- The Second Hospital of Jiaxing, Jiaxing, China
Objective: This study aimed to evaluate the methodological quality and measurement attribute quality of the post-stroke fatigue measurement scale, so as to provide some basis for the clinical application and promotion of related scales.
Methods: The Chinese National Knowledge Infrastructure, the Wanfang Data Knowledge Service Platform, the China Science and Technology Journal Database, the Chinese Medical Journal Full-text Database, the Chinese Biology Medicine, PubMed, Embase, Medline, the Cochrane Library, the Web of Science, CINAHL, and PsycINFO databases were searched for literature on the post-stroke fatigue measurement scale up to June 2022. Literature screening and data extraction were carried out independently by two researchers, and in the case of disagreement, discussions were held with a third investigator to reach an agreement, and the COSMIN checklist and criteria were used to systematically evaluate the attributes of the measurement scale.
Results: A total of 17 studies were included, involving 10 post-stroke fatigue measurement scales. The content validity of FSS-7, FACIT-F, NRS-FRS, and MFI-20 was “not mentioned,” and the remaining scales were “uncertain.” In terms of construct validity, MFS was “adequate”; FSS-7, FACIT-F, and NRS-FRS were “not mentioned”; and the remaining scales were “uncertain.” In terms of internal consistency, NRS-FRS was “not mentioned”; FSS and MFS were “adequate”; and the remaining scales were “uncertain.” In terms of hypothesis testing, CIS and FACIT-F were “not mentioned,” NRS-FRS was “adequate,” and the remaining scales were “uncertain.” The stability of FSS-7, CIS, FACIT-F, and MFI-20 was “not mentioned,” and the remaining scales were “adequate.” The cross-cultural validity of FSS-7 was “adequate,” and the remaining scales were “not mentioned.” All 10 scales were given a recommendation grade of “B”.
Conclusion: For the time being, the FSS can be recommended to measure post-stroke fatigue, but it still needs to be tested for more relevant measurement properties in order to gain more support from high-quality evidence. For a more comprehensive assessment of post-stroke fatigue, the FIS, FAS, and NFI-stroke should perhaps be considered, as the FSS is a one-dimensional scale that can only measure physical fatigue in patients; however, these scales also need to be tested for more relevant measurement properties to verify their clinical applicability.
1 Introduction
Post-stroke fatigue is not related to tension and is a subjective feeling of stroke survivors about weakness and tiredness (1, 2). PSF arises not only from physical activities but also from mental or social activities. As one of the common complications after stroke, it has a high incidence, which will make it difficult or impossible for patients to maintain daily activities, thus causing a certain degree of adverse effects on their quality of life (3, 4). Accurate measurement of PSF is the premise and basis for the timely and effective treatment of the disease. There are many scales used to measure PSF, such as the fatigue severity scale (FSS) (5), fatigue impact scale (FIS) (6), and fatigue assessment scale (FAS) (7). Kjeverud et al. (8) explored the frequency and overlap of PSF by using scales, such as FSS, and the results showed that different scales produced different results. Blackwell et al. (9) noted that there are currently no corresponding guidelines to assess fatigue management in patients with PSF in fatigue management and that there are no established guidelines yet. Thus it can be seen different measurement focus of different scales, it has not been able to determine whether these scales have good measurement properties, and few studies to systematically evaluate these measurement properties. The guidelines for the selection criteria for health measurement tools (COSMIN) (10) can assess the methodological quality and measurement attribute quality of the scale, and the best scale for the purpose of the study can be selected. In this study, a systematic evaluation of PSF measurement scales using COSMIN quality standards was carried out to clarify the methodological quality and measurement attribute quality of relevant scales. It aimed to comprehensively evaluate the evidence level of each measurement attribute, leading to the final recommendation and providing certain evidence-based support for the application and promotion of relevant scales in clinical practice.
2 Materials and methods
2.1 Inclusion criteria and exclusion criteria
Inclusion criteria were as follows: ① study subjects were stroke patients; ② the study includes the measurement performance evaluation of the PSF measurement scale; ③ at least one measurement attribute was evaluated on the scale; ④ access to the full text of the Chinese and English literature, where nationality is not limited. Exclusion criteria were as follows: ① review, systematic evaluation, conference, animal experiments, qualitative research, cases, and other types of literature; ② the evaluation tool is only used to study the current status of its application and collect research subject data or the literature measuring outcome indicators.
2.2 Literature retrieval strategy
Literature on PSF measurement published from the database until June 2022 in the Chinese Journal Full-text Database, the Wanfang Data Knowledge Service Platform, the VIP Database, the Chinese Medical Journal Full-text Database, the Chinese Biomedical Literature Database, PubMed, Embase, Medline, the Cochrane Library, the Web of Science, CINAHL, and PsycINFO databases were being retrieved using a computer. The literature search was completed by the combination of subject words and free words, and the gray literature search was performed.
2.3 Literature screening and data extraction
Two researchers who had participated in the relevant training and fully mastered the COSMIN evaluation criteria independently completed the literature screening and data extraction according to the inclusion and exclusion criteria and cross-checked the results. Once a disagreement occurs, they discuss it with the third investigator to reach a consensus. The contents of data extraction include those as follows: the first author, year of publication, country, scale name, sample size, scale dimension, scoring method used for each item, scale evaluation time, and retest time.
2.4 Evaluation steps
Two investigators independently completed the quality of PSF, the quality of measurement attributes, and the level of evidence using the COSMIN risk of bias tool (10) and performed a cross-check. In the case of disagreement, they discussed it with the third investigator to reach an agreement. The contents of data extraction include those as follows: the first author, year of publication, country, scale name, sample size, scale dimension, scoring method used for each item, scale evaluation time, and retest time.
2.5 Study tools
2.5.1 Methodological quality evaluation
The methodological quality of the included scale was assessed according to the COSMIN risk of bias checklist (11). A total of 10 modules need to be evaluated, namely, content validity scale development, content validity, structure validity, internal consistency, cross-cultural validity or measurement invariance, stability, measurement error, validity and criterion validity, hypothesis testing, and responsiveness. The risk of bias of each item in the module was evaluated with the result of “very good” “adequate” or “doubtful” or “inadequate,” and then the minimum evaluation of all the entries in a module was taken as the total evaluation result of the module.
2.5.2 Quality evaluation of the measurement properties
The quality of the nine measurement attributes of content validity, construct validity, internal consistency, stability, measurement error, hypothesis testing, cross-cultural validity or measurement invariance, criterion validity, and responsiveness were evaluated according to the COSMIN quality specification (12), and the evaluation rating is “sufficient (+)” or “inadequate (−)” or “uncertain (?).” When a measurement attribute of a scale is “sufficient (+)” or “inadequate (−)” or “uncertain (?),” the overall rating of this measurement attribute is also “full (+)” or “inadequate (−) “or “unsure (?).” In the meantime, when a measurement attribute of the scale is not evaluated consistently among studies, and the reason cannot be explained, then the overall rating of the measurement attribute is also “inconsistent (±).”
2.5.3 Evaluation of the evidence grade
The inclusion scale was assessed according to GRADE (13), evaluating it based on the risk of bias, inconsistency, imprecision, and indirectness. COSMIN first identified the measurement attributes of the measurement scale as “high quality,” then downgraded according to the above four aspects, and divided the level of evidence into “high” or “medium” or “low” or “extremely low.” Subsequently, opinions on the recommended strength of the scale were formed based on the evidence evaluation results. The recommended strength of the scale is “A” or “B” or “C”; “A” is recommended, “C” is not recommended, and “B” is between “A” and “C,” indicating that the scale has some potential, but more studies need to be conducted to verify its effectiveness. The content validity of the recommended strength “Grade A” is “sufficient” and the internal consistency level is not “low”; “Grade C” is proven insufficient; and “Grade B” corresponds to neither “Grade A” nor “Grade C”
3 Results
3.1 Literature search results
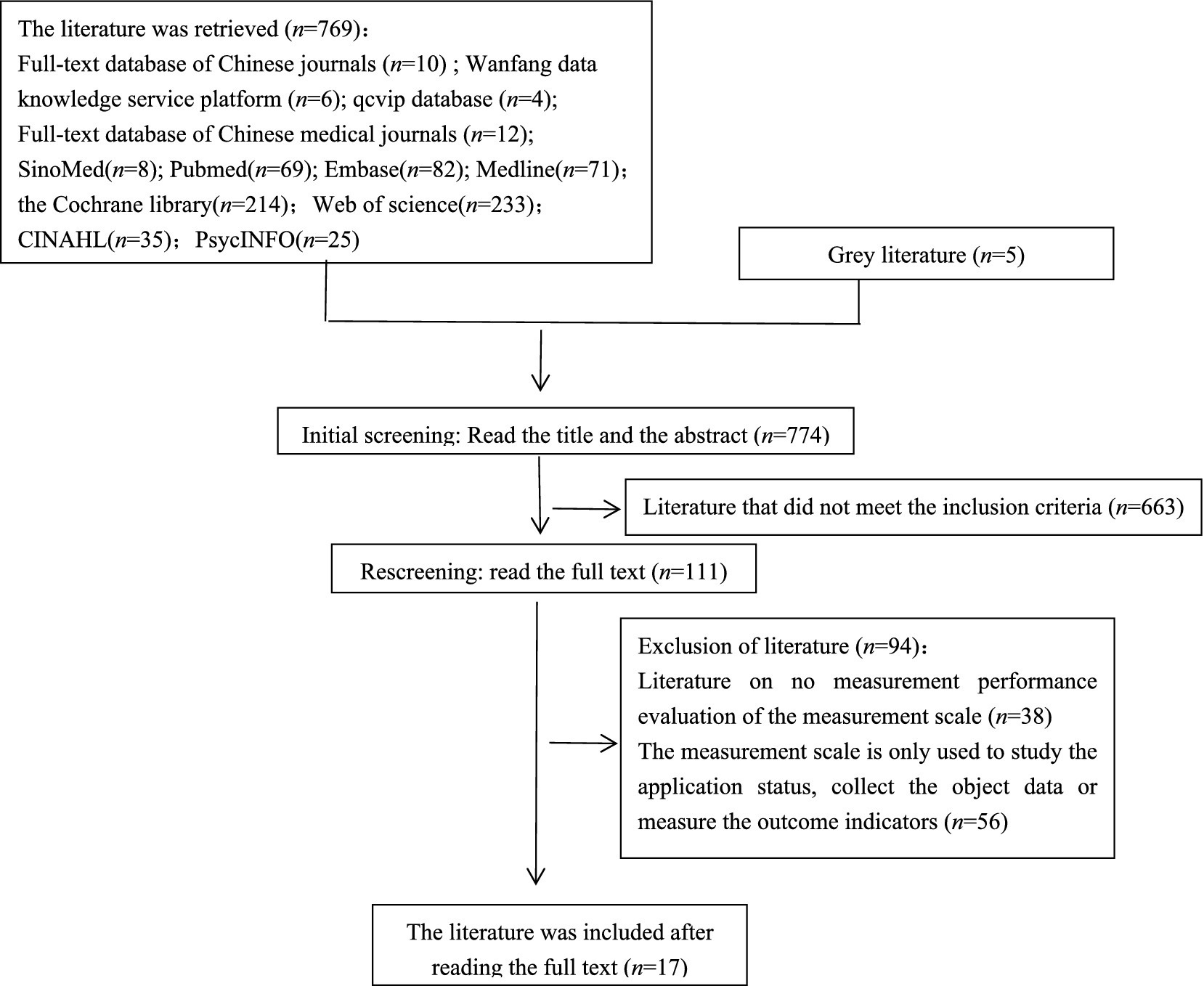
In total, 774 articles were screened according to inclusion and exclusion criteria, and 17 articles were finally included. The literature screening process is shown in Figure 1.
3.2 Basic characteristics of the included literature
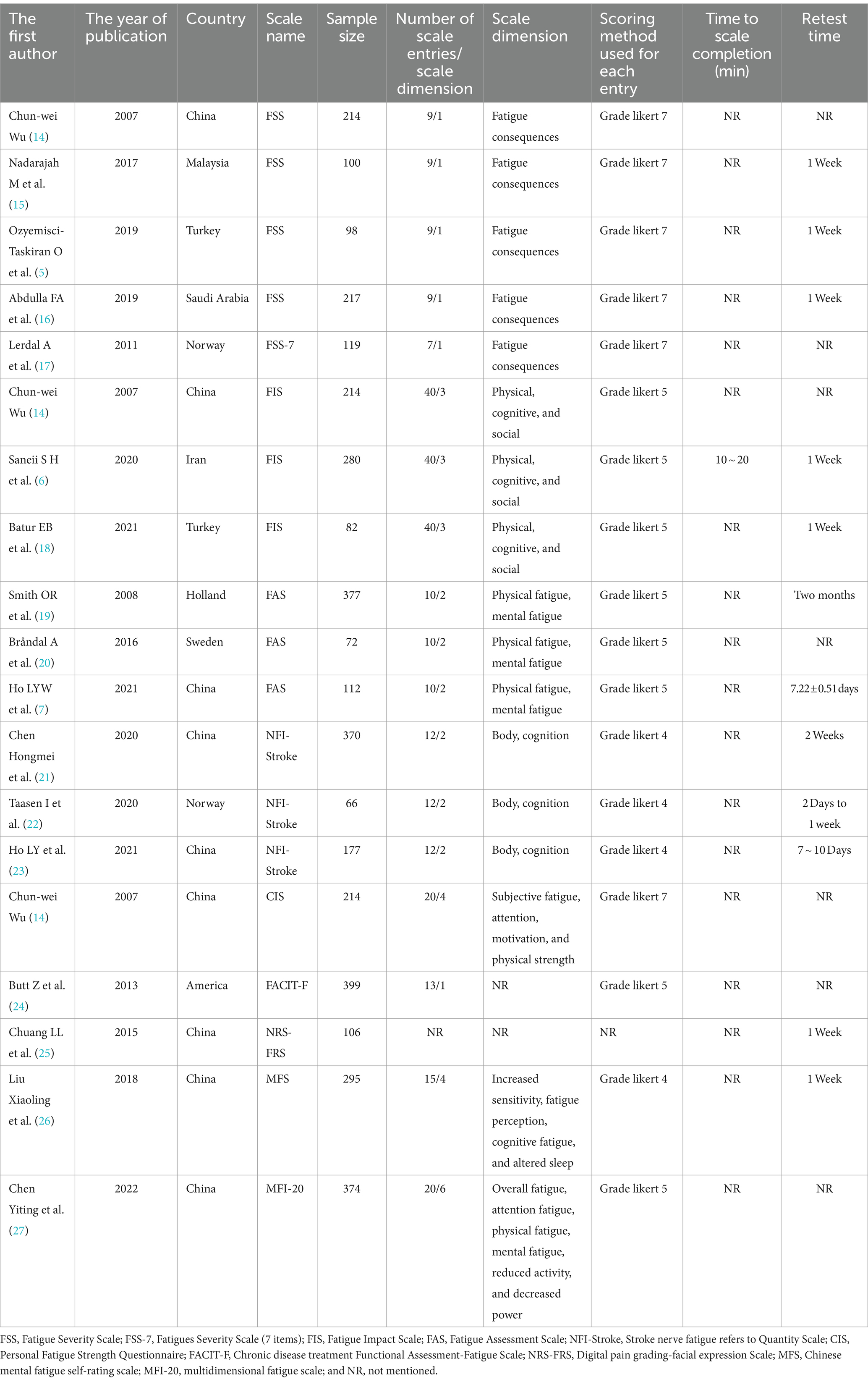
A total of 17 literature articles were included in this study (5–7, 14–27), involving 10 PSF-related measurement scales, namely, the Fatigue Severity Scale (FSS), Fatigue Severity Scale (7 entries) (FSS-7), Fatigue Impact Scale (FIS), Fatigue Assessment Scale (FAS), Stroke nerve fatigue Index Scale (NFI-Stroke), Personal Fatigue Strength Questionnaire (CIS), Functional Assessment of Chronic Disease Treatment-Fatigue Scale (FACIT-F), Digital Pain Scale-Facial expression Scale (NRS-FRS), Chinese version of Self-rating scale of mental fatigue (MFS), and The Chinese version of the multidimensional fatigue directory (MFI-20). Among them, the most evaluated scale was FSS with nine entries, while FSS-7 had two fewer entries with seven items. The NRS-FRS does not explicitly mention the scale dimensions, and FSS, FSS-7, and FACIT-F are all one-dimensional scales, while the rest are multidimensional scales. The scoring methods used for each item included Likert 4 scoring, Likert 5 scoring, and Likert 7 scoring; Likert 5 scoring was the most commonly used item scoring method in the included studies. The interval between the two measurements of the scale ranged from 2 days to 2 months. The basic characteristics of the included PSF correlation measurement scales are detailed in Table 1.
3.3 Methodological quality and measurement attributes quality evaluation results
The 17 included papers (5–7, 14–27) evaluated the content validity, structural validity, internal consistency, hypothesis testing, stability, cross-cultural validity, or measurement invariance of the scales in terms of methodological quality and quality of measurement attributes. (1) Content validity: eight studies (6, 7, 14, 20–23, 26) completed the evaluation of the scale content validity by consulting with experts. However, due to the insufficient description of the evaluation methods and processes adopted by the experts, the methodology quality assessment is “fuzzy” and the content validity is “uncertain.” Five studies (6, 7, 21, 23, 26) asked about patients’ understanding of the content of the scale, and two studies (6, 7) conducted face-to-face interviews with the patients and adjusted the content of the scale based on the interview results, but the methodology quality was assessed as “good” and “good” while the content validity was “sufficient”; the other three studies (21, 23, 26) did not perform qualitative analysis, the methodology quality and content validity corresponded to “fuzzy” and “uncertain.” (2) Structural validity: eight studies (5, 7, 14, 16, 21, 23, 26, 27) reported the test results of the structural validity of the scale. Among them, four studies (14, 16, 21, 27) used only the exploratory factor analysis to evaluate the structural validity of the scale, and the methodological quality was “good”; four studies (5, 7, 23, 26) performed confirmatory factor analysis, one study (5) was “good” due to insufficient sample size, and the other three studies (7, 23, 26) was “very good.” Two studies (23, 26) also reported the comparative fit coefficient of the scale (0.97), thus its construct validity was “sufficient.” (3) Internal consistency: 16 studies (5–7, 14–24, 26, 27) evaluated the internal consistency of the scale; of which, 8 studies (6, 15, 17–20, 22, 24) did not test the structural validity of the scale, the methodological quality corresponds to “fuzzy,” another 8 studies (5, 7, 14, 16, 21, 23, 26, 27) in addition to containing the test results of structural validity, also includes the Cronbach’s α coefficient of each dimension of the scale, thus the methodological quality is “very good.” In one study (23), Cronbach’s α coefficient is <0.7, its internal consistency corresponds to “inadequate.” (4) Hypothesis testing: The methodological quality of 11 studies (5, 7, 15, 16, 18, 20, 21, 23, 25–27) was “good”; 2 studies (6, 17) failed measurement properties or statistical analysis methods. A total of 10 studies (5–7, 15, 17, 18, 21, 23, 26, 27) did not make the hypothesis test as “uncertain,” and the (16, 20, 25) was “sufficient” in the remaining three studies. (5) Stability: 10 studies (5–7, 15, 16, 18, 20, 23, 25, 26) assessed the stability of the scale by test-retest reliability, of which 5 studies (7, 16, 18, 20, 23) were “fuzzy” in terms of methodological quality as they did not specify the measurement situation or retest time. In total, 10 studies (5–7, 15, 16, 18, 20, 23, 25, 26) all had a within-group correlation coefficient of >0.7, thus their stability was “sufficient.” (6) Cross-cultural validity or measurement invariance: only one study (17) conducted differential item functioning (DIF) analysis and the results showed that there is no DIF entry in FSS-7, thus the methodological quality is “very good,” and cross-cultural validity or measurement invariance is “sufficient.” The results of the methodological quality and measurement attribute quality assessment of the PSF measurement scale are shown in Tables 2, 3.
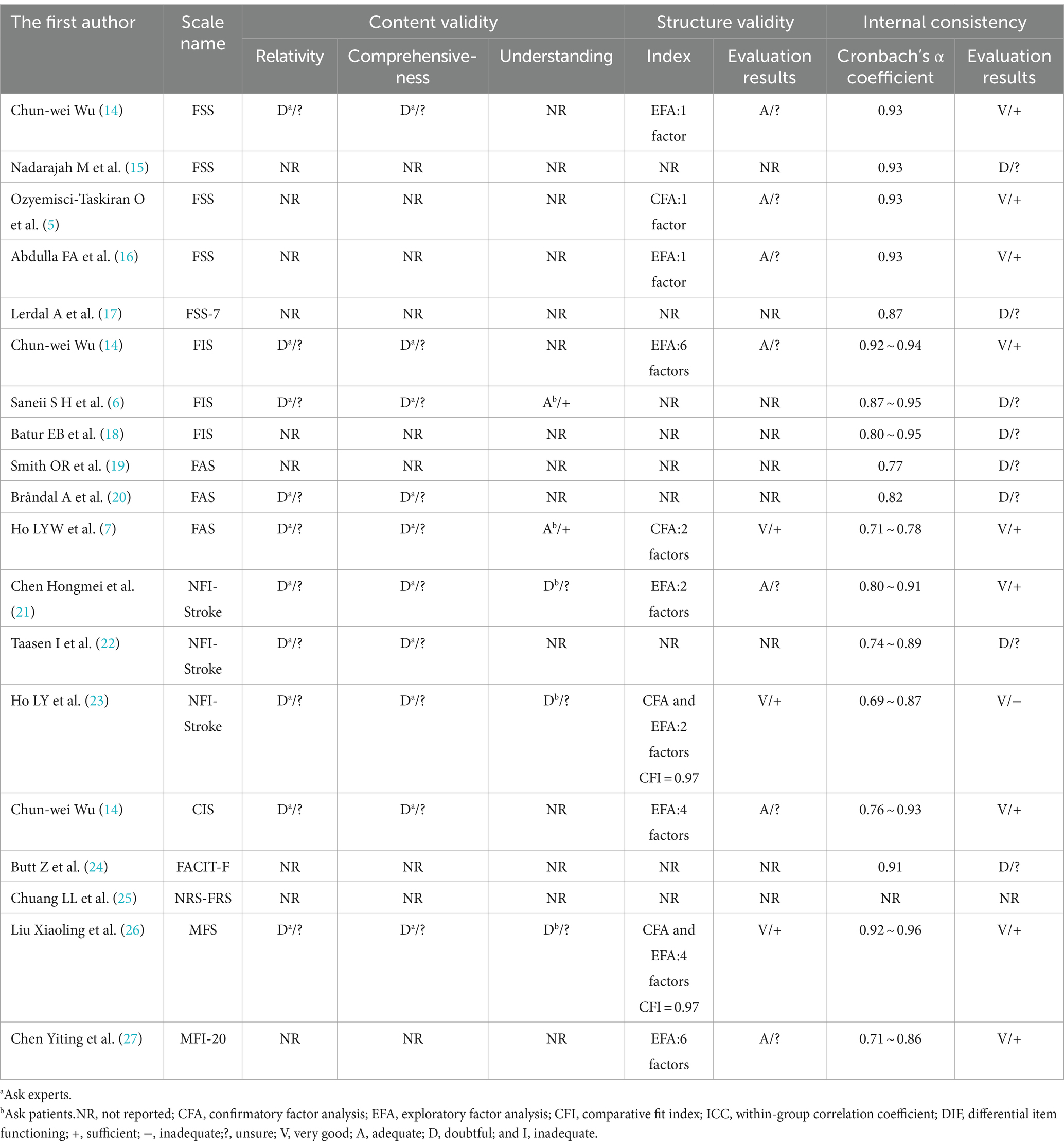
Table 2. The evaluation of content validity, structural validity, and internal consistency of PSF measurement scales.
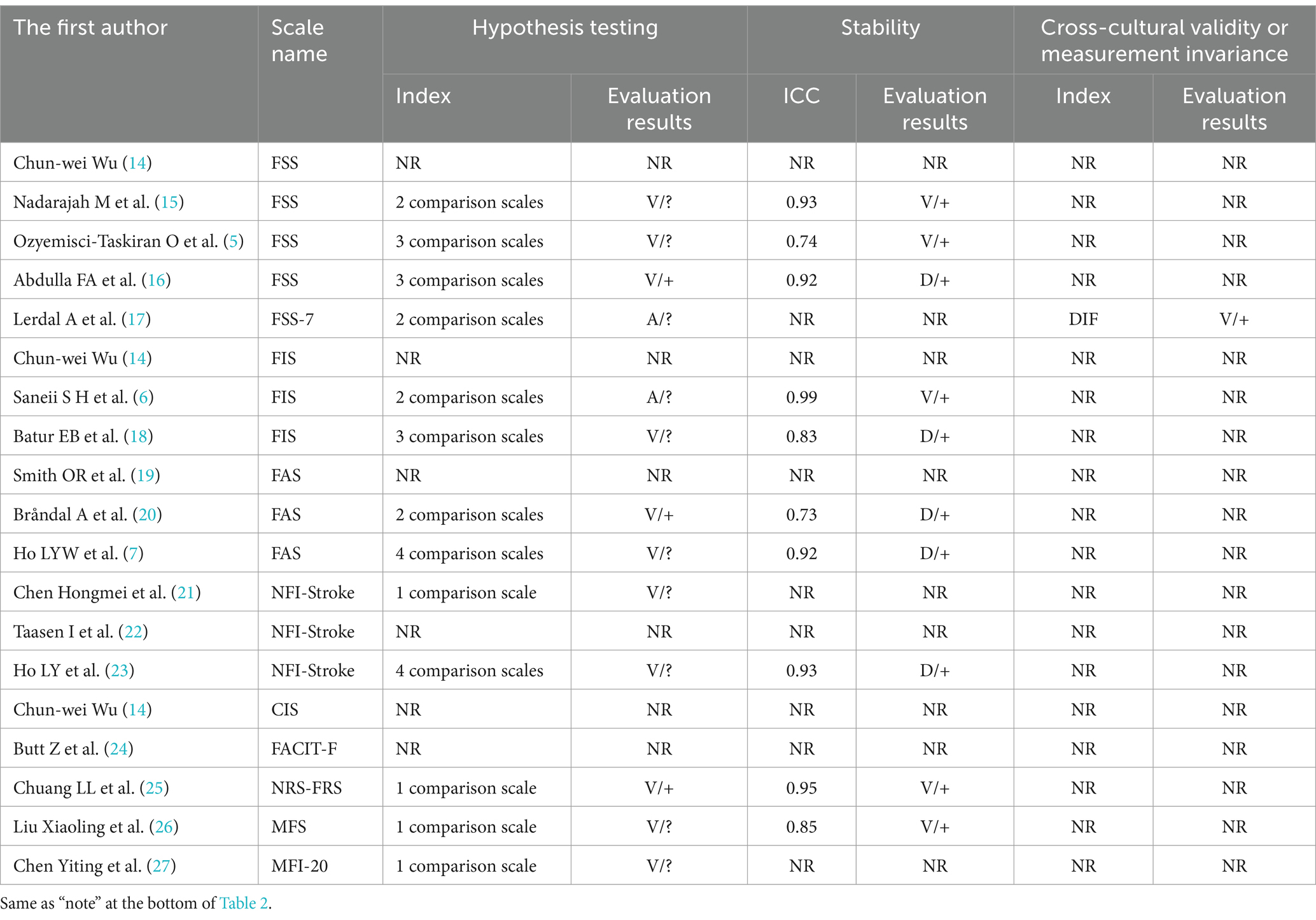
Table 3. The evaluation of hypothesis testing, stability, and cross-cultural validity or measurement invariance of PSF measurement scales.
3.4 Measurement attribute synthesis results and recommendations
For content validity, FSS-7, FACIT-F, NRS-FRS, and MFI-20 were “not mentioned” and the remaining scales were “uncertain.” The construct validity of the MFS was “sufficient,” the FSS-7, FACIT-F, and NRS-FRS were “not mentioned,” and the remaining scales were “uncertain.” The internal consistency of the NRS-FRS was “not mentioned,” the FSS and MFS were “sufficient,” and the internal consistency of the remaining scales was”uncertain.” The hypothesis tests for CIS and FACIT-F were “not mentioned,” NRS-FRS “sufficient,” and the remaining scales were “uncertain.” For stability, FSS-7, CIS, FACIT-F, and MFI-20 are “not mentioned” and the remaining scales are “sufficient.” Cross-cultural validity or measurement invariance of the FSS-7 was “full,” and the rest of the scales were “not mentioned.” Due to the risk of bias, the quality of evidence for the measurement attributes included in this study is mainly “medium” or “low,” and the recommendation grade is “B.” The synthetic results and recommendations of the PSF measurement scale are shown in Table 4.
4 Discussion
This study included 17 studies involving 10 PSF measurement scales. Although there are many scales available to measure PSF, they have different priorities for evaluating PSF, and the quality of their measurement properties is uneven, thus the corresponding test methods also have some problems. In this study, the existing issues in the included scale were analyzed and summarized from both scale validity and reliability, and relevant recommendations combined with the scale dimension were made, aiming to provide some theoretical basis for the selection, verification, or development of PSF-related measurement scales in the future.
Content validity, as the most important measurement attribute of a scale, can directly affect the level of evidence scale. However, most of the included studies did not explicitly mention the evaluation of the content validity of the scale, and only a small number of studies focused on the understanding of the scale. Based on this, in future research involving the development or verify the validity of the scale, in addition to the evaluation of scale validity, the researchers should also try to implement face-to-face interviews with the subjects, so as to intuitively understand the patient’s understanding of the scale, and according to the results of the scale content, in order to improve the agreement between the content of the scale and the tested constructs (28). Most of the included studies did not assess the structural validity of the scales. Furthermore, future studies need to present hypotheses between comparative scales before hypothesis testing; cross-cultural validity or measurement invariance should be evaluated. The scale reliability involved in this study included internal consistency and stability. It should be noted that when evaluating the internal consistency of multidimensional scales, the Cronbach’s α coefficient of the total scale and each subscale should be calculated simultaneously to clarify the reliability of each dimension of the scale. The stability of the scales included in this study was responded to by test–retest reliability. At present, researchers choose the retest time based on the hospitalization time of patients or from their own experience. There is no unified standard, which will affect the retest reliability of the scale to some extent. Therefore, future relevant studies should not only clarify the time interval between the two measurements but also clarify the basis for the selection of the retest time.
None of the scales included in this study evaluated responsiveness, measurement error, and criterion validity. Because the causal mechanism and specific features of PSF are still largely unknown (29), there is no golden standard for measuring PSF, thus it is impossible to evaluate the validity of PSF-related measurement scales. The FSS-7 included in this study was obtained after deleting two entries from the original FSS, but the study did not compare it with the original FSS to evaluate standard validity. Although Lenaert et al. (30) highlighted that there was a weak to moderate and strong correlation between the fatigue experience of patients with PSF and the FSS and FSS-7 scales, it also did not mention the content related to validity. Based on this, when the new scale is developed based on the original scale in the future, the new scale can be compared with the original scale, so as to complete the evaluation of the validity standard.
This study showed that FSS was the most evaluated scale, followed by FIS, FAS, and NFI-stroke, and none of these scales were evaluated for cross-cultural validity or measurement invariance. Although FSS-7 evaluated cross-cultural validity or measurement invariance, only one included study evaluated CIS, FACIT-F, NRS-FRS, MFS, and MFI-20, and more studies are needed to test whether the above scale is clinically applicable in the future. FSS has 9 items, and the evidence quality level of content validity and internal consistency corresponds to “medium” and “high,” respectively, which are also widely used in clinical practice. This is consistent with the findings of Kjeverud et al. (8). More research is needed in the future to clarify and harmonize the measurement tools for PSF. Therefore, this study temporarily recommends FSS for PSF measurement, but more tests still need to be conducted on its relevant measurement attributes, especially the cross-cultural validity or measurement invariance. In addition, the FSS is a unidimensional scale that can only assess somatic fatigue. For a multidimensional PSF assessment, perhaps FIS, FAS, and NFI-stroke should be considered. However, the above measurement scale should also be tested with more relevant measurement properties to verify its clinical applicability. This study also has some limitations as follows: ① only Chinese and English literature are included, there may be language bias; ② PSF scale has only one study included in the evaluation, which may affect the results of this study to some extent; ③ some scales have not been studied with large samples, thus the results of this study need to be interpreted carefully.
5 Conclusion
In summary, this study temporarily recommended FSS to measure PSF. To assess the PSF more comprehensively, the use of FIS, FAS, and NFI-stroke should be considered. All 10 PSF measurement scales involved in this study need to be studied to verify their validity. In future, when selecting, validating, or developing PSF-related measurement scales, the relevant assessment problems of the inclusion scales mentioned in this study should be avoided as far as possible, in order to get more high-quality evidence support and more scientific and standardized measurement tools.
Author contributions
LW: Methodology, Software, Writing – original draft, Writing – review & editing. HJ: Data curation, Software, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ulrichsen, KM, Kolskår, KK, Richard, G, Alnæs, D, Dørum, ES, Sanders, AM, et al. Structural brain disconnectivity mapping of post-stroke fatigue. Neuroimage Clin. (2021) 30:102635. doi: 10.1016/j.nicl.2021.102635
2. de, W, Dantzer, R, Ormstad, H, and Kuppuswamy, A. Mechanisms of poststroke fatigue. J Neurol Neurosurg Psychiatry. (2018) 89:287–93. doi: 10.1136/jnnp-2017-316007
3. De Doncker, W, Ondobaka, S, and Kuppuswamy, A. Effect of transcranial direct current stimulation on post-stroke fatigue. J Neurol. (2021) 268:2831–42. doi: 10.1007/s00415-021-10442-8
4. Klinedinst, NJ, Schuh, R, Kittner, SJ, Regenold, WT, Kehs, G, Hoch, C, et al. Post-stroke fatigue as an indicator of underlying bioenergetics alterations. J Bioenerg Biomembr. (2019) 51:165–74. doi: 10.1007/s10863-018-9782-8
5. Ozyemisci-Taskiran, O, Batur, EB, Yuksel, S, Cengiz, M, and Karatas, GK. Validity and reliability of fatigue severity scale in stroke. Top Stroke Rehabil. (2019) 26:122–7. doi: 10.1080/10749357.2018.1550957
6. Saneii, SH, Heidari, M, Zaree, M, and Akbarfahimi, M. Psychometric features of the Persian version of the fatigue impact scale in Iranian stroke patients. J Adv Med Biomed Res. (2020) 28:111–8. doi: 10.30699/jambs.28.127.111
7. Ho, LYW, Lai, CKY, and Ng, SSM. Measuring fatigue following stroke: the Chinese version of the fatigue assessment scale. Disabil Rehabil. (2021) 43:3234–41. doi: 10.1080/09638288.2020.1730455
8. Kjeverud, A, Andersson, S, Lerdal, A, Schanke, AK, and Østlie, K. A cross-sectional study exploring overlap in post-stroke fatigue caseness using three fatigue instruments: fatigue severity scale, fatigue questionnaire, and the Lynch's clinical interview. J Psychosom Res. (2021) 150:110605. doi: 10.1016/j.jpsychores.2021.110605
9. Blackwell, S, Crowfoot, G, Davey, J, Drummond, A, English, C, Galloway, M, et al. Management of post-stroke fatigue: an Australian health professional survey. Disabil Rehabil. (2023) 45:3893–9. doi: 10.1080/09638288.2022.2143578
10. Mokkink, LB, Boers, M, van der Vleuten, CPM, Bouter, LM, Alonso, J, Patrick, DL, et al. COSMIN risk of Bias tool to assess the quality of studies on reliability or measurement error of outcome measurement instruments: a Delphi study. BMC Med Res Methodol. (2020) 20:293. doi: 10.1186/s12874-020-01179-5
11. Mokkink, LB, De Vet, HC, Prinsen, CA, Patrick, DL, Alonso, J, Bouter, LM, et al. COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res. (2018) 27:1171–9. doi: 10.1007/s11136-017-1765-4
12. Terwee, CB, Prinsen, CAC, Chiarotto, A, Westerman, MJ, Patrick, DL, Alonso, J, et al. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res. (2018) 27:1159–70. doi: 10.1007/s11136-018-1829-0
13. Alhazzani, W, and Guyatt, G. An overview of the GRADE approach and a peek at the future. Med J Australia. (2018) 209:291–2. doi: 10.5694/mja18.00012
14. Wu, CW . Post-stroke fatigue: the application and evaluation of the scale and the analysis of related factors. Cap Med Univ. (2007)
15. Nadarajah, M, Mazlan, M, Abdul-Latif, L, and Goh, HT. Test-retest reliability, internal consistency and concurrent validity of fatigue severity scale in measuring post-stroke fatigue. Eur J Phys Rehabil Med. (2017) 53:703–9. doi: 10.23736/S1973-9087.16.04388-4
16. Abdulla, FA, Al-Khamis, FA, Alsulaiman, AA, and Alshami, AM. Psychometric properties of an Arabic version of the fatigue severity scale in patients with stroke. Top Stroke Rehabil. (2019) 26:448–55. doi: 10.1080/10749357.2019.1628465
17. Lerdal, A, and Kottorp, A. Psychometric properties of the fatigue severity scale-Rasch analyses of individual responses in a Norwegian stroke cohort. Int J Nurs Stud. (2011) 48:1258–65. doi: 10.1016/j.ijnurstu.2011.02.019
18. Batur, EB, Ozyemisçi-Taskiran, O, Yuksel, S, Cengiz, M, and Karatas, GK. Validity and reliability of the fatigue impact scale in stroke. Top Stroke Rehabil. (2021) 20:1–12. doi: 10.1080/10749357.2021.1978629
19. Smith, OR, van den Broek, KC, Renkens, M, and Denollet, J. Comparison of fatigue levels in patients with stroke and patients with end-stage heart failure: application of the fatigue assessment scale. J Am Geriatr Soc. (2008) 56:1915–9. doi: 10.1111/j.1532-5415.2008.01925.x
20. Bråndal, A, Eriksson, M, Wester, P, and Lundin-Olsson, L. Reliability and validity of the Swedish fatigue assessment scale when self-administrered by persons with mild to moderate stroke. Top Stroke Rehabil. (2016) 23:90–7. doi: 10.1080/10749357.2015.1112057
21. Chen, HM, Zhang, S, Wang, YG, Yuan, Y, Tang, XM, Zhang, XJ, et al. Chinese version of neurological fatigue index for stroke and its reliability and validity test. Nurs Res. (2020) 34:4. doi: 10.12102/j.issn.1009-6493.2020.23.003
22. Taasen, I, Loureiro, AP, and Langhammer, B. The neurological fatigue index for stroke. Reliability of a Norwegian version. Physiother. Theory Pract. (2020) 24:1–8. doi: 10.1080/09593985.2020.1825581
23. Ho, LY, Lai, CK, and Ng, SS. Testing the psychometric properties of the Chinese version of the neurological fatigue index-stroke. Clin Rehabil. (2021) 35:1329–40. doi: 10.1177/02692155211001684
24. Butt, Z, Lai, JS, Rao, D, Heinemann, AW, Bill, A, and Cella, D. Measurement of fatigue in cancer, stroke, and HIV using the functional assessment of chronic illness therapy—fatigue (FACIT-F) scale. J Psychosom Res. (2013) 74:64–8. doi: 10.1016/j.jpsychores.2012.10.011
25. Chuang, LL, Lin, KC, Hsu, AL, Wu, CY, Chang, KC, Li, YC, et al. Reliability and validity of a vertical numerical rating scale supplemented with a faces rating scale in measuring fatigue after stroke. Health Qual Life Outcomes. (2015) 30:91. doi: 10.1186/s12955-015-0290-9
26. Liu, XL, and Ma, SH. Sinicization and reliability and validity tests of the mental fatigue scale. Chinese J Rehabilitation Med. (2018) 33:6. doi: 10.3969/j.issn.1001-1242.2018.08.014
27. Chen, YT, Shi, J, Xia, H, and Li, Z. Reliability and validity of the Chinese version of multidimensional fatigue inventory in stroke patients. Shanghai Nurs. (2022) 22:5. doi: 10.3969/j.issn.1009-8399.2022.04.004
28. Yan, X, Liu, QQ, Su, YJ, Dou, XM, and Wei, SQ. Systematic review of universal supportive care needs scale for Cancer patients based on COSMIN guidelines. Gen Pract China. (2022) 25:408–15. doi: 10.12114/j.issn.1007-9572.2021.00.349
29. Kruithof, N, Van Cleef, MH, Rasquin, SM, and Bovend’Eerdt, TJ. Screening Poststroke fatigue; feasibility and validation of an instrument for the screening of Poststroke fatigue throughout the rehabilitation process. J Stroke Cerebrovasc Dis. (2016) 25:188–96. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.015
Keywords: post-stroke fatigue, measurement scales, COSMIN guide, systematic review, fatigue severity scale
Citation: Wu L and Jin H (2024) A systematic review of post-stroke fatigue measurement scale based on COSMIN guidelines. Front. Neurol. 15:1411472. doi: 10.3389/fneur.2024.1411472
Edited by:
Hideki Nakano, Kyoto Tachibana University, JapanReviewed by:
Jose Antonio López-Pina, University of Murcia, SpainRita Pilar Romero-Galisteo, University of Malaga, Spain
Copyright © 2024 Wu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingsha Wu, MTczNzc2MjA2MjZAMTYzLmNvbQ==
 Lingsha Wu
Lingsha Wu Haiqin Jin
Haiqin Jin