- 1Department of Rehabilitation Science, University of Franche-Comte, Besançon, France
- 2Integrative and Clinical Neurosciences UMR 1322 INSERM, University of Franche-Comte, Besançon, France
- 3ERCOS Group, ELLIADD Laboratory EA4661, UTBM University of Franche-Comte University, Besançon, France
- 4Postgraduate Programme in Health and Environment - University of Joinville Region, Joinville, Brazil
Background: Motor imagery (MI) has emerged as a promising therapeutic approach for Parkinson’s disease (PD). MI entails mentally rehearsing motor actions without executing them. This cognitive process has garnered attention due to its potential benefits in aiding motor function recovery in patients. The purpose of this review was to highlight the findings observed in motor symptoms, balance, gait, and quality of life.
Methods: A literature search was carried out in Medline, Embase, Cochrane, and Physiotherapy Evidence Database (PEDro), from the first publication to February 2024. Studies with at least one keyword to PD and MI in the title were included.
Results: The analysis included 53 studies out of the 262 identified. These comprised 12 randomized controlled trials (RCTs) with an average PEDro score of 6.6 out of 10, as well as 41 non-RCT studies. Notably, the majority of the RCTs focused on balance, gait, and lower limb exercises. The experimental group found an 85.2% improvement on the Timed Up and Go (TUG) with a cognitive task (p < 0.02), 5.8% improvement on the TUG (p < 0.05), and 5.1% improvement in walking speed (p < 0.05). Other variables did not show significant improvement. In descriptive and non-RCT studies, there were various tasks and outcomes for the lower and upper limbs. It has been demonstrated that there was no difference in execution time in MI between patients and healthy subjects (HS), whereas motor execution was slower in patients. Several tasks were analyzed for the upper limb, including thumb opposition, joystick movements, and writing tasks with variable results. RCTs were more focused on balance, lower limbs, and walking. There was no specific outcome regarding the upper limb or speech. Additionally, the heterogeneity of tasks and outcomes across studies is also a limitation.
Conclusion: Current research on walking disorders in PD shows promise, but further investigations are crucial, particularly with an emphasis on upper limb function and speech. Studies with larger sample sizes and more precise methodologies are needed to enhance our understanding of the potential benefits of MI within the framework of comprehensive PD rehabilitation.
1 Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease and a major cause of disability among the elderly. Although advancing age is linked to a heightened risk of PD, it remains uncertain whether this increase follows a linear or exponential pattern. A recent study underscored the need for higher-quality epidemiological data to ensure equitable representation across race, ethnicity, geography, sex, and gender (1). PD is caused by the loss of dopaminergic neurons, resulting in both motor and non-motor symptoms (2, 3). In PD patients, there are four primary clinical aspects: bradykinesia or akinesia, resting tremor, rigidity, and postural instability (2–10) whereas the non-motor symptoms include sleep disorders, depression, and digestive disorders (11). PD impacts sensorimotor functions such as walking, balance, and posture, leading to a decrease in the patient’s independence and participation in societal activities (12).
Parkinson’s disease (PD) presents various treatment options, with pharmacological approaches being the most prevalent. These treatments primarily focus on dopamine and its derivatives to manage symptoms (4). Although levodopa is widely recognized as the most effective medication for treating motor symptoms, there exist other medications such as monoamine oxidase type B inhibitors, amantadine, anticholinergics, β-blockers, or dopamine agonists. Its utilization is conditioned by the symptoms exhibited by the patient (13). Although this treatment is the most used, adverse effects such as dyskinesias and motor complications can be observed (14). This is one of the main reasons why other forms of symptomatic treatment have been researched. Among non-pharmacological treatments, physiotherapy has shown beneficial effects in the management of PD (5). Recent studies have shown positive effects on motor symptoms (5), quality of life (15), walking, and balance (5, 16, 17).
Among physiotherapy techniques, motor imagery (MI) was proposed more than 30 years ago as a potential tool of rehabilitation (18). It is defined as a mental process in which a person simulates a mental simulation of a motor act without making any movement (7, 8). This approach relies on the premise that MI and actual motor execution elicit activation in overlapping brain regions (19). Consequently, enhancing the engagement of motor regions in the brain (9) is a central objective of this technique.
MI, a recently developed approach for the rehabilitation of patients with PD, is supported and promoted for implementation in rehabilitation protocols as a promising approach (6, 20, 21). Several studies have demonstrated that combining MI with physiotherapy can be effective for patients with PD (6, 22). MI can be performed from a first-or third-person perspective (7, 23) and can be used for different modalities such as upper limb, lower limb, walking, and others. There are also numerous MI protocols based on distinct sensorimotor tasks (24–29), such as the goal-directed task and the Box and Block Test (BBT) (26), the MI of walking along a straight course (24), and the MI of walking forward, backward, and turning (25). Considering these different MI modalities, choosing the best MI protocol for a clinical application seems difficult, especially considering the procedures and possible expected benefits. Only one study has proposed a framework for motivational interviewing to help physiotherapists integrate MI into their clinical practice (27). In alignment with the imperative to optimize the clinical use of MI as a rehabilitation tool, this scoping review aimed to achieve two primary objectives. First, it was aimed to provide a comprehensive summary of the diverse MI protocols designed for patients with Parkinson’s disease (PD), to provide guidance and facilitate their application in clinical practice. Second, the review sought to highlight the key findings observed in these studies regarding motor symptoms, balance, gait, and quality of life.
2 Materials and methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines (Annex I). Based on our previous research, there is no existing scoping review on this subject.
2.1 Data sources and searches
Prospective research was carried out on four different databases, namely MEDLINE (PubMed), Embase, Cochrane (Cochrane library), and Physiotherapy Evidence Database (PEDro), from the initial publication until February 2024. To identify relevant articles, the following keywords and operators were used: “Parkinson disease”* OR “Parkinson Disease” OR “Parkinson’s disease”* AND “motor imagery”* OR “motor imagery practice”* OR “mental practice”*. In order to enhance the comprehensiveness of the potential articles included, the search was conducted using Medical Subject Headings (MeSH) terms and non-MeSH terms (identified by an asterisk).
2.2 Study selection
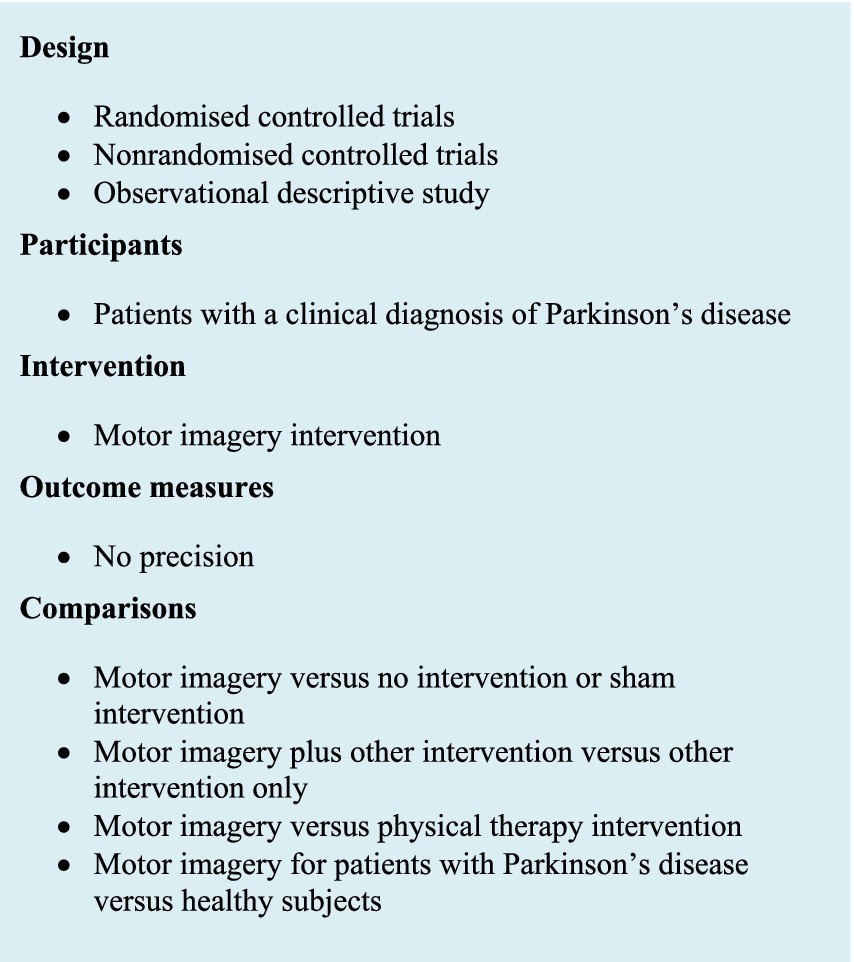
First, all articles with at least one keyword regarding PD and MI in the title were included in this phase. Duplicated articles were removed.
The eligibility criteria (Figure 1) for this phase of selection were applied to the title and abstract of the articles. Exclusion criteria were articles that were neither in English nor in French, feasibility and pilot studies, conference abstracts, and articles that did not focus on the specific effectiveness of MI. Full text was directly reviewed with eligibility criteria when the abstract did not provide sufficient information. Then, eligibility criteria were applied to the full text.
2.3 Data extraction and quality assessment
For this review, the articles were selected and read by two reviewers, MM and ET. Disagreements in this phase were resolved by consulting a third evaluator (YS).
The methodological quality of the randomized controlled studies (RCTs) was assessed with the PEDro scale. This is an 11-item scale. It is used to assess the external validity (criterion 1), internal validity (criterion 2–9), and interpretability of the findings (criterion 10 and 11) of a clinical trial or group comparison study. The PEDro scale is scored on a 10-point system, where 0 indicates very poor methodological quality and 10 signifies excellent methodological quality.
2.4 Data synthesis and analysis
Reviewers extracted the following key data from each article: the type of study, population characteristics, inclusion/exclusion criteria, intervention/protocol, variable of interest, and PEDro score. The mean (± Standard Deviation [SD]) values for all variables, p values, and modifications in percentage (comparisons among interventions and groups) were collected.
3 Results and comments
3.1 Selection of articles
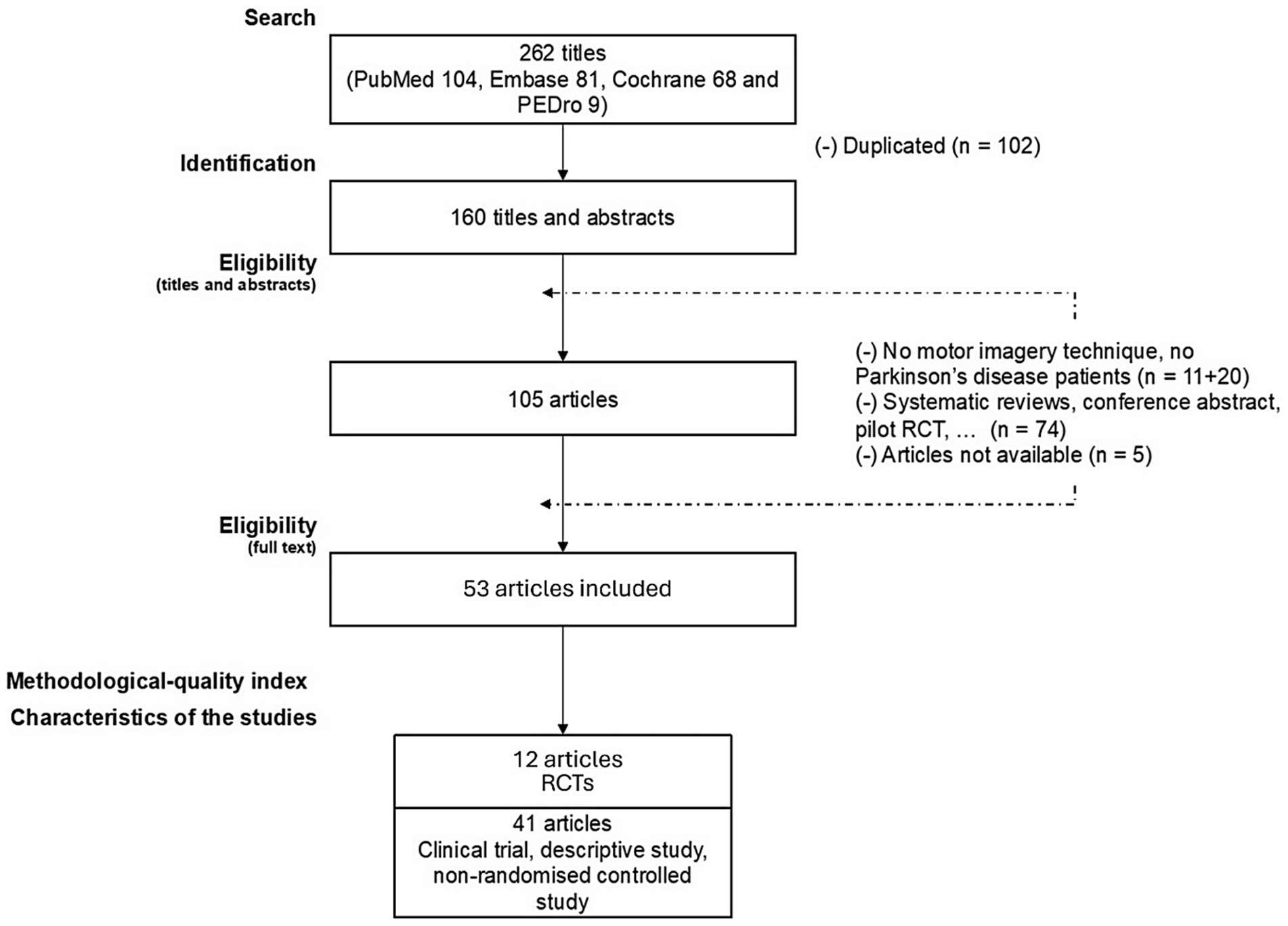
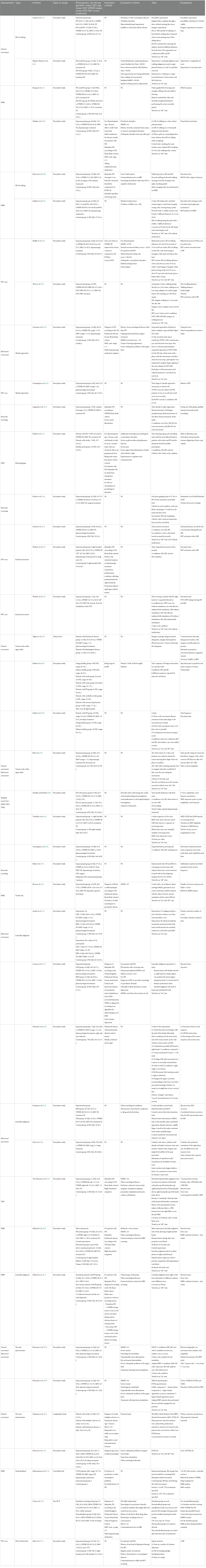
Figure 2 shows the article selection process for this review. From the four databases combined, 262 articles were identified. A total of 53 articles were included, with 12 RCTs and 41 non-RCTs, as well as descriptive studies.
Methodological quality as assessed by the mean PEDro score for RCTs was 6.6/10, with only one being lower than 3/10 (30). All eligibility criteria, random allocation, baseline intragroup similarity, and between-group statistical comparison were respected for all studies. Although this was the case for the majority of RCTs, the blinding of participants and therapists was not consistently maintained.
3.2 RCT: effects of MI intervention
3.2.1 Participants’ characteristics
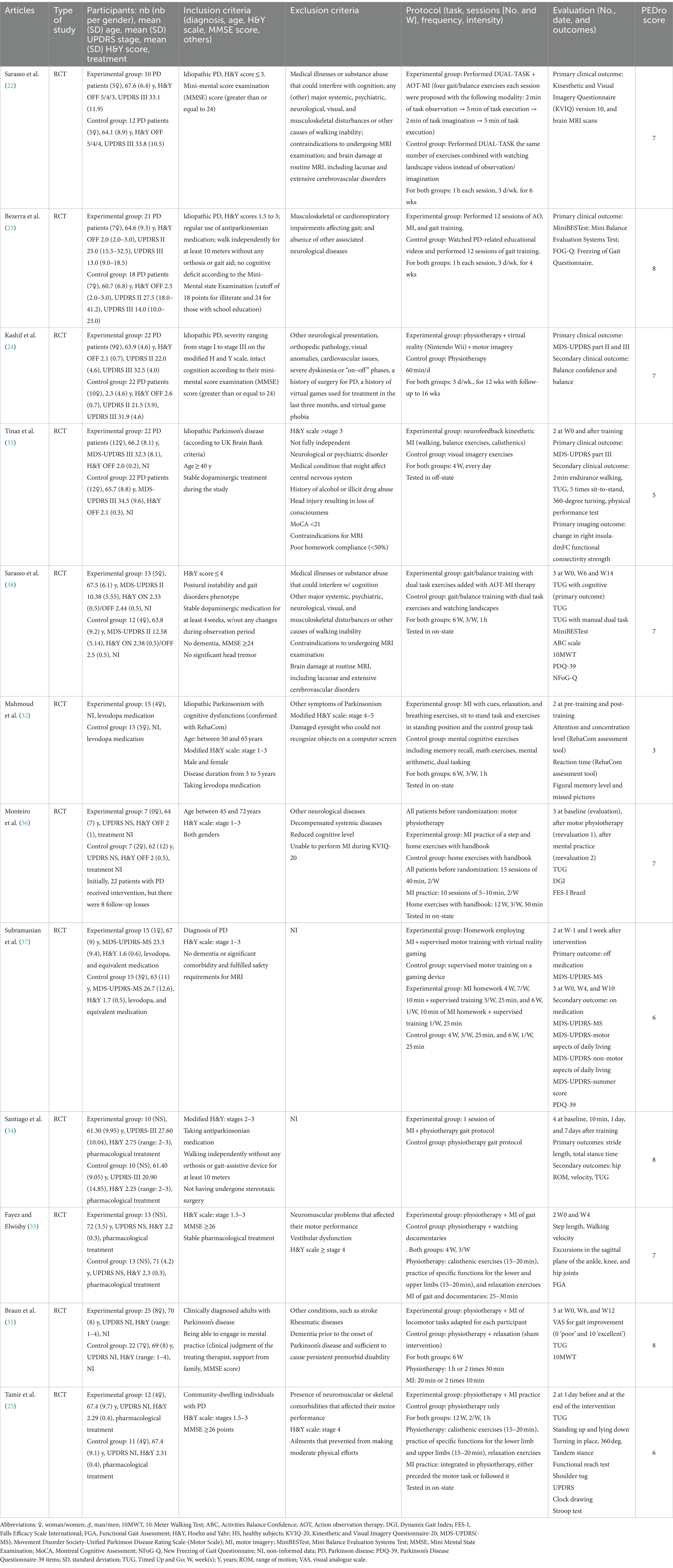
The characteristics of RCTs are presented in Table 1. Participants’ characteristics were based on the diagnosis of PD. The mean (SD) number of participants per study was 29.9 (±10.5), with a mean age of 66.2 (±8.3) years. Groups were composed of an average of 30.7% of women and 69.3% of men. The mean (SD) Hoehn and Yahr (H&Y) score was 2.2 (0.5), with an off-phase score taken when it was specified.
The majority of studies had as inclusion criteria a Hoehn and Yahr (H&Y) score ≤ 3 (23–25, 31–37), except for Sarasso et al. (38), who included patients with a H&Y score ≤ 4. One study failed to report eligibility criteria related to an H&Y score, and another study excluded patients with an H&Y score > 3 (31). For the exclusion criteria, in most studies, patients with neuromuscular, psychiatric, or neurological pathologies other than PD were excluded.
3.2.2 Protocols
Regarding the 12 RCTs, the mean protocol duration was 7 weeks, ranging from a single session to 12 weeks with a mean number of sessions per week of 3 (range: 1–7). The duration of the interventions was determined for 7 studies, with a mean duration of 55 min for the experimental group (range: 35–80) and 52 min for the control group (range: 25–80). All studies performed a pre-intervention and post-intervention assessment, and 3 studies (28, 30, 32) included a follow-up intervention ranging from 1 week to 8 weeks after the end of the protocol. Regarding the types of exercises, eight studies (22–24, 28–30, 32, 38) used an MI protocol of gait and balance exercises or gait exercises only. One study (33) comprised a single-step protocol for MI.
3.2.3 Outcomes
In terms of motor symptoms, two studies (32, 35) used the Movement Disorder Society’s (MDS) Unified Parkinson’s Disease Rating Scale (UPDRS) as the primary outcome. They compared Part III of UPDRS. Regarding the assessment of quality of life, only 4 studies (23, 24, 31, 35) assessed this parameter using the Parkinson’s Disease Questionnaire-39 (PDQ-39). The walking and balance abilities were assessed, including walking speed, step length, Timed Up and Go (TUG), Dynamic Gait Index (DGI), Functional Gait Assessment (FGA), 10-Meter Walk Test (10MWT), 2-min endurance walking test, sit-to-stand, or a balance test (23–25, 31, 33–36, 38). Six studies have focused on balance (23–25, 33, 34, 38). Lower limb range of motion (ROM) was also assessed in two studies, one (34) focusing on the hip and the other (35) evaluating the hip, knee, and ankle. No specific upper limb or speech outcomes have been assessed.
3.2.4 Results of RCT
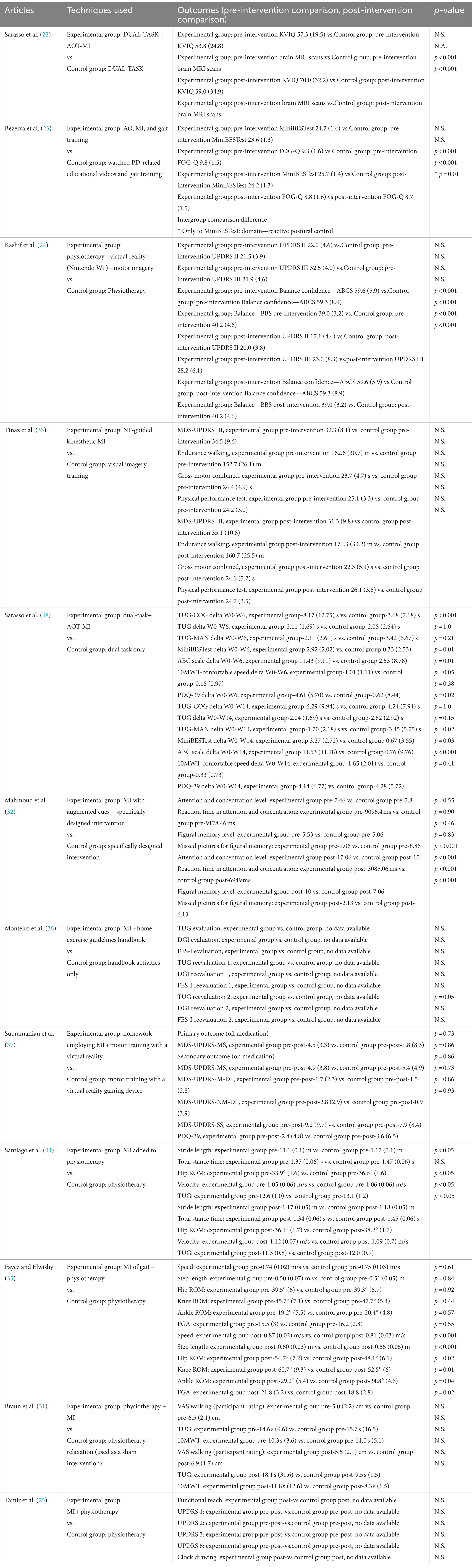
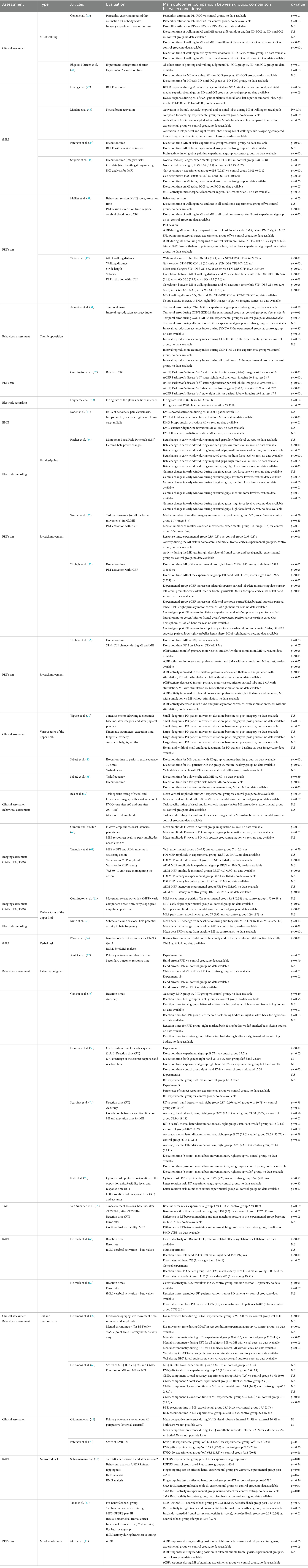
Among the 12 studies, there was a substantial range in the significance of intergroup differences. Out of these, 10 studies demonstrated a significant difference between groups after the intervention (Table 2).
Regarding the studies with gait and balance MI exercises, Sarasso et al. (38) reported a significant improvement in TUG with a cognitive task (primary outcome) compared to the control group. An improvement of 122% (p < 0.001) was found in week 6, and 48.3% (p = 0.02) in week 14. Furthermore, Santiago et al. (34) found an improvement in the TUG for the experimental group (5.8%; p < 0.05). Sarasso et al. (38) demonstrated an improvement of 388.05% (p = 0.02) during week 14 for the experimental group for the Mini Balance Evaluation System Test, as well as an improvement of 1417.1% (p = 0.03) for the Activities-specific Balance Confidence Scale. Mahmoud et al. (32) examined concentration parameters of motor learning. For the attention and concentration program, they used a questionnaire based on reaction time to identify matched figures. The motor learning test was based on a computer-based cognitive assessment device (RehaCom). The degree of attention and concentration was significantly improved by 70.6% (p < 0.001). The reaction time of the previous test was also improved by 55% (p < 0.001). Two other variables on figural memory were also improved (range: 42–65%; p < 0.001). Fayez and Elwishi (35) observed a significant difference in hip, knee, and ankle ROM in the experimental group (range: 13.7–17.7%; p < 0.01–0.04). For the spatiotemporal parameters, Fayez and Elwishi (35) showed a significant improvement in walking speed by 7.4% (p < 0.001), step length by 9.1% (p < 0.001), and FGA by 16% (p < 0.02) in the experimental group. Santiago et al. (34) observed a significant improvement in walking speed (2.8%; p < 0.05) in the experimental group. Sarasso et al. (38) reported an improvement of 400% at week 14 for the 10MWT. Monteiro et al. (36) studied MI with only one-step execution and found a significant difference for the TUG test at 14 weeks (difference not specified; p = 0.05).
Another noteworthy result was discovered by Sarasso et al. (38), wherein the MI was assessed using a Kinesthetic and Visual Imagery Questionnaire (KVIQ) and a MI functional magnetic resonance imaging (fMRI) task. During the fMRI, the participants, 25 PD patients and 23 healthy people, were asked to watch videos in the first-person perspective depicting gait/balance tasks and mentally simulate their execution. They demonstrated that action observation therapy and MI training (AOT-MI) in PD patients promoted functional plasticity in the brain areas involved in MI processes and gait/balance control (22).
There are no outcomes available for the upper limb or speech, as no specific outcomes were assessed.
3.3 Non-RCTs and descriptive studies: assessment of MI and main results
The results of the subsequent studies should be interpreted with the utmost caution, as we solely focused on their main results. We have organized the results according to this logic: first, the difference between patients with PD and healthy subjects (HS) in terms of MI (PD/HS-MI); second, the difference between patients with PD and HS in terms of ME (motor execution) (PD/HS-ME); and finally, the difference between ME and MI (MI/ME) for the same group of patients. The characteristics of the descriptive and non-RCT studies are shown in Table 3, and the main results are shown in Table 4.
3.3.1 Participants’ characteristics
In most of these studies (39–41), patients with PD were compared with HS of the same age. The mean (SD) number of participants per study was 30 (±18) and the mean age was 61 (±8). The groups were comprised of an average of 35.5% women and 64.5% men. For patients with PD, the main inclusion criteria were a diagnosis of idiopathic PD (10 studies specified that the diagnosis was made using the UK brain bank criteria) and an H&Y score. A total of 21 out of 41 studies did not mention the inclusion criteria. Four studies included patients with other neurological conditions, such as stroke, multiple sclerosis, and Huntington’s disease (39–42).
The Kinesthetic and Visual Imagery Questionnaire (KVIQ) was used to evaluate the ability of subjects to imagine from a first-person perspective by assessing the clarity of the image (visual: V subscale) and the intensity of the sensations (kinesthetic: K subscale) (28, 29, 42).
3.3.2 Protocols
We have grouped the studies according to whether they concern the lower limb, the upper limb, or language-related MI exercises. Subgroups were created within each category.
Eight studies focused on the lower limb using the MI of walking. Among these studies, the protocols were heterogeneous. Five studies tested MI walking in a straight line with different distances ranging from 2 to 15 m; 2 studies tested MI walking in a straight line, turning, turning back; and 1 study tested walking on an obstacle path.
The upper limb was involved in 16 studies. Three studies tested a thumb opposition task, 2 studies tested hand gripping, 3 studies tested joystick movement, and 8 studies tested various upper limb tasks with 8 different interventions.
Language-related tasks (verbal tasks) were used in only one study. Finally, other studies did not fit into the three categories mentioned above. Eight studies performed lateral judgment tasks, five used MI tests and questionnaires, two tested neurofeedback, and one tested whole-body MI.
Not all studies have evaluated patients with PD under the same conditions. Eleven studies evaluated patients during their off phase, 10 during the on phase, 6 during both phases, and 14 did not mention this information.
3.3.3 Outcomes for lower limb
Of these studies, 2 assessed walking in clinical conditions (40, 41); execution time was also used (7 studies) during different tasks (28, 43–48); and 6 assessed brain activity with regional Cerebral Blood Flow (rCBF) using a Positron Emission Tomography (PET) scan (45, 49) as well as using functional Magnetic Resonance Imaging (fMRI) (25, 44, 45, 49).
3.3.4 Outcomes for upper limb
In the thumb-opposition studies, Dominey et al. (50) evaluated the execution time for MI and ME. Avanzino et al. (51) assessed the timing error rate. Cunnington et al. (52) performed this task under a PET scan and compared the rCBF. Leiguarda et al. (53) analyzed the firing rate of the globus pallidus internus using microelectrode recording.
For hand gripping, muscle activation by electromyography (EMG) and monopolar local field potentials were evaluated (41, 54).
All joystick movement studies were conducted using a PET scan (55–57). In addition, two of them evaluated the execution time (55, 56).
For studies with varied upper limb tasks, the evaluations were also heterogeneous. The execution time was evaluated in three studies (39, 40, 58); KVIQ was assessed in one study (56); F-waves were assessed by EMG (59, 60); the amplitude of motor evoked potential by transcranial magnetic stimulation (TMS) (60, 61); movement-related potentials by electroencephalogram (62); and local field potentials by electrode recording (63).
3.3.5 Outcomes for verbal tasks
Péran et al. (64) used the number of correct responses and an fMRI as a means of assessment.
3.3.6 Outcomes for laterality judgment
Reaction time and error rate were measured for all these studies. The motor evoked potentials (MEP) amplitude was measured using TMS (65). An fMRI was used in two studies (66, 67).
3.3.7 Outcomes for MI tests and questionnaire
Several tests were used in the various studies. The score of these studies was used as an outcome. The KVIQ, Motor Imagery Questionnaire-Revised (MIQ-R), the Gait Imagery Questionnaire (GIQ), and the Chaotic Motor Imagery Assessment were used. The execution time was also measured for the BBT (29, 68).
3.3.8 Outcomes for neurofeedback intervention
In these non-RCT studies, the fMRI and UPDRS scores were used (69, 70).
3.3.9 Outcomes for MI of the whole body
The rCBF was assessed by using a PET scan (71).
3.3.10 Main results for lower limb (8 studies: 257 participants)
First, regarding imagined execution of walking time, three studies showed that there was no significant difference between PD and HS-MI (28, 44, 46). Cohen et al. (43) also found no significant difference between patients with PD with and without freezing of gait (FOG).
Second, regarding execution time of walking for PD/HS-ME, Peterson et al. (28) showed that patients with PD are slower than patients with HS (p < 0.001). It has been shown that patients with FOG were slower than patients without FOG in normal walking (p = 0.03) and when walking through a narrow doorway (p < 0.001) (43, 44).
Maillet et al. (45) investigated the influence of levodopa on the neural networks involved in the MI of gait in advanced PD and found that patients in the off phase had significantly different durations during the MI of gait compared to HS (p < 0.03), while in the on phase there was no significant difference when compared to HS. Weiss et al. (49) assessed the disparity between active and inactive transcranial stimulation in patients. When stimulation was active and for the MI condition, patients walked 51% further (p < 0.001), 57% faster (p < 0.001), and took 30% longer steps (p < 0.001).
Regarding brain activity, Maillet et al. (45) observed that MI of walking in patients with PD compared to HS increased brain activation in the premotor-parietal cortices and pontomesencephalic tegmentum and decreased brain activation in the motor and frontal associative areas, basal ganglia, thalamus, and cerebellum. Maidan et al. (48) found that compared to HS, patients with PD had higher activation in the frontal, parietal, temporal, and occipital lobes during MI of usual walking (p < 0.04). Huang et al. (47) demonstrated that during walking with MI, compared to controls, patients with PD without FOG had more brain activity in bilateral supplementary area, right superior temporal, and right medial superior frontal gyrus (p < 0.04). Weiss et al. (49) showed that, with or without deep brain stimulation in the subthalamic nucleus, the MI of walking induced activity in the supplementary motor area and the right superior parietal lobule against a rest condition (p < 0.05). In terms of the difference in FOG, Snijders et al. (46) found that FOG patients exhibited increased brain activity on fMRI in the mesencephalic locomotor region during MI of gait compared to non-FOG patients (p < 0.05).
3.3.11 Main results for the thumb-opposition task (4 studies: 52 participants)
The Dominey et al. (50) study showed that patients with PD were 69.8% slower compared to HS in the execution time of the thumb-opposition task (MI and ME data combined) (p < 0.001). Avanzino et al. (51) found that when the task was performed in a 0.5 Hz timing and the auditory cue was removed, patients with PD made more errors when continuing the task in both MI (p = 0.04) and ME (p = 0.05) conditions, which was not the case for a 1.5 Hz timing. In the study by Cunnington et al. (52), it was observed that the level of activation in the supplementary motor area followed a typical pattern in patients with PD when they were both in the “off” and “on” medication states during MI compared to the resting state (p < 0.001).
3.3.12 Main results for hand gripping task (2 studies: 32 participants)
Kobelt et al. (41) conducted a study on patients with stroke and PD by measuring their muscle activity by EMG. Their findings showed a significant activation of the deltoideus pars clavicularis (p < 0.001) and biceps brachii (p = 0.01) during the hand gripping task in MI in comparison to a resting state. There was, however, no significant difference in activation between MI and rest in the extensor digitorum and flexor carpi radialis muscles. Fischer et al. (54) recorded local field potentials with TMS in PD patients. They found that beta activity decreased significantly for MI at the two highest force levels compared to rest (range: p < 0.01–0.05) and for ME at all force levels (p < 0.001); gamma activity increased significantly at MI at the two highest force levels again compared to rest (range: p < 0.01–0.05) and for ME at all force levels (range: p < 0.01–0.05).
3.3.13 Main results for joystick movement (3 studies: 35 participants)
Thobois et al. (55) observed that patients with PD performed the joystick movement task slower with their more affected side than with their other side in both the MI and ME conditions (range: 10.8–13.7%, p < 0.05). Another study by Thobois et al. (56) found no significant difference in execution time between MI and ME. Samuel et al. (57) demonstrated that when performing the task, patients with PD compared to HS in the MI group showed a decrease in activity in the dorsolateral and mesial frontal cortex (p < 0.01), whereas in the ME group, there was a decrease in the right dorsolateral frontal cortex and basal ganglia (p < 0.01). The ability to retain previously made movements in MI as well as in ME was not different between PD and HS groups (57).
3.3.14 Main results for varied upper limb tasks (6 studies: 223 participants)
Yágüez et al. (39) conducted a pre-post-clinical trial with patients with PD. They examined the writing movement and execution time to perform ideograms. The intervention was first a practice phase in MI and then a phase in ME. A significant difference was observed in execution time between the baseline and post-ME practice sessions (p = 0.01) as well as between the post-MI and post-ME sessions, with an improvement after the ME practice phase (p = 0.03).
Sabaté et al. (40) demonstrated that sequential finger movements took 70% (p < 0.001) longer in MI and 80% (p < 0.001) longer in ME for patients with PD when compared to HS. Regarding the difference between MI and ME in patients with PD, Sabaté et al. (58) found a significant difference in favor of ME in execution time for a fast cyclic (p < 0.001) and a slow continuous movement task (p < 0.001), but no significant difference was found for a slow cyclic movement task. Bek et al. (59) demonstrated that action observation influences hand movement amplitude in PD patients, and MI increases the effects of action observation in these patients. People with PD may benefit from interventions that combine action observation with MI.
Gündüz and Kiziltan (60) analyzed F-waves during thumb abduction. They found that the average amplitude of F-waves significantly increased during MI and ME compared to rest conditions in both patients with PD non-apraxia (p < 0.001) and HS (p = 0.01) groups. Tremblay et al. (61) measured the MEP amplitude of two hand muscles both during the resting state and during the MI of a scissors-cutting task. No significant change was detected between conditions in patients with PD, while a significant difference was found in patients with HS (p < 0.05).
3.3.15 Main results for verbal task (1 study: 10 participants)
Péran et al. (64) compared three tasks in patients with PD: object naming, an action word related to the object, and a mental simulation of the action with the object. They found that in contrast to object naming, mental simulation demonstrated a greater degree of activation in the prefrontal cortex bilaterally and in the parietal-occipital junction bilaterally (p < 0.001).
3.3.16 Main results for the laterality judgment task (5 studies: 228 participants)
The task of lateral judgment involves an implicit MI process. Four studies (50, 72–74) divided the participants into groups based on their most affected side. Amick et al. (72) found that patients in the PD right-sided symptoms group made more errors than the HS in judging laterality (p = 0.01), but the left-sided symptoms group did not show a significant difference in error rates compared to the HS group. The results of Conson et al. (73) showed that patients with PD had a greater reaction time to determine the laterality of a body that corresponded to their most affected side compared to the other side (range: p < 0.01–0.03). However, no significant difference was found in terms of reaction time or accuracy between patients with right-sided symptoms and patients with left-sided symptoms (73). In the Dominey et al. (50) study, patients with PD were slower than patients with HS in determining letter symmetry and hand laterality (p < 0.001). Scarpina et al. (74) and Helmich et al. (67) conducted a similar protocol and found no significant differences in reaction time and accuracy among patients with PD with right-sided symptoms and HS, patients with PD with left-sided symptoms and HS, and between patients with PD with and without tremor and HS. Additionally, patients with PD with tremors demonstrated higher levels of imagery-related activity in the somatosensory area 3a when compared to both patients with PD without tremors and HS (p < 0.01) (67).
3.3.17 Main results for MI tests and questionnaire (6 studies: 252 participants)
Heremans et al. (29, 68) used an adapted version of the BBT, consisting of wooden blocks measuring 2.5 cm2 and a box that was divided into 2 equal partitions measuring 18-cm high. Participants were instructed to transport 20 blocks as fast as possible from one side of the box to the other. This task was performed under four conditions: (a) ME, (b) MI with visual cues, (c) MI with auditory cues, and (d) MI without cues. Each condition was repeated three times in a random order. During execution, the box was placed at the participants’ midline, with the compartment holding the blocks pointing toward the hand being tested. During MI with visual cues, free vision of the box and blocks was provided. During MI with auditory cues, the box was removed from the participant’s sight. Instead, auditory cues were provided by a metronome at a rate of 0.5 Hz, and the participants were instructed to align every tic with the imagined pick-up of one block. During MI without cues, no visual or auditory information was provided. They found that patients with PD were slower on the BBT in MI and ME compared to HS (range: 16.7–30.4%; p < 0.01–0.02). Regarding the impact of cues in BBT, wherein the time required to transport 20 blocks was assessed using a mental chronometry paradigm, the execution time revealed no significant difference between MI with cues and ME. However, MI without cues was significantly slower than ME (p < 0.05).
Several studies used MI tests and questionnaires. There was no significant disparity observed between patients with PD and HS for the MIQ-R, KVIQ-20, Chaotic Motor Imagery Assessment (CMIA), and GIQ. Heremans et al. (68) and Peterson et al. (75) investigated KVIQ in patients with PD phase on, off, and HS, and no significant difference was found among groups. For the GIQ, no significant distinction was found between patients with PD with FOG and without FOG (73).
Kobelt et al. (41) used the short version of the KVIQ (KVIQ-10), which contains 10 items. There are three subscales: KVIQ visual (5–25), KVIQ kinesthetic (5–25), and KVIQ kinesthetic + visual (10–50). The scales are defined as both visual and a kinesthetic 5-point Likert scales ranging from 1 to 5 (1 = “no image”/“no sensation,” 5 = “image as clear as seeing it”/“as intense as moving”). The mean scores of the subscales were calculated. The five participating PD patients scored an average of 3.3 points higher on the visual subscale of the KVIQ-10 than on the kinesthetic subscale (41).
In order to evaluate MI perspectives in patients, Gäumann et al. (42) used two photographs of each item of the KVIQ: one photograph representing the internal perspective and one representing the external perspective. After each KVIQ item, patients were asked to identify which photograph represented their preferred perspective. Among patients with PD, 71.5% preferred an internal perspective (a first-person view), 26.3% chose an external perspective (a third-person view), 0.4% selected both perspectives, and 2.3% were unable to choose a perspective. When assessed with the KVIQ kinesthetic subscale, which measures the intensity of sensations, 73.3% of patients with PD preferred an internal perspective, 25.2% preferred an external perspective, 0.3% preferred both perspectives, and 1.4% did not select any perspective.
In the study conducted by Bek et al. (59), no significant differences were observed between the two groups on either the visual or kinesthetic subscales of the KVIQ. Additionally, task-specific ratings of visual and kinesthetic imagery were similar between the groups both before and after MI instructions (see Table 3). Both groups, however, exhibited a significant increase in the use of kinesthetic imagery (PD: Z = 2.73, p = 0.01; control: Z = 3.47, p < 0.001) and visual imagery (PD: Z = 2.45, p = 0.01; control: Z = 3.15, p < 0.001) following MI instructions. The control group also reported enhanced vividness of sensations (Z = 2.14, p = 0.03) and images (Z = 2.35, p = 0.02) after instructions, whereas the PD group exhibited no significant alteration in the vividness of either sensations or images.
3.3.18 Main results for neurofeedback intervention (2 studies: 28 participants)
Tinaz et al. (69) found that the intensity and quality of body sensations evoked during MI and the emotional and motivational context of MI determined the direction (i.e., negative or positive) of the insula-dorsomedial frontal cortex’s functional connectivity. After 10–12 neurofeedback sessions with successful MI strategies, all subjects showed a significant increase in the insula-dorsomedial frontal cortex’s functional connectivity. The MI strategies encompassed movements associated with diverse activities and exercise routines, such as walking, running, lifting weights, and swimming. There was no significant difference in patients with PD between pre-and post-intervention on the MDS-UDPRS-III score. Subramanian et al. (70) demonstrated in a study involving PD patients an early stage of the disease. Out of 10 participants, 5 were in the experimental group (with feedback), and the remaining 5 were in the control group (without feedback). There was a significant improvement of 37% (p = 0.04) in the UPDRS score between pre-and post-intervention in the experimental group, whereas the control group showed no significant difference.
3.3.19 Main results for MI of the whole body (1 study: 22 participants)
Mori et al. (71) measured rCBF in patients with PD and HS while in a standing position. During MI, no significant difference was shown between groups. During ME, patients with PD against HS exhibited a significant increase in the right cerebellar vermis and left paracentral gyrus and a significant decrease in the bilateral middle frontal gyrus.
4 Discussion
Since the 1980s, motor imagery has been used in sport and performance activities and has attracted considerable interest (76). This technique has been adapted to PD patients’ rehabilitation with promising results, despite the limited number of RCT studies published (22–25, 31–38). Among the 53 included studies, there were few RCTs (12 studies) with an average PEDro score of 6.6, which can be considered as medium to high quality. The protocol and outcomes measured were heterogeneous, and there were no RCTs with specific outcomes for upper limbs or speech other than the UPDRS score. The population of RCTs and descriptive studies was relatively young with a low severity level (i.e., H&Y score). In fact, most RCTs excluded patients with scores greater than 3. Therefore, it is not possible to conclude the applicability of MI in patients with PD who have a higher severity. Hence, MI should be used as early as possible before cognitive impairment prevents its use. Taking these aspects into account, the results should be treated with caution, as methodological biases must be resolved before conclusions can be drawn.
In addition to RCTs, we also investigated descriptive and non-RCTs to determine how MI has been used in the PD population. It is also found that patients with PD have similar scores to HS in MI questionnaires (such as KVIQ, MIQ-R, and GIQ), which means that they can practice MI. The presence of cues (visual and auditory) was also found to improve the abilities of patients with PD in MI.
The MI of walking can be employed along a corridor of different lengths, using the time taken for execution as a method of measurement. Walking speed and TUG can be interesting outcomes to be assessed at regular intervals to monitor progress.
Motor symptoms assessed by the UPDRS showed no significant difference between the two groups (intervention vs. control) in the RCTs. However, Part 3 of the UPDRS comprises items for both the upper and lower limbs, and it has been observed that the RCTs were specifically directed toward the lower limbs. As the MI protocol did not encompass all aspects evaluated in the UPDRS, this may explain why there was no change (77).
Even though we did not establish date limits, we were unable to include many studies. Indeed, this is a recent topic of interest, as the initial study included herein was published in 1997, while the initial RCT included in this review dates from 2007. Among the studies that were excluded, there were 21 ongoing clinical trials whose results have not yet been fully published. Additional details regarding these studies are expected to be made available in the near future. This study aimed to guide and facilitate the use of MI in clinical practice, as well as to highlight the main results observed in these studies in terms of improvements in motor symptoms, balance, gait, and quality of life. Indeed, MI is a technique that does not necessitate any equipment, is easy and safe to set up, and merely requires a learning phase beforehand. In a context where the prevalence of PD is increasing, it is important to empower patients and provide them with tools they can use at home to complete other treatments.
The main limitation of this study was the fact that, in descriptive and non-RCT studies, only the main tasks and outcomes of MI were analyzed. Our primary emphasis was on the tasks and outcomes most commonly used in MI-related clinical research. However, there may be other fascinating areas that remain unexplored, such as activities that involve the dual-task paradigm, where motor and cognitive tasks are performed simultaneously. Additionally, a noteworthy limitation of this review is that the most significant studies, particularly RCTs, did not include patients with the most severe forms of PD. Consequently, it remains unclear whether the recommendations provided here apply to individuals with more advanced stages of the disease.
Despite the limited number of RCTs focusing on MI in patients with PD, combined with diverse protocols, outcomes, and potential biases, the findings offer a promising outlook, particularly in addressing walking and balance impairments. However, research into upper limb function or speech remains scarce. Future studies in this field must involve larger cohorts of participants and adopt more precise protocols tailored to the unique challenges posed by upper limb impairments. The criteria for assessing outcomes related to walking and balance align with recommendations from the French National Authority for Health, which provides a valuable standard for evaluating MI interventions in PD.
In conclusion, it is imperative to acknowledge that this scoping review underscores the necessity for further research and revisions in the forthcoming years. The ongoing RCTs registered in clinical trial databases highlight the evolving landscape of MI interventions for PD, suggesting that a comprehensive and updated systematic review will be essential to capture the latest advancements and insights in this field.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
MM: Data curation, Investigation, Methodology, Writing – original draft. ET: Data curation, Investigation, Methodology, Writing – original draft. MB: Writing – review & editing. EM: Writing – review & editing. NG: Funding acquisition, Supervision, Validation, Writing – review & editing. AS: Investigation, Methodology, Writing – original draft. YS: Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the “ANER” program from Région Bourgogne Franche Comté (contract ANER PARK-IMAGE, 2021Y-08279).
Acknowledgments
The authors would like to thank Alexander Hansen for his editorial assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ben-Shlomo, Y, Darweesh, S, Llibre-Guerra, J, Marras, C, San Luciano, M, and Tanner, C. The epidemiology of Parkinson's disease. Lancet. (2024) 403:283–92. doi: 10.1016/S0140-6736(23)01419-8
2. Balestrino, R, and Schapira, AHV. Parkinson disease. Eur J Neurol. (2020) 27:27–42. doi: 10.1111/ene.14108
3. Sveinbjornsdottir, S. The clinical symptoms of Parkinson's disease. J Neurochem. (2016) 139:318–24. doi: 10.1111/jnc.13691
4. Jagadeesan, AJ, Murugesan, R, Vimala Devi, S, Meera, M, Madhumala, G, Vishwanathan Padmaja, M, et al. Current trends in etiology, prognosis and therapeutic aspects of Parkinson's disease: a review. Acta Biomed. (2017) 88:249–62. doi: 10.23750/abm.v88i3.6063
5. Radder, DLM, Silva, L, de Lima, A, Domingos, J, Keus, SHJ, van Nimwegen, M, et al. Physiotherapy in Parkinson's disease: a meta-analysis of present treatment modalities. Neurorehabil Neural Repair. (2020) 34:871–80. doi: 10.1177/1545968320952799
6. Abbruzzese, G, Avanzino, L, Marchese, R, and Pelosin, E. Action observation and motor imagery: innovative cognitive tools in the rehabilitation of Parkinson’s disease. Neurorehabil Neural Repair. (2020) 34:871–80. doi: 10.1155/2015/124214
7. Hanakawa, T. Organizing motor imageries. Neurosci Res. (2016) 104:56–63. doi: 10.1016/j.neures.2015.11.003
8. MacIntyre, TE, Madan, CR, Moran, AP, Collet, C, and Guillot, A. Motor imagery, performance and motor rehabilitation. Prog Brain Res. (2018) 240:141–59. doi: 10.1016/bs.pbr.2018.09.010
9. Jeannerod, M. The representing brain: neural correlates of motor intention and imagery. Behav Brain Sci. (1994) 17:187–202. doi: 10.1017/S0140525X00034026
10. Moustafa, AA, Chakravarthy, S, Phillips, JR, Gupta, A, Keri, S, Polner, B, et al. Motor symptoms in Parkinson's disease: a unified framework. Neurosci Biobehav Rev. (2016) 68:727–40. doi: 10.1016/j.neubiorev.2016.07.010
11. Schapira, AHV, Chaudhuri, KR, and Jenner, P. Non-motor features of Parkinson disease. Nat Rev Neurosci. (2017) 18:435–50. doi: 10.1038/nrn.2017.62
12. Armstrong, MJ, and Okun, MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. (2020) 323:548–60. doi: 10.1001/jama.2019.22360
13. Connolly, BS, and Lang, AE. Pharmacological treatment of Parkinson disease: a review. JAMA. (2014) 311:1670–83. doi: 10.1001/jama.2014.3654
14. Espay, AJ, Morgante, F, Merola, A, Fasano, A, Marsili, L, Fox, SH, et al. Levodopa-induced dyskinesia in Parkinson disease: current and evolving concepts. Ann Neurol. (2018) 84:797–811. doi: 10.1002/ana.25364
15. Chen, K, Tan, Y, Lu, Y, Wu, J, Liu, X, and Zhao, Y. Effect of exercise on quality of life in Parkinson’s disease: a systematic review and meta-analysis. Parkinsons Dis. (2020) 2020:1–10. doi: 10.1155/2020/3257623
16. van der Kolk, NM, and King, LA. Effects of exercise on mobility in people with Parkinson's disease. Mov Disord. (2013) 28:1587–96. doi: 10.1002/mds.25658
17. Tomlinson, CL, Patel, S, Meek, C, Herd, CP, Clarke, CE, Stowe, R, et al. Physiotherapy versus placebo or no intervention in Parkinson's disease. Cochrane Database Syst Rev. (2013) 2013:CD002817. doi: 10.1002/14651858.CD002817.pub4
18. Warner, L, and McNeill, ME. Mental imagery and its potential for physical therapy. Phys Ther. (1988) 68:516–21. doi: 10.1093/ptj/68.4.516
19. Loporto, M, McAllister, C, Williams, J, Hardwick, R, and Holmes, P. Investigating central mechanisms underlying the effects of action observation and imagery through transcranial magnetic stimulation. J Mot Behav. (2011) 43:361–73. doi: 10.1080/00222895.2011.604655
20. Abbruzzese, G, Marchese, R, Avanzino, L, and Pelosin, E. Rehabilitation for Parkinson's disease: current outlook and future challenges. Parkinsonism Relat Disord. (2016) 22:S60–4. doi: 10.1016/j.parkreldis.2015.09.005
21. Mirelman, A, Maidan, I, and Deutsch, JE. Virtual reality and motor imagery: promising tools for assessment and therapy in Parkinson's disease. Mov Disord. (2013) 28:1597–608. doi: 10.1002/mds.25670
22. Sarasso, E, Gardoni, A, Zenere, L, Canu, E, Basaia, S, Pelosin, E, et al. Action observation and motor imagery improve motor imagery abilities in patients with Parkinson's disease – a functional MRI study. Parkinsonism Relat Disord. (2023) 116:105858. doi: 10.1016/j.parkreldis.2023.105858
23. Bezerra, PT, Santiago, LM, Silva, IA, Souza, AA, Pegado, CL, Damascena, CM, et al. Action observation and motor imagery have no effect on balance and freezing of gait in Parkinson's disease: a randomized controlled trial. Eur J Phys Rehabil Med. (2022) 58:715–22. doi: 10.23736/S1973-9087.22.07313-0
24. Kashif, M, Ahmad, A, Bandpei, MAM, Gilani, SA, Hanif, A, and Iram, H. Combined effects of virtual reality techniques and motor imagery on balance, motor function and activities of daily living in patients with Parkinson's disease: a randomized controlled trial. BMC Geriatr. (2022) 22:381. doi: 10.1186/s12877-022-03035-1
25. Tamir, R, Dickstein, R, and Huberman, M. Integration of motor imagery and physical practice in group treatment applied to subjects with Parkinson's disease. Neurorehabil Neural Repair. (2007) 21:68–75. doi: 10.1177/1545968306292608
26. Humphries, S, Holler, J, Crawford, TJ, Herrera, E, and Poliakoff, E. A third-person perspective on co-speech action gestures in Parkinson's disease. Cortex. (2016) 78:44–54. doi: 10.1016/j.cortex.2016.02.009
27. Nascimento, IAPDS, Santiago, LMM, de Souza, AA, Pegado, CL, Ribeiro, TS, and Lindquist, ARR. Effects of motor imagery training of Parkinson's disease: a protocol for a randomized clinical trial. Trials. (2019) 20:626. doi: 10.1186/s13063-019-3694-8
28. Peterson, DS, Pickett, KA, Duncan, RP, Perlmutter, JS, and Earhart, GM. Brain activity during complex imagined gait tasks in Parkinson disease. Clin Neurophysiol. (2014) 125:995–1005. doi: 10.1016/j.clinph.2013.10.008
29. Heremans, E, Nieuwboer, A, Feys, P, Vercruysse, S, Vandenberghe, W, Sharma, N, et al. External cueing improves motor imagery quality in patients with Parkinson disease. Neurorehabil Neural Repair. (2012) 26:27–35. doi: 10.1177/1545968311411055
30. Malouin, F, Jackson, PL, and Richards, CL. Towards the integration of mental practice in rehabilitation programs. A critical review. Front Hum Neurosci. (2013) 7:576. doi: 10.3389/fnhum.2013.00576
31. Braun, S, Beurskens, A, Kleynen, M, Schols, J, and Wade, D. Rehabilitation with mental practice has similar effects on mobility as rehabilitation with relaxation in people with Parkinson's disease: a multicentre randomised trial. J Physiother. (2011) 57:27–34. doi: 10.1016/S1836-9553(11)70004-2
32. Mahmoud, LSED, Abu Shady, NAER, and Hafez, ES. Motor imagery training with augmented cues of motor learning on cognitive functions in patients with parkinsonism. Int J Ther Rehabil. (2018) 25:13–9. doi: 10.12968/ijtr.2018.25.1.13
33. Tinaz, S, Kamel, S, Aravala, SS, Elfil, M, Bayoumi, A, Patel, A, et al. Neurofeedback-guided kinesthetic motor imagery training in Parkinson's disease: randomized trial. Neuroimage Clin. (2022) 34:102980. doi: 10.1016/j.nicl.2022.102980
34. Santiago, LM, de Oliveira, DA, de Macêdo Ferreira, LG, de Brito Pinto, HY, Spaniol, AP, de Lucena Trigueiro, LC, et al. Immediate effects of adding mental practice to physical practice on the gait of individuals with Parkinson's disease: randomized clinical trial. NeuroRehabilitation. (2015) 37:263–71. doi: 10.3233/NRE-151259
35. Fayez, E, and Elwishi, A. Effect of locomotor imagery training added to physical therapy program on gait performance in Parkinson patients: a randomized controlled study. Egypt J Neurol Psychiat Neurosurg. (2013) 50:31–7.
36. Monteiro, D, da Silva, LP, de Sá, PO, de Oliveira, AR, Coriolano, MWS, and Lins, OG. Mental practice after physiotherapy maintains functional mobility of people with Parkinson’s disease. Fisioter Pesqui. (2018) 25:65–73. doi: 10.1590/1809-2950/17192425012018
37. Subramanian, L, Morris, MB, Brosnan, M, Turner, DL, Morris, HR, and Linden, DE. Functional magnetic resonance imaging neurofeedback-guided motor imagery training and motor training for Parkinson's disease: randomized trial. Front Behav Neurosci. (2016) 10:111. doi: 10.3389/fnbeh.2016.00111
38. Sarasso, E, Agosta, F, Piramide, N, Gardoni, A, Canu, E, Leocadi, M, et al. Action observation and motor imagery improve dual task in Parkinson's disease: a clinical/fMRI study. Mov Disord. (2021) 36:2569–82. doi: 10.1002/mds.28717
39. Yágüez, L, Canavan, AG, Lange, HW, and Hömberg, V. Motor learning by imagery is differentially affected in Parkinson's and Huntington's diseases. Behav Brain Res. (1999) 102:115–27. doi: 10.1016/s0166-4328(99)00005-4
40. Sabaté, M, González, B, and Rodríguez, M. Adapting movement planning to motor impairments: the motor-scanning system. Neuropsychologia. (2007) 45:378–86. doi: 10.1016/j.neuropsychologia.2006.06.025
41. Kobelt, M, Wirth, B, and Schuster-Amft, C. Muscle activation during grasping with and without motor imagery in healthy volunteers and patients after stroke or with Parkinson's disease. Front Psychol. (2018) 9:597. doi: 10.3389/fpsyg.2018.00597
42. Gäumann, S, Gerber, RS, Suica, Z, Wandel, J, and Schuster-Amft, C. A different point of view: the evaluation of motor imagery perspectives in patients with sensorimotor impairments in a longitudinal study. BMC Neurol. (2021) 21:297. doi: 10.1186/s12883-021-02266-w
43. Cohen, RG, Chao, A, Nutt, JG, and Horak, FB. Freezing of gait is associated with a mismatch between motor imagery and motor execution in narrow doorways, not with failure to judge doorway passability. Neuropsychologia. (2011) 49:3981–8. doi: 10.1016/j.neuropsychologia.2011.10.014
44. Ehgoetz Martens, KA, Ellard, CG, and Almeida, QJ. A closer look at mechanisms underlying perceptual differences in Parkinson's freezers and non-freezers. Neuroscience. (2014) 274:162–9. doi: 10.1016/j.neuroscience.2014.05.022
45. Maillet, A, Thobois, S, Fraix, V, Redouté, J, Le Bars, D, Lavenne, F, et al. Neural substrates of levodopa-responsive gait disorders and freezing in advanced Parkinson's disease: a kinesthetic imagery approach. Hum Brain Mapp. (2015) 36:959–80. doi: 10.1002/hbm.22679
46. Snijders, AH, Leunissen, I, Bakker, M, Overeem, S, Helmich, RC, Bloem, BR, et al. Gait-related cerebral alterations in patients with Parkinson's disease with freezing of gait. Brain. (2011) 134:59–72. doi: 10.1093/brain/awq324
47. Huang, HC, Chen, CM, Lu, MK, Liu, BL, Li, CI, Chen, JC, et al. Gait-related brain activation during motor imagery of complex and simple ambulation in Parkinson's disease with freezing of gait. Front Aging Neurosci. (2021) 13:731332. doi: 10.3389/fnagi.2021.731332
48. Maidan, I, Rosenberg-Katz, K, Jacob, Y, Giladi, N, Deutsch, JE, Hausdorff, JM, et al. Altered brain activation in complex walking conditions in patients with Parkinson's disease. Parkinsonism Relat Disord. (2016) 25:91–6. doi: 10.1016/j.parkreldis.2016.01.025
49. Weiss, PH, Herzog, J, Pötter-Nerger, M, Falk, D, Herzog, H, Deuschl, G, et al. Subthalamic nucleus stimulation improves parkinsonian gait via brainstem locomotor centers. Mov Disord. (2015) 30:1121–5. doi: 10.1002/mds.26229
50. Dominey, P, Decety, J, Broussolle, E, Chazot, G, and Jeannerod, M. Motor imagery of a lateralized sequential task is asymmetrically slowed in hemi-Parkinson's patients. Neuropsychologia. (1995) 33:727–41. doi: 10.1016/0028-3932(95)00008-q
51. Avanzino, L, Pelosin, E, Martino, D, and Abbruzzese, G. Motor timing deficits in sequential movements in Parkinson disease are related to action planning: a motor imagery study. PLoS One. (2013) 8:e75454. doi: 10.1371/journal.pone.0075454
52. Cunnington, R, Egan, GF, O'Sullivan, JD, Hughes, AJ, Bradshaw, JL, and Colebatch, JG. Motor imagery in Parkinson's disease: a PET study. Mov Disord. (2001) 16:849–57. doi: 10.1002/mds.1181
53. Leiguarda, R, Cerquetti, D, Tenca, E, and Merello, M. Globus pallidus internus firing rate modification after motor-imagination in three Parkinson's disease patients. J Neural Transm (Vienna). (2009) 116:451–5. doi: 10.1007/s00702-009-0203-3
54. Fischer, P, Pogosyan, A, Cheeran, B, Green, AL, Aziz, TZ, Hyam, J, et al. Subthalamic nucleus beta and gamma activity is modulated depending on the level of imagined grip force. Exp Neurol. (2017) 293:53–61. doi: 10.1016/j.expneurol.2017.03.015
55. Thobois, S, Dominey, PF, Decety, J, Pollak, PP, Gregoire, MC, Le Bars, PD, et al. Motor imagery in normal subjects and in asymmetrical Parkinson's disease: a PET study. Neurology. (2000) 55:996–1002. doi: 10.1212/wnl.55.7.996
56. Thobois, S, Dominey, P, Fraix, V, Mertens, P, Guenot, M, Zimmer, L, et al. Effects of subthalamic nucleus stimulation on actual and imagined movement in Parkinson's disease: a PET study. J Neurol. (2002) 249:1689–98. doi: 10.1007/s00415-002-0906-y
57. Samuel, M, Ceballos-Baumann, AO, Boecker, H, and Brooks, DJ. Motor imagery in normal subjects and Parkinson's disease patients: an H215O PET study. Neuroreport. (2001) 12:821–8. doi: 10.1097/00001756-200103260-00040
58. Sabaté, M, Llanos, C, and Rodríguez, M. Integration of auditory and kinesthetic information in motion: alterations in Parkinson's disease. Neuropsychology. (2008) 22:462–8. doi: 10.1037/0894-4105.22.4.462
59. Bek, J, Gowen, E, Vogt, S, Crawford, TJ, and Poliakoff, E. Combined action observation and motor imagery influences hand movement amplitude in Parkinson's disease. Parkinsonism Relat Disord. (2019) 61:126–31. doi: 10.1016/j.parkreldis.2018.11.001
60. Gündüz, A, and Kiziltan, ME. F-wave and motor-evoked potentials during motor imagery and observation in apraxia of Parkinson disease. Muscle Nerve. (2015) 52:1072–7. doi: 10.1002/mus.24663
61. Tremblay, F, Léonard, G, and Tremblay, L. Corticomotor facilitation associated with observation and imagery of hand actions is impaired in Parkinson's disease. Exp Brain Res. (2008) 185:249–57. doi: 10.1007/s00221-007-1150-6
62. Cunnington, R, Iansek, R, Johnson, KA, and Bradshaw, JL. Movement-related potentials in Parkinson's disease motor imagery and movement preparation. Brain. (1997) 120:1339–53. doi: 10.1093/brain/120.8.1339
63. Kühn, AA, Doyle, L, Pogosyan, A, Yarrow, K, Kupsch, A, Schneider, GH, et al. Modulation of beta oscillations in the subthalamic area during motor imagery in Parkinson's disease. Brain. (2006) 129:695–706. doi: 10.1093/brain/awh715
64. Péran, P, Nemmi, F, Méligne, D, Cardebat, D, Peppe, A, Rascol, O, et al. Effect of levodopa on both verbal and motor representations of action in Parkinson's disease: a fMRI study. Brain Lang. (2013) 125:324–9. doi: 10.1016/j.bandl.2012.06.001
65. van Nuenen, BF, Helmich, RC, Buenen, N, van de Warrenburg, BP, Bloem, BR, and Toni, I. Compensatory activity in the extrastriate body area of Parkinson's disease patients. J Neurosci. (2012) 32:9546–53. doi: 10.1523/JNEUROSCI.0335-12.2012
66. Helmich, RC, de Lange, FP, Bloem, BR, and Toni, I. Cerebral compensation during motor imagery in Parkinson's disease. Neuropsychologia. (2007) 45:2201–15. doi: 10.1016/j.neuropsychologia.2007.02.024
67. Helmich, RC, Bloem, BR, and Toni, I. Motor imagery evokes increased somatosensory activity in Parkinson's disease patients with tremor. Hum Brain Mapp. (2012) 33:1763–79. doi: 10.1002/hbm.21318
68. Heremans, E, Feys, P, Nieuwboer, A, Vercruysse, S, Vandenberghe, W, Sharma, N, et al. Motor imagery ability in patients with early-and mid-stage Parkinson disease. Neurorehabil Neural Repair. (2011) 25:168–77. doi: 10.1177/1545968310370750
69. Tinaz, S, Para, K, Vives-Rodriguez, A, Martinez-Kaigi, V, Nalamada, K, Sezgin, M, et al. Insula as the Interface between body awareness and movement: a neurofeedback-guided kinesthetic motor imagery study in Parkinson's disease. Front Hum Neurosci. (2018) 12:496. doi: 10.3389/fnhum.2018.00496
70. Subramanian, L, Hindle, JV, Johnston, S, Roberts, MV, Husain, M, Goebel, R, et al. Real-time functional magnetic resonance imaging neurofeedback for treatment of Parkinson's disease. J Neurosci. (2011) 31:16309–17. doi: 10.1523/JNEUROSCI.3498-11.2011
71. Mori, Y, Yoshikawa, E, Futatsubashi, M, and Ouchi, Y. Neural correlates of standing imagery and execution in parkinsonian patients: the relevance to striatal dopamine dysfunction. PLoS One. (2020) 15:e0240998. doi: 10.1371/journal.pone.0240998
72. Amick, MM, Schendan, HE, Ganis, G, and Cronin-Golomb, A. Frontostriatal circuits are necessary for visuomotor transformation: mental rotation in Parkinson's disease. Neuropsychologia. (2006) 44:339–49. doi: 10.1016/j.neuropsychologia.2005.06.002
73. Conson, M, Trojano, L, Vitale, C, Mazzarella, E, Allocca, R, Barone, P, et al. The role of embodied simulation in mental transformation of whole-body images: evidence from Parkinson's disease. Hum Mov Sci. (2014) 33:343–53. doi: 10.1016/j.humov.2013.10.006
74. Scarpina, F, Magnani, FG, Tagini, S, Priano, L, Mauro, A, and Sedda, A. Mental representation of the body in action in Parkinson's disease. Exp Brain Res. (2019) 237:2505–21. doi: 10.1007/s00221-019-05608-w
75. Peterson, DS, Pickett, KA, and Earhart, GM. Effects of levodopa on vividness of motor imagery in Parkinson disease. J Parkinsons Dis. (2012) 2:127–33. doi: 10.3233/JPD-2012-12077
76. Feltz, DL, and Landers, DM. The effects of mental practice on motor skill learning and performance: a meta-analysis. J Sport Psychol. (1983) 5:25–57. doi: 10.1123/jsp.5.1.25
77. Goetz, CG, Tilley, BC, Shaftman, SR, Stebbins, GT, Fahn, S, Martinez-Martin, P, et al. Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. (2008) 23:2129–70. doi: 10.1002/mds.22340
Keywords: Parkinson’s disease, motor imagery therapy, mental practice, neurorehabilitation, rehabilitation
Citation: Michel M, Terragno E, Bereau M, Magnin E, Gueugneau N, Soares AV and Sagawa Y (2024) Exploring motor imagery as a therapeutic intervention for Parkinson’s disease patients: a scoping review. Front. Neurol. 15:1422672. doi: 10.3389/fneur.2024.1422672
Edited by:
Simona Bonavita, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Giorgio Maggioni, Sant’Isidoro Hospital Ferb Onlus Trescore Balneario, ItalyJudith Bek, University College Dublin, Ireland
Copyright © 2024 Michel, Terragno, Bereau, Magnin, Gueugneau, Soares and Sagawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicolas Gueugneau, bmljb2xhcy5ndWV1Z25lYXVAdW5pdi1mY29tdGUuZnI=
 Maxime Michel1
Maxime Michel1 Elena Terragno
Elena Terragno Matthieu Bereau
Matthieu Bereau Eloi Magnin
Eloi Magnin Nicolas Gueugneau
Nicolas Gueugneau Antonio Vinicius Soares
Antonio Vinicius Soares