
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 28 June 2024
Sec. Sleep Disorders
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1425327
This article is part of the Research Topic Novel Technologies in the Diagnosis and Management of Sleep-Disordered Breathing, volume II View all 13 articles
 Zhe Yang1†
Zhe Yang1† Tian Lv2†
Tian Lv2† Linna Jin1
Linna Jin1 Xiaoheng Lv1
Xiaoheng Lv1 Xiaoying Zhu1
Xiaoying Zhu1 Xiaoling Wang3
Xiaoling Wang3 Lisan Zhang3
Lisan Zhang3 Chenghan Tu4
Chenghan Tu4 Shiqin Chen5
Shiqin Chen5 Xiongwei Yan6*
Xiongwei Yan6*Objectives: Obstructive sleep apnea (OSA) and osteoarthritis (OA) are common comorbidities that significantly impact individuals’ quality of life. However, the relationship between OSA and OA remains unclear. This study aims to explore the connection between OSA and OA and evaluate causality using Mendelian randomization (MR).
Methods: A total of 12,454 participants from the National Health and Nutrition Examination Survey (2009–2012) were included. OSA participants were identified based on self-reported interviews. The association between OA and OSA was assessed through multivariable logistic regression analysis. A two-sample MR was employed to investigate the relationship between OSA and OA, specifically hip OA and knee OA, utilizing the inverse variance-weighted (IVW) approach.
Results: Based on the observational study, individuals with OSA exhibited a higher risk of OA (OR = 1.67, 95% CI = 1.40–1.98). IVW demonstrated that the risk of OA (OR = 1.13, 95% CI: 1.05–1.21, p = 0.001), hip OA (OR = 1.11, 95% CI: 1.04–1.18, p = 0.002), and knee OA (OR = 1.08, 95% CI: 1.02–1.14, p = 0.005) was significantly associated with OSA. Reverse MR analyses indicated no effect of OA on OSA. Additionally, body mass index (BMI) was found to mediate 36.9% (95% CI, 4.64–73.2%, p = 0.026) of the OSA effects on OA risk.
Conclusion: The cross-sectional observational analysis unveiled noteworthy associations between OSA and OA. Meanwhile, findings from the MR study provide support for a causal role.
OSA is characterized by recurrent upper respiratory tract obstructions during sleep, leading to a reduction or cessation of airflow (1). A total of 936 million individuals are affected by OSA worldwide, with 425 million individuals experiencing moderate-to-severe cases (2). OSA patients endure chronic intermittent hypoxemia and metabolic disorders (3), often accompanied by inflammatory diseases (4). Studies indicate common risk factors associated with OSA and OA patients (5). OA, the most prevalent musculoskeletal disease, affects synovial joints, causing joint pain, decreased mobility, and a diminished quality of life (6). In 2020, the global prevalence of OA affecting approximately 7.6% of the population has been increasing in recent years (7, 8), making OA the fourth leading global cause of disability (9, 10). The medical burden associated with OA is steadily increasing worldwide (11).
Both OSA and OA significantly impact individuals’ quality of life. OSA disrupts sleep quality, leading to fragmentation, while OA induces pain and discomfort, particularly during the night. Current drug efficacy for both diseases is limited (5), necessitating urgent and effective treatment strategies. Exploring the correlation and potential mediator between OSA and OA provides valuable insights into disease mechanisms and symptom exacerbation. This understanding can enhance strategies for managing symptoms and finally improve outcomes for patients affected by both OSA and OA.
MR is an innovative epidemiological approach employing genetic variables as instrumental variables (IV) to assess causal effects on outcomes; this approach is less susceptible to biases from confounding factors and reverse causality (12).
This study integrates an observational investigation within the National Health and Nutrition Examination Survey (NHANES) with MR techniques to elucidate the causal relationship between OSA and OA.
The NHANES, a comprehensive research initiative evaluating the wellbeing and dietary status of individuals in the United States, comprises five core components: demographic details, dietary data, physical examinations, laboratory discoveries, and questionnaires. Ethical approval for NHANES protocols was duly granted by the National Center for Health Statistics Research Ethics Review Board, and all participants provided informed consent. This research included a total of 39,722 individuals from four NHANES cycles (2005–2006, 2007–2008, 2015–2016, and 2017–2018). The analysis excluded participants with missing OA data (n = 19,538) or OSA data (n = 7), as well as those with rheumatoid arthritis or other non-osteoarthritis forms (n = 1,959). Additionally, participants with missing covariate data were excluded, including family income-to-poverty ratio (PIR) data (n = 1,447), educational data (n = 4), marital status data (n = 3), smoking status data (n = 6), alcohol consumption data (n = 1,713), BMI data (n = 91), chronic kidney disease (CKD) data (n = 88), diabetes mellitus (DM) data (n = 390), stroke data (n = 9), hemoglobin (HB) data (n = 1,766), serum alkaline phosphatase (ALP) data (n = 200), serum calcium data (n = 2), and alanine aminotransferase (Alt) data (n = 45). Consequently, the analysis encompassed a total of 12,454 individuals, as illustrated in Figure 1 through a flow chart.
The exposure variable in this study was OSA, while the outcome variable was OA. OSA determination relied on affirmative responses to three yes-or-no questions related to snoring frequency, snorting or cessation of breathing occurrences, and daytime sleepiness. Individuals with positive responses to any of these questions were considered to display symptoms suggestive of OSA (13). OA participants in this study were identified based on self-reported personal interview data on various health conditions.
Demographics, medical conditions, and laboratory examinations were used to classify covariates. During home interviews, data on demographic characteristics, such as age, gender, marital status, educational attainment, PIR, and race, were gathered, along with information on medical conditions such as DM, hypertension, hyperlipidemia, and CKD. As a part of the NHANES laboratory examination, serum samples were collected, including serum calcium, Alt, ALP, creatinine, white blood cell count (WBC), and HB levels.
For this research, we obtained genome-wide association study (GWAS) data on OSA from the Finnegan dataset population, which can be accessed at https://storage.googleapis.com/finngen-public-data-r9/summary_stats/finngen_R9_G6_SLEEPAPNO.gz. The study involved a total of 375,657 individuals, comprising 38,998 individuals with OSA and 336,659 individuals as controls. The GWAS catalog dataset (https://www.ebi.ac.uk/gwas/downloads/summary-statistics.ID GCST90044591) provided genetic data associated with OA from Jiang (14), consisting of 8,952 individuals diagnosed with OA and 447,396 control individuals without OA. The GWAS dataset for OA in the knees and hips was acquired from Tachmazidou et al. (15). BMI summary statistics were obtained from MRC-IEU, involving 461,460 samples (IEU GWAS ID ukb-b-19553). To mitigate population stratification bias, only studies including individuals of European descent were used to retrieve all summary data.
All GWAS studies included in this research received approval from the relevant ethical review boards, and participants provided written informed consent. The research adhered to the STROBE MR guideline (16).
We selected instrumental variables (IVs) for OSA, OA, OA of the hip and knee, potential mediator [fasting insulin, Homeostasis Model Assessment of Insulin Resistance, Modified Stumvoll Insulin Sensitivity Index, and Modified Stumvoll Insulin Sensitivity Index (model adjusted for BMI)] GWAS data with a p-value of <5 × 10−6, ensuring independence (r2 < 0.001, kb = 10,000). The IVs for the potential mediator (BMI, waist circumference, hip circumference, waist-to-hip ratio adjusted for BMI, and waist-hip ratio) GWAS data were chosen based on a p-value of less than 5 × 10−8, ensuring independence (r2 < 0.001, kb = 10,000). The F statistic for each single nucleotide polymorphism (SNP) was computed using the formula Beta2/SE2.
This study meticulously incorporated intricate sampling designs and weights following the NHANES analytic guidelines, using mobile examination center (MEC) weights for all analyses. Continuous variables are presented as means and standard errors (SEs), while categorical variables are expressed as proportions. The examination of the relationship between OSA and OA involved a multivariate binary logistic regression model to calculate the odds ratio (OR) and 95% confidence intervals (CI). Three models were constructed for statistical inference. Model 1 solely included OSA, while Model 2 expanded to incorporate gender, age, ethnicity, marital status, and educational background. Model 3, an augmented version of Model 2, encompassed additional factors such as creatinine levels, alcohol consumption, BMI, smoking habits, serum calcium, serum Alt, ALP, serum creatinine, WBC, and medical history of hypertension, DM, CKD, stroke, and hyperlipidemia.
Subgroup analyses aimed to explore potential modifications in the impact of OSA on OA. These analyses considered age (<60, ≥60), sex, BMI (<30, ≥30), CKD status (yes, no), hyperlipidemia (yes, no), DM status (yes, no), hypertension (yes, no), and stroke status (yes, no), adjusting for Model 3.
MR analyses included the computation of F statistics to gauge the strength of each instrument. An overview of the MR research design is displayed in Figure 2. The primary method, IVW, assessed the association of genetically predicted OSA and OA. Supplementary MR models, such as weighted mode, weighted median (WM), MR-Egger, and simple mode, were used. Cochrane Q test and MR-Egger intercept were used to examine potential heterogeneity and directional pleiotropy. A leave-one-out analysis identified significant single nucleotide polymorphisms (SNPs) and assessed the robustness of findings.
Furthermore, we used the IEU Open GWAS Project website1 to explore whether the genetic variants associated with OSA were also connected to other prevalent risk factors that might affect the results obtained from Mendelian randomization, including BMI, arthropathies, bone mineral density (BMD), vitamin D, and smoke (17, 18).
Reverse MR analyses, treating OA as the exposure and OSA as the outcome, were conducted to explore bidirectional causality, using the same GWAS datasets. The IVW examination was duplicated using an alternative osteoarthritis GWAS dataset from IEU GWAS, followed by a meta-analysis to consolidate outcomes.
To determine whether the observed association between OSA and OA was a direct association, we assessed the relationship between genetically previously established risk factors for OA (19) (BMI, waist circumference, hip circumference, waist-to-hip ratio adjusted for BMI, waist-hip ratio, fasting insulin, Homeostasis Model Assessment of Insulin Resistance, Modified Stumvoll Insulin Sensitivity Index, and Modified Stumvoll Insulin Sensitivity Index [model adjusted for BMI)] in MR analyses (GWAS dataset in Supplementary Table S1) (5). For significant associations, potential mediation effects (the exposure-mediator-outcome pathway) may exist. To explore the potential mediator between OSA and OA, a mediator MR analysis was conducted. This involved estimating the overall effect of OSA on OA (α), the effect of the potential mediator on OA (β2), and the effect of OSA on the potential mediator OA (β1). The direct impact of OSA on OA was calculated as α - β1*β2. Statistical analyses were conducted using R Studio 4.2.0 and the R package “Two Sample MR,” with a significance level set at a p-value of <0.05. Meta-analyses were carried out using RevMan 4.3.
The dataset under scrutiny included 12,454 participants. Table 1 presents the baseline characteristics categorized by OA. Of the participants, 1,560 (5.28%) had OA, and 3,628 (29.53%) had OSA. OSA participants exhibited a higher prevalence of OA (630, 39.84%) than those without OSA (2,998, 27.95%). The OA group was characterized by a higher likelihood of being male, former smokers, and having a history of DM, stroke, CKD, hyperlipidemia, hypertension, elevated serum creatinine, and BMI.
Table 2 displays the outcomes of logistic regression analyses with multiple adjustments. After meticulous adjustments for various factors, Model 1 resulted in OR = 1.71, 95% CI = 1.46–2.00, and p < 0.0001; Model 2 showed OR = 1.98, 95% CI = 1.67–2.33, and p < 0.0001; and Model 3 revealed OR = 1.67, 95% CI = 1.40–1.99, and p < 0.0001.
Subgroup analyses, considering variables such as age, sex, smoking status, history of CKD, hyperlipidemia, DM, hypertension, and stroke, consistently showed results with no significant interaction (all p interaction >0.05, Figure 1).
The genetic instrument for OSA (Supplementary Table S1) comprised 101 SNPs with F values exceeding 10 (see Supplementary Table S2). Employing the IVW method, the analysis indicated that OSA was associated with an increased likelihood of OA (OR = 1.13, 95% CI 1.05–1.21, p = 0.001), hip OA (OR = 1.11, 95% CI 1.04–1.18, p = 0.002), and knee OA (OR = 1.08, 95% CI 1.02–1.14, p = 0.005). Figure 3 visually represents outcomes from IVW, MR-Egger, weighted mode, weighted median, and simple mode.
The MR-Egger intercept test showed no horizontal pleiotropy regarding the impact of OSA on OA (intercept = 0.00046; p = 0.915), hip OA (intercept = 0.0038; p = 0.500), and knee OA (intercept = −0.00134; p = 0.861). The Cochrane Q test revealed no heterogeneity regarding the impact of OSA on OA (IVWQ 83.78, p = 0.691) but significant heterogeneity regarding OSA effects on hip OA (IVWQ 143.23, p = 0.00244) and knee OA (IVWQ 161.48, p = 7.45 10−5). The leave-one-out analysis found no SNP significantly influencing results (Figure 2–4).
After reviewing the IEU Open GWAS Project website, we discovered that 45 SNPs were linked to confounding factors [BMI (28), weight (3), obesity (1), waist circumference (1), arthropathies (6), BMD (2), vitamin D deficiency (2), and smoking (2)]. Then, we removed these 45 SNPs and found that the causality remained the same (OA IVW OR = 1.151, 95% CI: 1.042–1.273, p = 0.006, knee OA IVW OR = 1.064, 95% CI: 1.002–1.130, p = 0.04146917, hip OA IVW OR = 1.086, 95% CI: 1.002–1.178, p = 0.043).
Reverse MR analyses indicated no evidence of a causal relationship between OSA and OA. Odds ratios (OR) for OA, knee OA, and hip OA were 1.00 [95%CI (0.97–1.03), p = 0.89], 1.04 (95% CI: 0.99–1.09, p = 0.08), and 0.98 (95% CI: 0.91–1.06, p = 0.67), respectively. Replication analysis used OA GWAS data from Zengini et al. (20) (IVW OR = 1.04, 95% CI = 0.93–1.15, p = 0.493), meta-analyses showing increased OA risk with a genetic predisposition for elevated OSA levels (OR = 1.10, 95% CI = 1.03–1.17, p = 0.002) (Figure 4).
Given that waist circumference, hip circumference, BMI, and insulin resistance are well-established risk factors for OA, they could potentially mediate the effect of OSA on the risk of developing OA (Supplementary Figure S5 and Supplementary Table S5). Among the three potential mediators, we only identified BMI as a mediator between OSA and OA (Supplementary Table S3).
A total of 458 independent SNPs served as IVs for BMI, all with F statistics >10 (see Supplementary Table S4). Mediator MR analysis revealed that BMI (IVW: OR 1.49, 95% CI 1.35–1.64, p = 3.16e-15) was associated with an increased overall risk of OA (indirect effect β2). An increased BMI risk (indirect effect β1) was observed in relation to OSA (IVW OR 1.12, 95% CI 1.08–1.17, p = 2.46e-08). Furthermore, OSA demonstrated a causal association with heightened OA susceptibility (IVW OR = 1.13, 95% CI 1.05–1.21, p = 0.001) (overall effect α). The percentage of the impact of OSA on OA influenced by BMI was 36.9% (95% CI: 4.64–73.2%, p = 0.026) (Figure 5).
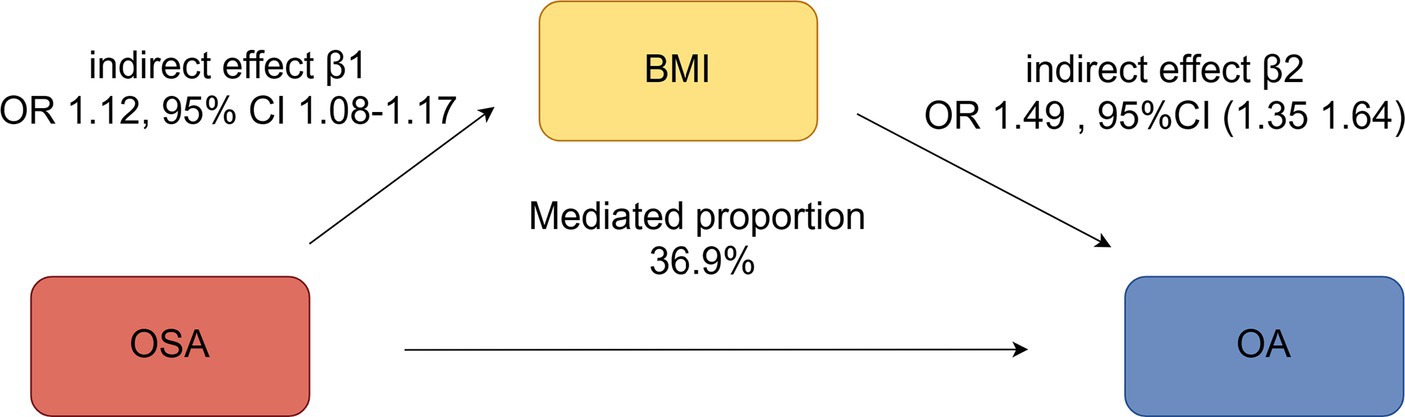
Figure 5. Mediation analysis of the effect of OSA on OA via BMI under a two-step Mendelian randomization analysis.
This study represents the first comprehensive investigation into the relationship between OSA and OA through MR analysis, utilizing large-scale observational study data and genetic datasets. Cross-sectional observational analysis identified significant relationships between OSA and OA, while findings based on the MR study did support a causal role. The analysis of potential mediating factors found that BMI was an important factor between OSA and OA.
OSA and OA are common diseases. Previous studies found that the prevalence of OSA among individuals with OA was significantly higher than that in the general population, with figures standing at 66% versus 17%, respectively (21).
Sleep, as a period of physiological recovery, creates an environment conducive to cell proliferation, migration, and differentiation, with cell division and protein synthesis peaking during sleep (22, 23). OSA causes airway collapse, leading to oxygen deprivation and disruption of sleep rhythm (24–26). Previous studies revealed that OSA can cause increases in inflammatory, oxidative stress, and metabolic abnormalities, such as abnormal blood lipids, uric acid, and blood sugar (22, 23), which may lead to the future development of OA.
In this study, our MR analysis provided evidence of a causal relationship between OSA and OA, substantiated by rigorous examinations of horizontal pleiotropy and heterogeneity. These findings were further reinforced by two-way MR analysis, eliminating the possibility of reverse causality.
Obesity and inflammation are potential pathogenic mechanisms by which OSA leads to OA. Previous studies, through bioinformatics analysis, have found that shared inflammation genes between OSA and OA are significantly enriched in the TNF pathway and the IL-17 pathway (27).
The correlation between OSA and BMI has been a focal point of research for quite some time. The sleep fragmentation due to OSA can result in a state of effective sleep deprivation, which, in turn, can cause daytime drowsiness, a reduction in physical activity, and, consequently, an increase in body weight (28). One key factor contributing to weight gain in individuals with OSA is insulin resistance. Studies have indicated that the development of insulin resistance in the context of sustained intermittent hypoxia is closely tied to the disruption of leptin signaling pathways (28, 29).
In this study, we observed significant mediated effects of BMI on the associations between OSA and OA risk. In particular, other obesity and insulin resistance markers mediated no association between OSA and OA risk, among which the mediated proportion of BMI was 36.9%, consistent with previous research (30). These findings suggested that OSA may increase obesity and finally aggravate OA.
A high BMI or obesity leads to overloading the joints due to excess weight, which, in turn, leads to the destruction of articular cartilage (31). Others attributed it to excess fat tissue, which secretes hormones and proinflammatory cytokines, contributing to low-grade systemic inflammation (32). Given that BMI is a relatively controllable mediating factor (33), effectively managing BMI in OSA patients could potentially reduce the incidence of OA.
This research integrates a cross-sectional approach with Mendelian randomization, offering a foundational exploration to ascertain preliminary associations. Subsequently, the Mendelian randomization study reinforces these findings by substantiating the underlying causal mechanisms. Moreover, this methodology effectively mitigates the risk of false positives inherent in Mendelian studies, thereby enhancing the credibility of our results (34). The limitations of the study include the identification of OSA based on participants’ self-reported interviews. This methodology might have resulted in an inflated estimation of the true incidence of OSA. Furthermore, the absence of granular individual-level data from the GWAS precluded us from discerning whether the condition could introduce any inherent biases into our analysis. Finally, the applicability of our findings to diverse ethnic groups may be constrained, given that our analysis was focused on individuals of European descent, thus limiting the generalizability of our conclusions.
The study identified a causal relationship between OSA and OA and uncovered BMI as a mediator, laying a foundation for future research avenues and clinical interventions in the realm of sleep-related musculoskeletal disorders.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by NCHS Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
ZY: Data curation, Methodology, Visualization, Writing – original draft, Writing – review & editing. TL: Data curation, Methodology, Visualization, Writing – original draft. LJ: Data curation, Supervision, Writing – original draft. XL: Data curation, Methodology, Supervision, Writing – original draft. XZ: Data curation, Methodology, Writing – original draft. XW: Data curation, Methodology, Writing – original draft. LZ: Data curation, Supervision, Validation, Visualization, Writing – original draft. CT: Data curation, Writing – original draft. SC: Data curation, Methodology, Writing – original draft. XY: Data curation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1425327/full#supplementary-material
1. Brodie, KD, and Goldberg, AN. Obstructive sleep apnea: a surgeon’s perspective. Med Clin North Am. (2021) 105:885–900. doi: 10.1016/j.mcna.2021.05.010
2. Benjafield, AV, Ayas, NT, Eastwood, PR, Heinzer, R, Ip, MSM, Morrell, MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. (2019) 7:687–98. doi: 10.1016/S2213-2600(19)30198-5
3. Giampá, SQC, Lorenzi-Filho, G, and Drager, LF. Obstructive sleep apnea and metabolic syndrome. Obesity. (2023) 31:900–11. doi: 10.1002/oby.23679
4. Labarca, G, Vena, D, Hu, WH, Esmaeili, N, Gell, L, Yang, HC, et al. Sleep apnea physiological burdens and cardiovascular morbidity and mortality. Am J Respir Crit Care Med. (2023) 208:802–13. doi: 10.1164/rccm.202209-1808OC
5. Gaspar, LS, Sousa, C, Álvaro, AR, Cavadas, C, and Mendes, AF. Common risk factors and therapeutic targets in obstructive sleep apnea and osteoarthritis: an unexpectable link? Pharmacol Res. (2021) 164:105369. doi: 10.1016/j.phrs.2020.105369
6. Hunter, DJ, and Bierma-Zeinstra, S. Osteoarthritis. Lancet. (2019) 393:1745–59. doi: 10.1016/S0140-6736(19)30417-9
7. GBD 2021 Osteoarthritis Collaborators . Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. (2023) 5:e508–22. doi: 10.1016/S2665-9913(23)00163-7
8. Turkiewicz, A, Petersson, IF, Björk, J, Hawker, G, Dahlberg, LE, Lohmander, LS, et al. Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarthr Cartil. (2014) 22:1826–32. doi: 10.1016/j.joca.2014.07.015
9. National Institute for Public Health and the Environment . Public health foresight study 2018 (VTV-2018): diseases. (2018). Available at: https://www.vtv2018.nl/en. (Accessed October 27, 2023).
10. Woolf, AD, and Pfleger, B. Burden of major musculoskeletal conditions. Bull World Health Organ. (2003) 81:646–56.
11. Safiri, S, Kolahi, AA, Smith, E, Hill, C, Bettampadi, D, Mansournia, MA, et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. (2020) 79:819–28. doi: 10.1136/annrheumdis-2019-216515
12. Kessler, RC, and Bromet, EJ. The epidemiology of depression across cultures. Annu Rev Public Health. (2013) 34:119–38. doi: 10.1146/annurev-publhealth-031912-114409
13. Cavallino, V, Rankin, E, Popescu, A, Gopang, M, Hale, L, and Meliker, JR. Antimony and sleep health outcomes: NHANES 2009–2016. Sleep Health. (2022) 8:373–9. doi: 10.1016/j.sleh.2022.05.005
14. Jiang, L, Zheng, Z, Fang, H, and Yang, J. A generalized linear mixed model association tool for biobank-scale data. Nat Genet. (2021) 53:1616–21. doi: 10.1038/s41588-021-00954-4
15. Tachmazidou, I, Hatzikotoulas, K, Southam, L, Esparza-Gordillo, J, Haberland, V, Zheng, J, et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet. (2019) 51:230–6. doi: 10.1038/s41588-018-0327-1
16. Skrivankova, VW, Richmond, RC, Woolf, BAR, Yarmolinsky, J, Davies, NM, Swanson, SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326:1614–21. doi: 10.1001/jama.2021.18236
17. Zhu, S, Ji, L, He, Z, Zhang, W, Tong, Y, Luo, J, et al. Association of smoking and osteoarthritis in US (NHANES 1999–2018). Sci Rep. (2023) 13:3911. doi: 10.1038/s41598-023-30644-6
18. Palazzo, C, Nguyen, C, Lefevre-Colau, MM, Rannou, F, and Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. (2016) 59:134–8. doi: 10.1016/j.rehab.2016.01.006
19. Ho, J, Mak, C, Sharma, V, To, K, and Khan, W. Mendelian randomization studies of lifestyle-related risk factors for osteoarthritis: a PRISMA review and Meta-analysis. Int J Mol Sci. (2022) 23:11906. doi: 10.3390/ijms231911906
20. Zengini, E, Hatzikotoulas, K, Tachmazidou, I, Steinberg, J, Hartwig, FP, Southam, L, et al. Genome-wide analyses using UK biobank data provide insights into the genetic architecture of osteoarthritis. Nat Genet. (2018) 50:549–58. doi: 10.1038/s41588-018-0079-y
21. Taylor, SS, Hughes, JM, Coffman, CJ, Jeffreys, AS, Ulmer, CS, Oddone, EZ, et al. Prevalence of and characteristics associated with insomnia and obstructive sleep apnea among veterans with knee and hip osteoarthritis. BMC Musculoskelet Disord. (2018) 19:79. doi: 10.1186/s12891-018-1993-y
22. Yang, Z, Lv, T, Lv, X, Wan, F, Zhou, H, Wang, X, et al. Association of serum uric acid with all-cause and cardiovascular mortality in obstructive sleep apnea. Sci Rep. (2023) 13:19606. doi: 10.1038/s41598-023-45508-2
23. Kanbay, A, Köktürk, O, Pihtili, A, Ceylan, E, Tulu, S, Madenci, E, et al. Obstructive sleep apnea is a risk factor for osteoarthritis. Tuberk Toraks. (2018) 66:304–11. doi: 10.5578/tt.57403
24. Adamovich, Y, Ladeuix, B, Golik, M, Koeners, MP, and Asher, G. Rhythmic oxygen levels reset circadian clocks through HIF1α. Cell Metab. (2017) 25:93–101. doi: 10.1016/j.cmet.2016.09.014
25. Manella, G, Aviram, R, Bolshette, N, Muvkadi, S, Golik, M, Smith, DF, et al. Hypoxia induces a time- and tissue-specific response that elicits intertissue circadian clock misalignment. Proc Natl Acad Sci USA. (2020) 117:779–86. doi: 10.1073/pnas.1914112117
26. Gabryelska, A, Turkiewicz, S, Karuga, FF, Sochal, M, Strzelecki, D, and Białasiewicz, P. Disruption of circadian rhythm genes in obstructive sleep apnea patients-possible mechanisms involved and clinical implication. Int J Mol Sci. (2022) 23:709. doi: 10.3390/ijms23020709
27. Weng, L, Luo, X, Luo, Y, Zhang, Q, Yao, K, Tan, J, et al. Association between sleep apnea syndrome and osteoarthritis: insights from bidirectional Mendelian randomization and bioinformatics analysis. Nat Sci Sleep. (2024) 16:473–87. doi: 10.2147/NSS.S461010
28. Polotsky, VY, Li, J, Punjabi, NM, Rubin, AE, Smith, PL, Schwartz, AR, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. (2003) 552:253–64. doi: 10.1113/jphysiol.2003.048173
29. Pillar, G, and Shehadeh, N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care. (2008) 31:S303–9. doi: 10.2337/dc08-s272
30. Lei, T, Li, M, Qian, H, Yang, J, Hu, Y, and Hua, L. The effect of sleep on metabolism, musculoskeletal disease, and mortality in the general US population: analysis of results from the National Health and Nutrition Examination Survey. JMIR Public Health Surveill. (2023) 9:e46385. doi: 10.2196/46385
31. Nedunchezhiyan, U, Varughese, I, Sun, ARJ, Wu, X, Crawford, R, and Prasadam, I. Obesity, inflammation, and immune system in osteoarthritis. Front Immunol. (2022) 13:907750. doi: 10.3389/fimmu.2022.907750
32. Lerman, SF, Finan, PH, Smith, MT, and Haythornthwaite, JA. Psychological interventions that target sleep reduce pain catastrophizing in knee osteoarthritis. Pain. (2017) 158:2189–95. doi: 10.1097/j.pain.0000000000001023
33. Vitiello, MV, McCurry, SM, Shortreed, SM, Baker, LD, Rybarczyk, BD, Keefe, FJ, et al. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain. (2014) 155:1547–54. doi: 10.1016/j.pain.2014.04.032
Keywords: obstructive sleep apnea, osteoarthritis, Mendelian randomization, NHANES (National Health and Nutrition Examination Survey), BMI—body mass index
Citation: Yang Z, Lv T, Jin L, Lv X, Zhu X, Wang X, Zhang L, Tu C, Chen S and Yan X (2024) The relationship between obstructive sleep apnea and osteoarthritis: evidence from an observational and Mendelian randomization study. Front. Neurol. 15:1425327. doi: 10.3389/fneur.2024.1425327
Received: 29 April 2024; Accepted: 12 June 2024;
Published: 28 June 2024.
Edited by:
Victor B. Fenik, Albany Medical College, United StatesCopyright © 2024 Yang, Lv, Jin, Lv, Zhu, Wang, Zhang, Tu, Chen and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiongwei Yan, eWFueGlvbmd3ZWk3Nzg5QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.