- 1Department of Neurology, State University of New York Downstate Health Sciences University, Brooklyn, NY, United States
- 2Department of Neurology, University Hospital Carl Gustav Carus, Technical University Dresden, Dresden, Germany
Background: Rapid and accurate acute ischemic stroke (AIS) diagnosis is needed to expedite emergent thrombolytic and mechanical thrombectomy treatment. Changes in blood-based protein biomarkers during the first 24 h of AIS, the time window for treatment, could complement imaging techniques and facilitate rapid diagnosis and treatment.
Methods: We performed a systematic review according to PRISMA guidelines. MEDLINE, EMBASE, Cochrane Library, and Web of Science databases were searched for eligible studies comparing levels of blood-based protein biomarkers in AIS patients with levels in healthy controls and stroke mimics. Protein biomarkers from the following pathophysiological categories were included: neurovascular inflammation (MMP-9, TNF-alpha), endothelial integrity (VCAM-1, ICAM-1), cell migration (E-Selectin, P-Selectin, L-Selectin), markers of glial and neuronal origin (GFAP, S100, S100B, NSE), and cardiac dysfunction (BNP, NT-proBNP). The literature search was limited to English-language publications before November 7th, 2023.
Results: A total of 61 studies from 20 different countries were identified, which included in total, 4,644 AIS patients, 2,242 stroke mimics, and 2,777 controls. Studies investigating TNF-alpha, MMP-9, VCAM-1, ICAM-1, E-Selectin, L-Selectin, GFAP, NSE, and S100B showed pronounced methodological heterogeneity, making between-study comparisons difficult. However, in 80% of NT-proBNP and BNP studies, and all P-selectin studies, higher biomarker levels were observed in AIS patients compared to healthy controls and/or patients with stroke mimics.
Conclusion: None of the biomarkers included showed sufficient evidence for additional diagnostic benefit for AIS. Comprehensive standardized global multicenter studies are needed to (1) permit comparability, (2) enable valid statements about protein-based biomarkers, and (3) reflect real-world scenarios.
1 Introduction
Stroke is the third leading cause of long-term disability and the second leading cause of death worldwide (1). The two major stroke subtypes are ischemic and hemorrhagic stroke, with 87% of all strokes being ischemic (2). The detection of intracerebral hemorrhagic stroke with non-contrast computerized tomography (CT) has a sensitivity approaching 100% when performed within 6 h of stroke symptoms onset (3). However the early detection of acute ischemic stroke (AIS) with CT has a much lower sensitivity, ranging from 57 to 71%, during the first hours after symptom onset (3–5). Although brain magnetic resonance imaging (MRI) has a 79.8% sensitivity for AIS detection in the hyperacute phase, MRI not only takes more time but is also not widely used in emergency or under-resourced settings (6). Rapid and accurate diagnosis is required in the first 24 h of AIS, since patients may be eligible for reperfusion therapy with intravenous recombinant tissue plasminogen activator (rt-PA) within the first 4.5 h, and endovascular therapy (EVT) up to 24 h in some patients with large vessel occlusions (7). In addition, economic and logistical factors including availability of brain MRI or interventional radiology resources for EVT, infrastructure, and fast biomarker detection devices play key roles in acute diagnosis and intervention decisions (8).
Blood-based protein biomarkers could complement the diagnosis of AIS because of their relative ease and rapidity of use, and low cost (9–11). To be clinically applicable, blood-based biomarkers need to be specific, sensitive, and reliable (8). However, no diagnostically accurate protein biomarkers from blood have yet been proven to have the required diagnostic accuracy for clinical application and decision-making in AIS. This may change with the continued and accelerated development of sensitive and accurate assay methods for the detection of blood-based protein biomarkers. For example, compared to Enzyme-linked Immunosorbent Assay (ELISA), Single Molecule Array (SIMOA) provides enhanced measurement resolution to as little as femtogram/ml concentrations. Furthermore, Point-of-care technologies are continually evolving (12–14). SIMOA revitalized the investigation of blood-based glial fibrillary acid protein (GFAP) and matrix-metalloproteinase 9 (MMP-9) (15, 16). With SIMOA and other constantly improving measurement methods such as point of care (POCT)-devices, the question arises as to whether these more sensitive and specific techniques may lead to new insights regarding the utility of circulating protein biomarkers in AIS diagnosis. Furthermore, now that an extended time window for EVT has been achieved in AIS patients suffering from large vessel occlusions (LVO), with large, salvageable ischemic penumbras compared to small cores, validated blood tests could be used in the field to transfer patients to appropriate stroke facilities for thrombolysis or thrombectomy-capable centers for EVT.
When a blood vessel is blocked in ischemic stroke, the primary blood supply to a circumscribed area of the brain is compromised. This leads to a reduced supply of oxygen and glucose and thus depletes energy/adenosine triphosphate to that brain area, as well as increasing metabolic end products such as carbon dioxide and lactate. This results in an acidotic shift (17–19). Evolving ischemia and reduced cellular energy impair metabolic pumps, damage cells, and activate the innate immune system in the microvessels and brain overall (20). Local neurovascular inflammation is largely driven by inflammatory signaling, inflammatory molecules such as metalloproteinases, and cytokines such as tumor necrosis factor-alpha (TNF-alpha). This activates immune cells and damages the extracellular matrix and the blood–brain barrier, resulting in the entry of toxic blood constituents including activated leukocytes (20–23). Neurovascular inflammatory processes involve interactions among E-Selectin, P-Selectin, and L-Selectin (i.e., small protein molecules expressed on leukocytes/endothelium) that mediate the first contact between stimulated endothelial cells and leukocytes. Selectins are involved in initial interactions/rolling of leukocytes. P-selectin facilitates the rolling of platelets and leukocytes on activated endothelial cells. Upon platelet activation, P-selectin translocates from intracellular granules to the outer membrane, while fibrinogen aggregates platelets by binding glycoprotein (GP) IIb/IIIa between adjacent platelets. Up-regulated integrin and Ig superfamily cell adhesion molecules provide firm adhesion and their subsequent extravasation into the brain from the vascular endothelium to initiate immune cell homing, platelet binding, and neutrophil extravasation. Thus, Vascular Cell Adhesion Molecule-1 (VCAM-1) and Intercellular Adhesion Molecule-1 (ICAM-1) are responsible for cell adhesion of circulating immune cells enabling leukocyte migration from the leaky vessels into the brain (24, 25). The evolution of brain injury continues to increase with migration of leukocytes, glial activation and inflammation, and accumulation of necrotic and apoptotic cells (i.e., increased cell death and an expanding brain infarction) (24).
In AIS, these pathophysiological processes lead to cell damage and death and cause the release of cell proteins inside and on the surface of the brain and vascular cells into the bloodstream. Glial fibrillary acid protein (GFAP), which is found in astrocytes, is responsible for the stability of the intracellular structure (26). Neuron-specific enolase (NSE) is involved in the energy metabolism of neurons via catalysis of 2-phosphoglycerate to phosphoenolpyruvate (27). The S100 protein family consists of at least 20 different, multifunctional signaling proteins involved in regulating various cellular processes such as motility, differentiation, cell cycle progression, and cell growth (28). One member of the S100 family, S100B, is found in astrocytes, and oligodendrocytes. These proteins are released into the blood when cells are injured by a disruption of the blood–brain barrier, as in AIS (29).
Vascular occlusion in AIS not only leads to brain insults but also has consequences on the autonomic nervous and cardiovascular systems (30). The interaction between brain and heart is often referred to as stroke-heart syndrome, which is associated with a vegetative derailment that primarily affects blood pressure and heart rate, but can also include cardiac arrhythmias or cardiac shock (31). Proteins such as N-Terminal pro-Brain Natriuretic Peptide (NT-proBNP) or Brain Natriuretic Peptide (BNP) are increasingly released, when the heart is being stressed, as in AIS (32–34).
In this systematic review, we summarize the literature on blood-based protein biomarkers into the following pathophysiological classes: neurovascular inflammation (MMP-9, TNF-alpha), endothelial function (VCAM-1, ICAM-1), cell migration (E-selectin, P-selectin, L-selectin), markers of glial, astrocytic neuronal origin (NSE, GFAP, S100, S100B) and cardiac dysfunction (BNP, NT-proBNP). These biomarkers play key roles in the progressive ischemic brain injury and pathophysiology that occurs after AIS. Our review includes studies reporting blood-based biomarker levels measured within 24 h after the onset of AIS symptoms to provide an up-to-date overview of individual potential blood-based protein biomarkers that may aid acute AIS diagnosis. A secondary objective was to screen and analyze included studies that measured more than one of these biomarkers in AIS patients and to summarize these results.
2 Materials and methods
2.1 Study criteria
The clinical research question (i.e., Population, Intervention, Controls, Outcome, and Time; PICOT) and the following inclusion criteria were: (1) Population: patients with AIS, age ≥ 18 years; (2) Intervention: measurement of blood MMP-9, TNF-alpha, VCAM-1, ICAM-1, E-Selectin, P-Selectin, L-Selectin, NSE, GFAP, S100, S100B, BNP and NT-proBNP levels; (3) Inclusion of a control group: healthy controls (HC), matched controls, and/or stroke mimics as indicated by the authors of the included studies (i.e., syncope, migraine, neuropathy, brain tumor, toxic-metabolic disturbances, systemic infections, postictal state, headache disorders, vestibular disorders, seizure, symptomatic aggravation of known neurodegenerative disorders, peripheral neuropathy, syncope, brain tumor, metabolic disorders, functional disorders, infections, transient global amnesia, hearing loss, etc.); (4) Outcomes included comparison of biomarker levels between AIS patients and controls; (5) Time interval: studies up to November 2023; (6) Study design: randomized controlled trials, prospective cohort studies, case–control studies, retrospective studies; and (7) English language.
Exclusion criteria were: (1) Published whole text language other than English; (2) Missing control group; (3) Animal studies; and (4) Case reports, case series, reviews, and letters to the editor.
2.2 Data sources and search strategy
This systematic review followed the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines (35). The literature search was performed by two independent investigators (JR, AC) using EMBASE, The Web of Science, The Cochrane Library, and MEDLINE up to November 2023.
Combinations of search strings included “stroke,” “protein,” “biomarker,” “NSE,” neuron specific enolase,” “S100,” “S100B,” “GFAP,” “glial fibrillary acid protein,” MMP-9″, “metalloproteinase,” “ICAM-1″,” intercellular adhesion molecule,” “VCAM-1″, “vascular cell adhesion molecule,” “Selectin,” “BNP,” “B-type natriuretic peptide,” “NT-proBNP,” “TNF-alpha,” “tumor necrosis factor” with the Boolean operators “AND” and “OR.” The Boolean operator “NOT” was used for the terms, “mice,” “mouse,” “rat,” “rats,” and “animal.” (36) Reference lists of identified articles were screened for additional sources. Furthermore, relevant meta-analyses and systematic reviews were screened manually to ensure a comprehensive literature review. The complete search strategy can be found in the “Appendix_Literature_Search_Strategy.” Zotero was used to screen literature search results and remove duplicates (37).
2.3 Data extraction
The literature review was performed by two independent investigators (JR, AC). A third reviewer (FCB) was involved in case of disagreement between the two primary reviewers. The following data were extracted from the publications: (1) study characteristics (type of study, time of investigation, number of included AIS and controls); (2) participants’ baseline characteristics (age, sex/gender); (3) biomarker measurement methods used, units of measurements; (4) blood drawing time (range); (5) study outcome (biomarker comparisons between AIS patients and control groups. If outcome-related data could not be obtained by searching the articles for text, manuscript tables, and supplemental tables, data were extracted using the WebPlotDigitizer if an adequate graphic was provided) (38). If not otherwise specified, significance levels of the included studies were p-value of ≤0.05. All results reported in this review were taken from the original articles and are presented according to their specifications.
2.4 Methodological quality assessment—risk of bias
The quality of case–control and cohort studies was evaluated according to the “Newcastle-Ottawa Scale” (NOS). The developers of the NOS established a “star system” in which studies are rated in the categories “Selection, Comparability, and Exposure/Outcome.” (39) A maximum of 9 points can be achieved. In line with the current literature the studies were classified as follows: ≥7 stars were considered as “good-quality,” 2–6 stars, “fair-quality,” and ≤ 1 star, “poor-quality.”
3 Results
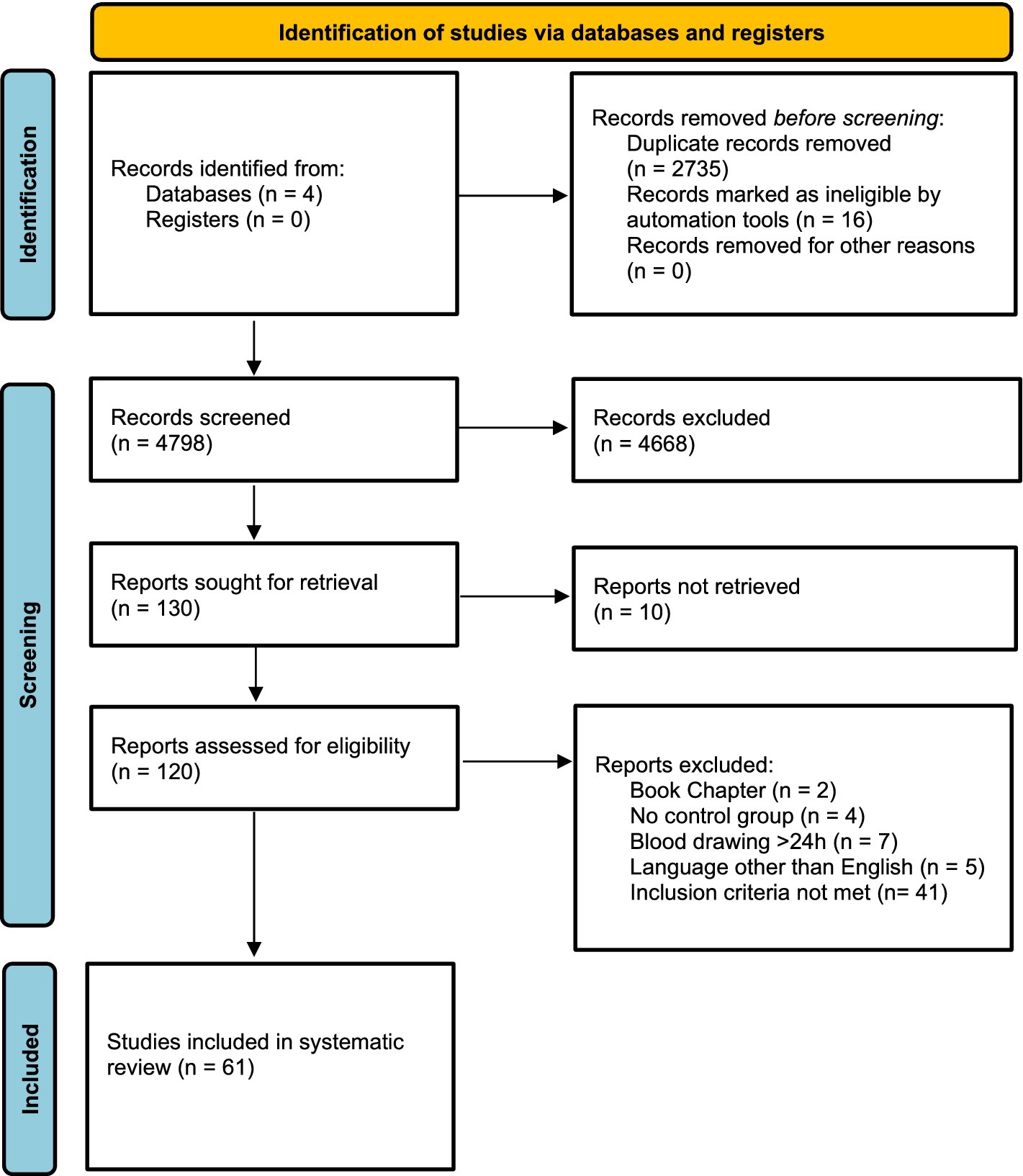
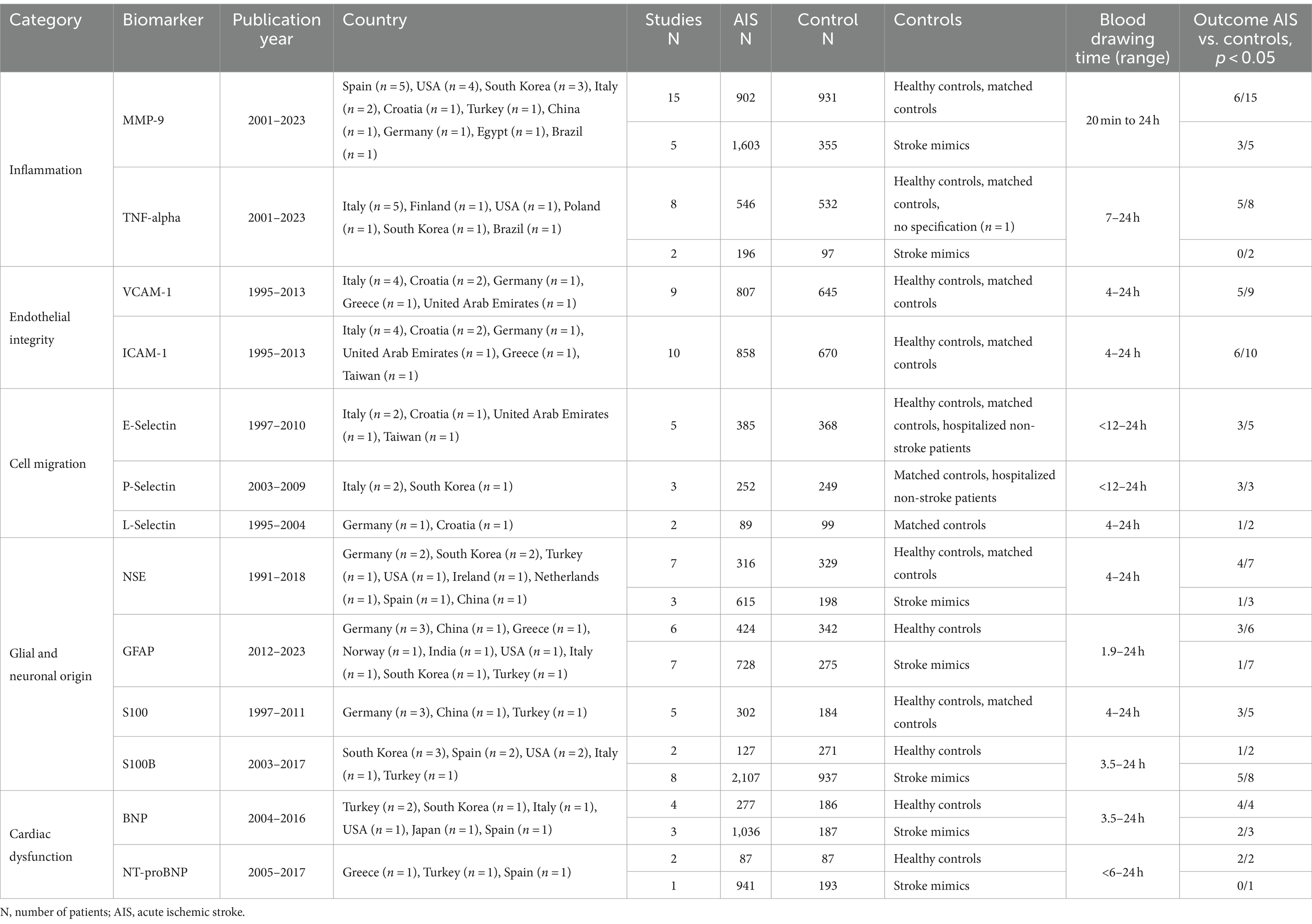
The initial search used EMBASE, The Web of Science, The Cochrane Library, and MEDLINE and resulted in 7,533 publications. After removing duplicates, 4,798 publications were screened. Of those, 130 were eligible for a full-text search. Subsequently, 69 were excluded, 10 could not be retrieved, two were book chapters, four did not include a control group, five were written in a language other than English, and 48 did not meet our inclusion criteria (e.g., investigation of other biomarkers than stated in the inclusion criteria, blood sampling >24 h after symptom onset or the timing of blood sampling was uncertain) (Figure 1). Considering that individual studies sometimes investigated more than one biomarker, we were able to detect a total of 61 unique studies that reported, in total, on 4,644 AIS patients and 2,777 participants in a control group. The number of studies reporting on each of the biomarkers is as follows: MMP-9, TNF-alpha, n = 10; n = 20; VCAM-1, n = 9; ICAM-1, n = 10, E-Selectin, n = 5, P-Selectin, n = 3, L-Selectin, n = 2, NSE, n = 10, GFAP, n = 11, S100, n = 5, S100B, n = 9, BNP, n = 7, NT-proBNP n = 3, biomarker panels, n = 8. Most studies were carried out in Italy (14.8%), Germany (11.5%), and Turkey (11.5%). Interestingly, 20 different countries were represented in the included studies (Table 1; Supplementary Figure S1). Overall, the NOS bias assessment showed that most of the studies evidenced a fair quality (70.5%). Good quality was present in 29.5%. Poor quality was not detected. On average, a value of 5.36 (standard deviation, SD: 1.752) stars was observed (Supplementary Tables S1–S5).
3.1 Biomarkers of neurovascular inflammation
3.1.1 MMP-9
Twenty studies in which MMP-9 was measured were included and comprised 2,515 AIS patients and 1,286 controls (HC/matched controls: n = 931, stroke mimics: n = 355) (22, 34, 40–57). Most investigations occurred in Spain (n = 5) followed by the USA (n = 4), South Korea (n = 3), Italy (n = 2), Croatia (n = 1), Turkey (n = 1), China (n = 1), Germany (n = 1), Egypt (n = 1), and Brazil (n = 1). ELISA was the measurement platform in 76%. However, POCT systems as well as heat shock protein pull-down assays and antibody-based arrays other than ELISA were used. Studies were published between 2001 and 2023. Blood drawing time among studies ranged from 20 min to <24 h after symptom onset (Table 2). Seventy percent of the included studies showed good quality according to the NOS, while 30% of the studies were of fair quality (Supplementary Table S1).
3.1.1.1 AIS vs. healthy controls/matched controls
Most of the studies measuring MMP-9 (n = 15; 80%) compared AIS patients to HC/matched controls. Higher MMP-9 levels in AIS patients compared to HC were observed generally in 47% of studies (22, 43–45, 52, 54, 56) (Table 1). In 20% of studies there was a significant difference (p < 0.05) (47–49). No statistical comparison was made between AIS and HC in 33% of the studies, yet 80% of those studies reported at least twice as high MMP-9 levels in AIS patients compared to HC (34, 40, 41, 44, 46, 57).
3.1.1.2 AIS vs. stroke mimics
Patients with stroke mimics as a control group were observed in 20% of studies. Three of them showed higher MMP-9 levels in AIS patients compared to stroke mimics (p ≤ 0.05), although two studies did not perform statistical comparisons between AIS and stroke mimic patients (Table 1) (42, 51, 53). In two studies, almost similar MMP-9 levels were observed in AIS and stroke mimic patients (175 ng/mL ± 149 vs. 175 ng/mL ± 227, p = 0.980; and median 11.6 pg/mL, IQR: 11.2–12.3 vs. 11.8 pg/mL, IQR: 11.3–12.2) (50, 55). In a receiver operator curve (ROC) analysis to differentiate between AIS and stroke mimics based on MMP-9 levels, a cut-off value of 199 ng/mL showed a sensitivity of 65% and a specificity of 53%. The odds ratio (OR) of AIS versus stroke mimic was 1.66 (95% CI: 1.01–2.73, p = 0.046) (42). In another study, analyzing MMP-9 using a bootstrap method there was moderate predictive power for AIS, ranging from 42.4% (backward selection) to 75.5% (forward selection).
3.1.1.3 Etiology-, outcome-, severity related MMP-9 levels in AIS patients
Compared to HC, higher MMP-9 levels were reported in one study investigating patients experiencing large artery atherosclerosis strokes (LAAS) 1137.0 ng/mL [IQR: 809.0–1813.0], lacunar stroke (LAC) 1367.0 ng/mL [IQR: 763.5–1775.0], and cardioembolic infarcts (CEI) 1307.0 ng/mL [IQR: 814.5–1750.0] vs. 686.4 ng/mL [IQR: 544.8–1048.0]; (p ≤ 0.05) (54). Studying MMP-9 levels in patients with unoccluded middle cerebral artery (MCA), proximal occlusion of the MCA, or distal occlusion of the MCA using transcranial Doppler revealed the more distal the pathology, the higher the MMP-9 levels (unoccluded MCA: 99.4 ng/mL, SD: 102.4; proximal occlusion: 236.4 ng/mL ± 71.1; distal occlusion: 188.1 ng/mL ± 149.3, Kruskal-Wallis-test p = 0.032) (40). Moderate to high correlations between infarct volume measured by brain CT or MRI on admission and MMP-9 concentrations were reported (r = 0.35–0.89, p < 0.001, respectively) (22, 43, 45, 47, 52). However, two studies reported correlations p > 0.05, albeit early signs of ischemia on brain CT were associated with higher levels of MMP-9 (163 ng/mL [IQR: 110–193] vs. 54 ng/mL [IQR: 38–74], p < 0.001) (40, 43). MMP-9 concentrations have also correlated positively with the severity of stroke as determined by the National Institutes of Health and Stroke Scale (NIHSS) after 12 h (r = 0.46, p = 0.01), but not with the NIHSS measured on admission (r = 0.28, p = 0.12) (52). Interestingly, mean MMP-9 levels did not differ in the presence versus absence of hypertension (118.3 ng/mL versus 104.1 ng/mL, p = 0.58) (41). However, one study reported a positive correlation between plasma MMP-9 levels and both systolic (r = 0.70) and diastolic blood pressure (r = 0.63, p ≤ 0.05) (43).
3.1.2 TNF-alpha
Ten eligible studies measuring TNF-alpha were found comprising 742 AIS patients and 629 controls (HC/matched controls: n = 532 stroke mimics: n = 97) (53, 54, 57–64). Half of the studies were carried out in Italy (n = 5) followed by Finland (n = 1), USA (n = 1), Poland (n = 1), South Korea (n = 1), and Brazil (n = 1). Studies were published between 2001 and 2023 and in all studies, ELISA was used to measure TNF-alpha. Only two studies used stroke mimics as a control group while the others included age-, and sex-matched healthy controls or patients admitted to the hospital for a cause other than acute cardiovascular or cerebrovascular events. Blood collection among studies ranged from 7 h to <24 h after symptom onset (Table 2). The quality of the studies was found to be good in 50% of the studies and fair in 50% of the included studies (Supplementary Table S1).
3.1.2.1 AIS vs. healthy controls/matched control patients
More than half of the included studies (62.5%) showed higher levels of TNF-alpha in AIS patients compared to the control group, 25% revealed lower TNF-alpha levels and one study did not provide a statistical comparison (58, 60–64).
3.1.2.2 AIS vs. stroke mimics
One study revealed no difference in TNF-alpha levels between AIS patients and stroke mimics (2.2 pg/mL, IQR: 0–8.9 vs. 2.6 pg/mL, IQR: 0–7.4, p = 0.541) (53). Comparable results were obtained by another study (0.47 pg/mL, IQR: 0.34–0.74 vs. 0.55 pg/mL, IQR: 0.41–5.70) (57).
3.1.2.3 Etiology-, outcome-, severity related TNF-levels in AIS patients
Among AIS classified as CEI, compared to LAAS, LAC, or other determined etiology (ODE), higher median TNF-alpha plasma levels were reported (p < 0.0001) (61, 62). Contrary results were reported by one study that did not find differences by AIS etiology (LAAS, LAC, CEI) and age-, ethnicity-, and body mass index-matched healthy controls (54). No differences in TNF-alpha levels were found in another study (30.1 pg/mL, SD:12.5 vs. 29.0 pg/mL, SD: 13.9, p = 0.746) (59). Among patients with LAC and without a lacunar detectable lesion on neuroimaging, no difference in TNF-alpha levels was found (p = 0.87) (61). Severely affected stroke patients measured via the NIHSS had higher TNF-alpha levels compared to less severely affected patients (NIHSS>30; 225.32 pg/mL, SD: 163.6 vs. NIHSS ≤30 52.744 pg/mL, SD: 26.86, p = 0.0001) (64). A multivariate analysis showed that gender, age, vascular risk factors and the occurrence of inflammatory complications had no influence on TNF-α levels (59).
3.2 Biomarkers of endothelial integrity
3.2.1 VCAM-1
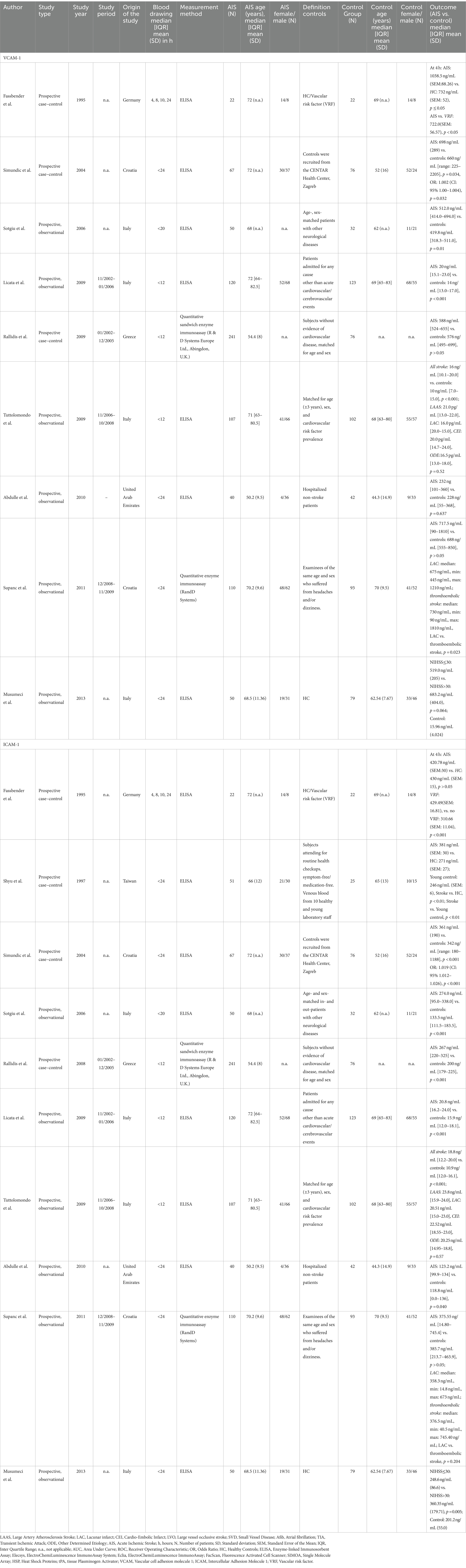
Nine studies measured VCAM-1 comprised of 807 AIS patients and 645 controls (60–62, 64–69). Most studies were carried out in Italy (n = 4, 44%), followed by Croatia (n = 2), Germany (n = 1), Greece (n = 1), and United Arab Emirates (n = 1). Year of publication ranged between 1995 and 2013 and ELISA was the measurement of choice. Blood collection time was reported with a range of 4 h to <24 h after stroke onset (Table 3). Five of the studies showed a fair quality while 4 revealed a good quality. Poor quality was not observed (Supplementary Table S2).
3.2.1.1 AIS vs. healthy controls/matched control patients
Compared to AIS patients, lower VCAM-1 levels were found in 66.67% of control patients (60–62, 65, 66). Contrary results were found in three studies (67–69). The highest measured value (462 ng/mL) was reported in an 86-year-old woman with a severe course of AIS that ended in death (66). Another patient with a high value (1,820 ng/mL) was measured in a 70-year-old man with no history of smoking, myocardial infarction, or hypertension but pre-existing hypercholesterolemia and diabetes mellitus. Surprisingly, his brain CT revealed multiple chronic lacunar ischemic lesions. However, VCAM-1 levels were not associated with age, diabetes mellitus, hypercholesterolemia, hypertension, or degree of arteriosclerosis (66).
3.2.1.2 Etiology-, outcome-, severity related VCAM-1 levels in AIS patients
No differences in median VCAM-1 levels were reported for patients who experienced LAAS, CEI, ODE, or LAC (21 ng/mL, IQR: 13–22; 20 ng/mL, IQR: 14.7–24; 16.5 ng/mL, IQR: 13–18; 16 ng/mL, IQR: 20–15, respectively, Kruskal-Wallis test p = 0.52) (62). However, a difference was observed when comparing controls to thromboembolic strokes while no difference was observed for lacunar stroke patients (69). No differences were found in VCAM-1 levels in AIS patients by NIHSS severity (mild, moderate, severe stroke) or outcome measured by the Barthel-Index (69). Nevertheless, higher levels of VCAM-1 were suggested among those with an NIHSS ≥30 compared to those with an NIHSS<30 (683.2 ng/mL, SD: 404 vs. 519 ng/mL, SD: 205, p = 0.064) (64). Furthermore, both groups had higher VCAM-1 levels compared to healthy controls (15.96 ng/mL, SD: 4.02) (64). VCAM-1 has been positively correlated with the NIHSS score (r = 0.73, p < 0.01) and inversely correlated with the Glasgow Coma Scale (GCS) (r = −0.64, p < 0.01) (60).
3.2.2 ICAM-1
ICAM-1 was measured in 10 studies with a total of 858 AIS patients and 670 controls. There were three studies with healthy controls, four studies with non-stroke patient controls, and three studies with matched controls (60–62, 64–70). Most studies were carried out in Italy (n = 4), followed by Croatia (n = 2), Germany (n = 1), United Arab Emirates (n = 1), Greece (n = 1), and Taiwan (n = 1). Publication years ranged between 1997 and 2013 and the ICAM-1 measurement of choice was ELISA. The blood draw was performed between 4 and 24 h after stroke onset (Table 3). Six of the included studies revealed fair quality and four studies had good quality per the NOS (Supplementary Table S2).
3.2.2.1 AIS vs. healthy controls/matched control patients
Higher ICAM-1 concentrations among patients with AIS compared to the controls were reported in 70% of studies (60–62, 66–68, 70). No difference was found in 20%, while one study did not perform a statistical comparison (64, 65, 69).
3.2.2.2 Etiology-, outcome-, severity related ICAM-1 levels in AIS patients
Four studies did not find differences in ICAM-1 levels by AIS etiology or severity measured using the NIHSS. Nevertheless, one study showed higher ICAM-1 levels in AIS patients who suffered more severe strokes measured by NIHSS (60–62, 64, 69).
ICAM-1 concentrations and involvement of different stroke territories have also been shown to not differ from the control group and no correlations between the extent of brain injury and ICAM-1 levels were observed (61, 70). One study reported that a 10 ng/mL higher ICAM-1 level led to a 9% higher risk of death (60, 64, 67). ICAM-1 levels have been reported to be independent of age, sex, diabetes mellitus, and the degree of atherosclerosis (66). However, one study observed higher ICAM-1 levels in patients with arteriosclerosis (65). In contrast, one study found higher ICAM-1 levels in patients with abnormal carotid ultrasound findings, although the differences were not significant (p > 0.05) (70).
3.3 Biomarkers of cell migration
3.3.1 E-Selectin
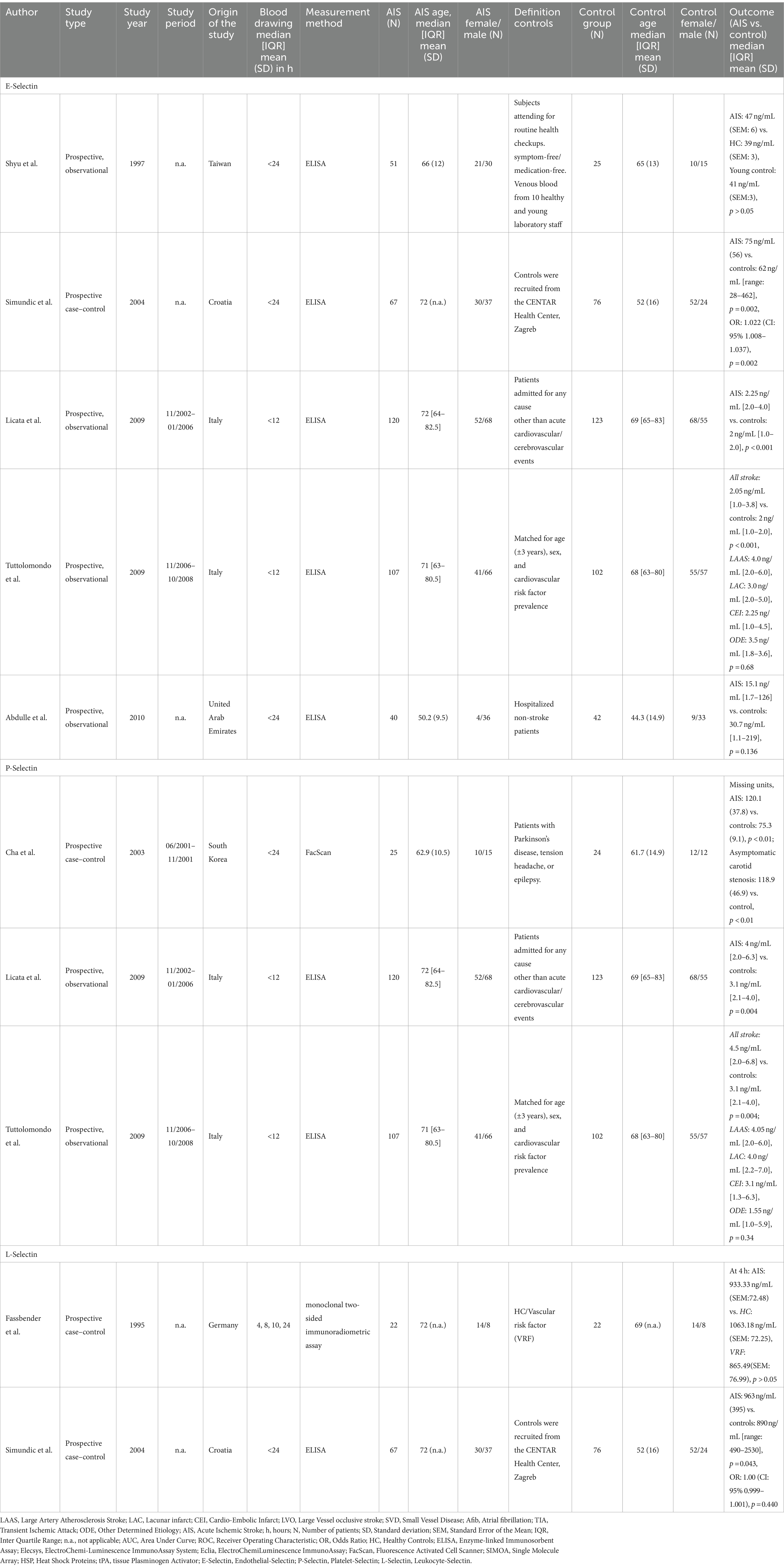
E-Selectin was measured among five studies comprised of 385 stroke patients and 368 controls (61, 62, 66, 68, 70). Eighty percent of studies included patients with conditions other than a neurologic disease, and one study included matched controls (on age, sex, and prevalence of cardiovascular risk factors). Studies were carried out in Italy (n = 2), United Arab Emirates (n = 1), Croatia (n = 1), and Taiwan (n = 1). All studies used ELISA for measuring E-Selectin level and dates of publications were between 1997 and 2010. The time of blood sampling after stroke onset was reported as from within <12 h to within <24 h (Table 4). Two studies showed good quality while three studies showed fair quality assessed by the NOS (Supplementary Table S3).
Higher P-Selectin values compared to controls were observed among 60% of studies, while 40% showed no difference (61, 62, 66). No differences between reported median E-Selectin levels were found for patients suffering LAAS, LAC, CEI, ODE (4.0 ng/mL, IQR: 2.0–6.0; 3.0 ng/mL, IQR: 2.0–5.0; CEI: 2.25 ng/mL, IQR: 1.0–4.5; ODE: 3.5 ng/mL, IQR: 1.8–3.6, respectively, Kruskal-Wallis test p = 0.68) (62) (Table 4).
3.3.2 P-Selectin
Three studies measured P-Selectin and included 252 AIS patients and 249 controls who were admitted for reasons other than cerebrovascular events, i.e., Parkinson’s disease, tension headache, and epilepsy, age, sex, and presence of cardiovascular risk factors matched patients (61, 62, 71). Studies were published between 2003 and 2009 and mean blood draw time ranged between from within <12 h to within <24 h after symptom onset. Measurement of P-Selectin was accomplished using ELISA in two studies carried out in Italy, whereas a South Korean study used Fluorescence-activated cell sorting (FacScan) (Table 4). Two of the studies were of good quality and one study revealed fair quality (Supplementary Table S3).
All studies reported higher P-Selectin values in stroke patients compared to controls (61, 62, 71). However, no differences between median P-Selectin levels were found for different stroke etiologies (LAAS: 4.05 ng/mL, IQR: 2.0–6.0; LAC: 4.0 ng/mL, IQR: 2.2–7.0; CEI: 3.1 ng/mL, IQR: 1.3–6.3; ODE: 1.55 ng/mL, IQR: 1.0–5.9, Kruskal-Wallis p = 0.34) (61, 62). P-Selectin was also reported as elevated in AIS patients, as well as in asymptomatic atherosclerotic stenosis patients compared to healthy controls (p < 0.01) (71). P-Selectin levels did not differ between AIS patients and asymptomatic carotid stenosis patients in the same study (71).
3.3.3 L-Selectin
Through our literature research, two articles were found on L-Selectin (65, 66). (Table 3) In total, 89 AIS patients and 98 healthy control patients were recruited from the CENTAR, Health Center Zagreb, Croatia (Table 4). Both studies showed fair quality according to the NOS (Supplementary Table S3).
One study measured blood L-Selectin levels in patients experiencing stroke and HC using ELISA samples drawn within 24 h of AIS symptom onset. Higher concentrations of L-selectin in AIS patients compared to the controls were observed (963 ng/mL, SD: 395 vs. 890 ng/mL, range: 490–2530, p = 0.043) (66). However, the other study reported contrary results using a monoclonal two-sided immunoradiometric assay. No differences in L-Selectin levels between AIS patients and healthy controls at 4 h after symptom onset were observed (933.33 ng/mL, standard error of the mean (SEM): 72.48 vs. 1063.18 ng/mL, SEM: 72.25, p > 0.05). Furthermore, slight changes in L-Selectin levels were observed 8 and 10 h after the onset of symptoms while no differences between various time points and HC were observed (65). One study showed that L-Selectin levels decreased with a higher number of risk factors (smoking, hypertension, diabetes mellitus, or hypercholesterolemia) one risk factor: 777.35 ng/mL (SEM:114.03) vs. four risk factors: 692.75 ng/mL (SEM: 112.60) (65).
3.4 Glial and neuronal markers
3.4.1 NSE
Ten studies evaluating NSE comprising 931 AIS patients and 527 control subjects (HC/matched controls: n = 329, stroke mimics: n = 198) were included in this review. The controls encompassed five studies with HC, three studies with control patients with conditions other than neurological diseases, and two studies with stroke mimics as the controls (53, 55, 72–79) (Table 4). Most of the studies were carried out in Germany (n = 2) and South Korea (n = 2), followed by Turkey (n = 1), USA (n = 1), Ireland (n = 1), Netherlands (n = 1), Spain (n = 1), and China (n = 1). Publication years ranged between 1997 and 2018. Reported blood drawing time ranged from 4 h to <24 h. In 60% of studies, NSE was measured using ELISA. Other techniques applied were radioimmunoassay as well as antibody-based arrays and monoclonal two-sided immunoradiometric assay (Table 5). Study quality, evaluated with the NOS was found to be of good quality in 20%, and fair quality in 80% of studies (Supplementary Table S4).
3.4.1.1 AIS vs. healthy controls/patients with other than neurological diseases
Higher NSE concentrations in AIS patients compared to controls were reported in 57% of the studies (74, 76, 77, 79). In two studies, no significant differences were found between AIS patients and controls (72, 75). One study described that NSE levels in AIS patients were elevated at 8, 10, and 24 h after symptom onset (p ≤ 0.05), but not 4 h after symptom onset (73).
3.4.1.2 AIS vs. controls and stroke mimics
Two studies revealed no differences in NSE levels between AIS patients and controls (53, 55). One study showed higher NSE levels in AIS patients compared to stroke mimics (32.671 mg/L, SD: 30.42 vs. 17.417 mg/L, SD: 7.08, p = 0.005) (78). An ROC analysis revealed an area under curve (AUC) of 0.67 for NSE (bootstrap 95% CI: 0.55–0.80). The best cut-off value for NSE was found to be 18 μ/L, with a sensitivity of 61%, specificity of 53%, negative predictive value (NPV) of 52%, and positive predictive value (PPV) of 62% (78).
3.4.1.3 Etiology-, outcome-, severity related NSE levels in AIS patients
In AIS patients with cortical and non-cortical infarcts, contrasting results in NSE values were found by infarct location (73). One study reported a difference in NSE levels, whereas another study showed no difference (73, 77). However, patients with total anterior cerebral infarction (TACI) had higher serum NSE levels on admission compared to patients with partial anterior cerebral infarction (PACI) but not LAC (10.3 ng/mL, SEM: 1.0; 7.6 ng/mL, SEM: 0.5; 7.8 ng/mL, SEM: 0.6, respectively) (75). Another study reported higher NSE levels in 71% of patients with LAC as well as 53% of TACI patients compared to controls (76). Additionally, patients with cortical infarctions presented with higher NSE levels when hyperglycemia was persistent (11.2 ng/mL, SEM: 0.8 vs. 7.2 ng/mL, SEM: 0.5, p = 0.0008). This was not seen in patients experiencing lacunar stroke (75). Three studies showed a positive correlation between NSE levels and lesion volume on brain imaging (p ≤ 0.05) (72, 74, 76). When comparing infarct volume of less than 5 cm (3) with >5 cm (3), no difference in NSE concentrations was observed (73). Patients with chronic atrial fibrillation showed higher day one NSE concentrations than patients without (37.39 ng/mL, SD: 9.37 vs. 24.49 ng/mL, SD: 23.16, p = 0.0018) (79).
3.4.2 GFAP
For the analysis of GFAP, 11 studies were eligible for inclusion comprising 1,152 AIS patients and 617 control patients (HC: n = 342, stroke mimics: n = 275) (15, 16, 57, 80–85) (Table 4). Most of the studies were carried out in Germany (n = 3) followed by China (n = 1), Greece (n = 1), Norway (n = 1), India (n = 1), USA (n = 1), Italy (n = 1), South Korea (n = 1), and Turkey (n = 1) and publication years ranged between the 2013 and 2023. Almost half of the studies (45.5%) of studies used ELISA, 36.4% used SIMOA, and two used electro-chemiluminometric immune assay. Blood drawing time ranged between 1.9 h and < 24 h after symptom onset (Table 5). Risk of bias assessment revealed four studies with good quality while seven studies were found to be of fair quality. Poor quality was not observed (Supplementary Table S4).
3.4.2.1 AIS vs. healthy controls
Six studies compared AIS patients with patients who experienced stroke albeit two studies investigated both healthy controls and stroke mimics. Half of the studies showed higher GFAP levels in AIS patients compared to controls (p ≤ 0.05) (81, 85, 86). One study showed higher levels of GFAP in AIS patients compared to HC but statistical testing for differences was not performed (195.22 pg./mL, range: 52.77–1526.74 vs. 80.37 pg/mL, range: 56.43–132.86) (57). Two studies showed comparable GFAP concentrations between both groups (82, 83). GFAP levels were higher in brain CT image-confirmed AIS patients as well as patients with clinically suspected stroke and normal CT, than in HC. In addition, it was reported that no difference was found in patients with suspected stroke without brain CT imaging signs of stroke compared to patients with positive CT findings (86). When applying a cut-off value of 33.24 ng/mL for GFAP, ROC analysis showed a sensitivity of 70.59% and a specificity of 70% (AUC = 0.684, 95% CI: 0.558–0.792) for the differentiation between AIS and HC. Furthermore, no association between stroke severity and GFAP levels was reported (r = 0.164, p = 0.311) (86).
3.4.2.2 AIS vs. stroke mimics
Seven studies compared AIS patients with stroke mimics (15, 16, 53, 80, 82–84). Interestingly, lower GFAP concentrations in AIS versus stroke mimic patients on admission were observed in one study (0.08 μg/L, IQR: 0.02–0.14 vs. 0.19 μg/L, IQR: 0.16–0.21) (80). Contrary results were obtained in another study (p = 0.029) (53). Higher GFAP concentrations were observed in two studies, however, no statistical comparisons of the two groups were reported (16, 84). Three studies reported comparable GFAP levels in AIS patients and stroke mimic patients (15, 82, 83). GFAP was not found to differentiate AIS from stroke mimics per a reported AUC of 0.47 (95% CI: 0.40–0.55) (16).
3.4.3 S100
Five studies measuring S100 were eligible for this review (73, 74, 87–89). In total, 302 AIS patients and 184 HC/matched controls were included and reported blood drawing time between 4 h and < 24 h after symptom onset. Sixty percent of the studies were conducted in Germany (n = 3), followed by China (n = 1) and Turkey (n = 1). S100 was measured using ELISA (n = 3) or a two-site radioimmunoassay (n = 2). The studies were published between 1997 and 2011 (Table 5). Two studies revealed good quality and three studies fair quality when evaluated with NOS (Supplementary Table S4).
In 60% of the studies, higher serum S100 protein levels were observed in AIS patients compared to HC and/or an age- and sex-matched control group (73, 74, 87). Patients with CEI and atherothrombotic stroke had higher S100 values than age and sex-matched controls (0.47 ng/mL, SD: 0.31; 0.47 ng/mL, SD: 0.45 vs. 0.05 μg/L, SD: 0.02, respectively, p < 0.001) (89). A positive correlation between S100 concentrations and infarct size was detected (r = 0.75, p < 0.001) (74). A higher S100 concentration in MCA infarction patients compared to HC was found in another study (87). Initially higher concentrations of S100 were more associated with a worse functional outcome albeit a significant correlation was not reported (87).
S100 protein emerged as a predictor of post-stroke mortality as an increase of 0.735 μg/L led to an increased post-stroke mortality of 110% (88). However, one study showed increasing S100 in stroke patients depending on the time of blood collection (12.5% at hour 4; 20.8% at hour 8, 37.5% at hour 10, 60.9% at hour 24, and 57.1% at hour 72) (73).
3.4.4 S100B
Our literature search revealed 10 studies on S100B (34, 42, 46, 50, 51, 53, 55, 78, 90, 91). In total, 2,234 patients experiencing stroke and 1,208 controls (HC: n = 271, stroke mimics: n = 937) were included. Control groups included stroke mimics (80% of studies) and two studies enrolled HC. To measure S100B, ELISA was used in six studies, a POCT in three studies, and an antibody array in one study. Blood samples were taken between 3.5 h and < 24 h after the onset of stroke symptoms. Most of the studies were carried out in South Korea (n = 3) followed by Spain (n = 2), USA (n = 3), Italy (n = 1), and Turkey (n = 1) (Table 5). In the risk of bias assessment, three of the included studies showed good quality and seven showed fair quality (Supplementary Table S4).
3.4.4.1 AIS vs. healthy controls
The literature search revealed two studies with contrary results as one study reported lower S100B levels in AIS patients compared to controls (103.1 pg/mL, SD: 13.6 vs. 188.6 pg/mL, SD: 14.1, p < 0.001) while another study found higher mean S100B levels in AIS patients compared to controls (44.6 ng/L, IQR: 5.46–96.56 vs. 19.98 ng/L, IQR: 0.0–67.92) (34, 46).
3.4.4.2 AIS vs. stroke mimics
Five studies showed higher S100B levels in AIS patients compared to stroke mimics (51, 53, 78, 90, 91). However, two studies did not reveal any differences in S100B levels between the two groups while one study reported similar GFAP levels between the groups (42, 50, 55). One study showed a weak to moderate predictive value of S100B for the discrimination of AIS (AUC: 0.70, cut-off value: 23.5 pg./mL, sensitivity: 54.0%, specificity: 83.5%, p < 0.001) (90). However, excellent discrimination was found by another study using a cut-off value of 65 μg/L resulting in an AUC of 0.89 (bootstrap 95% CI: 0.81–0.96), a sensitivity of 87%, a specificity of 72%, and an NPV as well as PPV of 81% (78). Stroke severity measured with the NIHSS was positively correlated with S100B levels (r = 0.45, p ≤ 0.05) in the study with moderate predictive value (90).
3.5 Biomarkers of cardiac dysfunction
3.5.1 BNP
BNP was measured in seven studies, two of which were performed in Turkey, and one each in South Korea, Italy, USA, Japan, and Spain (34, 42, 50, 51, 92–94). There were a total of 1,313 AIS patients and 373 control patients (HC: n = 2286, stroke mimics: n = 187). Of those, HC were examined in three studies, three studies included stroke mimics and one study included control patients with hypertension. Blood drawing time ranged between 3.5 and < 24 h after the onset of symptoms. Publication years ranged between 2004 and 2011. The most used method for measuring BNP was a POCT followed by ELISA, microparticle immunoassay, and a highly sensitive two-site immunoradiometric assay (Table 6). The quality of studies was found to be good in one study and six studies showed fair quality according to the NOS (Supplementary Table S5).
3.5.1.1 AIS vs. healthy controls/patients with other than neurological diseases; etiology-, outcome-, severity related NSE levels in AIS patients
Higher BNP levels in AIS patients compared to controls were found in all of the studies (34, 92–94). BNP levels were not correlated with infarct size or stroke severity measured by the NIHSS but positively correlated with mean arterial pressure (MAP) (94). Also, in one study, a positive correlation between MAP and BNP levels but no correlation between stroke severity and BNP levels or infarct size were detected (94). Positive correlations between mean BNP levels and age as well as MAP were also found in one study, whereas no correlation between BNP levels and ventricular ejection fraction was detected (93).
Furthermore, BNP levels in AIS patients with atrial fibrillation (AF) as well as AIS patients without AF were compared to healthy controls. The highest BNP concentrations were found in AIS + AF patients while AIS patients without AF and AF patients without AIS had similar BNP levels (AIS + AF: 186.3 pg/mL, SD: 153.6; AIS without AF: 59.5 pg/mL, SD: 49.3 vs. AF without AIS: 68.1 pg/mL, SD: 53.5). However when comparing these subgroups with HC, BNP levels were lower in HC (92). In addition, BNP levels and MAP on admission were positively correlated in AIS patients without AF (r = 0.34). In contrast, an inverse correlation of BNP and MAP in AIS patients with AF was reported (r = −0.326, p = 0.0147) (92).
3.5.1.2 AIS vs. stroke mimics
Two studies showed higher BNP levels in AIS patients compared to stroke mimics (50, 51). Only one of the studies showed no difference in BNP levels between AIS and stroke mimics (42). BNP was not predictive for AIS (OR: 2.17, IQR: 0.90–5.24, p = 0.084) (51).
3.5.2 NT-proBNP
In contrast, three studies measured NT-proBNP and included 1,028 AIS patients and 280 controls (two studies with age- and sex-matched HC, and one study with stroke mimics) (32, 33, 55). Studies were published between 2005 and 2017 and carried out in Greece, Spain, and Turkey. The time of blood sampling was from within <6 h to within <24 h after symptom onset. NT-proBNP concentrations were measured with electrochemiluminescence systems, enzyme immunoassay, and an antibody-based array (Table 6). The risk of bias assessment revealed good quality in one study and moderate quality in two studies.
All studies reported higher NT-proBNP levels in patients with AIS compared to controls. Additionally, AIS patients with an infarct diameter > 3 cm showed elevated NT-proBNP concentrations when compared to controls (logNT-proBNP: 7.96 ng/mL, SD: 1.66 vs. 6.52 ng/mL, SD: 1.6, p = 0.002) (33). However, AIS patients with electrocardiogram changes had higher NT-proBNP levels compared to those without changes, especially, patients with impaired left ventricular ejection fraction as well as impaired left ventricular end-diastolic diameter (p = 0.019, p = 0.011) (33). Interestingly, NT-proBNP levels did not differ whether strokes occurred in the carotid or vertebrobasilar location (32). No correlation between stroke severity or infarct size was observed (32). However, on the day of admission, NT-proBNP concentration between CEI and atherothrombotic-caused stroke differed (166.3 fmol/mL, SEM: 25.3 vs. 108.4 fmol/mL, SEM: 8.3, p ≤ 0.05) (32). In a study of 941 AIS patients and 193 stroke mimic patients, a ROC analysis at a cut-off value of 4.685 pg./mL showed a sensitivity of 76.9% and a specificity of 43.5% (AUC = 0.742, 95% CI: 0.686–0.797, p < 0.0001) (55).
3.6 Biomarker panels
In addition to the examination of individual biomarkers, eight studies created panels to predict AIS. Those studies were published between 2003 and 2014 (Table 7) (34, 42, 46, 50, 53, 78, 90, 91).
One study investigated 293 AIS patients and 361 stroke mimics, some evaluated with brain CT lesions and some without. The median time from ‘last seen normal’ to blood draw was 9.3 h (IQR: 4.5–18.2) (91). A logistic regression model including the predictors BNP, MMP-9, S100B, and D-dimer provided modest discriminative capacity (c-statistics = 0.67). The highest capacity was achieved at 3 h after symptom onset (c = 0.73), and still considered modest. OR were reported for the 3rd and 4th quartiles of this panel (Q3: 0.48–0.62; Q4: >0.62, p < 0.0001). OR ranged between 3.18 and 5.23 evaluated by probability quartiles. Additionally, the NIHSS correlated positively with logistic regression values (r = 0.431, p < 0.0001). For the detection of stroke within 3 h, logistic regression analyses showed a sensitivity ranging between 27 and 91% (75th and 25th percentile) and a specificity between 45 and 89% (25th and 75th percentile). BNP concentration contributed the most and, S100B, the least. However, the panel showed a positive correlation with ischemic lesions on CT (p ≤ 0.05) (91).
Another study investigated MMP-9, BNP, S100B, and D-dimer. The derived multimarker index level was higher in AIS patients compared to stroke mimics (50). Evaluating single biomarkers, it was shown that D-dimer and BNP differed whereas S100B and MMP-9 showed conflicting results. The best cut-off value for this panel was determined to be an index of 4.5, with a PPV and NPV of 0.76 (95% CI: 0.68–0.83) and 0.61 (95% CI: 0.54–0.67), respectively. A ROC curve was generated which showed an AUC of 0.85 (95% CI: 0.67–0.94) when the blood draw was performed <3 h (p < 0.001) after symptom onset. Later measurements were less robust [AUC: 0.68 (95% CI: 0.52–0.81), p = 0.026]. The combination of the biomarker panel index and stroke severity measured by the Cincinnati Prehospital Stroke Scale enhanced the ROC analysis results [0.86 (95% CI: 0.79–0.91), p < 0.01] (50).
Another biomarker panel included NSE, S100B, and ischemia-modified albumin. Using these combined biomarkers, a sensitivity of 97% was achieved however specificity was found to be 23% with an NNPV of 88% and a PPV of 62% for discrimination of AIS versus stroke mimics. Combining the most promising biomarkers in the panel, i.e., ischemia-modified albumin and S100B gave the best results with a sensitivity of 97%, specificity of 37%, NPV of 66%, and a PPV of 92% (78).
A study of 188 AIS patients and 90 stroke mimics reported on a biomarker panel including IL-6, S100B, and MMP-9 which was incorporated in a clinical data assessment (age, AF, Face-Arm-Speech-Test results). It was shown that ROC analysis did not yield better results if biomarkers were added to the analysis (AUC: 0.865 vs. 0.837 with biomarkers, p = 0.069). Interestingly the clinical assessment showed better results compared to the biomarkers alone (AUC: 0.837 vs. 0.749, p = 0.017) (53).
Another study investigated 915 AIS patients and 90 stroke mimics and reported that the best sensitivity and specificity calculated by ROC analysis were found for caspase-3, D-dimer, soluble Receptor for Advanced Glycation End Products (sRAGE), Chimerin-II, secretagogin, and MMP-9, which formed the basis of the biomarker panel. Calculated ROC including all six biomarkers revealed that if caspase-3, d-dimer, and sRAGE were high and the other biomarkers were low, a stroke probability of 100% was predicted. However, discrimination of ischemic strokes from stroke mimics with blood sampling <24 h from symptom onset, illustrated that the AUC was moderate [0.759 (95% CI: 0.705–0.813)] (42).
A biomarker panel examined among 89 patients with stroke and 38 HC consisting of BNP, D-dimer, MMP-9, and S100B, and a derived multimarker index, showed higher values in stroke patients compared to HC. An AUC of 0.714 was calculated for the diagnosis of AIS. When applying a cut-off value of 1.3, sensitivity was 91%, and specificity 21.5%. However, when applying the upper quartile cut-off value of 5.9, sensitivity slightly increased whereas specificity sharply decreased (sensitivity: 93.5%, specificity: 5.9%) (34).
A biomarker panel comprised of S100B, and high fatty acid binding protein (H-FABP) was conducted among 111 AIS patients, and 127 stroke mimics. However, individually, each of them showed moderate diagnostic value referring to the ROC analysis (H-FABP: AUC: 0.71, cut-off value: 9.7 ng/mL, sensitivity: 59.5%, specificity: 79.5%; S100B: AUC: 0.70, cut off value 23.5 pg/mL, sensitivity: 54.0%, specificity: 83.5%). The combination of the two biomarkers did not increase the diagnostic value (AUC: 0.75, sensitivity: 66.8%, specificity: 73.2%) (90).
A biomarker panel conducted among 54 AIS patients and 214 stroke mimics that included a combination of von Willebrand factor, MMP-9, S100B, Monocyte chemoattractant protein-1, B-type neurotrophic growth factor yielded a sensitivity of 90.7%, and a specificity of 96.7% for detection of ischemic stroke within 12 h (46).
4 Discussion
Stroke remains the second most common cause of death worldwide, with an incidence of approximately 15 million patients experiencing AIS every year. A rising trend from 1990 to 2019 was observed, which may be linked to increasing prosperity, rising life expectancy, improved medical care, and new therapies for once-incurable communicable and non-communicable diseases (95, 96). For example, people with HIV, have a life expectancy similar to those without HIV when receiving the best medical care (97, 98). To adequately meet the challenge of the increasing number of strokes globally and global variation in stroke subtypes, we need tools that enable reliable indication of a stroke.
We conducted a systematic review of 13 blood-based protein biomarkers for the diagnosis of AIS, from 61 studies that collected biomarker data within 24 h of symptom onset. The major strength of this review is that we focused on the predictive value of blood-based protein biomarkers for the diagnosis of AIS across a variety of pathologic mechanisms including neurovascular inflammation (MMP-9, TNF-alpha), endothelial integrity (VCAM-1, ICAM-1), cell migration (E-Selectin, P-Selectin, L-Selectin), and glial and astrocytic neuronal biomarker (NSE, GFAP, S100, S100B), and cardiac dysfunction (BNP, NT-proBNP). S100, P-Selectin, and BNP showed overall positive results in differentiating AIS from matched controls and HC (34, 50, 51, 61, 71, 73, 74, 87, 89, 92, 93).
Interestingly, BNP was the only blood-based protein biomarker that showed good differentiability when comparing AIS to stroke mimics which might be partly explained by a high cardiac burden in AIS (50, 51). However, it should be considered that AIS patients may already have cardiovascular risk factors or have even suffered a cardiovascular event, which may have led to pre-existing higher BNP values. Preexisting AF or hypertension is accompanied by higher BNP levels which is reflected by the results of some of the included studies herein (99–102). This also suggests that stroke mimics better reflect reality, as they show stroke-like symptoms, especially in the acute phase, making it difficult to distinguish them from AIS patients. However, only 26.4% of studies examined stroke mimics as a control group. This represents a major limitation related to generalizability. It is not the healthy patient who is admitted to the hospital, but symptomatic patients in whom a stroke is suspected. It is of utmost importance to include a cohort of stroke mimics, as it is often challenging to differentiate them using acute diagnostics. For example, some studies investigating VCAM-1, ICAM-1, and P-Selectin, only examined healthy or matched controls, and most studies were positively predictive for AIS (60–62, 64–68, 70). All studies on P-Selectin showed significant differences in protein levels between AIS patients and controls (61, 62, 71). However, no stroke mimics were investigated. Unfortunately, this approach has not been pursued for VCAM-1, ICAM-1, and P-Selectin since 2013.
Nevertheless, comparisons of AIS patients with stroke mimics should be treated with caution for several reasons. Firstly, in all studies that had stroke mimics as a control group, blood samples were only taken at one point in time and therefore possible short-term changes in protein levels were not represented. Secondly, unmapped kinetics of blood protein levels could potentially provide additional information and allow differentiation between AIS and stroke mimics. This is of great importance, as patients with AIS can, according to current guidelines, only benefit from systemic lysis therapy within 4.5 h or, under certain circumstances, from endovascular therapy within 24 h (7, 103–105). Thirdly, there are only four published studies that have investigated AIS and stroke mimics in the last 5 years (15, 16, 57, 84).
Further limitations for the generalizability of published studies lie in the high heterogeneity of these studies. First, studies included in this review show a high geographical variability. Of 61 studies, 20 countries were represented, however, there is only one Low and Middle Income Country represented for which blood-based biomarkers are important given the scarcity of brain imaging (106, 107). Therefore, regional variations in race and ethnicity, diagnostic criteria and brain imaging modalities, access to healthcare generally, and access to and degree of technology for biomarker measurements, in addition to other differences are important for the interpretation of these data. This must also be considered for future studies, as possible race/ethnic differences may contribute to different protein kinetics in the acute phase, and thus may have affected the results in the studies we included. Second, all the evaluated blood-based protein biomarkers are not exclusively observed in the condition of stroke. For example, elevated S100B levels are also observed in patients with multiple sclerosis, amyotrophic lateral sclerosis, or cancer, as S100B is found in all neuronal tissue thus leading to a low specificity (108–111). This is also the case with GFAP and NSE and although BNP is a cardiac peptide, it can also be elevated in diseases that cause secondary cardiac stress (e.g., pulmonary hypertension, stroke, renal insufficiency) (112–118).
GFAP is one of the most studied biomarkers in stroke but is also broadly investigated in differentiating AIS from intracerebral bleeding (15, 16, 119). However, few studies dealt with the diagnostic value of GFAP in AIS patients within the first 24 h (15, 120). The majority of studies included in this analysis showed higher GFAP levels in AIS patients compared to HC/matched controls but not to stroke mimics, possibly due to its low specificity. Elevated GFAP concentrations can also be found in multiple sclerosis, brain tumors, encephalitis, amyotrophic lateral sclerosis, and Alzheimer’s Disease which was reflected by an AUC ranging between 0.67 and 0.71 and a maximum specificity of 70% (116–118, 121).
Additionally, we searched included studies on whether developed biomarker panels were eligible for AIS discrimination (Table 7). Investigated panels consisted of different combinations of MMP-9, BNP, D-dimer, NSE, ischemia-modified albumin, S100B, and HFABP. A promising panel included S100B, MMP-9, BNP, and D-dimer, which, in combination with the clinical characteristics of the patients, achieved a high AUC (50). However, the individual biomarkers did not perform well on their own and their specificity was rather low. Nevertheless, this approach may be the most promising compared to other panels studied, which were rather unspecific (42, 90, 91). Other panels, which also examined stroke mimic patients without taking clinical characteristics into account, were found to have low specificity (34, 42, 53, 78, 90, 91).
Overall, blood-based protein biomarkers may be value-added for acute diagnostics in patients with AIS in the future. However, there remains a lack of systematic, large-scale studies investigating the prognostic value of blood-based protein biomarkers. Many issues remain. First, there has been a lack of inclusion of stroke mimics, and blood-based protein biomarker kinetics in 21st century published studies. Second, comprehensive standardized global multicenter studies are needed. Third, a further limitation of the included studies is the lack of ethnic representation. Recent studies have demonstrated an additional benefit of incorporating the variable of race. For instance, the implementation of race-adjusted scales was demonstrated to markedly enhance the predictability of disease classification, occupational eligibility, and disability compensation in patients undergoing spirometric testing (122). A comparable impact may also be observed with regard to the predictive accuracy of individual biomarkers, which should be subjected to further investigation in the future. Only two of the included studies presented data on different entheses, therefore no conclusions could be drawn in this review. Fourth, blood-based biomarkers for central nervous system disorders may be elevated in peripheral nervous system disorders, e.g., peripheral neuropathies, trauma, and infectious diseases such as HIV. Fifth, another limitation of this review is the paucity of data regarding the specificity and sensitivity of the biomarkers included in this study. Consequently, a sufficient comparative presentation was not possible. Sixth, ultrasensitive assays, such as SIMOA are expensive and not universally available, nor are all protein assays available in individual clinic settings. Despite this, technical developments will bring this into the clinic at affordable prices. Finally, the measurement of blood-based protein biomarkers is less expensive than brain imaging techniques such as brain CT or brain MRI.
The present review has limitations. It is possible that studies were overlooked in the literature search. To minimize this, we examined reference lists of the included studies as well as other systematic reviews and meta-analyses. Also, we extracted values for blood-based protein biomarkers that were not shown in the text or diagrams using the WebPlotDigitizer, which may have led to bias (38). Nonetheless, to the authors’ knowledge, this represents the most comprehensive systematic review of blood-based protein biomarkers for stroke: MMP-9, TNF-alpha, VCAM-1, ICAM-1, E-Selectin, P-Selectin, L-Selectin, NSE, GFAP, S100, S100B, BNP, and NT-proBNP.
5 Conclusion
In summary, the blood-based protein biomarker studies examined in this review do not provide sufficient evidence to assist in the diagnosis of AIS. This is due to the limitations of the studies themselves, as well as inadequate comparability between studies, the lack of studies with stroke mimics as controls, and available protein kinetic changes in the acute AIS phase. Therefore, not only biomarkers such as BNP, S100B, and P-Selectin (i.e., which have shown promise in this review), but also biomarkers such as MMP-9, TNF-alpha, VCAM-1, ICAM-1, E-Selectin, L-Selectin, NSE, GFAP, S100, and NT-proBNP should be further investigated. Considering repeated measurements to map the protein kinetic changes over time in the acute setting (<24 h), and inclusion of stroke mimics to reflect the real-world scenario would enhance clinical diagnostics.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AC: Data curation, Investigation, Validation, Writing – review & editing. AS: Validation, Visualization, Writing – review & editing. FB: Conceptualization, Funding acquisition, Methodology, Supervision, Validation, Writing – review & editing. DG: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing. AB: Conceptualization, Funding acquisition, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Institutes of Health, R01-EB031579 (AB), MACS/WIHS Combined Cohort Study, Brooklyn Clinical Research Site, National Institutes of Health U01-HL146202 (DG), and DHSU Senior Vice President for Research Office Seed Grant Funding 2023–2024 (FB, AB).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1411307/full#supplementary-material
References
1. The World Stroke Organization. World stroke organization. (2023). Available at: https://www.world-stroke.org/what-we-do#:~:text=Stroke%20is%20the%20second%20leading,factors%20and%20increased%20public%20awareness (Accessed August 15, 2023).
2. Johns Hopkins Medicine. (2022). Available at: https://www.hopkinsmedicine.org/health/conditions-and-diseases/stroke/types-of-stroke (Accessed August 15, 2023).
3. Perry, JJ, Stiell, IG, Sivilotti, MLA, Bullard, MJ, Emond, M, Symington, C, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. (2011) 343:d4277. doi: 10.1136/bmj.d4277
4. Lev, MH, Farkas, J, Gemmete, JJ, Hossain, ST, Hunter, GJ, Koroshetz, WJ, et al. Acute stroke: improved nonenhanced CT detection—benefits of soft-copy interpretation by using variable window width and center level settings. Radiology. (1999) 213:150–5. doi: 10.1148/radiology.213.1.r99oc10150
5. Kalafut, MA, Schriger, DL, Saver, JL, and Starkman, S. Detection of early CT signs of >1/3 middle cerebral artery infarctions: interrater reliability and sensitivity of CT interpretation by physicians involved in acute stroke care. Stroke. (2000) 31:1667–71. doi: 10.1161/01.STR.31.7.1667
6. Shah, VP, Silva, LOJE, Farah, W, Seisa, M, Kara Balla, A, Christensen, A, et al. Diagnostic accuracy of neuroimaging in emergency department patients with acute vertigo or dizziness: a systematic review and meta-analysis for the guidelines for reasonable and appropriate care in the emergency department. Acad Emerg Med. (2023) 30:517–30. doi: 10.1111/acem.14561
7. Nogueira, RG, Jadhav, AP, Haussen, DC, Bonafe, A, Budzik, RF, Bhuva, P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
8. Plebani, M, Zaninotto, M, and Mion, MM. Requirements of a good biomarker: translation into the clinical laboratory. In: Van Eyk, JE, and Dunn, MJ. editors. Clinical proteomics.
9. Maestrini, I, Ducroquet, A, Moulin, S, Leys, D, Cordonnier, C, and Bordet, R. Blood biomarkers in the early stage of cerebral ischemia. Rev Neurol. (2016) 172:198–219. doi: 10.1016/j.neurol.2016.02.003
10. Bsat, S, Halaoui, A, Kobeissy, F, Moussalem, C, el Houshiemy, MN, Kawtharani, S, et al. Acute ischemic stroke biomarkers: a new era with diagnostic promise? Acute Med Surg. (2021) 8:e696. doi: 10.1002/ams2.696
11. Dagonnier, M, Donnan, GA, Davis, SM, Dewey, HM, and Howells, DW. Acute stroke biomarkers: are we there yet? Front Neurol. (2021) 12:619721. doi: 10.3389/fneur.2021.619721
12. Kuhle, J, Barro, C, Andreasson, U, Derfuss, T, Lindberg, R, Sandelius, Å, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med. (2016) 54:1655–61. doi: 10.1515/cclm-2015-1195
13. Kinnamon, DS, Heggestad, JT, Liu, J, and Chilkoti, A. Technologies for Frugal and Sensitive Point-of-Care Immunoassays. Annu Rev Anal Chem (Palo Alto, Calif). (2022) 15:123–49. doi: 10.1146/annurev-anchem-061020-123817
14. Wilson, CS, Vashi, B, Genzor, P, Gregory, MK, Yau, J, Wolfe, L, et al. Point-of-care biomarker assay for rapid multiplexed detection of CRP and IP-10. SLAS Technol. (2023) 28:442–8. doi: 10.1016/j.slast.2023.10.002
15. Kalra, L-P, Khatter, H, Ramanathan, S, Sapehia, S, Devi, K, Kaliyaperumal, A, et al. Serum GFAP for stroke diagnosis in regions with limited access to brain imaging (BE FAST India). Eur Stroke J. (2021) 6:176–84. doi: 10.1177/23969873211010069
16. Jæger, HS, Tranberg, D, Larsen, K, Valentin, JB, Blauenfeldt, RA, Luger, S, et al. Diagnostic performance of glial fibrillary acidic protein and prehospital stroke scale for identification of stroke and stroke subtypes in an unselected patient cohort with symptom onset < 4.5 h. Scand J Trauma Resusc Emerg Med. (2023) 31:1. doi: 10.1186/s13049-022-01065-7
17. Siesjö, BK. Mechanisms of ischemic brain damage. Crit Care Med. (1988) 16:954–63. doi: 10.1097/00003246-198810000-00006
18. Scheinberg, P. The biologic basis for the treatment of acute stroke. Neurology. (1991) 41:1867–73. doi: 10.1212/WNL.41.12.1867
19. Gusev, E, and Skvortsova, VI. Metabolic acidosis and ischemic damage In: Brain Ischemia. Boston, MA: Springer US. 95–9.
20. Jayaraj, RL, Azimullah, S, Beiram, R, Jalal, FY, and Rosenberg, GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. (2019) 16:142. doi: 10.1186/s12974-019-1516-2
21. Clausen, BH, Degn, M, Martin, NA, Couch, Y, Karimi, L, Ormhøj, M, et al. Systemically administered anti-TNF therapy ameliorates functional outcomes after focal cerebral ischemia. J Neuroinflammation. (2014) 11:203. doi: 10.1186/s12974-014-0203-6
22. Abdelnaseer, MM, Elfauomy, NM, Esmail, EH, Kamal, MM, and Elsawy, EH. Matrix Metalloproteinase-9 and recovery of acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:733–40. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.043
23. Zhou, X, Yu, F, Feng, X, Wang, J, Li, Z, Zhan, Q, et al. Immunity and inflammation predictors for short-term outcome of stroke in young adults. Int J Neurosci. (2018) 128:634–9. doi: 10.1080/00207454.2017.1408614
24. Edwards, DN, and Bix, GJ. The inflammatory response after ischemic stroke: targeting β2 and β1 Integrins. Front Neurosci. (2019) 13:540. doi: 10.3389/fnins.2019.00540
25. Yang, C, Hawkins, KE, Doré, S, and Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Phys Cell Phys. (2019) 316:C135–53. doi: 10.1152/ajpcell.00136.2018
26. Dvorak, F, Haberer, I, Sitzer, M, and Foerch, C. Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral Haemorrhage and Ischaemic stroke. Cerebrovasc Dis. (2008) 27:37–41. doi: 10.1159/000172632
27. Haque, A, Polcyn, R, Matzelle, D, and Banik, NL. New insights into the role of neuron-specific enolase in neuro-inflammation, neurodegeneration, and neuroprotection. Brain Sci. (2018) 8:33. doi: 10.3390/brainsci8020033
28. Marenholz, I, Heizmann, CW, and Fritz, G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature). Biochem Biophys Res Commun. (2004) 322:1111–22. doi: 10.1016/j.bbrc.2004.07.096
29. Heizmann, CW, Fritz, G, and Schäfer, BW. S100 proteins: structure, functions and pathology. Front Biosci. (2002) 7:d1356–68. doi: 10.2741/A846
30. De Raedt, S, De Vos, A, and De Keyser, J. Autonomic dysfunction in acute ischemic stroke: an underexplored therapeutic area? J Neurol Sci. (2015) 348:24–34. doi: 10.1016/j.jns.2014.12.007
31. Scheitz, JF, Sposato, LA, Schulz-Menger, J, Nolte, CH, Backs, J, and Endres, M. Stroke-heart syndrome: recent advances and challenges. J Am Heart Assoc. (2022) 11:e026528. doi: 10.1161/JAHA.122.026528
32. Giannakoulas, G, Hatzitolios, A, Karvounis, H, Koliakos, G, Charitandi, A, Dimitroulas, T, et al. N-terminal pro-brain natriuretic peptide levels are elevated in patients with acute ischemic stroke. Angiology. (2005) 56:723–30. doi: 10.1177/000331970505600610
33. Iltumur, K, Karabulut, A, Apak, I, Aluclu, U, Ariturk, Z, and Toprak, N. Elevated plasma N-terminal pro-brain natriuretic peptide levels in acute ischemic stroke. Am Heart J. (2006) 151:1115–22. doi: 10.1016/j.ahj.2005.05.022
34. Kim, MH, Kang, SY, Kim, MC, and Lee, WI. Plasma biomarkers in the diagnosis of acute ischemic stroke. Ann Clin Lab Sci. (2010) 40:336–41.
35. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
36. Higgins, JP, and Green, S. Cochrane handbook for systematic reviews of interventions. (2011). Available at: https://handbook-5-1.cochrane.org/chapter_6/6_4_7_boolean_operators_and_or_and_not.htm (Accessed August 17, 2023).
37. Ivey, C, and Crum, J. Choosing the right citation management tool: endnote, Mendeley, Refworks, or Zotero. J Med Libr Assoc. (2018) 106:399–403. doi: 10.5195/jmla.2018.468
38. Rohatgi, A. Webplotdigitizer: Version 4.6. Available at: https://automeris.io/WebPlotDigitizer (2022).
39. Wells, G, Shea, B, O’Connell, D, Peterson, JE, Welch, V, Losos, M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. (2000). Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed April 21, 2024).
40. Montaner, J, Alvarez-Sabín, J, Molina, C, Anglés, A, Abilleira, S, Arenillas, J, et al. Matrix metalloproteinase expression after human Cardioembolic stroke. Stroke. (2001) 32:1759–66. doi: 10.1161/01.STR.32.8.1759
41. Montaner, J, Molina, CA, Monasterio, J, Abilleira, S, Arenillas, JF, Ribó, M, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. (2003) 107:598–603. doi: 10.1161/01.CIR.0000046451.38849.90
42. Montaner, J, Mendioroz, M, Ribó, M, Delgado, P, Quintana, M, Penalba, A, et al. A panel of biomarkers including caspase-3 and D-dimer may differentiate acute stroke from stroke-mimicking conditions in the emergency department. J Intern Med. (2010) 270:166–74. doi: 10.1111/j.1365-2796.2010.02329.x
43. Castellanos, M, Leira, R, Serena, J, Pumar, J́M, Lizasoain, I, Castillo, J́, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. (2003) 34:40–6. doi: 10.1161/01.STR.0000046764.57344.31
44. Heo, JH, Kim, SH, Lee, KY, Kim, EH, Chu, CK, and Nam, JM. Increase in plasma matrix metalloproteinase-9 in acute stroke patients with thrombolysis failure. Stroke. (2003) 34:e48–50. doi: 10.1161/01.STR.0000073788.81170.1C
45. Horstmann, S, Kalb, P, Koziol, J, Gardner, H, and Wagner, S. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke. (2003) 34:2165–70. doi: 10.1161/01.STR.0000088062.86084.F2
46. Reynolds, MA, Kirchick, HJ, Dahlen, JR, Anderberg, JM, McPherson, PH, Nakamura, KK, et al. Early biomarkers of stroke. Clin Chem. (2003) 49:1733–9. doi: 10.1373/49.10.1733
47. Ning, M, Furie, KL, Koroshetz, WJ, Lee, H, Barron, M, Lederer, M, et al. Association between tPA therapy and raised early matrix metalloproteinase-9 in acute stroke. Neurology. (2006) 66:1550–5. doi: 10.1212/01.wnl.0000216133.98416.b4
48. Vukasovic, I, Tesija-Kuna, A, Topic, E, Supanc, V, Demarin, V, and Petrovcic, M. Matrix metalloproteinases and their inhibitors in different acute stroke subtypes. Clin Chem Lab Med. (2006) 44:428–34. doi: 10.1515/CCLM.2006.079
49. Lucivero, V, Prontera, M, Mezzapesa, DM, Petruzzellis, M, Sancilio, M, Tinelli, A, et al. Different roles of matrix metalloproteinases-2 and -9 after human ischaemic stroke. Neurol Sci. (2007) 28:165–70. doi: 10.1007/s10072-007-0814-0
50. Vanni, S, Polidori, G, Pepe, G, Chiarlone, M, Albani, A, Pagnanelli, A, et al. Use of biomarkers in triage of patients with suspected stroke. J Emerg Med. (2011) 40:499–505. doi: 10.1016/j.jemermed.2008.09.028
51. Glickman, SW, Phillips, S, Anstrom, KJ, Laskowitz, DT, and Cairns, CB. Discriminative capacity of biomarkers for acute stroke in the emergency department. J Emerg Med. (2011) 41:333–9. doi: 10.1016/j.jemermed.2010.02.025
52. Demir, R, Ulvi, H, Özel, L, Özdemir, G, Güzelcik, M, and Aygül, R. Relationship between plasma metalloproteinase-9 levels and volume and severity of infarct in patients with acute ischemic stroke. Acta Neurol Belg. (2012) 112:351–6. doi: 10.1007/s13760-012-0067-4
53. An, S-A, Kim, J, Kim, O-J, Kim, JK, Kim, NK, Song, J, et al. Limited clinical value of multiple blood markers in the diagnosis of ischemic stroke. Clin Biochem. (2013) 46:710–5. doi: 10.1016/j.clinbiochem.2013.02.005
54. Lehmann, MF, Kallaur, AP, Oliveira, SR, Alfieri, DF, Delongui, F, de Sousa Parreira, J, et al. Inflammatory and metabolic markers and short-time outcome in patients with acute ischemic stroke in relation to TOAST subtypes. Metab Brain Dis. (2015) 30:1417–28. doi: 10.1007/s11011-015-9731-8
55. Bustamante, A, López-Cancio, E, Pich, S, Penalba, A, Giralt, D, García-Berrocoso, T, et al. Blood biomarkers for the early diagnosis of stroke. Stroke. (2017) 48:2419–25. doi: 10.1161/STROKEAHA.117.017076
56. Li, Y, Han, X, Luo, S, Huang, H, Huang, X, Li, M, et al. Predictive value of longitudinal changes of serum matrix metalloproteinase-9 and brain-derived neurotrophic factor in acute ischemic stroke. Front Aging Neurosci. (2022) 14:952038. doi: 10.3389/fnagi.2022.952038
57. Kowalski, RG, Ledreux, A, Violette, JE, Neumann, RT, Ornelas, D, Yu, X, et al. Rapid activation of neuroinflammation in stroke: plasma and extracellular vesicles obtained on a Mobile stroke unit. Stroke. (2023) 54:e52–7. doi: 10.1161/STROKEAHA.122.041422
58. Zaremba, J, Skrobanski, P, and Losy, J. Tumour necrosis factor-alpha is increased in the cerebrospinal fluid and serum of ischaemic stroke patients and correlates with the volume of evolving brain infarct. Biomed Pharmacother. (2001) 55:258–63. doi: 10.1016/S0753-3322(01)00058-0
59. Intiso, D, Zarrelli, MM, Lagioia, G, di Rienzo, F, Checchia de Ambrosio, C, Simone, P, et al. Tumor necrosis factor alpha serumlevels and inflammatory response in acute ischemic strokepatients. Neurol Sci. (2004) 24:390–6. doi: 10.1007/s10072-003-0194-z
60. Sotgiu, S, Zanda, B, Marchetti, B, Fois, ML, Arru, G, Pes, GM, et al. Inflammatory biomarkers in blood of patients with acute brain ischemia. Eur J Neurol. (2006) 13:505–13. doi: 10.1111/j.1468-1331.2006.01280.x
61. Licata, GT, Tuttolomondo, A, Raimondo, DD, Corrao, S, Sciacca, RD, and Pinto, A. Immuno-inflammatory activation in acute cardio-embolic strokes in comparison with other subtypes of ischaemic stroke. Thromb Haemost. (2009) 101:929–37. doi: 10.1160/TH08-06-0375
62. Tuttolomondo, A, di Sciacca, R, di Raimondo, D, Serio, A, D'Aguanno, G, la Placa, S, et al. Plasma levels of inflammatory and thrombotic/fibrinolytic markers in acute ischemic strokes: relationship with TOAST subtype, outcome and infarct site. J Neuroimmunol. (2009) 215:84–9. doi: 10.1016/j.jneuroim.2009.06.019
63. Cevik, O, Adiguzel, Z, Baykal, AT, Somay, G, and Sener, A. The apoptotic actions of platelets in acute ischemic stroke. Mol Biol Rep. (2013) 40:6721–7. doi: 10.1007/s11033-013-2787-9
64. Musumeci, M, Sotgiu, S, Persichilli, S, Arru, G, Angeletti, S, Fois, ML, et al. Role of SH levels and markers of immune response in the stroke. Dis Markers. (2013) 35:246205:141–7. doi: 10.1155/2013/246205
65. Fassbender, K, Mössner, R, Motsch, L, Kischka, U, Grau, A, and Hennerici, M. Circulating selectin- and immunoglobulin-type adhesion molecules in acute ischemic stroke. Stroke. (1995) 26:1361–4. doi: 10.1161/01.STR.26.8.1361
66. Simundic, A-M, Basic, V, Topic, E, Demarin, V, Vrkic, N, Kunovic, B, et al. Soluble adhesion molecules in acute ischemic stroke. Clin Invest Med. (2004) 27:86–92. doi: 10.1007/978-3-642-18480-2_11
67. Rallidis, LS, Zolindaki, MG, Vikelis, M, Kaliva, K, Papadopoulos, C, and Kremastinos, DT. Elevated soluble intercellular adhesion molecule-1 levels are associated with poor short-term prognosis in middle-aged patients with acute ischaemic stroke. Int J Cardiol. (2009) 132:216–20. doi: 10.1016/j.ijcard.2007.11.031
68. Abdulle, AM, Pathan, JY, Moussa, N, and Gariballa, S. Association between homocysteine and endothelial dysfunction markers in stroke disease. Nutr Neurosci. (2010) 13:2–6. doi: 10.1179/147683010X12611460763562
69. Supanc, V, Biloglav, Z, Kes, VB, and Demarin, V. Role of cell adhesion molecules in acute ischemic stroke. Ann Saudi Med. (2011) 31:365–70. doi: 10.4103/0256-4947.83217
70. Shyu, KG, Chang, H, and Lin, CC. Serum levels of intercellular adhesion molecule-1 and E-selectin in patients with acute ischaemic stroke. J Neurol. (1997) 244:90–3. doi: 10.1007/s004150050055
71. Cha, J-K, Jeong, M-H, Jang, J-Y, Bae, HR, Lim, YJ, Kim, JS, et al. Serial measurement of surface expressions of CD63, P-selectin and CD40 ligand on platelets in atherosclerotic ischemic stroke. A possible role of CD40 ligand on platelets in atherosclerotic ischemic stroke. Cerebrovasc Dis. (2003) 16:376–82. doi: 10.1159/000072560
72. Cunningham, RT, Young, IS, Winder, J, O'Kane, MJ, McKinstry, S, Johnston, CF, et al. Serum neurone specific enolase (NSE) levels as an indicator of neuronal damage in patients with cerebral infarction. Eur J Clin Investig. (1991) 21:497–500. doi: 10.1111/j.1365-2362.1991.tb01401.x
73. Fassbender, K, Schmidt, R, Schreiner, A, Fatar, M, Mühlhauser, F, Daffertshofer, M, et al. Leakage of brain-originated proteins in peripheral blood: temporal profile and diagnostic value in early ischemic stroke. J Neurol Sci. (1997) 148:101–5. doi: 10.1016/S0022-510X(96)05351-8
74. Missler, U, Wiesmann, M, Friedrich, C, and Kaps, M. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. (1997) 28:1956–60. doi: 10.1161/01.STR.28.10.1956
75. Sulter, G, Elting, JW, and De Keyser, J. Increased serum neuron specific enolase concentrations in patients with hyperglycemic cortical ischemic stroke. Neurosci Lett. (1998) 253:71–3. doi: 10.1016/S0304-3940(98)00595-3
76. Oh, S-H, Lee, J-G, Na, S-J, Park, JH, Choi, YC, and Kim, WJ. Prediction of early clinical severity and extent of neuronal damage in anterior-circulation infarction using the initial serum neuron-specific enolase level. Arch Neurol. (2003) 60:37–41. doi: 10.1001/archneur.60.1.37
77. Wu, YC, Zhao, YB, Lu, CZ, Qiao, J, and Tan, YJ. Correlation between serum level of neuron-specific enolase and long-term functional outcome after acute cerebral infarction: prospective study. Hong Kong Med J. (2004) 10:251–4.
78. Cakmak, VA, Gunduz, A, Karaca, Y, Alioğlu, Z, Menteşe, A, and Topbaş, M. Diagnostic significance of ischemia-modified albumin, S100b, and neuron-specific enolase in acute ischemic stroke. J Acad Emerg Med. (2014) 13:112+. doi: 10.5152/jaem.2014.37980
79. Gójska-Grymajło, A, Zieliński, M, Wardowska, A, Gąsecki, D, Pikuła, M, and Karaszewski, B. CXCR7+ and CXCR4+ stem cells and neuron specific enolase in acute ischemic stroke patients. Neurochem Int. (2018) 120:134–9. doi: 10.1016/j.neuint.2018.08.009
80. Foerch, C, Niessner, M, Back, T, Bauerle, M, de Marchis, GM, Ferbert, A, et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem. (2012) 58:237–45. doi: 10.1373/clinchem.2011.172676
81. Ren, C, Kobeissy, F, Alawieh, A, Li, N, Li, N, Zibara, K, et al. Assessment of serum UCH-L1 and GFAP in acute stroke patients. Sci Rep. (2016) 6:24588. doi: 10.1038/srep24588
82. Katsanos, AH, Makris, K, Stefani, D, Koniari, K, Gialouri, E, Lelekis, M, et al. Plasma glial fibrillary acidic protein in the differential diagnosis of intracerebral hemorrhage. Stroke. (2017) 48:2586–8. doi: 10.1161/STROKEAHA.117.018409
83. Luger, S, Witsch, J, Dietz, A, Hamann, GF, Minnerup, J, Schneider, H, et al. Glial fibrillary acidic protein serum levels distinguish between intracerebral hemorrhage and cerebral ischemia in the early phase of stroke. Clin Chem. (2017) 63:377–85. doi: 10.1373/clinchem.2016.263335
84. Luger, S, Jæger, HS, Dixon, J, Bohmann, FO, Schaefer, J, Richieri, SP, et al. Diagnostic accuracy of glial fibrillary acidic protein and ubiquitin Carboxy-terminal hydrolase-L1 serum concentrations for differentiating acute intracerebral hemorrhage from ischemic stroke. Neurocrit Care. (2020) 33:39–48. doi: 10.1007/s12028-020-00931-5
85. Ferrari, F, Rossi, D, Ricciardi, A, Morasso, C, Brambilla, L, Albasini, S, et al. Quantification and prospective evaluation of serum NfL and GFAP as blood-derived biomarkers of outcome in acute ischemic stroke patients. J Cereb Blood Flow Metab. (2023) 43:1601–11. doi: 10.1177/0271678X231172520
86. Ekingen, E, Yilmaz, M, Yildiz, M, Atescelik, M, Goktekin, MC, Gurger, M, et al. Utilization of glial fibrillary acidic protein and galectin-3 in the diagnosis of cerebral infarction patients with normal cranial tomography. Niger J Clin Pract. (2017) 20:433–7. doi: 10.4103/1119-3077.187311
87. Büttner, T, Weyers, S, Postert, T, Sprengelmeyer, R, and Kuhn, W. S-100 protein: serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke. (1997) 28:1961–5. doi: 10.1161/01.STR.28.10.1961
88. Rainer, TH, Wong, KS, Lam, W, Lam, NYL, Graham, CA, and Lo, YMD. Comparison of plasma beta-globin DNA and S-100 protein concentrations in acute stroke. Clin Chim Acta. (2007) 376:190–6. doi: 10.1016/j.cca.2006.08.025
89. Üstündağ, M, Orak, M, Güloğlu, C, Tamam, Y, Sayhan, MB, and Kale, E. The role of serum osteoprotegerin and S-100 protein levels in patients with acute ischaemic stroke: determination of stroke subtype, severity and mortality. J Int Med Res. (2011) 39:780–9. doi: 10.1177/147323001103900310
90. Park, S-Y, Kim, M-H, Kim, O-J, Ahn, HJ, Song, JY, Jeong, JY, et al. Plasma heart-type fatty acid binding protein level in acute ischemic stroke: comparative analysis with plasma S100B level for diagnosis of stroke and prediction of long-term clinical outcome. Clin Neurol Neurosurg. (2013) 115:405–10. doi: 10.1016/j.clineuro.2012.06.004
91. Laskowitz, DT, Kasner, SE, Saver, J, Remmel, KS, and Jauch, EC. Clinical usefulness of a biomarker-based diagnostic test for acute stroke. Stroke. (2009) 40:77–85. doi: 10.1161/STROKEAHA.108.516377
92. Nakagawa, K, Yamaguchi, T, Seida, M, Yamada, S, Imae, S, Tanaka, Y, et al. Plasma concentrations of brain natriuretic peptide in patients with acute ischemic stroke. Cerebrovasc Dis. (2005) 19:157–64. doi: 10.1159/000083249
93. Cakir, Z, Saritas, A, Emet, M, Aslan, S, Akoz, A, and Gundogdu, F. A prospective study of brain natriuretic peptide levels in three subgroups: stroke with hypertension, stroke without hypertension, and hypertension alone. Ann Indian Acad Neurol. (2010) 13:47–51. doi: 10.4103/0972-2327.61277
94. Sayan, S, and Kotan, D. Levels of brain natriuretic peptide as a marker for the diagnosis and prognosis of acute ischemic stroke. Arch Med Sci Atheroscler Dis. (2016) 1:e16–22. doi: 10.5114/amsad.2016.59751
95. Tsao, CW, Aday, AW, Almarzooq, ZI, Anderson, CAM, Arora, P, Avery, CL, et al. Heart disease and stroke Statistics-2023 update: a report from the American Heart Association. Circulation. (2023) 147:e93–e621. doi: 10.1161/CIR.0000000000001123
96. WHO EMRO. Stroke, cerebrovascular accident. Health topics. Available at: https://www.emro.who.int/health-topics/stroke-cerebrovascular-accident/index.html (accessed 9 March 2024).
97. Trickey, A, Sabin, CA, Burkholder, G, Crane, H, d'Arminio Monforte, A, Egger, M, et al. Life expectancy after 2015 of adults with HIV on long-term antiretroviral therapy in Europe and North America: a collaborative analysis of cohort studies. Lancet HIV. (2023) 10:e295–307. doi: 10.1016/S2352-3018(23)00028-0
98. Jarrín, I, Rava, M, Raposo, JDR, Rivero, A, Guerrero, JDR, De Lagarde, M, et al. Life expectancy of people with HIV on antiretroviral therapy in Spain. AIDS. (2023) 38:387–95. doi: 10.1097/QAD.0000000000003772
99. Takeda, T, and Kohno, M. Brain natriuretic peptide in hypertension. Hypertens Res. (1995) 18:259–66. doi: 10.1291/hypres.18.259
100. Tsuchida, K, and Tanabe, K. Influence of paroxysmal atrial fibrillation attack on brain natriuretic peptide secretion. J Cardiol. (2004) 44:1–11.
101. Casserly, B, and Klinger, JR. Brain natriuretic peptide in pulmonary arterial hypertension: biomarker and potential therapeutic agent. Drug Des Devel Ther. (2009) 3:269–87. doi: 10.2147/DDDT.S4805
102. Brady, PF, Chua, W, Nehaj, F, Connolly, DL, Khashaba, A, Purmah, YJV, et al. Interactions between atrial fibrillation and natriuretic peptide in predicting heart failure hospitalization or cardiovascular death. J Am Heart Assoc. (2022) 11:e022833. doi: 10.1161/JAHA.121.022833
103. Albers, GW, Marks, MP, Kemp, S, Christensen, S, Tsai, JP, Ortega-Gutierrez, S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
104. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
105. Berge, E, Whiteley, W, Audebert, H, de Marchis, GM, Fonseca, AC, Padiglioni, C, et al. European stroke organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. (2021) 6:I–LXII. doi: 10.1177/2396987321989865
106. Lam, BYK, Cai, Y, Akinyemi, R, Biessels, GJ, van den Brink, H, Chen, C, et al. The global burden of cerebral small vessel disease in low- and middle-income countries: a systematic review and meta-analysis. Int J Stroke. (2023) 18:15–27. doi: 10.1177/17474930221137019
107. Babulal, GM, Quiroz, YT, Albensi, BC, Arenaza-Urquijo, E, Astell, AJ, Babiloni, C, et al. Perspectives on ethnic and racial disparities in Alzheimer’s disease and related dementias: update and areas of immediate need. Alzheimers Dement. (2019) 15:292–312. doi: 10.1016/j.jalz.2018.09.009
108. Michetti, F, Massaro, A, Russo, G, and Rigon, G. The S-100 antigen in cerebrospinal fluid as a possible index of cell injury in the nervous system. J Neurol Sci. (1980) 44:259–63. doi: 10.1016/0022-510X(80)90133-1
109. Ponath, G, Schettler, C, Kaestner, F, Voigt, B, Wentker, D, Arolt, V, et al. Autocrine S100B effects on astrocytes are mediated via RAGE. J Neuroimmunol. (2007) 184:214–22. doi: 10.1016/j.jneuroim.2006.12.011
110. Zhang, J, Gu, Y, Liu, X, Rao, X, Huang, G, and Ouyang, Y. Clinicopathological and prognostic value of S100A4 expression in non-small cell lung cancer: a meta-analysis. Biosci Rep. (2020) 40:BSR20201710. doi: 10.1042/BSR20201710
111. Tree, K, Kotecha, K, Mehta, S, Fuchs, TL, Toon, CW, Gill, AJ, et al. Granular cell tumour of the pancreas: a case report and systematic review. Langenbeck's Arch Surg. (2023) 408:64. doi: 10.1007/s00423-023-02761-3
112. Schaf, DV, Tort, ABL, Fricke, D, Schestatsky, P, Portela, LVC, Souza, DO, et al. S100B and NSE serum levels in patients with Parkinson’s disease. Parkinsonism Relat Disord. (2005) 11:39–43. doi: 10.1016/j.parkreldis.2004.07.002
113. Tian, Z, Liang, C, Zhang, Z, Wen, H, Feng, H, Ma, Q, et al. Prognostic value of neuron-specific enolase for small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol. (2020) 18:116. doi: 10.1186/s12957-020-01894-9
114. Han, XD, Lu, RQ, Hu, HY, and Guo, L. Application of serum markers in medullary thyroid carcinoma. Zhonghua Yu Fang Yi Xue Za Zhi. (2021) 55:1468–74. doi: 10.3760/cma.j.cn112150-20210308-00229
115. Fuksiewicz, M, Kowalska, M, Kolasinska-Cwikla, A, and Kotowicz, B. Serum levels of neuron-specific enolase as a prognostic factor for disease progression in patients with GET/NEN in the pancreas and the small intestine. Endocr Connect. (2022) 11:e210647. doi: 10.1530/EC-21-0647
116. Benninger, F, Glat, MJ, Offen, D, and Steiner, I. Glial fibrillary acidic protein as a marker of astrocytic activation in the cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J Clin Neurosci. (2016) 26:75–8. doi: 10.1016/j.jocn.2015.10.008
117. van Bodegraven, EJ, van Asperen, JV, Robe, PAJ, and Hol, EM. Importance of GFAP isoform-specific analyses in astrocytoma. Glia. (2019) 67:1417–33. doi: 10.1002/glia.23594
118. Ayrignac, X, Le Bars, E, Duflos, C, Hirtz, C, Maceski, AM, Carra-Dallière, C, et al. Serum GFAP in multiple sclerosis: correlation with disease type and MRI markers of disease severity. Sci Rep. (2020) 10:10923. doi: 10.1038/s41598-020-67934-2
119. Kumar, A, Misra, S, Yadav, AK, Sagar, R, Verma, B, Grover, A, et al. Role of glial fibrillary acidic protein as a biomarker in differentiating intracerebral haemorrhage from ischaemic stroke and stroke mimics: a meta-analysis. Biomarkers. (2020) 25:1–8. doi: 10.1080/1354750X.2019.1691657
120. Mattila, OS, Ashton, NJ, Blennow, K, Zetterberg, H, Harve-Rytsälä, H, Pihlasviita, S, et al. Ultra-early differential diagnosis of acute cerebral ischemia and hemorrhagic stroke by measuring the prehospital release rate of GFAP. Clin Chem. (2021) 67:1361–72. doi: 10.1093/clinchem/hvab128
121. Joo, JY, Yoo, D, and Ahn, T-B. Parainfectious anti-glial fibrillary acidic protein-associated meningoencephalitis. J Mov Disord. (2022) 15:66–70. doi: 10.14802/jmd.21115
122. Diao, JA, He, Y, Khazanchi, R, Nguemeni Tiako, MJ, Witonsky, JI, Pierson, E, et al. Implications of race adjustment in lung-function equations. N Engl J Med. (2024) 390:2083–97. doi: 10.1056/NEJMsa2311809
Glossary
Keywords: ischemic stroke, protein-based biomarkers, diagnosis, prediction, blood biomarkers
Citation: Rahmig J, Chanpura A, Schultz A, Barone FC, Gustafson D and Baird AE (2024) Blood-based protein biomarkers during the acute ischemic stroke treatment window: a systematic review. Front. Neurol. 15:1411307. doi: 10.3389/fneur.2024.1411307
Edited by:
Christopher Paul Kellner, Mount Sinai Health System, United StatesReviewed by:
Frederik Denorme, Washington University in St. Louis, United StatesGeoffrey Alan Donnan, University of Melbourne, Australia
Copyright © 2024 Rahmig, Chanpura, Schultz, Barone, Gustafson and Baird. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alison E. Baird, YWxpc29uLmJhaXJkQGRvd25zdGF0ZS5lZHU=
†These authors have contributed equally to this work and share senior authorship
 Jan Rahmig
Jan Rahmig Aditya Chanpura1
Aditya Chanpura1 Aaliyah Schultz
Aaliyah Schultz Frank C. Barone
Frank C. Barone Deborah Gustafson
Deborah Gustafson Alison E. Baird
Alison E. Baird