
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 05 July 2024
Sec. Endovascular and Interventional Neurology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1414898
This article is part of the Research TopicAdvances and controversies in ischemic stroke management: from prevention to diagnosis and acute treatmentView all 95 articles
 Guangzhi Liu1,2†
Guangzhi Liu1,2† Jianghui Cao3†
Jianghui Cao3† Peiyang Zhou2
Peiyang Zhou2 Dong Sun1
Dong Sun1 Zhiming Kang1
Zhiming Kang1 Ruixue Fan2
Ruixue Fan2 Bin Mei1*
Bin Mei1* Junjian Zhang1*
Junjian Zhang1*Background: The density of contrast medium in digital subtraction angiography (DSA) have been used to evaluate the cerebral circulation function. Our aim was to study the effect of difference in arteriovenous peak optical density (POD) after thrombectomy on functional outcomes.
Methods: Consecutive patients with acute ischemic stroke due to large vessel occlusion who underwent thrombectomy were reviewed. We processed DSA images with ImageJ software to measure the POD of internal carotid artery (ICA) and cortical veins. The average POD of cortical veins (PODVA) and the POD difference between ICA and cortical veins (PODICA-CV) were calculated. Primary outcome was good functional outcome (modified Rankin scale score of 0–2 at 90 days).
Results: One hundred sixty-six patients were finally included in the study. Patients with good functional outcome had lower ipsilateral PODVA (median [interquartile range (IQR)], 257.198 [216.623–296.631] vs. 290.944 [248.647–338.819], p < 0.001) and lower ipsilateral PODICA-CV (median [IQR], 128.463 [110.233–153.624] vs. 182.01 [146.621–211.331], p < 0.001). Multivariable logistic regression analyses showed that ipsilateral PODVA (odds ratio [OR] 0.991, 95% confidence interval [CI] 0.984–0.999, p = 0.019) and ipsilateral PODICA-CV (OR 0.975, 95% CI 0.963–0.986, p < 0.001) were associated with good functional outcome. The predictive ability was significantly enhanced in the model including ipsilateral PODICA-CV (0.893 vs. 0.842, p = 0.027). No correlation was found between ipsilateral PODICA-CV and expanded Thrombolysis in Cerebral Infarction grades (r = −0.133, p = 0.099).
Conclusion: Ipsilateral PODICA-CV is an additional indicator of cerebral reperfusion status and predicts functional outcomes after thrombectomy.
Thrombectomy has been identified as an effective treatment for patients with acute ischemic stroke due to large vessel occlusion (AIS-LVO) (1). However, despite the high rate of successfully reopening the blocked arteries and the use of various predictors such as arterial collaterals, initial core infarction, and penumbra tissue in patient selection, only around half of the patients actually benefit from thrombectomy (2, 3). Successful arterial recanalization is not enough to ensure adequate cerebral tissue reperfusion. The CHOICE trial has shown that using alteplase directly into the artery after successful thrombectomy can further improve blood flow in the small vessels and reduce the risk of hypoperfusion (4). In previous animal studies, downstream vascular dysfunction caused by microclots, compression, contraction, inflammation and reactive oxygen species has been verified as a major factor contributing to poor microcirculatory reperfusion, leading to the phenomenon known as “non-reflow” (5, 6). Therefore, it is necessary to evaluate the condition of the downstream vessels after thrombectomy to accurately assess the success of the procedure.
However, current methods for assessing downstream vascular function are not uniform. Even if patients show signs of poor downstream vascular function, they are less likely to receive timely treatment (5). There is a crucial need for a method that can quickly and effectively assess the status of downstream vessels after thrombectomy. One promising approach is to assess the cerebral venous outflow (VO). Since all blood in the brain eventually drains into the venous system, changes in cerebral VO can occur following a blockage in the upstream vessels. These changes have been observed on computed tomography angiography (CTA) images in patients with AIS-LVO and correlated with the hypoperfusion intensity ratio which is superior to arterial collateral in the assessment of tissue perfusion (7). After successful arterial recanalization, the microcirculatory disturbance can also be reflected by VO. The postoperative time to peak density (TTP) of contrast medium in the cortical vein has been found as an independent predictor of good functional outcome. Moreover, the arteriovenous TTP difference significantly improved the predictive ability for outcomes after thrombectomy (8). These results emphasize the effectiveness of comprehensive evaluation including arterial and venous circulation in gathering more information about the cerebral reperfusion. However, it is important to note that this method evaluates a distal branch of middle cerebral artery and a specific vein, resulting in a limited assessment of brain tissue. Furthermore, the processing of data obtained through this method requires the use of commercially available software.
To address this issue, we introduced a novel method to reflect the postoperative vascular function. We used ImageJ software to measure the venous peak optical density (POD) and the difference with arterial POD on digital subtraction angiography (DSA) images immediately after thrombectomy in patients with different prognosis. Our hypothesis is that the POD difference can serve as a reliable indicator to assess the effectiveness of cerebral reperfusion therapy and predict functional outcomes in patients with AIS-LVO.
This study was approved by our institutional ethical committee. The study included consecutive patients with Acute Ischemic Stroke with Large Vessel Occlusion (AIS-LVO) who underwent thrombectomy at our center between July 2018 and July 2022. The patients were reviewed retrospectively. We included patients who met the following criteria: (1) had occlusion in the terminal internal carotid artery (ICA) or proximal middle cerebral artery (MCA); (2) received thrombectomy within 24 h after the onset of symptoms; (3) achieved successful recanalization with an expanded Thrombolysis in Cerebral Infarction grade (eTICI) of 2b-3; (4) had baseline head nonenhanced computed tomography (NCCT) images; (5) had interpretable digital subtraction angiography (DSA) images for assessing collateral circulation, arterial recanalization grades, and POD; and (6) had essential demographic and clinical data. Patients with posterior circulation or only anterior cerebral artery occlusion, a modified Rankin Scale (mRS) score of ≥2 before stroke onset, no follow-up mRS at 90 days, or poor image quality were excluded from the study. The flowchart of patient selection is shown in Figure 1.
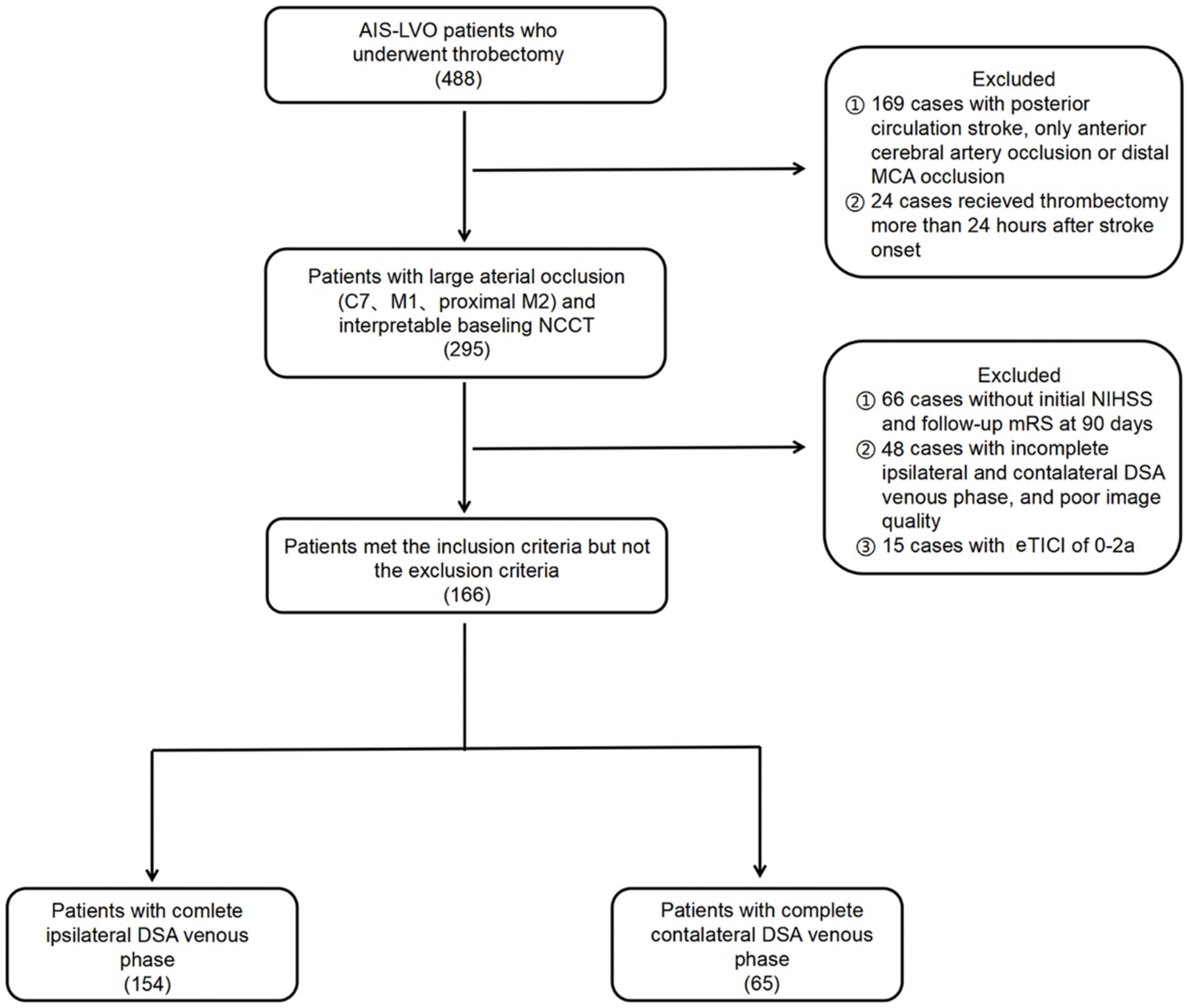
Figure 1. Flow chart of this study. AIS-LVO, acute ischemic stroke due to large vessel occlusion; MCA, middle cerebral artery; NCCT, nonenhanced computed tomography; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale; DSA, digital subtraction angiography; eTICI, expanded Thrombolysis in Cerebral Infarction.
Demographic and clinical information were obtained from the electronic medical records including age, gender, medical history (hypertension, diabetes mellitus, hyperlipidemia, coronary heart disease, atrial fibrillation, smoking, drinking), initial National Institute of Health Stroke Scale (NIHSS) score, the Trial of ORG 10,172 in Acute Stroke Treatment (TOAST) classification, intravenous thrombolysis treatment (IVT), onset to puncture time (OPT), puncture to recanalization time (PRT) and onset to recanalization time (ORT).
All imaging results were independently assessed by two neuroradiologists who were blinded to the clinical information. In cases of disagreement, a third reviewer provided the final decision. The extent of baseline cerebral infarction was evaluated using the Alberta Stroke Program Early CT score (ASPECTS) on baseline nonenhanced CT images. Hemorrhagic transformation (HT) and infarct volume were evaluated within 48 h after thrombectomy using follow-up CT or magnetic resonance (MR) images. The infarct volume was measured using ITK-SNAP software version 3.8.0.
Two-dimensional DSA images including complete venous phase were acquired on an angiographic system (PHILIPS. UNIQ FD20, Holland) at a rate of 6 frames per second in the anterior–posterior and lateral positions separately. On the normal side, 8 mL contrast medium (iodixanol, Yangtze River Pharmaceutical Group, China) was injected with a power injector through a guiding catheter at terminal common carotid artery with 5 mL/s speed. This procedure was uniform in our department. The location of the catheter and injection parameters on the affected side were determined by the interventionalists during operation. The grades of collateral circulation were determined using the American Society of Intervention and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) score based on pre-thrombectomy DSA images. After the final injection of contrast medium, consecutive DSA images were obtained to assess arterial recanalization status using eTICI grade and were processed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The superficial middle cerebral vein, vein of Trolard, and vein of Labbé were specifically identified as the target veins for analysis because they were dominant in venous drainage from the MCA territory, easily recognizable on radiological images, and have been extensively studied in previous research (7, 9–12). Additionally, the terminal ICA was chosen as the target artery. Circle regions of interest (ROIs) were positioned on these vessels based on following criteria: (1) in anterior–posterior position; (2) with a diameter slightly smaller than the width of the vessels; (3) without other overlapping vessels presenting in corresponding phases; (4) only on main veins; and (5) as close to the midpoint of the target veins as possible. The Peak Optical Density (POD) of each ROI was measured using the Time Series Analyzer V3 plugin. As all DSA images were processed in the original DICOM format and without color inversion, a lower POD value meant higher the density. The venous average POD (PODVA) was calculated by adding up the individual POD values of all visible target veins and then dividing this sum by the total number of veins. The difference value of peak optical density between ICA and cortical veins (PODICA-CV) was calculated by subtracting the POD of ICA (PODA) from PODVA. Figure 2 provided an example of the procedure.
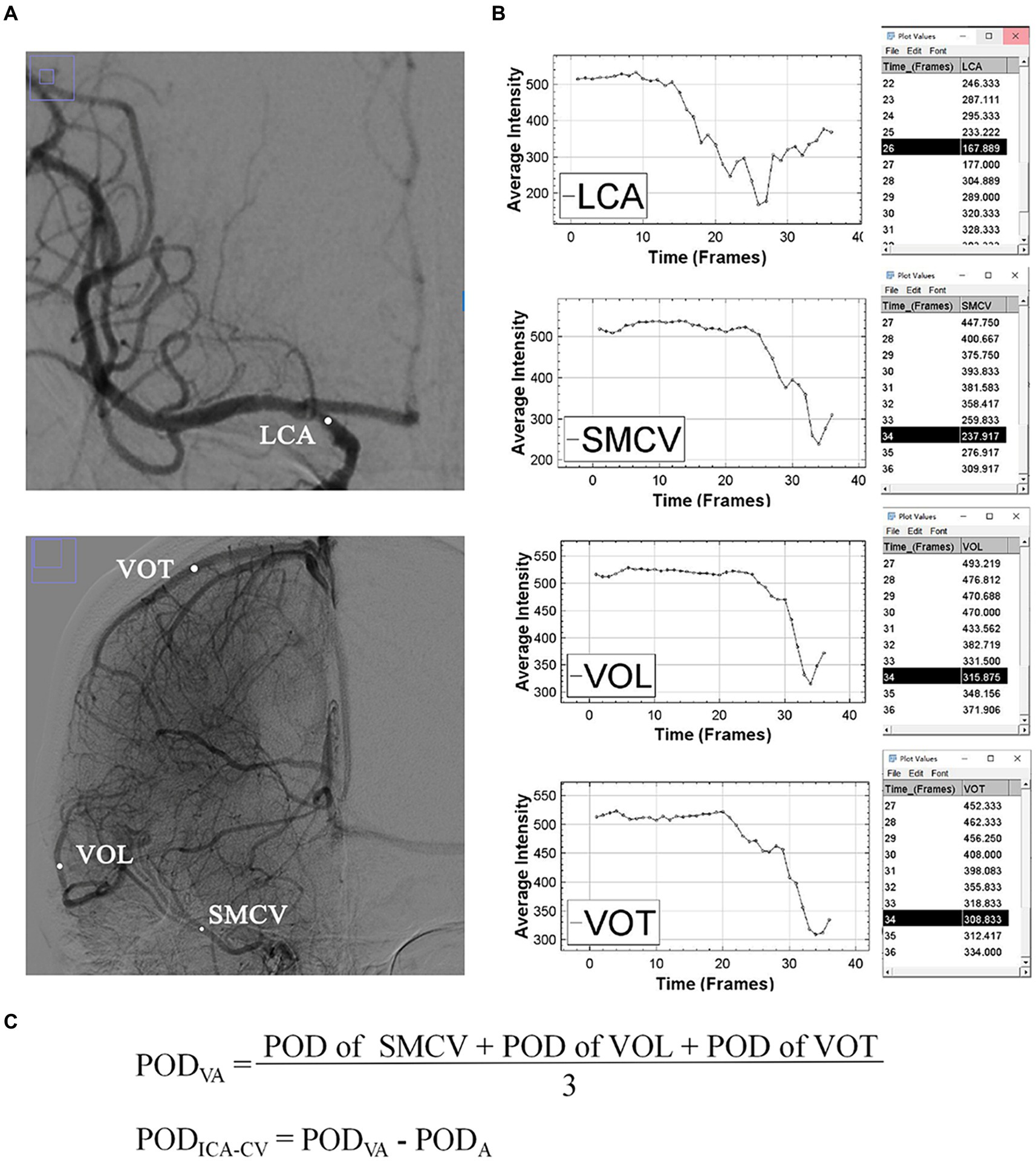
Figure 2. An example of the measurement of POD. (A) Shows the regions of interest (white dots) of superficial middle cerebral vein, vein of Labbé and vein of Trolard in the arterial and venous phases. (B) Shows the time-density curves and POD values of target vessels. (C) Shows the formulas for PODVA and PODICA-CV. ICA, internal carotid artery; SMCV, superficial middle cerebral vein; VOL, vein of Labbé; VOT, vein of Trolard; POD, peak optical density; PODVA, cortical venous average POD; PODA, POD of terminal internal carotid artery; PODICA-CV, difference between PODVA and PODA.
The functional outcomes were evaluated using the mRS scores at 90 days after thrombectomy. In our center, it is standard practice to conduct a prospective follow-up investigation of mRS scores in patients who underwent thrombectomy. The primary outcome was a good functional outcome, defined as an mRS score of 0–2 at 90 days. The secondary outcome was hemorrhagic transformation (HT) observed on follow-up CT or MR images.
The distribution of continuous variables was assessed by the Kolmogorov–Smirnov test. Continuous variables were analyzed using Wilcoxon rank-sum tests, while categorical variables were analyzed using the chi-square test or the Fisher’s exact test. The level of agreement for POD between different observers was measured using the intraclass correlation coefficient (ICC) analysis. A low POD was defined as a POD value lower than the cutoff value determined by receiver operating characteristic curves (ROC) analysis with the mRS score of 0–2 at 90 days as the outcome. The variables associated with good functional outcome, low PODVA, low PODICA-CV and HT in the univariate analyses were adjusted in multivariable logistic regression analyses. Delong tests were performed to compare the predictive abilities of three regression models: (1) model 1 adjusted for age, history of coronary heart disease, ASPECTS, ASITN/SIR score, initial NIHSS score, eTICI grade of 3, HT and infarct volume; (2) model 2 adjusted for the variables in model 1 and ipsilateral PODVA; (3) model 3 adjusted for the variables in model 1 and ipsilateral PODICA-CV. Spearman correlation coefficient was used to analyze the associations among ipsilateral PODICA-CV, mRS score at 90 days, eTICI grade, grade of collateral circulation and infarct volume. Mediation analysis using Model 4 in the PROCESS Marco was performed to assess whether the association between the grades of collateral circulation and mRS scores was mediated by PODICA-CV. The number of bootstrap samples was 5,000. Indirect effect was considered significant when 95% CI did not include zero. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 25.0 (IBM, Armonk, New York, USA) and MedCalc version 20.218 (MedCalc Software, Ostend, Belgium).
A total of 488 patients who underwent thrombectomy were reviewed in this study. Finally, a total of 166 patients were included based on inclusion and exclusion criteria. The median (interquartile range [IQR]) age of these patients was 68 (57–73) years and 104 (62.7%) patients were male. The median (IQR) initial NIHSS was 17 (13–21). After thrombectomy, 95 (57.2%) of the patients achieved eTICI grade of 3, 36 (21.7%) of the patients attained an eTICI grade of 2c, while 14.5 and 6.6% achieved eTICI grades of 2b67 and 2b50, respectively. HT and infarct volume were not assessed in 7 patients due to the missing of follow-up images. Overall, 34 (20.5%) of the patients experienced HT and the median (IQR) infarct volume was 14.75 (3.77–61.53) ml. Additionally, 90 (54.2%) of the patients achieved a mRS score of 0–2 at 90 days.
POD data were compared between the two hemispheres in different patients to clarify the differences. Overall, POD was measured in 154 (92.2%) patients for the ipsilateral hemisphere and in 65 (38.9%) patients for the contralateral hemisphere. The reproducibility for POD measurements between observers was found to be excellent. Specifically, the ICC for ipsilateral PODA was 0.928, for ipsilateral PODVA it was 0.901, and for ipsilateral PODICA-CV it was 0.834. On the contralateral side, the ICC for PODA was 0.938, for PODVA it was 0.892, and for PODICA-CV it was 0.877.
The contralateral PODA was found to be higher than the ipsilateral PODA (median [IQR], 173.987 [144.813–202.975] vs. 118.668 [84.659–159.686], p < 0.001). However, no significant difference was found between the ipsilateral and contralateral PODVA (median [IQR], 278.317 [233.298–311.679] vs. 272.926 [239.371–296.095], p = 0.53). Ipsilateral PODICA-CV was higher than contralateral PODICA-CV (median [IQR], 150.724 [119.583–186.023] vs. 101.744 [74.941–123.314], p < 0.001).
The baseline characteristics between good and poor functional outcome groups were summarized in Table 1. For the patients with good functional outcomes, both ipsilateral PODVA (median [IQR], 257.198 [216.623–296.631] vs. 290.944 [248.647–338.819], p < 0.001) and ipsilateral PODICA-CV (median [IQR], 128.463 [110.233–153.624] vs. 182.01 [146.621–211.331], p < 0.001) were lower than the patients with poor functional outcome. The ipsilateral PODA between good and poor outcome groups was similar (median [IQR], 119.914 [91.619–166.566] vs. 115.239 [83.554–152.824], p = 0.128). Compared with the ipsilateral PODICA-CV in good outcome group, contralateral PODICA-CV was lower (p < 0.001). The comparation of POD are shown in Figure 3.
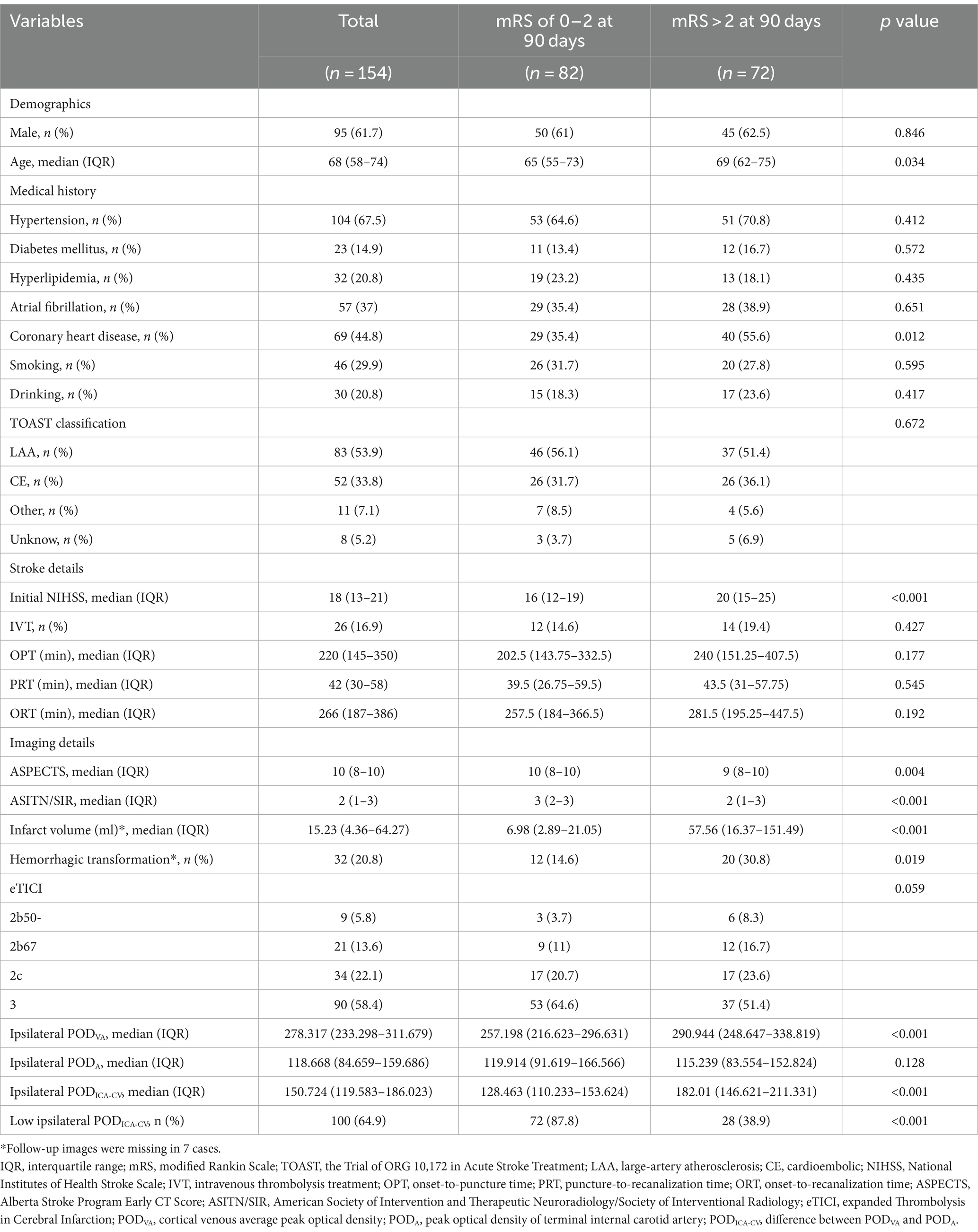
Table 1. Baseline characteristics in patients with ipsilateral POD (comparation between different prognosis).

Figure 3. The boxplots show the POD differences among different groups. (A) Shows significant difference in ipsilateral PODVA between good and poor functional outcome groups, but no difference between PODVA in different hemispheres. (B) Shows significant difference in PODA between bilateral hemispheres, but no difference between ipsilateral PODA in different functional outcome groups. (C) Shows significant differences in PODICA-CV between bilateral hemispheres and ipsilateral PODICA-CV between good and poor functional outcome groups. POD, peak optical density; PODVA, cortical venous average POD; PODA, POD of terminal internal carotid artery; PODICA-CV, difference between PODVA and PODA.
Table 2 summarizes the results of univariate and multivariable analyses. After adjusting for age, history of coronary heart disease, ASPECTS, ASITN/SIR score, initial NIHSS score, eTICI grade of 3, HT and infarct volume related to good functional outcome with p < 0.1, multivariable logistic regression analyses showed that both ipsilateral PODVA (odds ratio [OR] 0.991, 95% confidence interval [CI] 0.984–0.999, p = 0.019) and ipsilateral PODICA-CV (OR 0.975, 95% CI 0.963–0.986, p < 0.001) were significantly associated with good functional outcome. Moreover, ipsilateral PODA was not an independent predictor of good functional outcome (OR 1.006, 95% CI 0.998–1.015, p = 0.137). Ipsilateral PODICA-CV was positive correlated with mRS score at 90 days (r = 0.456, p < 0.001).
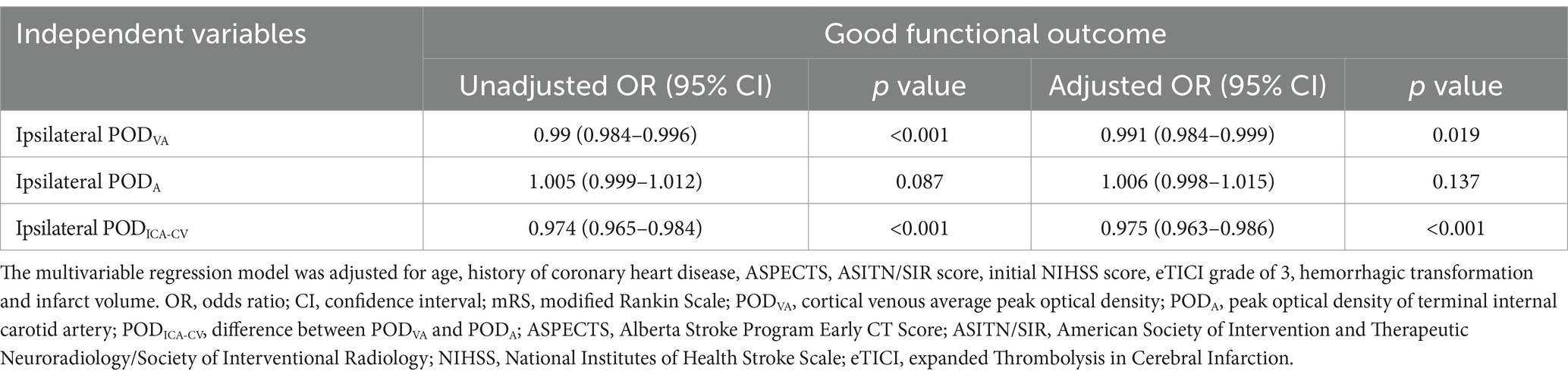
Table 2. Univariable and multivariable logistic regression analyses for good functional outcome (mRS of 0–2 at 90 days).
The predictive ability for good functional outcomes was assessed using area under the curve (AUC). The AUC for ipsilateral PODVA was 0.667 (95% CI 0.581–0.753, p < 0.001) with the cutoff value of 259.915, sensitivity of 73.6% and specificity of 53.7%. While the AUC for ipsilateral PODICA-CV was 0.787 (95% CI 0.713–0.861, p < 0.001) with the cutoff value of 163.088, sensitivity of 61.1% and specificity of 87.8%.
When including ipsilateral PODICA-CV in the baseline model, there was a significant increase in AUC (0.893 vs. 0.842, p = 0.027). Compared with baseline model, the enhancement of predictive ability of model 2 adjusted for ipsilateral PODVA and the variables in baseline model was not significant (0.865 vs. 0.842, p = 0.107). The ROCs comparation were shown in Figure 4.
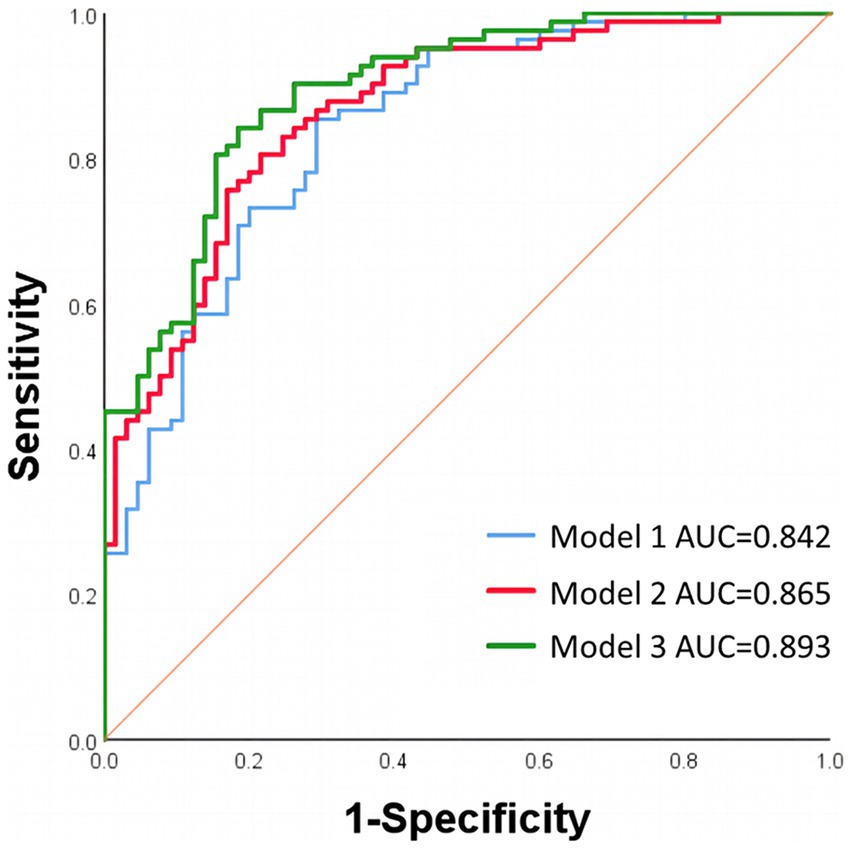
Figure 4. ROCs of different regression models. Model 1 was adjusted for age, history of coronary heart disease, ASPECTS, ASITN/SIR score, initial NIHSS score, eTICI grade of 3, hemorrhagic transformation and infarct volume. Model 2 was adjusted for the variables in model 1 and ipsilateral PODVA. Model 3 was adjusted for the variables in model 1 and ipsilateral PODICA-CV. ROCs, receiver operating characteristic curves; AUC, area under the curve; ASPECTS, Alberta Stroke Program Early CT Score; ASITN/SIR, American Society of Intervention and Therapeutic Neuroradiology/Society of Interventional Radiology; NIHSS, National Institutes of Health Stroke Scale; eTICI, expanded Thrombolysis in Cerebral Infarction; PODVA, cortical venous average peak optical density; PODICA-CV, difference between PODVA and peak optical density of terminal internal carotid artery.
In 154 patients with ipsilateral POD, 63 (40.9%) of them had low PODVA and 100 (64.9%) of them had low PODICA-CV. Both ASITN/SIR score (OR 1.183, 95% CI 0.907–1.543, p = 0.216) and eTICI grade (OR 0.831, 95% CI 0.588–1.177, p = 0.298) had no relationship with low ipsilateral PODVA in univariate analysis. After adjusted for age, initial NIHSS score and eTICI grade, it was found that only the ASITN/SIR score was significantly associated with low ipsilateral PODICA-CV (OR 1.391, 95% CI 1.023–1.891, p = 0.035) in multivariable logistic regression analysis. This founding was shown in Table 3. Moreover, there was a negative correlation between ipsilateral PODICA-CV and ASITN/SIR score (r = −0.193, p = 0.016). No linear relationship was found between eTICI grade and PODICA-CV (r = 0.133, p = 0.099).
The effects of ASITN/SIR scores on PODICA-CV (β = −0.209, p = 0.093) and PODICA-CV on mRS scores (β = 0.401, p < 0.001) were statistically significant in the mediation analysis. Partial mediation effect of PODICA-CV on the relationship between the collateral circulation and mRS scores was confirmed, accounting for 24.2% of the total effect (indirect effect −0.151, 95% CI −0.282–−0.034; total effect −0.623, 95% CI −0.893–−0.352). These results are shown in Figure 5.
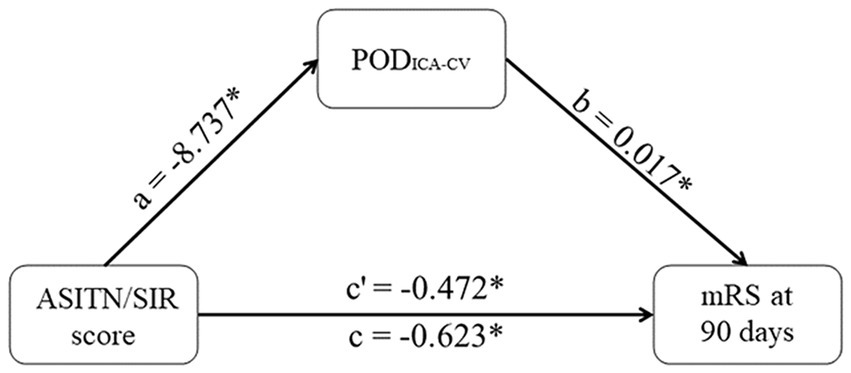
Figure 5. Mediation effect of PODICA-CV. PODICA-CV partly mediates the effects of ASITN/SIR scores on mRS at 90 days. The coefficient c is the total effect and c’ is the direct effect. All coefficients are unstandardized. *p < 0.05. PODICA-CV, difference between PODVA and peak optical density of terminal internal carotid artery; ASITN/SIR, American Society of Intervention and Therapeutic Neuroradiology/Society of Interventional Radiology; mRS, modified Rankin Scale.
Ipsilateral PODVA was not associated with HT in univariate analysis (OR 1.001, 95% CI 0.995–1.008, p = 0.715). Table 4 shows a significant association between ipsilateral PODICA-CV and HT (OR 1.008, 95% CI 1.0004–1.016, p = 0.04) after adjusting for diabetes mellitus history. A positive correlation was found between ipsilateral PODICA-CV and infarct volume (r = 0.361, p < 0.001).
In our study, we conducted a novel approach to quantify the VO on DSA images and reflect the status of microcirculation and venous circulation after successful arterial recanalization which may involve in the “non-reflow” phenomenon (6). We found that the PODA taken as the background value showed no difference in patients with different prognosis, but the higher ipsilateral PODVA and higher ipsilateral PODICA-CV were more likely to occur in poor functional outcome group. Both ipsilateral PODVA and ipsilateral PODICA-CV were independent predictors of functional outcomes. Furthermore, contralateral PODICA-CV was significantly less than ipsilateral PODICA-CV. Our findings suggest that POD is an effective DSA parameter for assessing cerebral circulatory function and further demonstrate that the importance of VO in the postoperative evaluation.
VO is under the influence of upstream circulatory interaction. In animal stroke models, the reduction of VO has been observed following the occlusion of MCA, and animals with poor collaterals are more likely to have complete absence of VO which is associated with severe functional deficits and larger final infarction (13). The VO changes can also be observed on CTA images in AIS-LVO patients. This CTA VO changes is related with the ratio of brain tissue with Tmax >10 s/Tmax >6 s which is an important indicator for cerebral microcirculatory perfusion (7). With the increasing of the related researches, the pre-thrombectomy VO has been considered as a strong predictor of successful arterial recanalization, clinical outcomes and brain edema (14–16). As a result, evaluating pre-thrombectomy VO can provide a more precise information for identifying patients who would benefit from thrombectomy, thereby preventing unnecessary surgical procedures and the development of complications. This is particularly important in the cases that perfusion imaging is not available.
After thrombectomy, the abnormal VO usually represents poor cerebral reperfusion. For instance, luxury perfusion, a condition characterized by early venous filling, has been observed to frequently occur after thrombectomy and can lead to HT (17). However, quantifying premature venous filling is difficult and its effect on functional outcomes is still a matter of debate. Only one study has successfully used a quantitative method to evaluate postoperative VO by measuring the TTP of rolandic vein and has shown that a shorter TTP difference between distal MCA and rolandic vein is a marker of sufficient and effective microcirculatory reperfusion (8). This finding highlights the importance of evaluating both arterial and venous features in assessing cerebral circulation, rather than focusing solely on arteries or veins. In our study, we further confirmed this finding by comparing the AUCs of different regression models using the Delong test. We found that including ipsilateral PODICA-CV in the baseline model significantly improved its predictive ability compared to including ipsilateral PODVA. Furthermore, different from the previous study that investigated only a single cortical vein (8), we evaluated a wider range from the ICA to three dominant cortical veins, resulting in the ipsilateral PODICA-CV becoming a more comprehensive indicator of cerebral reperfusion and providing an additional potential for predicting HT. This insight is supported by a recent study that demonstrates persistent dysfunction between the ICA and distal small vessels, even with a mTICI score of 2b-3 after thrombectomy (18).
Collateral circulation is considered as a crucial factor in determining outcomes of patients with acute ischemic stroke (19). Even in the late time window, robust collaterals can prevent stroke patients from the progression of ischemic core and severe functional deficits (20). The AIS-LVO patients with robust collaterals always have a superior response to thrombectomy and better outcomes (21). In our study, we found that collateral circulation was the only independent predictor of low ipsilateral PODICA-CV, and patients with lower ipsilateral PODICA-CV were more likely to have robust collaterals, small infarct volume, and better functional outcomes. The relationship between grades of collateral circulation and mRS scores was partially mediated by PODICA-CV (mediation proportion 24.2%). As a functional indicator of cerebral circulation, ipsilateral PODICA-CV may be helpful to explain the patients with good collateral circulation but poor prognosis after stroke.
Unlike previous studies (8), our study yielded different findings as it did not observe any impact of expanded recanalization grade on prognosis. Although a higher proportion of eTICI grade of 3 was found in favorable prognosis patients, it did not predict outcomes in multivariate analysis. Similarly, the effect of various expanded recanalization grades on ipsilateral PODICA-CV was also not detected. Moreover, we identified that both ipsilateral PODICA-CV and infarct volume, which was a crucial influential factor of outcomes (22), served as independent predictors of outcome. Our results provide additional insight into the notion that the “non-reflow” phenomenon cannot be solely attributed to incomplete recanalization (6).
Quantitative characteristics of cerebral circulation on DSA images have been successfully utilized to predict the stroke risk in the patients (18), hyperperfusion after carotid artery stenting or angioplasty (23), post-thrombectomy HT (24) and clinical outcomes (8, 25, 26). Nevertheless, there is no standardized method to quantify these characteristics. In the balloon test occlusion study, the DSA frame numbers of the starting of venous phases were recorded to analyze the difference between bilateral cerebral circulation (27). The similar frame numbers counting method was adopted in two previous studied to measure cerebral circulation time (18, 28). Additionally, there are several studies using a variety of software to quantify cerebral circulatory function (8, 24, 25, 29, 30). Most of the methods have limitation of generalizability because of the non-open source software or high technical barrier. To better evaluate the cerebral circulatory function quantitatively, our study performed open-source ImageJ software to process DSA images. ImageJ is a free and open-source image processing software primarily used for scientific research and biomedical fields. It offers a range of powerful image processing and analysis tools available to measure and segment a stack of dynamic images. As a parameter of quantified DSA, the vascular peak density is investigated rarely (31). However, Liu et al. (25) pointed out that it has an advantage over TTP in predicting outcomes after thrombectomy. For this reason, we used POD to assess VO and the function of cerebral circulation.
Our study has several limitations. First, it was a retrospective single-center design with a relatively small sample size, which may introduce selection bias. Second, due to the small number of patients with complete contralateral venous phase on DSA images, we could not use the method referred in the previous study to measure cerebral circulation time and compare it with our parameters. Third, our method for evaluating VO cannot be applied in patients whose target veins are not opacified after thrombectomy. The absence of venous opacification is usually caused by the failed arterial recanalization or severe obstruction in downstream vessels. Our results should be confirmed in the prospective intervention trials in the future.
The ipsilateral PODICA-CV easily acquired from DSA images reflected more comprehensive cerebral circulatory function. It was associated with function outcomes after thrombectomy and proposed as an indicator to assess effectiveness of thrombectomy. The further reduction of ipsilateral PODICA-CV after successful arterial recanalization may lead to a better prognosis in AIS-LVO patients.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Institutional Review Board of Xiangyang No.1 People’s Hospital, Hubei University of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin due to the retrospective nature of the study.
GL: Conceptualization, Formal analysis, Methodology, Software, Validation, Writing – original draft. JC: Data curation, Methodology, Software, Writing – original draft. PZ: Investigation, Methodology, Validation, Writing – original draft. DS: Formal analysis, Investigation, Writing – original draft. ZK: Formal analysis, Validation, Writing – original draft. RF: Investigation, Writing – original draft. BM: Funding acquisition, Methodology, Project administration, Software, Validation, Writing – review & editing. JZ: Conceptualization, Project administration, Supervision, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Translational Medicine and Interdisciplinary Research Joint Fund of Zhongnan Hospital of Wuhan University (Grant number: ZNJC201924) and Hubei Province health and family planning scientific research project (Grant number: WJ2023M056).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mead, GE, Sposato, LA, Sampaio Silva, G, Yperzeele, L, Wu, S, Kutlubaev, M, et al. A systematic review and synthesis of global stroke guidelines on behalf of the world stroke organization. Int J Stroke. (2023) 18:499–531. doi: 10.1177/17474930231156753
2. Jovin, TG, Nogueira, RG, Lansberg, MG, Demchuk, AM, Martins, SO, Mocco, J, et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): a systematic review and individual patient data meta-analysis. Lancet. (2022) 399:249–58. doi: 10.1016/s0140-6736(21)01341-6
3. Mitchell, PJ, Yan, B, Churilov, L, Dowling, RJ, Bush, SJ, Bivard, A, et al. Endovascular thrombectomy versus standard bridging thrombolytic with endovascular thrombectomy within 4·5 h of stroke onset: an open-label, blinded-endpoint, randomised non-inferiority trial. Lancet. (2022) 400:116–25. doi: 10.1016/s0140-6736(22)00564-5
4. Renú, A, Millán, M, San Román, L, Blasco, J, Martí-Fàbregas, J, Terceño, M, et al. Effect of intra-arterial alteplase vs placebo following successful thrombectomy on functional outcomes in patients with large vessel occlusion acute ischemic stroke: the CHOICE randomized clinical trial. JAMA. (2022) 327:826–35. doi: 10.1001/jama.2022.1645
5. Zhang, Y, Jiang, M, Gao, Y, Zhao, W, Wu, C, Li, C, et al. "No-reflow" phenomenon in acute ischemic stroke. J Cereb Blood Flow Metab. (2024) 44:19–37. doi: 10.1177/0271678X231208476
6. Mujanovic, A, Ng, F, Meinel, TR, Dobrocky, T, Piechowiak, EI, Kurmann, CC, et al. No-reflow phenomenon in stroke patients: a systematic literature review and meta-analysis of clinical data. Int J Stroke. (2023) 19:58–67. doi: 10.1177/17474930231180434
7. Faizy, TD, Kabiri, R, Christensen, S, Mlynash, M, Kuraitis, GM, Broocks, G, et al. Favorable venous outflow profiles correlate with favorable tissue-level collaterals and clinical outcome. Stroke. (2021) 52:1761–7. doi: 10.1161/STROKEAHA.120.032242
8. Ji, Y, Shi, B, Yuan, Q, Wu, K, Fang, J, Wang, H, et al. Effect of prolonged microcirculation time after thrombectomy on the outcome of acute stroke. J Neurointerv Surg. (2023) 15:1078–83. doi: 10.1136/jnis-2022-019566
9. van Horn, N, Heit, JJ, Kabiri, R, Mader, MM, Christensen, S, Mlynash, M, et al. Cerebral venous outflow profiles are associated with the first pass effect in endovascular thrombectomy. J Neurointerven Surgery. (2022) 14:1056–61. doi: 10.1136/neurintsurg-2021-018078
10. Drozdov, AA, Arora, M, Sheikhy, A, Leon Guerrero, CR, and Taheri, MR. Anterior ischemic stroke: analysis of the multivariable CT-based models for prediction of clinical outcome. J Stroke Cerebrovasc Dis. (2023) 32:107242. doi: 10.1016/j.jstrokecerebrovasdis.2023.107242
11. Singh, N, Bala, F, Kim, BJ, Najm, M, Ahn, SH, Fainardi, E, et al. Time-resolved assessment of cortical venous drainage on multiphase CT angiography in patients with acute ischemic stroke. Neuroradiology. (2021) 64:897–903. doi: 10.1007/s00234-021-02837-1
12. Li, S, Hong, L, Yang, W, Liu, X, Zhang, Y, Ling, Y, et al. The benefit of favorable venous outflow profile is mediated through reduced microvascular dysfunction in acute ischemic stroke. Eur Stroke J. (2024) 9:432–40. doi: 10.1177/23969873231224573
13. Sasaki, M, Honmou, O, Radtke, C, and Kocsis, JD. Development of a middle cerebral artery occlusion model in the nonhuman primate and a safety study of i.v. infusion of human mesenchymal stem cells. PLoS One. (2011) 6:e26577. doi: 10.1371/journal.pone.0026577
14. Faizy, TD, Kabiri, R, Christensen, S, Mlynash, M, Kuraitis, G, Meyer, L, et al. Venous outflow profiles are linked to cerebral edema formation at noncontrast head CT after treatment in acute ischemic stroke regardless of collateral vessel status at CT angiography. Radiology. (2021) 299:682–90. doi: 10.1148/radiol.2021203651
15. Myint, MZ, Yeo, LLL, Tan, BYQ, The, EZ, Lim, MC, Sia, C-H, et al. Internal cerebral vein asymmetry is an independent predictor of poor functional outcome in endovascular thrombectomy. J Neurointerven Surgery. (2022) 14:683–7. doi: 10.1136/neurintsurg-2021-017684
16. Faizy, TD, Kabiri, R, Christensen, S, Mlynash, M, Kuraitis, G, Mader, MM, et al. Association of Venous Outflow Profiles and Successful Vessel Reperfusion after Thrombectomy. Neurology. (2021) 96:e2903–11. doi: 10.1212/WNL.0000000000012106
17. Li, Y, Cao, W, Xu, X, Li, T, Chen, Y, Wang, Y, et al. Early venous filling after mechanical thrombectomy in acute ischemic stroke due to large vessel occlusion in anterior circulation. J Neurointerv Surg. (2024) 16:248–52. doi: 10.1136/jnis-2023-020336
18. Wang, YJ, Wang, JQ, Qiu, J, Li, W, Sun, XH, Zhao, YG, et al. Association between collaterals, cerebral circulation time and outcome after thrombectomy of stroke. Ann Clin Trans Neurol. (2022) 10:266–75. doi: 10.1002/acn3.51718
19. Uniken Venema, SM, Dankbaar, JW, van der Lugt, A, Dippel, DWJ, and van der Worp, HB. Cerebral collateral circulation in the era of reperfusion therapies for acute ischemic stroke. Stroke. (2022) 53:3222–34. doi: 10.1161/strokeaha.121.037869
20. Liebeskind, DS, Saber, H, Xiang, B, Jadhav, AP, Jovin, TG, Haussen, DC, et al. Collateral circulation in thrombectomy for stroke after 6 to 24 hours in the DAWN trial. Stroke. (2022) 53:742–8. doi: 10.1161/strokeaha.121.034471
21. Maguida, G, and Shuaib, A. Collateral circulation in ischemic stroke: an updated review. J Stroke. (2023) 25:179–98. doi: 10.5853/jos.2022.02936
22. Wong, KK, Cummock, JS, Li, G, Ghosh, R, Xu, P, Volpi, JJ, et al. Automatic segmentation in acute ischemic stroke: prognostic significance of topological stroke volumes on stroke outcome. Stroke. (2022) 53:2896–905. doi: 10.1161/strokeaha.121.037982
23. Yamauchi, K, Enomoto, Y, Otani, K, Egashira, Y, and Iwama, T. Prediction of hyperperfusion phenomenon after carotid artery stenting and carotid angioplasty using quantitative DSA with cerebral circulation time imaging. J Neurointerv Surg. (2018) 10:576–9. doi: 10.1136/neurintsurg-2017-013259
24. Kosior, JC, Buck, B, Wannamaker, R, Kate, M, Liapounova, NA, Rempel, JL, et al. Exploring reperfusion following endovascular thrombectomy. Stroke. (2019) 50:2389–95. doi: 10.1161/STROKEAHA.119.025537
25. Wen, WL, Fang, YB, Yang, PF, Zhang, YW, Wu, YN, Shen, H, et al. Parametric digital subtraction angiography imaging for the objective grading of collateral flow in acute middle cerebral artery occlusion. World Neurosurg. (2016) 88:119–25. doi: 10.1016/j.wneu.2015.12.084
26. Prasetya, H, Ramos, LA, Epema, T, Treurniet, KM, Emmer, BJ, van den Wijngaard, IR, et al. qTICI: quantitative assessment of brain tissue reperfusion on digital subtraction angiograms of acute ischemic stroke patients. Int J Stroke. (2020) 16:207–16. doi: 10.1177/1747493020909632
27. Abud, DG, Spelle, L, Piotin, M, Mounayer, C, Vanzin, JR, and Moret, J. Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol. (2005) 26:2602–9.
28. Chen, Z, Li, M, Wu, Z, Zhang, M, Weng, G, Li, M, et al. Cerebral circulation time is a potential predictor of disabling ischemic cerebrovascular events in patients with non-disabling middle cerebral artery stenosis. Front Neurol. (2021) 12:653752. doi: 10.3389/fneur.2021.653752
29. Loo, JK, Hu, YS, Lin, TM, Lin, CJ, Lirng, JF, Wu, HM, et al. Shortened cerebral circulation time correlates with seizures in brain arteriovenous malformation: a cross-sectional quantitative digital subtraction angiography study. Eur Radiol. (2022) 32:5402–12. doi: 10.1007/s00330-022-08690-x
30. Naraoka, M, Matsuda, N, Shimamura, N, and Ohkuma, H. Role of microcirculatory impairment in delayed cerebral ischemia and outcome after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. (2022) 42:186–96. doi: 10.1177/0271678x211045446
Keywords: ischemic stroke, peak optical density, cerebral reperfusion, functional outcomes, thrombectomy
Citation: Liu G, Cao J, Zhou P, Sun D, Kang Z, Fan R, Mei B and Zhang J (2024) Difference between arterial and venous peak optical density after thrombectomy is associated with functional outcomes. Front. Neurol. 15:1414898. doi: 10.3389/fneur.2024.1414898
Received: 09 April 2024; Accepted: 25 June 2024;
Published: 05 July 2024.
Edited by:
Raffaele Ornello, University of L’Aquila, ItalyReviewed by:
Hye-Yeon Choi, Gangnam Severance Hospital, Republic of KoreaCopyright © 2024 Liu, Cao, Zhou, Sun, Kang, Fan, Mei and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjian Zhang, emhhbmdqakB3aHUuZWR1LmNu; Bin Mei, bmV1cm9tZWkyMEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.