- 1Department of Encephalopathy, the First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, China
- 2The First Clinical Medical College of Henan University of Traditional Chinese Medicine, Zhengzhou, China
Background: Thromboelastography (TEG) can objectively reflect the formation, development and rupture process of thrombosis in patients, but there are limited data on whether TEG can be used as a predictive tool for recurrence in patients with acute ischemic stroke.
Objective: To explore the TEG risk of recurrence in patients with acute ischemic stroke predictive value.
Methods: A total of 441 patients with acute ischemic stroke who met the research criteria in the First Affiliated Hospital of Henan University of Traditional Chinese Medicine from January 2020 to December 2021 were selected as the research objects. TEG was measured in all patients, and the main parameters of TEG (R value, indicating coagulation reaction time; K value and Angle, the rate of blood clot formation; MA value, indicating the maximum amplitude). The primary outcome of this study was ischemic stroke recurrence. Recurrent events included cerebral infarction, cerebral hemorrhage, TIA, and were determined by combining imaging events and clinical events. Logistic regression analysis was used to explore the influencing factors of recurrence in patients with acute ischemic stroke.
Results: Fifty-six patients (12.7%) had recurrence. Multivariate Logistic regression analysis showed that: Age [OR = 1.078, 95%CI(1.024, 1.135)], triglyceride [OR = 1.541, 95%CI(1.033, 2.298)], glycosylated hemoglobin [OR = 1.401, 95%CI(1.097, 1.790)], history of hypertension [OR = 16.046, p < 0.05], 95%CI(4.726, 54.489), R value [OR = 0.533, 95%CI(0.351, 0.809)], MA value [OR = 1.399, 95%CI(1.004, 1.949)] were independent influencing factors for hemorrhagic transformation in patients with acute ischemic stroke.
Conclusion: TEG has some value in predicting recurrence in patients with acute ischemic stroke, and the MA value in TEG [AUC = 0.806 (95%CI:0.747–0.867), with a sensitivity of 78.6% and a specificity of 70.4%], predicted the most significant efficiency of AIS recurrence.
1 Introduction
Acute ischemic stroke (AIS) is one of the most common cerebrovascular diseases worldwide, and it is also one of the most important causes of disability and death worldwide, which has caused a significant burden on individual health, family and society (1). According to the World Health Organization, about 15 million people suffer from stroke every year, about one third of them will die, and another third will suffer severe disability (2). AIS has a high recurrence rate, and studies have shown that about a quarter of patients experience recurrence within 5 years after initial stroke (3). Despite significant progress in the acute management and preventive measures of AIS in recent years, the risk of AIS recurrence remains high, and the mortality and disability rates after recurrence are significantly increased. Therefore, effective prediction of the recurrence of AIS is essential to implement preventive measures and improve patient outcomes.
Numerous scientific studies have established that the recurrence of ischemic stroke is associated with risk factors such as age, gender, hypertension, diabetes, coronary heart disease, family history, unhealthy lifestyle habits, serum protein levels, total cholesterol, high-density lipoprotein cholesterol, and coagulation function (4–6). These studies have formed a solid foundation for predicting the risk of recurrent ischemic stroke. Currently in clinical practice, various scales are primarily used to predict the risk of recurrent ischemic stroke; examples include the Essen scale (7) and SP-IIscale (8). Despite achieving some degree of effectiveness in predicting the risk of recurrent ischemic stroke based on current domestic and international research status using these scales and predictive factors adopted thus far; most variables utilized in these studies are predominantly traditional indicators such as personal history and past records. As a result their predictive accuracy is relatively low and they are unable to explain or verify potential relationships between variables. In recent years with advancements in medical technology however thromboelastography (TEG) has seen increasing clinical application across multiple fields including surgery, liver disease cardiovascular disease, and trauma. This demonstrates its potential value in predicting and managing patients’ coagulation function (9). The unique feature of TEG lies in its ability to comprehensively assess a patient’s coagulation status including parameters such as coagulation time, clot formation speed, clot strength, and fibrinolysis etc. (10), which holds particular significance for stroke patients given that their coagulation function is related to recurrence risks (11). Traditional coagulation indicators like PT, TI, and APTT fail to effectively reflect overall clotting function or thrombus strength information. Moreover they are easily influenced by anticoagulant drugs thereby limiting their predictive value for thrombus formation and prognosis after strokes (12). Despite demonstrating potential applications within these areas, TGE’s role efficacy remains insufficiently researched when it comes to predicting AIS recurrences.
Therefore, the aim of this study was to explore the potential value and application of TEG in predicting AIS recurrence. By detecting TEG in AIS patients and tracking their recurrence, we hope to identify the TEG parameters associated with the risk of recurrence, and then provide more personalized risk assessment and prevention strategies for AIS patients.
2 Materials and methods
2.1 General data
The case data in this study were derived from 441 AIS patients who were treated in the First Affiliated Hospital of Henan University of Traditional Chinese Medicine from January 2020 to December 2021. All the enrolled patients had undergone TEG measurement. Inclusion criteria: ① Conform to the guidelines of diagnosis and treatment of acute ischemic stroke in China 2018 (13) midbrain stroke related diagnostic criteria; ② Diagnosis of acute ischemic stroke according to medical history and CT/MRI; ③ no hepatitis B, hepatitis C, AIDS, syphilis and other infectious diseases; ④ age ≥ 18 years old; Patients or their legal guardians provided written informed consent. Exclusion criteria: ① Transient ischemic attack (TIA), hemorrhagic stroke and mixed stroke diagnosed by western medicine; ② Ischemic stroke diagnosed by western medicine but with disturbance of consciousness (including coma, lethargy, drowsiness, confusion and delirium), TCM diagnosis belongs to the viscera of stroke; ③ Severe liver and kidney failure, malignant tumor, gastrointestinal bleeding; ④ unable to cooperate with clinical data collection; ⑤ pregnant and lactating women; ⑥ patients with serious missing clinical data (data missing rate > 20%). TEG and related examinations were performed after admission, and the patients were followed up by outpatient examination, telephone and other methods for 1 year. Patients with recurrence were enrolled as observation group, and patients without recurrence were enrolled as control group. The follow-up time was 1 year. The study was approved by the ethics committee of the investigator’s hospital (approval number: 2022HL-088-01).
2.2 Methods
This study was a prospective clinical registry study. According to literature research and expert consultation in the early stage of the project, we developed a clinical data collection table. The relevant data of the selected patients included: General demographic data (age, gender), personal history (continuous smoking history, continuous drinking history, exercise), condition at onset (BMI index, systolic blood pressure at admission, diastolic blood pressure at admission), past history (TIA, hypertension, diabetes, coronary heart disease, hyperlipidemia, hyperhomocysteinemia), laboratory biochemical indicators (red blood cell count, white blood cell count) number, platelet count, hemoglobin concentration, total cholesterol, triglyceride, low density lipoprotein, high density lipoprotein, apolipoprotein A1, apolipoprotein B, plasma prothrombin time, fibrinogen content, activated partial thromboplastin time, thrombin time measurement, glycosylated hemoglobin, creatinine, uric acid, homocysteine, TOAST classification (large artery porgrage) Arteriosclerosis type, arteriole type (including lacunar), cardiac origin, other causes), related scales (mRS Total score, NIHSS total score).
After AIS patients were admitted to hospital, the median cubital vein blood was taken by the nurses in the Department of encephalopathy, placed in the sodium citrate anticoagulant tube, and sent to the test within 2 h. The test was performed by the laboratory physician in the thrombelastography laboratory of our hospital. The test process is fully automated. The specific parameters of TEG in patients: reaction time (R value), coagulation time (K value), coagulation Angle (Angle), maximum amplitude of thrombus (MA value) level.
The general demographic data, personal history, conditions at onset, past history, laboratory biochemical indexes, TOAST classification, NIHSS scale score, mRS Scale score and main parameters of TEG were compared between patients with and without recurrence after stroke, and the influencing factors of recurrence in patients with acute ischemic stroke were analyzed.
2.3 Study variables
2.3.1 Outcome indicators
The outcome indicators in this study included primary outcome indicators and secondary outcome indicators. The primary outcome was recurrent events during the ischemic stroke follow-up period, and the secondary outcome was neurological deterioration.
2.3.1.1 Primary outcome measure
Recurrent events include cerebral infarction, cerebral hemorrhage, and TIA (14, 15). The determination of recurrent events should be based on reliable imaging indicators and doctors’ clinical evaluation, combined with imaging events and clinical events.
2.3.1.2 Secondary outcome measure
Neurological function deterioration was demonstrated by experts according to CHANCE criteria (16).
2.3.2 Time of measurement
The study used three times of on-site follow-up and five times of telephone. On-site follow-up was required for each patient at 12, 24, and 48 weeks after enrollment, and telephone follow-up at 8, 20, 28, 36, and 44 weeks and at the end of follow-up. Predictors of relapse were measured and recorded at baseline enrollment and on-site follow-up. Outcomes are captured and recorded during follow-up and need to be supported by objective and valid clinical evidence (imaging reports or medical records).
2.3.3 Outcome evaluation
The end event evaluation expert group is composed of one group leader and four members, a total of five people. The evaluation method is as follows: The detailed clinical records and image data of recurrent ischemic stroke events are sorted out, and the information of the patient and the receiving doctor (including the information reflected in the image examination and physical and chemical examination) is hidden, and the final judgment and evaluation is made by the expert group of the end event.
2.4 Statistical methods
In the study, SPSS 25.0 statistical software was used for univariate analysis of general data, including t test or Mann–Whitney U test for quantitative data and two test for qualitative data. In this study, p < 0.05 was taken as the criterion to indicate a statistically significant difference.
3 Results
3.1 General situation
The results of this study showed that a total of 441 AIS patients were included. According to the recurrence within 1 year after discharge, the subjects were divided into two groups, including 56 cases in the recurrence group and 385 cases in the non-recurrence group, with a recurrence rate of 13.7%. The oldest patient was 88 years old and the youngest was 33 years old, with an average age of 63.97. There were 263 males (59.6%) and 178 females (40.3%).
3.2 Comparison of baseline data (general demographic data, past history, laboratory biochemical indexes, TOAST classification, exercise or not, mRS Scale score, NIHSS scale score) between recurrence and non-recurrence patients
The age, BMI index, history of hypertension and hyperhomocysteinemia in recurrent AIS patients were higher than those in non-recurrent AIS patients. The proportion of male patients with recurrence was higher than that of female patients. The proportion of non-recurrence in patients who adhered to exercise was higher than that in recurrent AIS patients. The proportion of recurrent patients with small arterial occlusive according to TOAST classification was higher than that of non-recurrent patients; The results showed that age, BMI index, history of hypertension, history of hyperhomocysteinemia, exercise, triglyceride, activated partial thromboplastin time, glycated hemoglobin A1c, TOAST classification of small artery occlusion were the risk factors of recurrence in AIS patients (p < 0.05).If AIS patients relapse factors: Gender, systolic blood pressure at onset, diastolic blood pressure at onset, TIA history, hyperlipidemia history, coronary heart disease history, continuous smoking history, continuous drinking history, NIHSS score, mRS Score, red blood cell count, white blood cell count, platelet count, hemoglobin concentration, total cholesterol, low density lipoprotein, high density lipoprotein, apolipoprotein A1, apolipoprotein B, plasma Prothrombin time, thrombin time, fibrinogen content determination, creatinine, uric acid, homocysteine, TOAST classification for the proportion of cardiac and other reasons, there was no statistically significant difference(p > 0.05), as presented in Table 1.
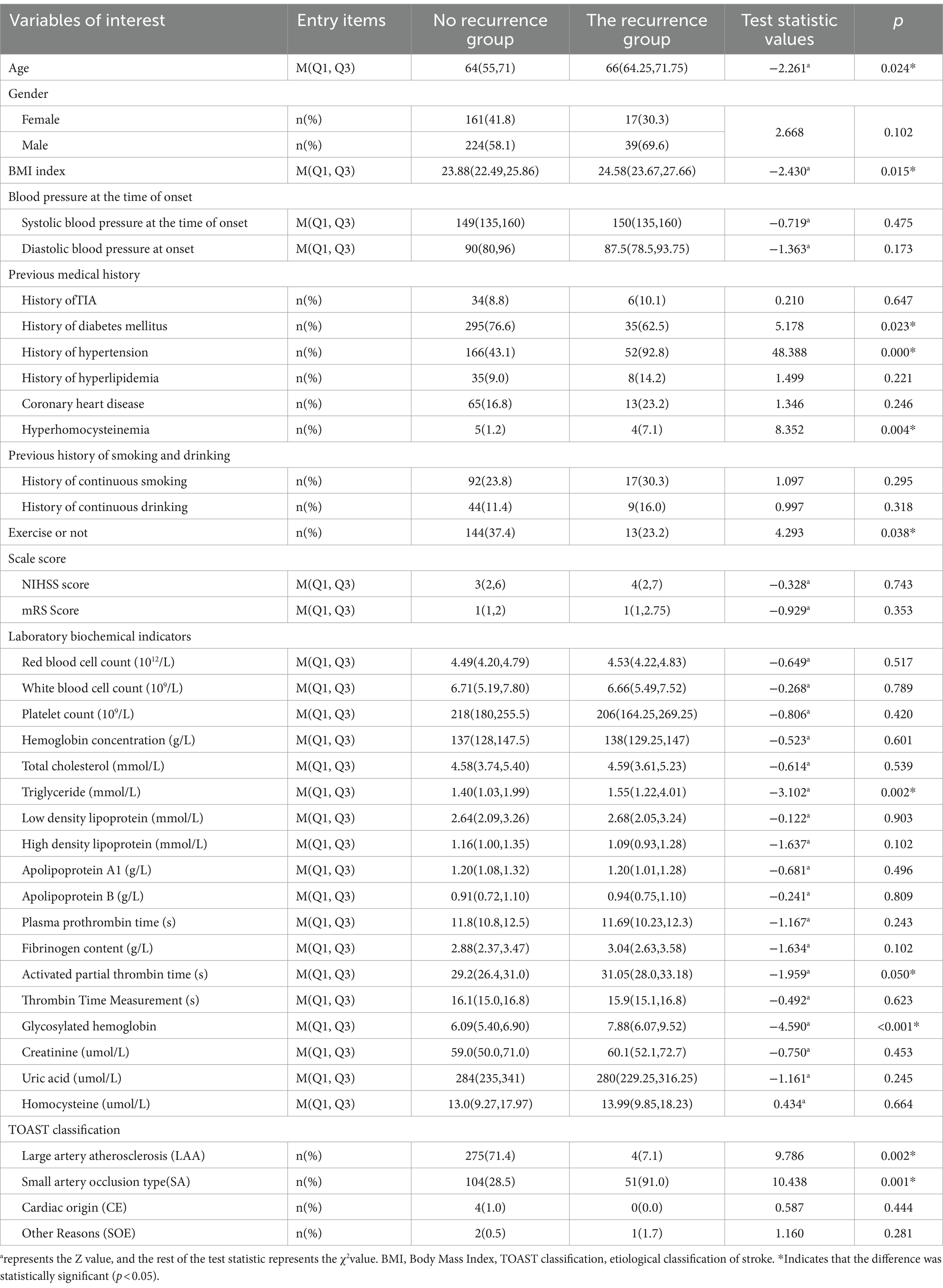
Table 1. The baseline data (general demographic data, past history, laboratory biochemical indexes, TOAST classification, exercise or not, mRS Scale score, NIHSS scale score) of patients with recurrence and without recurrence were compared.
3.3 Comparison of TEG parameters between patients with and without recurrence
The R value of recurrent AIS patients was shorter than that of non-recurrent AIS patients, and the Angle Angle and MA value of recurrent AIS patients were higher than those of non-recurrent AIS patients, and the differences were statistically significant (p < 0.05). There was no significant difference in K value between recurrent and non-recurrent AIS patients (p > 0.05), as shown in Table 2.
3.4 Univariate and multivariate logistic analysis of influencing factors of recurrence in AIS patients
With the presence or absence of AIS recurrence as the dependent variable (assignment: Yes =1, no =0) The variables with statistical differences in Tables 1, 2 were independent variables, and univariate Logistic regression analysis was performed. The results showed that age, triglyceride, glycosylated hemoglobin, history of diabetes, history of hypertension, history of hyperhomocysteinemia, exercise, TOAST classification of large artery atherosclerosis type and small artery occlusion type, R value, Angle Angle, MA value may be the influencing factors of AIS recurrence (p < 0.05), see Table 3.
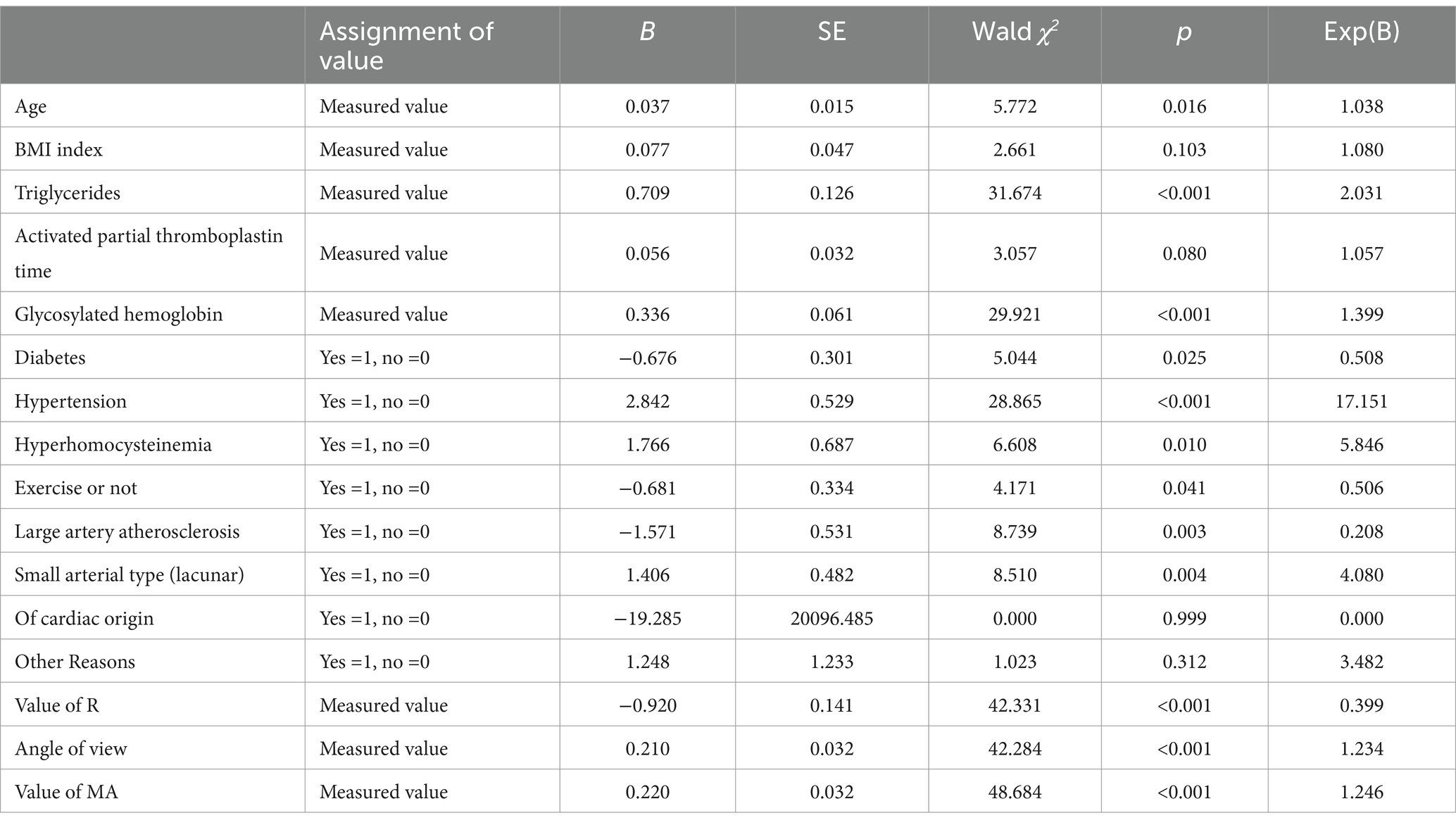
Table 3. Univariate logistic regression analysis of the influencing factors of recurrence in AIS patients.
The recurrence of AIS patients was taken as the dependent variable, and the variables with statistical differences in the univariate Logistic regression analysis were taken as independent variables to conduct multivariate Logistic regression analysis.The results showed that age, triglyceride, glycosylated hemoglobin, hypertension history, R value and MA value were independent risk factors for relapse in AIS patients (p < 0.05), as shown in Table 4.
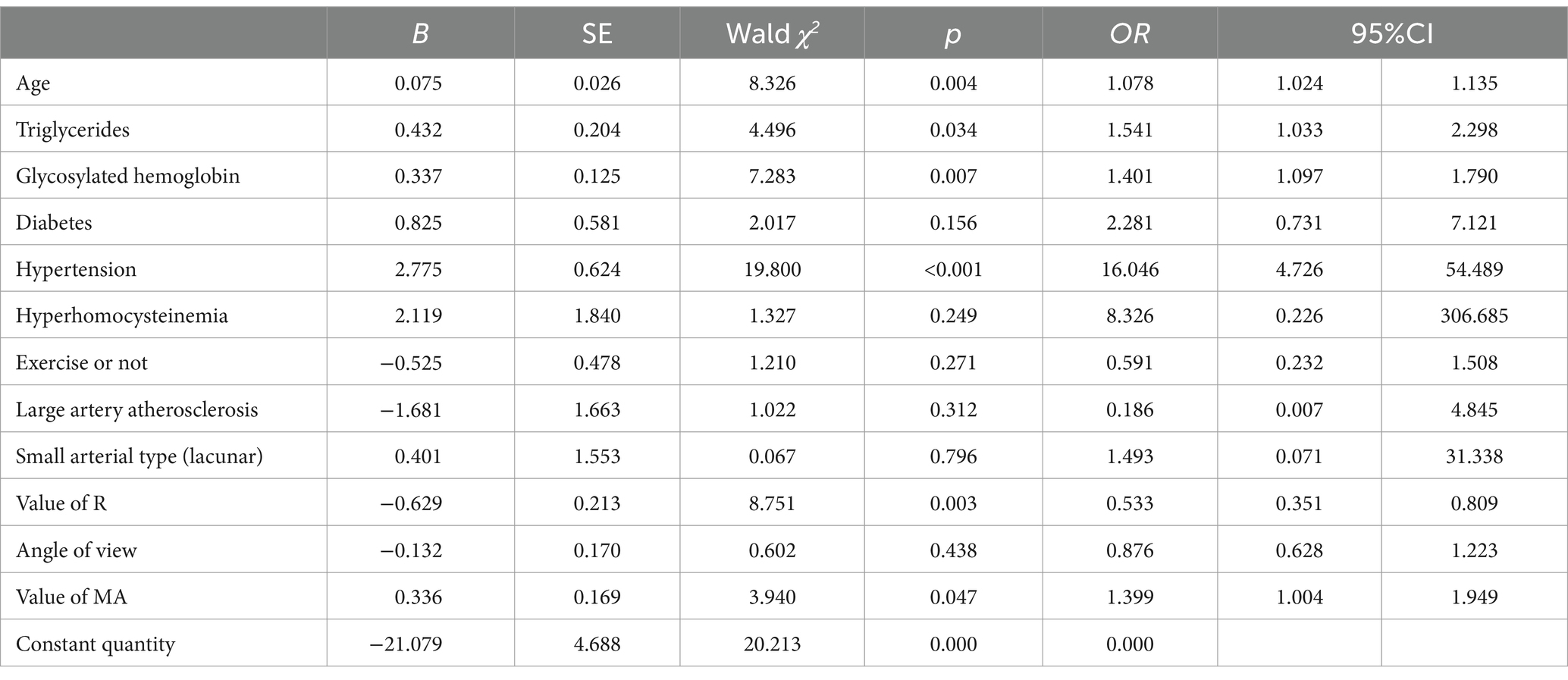
Table 4. Multivariate logistic regression analysis of factors influencing recurrence in AIS patients.
3.5 Evaluation of the clinical validity of the predictors
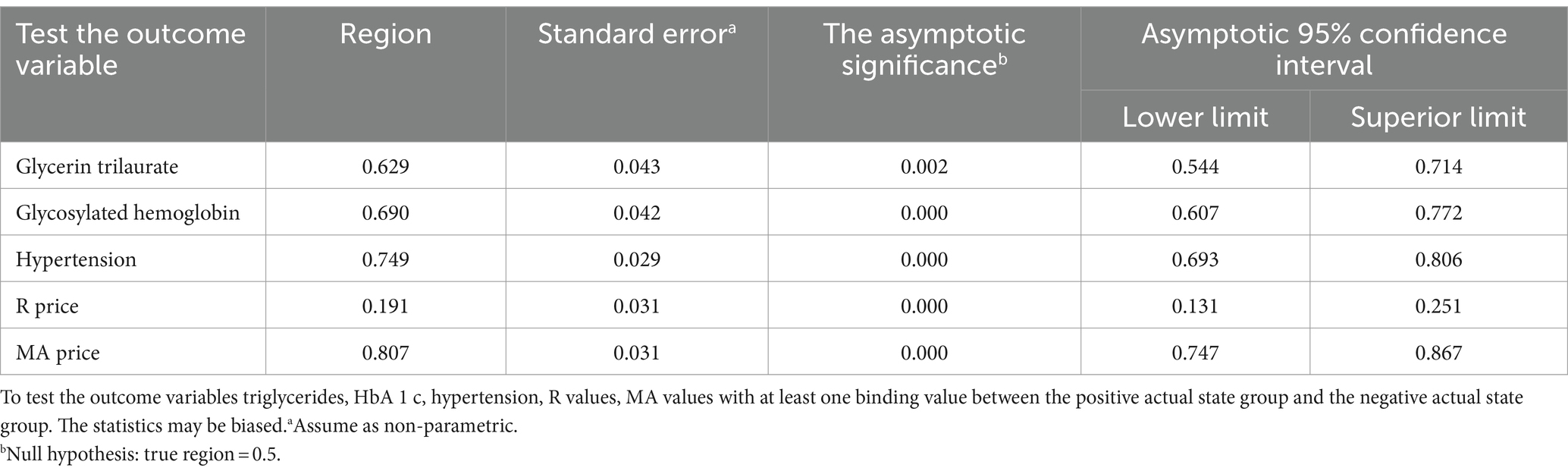
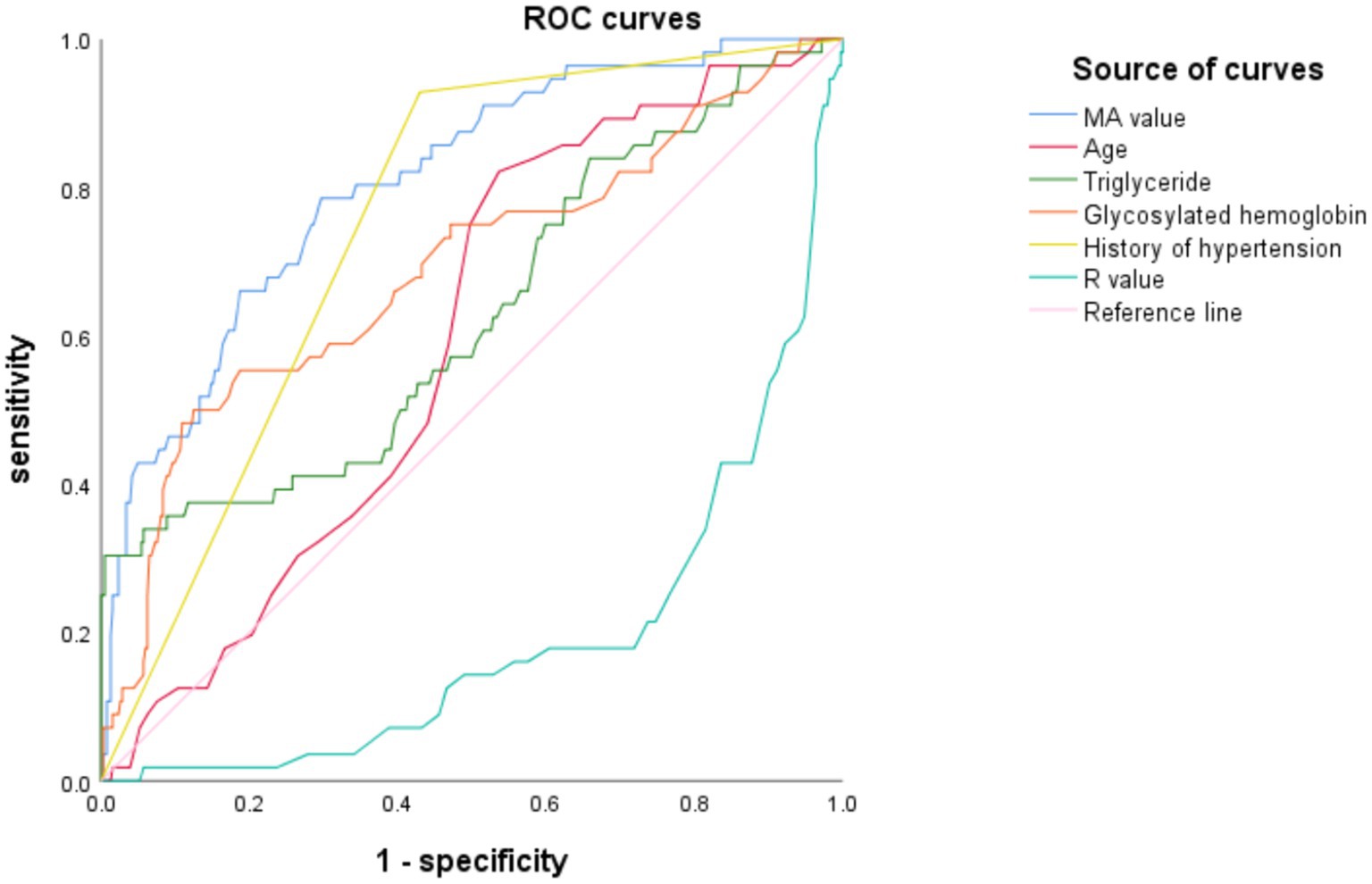
The receiver operating characteristic (ROC) curves were used to evaluate the independent risk factors of AIS recurrence as follows: Based on the multivariate Logistic regression analysis, the ROC curve analysis results of age, triglycerides, HbA1c, hypertension history, R value, and MA value showed p < 0.05, indicating that the above 5 independent risk factors have a significant ability to predict AIS recurrence. The AUC of MA value was 0.806 (95%CI: 0.747–0.867), the AUC of hypertension history was 0.749 (95%CI: 0.693–0.806), and the AUC of HbA1c was 0.690 (95%CI, 0.607–0.772) were the top three, indicating that the above three independent risk factors have a more significant ability to predict AIS recurrence. The results are shown in Table 5 and Figure 1.
The detection results show that the MA value has the most significant ability to predict AIS recurrence, and the critical value for predicting AIS with the MA value is 61.25, with a sensitivity of 78.6% and a specificity of 70.4%.
4 Discussion
Acute ischemic stroke (AIS) is a common neurological disease, and its etiology is related to the formation of atherosclerotic plaques, hemodynamic abnormalities, and coagulation/anticoagulation disorders. Its recurrence rate is relatively high, which seriously affects the quality of life and prognosis of patients (17). Abnormal coagulation function detected by conventional coagulation tests is associated with an increased risk of recurrent stroke (18). MAINO et al. found that increased thrombophilia was significantly associated with the risk of recurrent ischemic stroke (19). TEG can continuously, dynamically and quantitatively record the process of blood clot formation, comprehensively reflect the interaction of coagulation factors, platelets and FIB, and has important value in predicting bleeding tendency and thrombosis in patients (20). Studies have shown that TEG parameters are closely related to the coagulation status of patients with ischemic stroke in the acute phase, and may be associated with long-term prognosis (21).
Our findings are consistent with previous studies, which showed that specific TEG parameters, especially the maximum thrombus intensity (MA), were independent risk factors for AIS recurrence. MA mainly evaluates when blood clots reach their maximum intensity, which increases hypercoagulability and reduces platelet dysfunction, thrombocytopenia, or hypofibrinogenemia (22). Through TEG testing in 44 patients with acute ischemic stroke treated with thrombolysis, some scholars found that MA was associated with poor clinical score, that is, the lower the MA score with the progress of anticoagulation therapy, the better the clinical prognosis of patients (23).The positive association of MA and the risk of cardiovascular and cerebrovascular events was also reported in the study by Shi et al. (24). While our study strengthened the clinical relevance of this finding with a larger sample size and a longer follow-up period, further confirming the effectiveness of using TEG as a more dynamic and comprehensive assessment tool. Furthermore, the use of TEG may also help identify high-risk patient populations that are not detectable by conventional coagulation tests, providing a basis for personalized preventive treatment. For example, for patients with high thrombosis tendency indicated by TEG markers, physicians may choose a more aggressive anticoagulation strategy. This is in line with the study by Paciaroni et al., who found that a personalized anticoagulation regimen was effective in reducing the risk of recurrence in stroke patients (25).
However, there are some limitations to our study. First, as an observational study, we could not identify a direct link between TEG parameters and stroke recurrence from causality. Second, despite our relatively large sample size, the study remained limited to a single center, possibly limiting the general applicability of the results. Future studies will need to be conducted in multiple centers to validate our findings.
In conclusion, this study confirmed the potential value of TEG parameters, especially MA values, in predicting the risk of recurrent ischemic stroke. These findings provide a scientific basis for the clinical use of TEG as a risk assessment and management tool for patients with ischemic stroke. In the future, on the basis of previous studies, relevant imaging omics data and biomarker information of AIS patients should be further collected, and more studies on ischemic lesions should be conducted, such as studies on the identification of ischemic penumbral band in AIS patients by OEF map generated by quantitative magnetic resonance susceptibility mapping (QSM) (26). Further explore the application of TEG in different stroke subtypes, while considering individual patient differences, and build a recurrence risk assessment model based on machine learning to reduce the recurrence risk of ischemic stroke patients, and ultimately improve the prognosis and quality of life of patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Henan University of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RG: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. XS: Conceptualization, Supervision, Validation, Writing – review & editing. JL: Data curation, Writing – original draft. JZ: Data curation, Investigation, Writing – original draft. SH: Data curation, Investigation, Writing – original draft. RL: Supervision, Writing – review & editing. YX: Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the national natural science foundation of China (81173472, 81973618, and 88153422); Construction Project of National Famous Old Chinese Medicine Experts Inheritance Studio of State Administration of Traditional Chinese Medicine (National TCM Education and Development [2014] No. 20); and Henan Special Research Project of Traditional Chinese Medicine, No. 20-21ZYzD17.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Writing group of guidelines for the diagnosis and treatment of acute ischemic stroke from Chinese Stroke Society. China’s guidelines of diagnosis and treatment of acute ischemic stroke, 2010. J. Chin. Clin. doctors. (2011) 2:50–59. doi: 10.3969/j.iSSN.1674-7372.2010.04.012
2. Boursin, P, Paternotte, S, Dercy, B, Sabben, C, and Maier, B. Semantique, epidemiologie et semiologie des accidents vasculaires cerebraux [Semantics, epidemiology and semiology of stroke]. Soins. (2018) 63:24–7. doi: 10.1016/j.soin.2018.06.008
3. Smith, JA, Doe, ER, and James, ST. Long-term risk of stroke ecurrence: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2020) 29:104659. doi: 10.1016/j.jstrokecerebrovasdis.2020.104659
4. Zhang, Q . Study on the correlation between serum albumin and recurrence and adverse outcomes of acute ischemic stroke. China: Henan University (2017).
5. Zuo, RJ, Wang, P, and Ji, JG. Relationship between serum lipoprotein phospholipase A2 and early recurrence of vascular events after transient ischemic attack and mild stroke. Shandong Med. (2019) 59:78–81.
6. Li, YF, Wang, CH, and Sun, WX. The value of total cholesterol/high density lipoprotein cholesterol ratio in predicting stroke recurrence. Chinese J Practical Neurolog Dis. (2023) 9:771–5.
7. CAPRIE Steering Committee . A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE).CAPRIE steering committee. Lancet. (1996) 348:1329–39. doi: 10.1016/S0140-6736(96)09457-3
8. Johnston, SC, Rothwell, PM, and Nguyen-Huynh, MN. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. (2007) 369:283–92. doi: 10.1016/S0140-6736(07)60150-0
9. Zaky, A . Thromboelastometry versus rotational Thromboelastography in cardiac surgery. Semin Cardiothorac Vasc Anesth. (2017) 21:206–11. doi: 10.1177/1089253217697146
10. Othman, M, and Kaur, H. Thromboelastography (TEG). Methods Mol Biol. (2017) 1646:533–43. doi: 10.1007/978-1-4939-7196-1_39
11. Rao, Z, Zheng, H, Wang, F, Wang, A, Liu, L, Dong, K, et al. The association between high on-treatment platelet reactivity and early recurrence of ischemic events after minor stroke or TIA. Neurol Res. (2017) 39:719–26. doi: 10.1080/01616412.2017.1312793
12. Sun, BL, Hu, YJ, and Jin, W. Prognostic value of thromboelastogram parameters combined with platelet-leukocyte aggregate detection in early neurological deterioration and prognosis of patients with acute cerebral infarction. J Clin Prac Hospital. (2019) 19:26–9. doi: 10.3969/j.issn.1672-6170.2022.02.007
13. Zhong, D, Zhang, ST, and Wu, B. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chinese J Modern Neurolog Dis. (2019) 19:897–901. doi: 10.3969/j.issn.1672-6731.2019.11.015
14. Tian, Y, Jia, H, Li, S, Wu, Y, Guo, L, Tan, G, et al. The associations of stroke,transient ischemic attack,and/or stroke-related recurrent vascular events with lipoprotein-associated phospholipase A2. A systematic review and meta-analysis. Medicin (Baltimore). (2017) 96:e9413. doi: 10.1097/MD.0000000000009413
15. Chinese Society of Neurology, Chinese Society of Cerebrovascular Disease Wang, YJ, Liu, M, et al. Chinese guidelines for secondary prevention of ischemic stroke and transient ischemic attack 2014. Chin J Neurolog. (2015) 11:139–48.
16. Wang, Y, Wang, Y, Zhao, X, Liu, L, Wang, D, Wang, C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. (2013) 369:11–9. doi: 10.1016/j.jvs.2013.08.010
17. Chen, QP, and Kuang, X. Evaluation of short-term prognosis of patients with acute cerebral infarction by routine coagulation test and thromboelastography. J Practical Clin Med. (2021) 25:36–9. doi: 10.7619/jcmp.20200776
18. Park, S, Kwon, B, Oh, JK, Song, JK, Lee, JS, and Kwon, SU. Risk of recurrent ischemic stroke in patients with patent foramen ovale: the role of D-dimer. J Stroke erebrovasc Dis. (2023) 32:107246. doi: 10.1016/j.jstrokecerebrovasdis.2023.107246
19. Maino, A, Algra, A, Peyvandi, F, Rosendaal, FR, and Siegerink, B. Hypercoagulability and the risk of recurrence in young women with myocardial infarction or ischaemic stroke: a cohort study. BMC Cardiovasc Disord. (2019) 19:55. doi: 10.1186/s12872-019-1040-4
20. Bai, CY, and Dong, K. Relationship between thromboelastography and coagulation function in patients with acute cerebral infarction. Hainan Med. (2019) 31:1923–6. doi: 10.3969/j.issn.1003-6350.2020.15.006
21. Luo, SY, He, CL, and Chen, ZY. Evaluation value of thromboelastography combined with coagulation index in premature infants' blood coagulation function. Hainan Med. (2019) 31:2089–92. doi: 10.3969/j.issn.1003-6350.2020.16.013
22. Assab, TA, Raveh-Brawer, D, and Abramowitz, J. The predictive value of Thromboelastogram in the evaluation of patients with suspected acute venous thromboembolism. Acta Haematol. (2020) 143:272–8. doi: 10.1159/000502348
23. Ma, L, Chen, W, Pan, Y, Yan, H, Li, H, Meng, X, et al. Comparison of VerifyNow, thromboelastography, and PL-12 in patients with minor ischemic stroke or transient ischemic attack. Aging (Albany NY). (2021) 13:8396–407. doi: 10.18632/aging.202650
24. Shi, Z, Zheng, WC, Fu, XL, Fang, XW, Xia, PS, and Yuan, WJ. Hypercoagulation on Thromboelastography predicts early neurological deterioration in patients with acute ischemic stroke. Cerebrovasc Dis. (2018) 46:123–9. doi: 10.1159/000492729
25. Paciaroni, M, Agnelli, G, Falocci, N, Caso, V, Becattini, C, Marcheselli, S, et al. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF study. Stroke. (2015) 46:2175–82. doi: 10.1161/STROKEAHA.115.008891
Keywords: thromboelastography, recurrent stroke, influencing factors analysis, predictive value, acute ischemic stroke
Citation: Guo R, Shen X, Lu J, Zhou J, Hao S, Lan R and Xu Y (2024) Clinical value of thromboelastography in predicting the risk of recurrence of acute ischemic stroke. Front. Neurol. 15:1420915. doi: 10.3389/fneur.2024.1420915
Edited by:
Matteo Foschi, Azienda Unità Sanitaria Locale (AUSL) della Romagna, ItalyReviewed by:
Yuanyuan Wang, Beijing University of Chinese Medicine, ChinaYuto Uchida, Johns Hopkins Medicine, United States
Copyright © 2024 Guo, Shen, Lu, Zhou, Hao, Lan and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoming Shen, c3htZG9jQDE2My5jb20=
 Ruyue Guo
Ruyue Guo Xiaoming Shen1*
Xiaoming Shen1* Jiao Zhou
Jiao Zhou Yumin Xu
Yumin Xu