- 1Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2China National Clinical Research Center for Neurological Diseases, Beijing, China
- 3Department of Neurosurgery, Beijing Hospital, National Center of Gerontology, Beijing, China
- 4Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, China
Background: Systemic immune-inflammatory markers combine various individual inflammatory cell parameters to comprehensively explore their relationship with the development and long-term outcomes of cardiovascular, cerebrovascular, and oncological disorders. The systemic immune-inflammatory marker index has not been extensively studied in terms of its impact on the long-term prognosis following cerebral revascularization in MMD patients. Our research aims to address this gap and improve the prediction of long-term outcomes for these patients.
Methods: We included 851 patients with Moyamoya disease who underwent cerebral revascularization at our medical center from 2009 to 2021. Systemic immune-inflammatory markers were calculated based on routine blood test results at admission, and follow-up was conducted for over 6 months after surgery. During monitoring and upon release, we evaluated patient neurological condition by utilizing the modified Rankin Scale (mRS). We examined the correlation between alterations in mRS ratings and systemic immune-inflammatory markers.
Results: Comparing the unfavorable long-term prognosis group to the favorable long-term prognosis group, it was found that the NLR level was markedly higher (p = 0.037), while the LMR was lower in the unfavorable long-term prognosis group (p = 0.004). Results from logistic regression analysis revealed that the high-level LMR group had a lower risk of unfavorable long-term prognosis compared to the low-level group (T3: OR = 0.433, 95% CI [0.204–0.859], p = 0.026). The AUC of the model was 0.750 (95% CI [0.693–0.806]).
Conclusion: Lymphocyte-to-monocyte ratio levels are independently linked to an increased risk of unfavorable long-term prognosis, highlighting LMR as a new and effective predictor for postoperative Moyamoya patients.
Introduction
Moyamoya disease is a rare cerebrovascular condition characterized by progressive narrowing of the terminal segments of both internal carotid arteries. This leads to the formation of an abnormal vascular network with collateral circulation at the base of the brain, elevating the risk of ischemic or hemorrhagic stroke and potentially resulting in high morbidity and mortality rates (1). Moyamoya disease arises from a complex interaction of genetic, immune, inflammatory, and other factors. Several researches have emphasized the significant impact of persistent systemic inflammation on the development of Moyamoya disease (2). Regarding treatment, cerebral revascularization surgery is widely regarded as the most effective approach, which encompasses direct, indirect, and combined bypass techniques (3–5). Previous studies have indicated that advanced Suzuki stage, RNF213 p.R4810K variant, diabetes, and the nutritional index (PNI) are risk factors associated with long-term unfavorable long-term prognosis following cerebral revascularization (6–9). However, the factors influencing the long-term prognosis of patients with Moyamoya disease (MMD) after cerebral revascularization are still not fully elucidated.
Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune-inflammatory index (SII), and lymphocyte-to-monocyte ratio (LMR) are some readily accessible inflammatory markers in clinical practice. They are more indicative of systemic inflammation levels compared to standard neutrophil (Neut) or lymphocyte (Ly) counts alone. The NLR, PLR, LMR, and SII have been extensively employed in forecasting the long-term outcomes in individuals with different types of cancer such as breast cancer, hepatocellular carcinoma, stomach cancer, and tongue cancer (10–12). Recent research has shown that the neutrophil-to-lymphocyte ratio (NLR) can be a reliable indicator of poor long-term outcomes for individuals with cardiovascular conditions (13). Additionally, there has been a growing body of evidence suggesting a connection between markers of systemic immune inflammation and the likelihood of developing Moyamoya disease (MMD). These findings have significant implications for the management and treatment of patients with these health concerns (14). This study aims to explore the potential relationship between systemic immune-inflammatory marker levels upon admission and the long-term prognosis after cerebral revascularization surgery in patients with Moyamoya disease (MMD), and to establish an appropriate predictive model.
Methods
Study design and participants
We conducted a retrospective analysis of patients with Moyamoya disease (MMD) who visited Beijing Tiantan Hospital from 2009 to 2021 and underwent cerebral revascularization surgery. Confirmation of Moyamoya disease (MMD) was established through the utilization of digital subtraction angiography (DSA) as outlined in the 2012 Japanese guidelines. The criteria for exclusion were as follows: (1) individuals below the age of 18 or over the age of 60; (2) patients who have had a clearly identified source of infection within 1 month before admission, or cases where the source of infection is unclear but the white blood cell count is greater than or equal to 10 × 109/L; (3) patients with secondary moyamoya syndrome, including those with concurrent conditions such as atherosclerosis, vasculitis, neurofibromatosis, autoimmune diseases, and other metabolic diseases; (4) 28 patients lost to follow-up were excluded. Finally, 851 patients with MMD were included in our study, including 765 in the favorable long-term prognosis group and 86 in the unfavorable long-term prognosis group. The Ethics Committee at Beijing Tiantan Hospital approved all study protocols (Approval Number: KY2016-048-01) in accordance with the Helsinki Declaration principles. This research is recorded in the Chinese Clinical Trial Registry (ChiCTR2000031412). Informed consent was obtained from all participants (Figure 1).
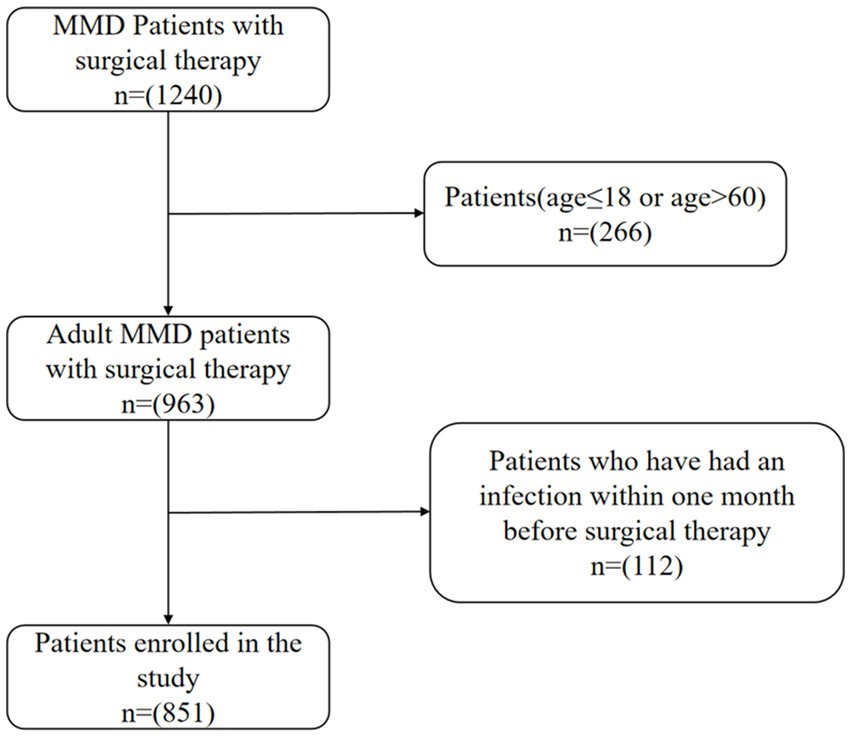
Figure 1. Flow diagram of the study participants. MMD indicates moyamoya disease.Infection is defined as a white blood cell (WBC) count greater than 10.0 ×109/L.
Baseline data collection
We collected baseline data from all participants, including age, gender, RNF213 p.R4810K variant, body mass index (BMI), Suzuki stage, surgical modality, and medical history, such as diabetes, hypertension, dyslipidemia, smoking, and alcohol consumption. We define Suzuki stages 1–3 as early stage and stages 4–6 as advanced stage. Based on the clinical phenotypes, patients were categorized into groups of transient ischemic attack (TIA), infarction, or hemorrhage. On the morning of the operation, peripheral blood samples were collected from all MMD patients after fasting for more than 12 h and analyzed using an automated hematology analyzer to measure white blood cells (WBC), lymphocytes (LY), neutrophils (NEUT), monocytes (MONO), platelets (PLT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), Calcium (Ca), Phosphorus(P), triglycerides (TG), total cholesterol (CHO), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A1 (ApoA1), apolipoprotein B (ApoB), and homocysteine (Hcy). Systemic immune-inflammatory markers, including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), and systemic immune-inflammatory index (SII), were calculated as described previously.
Treatment and changes in long-term prognosis
Our neurosurgical team performed three types of cerebral revascularization surgeries on patients: direct, indirect, and combined bypass. At our medical center, direct and combined bypass techniques were the preferred methods, with indirect bypass being selected in instances where the superficial temporal artery or middle cerebral artery was deemed too delicate for bypass surgery. Clinical presentation of patients was the main consideration for cerebral revascularization surgery, focusing on symptomatic hemispheres of the brain. Long-term prognosis were evaluated through face-to-face or telephone interviews conducted after discharge, with a median follow-up time of 29.27 months. Follow-up events included transient ischemic attack (TIA), ischemic stroke, and hemorrhagic stroke. We assessed patients’ neurological status using the modified Rankin Scale (mRS), where scores of 0–2 indicate favorable neurological status and 3–5 indicate unfavorable status. Two neurosurgery residents evaluated mRS scores of patients without knowledge of the surgical side or systemic immune-inflammatory markers and compared them with the mRS scores upon discharge. A stable or decreased mRS score compared to discharge was considered a favorable long-term prognosis, while an increased score was considered a unfavorable long-term prognosis.
Statistical analysis
We performed statistical analysis and graphical display using SPSS version 25.0 (IBM Corporation, Armonk, NY, United States), Empower(R) (www.empowerstats.com, X&Y Solutions, Inc., Boston, MA, United States), and Prism (GraphPad Prism Version 10.0 for Windows, GraphPad Software, Boston, MA, United States, www.graphpad). A two-tailed test with p < 0.05 was considered statistically significant. Baseline differences between the favorable and unfavorable long-term prognosis groups of patients with Moyamoya disease (MMD) were compared. Continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR), while categorical variables were expressed as frequencies. All participants were divided into three groups according to tertiles of systemic immune inflammatory markers: NLR (T1 ≤ 1.718, T2:1.718 to < 2.312, T3 ≥ 2.312); PLR (T1 ≤ 107.733, T2:107.733 to < 143.284, T3 ≥ 143.284); SII (T1 ≤ 391.057, T2:391.057 to <580.143, T3 ≥ 580.143); LMR (T1 ≤ 4.618, T2:4.618 to < 6.334, T3 ≥ 6.334).
Differences between continuous variables were analyzed using either a two-tailed Student’s t test or Mann–Whitney’s U test, while differences between categorical variables were assessed using either the Chi-square test or Fisher’s exact test. A logistic regression model was employed to identify independent factors influencing prognosis. The systemic immune inflammatory markers regression model served as the crude model, which was then adjusted in Model 1 to include age, gender, RNF213 p.R4810K variant, clinical phenotypes, Suzuki stage and surgical modality. Model 1 was further refined in Model 2 by incorporating hypertension, diabetes, hyperlipidemia, smoking, drinking, and homocysteine. Furthermore, the predictive performance of the model for long-term prognosis was evaluated by constructing a receiver operating characteristic (ROC) curve and calculating the area under the curve (AUC).
Results
Baseline characteristics of participants
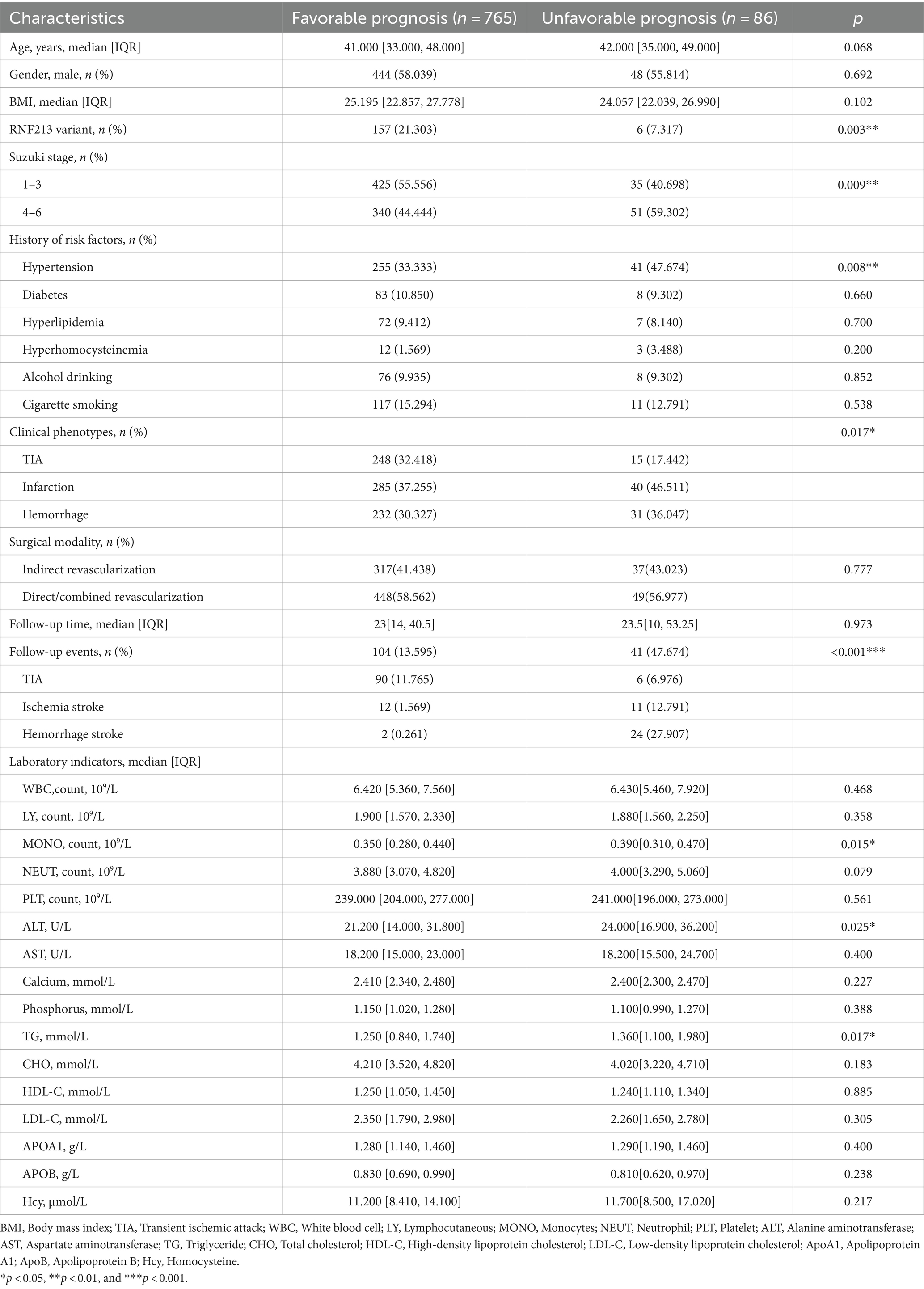
The baseline characteristics of Moyamoya disease (MMD) patients are presented in Table 1 and Supplementary material. Our study ultimately included 851 cases of MMD patients, with 765 cases classified as having favorable long-term prognosis (89.9%) and 86 cases classified as having unfavorable long-term prognosis (10.1%). Included in the study were a total of 851 participants, consisting of 359 men (42.2%) and 492 women (57.8%). The median age of the individuals was 41 years, with a range of 34–48 years. The presence of the RNF213 p.R4810K variant was more prevalent in the group with favorable long-term outcomes compared to those with unfavorable outcomes (21.303% vs. 7.317%, p = 0.003). There is a statistically significant difference in the risk of adverse prognosis between patients with early and advanced stages of Suzuki stage (p = 0.009). Hypertension, as a common risk factor for stroke, was more prevalent in the unfavorable long-term prognosis group compared to the favorable long-term prognosis group (47.674% vs. 33.333%, p = 0.008). Patients in the unfavorable long-term prognosis group had higher levels of MONO, ALT, and TG compared to the favorable long-term prognosis group (p = 0.015, p = 0.025, p = 0.017). Significant differences in long-term prognosis outcomes were observed among patients in the TIA group (p = 0.017), while no significant differences were observed in the ischemic and hemorrhagic groups. There were no significant statistical differences in the prevalence of smoking history, alcohol consumption, diabetes, dyslipidemia, or hyperhomocysteinemia between the two groups. Additionally, there were no significant statistical differences in WBC, LY, NEUT, PLT, AST, Ca, P, CHO, HDL-C, LDL-C, ApoA1, ApoB, or Hcy levels between the two groups.
The overall median follow-up time was 23 [13, 42] months, with no significant statistical difference in median follow-up time between the favorable and unfavorable long-term prognosis groups (23 [14, 40.5] vs. 23.5 [10, 53.25], p = 0.268). The rate of stroke events during follow-up was significantly higher in the unfavorable long-term prognosis group compared to the favorable long-term prognosis group (47.674% vs. 13.595%, p < 0.001).
Relationship between systemic immune-inflammatory marker levels and unfavorable long-term prognosis
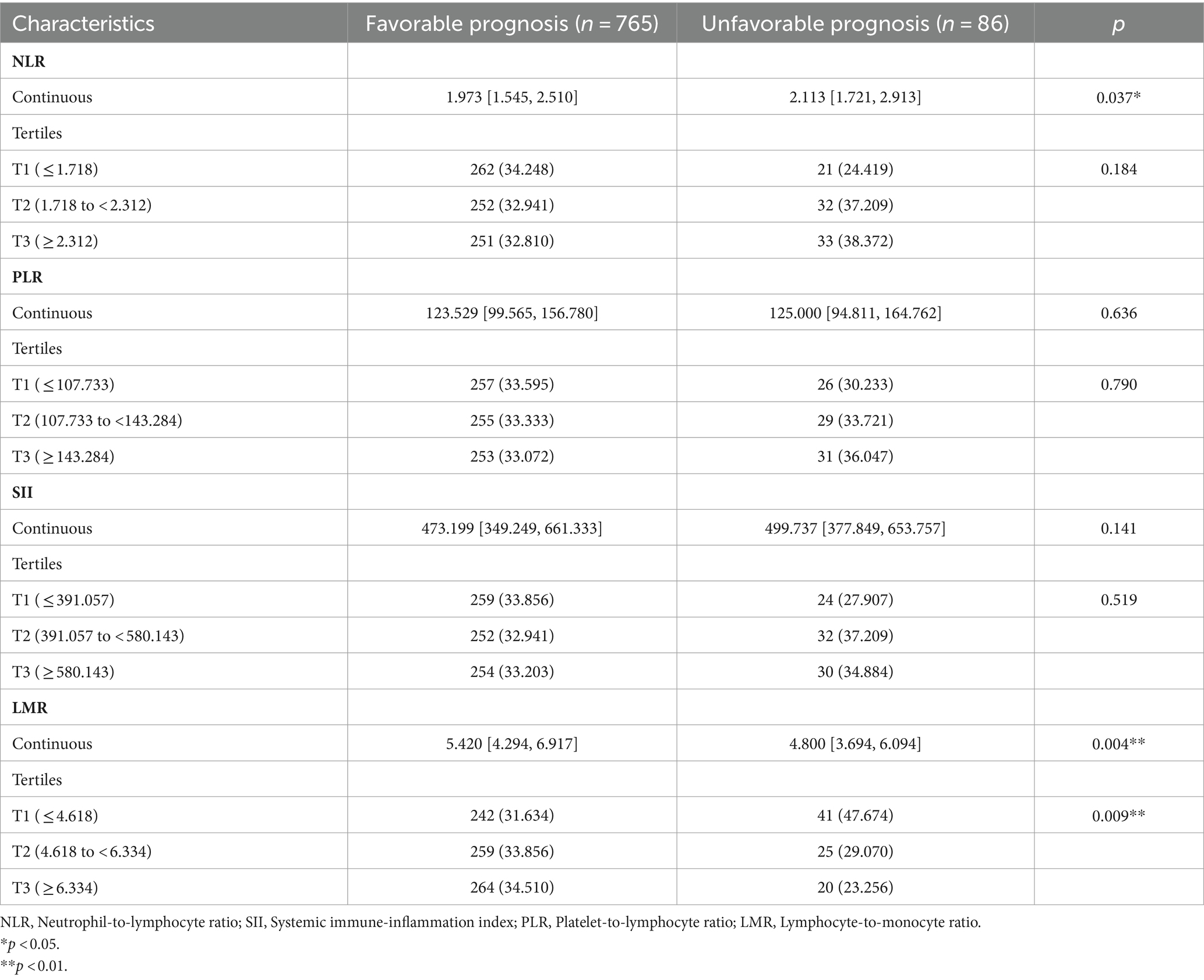
Figure 2 shows the differences in NLR, PLR, SII, and LMR between the two groups. In the unfavorable long-term prognosis group, NLR levels were significantly higher than those in the favorable long-term prognosis group (2.113 [1.721, 2.913] vs. 1.973 [1.545, 2.510], p = 0.037), while LMR levels were lower than those in the favorable long-term prognosis group (4.800 [3.694, 6.094] vs. 5.420 [4.294, 6.917], p = 0.004). There were no significant differences in SII and PLR values between the two groups (p > 0.05). The differences between systemic immune inflammatory marker levels in the favorable and unfavorable prognosis groups are shown in Table 2. In the low-level LMR group, 41 individuals had a poor mid- and long-term prognosis, representing 47.674% of the group. The medium-level group had 25 people with an unfavorable long-term prognosis, accounting for 29.070%, while the high-level group had 20 individuals with an unfavorable long-term prognosis, making up 23.256%. The statistical analysis showed a significant difference between the groups (p = 0.009), indicating a strong association between LMR levels and long-term prognosis. Interestingly, there was no disparity in the risk of unfavorable long-term prognosis across the NLR, SII, and PLR groups, suggesting a lack of clear relationship between these immune-inflammatory markers and long-term prognosis.
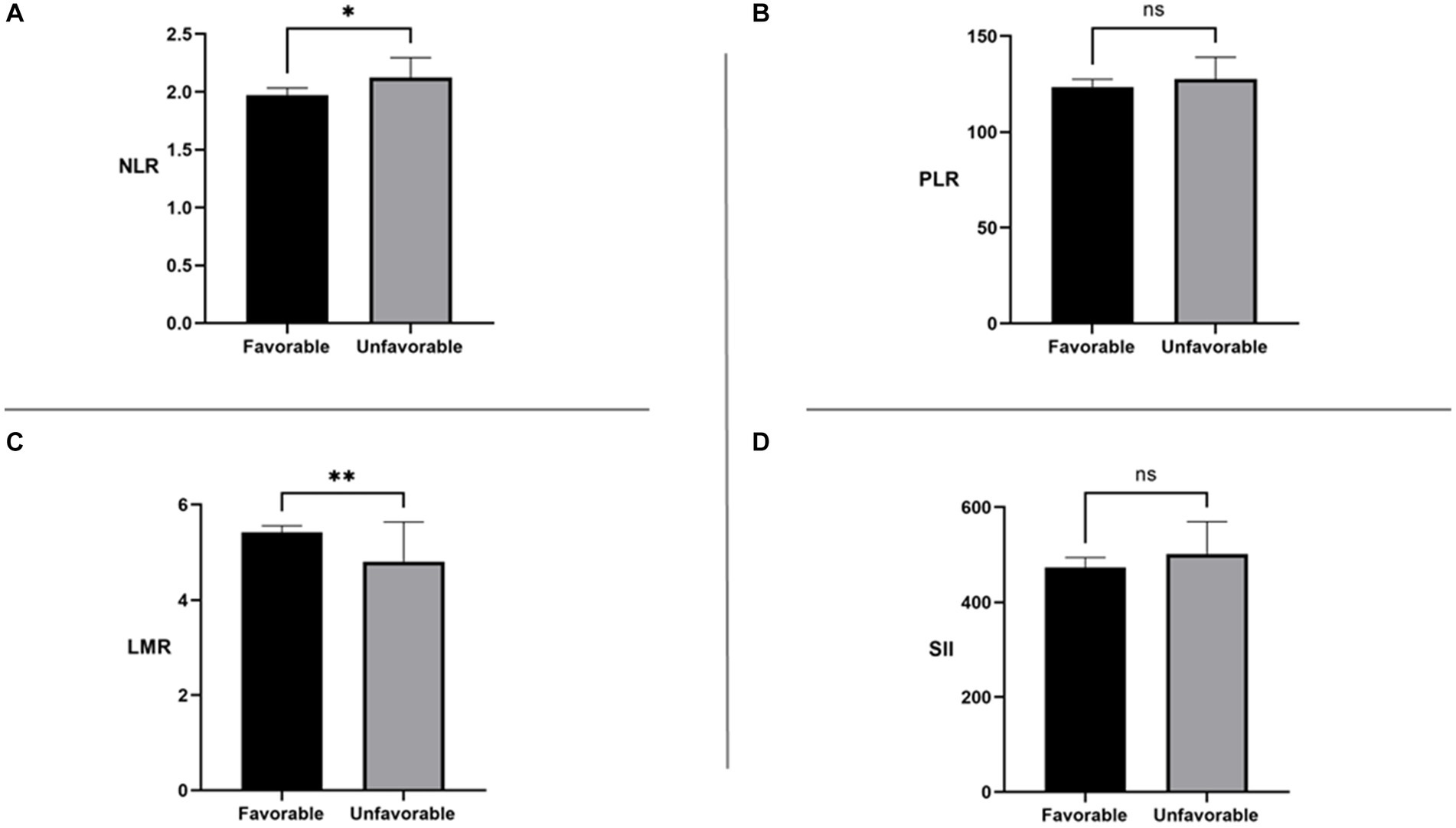
Figure 2. NLR: neutrophil-to-lymphocyte ratio; SII: systemic immune-inflammation index; PLR: platelet-to-lymphocyte ratio; LMR: lymphocyte-to-monocyte ratio. ns, No significance *p < 0.05 **p < 0.01.
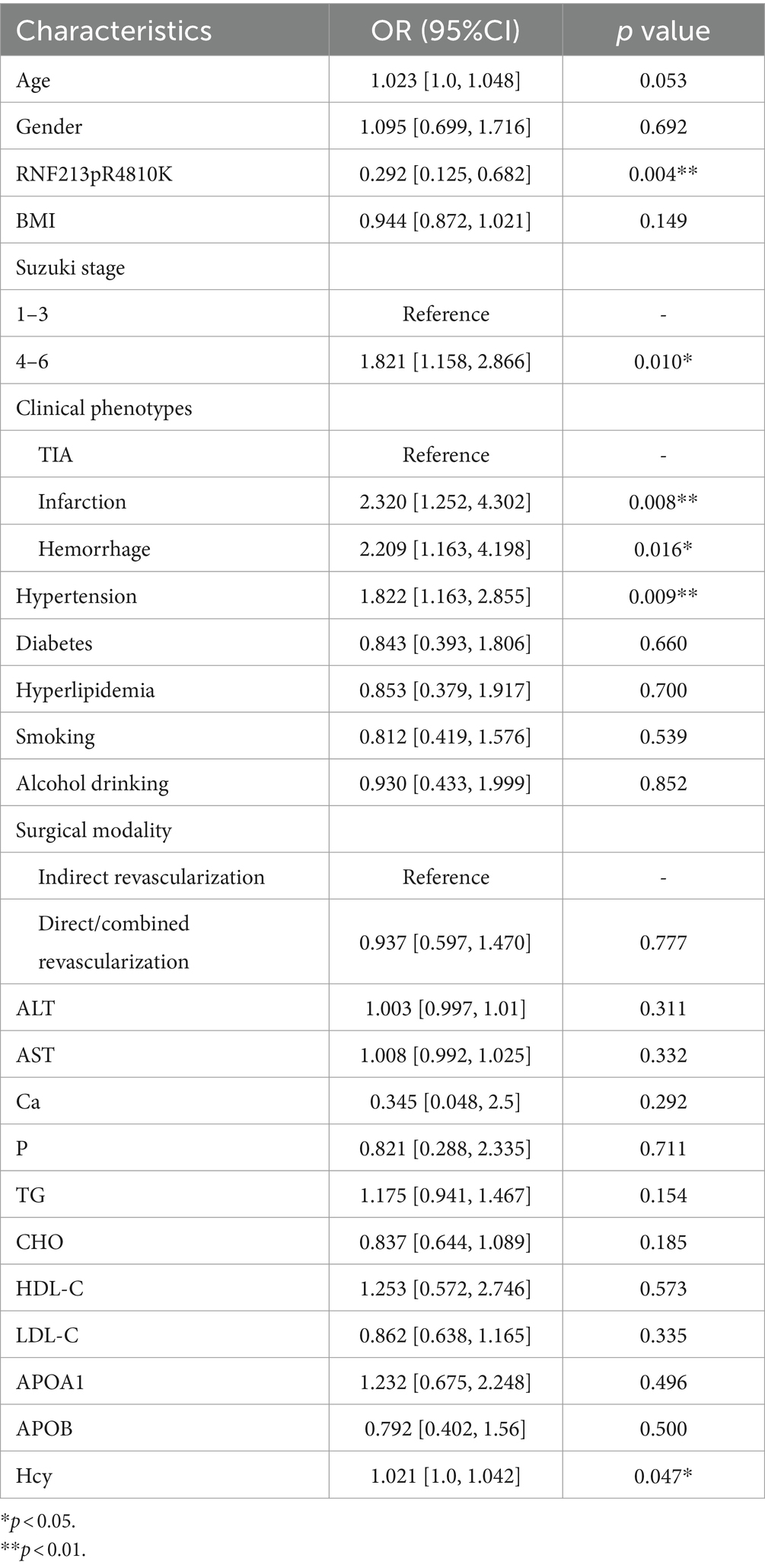
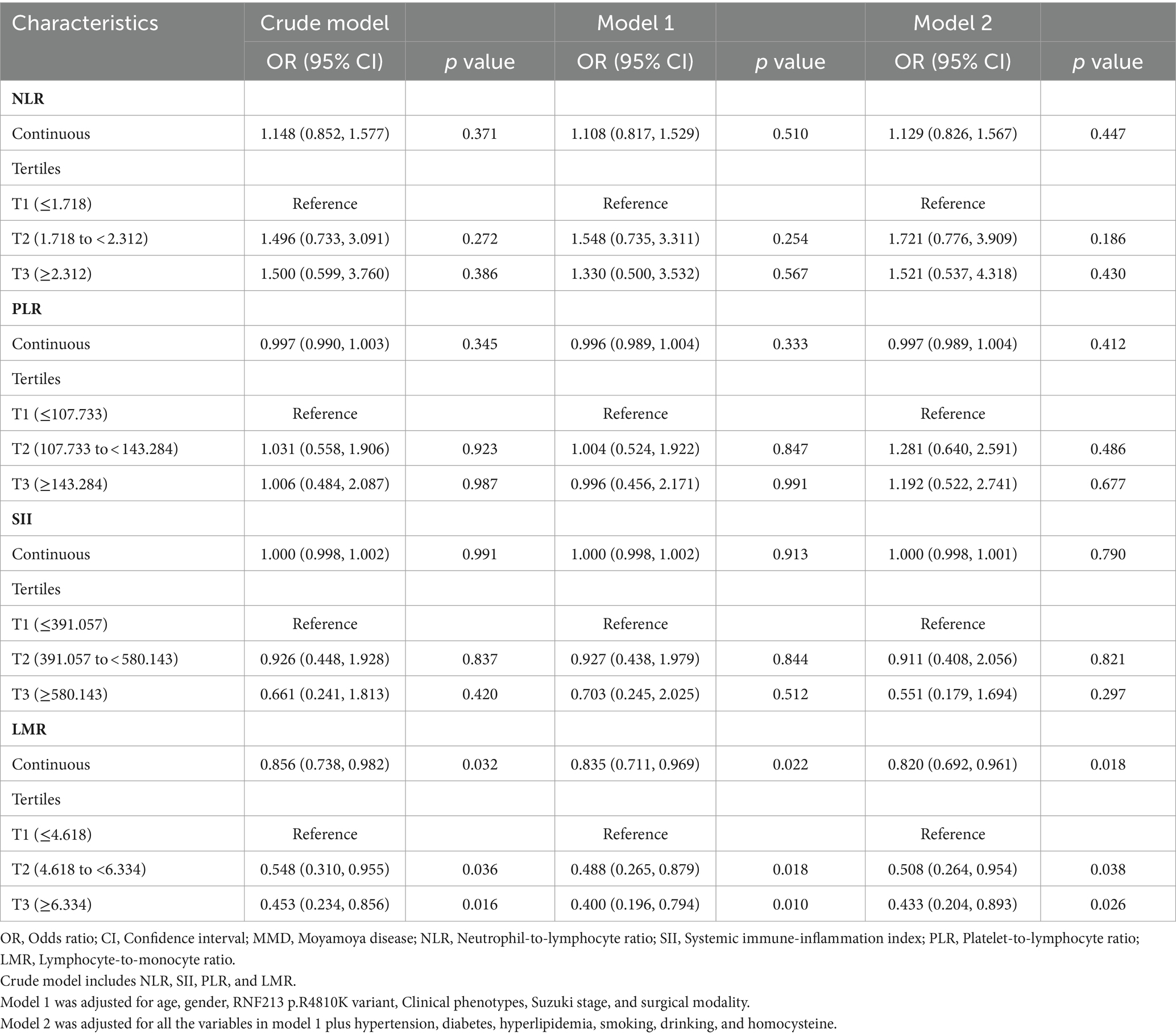
We performed univariate logistic regression analysis of systemic immune-inflammatory markers and some factors that may be associated with the risk of unfavorable prognosis (Tables 3, 4). Identify statistically significant factors and factors previously documented in the literature as being linked to poor prognosis, then conduct multifactor logistic regression analysis on them alongside systemic immune and inflammatory markers. Logistic regression analysis incorporated systemic immune and inflammatory markers as continuous variables (Table 5). The final adjusted model 2 indicated that elevated LMR levels are protective factors for long-term prognosis (OR = 0.820, 95% CI [0.692–0.961], p = 0.018), and patients with RNF213 mutations had a lower risk of unfavorable long-term prognosis compared to those without these mutations (OR = 0.301, 95% CI [0.102–0.707], p = 0.015). Additionally, homocysteine levels, clinical phenotypes, and Suzuki stage are associated with the risk of unfavorable long-term prognosis. No significant relationships were found between other systemic immune-inflammatory indicators and long-term prognosis. The AUC = 0.742 (95% CI [0.686–0.799]) (Figure 3).
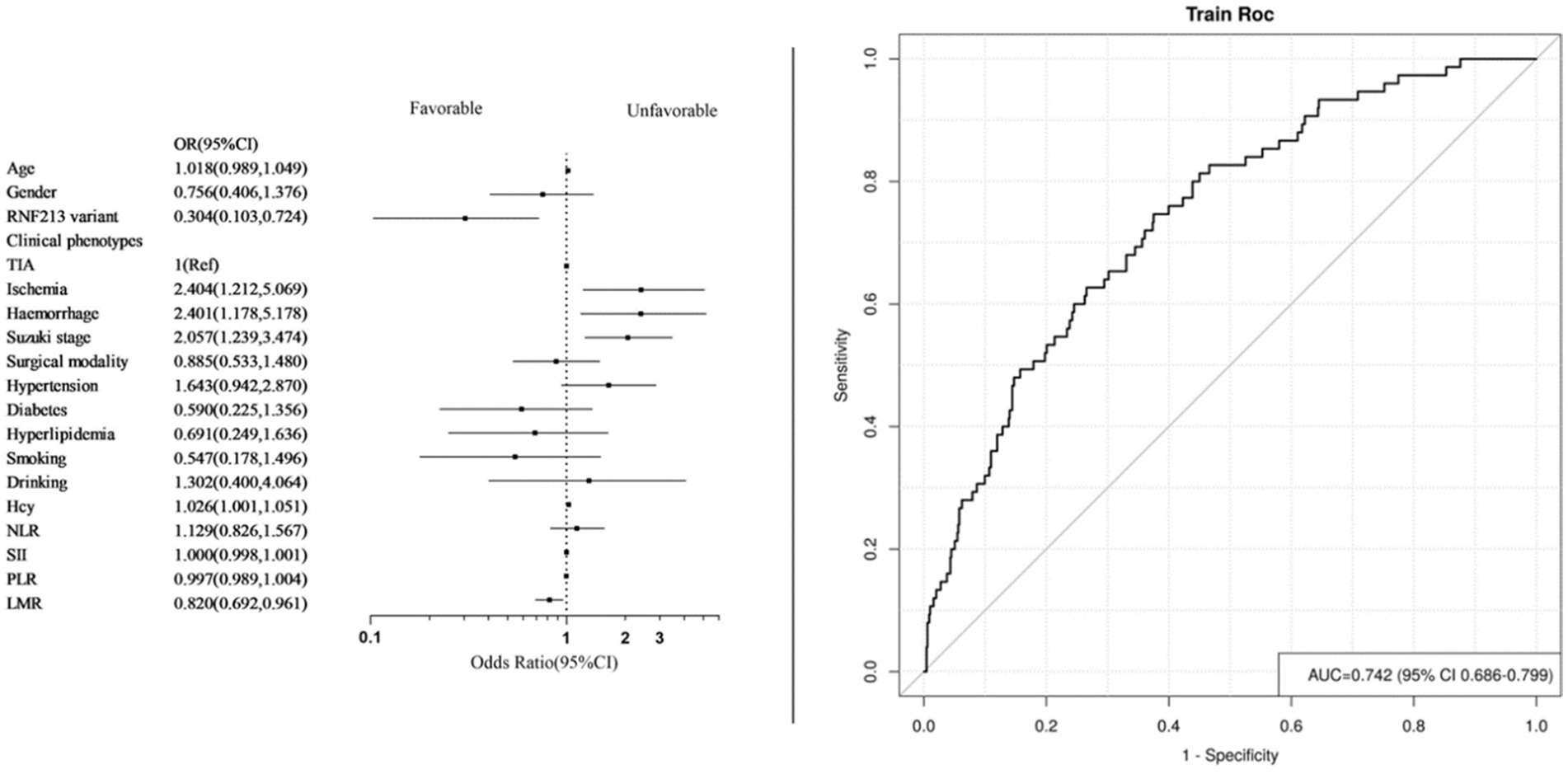
Figure 3. Forest plot and ROC curve of model 2. (Systemic immune inflammatory markers as continuous variables).
To address individual variability in systemic immune and inflammatory markers derived from blood tests, these markers were categorized into low (T1), medium (T2), and high levels (T3) for further analysis (Table 5). The final adjusted model 2 results revealed that compared with the T1 group, the increased LMR levels in the T3 and T2 groups are protective factors for long-term prognosis (T2: OR = 0.508, 95% CI [0.264–0.954], p = 0.038; T3: OR = 0.433, 95% CI [0.204–0.893], p = 0.026). The AUC = 0.750 (95% CI [0.693–0.806]) (Figure 4).
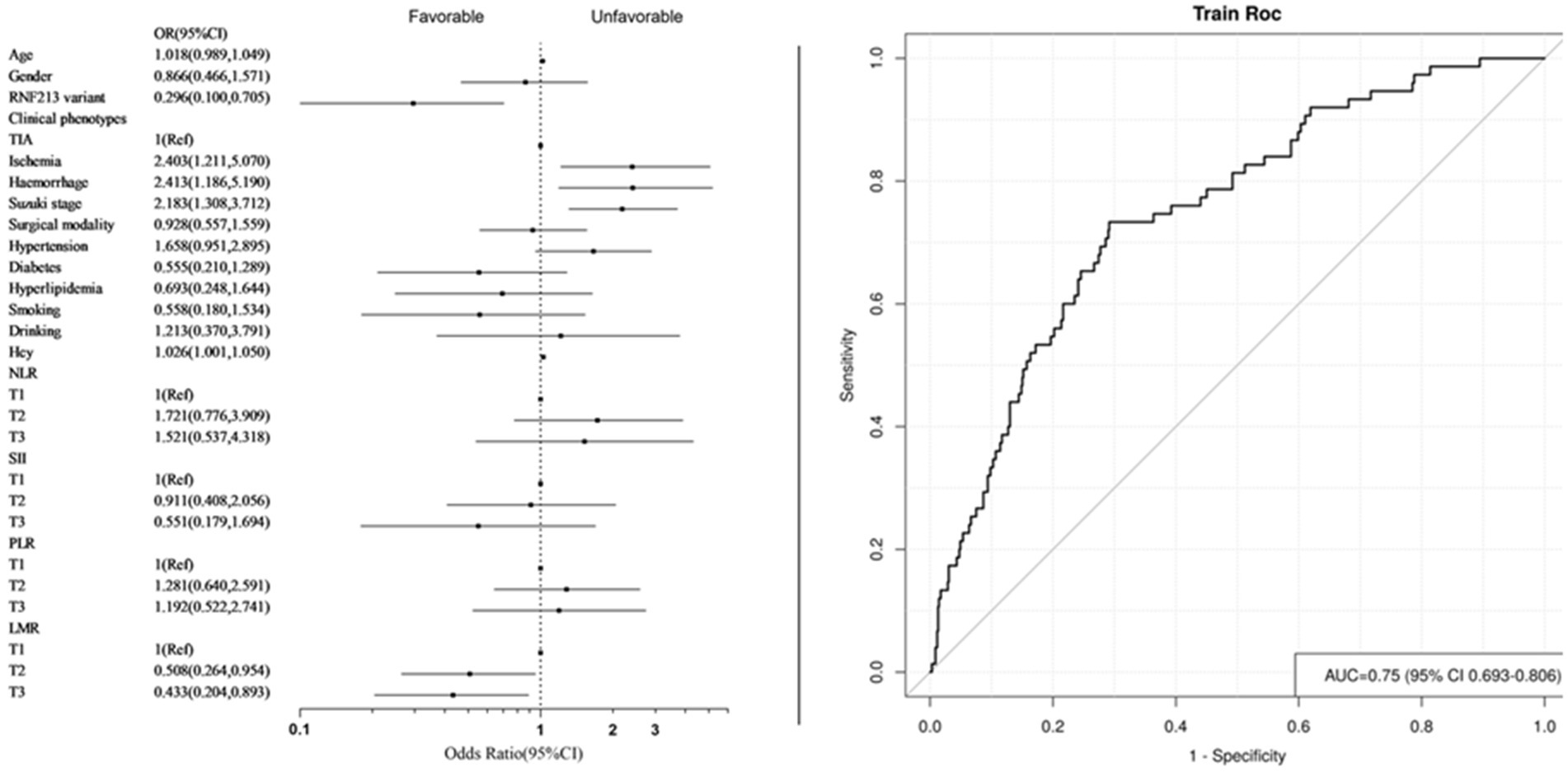
Figure 4. Forest plot and ROC curve of model 2. (Systemic immune inflammation markers as categorical variables).
Discussion
The etiology and pathogenesis of Moyamoya disease (MMD) remain unclear and are widely regarded as a multifactorial disease resulting from genetic, immune, inflammatory, and other factors. The primary treatment for MMD revolves around surgical intervention, with cerebral revascularization being the mainstay. Patients undergoing cerebral revascularization surgery experience a significant reduction in the risk of stroke occurrence (4). The long-term prognosis of patients with Moyamoya disease (MMD) after surgery is challenging to predict. Previous research has attempted to establish long-term prognosis prediction models based on genetic factors, clinical characteristics, and nutritional indices (6, 7, 9). However, insufficient consideration has been given to the impact of systemic immune-inflammatory factors on long-term prognosis.
This study included 851 subjects who underwent cerebral revascularization surgery and found significant differences in neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) between the groups with favorable and unfavorable long-term prognosis. NLR levels were higher in the unfavorable long-term prognosis group compared to the favorable group, while LMR levels were lower in the unfavorable long-term prognosis group. However, systemic immune-inflammatory index (SII) and platelet-to-lymphocyte ratio (PLR) showed no significant differences between the two groups. Logistic regression analysis revealed that LMR serves as an independent risk factor in predicting unfavorable long-term prognosis. Furthermore, as the level of LMR increases, the risk of unfavorable long-term prognosis decreases. No significant relationship was observed between other inflammation markers and long-term prognosis. Therefore, LMR is considered a novel indicator for predicting long-term prognosis after cerebral revascularization surgery in Moyamoya disease patients. The lymphocyte-to-monocyte ratio (LMR) is easily obtainable in clinical practice and has been widely used as a biomarker for predicting patient outcomes in various fields including cerebrovascular diseases, cancer, and neurology (12, 15–18). All suggest that lower LMR levels are indicative of worse long-term outcomes in patients, although the specific mechanisms behind this remain unclear.
In recent years, there has been a growing focus on neuroinflammation, with numerous studies demonstrating the significant impact of inflammatory processes on the initiation and progression of ischemic stroke (19–22). It is believed that lymphocyte counts have a neuroprotective effect and can enhance neurological function, while monocytes and neutrophils may exacerbate symptoms (23–25). Research has indicated that ischemia of the brain has the potential to inhibit the functioning of the lymphatic system. Consequently, this can elevate the likelihood of infections occurring after a stroke, which ultimately serves as a leading contributor to the mortality rate among individuals who have suffered a stroke (26). During the acute phase of ischemic stroke, the inflammatory response plays a dual role by inducing cell death and tissue damage while also triggering a preconditioning state in the brain to exert self-protective effects. Inflammatory mediators automatically restrain the progression of pathological processes and facilitate the repair of damaged tissues (27). The physiological reactions post-stroke may be ascribed to the extravasation of leukocytes and the discharge of various inflammatory agents, setting off a cascade of events that culminates in neuronal demise or programmed cell death, leading to unfavorable outcomes for stroke sufferers (28). An increase in lymphocyte levels can elevate anti-inflammatory proteins such as cytokine interleukin (IL)-10, while simultaneously inhibiting the expression of pro-inflammatory proteins like IL-6 and tumor necrosis factor-alpha (TNF-α), thereby producing neuroprotective effects (29, 30). Post-stroke systemic stress leads to a decrease in lymphocyte count, promoting the activation of the renin-angiotensin system, resulting in cortisol release, which induces lymphocyte apoptosis (31). A dip in lymphocyte levels post-acute ischemic stroke is linked to postponed brain harm and deteriorated functional results, ultimately resulting in adverse outcomes for stroke patients (32–34). However, our research discovered that there was no noteworthy variance in lymphocyte count between the group exhibiting favorable long-term prognosis and the group showing unfavorable long-term prognosis. On the other hand, the monocyte count was markedly elevated in the group with unfavorable long-term prognosis when juxtaposed with the group with favorable long-term prognosis, leading to a conspicuous contrast in LMR levels. This disparity in LMR between the two groups may be primarily attributed to the aforementioned factor.
Monocytes, serving as prime contributors to systemic inflammatory reactions, are classified into three principal groupings in the human populace and fulfill distinct roles in the pathophysiology of ischemia (35). In human, monocytes are regrouped in three main subsets based on their CD14 and CD16 expression levels, which are the classical subset (CD14++CD16−), the intermediate subset (CD14++CD16+), and the non-classical subset (CD14+CD16++). CD14++CD16− monocytes, identified by elevated CCR2 chemokine receptor levels and low CX3CR1 expression, add to inflammation by discharging cytokines like IL-1β, IL-6, and TNF-α, while CD14+CD16++ monocytes counteract inflammation by producing IL-10 (36). In the aftermath of a stroke event, an upsurge in classical monocytes is an autonomous forecaster of unfavorable outcomes at the three-month mark, whereas a greater proportion of non-conventional monocytes is linked to improved outcomes following the onset of acute ischemic stroke (37). Furthermore, there is a positive relationship between total monocyte counts and mRS scores at the three-month point, indicating that higher monocyte counts are related to unfavorable functional prognosis (38).
Moyamoya disease (MMD) is an important etiology of stroke in young adults in Southeast Asia. Therefore, integrating lymphocyte and monocyte counts into the lymphocyte-to-monocyte ratio (LMR) as a novel predictor of long-term prognosis after surgical treatment for MMD is highly meaningful. Our study revealed, for the first time, that LMR levels are independent risk factors for unfavorable long-term prognosis. Decreased LMR levels often lead to worse outcomes.
Nonetheless, our research encountered several constraints. Primarily, it was a retrospective investigation carried out at a sole research facility, exclusively involving grown-ups while omitting minors. This could potentially impede its effectiveness and introduce biases in selection. Secondly, we only calculated systemic immune-inflammatory markers at admission without dynamic monitoring and observation, which may affect the correlation between LMR in MMD patients and long-term prognosis. Thirdly, we only used the modified Rankin Scale (mRS) to assess functional outcomes, which may not provide a detailed definition of favorable and unfavorable long-term prognosis.
Conclusion
Systemic immune and inflammatory markers that can be easily obtained in a clinical setting play a crucial role in predicting the risk of Moyamoya disease and post-surgery prognosis. Our study shows that lymphocyte-to-monocyte ratio (LMR) levels are independently linked to an increased risk of unfavorable long-term prognosis, highlighting LMR as a new and effective predictor for postoperative moyamoya patients. It is essential to conduct future prospective studies with larger, multicenter cohorts to validate our results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee at Beijing Tiantan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study (KY2022-051-08).
Author contributions
SW: Conceptualization, Data curation, Writing – original draft. WL: Data curation, Methodology, Writing – original draft. YZ: Investigation, Writing – review & editing. CL: Investigation, Writing – review & editing. PG: Supervision, Writing – review & editing. DZ: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Key Research and Development Program of China, grant number 2021YFC2500502.
Acknowledgments
We acknowledge all individuals for their participation in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1418729/full#supplementary-material
References
1. Ihara, M, Yamamoto, Y, Hattori, Y, Liu, W, Kobayashi, H, Ishiyama, H, et al. Moyamoya disease: diagnosis and interventions. Lancet Neurol. (2022) 21:747–58. doi: 10.1016/S1474-4422(22)00165-X
2. Mikami, T, Suzuki, H, Komatsu, K, and Mikuni, N. Influence of inflammatory disease on the pathophysiology of Moyamoya disease and quasi-moyamoya disease. Neurol Med Chir (Tokyo). (2019) 59:361–70. doi: 10.2176/nmc.ra.2019-0059
3. Pandey, P, and Steinberg, GK. Neurosurgical advances in the treatment of Moyamoya disease. Stroke. (2011) 42:3304–10. doi: 10.1161/strokeaha.110.598565
4. Scott, RM, and Smith, ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. (2009) 360:1226–37. doi: 10.1056/NEJMra0804622
5. Yoshida, Y, Yoshimoto, T, Shirane, R, and Sakurai, Y. Clinical course, surgical management, and long-term outcome of moyamoya patients with rebleeding after an episode of intracerebral hemorrhage: an extensive follow-up study. Stroke. (1999) 30:2272–6. doi: 10.1161/01.str.30.11.2272
6. Ge, P, Ye, X, Liu, X, Deng, X, Wang, R, Zhang, Y, et al. Association between p.R4810K variant and long-term clinical outcome in patients with Moyamoya disease. Front Neurol. (2019) 10:662. doi: 10.3389/fneur.2019.00662
7. Yu, X, Ge, P, Zhai, Y, Wang, R, Zhang, Y, and Zhang, D. Hypo-high density lipoproteinemia is a predictor for recurrent stroke during the long-term follow-up after revascularization in adult moyamoya disease. Front Neurol. (2022) 13:891622. doi: 10.3389/fneur.2022.891622
8. Zhao, M, Deng, X, Gao, F, Zhang, D, Wang, S, Zhang, Y, et al. Ischemic stroke in young adults with Moyamoya disease: prognostic factors for stroke recurrence and functional outcome after revascularization. World Neurosurg. (2017) 103:161–7. doi: 10.1016/j.wneu.2017.03.146
9. Yu, X, Ge, P, Zhai, Y, Liu, W, Zhang, Q, Ye, X, et al. The prognostic nutrition index is a predictor for long-term outcomes after revascularization in adult moyamoya disease. Acta Neurochir. (2023) 165:3623–30. doi: 10.1007/s00701-023-05816-y
10. Graziano, V, Grassadonia, A, Iezzi, L, Vici, P, Pizzuti, L, Barba, M, et al. Combination of peripheral neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast. (2019) 44:33–8. doi: 10.1016/j.breast.2018.12.014
11. Mouchli, M, Reddy, S, Gerrard, M, Boardman, L, and Rubio, M. Usefulness of neutrophil-to-lymphocyte ratio (NLR) as a prognostic predictor after treatment of hepatocellular carcinoma. Ann Hepatol. (2021) 22:100249. doi: 10.1016/j.aohep.2020.08.067
12. Mei, P, Feng, W, Zhan, Y, and Guo, X. Prognostic value of lymphocyte-to-monocyte ratio in gastric cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol. (2023) 14:1321584. doi: 10.3389/fimmu.2023.1321584
13. Ferro, D, Matias, M, Neto, J, Dias, R, Moreira, G, Petersen, N, et al. Neutrophil-to-lymphocyte ratio predicts cerebral edema and clinical worsening early after reperfusion therapy in stroke. Stroke. (2021) 52:859–67. doi: 10.1161/STROKEAHA.120.032130
14. Liu, W, Liu, C, Yu, X, Zhai, Y, He, Q, Li, J, et al. Association between systemic immune-inflammatory markers and the risk of moyamoya disease: a case-control study. Ann Med. (2023) 55:2269368. doi: 10.1080/07853890.2023.2269368
15. Kandathil, SA, Peter Truta, I, Kadletz-Wanke, L, Heiduschka, G, Stoiber, S, Kenner, L, et al. Lymphocyte-to-monocyte ratio might serve as a prognostic marker in young patients with tongue squamous cell carcinoma. J Pers Med. (2024) 14. doi: 10.3390/jpm14020159
16. Li, F, Weng, G, Zhou, H, Zhang, W, Deng, B, Luo, Y, et al. The neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and neutrophil-to-high-density-lipoprotein ratio are correlated with the severity of Parkinson's disease. Front Neurol. (2024) 15:1322228. doi: 10.3389/fneur.2024.1322228
17. Shi, X, Yang, L, Bai, W, Jing, L, and Qin, L. Evaluating early lymphocyte-to-monocyte ratio as a predictive biomarker for delirium in older adult patients with sepsis: insights from a retrospective cohort analysis. Front Med. (2024) 11:1342568. doi: 10.3389/fmed.2024.1342568
18. Ren, H, Liu, X, Wang, L, and Gao, Y. Lymphocyte-to-monocyte ratio: a novel predictor of the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:2595–602. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.019
19. Chamorro, A, Dirnagl, U, Urra, X, and Planas, AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. (2016) 15:869–81. doi: 10.1016/S1474-4422(16)00114-9
20. Becker, KJ, and Buckwalter, M. Stroke, inflammation and the immune response: Dawn of a new era. Neurotherapeutics. (2016) 13:659–60. doi: 10.1007/s13311-016-0478-7
21. Shi, K, Tian, DC, Li, ZG, Ducruet, AF, Lawton, MT, and Shi, FD. Global brain inflammation in stroke. Lancet Neurol. (2019) 18:1058–66. doi: 10.1016/S1474-4422(19)30078-X
22. Rust, R, Gronnert, L, and Schwab, ME. Inflammation after stroke: a local rather than systemic response? Trends Neurosci. (2018) 41:877–9. doi: 10.1016/j.tins.2018.09.011
23. Gong, P, Xie, Y, Jiang, T, Liu, Y, Wang, M, Sun, H, et al. Neutrophil-lymphocyte ratio predicts post-thrombolysis early neurological deterioration in acute ischemic stroke patients. Brain Behav. (2019) 9:e01426. doi: 10.1002/brb3.1426
24. Duan, Z, Wang, H, Wang, Z, Hao, Y, Zi, W, Yang, D, et al. Neutrophil-lymphocyte ratio predicts functional and safety outcomes after endovascular treatment for acute ischemic stroke. Cerebrovasc Dis. (2018) 45:221–7. doi: 10.1159/000489401
25. Yamamoto, Y, Osanai, T, Nishizaki, F, Sukekawa, T, Izumiyama, K, Sagara, S, et al. Matrix metalloprotein-9 activation under cell-to-cell interaction between endothelial cells and monocytes: possible role of hypoxia and tumor necrosis factor-alpha. Heart Vessel. (2012) 27:624–33. doi: 10.1007/s00380-011-0214-5
26. Santos Samary, C, Pelosi, P, Leme Silva, P, and Rieken Macedo Rocco, P. Immunomodulation after ischemic stroke: potential mechanisms and implications for therapy. Crit Care. (2016) 20:391. doi: 10.1186/s13054-016-1573-1
27. Vidale, S, Consoli, A, Arnaboldi, M, and Consoli, D. Postischemic inflammation in acute stroke. J Clin Neurol. (2017) 13:1–9. doi: 10.3988/jcn.2017.13.1.1
28. Anrather, J, and Iadecola, C. Inflammation and stroke: an overview. Neurotherapeutics. (2016) 13:661–70. doi: 10.1007/s13311-016-0483-x
29. Liesz, A, Suri-Payer, E, Veltkamp, C, Doerr, H, Sommer, C, Rivest, S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. (2009) 15:192–9. doi: 10.1038/nm.1927
30. Li, P, Mao, L, Zhou, G, Leak, RK, Sun, BL, Chen, J, et al. Adoptive regulatory T-cell therapy preserves systemic immune homeostasis after cerebral ischemia. Stroke. (2013) 44:3509–15. doi: 10.1161/STROKEAHA.113.002637
31. Balci, KG, Balci, MM, Arslan, U, Açar, B, Maden, O, Selcuk, H, et al. Increased platelet-to-lymphocyte ratios and low relative lymphocyte counts predict appropriate shocks in heart failure patients with ICDs. Acta Cardiol Sin. (2016) 32:542–9. doi: 10.6515/acs20151012b
32. Kim, J, Song, TJ, Park, JH, Lee, HS, Nam, CM, Nam, HS, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis. (2012) 222:464–7. doi: 10.1016/j.atherosclerosis.2012.02.042
33. Chiu, NL, Kaiser, B, Nguyen, YV, Welbourne, S, Lall, C, and Cramer, SC. The volume of the spleen and its correlates after acute stroke. J Stroke Cerebrovasc Dis. (2016) 25:2958–61. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.012
34. Kleinschnitz, C, Kraft, P, Dreykluft, A, Hagedorn, I, Göbel, K, Schuhmann, MK, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. (2013) 121:679–91. doi: 10.1182/blood-2012-04-426734
35. ElAli, A, and Jean, LBN. The role of monocytes in ischemic stroke pathobiology: new avenues to explore. Front Aging Neurosci. (2016) 8:29. doi: 10.3389/fnagi.2016.00029
36. Naert, G, and Rivest, S. A deficiency in CCR2+ monocytes: the hidden side of Alzheimer's disease. J Mol Cell Biol. (2013) 5:284–93. doi: 10.1093/jmcb/mjt028
37. Urra, X, Villamor, N, Amaro, S, Gómez-Choco, M, Obach, V, Oleaga, L, et al. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab. (2009) 29:994–1002. doi: 10.1038/jcbfm.2009.25
Keywords: systemic immune-inflammatory markers, Moyamoya disease, long-term prognosis, lymphocyte-to-monocyte ratio (LMR), stroke
Citation: Wang S, Liu W, Zhai Y, Liu C, Ge P and Zhang D (2024) Systemic immune-inflammatory markers and long-term prognosis after revascularization in Moyamoya disease: a retrospective study. Front. Neurol. 15:1418729. doi: 10.3389/fneur.2024.1418729
Edited by:
Anders Lewén, Uppsala University, SwedenReviewed by:
Fei Ye, National University of Singapore, SingaporeJianming Cai, People’s Liberation Army General Hospital, China
Copyright © 2024 Wang, Liu, Zhai, Liu, Ge and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peicong Ge, Z2VwZWljb25nQDE2My5jb20=; Dong Zhang, emhhbmdkb25nMDY2MEBhbGl5dW4uY29t
†These authors have contributed equally to this work and share first authorship
 Shuang Wang
Shuang Wang Wei Liu
Wei Liu Yuanren Zhai
Yuanren Zhai Chenglong Liu
Chenglong Liu Peicong Ge
Peicong Ge Dong Zhang
Dong Zhang