- Department of Neurology, Medical University of South Carolina, Charleston, SC, United States
Stroke and traumatic brain injury (TBI) are a significant cause of death and disability nationwide. Both are considered public health concerns in rural communities in the state of South Carolina (SC), particularly affecting the African American population resulting in considerable morbidity, mortality, and economic burden. Stem cell therapy (SCT) has emerged as a potential intervention for both diseases with increasing research trials showing promising results. In this perspective article, the authors aim to discuss the current research in the field of SCT, the results of early phase trials, and the utilization of outcome measures and biomarkers of recovery. We searched PubMed from inception to December 2023 for articles on stem cell therapy in stroke and traumatic brain injury and its impact on rural communities, particularly in SC. Early phase trials of SCT in Stroke and Traumatic Brain injury yield promising safety profile and efficacy results, but the findings have not yet been consistently replicated. Early trials using mesenchymal stem cells for stroke survivors showed safety, feasibility, and improved functional outcomes using broad and domain-specific outcome measures. Neuroimaging markers of recovery such as Functional Magnetic Resonance Imaging (fMRI) and electroencephalography (EEG) combined with neuromodulation, although not widely used in SCT research, could represent a breakthrough when evaluating brain injury and its functional consequences. This article highlights the role of SCT as a promising intervention while addressing the underlying social determinants of health that affect therapeutic outcomes in relation to rural communities such as SC. It also addresses the challenges ethical concerns of stem cell sourcing, the high cost of autologous cell therapies, and the technical difficulties in ensuring transplanted cell survival and strategies to overcome barriers to clinical trial enrollment such as the ethical concerns of stem cell sourcing, the high cost of autologous cell therapies, and the technical difficulties in ensuring transplanted cell survival and equitable healthcare.
Introduction
Stroke is the 6th leading cause of mortality and a leading cause of morbidity in South Carolina and resulted in healthcare expenses of $1.3 billion in 2020 alone (1). The incidence of stroke varies by age, gender, race, and ethnicity. African American (AA) men are particularly vulnerable and have a 49 percent greater likelihood of dying from stroke than Caucasian Americans (CA) (2). A higher prevalence of stroke risk factors among AA and males compared to CA and females contributes to these disparities (2). A National Study of inpatient rehabilitation after the first stroke showed that AAs were younger and more disabled on admission, more likely to be discharged home and less likely to report independence on ADLs (3). Data from the Brain Attack Surveillance in Corpus Christi (BASIC) project also show that post-stroke Hispanic Americans scored worse on neurological, functional, and cognitive outcomes than CA (4).
Traumatic Brain Injury (TBI) is a significant cause of death and disability among the young population and is estimated to occur every 15 s in the United States (5). The economic impact of TBI is staggering, accruing an annual cost of over $77 billion in the United States (6). Between 2016 and 2018 about 4,310 TBI-related deaths were reported in South Carolina; this is 57.8% higher than the national average (7). These deaths were a combination of accidental, homicidal and suicidal causes. While the exact description of poor in South Carolina is unknown, health and economic barriers in this state may be more common than elsewhere. The degree of rurality played a role in higher incidences of TBI and increased barriers to emergency medical care (8). Racial and ethnic differences are apparent in acute and post-concussive management (9). During the early acute phase, there is a discrepancy in those taken to the hospital for evaluation (9). Afterward, there is a high risk of inadequate follow-up and management in the post-concussion period (10).
In addition, race and gender disparities in stroke and TBI care also play a significant role in patient outcomes. A report by the National Institute of Neurological Disorders and Stroke (NINDS) reveals that only 42% of the total population in clinical trials from 1985 to 2008 were women in acute stroke clinical trials (11, 12). Numerous studies report reduced access to emergency stroke care, delayed hospital arrivals, and limited rehabilitation resources for AA compared to CA (13). These disparities are echoed in clinical trials, with non-White minorities significantly underrepresented, which affects the validation and generalizability of clinical trial outcomes (11).
Treatment options for acute ischemic stroke are approved by the Food and Drug Administration (FDA) including intravenous thrombolytics (IVT) and mechanical endovascular thrombectomy (MT) (14). However the time-sensitive nature and strict selection criteria often exclude acute stroke patients from receiving these treatments. After stroke completion, dedicated rehabilitation for survivors is the only option with a proven long-term patient benefit (15). Of those who develop motor weakness after stroke, only 50% achieve functional independence at 6 months. Maximum rehabilitation benefit occurs within the first months after stroke (16). Similarly treatment options for TBIs outside neurosurgical intensive care units are limited. Lifestyle modifications, medication management, cognitive rehabilitation, and surgeries have been explored with mixed results (17).
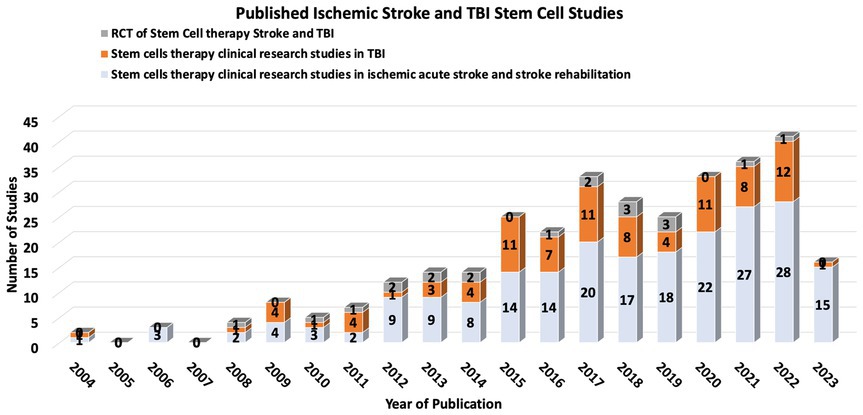
Stem cell therapy (SCT) has emerged as a potentially transformative intervention for ischemic stroke and TBI, with the ambitious aim of replacing or aiding the recovery of neurons and vascular cells affected by ischemic events. While there are no current FDA-approved SCT trials for stroke or TBI, increasing research over the past decade shows some promising trends (18–20) (Figure 1).
Stem cells in stroke and TBI clinical trials
Stem cell therapy is a potentially transformative intervention for ischemic stroke and TBI. Several clinical trials have addressed the utility of different stem cell types in ischemic stroke and TBI, including mesenchymal stem cells (MSCs), neural stem cells (NSCs), and induced pluripotent stem cells (iPSCs) (20, 21). These trials vary widely in the design of stem cell sources, dosages, delivery routes, and timing of post-stroke therapy.
Results of early-phase SCT clinical trials present a promising safety profile, with no significant adverse effects directly attributable to the therapy (22). Some trials have shown improvements in neurological function and reductions in lesion volume, but these findings have yet to be consistently replicated across a spectrum of studies. The Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPs) committee has been formed to guide and bridge the gap between basic and clinical studies (23).
One noteworthy example is the multipotent adult progenitor cells in acute ischemic stroke (MASTERS) clinical trial, a phase 2 study exploring multipotent adult progenitor cells (MAPCs) in acute ischemic stroke (24). This trial enrolled 129 patients, allocating them to either a low or high dose of the cells or a placebo. While the treatment was deemed safe, no significant differences were observed in global recovery.
Stem cell therapy also may represent a breakthrough for stroke survivors, especially when combined with rehabilitation therapy (25). The two most extensive Randomized controlled trials (RCTs) for stem cell therapy in stroke rehabilitation and recovery in the US evaluated the impact of MSC in patients with stroke more than 6 months prior with safety endpoints and functional recovery endpoints. Both trials showed safety, feasibility and improved functional outcomes (26).
Stem cells and outcome measures
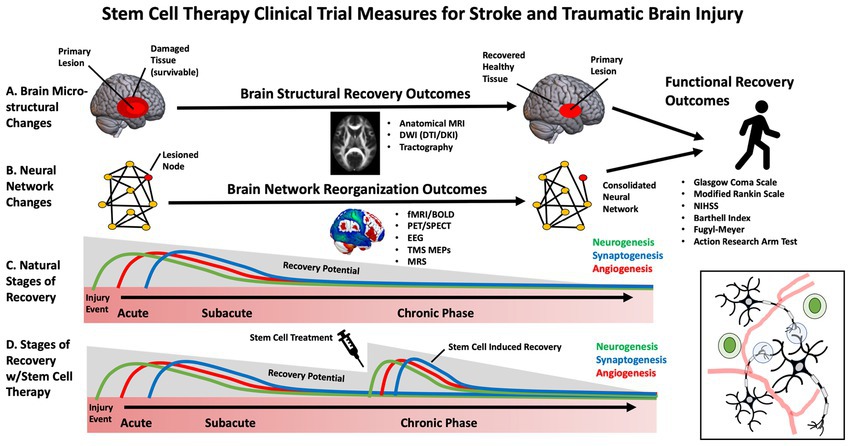
While early clinical trials for SCT in stroke have primarily focused on feasibility and safety, some studies have begun to evaluate efficacy (27). Selecting the appropriate patients and outcome measures to maximize stem-cell clinical trials, sensitivity, specificity and power is necessary. This is especially important in stroke and TBI, in which heterogeneous brain circuitry is affected, and plasticity is highly dynamic throughout various stages of the recovery process (e.g., acute, subacute, chronic) (28). The most frequently used outcome measures in stem cell clinical trials for stroke and TBI have included broad, domain-general actions of disability, such as the Glasgow Coma Scale (GCS), modified Rankin Scale (mRS), National Institutes of Health Stroke Scale (NIHSS) (29), European Stroke Scale (ESS) and Barthell Index (BI). These measures address broad aspects of functional impairment but lack specificity. Domain-specific outcome measures include the Fugl Meyer Assessment (FMA), Action Research Arm Test (ARAT), and performance on specific functional tasks. These measures may provide more targeted, sensitive measures of behavioral change (30) (Figure 2).
Biomarkers and mechanistic measures of brain recovery
While several stem-cell trials have focused on functional clinical outcome measures, there is an additional need to establish reliable biomarkers and mechanistic outcomes that capture brain-based changes during recovery (27). This enables effective translation between pre-clinical animal models and humans, allowing for more individualized and practical approaches to SCT (31). This is particularly important for disparities in stem cell clinical trials as it overcomes issues associated with language and cultural barriers that influence the reliability of subjective measures (13).
Currently there are no standardized or validated biomarkers for stroke or TBI stem cell treatments, making it difficult to determine which are optimal for clinical trials (32). Blood-based biomarkers have been investigated to measure growth factors and inflammation (33) which appear to be influenced by stem cell treatments in preclinical and clinical trials. Neurotrophic factors that support the survival and growth of brain tissue were explored in previous studies and included nerve growth factor (NGF), glial-derived neurotrophic factor (GDNF), and brain-derived neurotrophic factor (BDNF). Meanwhile, vascular endothelial growth factor (VEGF) and fibroblast growth factors (FGF) have been investigated as they may reflect vascular and tissue remodeling following injury (34). Serum-based inflammatory biomarkers can reflect the anti-inflammatory effects of stem cells and include inflammatory cytokines interleukins (IL; e.g., IL-2, IL-4, IL-6, IL1-beta, IL1-alpha, IL-10), tumor necrosis factor (TNF) alpha, and interferon-gamma among several others (34). Which of these growth factors and inflammatory biomarkers are the most sensitive and clinically meaningful within the context of stroke and TBI rehabilitation has yet to be determined still a matter of ongoing research (35). In addition to blood-based biomarkers, advances in brain imaging and non-invasive brain stimulation may prove to be useful tools in developing novel biomarkers for SCT clinical trials. These measures can be focused on changes to the primary site of injury or remote modifications, including reorganizing brain circuits affected by the injury. Recent advances in clinical neuroscience make it possible to non-invasively assess biological features of the brain, including structural integrity and neurophysiology (36). These novel tools, including neuroimaging and non-invasive brain stimulation to probe neural circuits, may be helpful in (1) developing more individualized approaches to stem cell treatment, (2) as an approach to stratify those who are most likely to benefit from a given therapy and (3) to understand how and where the cells are integrating into specific circuits or networks (37).
Brain imaging measures
Neuroimaging of the brain has undergone significant advancement over the past decades. These approaches measure the neural architecture and activity thought to underly functional recovery after stroke and TBI and may provide a more accurate measure of brain recovery than clinical assessment tools (38). Due to the non-invasive nature of these approaches and widespread accessibility across major medical centers, neuroimaging is one of the most widely used objective outcome measures for neurological clinical trials.
Structural neuroimaging can evaluate changes in the anatomical features of brain tissue, including volumetric measurements, morphology, and tissue microstructure. This has been primarily performed using MRI. Routine clinical scans including high-resolution T1 scans, diffusion-weighted imaging (DWI), susceptibility-weighted imaging (SWI) and T2 scans may be utilized to estimate gray and white matter volume, lesion volume, penumbra volume, and cortical gyrification indices and have been informative biomarkers in pre-clinical stem cell studies (39). These calculations can help monitor changes in lesion size and impact overall brain morphometry throughout the recovery period in future human trials (40). Meanwhile, advanced DWI sequences can track complex fiber pathways and detailed information about brain tissue microstructure (41). Early limitations associated with tractography derived from diffusion tensor imaging (DTI), such as complex fiber-crossing, have undergone rapid advancement with more sophisticated approaches, including diffusion kurtosis imaging (DKI) and constrained spherical deconvolution (CSD) (42). Many studies have examined how the integrity of the corticospinal tract measured using fractional anisotropy relates to motor impairment in the context of stroke (38). Although the precise biological correlates of these diffusion measures and their interpretation are still being investigated, these approaches hold promise as a sensitive measure of changes in the health of brain tissue in clinical trials with stem cell therapies.
There is a growing appreciation that brain injury and its functional consequences cannot simply be explained by damage to a single structure but rather by the connectivity of that structure to an integrated network (43). Neuroimaging is an effective tool to assess neural activity within these distributed brain networks. In the context of stroke and TBI, reorganization of neural networks may underly recover after rehabilitation (37). Thus, it will be essential to understand the impact of stem cell interventions on these large-scale networks. Determining neural activity-specific timescales and spatial resolutions for quantitative change provides a reliable measure of structural changes in the brain. Specific neuroimaging approaches can be tailored to brain assessments in the setting of stem cell infusion. Functional Magnetic Resonance Imaging (fMRI) and electroencephalography (EEG) are the most widely used approaches to study cortical networks. fMRI relies on an indirect measure of neural activation by assessing how blood oxygenation levels change over time. The resulting blood oxygen level-dependent signal (BOLD) is acquired by subtracting “resting state” activity from neural activity during or during task engagement. fMRI has been used to demonstrate neural plasticity within neural networks following brain injury (44). While less spatially precise, EEG can directly measure neuroelectric activity at a high temporal resolution which is easily scalable across medical centers. EEG may predict functional outcomes and may be correlated with mRS, the FM and the NIHSS (32). Transcranial Magnetic Stimulation (TMS) may be combined with other neuroimaging approaches to probe non-motor networks or with neurophysiological recordings to assess motor pathways. TMS motor evoked potentials (MEPs) have been used to assess corticospinal integrity following stroke and are a good prognostic indicator of the extent of functional recovery (45).
While the previously mentioned approaches may be effective at identifying network remodeling and neuroplasticity, these approaches cannot assess angiogenesis and neurogenesis associated with stem cell therapies. Imaging modalities such as Positron Emission Tomography (PET) can evaluate changes in vasculature and neuronal survivability by measuring regional cerebral blood flow (rCBF) and metabolic rate using radiotracers. Magnetic resonance spectroscopy (MRS) can also monitor changes in metabolite composition and concentration within brain tissue (46, 47). This approach may provide a surrogate marker for cellular repair mechanisms and metabolic changes in the recovery process.
Stem cells therapy challenges
The current challenges of stem cell therapy for stroke and TBI are multifactorial and significant. First, the best source of MSCs for stroke treatment has yet to be established (48, 49). Most preclinical studies used MSCs from healthy, young donors and about half of the clinical studies used autologous MSC (50). Harvesting stem cells from donors, especially neural stem cells (NSCs) or embryonic stem cells (ESCs), raises ethical concerns as well as concerns regarding the viability and effectiveness of stem cells from different donor types. Harvesting stem cells from donors, especially neural stem cells (NSCs) or embryonic stem cells (ESCs). Ethical issues arise primarily from the use of ESCs, which involves the destruction of embryos, and the use of NSCs, which often require fetal tissue. These ethical concerns can hinder research progress and limit the availability of stem cells for clinical use (51, 52). Although the use of autologous MSC addresses this issue, MSC are costly and require several months for optimal production; this delays administration beyond desired treatment windows (49, 53). The optimal timing for MSC administration is controversial; while very early transplantation within 48 h is recommended, some studies suggest benefits even 1 month post-stroke (54) (Supplementary Table 1). The administration route presents another hurdle: systemic approaches like intravenous (IV) and intra-arterial (IA), compared to direct intrathecal (IC) approaches carry potential risks and benefits. For instance, IV administration may lead to pulmonary trapping of cells, whereas IC administration poses risks of infection and bleeding (55). Technical challenges include Tracking transplanted cells to ensure survival and overcoming potential immune rejection. Imaging modalities including magnetic resonance imaging (MRI), positron emission tomography (PET), single-photon emission computed tomography (SPECT/CT), or bioluminescence imaging (BLI) using green fluorescent protein-Luciferase (GFP-Luc) may be utilized for stem cells in vivo tracking (53). Safety concerns persist, especially the risk of undesirable tissue differentiation and oncogenesis, exacerbated when genetic manipulation or reprogramming is employed to augment MSCs, potentially causing unregulated cell proliferation. This is underscored by instances where stem cell transplants have induced tumorigenesis (56, 57). Additionally, the survival and integration of transplanted cells into the host tissue remain significant hurdles. The hostile post-stroke environment, characterized by inflammation and scarring, can impede the survival and integration of transplanted cells (58).
Outcome evaluation measures should be clear and unified for patients receiving therapy whether functional, quality of life or cognitive preclinical and clinical study endpoints and outcome measurement methods were heterogeneous. Patient selection and treatment costs are other significant issues. Many stroke patients have comorbidities such as hypertension, diabetes and heart disease that may exert an impact on therapy efficacy (59, 60). In 2018, the costs of producing autologous cell therapies were estimated to be US$ 94 per million cells for a dose of 2 million cells per kg, which is calculated to be US$ 13,160 per dose for an average-weight adult, which raises the question of whether stem cell therapy would benefit only the better socio-economic group (61). Comprehensive clinical research is essential to establish a clear transplantation protocol, considering the timing, route, and dosage for optimal therapeutic outcomes. This includes addressing the technical challenges of cell tracking, survival, and integration and ensuring ethical practices and cost-effectiveness to make stem cell therapy a viable option for a broader patient population.
Challenges in South Carolina
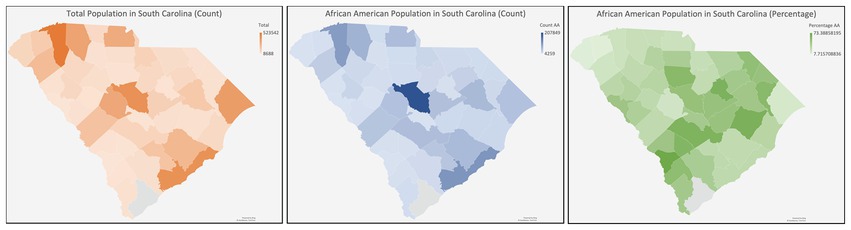
South Carolina (SC) is characterized by a predominantly rural demographic, with approximately 35% of its inhabitants residing in rural locales, a figure substantially higher than the national average (62). Health disparities are more frequent in rural populations due to diminished prevalence of health insurance coverage, inferior socioeconomic and educational strata, and distinct cultural and societal influences (63) Because there are barriers to clinical trial enrollment in these areas (64), continued efforts to determine the obstacles to CT enrollment in SC regarding accessibility (e.g., lack of awareness, physicians not broaching CT options, unavailability of health insurance) and cognitive/psychological impediments (e.g., deficits in subjective and objective knowledge, prevalent misconceptions, ingrained distrust, apprehensions, and perceived risk) are needed (65) (Figure 3).
Several strategies can be applied to address enrollment barriers, including involving local physicians, community engagement/education, active recruitment, and financial incentives and support. A study monitoring community engagement with surgeons in the US Midwest found that most surgeons needed to be made aware of available trials and had no experience with the trial referral process (66). Furthermore, when later surveying patients following education, they described a more positive experience with their surgeons. This same study identified that facility communication and collaboration improved patient continuity of care. However, prior to seeing their referring physician, awareness of potential options extends to community and civic involvement (66). A study surveying 212 African Americans and Caucasians across rural and urban communities found that increased participation of churches/schools and family/friend referrals were more effective in rural communities versus in urban it was schools, media, and family/friends (67). This emphasizes the need to develop grassroots relationships in communities to foster a collaborative approach to medical access. This is further supported by another trial that identified low recruitment rates among rural and black individuals for palliative care clinical trials (68). In that study, recruitment strategies developed by community advisory groups aided in directing a more targeted approach to increase access and awareness of available trials. Of the 2,879 participants involved, 228 were eligible for potential trials. Of those who were enrolled in trials, only 12.7% consented when only a study coordinator was available, versus 58.8% when a community advisory group member was also present. This underlines the importance of embedded community allies in improving facility-community relationships. An analysis of recruitment strategies on pediatric RCTs in rural primary care clinics in 2022 found that utilizing traditional methods (i.e., posters, social media, press releases) was needed to complete enrollment for recruitment participation. In contrast, active enrollment (EMR-generated lists with staff follow-up) did (69). Furthermore, it reports that time to enrollment was quicker with active versus traditional methods. Lastly, financial barriers are among the most significant between rural and urban enrollment populations, as trials are ordinarily run in urban areas- thus requiring a considerable time commitment and financial commitment via travel, to participate in studies. Financial incentives alleviated these concerns and proved to be a significant motivator (67). Moreover, AAs exhibited a discernible gap in subjective and objective knowledge regarding CTs and an amplified perception of risk upon participation (65, 70). Gender-specific data pertinent to CT enrollment remains limited; however, one survey delineating gender-related discrepancies in CT willingness unveiled that females exhibited an increased perception of potential harm from trials despite displaying heightened susceptibility to financial inducements (71). Subsequent studies discerned that clinician trust and the perceived prospective benefits (either personal or altruistic) notably influenced females’ participation in CTs (72).
Addressing these cognitive and psychological barriers requires culturally sensitive education and awareness campaigns. These campaigns should focus on dispelling misconceptions, building trust, and providing clear information about the benefits and risks of clinical trials.
Enrollment volumes for stem cell therapy clinical trials vary between states in the United States, influenced by infrastructure, funding, and public awareness. States with established stem cell research centers and robust healthcare infrastructure tend to have higher enrollment volumes. For example, California, home to the California Institute for Regenerative Medicine (CIRM) and multiple Alpha Stem Cell Clinics, sees a high volume of patient enrollment. These centers provide a collaborative infrastructure that accelerates the development and validation of stem cell therapies, making California a leader in this field. Significant state-specific funding can impact enrollment. For instance, the UC San Diego Alpha Stem Cell Clinic received a grant from CIRM to expand clinical trials, highlighting the state’s commitment to advancing stem cell research and increasing patient enrollment. States with leading academic and research institutions, such as Massachusetts and Texas often have more robust public awareness and better enrollment rates in clinical trials. Rural states or those with less developed healthcare infrastructures need help enrolling patients due to logistical issues like transportation and limited access to specialized healthcare facilities. Addressing these barriers through local physician involvement and community engagement is essential for improving enrollment rates.
Ethical considerations
In the United States, AAs have historically been subjected to experimental medical research while having limited access to quality healthcare (72). The unique socio-demographic landscape of SC, marked by its predominantly rural composition and higher-than-average AA population, necessitates a tailored approach to stem cell therapy research and application. It is not merely the impact of scientific innovation that must guide the research, but an acute understanding and acknowledgment of the existing health disparities that plague rural and AA communities in the state (73).The historical and systemic barriers these populations face, ranging from restricted access to healthcare, limited health insurance coverage, and socio-economic and educational challenges to deeply rooted cultural and societal norms, raise pivotal ethical questions (73).
Firstly, an ethical mandate is to ensure that the AA community is adequately represented in research trials, given its sizeable presence in SC. This is vital to ensure therapeutic efficacy and safety across the diverse genetic and socio-cultural landscapes. Beyond representation, the state also has higher rural demographics and inherent health disparities, emphasizing the need for equitable access. Given the intricate weave of socio-economic challenges, efforts must be undertaken to ensure that cost does not become a prohibitive barrier, particularly for the AA community and other marginalized groups in SC. Ethical considerations also extend to education and awareness campaigns. These campaigns must be culturally sensitive, addressing potential misconceptions and ensuring that the diverse populations of SC are informed and empowered to make decisions regarding stem cell therapies.
In conclusion, as SC navigates the promising terrain of stem cell research and application, it must do so with an ethical compass calibrated to its unique socio-demographic challenges. Only by doing so can the state ensure that the promise of stem cell therapies is a beacon of hope for all its residents, irrespective of race, socio-economic status, or geographical location.
Author contributions
GM: Methodology, Resources, Writing – original draft, Writing – review & editing. DL: Software, Writing – original draft, Writing – review & editing. PG: Writing – original draft, Writing – review & editing. MR: Writing – original draft, Writing – review & editing. JV: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank Dan Lackland and Mark Stacy for their advice and review of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1419867/full#supplementary-material
References
1. S.C. Department of Health and Environmental Control . (2020). *Stroke*. Retrieved from https://www.dph.sc.gov/diseases-conditions/conditions/heart-diseasestroke/stroke#:~:text=Stroke%20is%20South%20Carolina’s%20sixth,in%20South%20Carolina%20in%202020.
2. Feng, W, Nietert, PJ, and Adams, RJ. Influence of age on racial disparities in stroke admission rates, hospital charges, and outcomes in South Carolina. Stroke. (2009) 40:3096–101. doi: 10.1161/STROKEAHA.109.554535
3. Odonkor, CA, Esparza, R, Flores, LE, Verduzco-Gutierrez, M, Escalon, MX, Solinsky, R, et al. Disparities in health Care for Black Patients in physical medicine and rehabilitation in the United States: a narrative review. PM&R. (2021) 13:180–203. doi: 10.1002/pmrj.12509
4. Reeves, SL, Brown, DL, Baek, J, Wing, JJ, Morgenstern, LB, and Lisabeth, LD. Ethnic differences in Poststroke quality of life in the brain attack surveillance in Corpus Christi (BASIC) project. Stroke. (2015) 46:2896–901. doi: 10.1161/STROKEAHA.115.010328
5. Johnson, WD, and Griswold, DP. Traumatic brain injury: a global challenge. Lancet Neurol. (2017) 16:949–50. doi: 10.1016/S1474-4422(17)30362-9
6. Dewan, MC, Rattani, A, Gupta, S, Baticulon, RE, Hung, Y-C, Punchak, M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. (2019) 130:1080–97. doi: 10.3171/2017.10.JNS17352
7. Daugherty, J, Zhou, H, Sarmiento, K, and Waltzman, D. Differences in state traumatic brain injury–related deaths, by principal mechanism of injury, intent, and percentage of population living in rural areas — United States, 2016–2018. MMWR Morb Mortal Wkly Rep. (2021) 70:1447–52. doi: 10.15585/mmwr.mm7041a3
8. Gontkovsky, ST, Sherer, M, Nick, TG, Nakase-Thompson, R, and Yablon, SA. Effect of urbanicity of residence on TBI outcome at one year post-injury. Brain Inj. (2006) 20:701–9. doi: 10.1080/02699050600744103
9. Bazarian, JJ, Pope, C, McClung, J, Cheng, YT, and Flesher, W. Ethnic and racial disparities in emergency Department Care for Mild Traumatic Brain Injury. Acad Emerg Med. (2003) 10:1209–17. doi: 10.1197/S1069-6563(03)00491-3
10. Tang, AR, Wallace, J, Grusky, AZ, Hou, BQ, Hajdu, KS, Bonfield, CM, et al. Investigation of factors contributing to racial differences in sport-related concussion outcomes. World Neurosurg. (2023) 173:e755–65. doi: 10.1016/j.wneu.2023.03.009
11. Burke, JF, Brown, DL, Lisabeth, LD, Sanchez, BN, and Morgenstern, LB. Enrollment of women and minorities in NINDS trials. Neurol Int. (2011) 76:354–60. doi: 10.1212/WNL.0b013e3182088260
12. Carcel, C, and Reeves, M. Under-enrollment of women in stroke clinical trials. Stroke. (2021) 52:452–7. doi: 10.1161/STROKEAHA.120.033227
13. Cruz-Flores, S, Rabinstein, A, Biller, J, Elkind, MSV, Griffith, P, Gorelick, PB, et al. Racial-ethnic disparities in stroke care: the American experience. Stroke. (2011) 42:2091–116. doi: 10.1161/STR.0b013e3182213e24
14. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American stroke. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
15. Lee, KB, Lim, SH, Kim, KH, Kim, KJ, Kim, YR, Chang, WN, et al. Six-month functional recovery of stroke patients: a multi-time-point study. Int J Rehab Res Internationale Zeitschrift fur Rehabilitationsforschung Revue internationale de recherches de readaptation. (2015) 38:173–80. doi: 10.1097/MRR.0000000000000108
16. Hatem, SM, Saussez, G, Della Faille, M, Prist, V, Zhang, X, Dispa, D, et al. Rehabilitation of motor function after stroke: a multiple systematic review focused on techniques to stimulate upper extremity recovery. Front Hum Neurosci. (2016) 10:442. doi: 10.3389/fnhum.2016.00442
17. Kowalski, RG, Hammond, FM, Weintraub, AH, Nakase-Richardson, R, Zafonte, RD, Whyte, J, et al. Recovery of consciousness and functional outcome in moderate and severe traumatic brain injury. JAMA Neurol. (2021) 78:548–57. doi: 10.1001/jamaneurol.2021.0084
18. Kawabori, M, Shichinohe, H, Kuroda, S, and Houkin, K. Clinical trials of stem cell therapy for cerebral ischemic stroke. Int J Mol Sci. (2020) 21:7380. doi: 10.3390/ijms21197380
19. Negoro, T, Okura, H, Maehata, M, Hayashi, S, Yoshida, S, and Takada, N. Trends in clinical trials for stroke by cell therapy: data mining ClinicalTrials.gov and the ICTRP portal site. NPJ Regen Med. (2019) 4:20. doi: 10.1038/s41536-019-0082-7
20. Trounson, A, and McDonald, C. Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell. (2015) 17:11–22. doi: 10.1016/j.stem.2015.06.007
21. Prasad, K, Sharma, A, Garg, A, Mohanty, S, Bhatnagar, S, Johri, S, et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke. Stroke. (2018) 45:3618–24. doi: 10.1161/STROKEAHA.114.007028
22. Li, J, Zhang, Q, Wang, W, Lin, F, Wang, S, and Zhao, J. Mesenchymal stem cell therapy for ischemic stroke: a look into treatment mechanism and therapeutic potential. J Neurol. (2021) 268:4095–107. doi: 10.1007/s00415-020-10138-5
23. STEPS Participants . Stem cell therapies as an emerging paradigm in stroke (STEPS). Stroke. (2009) 40:510–5. doi: 10.1161/STROKEAHA.108.526863
24. Hess, DC, Wechsler, LR, Clark, WM, Savitz, SI, Ford, GA, Chiu, D, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blindF, placebo-controlled, phase 2 trial. Lancet Neurol. (2017) 16:360–8. doi: 10.1016/S1474-4422(17)30046-7
25. Savitz, SI, Cramer, SC, Wechsler, L, Aronowski, J, Boltze, J, Borlongan, C, et al. Stem cells as an emerging paradigm in stroke 3. Stroke. (2014) 45:634–9. doi: 10.1161/STROKEAHA.113.003379
26. Borlongan, CV . Concise review: stem cell therapy for stroke patients: are we there yet? Stem Cells Transl Med. (2019) 8:983–8. doi: 10.1002/sctm.19-0076
27. Balkaya, M, and Cho, S. Optimizing functional outcome endpoints for stroke recovery studies. J Cereb Blood Flow Metab. (2019) 39:2323–42. doi: 10.1177/0271678X19875212
28. Jordan, HT, Che, J, Byblow, WD, and Stinear, CM. Fast outcome categorization of the upper limb after stroke. Stroke. (2022) 53:578–85. doi: 10.1161/STROKEAHA.121.035170
29. Kwah, LK, and Diong, J. National Institutes of Health stroke scale (NIHSS). Aust J Phys. (2014) 60:61. doi: 10.1016/j.jphys.2013.12.012
30. Gladstone, DJ, Danells, CJ, and Black, SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. (2002) 16:232–40. doi: 10.1177/154596802401105171
31. Davis, KD, Aghaeepour, N, Ahn, AH, Angst, MS, Borsook, D, Brenton, A, et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat Rev Neurol. (2020) 16:381–400. doi: 10.1038/s41582-020-0362-2
32. Alt Murphy, M, Resteghini, C, Feys, P, and Lamers, I. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol. (2015) 15:29. doi: 10.1186/s12883-015-0292-6
33. Palà, E, Bustamante, A, Jolkkonen, J, Hommel, M, Rosell, A, and Montaner, J. Blood-based biomarkers and stem cell therapy in human stroke: a systematic review. Mol Biol Rep. (2020) 47:6247–58. doi: 10.1007/s11033-020-05627-9
34. Taguchi, A, Sakai, C, Soma, T, Kasahara, Y, Stern, DM, Kajimoto, K, et al. Intravenous autologous bone marrow mononuclear cell transplantation for stroke: Phase1/2a clinical trial in a homogeneous Group of Stroke Patients. Stem Cells Dev. (2015) 24:2207–18. doi: 10.1089/scd.2015.0160
35. Turnbull, MT, Zubair, AC, Meschia, JF, and Freeman, WD. Mesenchymal stem cells for hemorrhagic stroke: status of preclinical and clinical research. NPJ Regen Med. (2019) 4:10. doi: 10.1038/s41536-019-0073-8
36. Nishimura, K, Cordeiro, JG, Ahmed, AI, Yokobori, S, and Gajavelli, S. Advances in traumatic brain injury biomarkers. Cureus. (2022) 14:e23804. doi: 10.7759/cureus.23804
37. Castellanos, NP, Leyva, I, Buldú, JM, Bajo, R, Paúl, N, Cuesta, P, et al. Principles of recovery from traumatic brain injury: reorganization of functional networks. NeuroImage. (2011) 55:1189–99. doi: 10.1016/j.neuroimage.2010.12.046
38. Jeurissen, B, and Szczepankiewicz, F. Multi-tissue spherical deconvolution of tensor-valued diffusion MRI. NeuroImage. (2021) 245:118717. doi: 10.1016/j.neuroimage.2021.118717
39. Hartman, RE, Nathan, NH, Ghosh, N, Pernia, CD, Law, J, Nuryyev, R, et al. A biomarker for predicting responsiveness to stem cell therapy based on mechanism-of-action: evidence from cerebral injury. Cell Rep. (2020) 31:107622. doi: 10.1016/j.celrep.2020.107622
40. Gauthier, LV, Taub, E, Mark, VW, Barghi, A, and Uswatte, G. Atrophy of spared gray matter tissue predicts poorer motor recovery and rehabilitation response in chronic stroke. Stroke. (2012) 43:453–7. doi: 10.1161/STROKEAHA.111.633255
41. Cheng, B, Dietzmann, P, Schulz, R, Boenstrup, M, Krawinkel, L, Fiehler, J, et al. Cortical atrophy and transcallosal diaschisis following isolated subcortical stroke. J Cereb Blood Flow Metab. (2019) 40:611–21. doi: 10.1177/0271678X19831583
42. Jensen, JH, and Helpern, JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. (2010) 23:698–710. doi: 10.1002/nbm.1518
43. Price, CJ, Hope, TM, and Seghier, ML. Ten problems and solutions when predicting individual outcome from lesion site after stroke. NeuroImage. (2017) 145:200–8. doi: 10.1016/j.neuroimage.2016.08.006
44. Golestani, A-M, Tymchuk, S, Demchuk, A, and Goodyear, BG. Longitudinal evaluation of resting-state fMRI after acute stroke with hemiparesis. Neurorehabil Neural Repair. (2012) 27:153–63. doi: 10.1177/1545968312457827
45. Stinear, CM, Byblow, WD, Ackerley, SJ, Smith, M-C, Borges, VM, and Barber, PA. PREP2: a biomarker-based algorithm for predicting upper limb function after stroke. Ann Clin Transl Neurol. (2017) 4:811–20. doi: 10.1002/acn3.488
46. Paparella, I, Vanderwalle, G, Stagg, CJ, and Maquet, P. An integrated measure of GABA to characterize post-stroke plasticity. Neuroimage Clin. (2023) 39:103463. doi: 10.1016/j.nicl.2023.103463
47. Harston, GWJ, Okell, TW, Sheerin, F, Schulz, U, Mathieson, P, Reckless, I, et al. Quantification of serial cerebral blood flow in acute stroke using arterial spin labeling. Stroke. (2017) 48:123–30. doi: 10.1161/STROKEAHA.116.014707
48. Li, W, Shi, L, Hu, B, Hong, Y, Zhang, H, and Li, X. Mesenchymal stem cell-based therapy for stroke: current understanding and challenges. Front Cell Neurosci. (2021) 15:15. doi: 10.3389/fncel.2021.628940
49. Liu, H, Reiter, S, Zhou, X, Chen, H, Ou, Y, Lenahan, C, et al. Insight into the mechanisms and the challenges on stem cell-based therapies for cerebral ischemic stroke. Front Cell Neurosci. (2021) 15:15. doi: 10.3389/fncel.2021.637210
50. Block, TJ, Marinkovic, M, Tran, ON, Gonzalez, AO, Marshall, A, Dean, DD, et al. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res Ther. (2017) 8:239. doi: 10.1186/s13287-017-0688-x
51. Lo, B, and Parham, L. Ethical issues in stem cell research. Endocr Rev. (2009) 30:204–13. doi: 10.1210/er.2008-0031
52. Hyun, I . The bioethics of stem cell research and therapy. J Clin Invest. (2010) 120:71–5. doi: 10.1172/JCI40435
53. Manley, NC, and Steinberg, GK. Tracking stem cells for cellular therapy in stroke. Curr Pharm Des. (2012) 18:3685–93. doi: 10.2174/138161212802002643
54. Liaw, N, and Liebeskind, D. Emerging therapies in acute ischemic stroke. F1000Res. (2020) 9:9. doi: 10.12688/f1000research.21100.1
55. Levy, O, Kuai, R, Siren, EMJ, Bhere, D, Milton, Y, Nissar, N, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. (2024) 6:eaba6884. doi: 10.1126/sciadv.aba6884
56. Zhang, J, Huang, X, Wang, H, Liu, X, Zhang, T, Wang, Y, et al. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther. (2015) 6:234. doi: 10.1186/s13287-015-0240-9
57. Ollier, MP, and Hartmann, L. Bidimensional immunoelectrophoresis in three stages (semi micromethod). Biomedicine. (1976) 25:184–7.
58. Hess, DC, and Borlongan, CV. Cell-based therapy in ischemic stroke. Expert Rev Neurother. (2008) 8:1193–201. doi: 10.1586/14737175.8.8.1193
59. Cipolla, MJ, Liebeskind, DS, and Chan, S-L. The importance of comorbidities in ischemic stroke: impact of hypertension on the cerebral circulation. J Cereb Blood Flow Metab. (2018) 38:2129–49. doi: 10.1177/0271678X18800589
60. Chen, J, Ye, X, Yan, T, Zhang, C, Yang, X-P, Cui, X, et al. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. (2011) 42:3551–8. doi: 10.1161/STROKEAHA.111.627174
61. Krause, M, Phan, TG, Ma, H, Sobey, CG, and Lim, R. Cell-based therapies for stroke: are we there yet? Front Neurol. (2019) 10:10. doi: 10.3389/fneur.2019.00656
62. America’s Health Rankings analysis of U.S . (2023). Census Bureau, United Health Foundation, 485. Available at: AmericasHealthRankings.org. Accessed March 04, 2024.
63. U.S. Census Bureau . (2023). “U.S. Census Bureau.” Available at: https://www.census.gov/. Accessed March 04, 2024.
64. Bergeron, CD, Foster, C, Friedman, DB, Tanner, A, and Kim, SH. Clinical trial recruitment in rural South Carolina: a comparison of investigators’ perceptions and potential participant eligibility. Rural Remote Health. (2013) 13:171–84. doi: 10.3316/informit.311580564006072
65. Kim, S-H, Tanner, A, Friedman, DB, Foster, C, and Bergeron, CD. Barriers to clinical trial participation: a comparison of rural and urban communities in South Carolina. J Community Health. (2014) 39:562–71. doi: 10.1007/s10900-013-9798-2
66. Ellis, SD, Geana, M, Mackay, CB, Moon, DJ, Gills, J, Zganjar, A, et al. Science in the heartland: exploring determinants of offering cancer clinical trials in rural-serving community urology practices. Urologic Oncol: Seminars and Original Investigations. (2019) 37:529.e9–529.e18. doi: 10.1016/j.urolonc.2019.03.004
67. Friedman, DB, Foster, C, Bergeron, CD, Tanner, A, and Kim, S-H. A qualitative study of recruitment barriers, motivators, and community-based strategies for increasing clinical trials participation among rural and urban populations. Am J Health Promot. (2015) 29:332–8. doi: 10.4278/ajhp.130514-QUAL-247
68. Gazaway, S, Bakitas, M, Underwood, F, Ekelem, C, Duffie, M, McCormick, S, et al. Community informed recruitment: a promising method to enhance clinical trial participation. J Pain Symptom Manag. (2023) 65:e757–64. doi: 10.1016/j.jpainsymman.2023.02.319
69. Darden, PM II, Davis, AM, Lee, JY, Bimali, M, Simon, AE, Atz, AM, et al. Active vs traditional methods of recruiting children for a clinical trial in rural primary care clinics: a cluster-randomized clinical trial. JAMA Netw Open. (2022) 5:e2244040. doi: 10.1001/jamanetworkopen.2022.44040
70. Kim, S-H, Tanner, A, Friedman, DB, Foster, C, and Bergeron, C. Barriers to clinical trial participation: comparing perceptions and knowledge of African American and white south Carolinians. J Health Commun. (2015) 20:816–26. doi: 10.1080/10810730.2015.1018599
71. Lobato, L, Bethony, JM, Pereira, FB, Grahek, SL, Diemert, D, and Gazzinelli, MF. Impact of gender on the decision to participate in a clinical trial: a cross-sectional study. BMC Public Health. (2014) 14:1156. doi: 10.1186/1471-2458-14-1156
72. Chu, SH, Kim, EJ, Jeong, SH, and Park, GL. Factors associated with willingness to participate in clinical trials: a nationwide survey study. BMC Public Health. (2015) 15:10. doi: 10.1186/s12889-014-1339-0
Keywords: stem cells, stroke, traumatic brain injury, disparities, South Carolina
Citation: Mohamed GA, Lench DH, Grewal P, Rosenberg M and Voeks J (2024) Stem cell therapy: a new hope for stroke and traumatic brain injury recovery and the challenge for rural minorities in South Carolina. Front. Neurol. 15:1419867. doi: 10.3389/fneur.2024.1419867
Edited by:
Ulises Gomez-Pinedo, Health Research Institute of Hospital Clínico San Carlos, SpainReviewed by:
Alejandro A. Canales-Aguirre, CONACYT Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ), MexicoDoddy Denise Ojeda-Hernández, Complutense University of Madrid, Spain
Copyright © 2024 Mohamed, Lench, Grewal, Rosenberg and Voeks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ghada A. Mohamed, bW9oYW1lZGdAbXVzYy5lZHU=
 Ghada A. Mohamed
Ghada A. Mohamed Daniel H. Lench
Daniel H. Lench Parneet Grewal
Parneet Grewal Mark Rosenberg
Mark Rosenberg Jenifer Voeks
Jenifer Voeks