided statistically significant results that patients’ with positive ATTR average age is higher compared with those without ATTR deposits (11, 12, 15, 17). Eldhagen et al. (14) found that ATTR was significantly more prevalent in the 70- to 79-year age group than in the younger age groups. Yaseen et al. (13) showed that the results were positive for ATTR in the 51–60 and 61–70 age groups only, and negative in all other younger age groups. One study revealed that patients older than 70 were 4.8 times more likely to have amyloid in the ligamentum flavum (11).
Relation between ligamentum flavum thickness, systemic amyloidosis and/or other local amyloidosis and amyloid deposits in spinal cord
Five studies investigated and found significant relationship between the ligamentum flavum thickness and positive ATTR (11–13, 15, 18). Only one study investigated correlation between symptoms and quality of life and ATTR deposits but found no significant results (17). There was no statistically significant difference in the number of spinal levels that required operation between the patients with positive ATTR and patients with negative ATTR (11).
Five studies investigated cardiac involvement among patients with positive ATTR, three of them found cardiological abnormalities, but signs of cardiac amyloidosis were not found (10, 11, 14, 15, 17). Three studies noticed carpal tunnel syndrome among patients with positive ATTR (10–12, 14). One study showed that carpal tunnel syndrome was more frequent in patients with positive ATTR compared to patients with negative ATTR (12).
Summary of the results of reviewed articles are presented in Table 2.
Conclusion
ATTR deposits, especially wild type ATTR, are frequently found in older patients with spinal stenosis, especially in the lumbar region, as is the case with wild type ATTR anywhere. The presence of ATTR deposits is related to ligamentum flavum thickness. Moreover, patients with positive ATTR tend to have cardiac abnormalities and tunnel carpal syndrome, but more evidence is needed. However, there is lack of evidence about the impact of ATTR in ligamentum flavum on the severity of symptoms of spinal stenosis, quality of life, number of spinal levels that require surgery, motor function, gait stability, fall frequency and independent ambulation in an elderly patient population post operatively.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LT: Supervision, Writing – review & editing. DT: Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ATTR, Transthyretin; ATTR, Transthyretin amyloids; MeSH, Medical Subject Heading; MRI, Magnetic resonance images; SPECT, Single photon emission computed tomography; NT-proBNP, N-terminal pro–B-type natriuretic peptide.
References
1. Wechalekar, AD, Gillmore, JD, and Hawkins, PN. Systemic amyloidosis. Lancet. (2016) 387:2641–54. doi: 10.1016/S0140-6736(15)01274-X
2. Godara, A, Riesenburger, RI, Zhang, DX, Varga, C, Fogaren, T, Siddiqui, NS, et al. Association between spinal stenosis and wild-type ATTR amyloidosis. Amyloid. (2021) 28:226–33. doi: 10.1080/13506129.2021.1950681
3. Jensen, RK, Jensen, TS, Koes, B, and Hartvigsen, J. Prevalence of lumbar spinal stenosis in general and clinical populations: a systematic review and meta-analysis. Eur Spine J. (2020) 29:2143–63. doi: 10.1007/s00586-020-06339-1
4. Melancia, JL, Francisco, AF, and Antunes, JL. Spinal stenosis. Handb Clin Neurol. (2014) 119:541–9. doi: 10.1016/B978-0-7020-4086-3.00035-7
5. Katz, JN, Zimmerman, ZE, Mass, H, and Makhni, MC. Diagnosis and Management of Lumbar Spinal Stenosis: a review. JAMA. (2022) 327:1688–99. doi: 10.1001/jama.2022.5921
6. Cuddy, SAM, and Falk, RH. Amyloidosis as a systemic disease in context. Can J Cardiol. (2020) 36:396–407. doi: 10.1016/j.cjca.2019.12.033
7. Ueda, M. Transthyretin: its function and amyloid formation. Neurochem Int. (2022) 155:105313. doi: 10.1016/j.neuint.2022.105313
8. PRISMA. Transparent reporting of systematic reviews and meta-analyses. (2020) Available at: http://prisma-statement.org/. Accessed 20 Jan 2023.
9. National Heart, Lung and Blood Institute. Study quality assessment tools. (2021) Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools/. Accessed 20 Jan 2023.
10. George, KM, Dowd, RS, Nail, J, Yu, A, Mastroianni, M, Wang, AY, et al. Wild-type transthyretin amyloidosis occurring in the ligamentum Flavum of the cervicothoracic spine. World Neurosurg. (2020) 142:e325–30. doi: 10.1016/j.wneu.2020.06.228
11. George, KM, Hernandez, NS, Breton, J, Cooper, B, Dowd, RS, Nail, J, et al. Increased thickness of lumbar spine ligamentum flavum in wild-type transthyretin amyloidosis. J Clin Neurosci. (2021) 84:33–7. doi: 10.1016/j.jocn.2020.11.029
12. George, KM, Hernandez, NS, Breton, J, Cooper, B, Dowd, RS, Nail, J, et al. Lumbar ligamentum flavum burden: evaluating the role of ATTRwt amyloid deposition in ligamentum flavum thickness at all lumbar levels. Clin Neurol Neurosurg. (2021) 206:106708. doi: 10.1016/j.clineuro.2021.106708
13. Al Yaseen, M, Al Zahid, H, and Al-Haroon, S. Amyloid deposits in the ligamentum Flavum related to lumbar Spinal Canal stenosis and lumbar disc degeneration. Cureus. (2022) 14:e26221. doi: 10.7759/cureus.26221
14. Eldhagen, P, Berg, S, Lund, LH, Sörensson, P, Suhr, OB, and Westermark, P. Transthyretin amyloid deposits in lumbar spinal stenosis and assessment of signs of systemic amyloidosis. J Intern Med. (2021) 289:895–905. doi: 10.1111/joim.13222
15. Yanagisawa, A, Ueda, M, Sueyoshi, T, Okada, T, Fujimoto, T, Ogi, Y, et al. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. (2015) 28:201–7. doi: 10.1038/modpathol.2014.102
16. Westermark, P, Westermark, GT, Suhr, OB, and Berg, S. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Ups J Med Sci. (2014) 119:223–8. doi: 10.3109/03009734.2014.895786
17. Maurer, MS, Smiley, D, Simsolo, E, Remotti, F, Bustamante, A, Teruya, S, et al. Analysis of lumbar spine stenosis specimens for identification of amyloid. J Am Geriatr Soc. (2022) 70:3538–48. doi: 10.1111/jgs.17976
Keywords: ATTR, amyloidosis, spinal stenosis, lumbar stenosis, ligamentum flavum
Citation: Tamasauskas D and Tamasauskiene L (2024) Transthyretin amyloidosis in patients with spinal stenosis who underwent spinal surgery: a systematic review and meta-analysis. Front. Neurol. 15:1425862. doi: 10.3389/fneur.2024.1425862
Edited by:
Sasha Zivkovic, Yale University, United StatesReviewed by:
Jonas Wixner, Umeå University, SwedenChieko Suzuki, Hirosaki University, Japan
Sasha Zivkovic, Yale University, United States
Copyright © 2024 Tamasauskas and Tamasauskiene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Tamasauskiene, bGF1cmEudGFtYXNhdXNraWVuZUBsc211Lmx0
 Domantas Tamasauskas
Domantas Tamasauskas Laura Tamasauskiene
Laura Tamasauskiene