
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 16 July 2024
Sec. Pediatric Neurology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1411184
Aim: Our study aimed to assess the association between UCP2 gene 3’ untranslated region insertion/deletion (3’UTR I/D) and A55V (alanine/valine) polymorphisms and neural tube defects (NTDs) susceptibility.
Materials and methods: According to pre-determined inclusion and exclusion criteria, the article search was conducted to search articles published before October 2023. Two authors independently screened the included articles and extracted their basic characteristics. After quality evaluation, the meta-analysis and trial sequential analysis (TSA) were conducted using RevMan 5.4, Stata/MP 17, and TSA 0.9.5.10 Beta. Subgroup analysis was conducted based on country and case group composition. Sensitivity analysis was conducted using a one-by-one exclusion method. Begg’s and Egger’s tests were used to evaluate publication bias.
Results: A total of seven articles were included. Overall meta-analysis revealed significant heterogeneity among the included studies for 3’UTR I/D polymorphism of the UCP2 gene. Significant statistical data indicated that those with the DD genotype and D allele had higher chances of NTD compared to those with the II genotype and the I allele, respectively. The combined result of II vs. ID was not statistically significant. A55V variation showed no statistical significance in the risk of NTD, despite the absence of significant heterogeneity across the included studies. Most of the heterogeneity was resolved after subgrouping, and a higher risk of the ID genotype was found than the II genotype for Chinese people. Genotyping NTD patients or their mothers was not a factor affecting the heterogeneity. Sensitivity analysis and publication bias analysis suggested that positive findings supported our results.
Conclusion: The UCP2 gene 3’UTR I/D polymorphism increased the likelihood of developing NTDs in the Chinese population, with the D allele being the risk factor, which contributed to the understanding of the genetic basis of NTDs. TSA indicated that more high-quality original studies were needed in the future for further validation.
Neural tube defects (NTDs) are a class of congenital abnormalities distinguished by incomplete closure of the neural tube throughout the process of embryonic development (1). These abnormalities pose a serious global public health concern as they can result in serious neurological complications. NTDs have a complex etiology that includes environmental, dietary, and genetic factors (2, 3). Figuring out the genetic mechanisms that contribute to NTD susceptibility is essential for achieving a comprehensive understanding of their pathogenesis and creating effective prevention and treatment measures.
Gene polymorphisms, which represent specific variations in DNA sequence, are essential for modifying susceptibility to various diseases and conditions. These variations alter the expression and function of genes, contributing to the complex genetic mechanism underlying NTDs. For example, it has been confirmed that specific gene polymorphisms affect the susceptibility to NTDs (4, 5). As a member of the mitochondrial anion carrier protein family, uncoupling protein 2 (UCP2) is primarily responsible for regulating the reactive oxygen species that are produced by the mitochondria (6, 7). The UCP2 gene, which is located on chromosome 11q13, has been considered a potential candidate in the genetic landscape of NTDs (8, 9). UCP2 variants may play an important role in energy metabolism, weight regulation, and preventing the accumulation of reactive oxygen species. These conditions, primarily through obesity and diabetes, are considered risk factors for NTDs (10, 11). Numerous physiological processes, such as the regulation of oxidative stress and mitochondrial function, have been linked to the 3′ untranslated region (3’UTR) insertion/deletion (I/D) and A55V polymorphisms within the UCP2 gene (12–14). Many studies have been conducted to assess the link between these polymorphisms and the likelihood of suffering NTDs, but conflicting findings have left conclusions uncertain (10, 11, 15–19).
In order to provide a more robust and reliable estimate of the genetic contribution of UCP2 to NTD susceptibility by pooling data from relevant studies, we conducted a thorough meta-analysis by systematically evaluating the relationship between the polymorphisms of 3’UTR I/D and A55V in the UCP2 gene and the risk of NTDs. Additionally, to enhance the validity of our findings and control for potential false-positive results, trial sequential analysis (TSA) was performed as a statistical method designed to assess the cumulative evidence and determine whether further studies were warranted (20). In this way, our findings aimed to provide robust insights into the genetic basis of NTDs, guide future research, and influence clinical strategies for the prevention and management of these congenital anomalies.
Our meta-analysis was performed based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guideline (21). PROSPERO confirmed our meta-analysis registration under the ID CRD42023483551. The articles we included needed to be publicly published studies that investigated the connection between UCP2 gene polymorphism and NTD susceptibility. The research object of the original study needed to be humans, regardless of age, gender, country, or race. The original study needed to have a clear control group and case group, with the number of people in each group and genotype provided. Articles with unrelated topics would be excluded. If the data were incomplete and could not be resolved after contacting the corresponding author via email, it would also be excluded.
Article searching was conducted using Web of Science, PubMed, Embase, CNKI (Chinese), Wangfang Data (Chinese), and VIP (Chinese) databases. Two authors independently searched articles published from the establishment of the database to October 2023 and cross-checked the results. If there were different results, it would be consulted with third-party experts for advice. The search terms included UCP2, uncoupling protein 2, NTDs, NTDs, spina bifida, anencephaly, encephalocele, spinal dysraphism, hydranencephaly, iniencephaly, myelomeningocele, meningocele, schizencephaly, lipomeningocele, and craniorachischisis. Table 1 shows the search strategy using PubMed as an example.
Two authors independently conducted article screening and cross checked the results. Any different results would be resolved with the advice of third-party experts. Firstly, for articles that were repeatedly retrieved in multiple databases, only one of the records would be retained. Read the title and abstract of the articles firstly for preliminary screening, and then excluded those that obviously did not match the inclusion criteria. After then, read the full content for final screening and recorded the reasons for each piece that was excluded. Two authors independently extracted data for each original study, including the name of first author, publication year, country, the type of case group, and the number of people in each group and genotype.
Using the Newcastle–Ottawa scale, two authors independently assessed and cross-checked the quality of original studies. If there were different results, it would be consulted with third-party experts for advice. Articles evaluated as low quality would be excluded before being included in the meta-analysis.
Meta-analysis and TSA were conducted using Stata MP 17 (StataCorp LLC, Texas, United States), Review Manager 5.4.1 (The Cochrane Collaboration, London, United Kingdom), and TSA 0.9.5.10 Beta (Copenhagen Trial Unit, Copenhagen, Denmark) (α = 0.05). If the p-value of the heterogeneity test was greater than 0.1, it indicated that there was no significant heterogeneity, and a fixed effect model would be used for meta-analysis. If p < 0.1 was found, significant heterogeneity was indicated. The random-effects model will be used to attempt to explain and handle the heterogeneity through subgroup analysis. Combined odds ratios (ORs), 95% confidence intervals (CIs), and p-values were recorded. A strategy of one-by-one exclusion was used to conduct sensitivity analysis. To assess publication bias, Begg’s and Egger’s tests were applied. TSA was applied to evaluate whether the number of samples incorporated into the meta-analysis had reached the “required information size,” and whether sufficient samples had been included to provide a “definitive” conclusion. This method was particularly suited for our analysis as it provided a suggested endpoint for clinical original studies on this topic, ensuring that the conclusions drawn were robust and reliable. Additionally, for meta-analysis that included a limited number of studies, it indicated whether further research was needed to validate the conclusions (20).
A total of 60 records were retrieved through the article searching process. After the step-by-step screening, a total of seven articles met the final inclusion criteria (10, 11, 15–19). Among them, there were five studies in China and two studies in the United States of America (United States). For 3’UTR I/D, the original studies had a total of 1848 samples, including 864 in the case group and 984 in the control group. For A55V, the original studies had a total of 1,572 samples, including 781 in the case group and 791 in the control group. The flow diagram of article searching and screening is shown in Figure 1.
The result of the article quality evaluation indicated that the quality of all the articles mentioned above met the inclusion criteria for meta-analysis. The results of data extraction are shown in Tables 2, 3. For the study conducted by Mitchell A et al., the case groups of children and mothers shared the same control group. As the shared control group could not be reused, the sample size of each genotype in the control group would be halved when the case groups of children and mothers were included in the same meta-analysis (22).
By incorporating data from all individuals, the overall meta-analysis of the population was conducted, with the results represented in Table 4.
For 3’UTR I/D, all II vs. ID, II vs. DD, and I vs. D polymorphisms had a significant p-value of heterogeneity in the Q-test, indicating that high heterogeneity existed. Therefore, the random-effects model was applied. II vs. ID polymorphism showed that the OR = 0.48, with p = 0.14 and 95% CI [0.18, 1.27], indicating no statistical significance (Figure 2A). However, for II vs. DD and I vs. D polymorphisms, the combined ORs were 0.57 and 0.58, with 95% CI [0.33, 0.995] and [0.35, 0.98], respectively. This showed that individuals with the DD genotype would suffer a higher risk of NTDs than those with the II genotype, and individuals with the D allele would suffer a higher risk of NTDs than those with the I allele, with significant statistical evidence (Figures 2B,C).
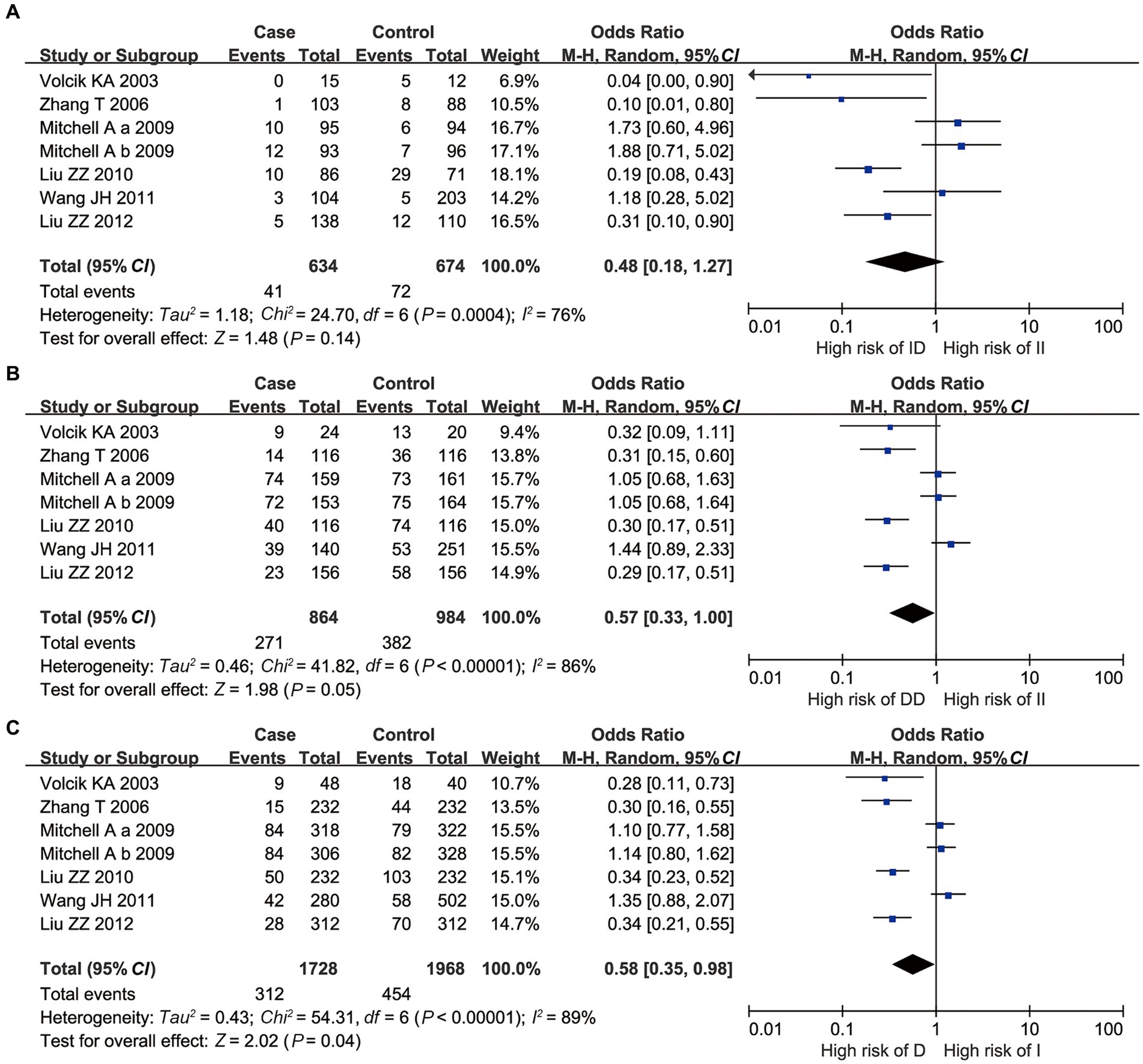
Figure 2. Overall meta-analysis results of the 3’UTR I/D polymorphism of UCP2 gene. (A) II vs. ID; (B) II vs. DD; and (C) I vs. D.
For A55V, all AA vs. AV, AA vs. VV, and A vs. V polymorphisms had no significant p-value of heterogeneity in the Q-test, indicating that low heterogeneity existed. Besides, all the p-values of the overall meta-analysis showed that no statistical significance was found under the fixed effect model (p > 0.05, Figure 3).
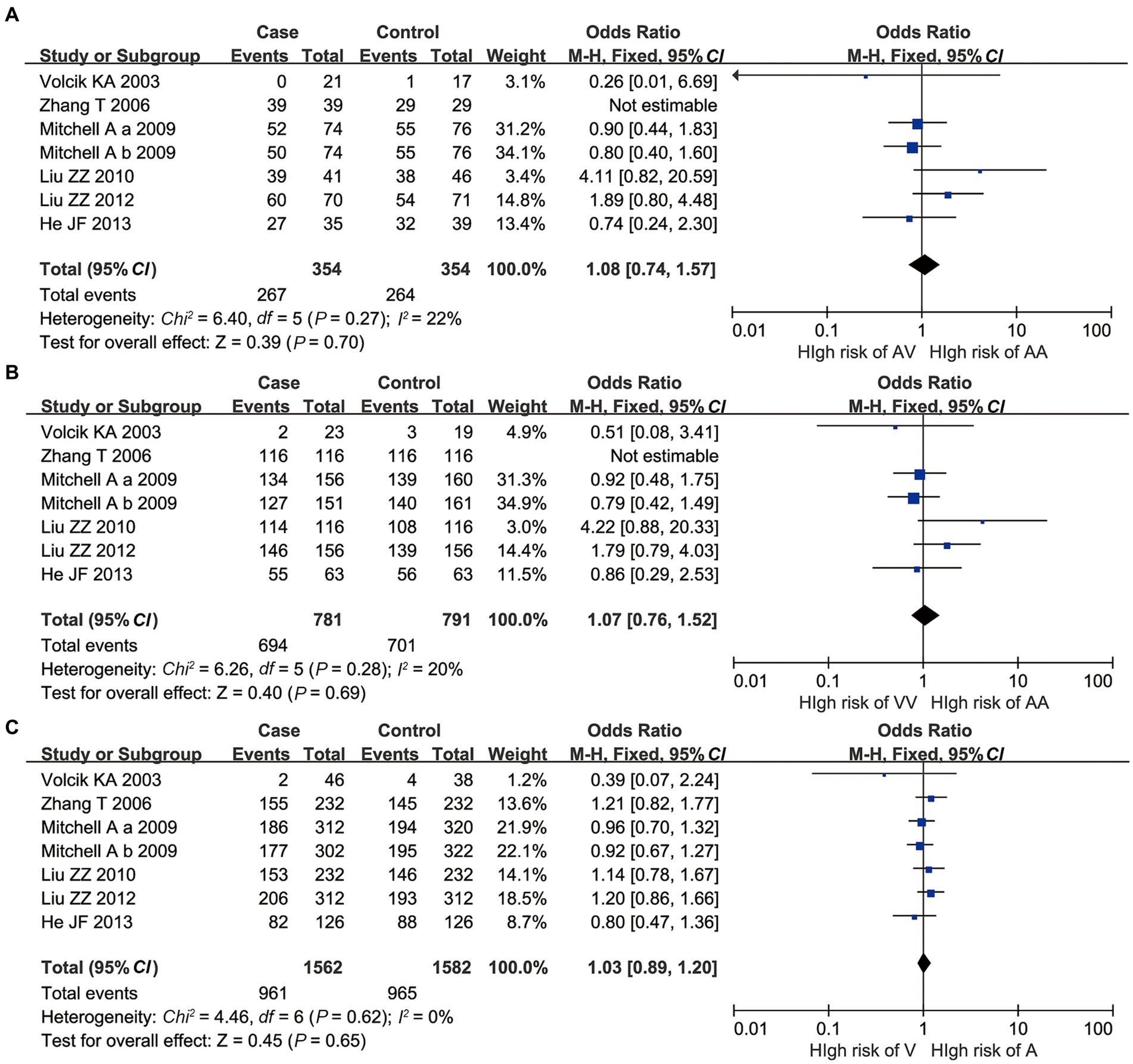
Figure 3. Overall meta-analysis results of A55V polymorphism of UCP2 gene. (A) AA vs. AV; (B) AA vs. VV; and (C) A vs. V.
Subgroup analysis was carried out based on country (Table 5). For 3’UTR I/D II vs. ID polymorphism, the heterogeneity was resolved after subgrouping, with a heterogeneity Q-test p-value of 0.13 > 0.1 in the Chinese subgroup. This situation was similar in II vs. DD polymorphism, with a heterogeneity Q-test p-value of 0.19 > 0.1 in the United States subgroup. For the Chinese population, the results of the II vs. ID meta-analysis showed OR = 0.27 and p < 0.0001, with 95% CI [0.15, 0.46], indicating that individuals carrying ID genotype had a higher risk of NTDs than those carrying II genotype, and the results were statistically significant. However, for the USA population, there was no statistical evidence to suggest an association between the risk of NTDs considering all II vs. ID, II vs. DD, and I vs. D polymorphisms (p > 0.05). These findings were different from the results of the overall meta-analysis.
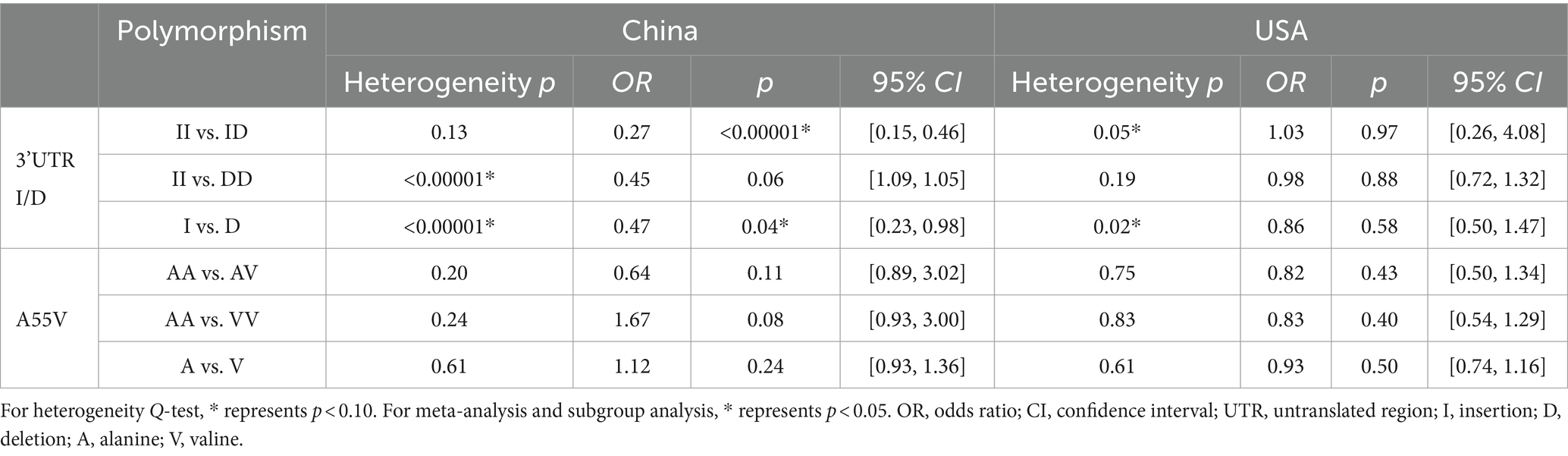
Table 5. Subgroup analysis results based on the country of the UCP2 gene 3’UTR I/D and A55V polymorphisms.
For A55V polymorphism, the findings after subgrouping were similar to overall meta-analysis results, indicating that no significant statistical evidence was found to support the association with NTD risk.
Subgroup analysis was carried out based on case group composition (Table 6). For both 3’UTR I/D and A55V polymorphisms, the heterogeneity seemed to have no significant change after subgrouping according to the case group composition (mother or children). The situations had not changed, presenting as p > 0.1 for II vs. ID, II vs. DD, I vs. D of 3’UTR I/D polymorphism, and AA vs. AV, AA vs. VV, and A vs. V of A55V polymorphism. This indicated that genotyping NTD patients or their mothers was not a factor affecting the heterogeneity of the meta-analysis. Besides, for 3’UTR I/D, significant II vs. DD and I vs. D polymorphisms in the overall meta-analysis did not show significant statistical differences after subgrouping based on case group composition.
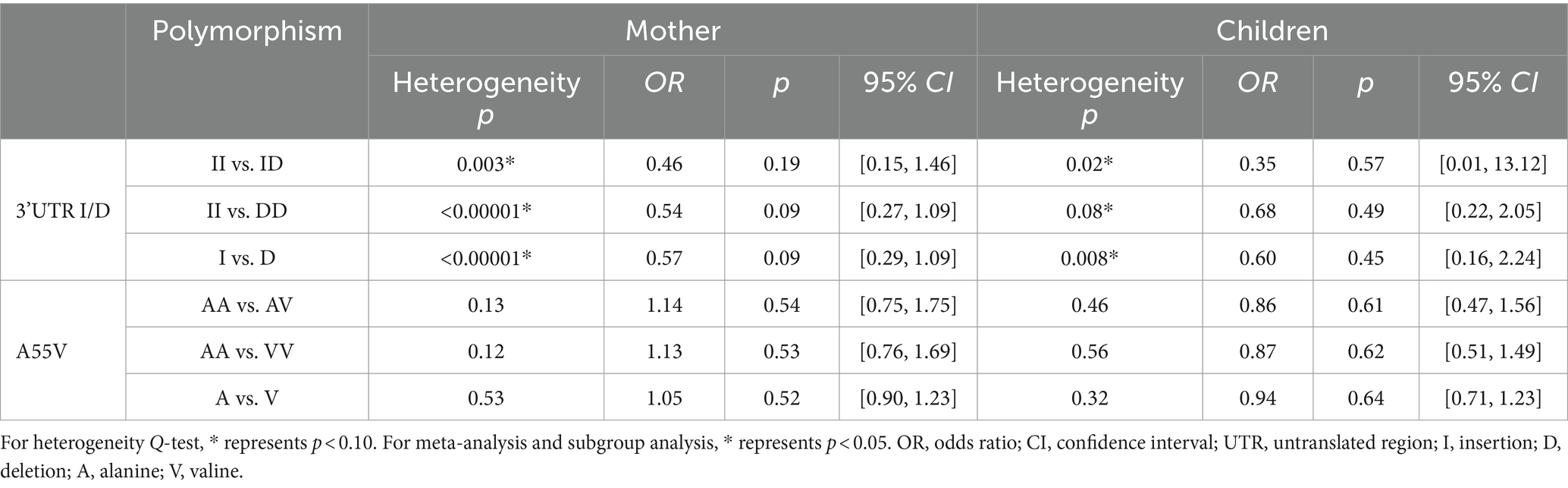
Table 6. Subgroup analysis results based on the case group composition of UCP2 gene 3’UTR I/D and A55V polymorphisms.
The one-by-one exclusion method was used for sensitivity analysis. For 3’UTR I/D polymorphism, taking II vs. ID as an example, the combined OR remained less than 1 and p remained higher than 0.05 after excluding any single original studies. It indicated that the meta-analysis results were relatively stable and less affected by changes in a single study. The situation remained the same for A55V polymorphism, taking AA vs. AV as an example.
According to Begg’s test, the qualitative funnel plot is shown in meta-analysis were reliab Figure 4. All the included studies presented in the plot, and distributed symmetrically along the axis, indicating low risk of publication bias. The quantitative data were calculated to reach more accurate conclusions, with all the p > 0.05, and the quantitative Egger’s test of all the polymorphisms showed p > 0.05 as well. All of the above indicated no significant publication bias, and the results of the meta-analysis were reliable. All the quantitative data are presented in Table 7.
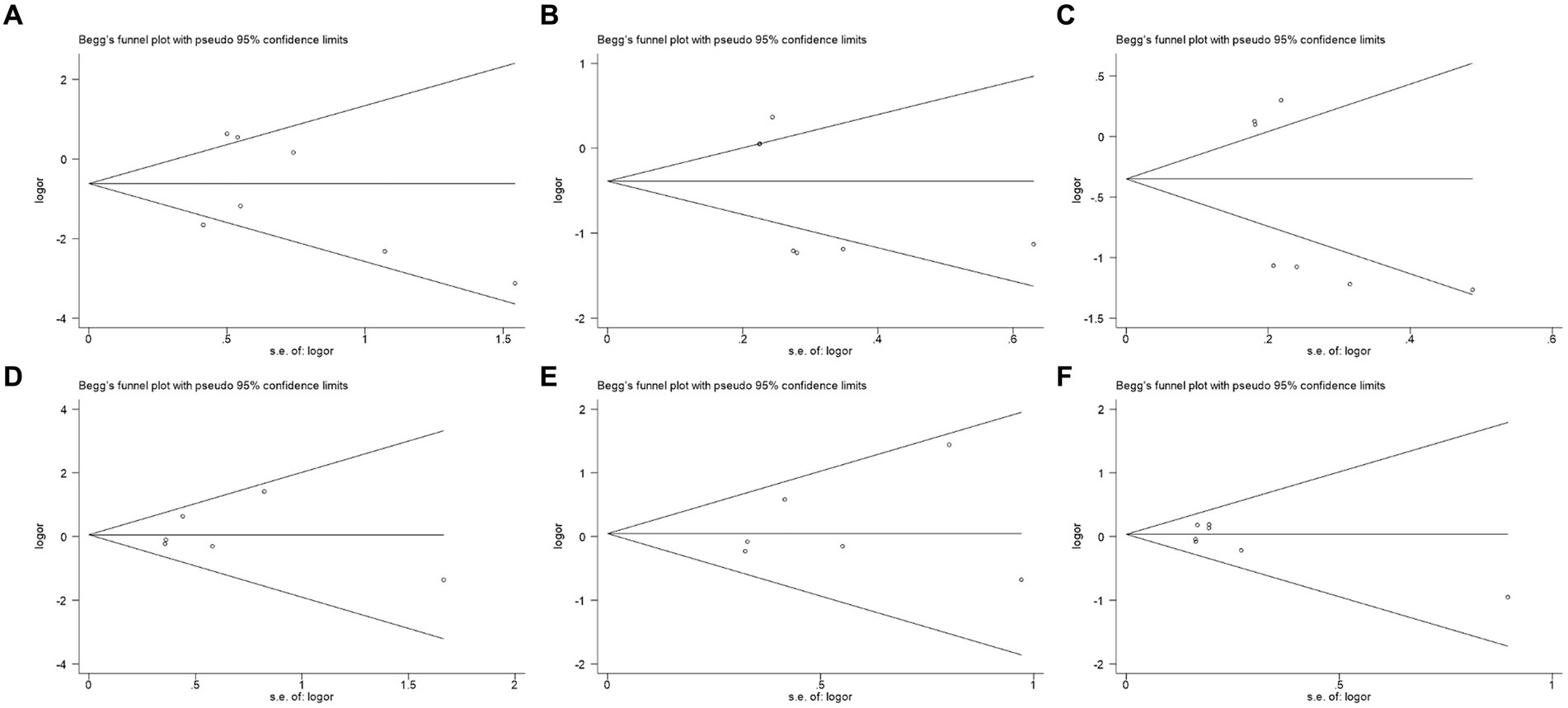
Figure 4. Funnel plots of publication bias analysis. (A) II vs. ID; (B) II vs. DD; (C) I vs. D; (D) AA vs. AV; (E) AA vs. VV; and (F) A vs. V.
The boundary type of hypothesis testing was set as two-sided tests, and Type I error was defined as 5%. For the required information size (RIS), statistical power was defined as 80%. Based on clinical experience, the relative risk reduction rate was defined as 35%, and the event rate in the control group was defined as 3%. The results of TSA are shown in Figure 5. All plots showed that the Z-curve neither intersects the threshold line nor reaches RIS. This showed that we did not have sufficient confidence that the results of the meta-analysis did not need to be verified by subsequent original studies; that is, the minimum amount of sample required to draw a “definitive” conclusion had not yet been reached, suggesting the necessity for further original studies to validate the conclusion of this meta-analysis in the future (20).
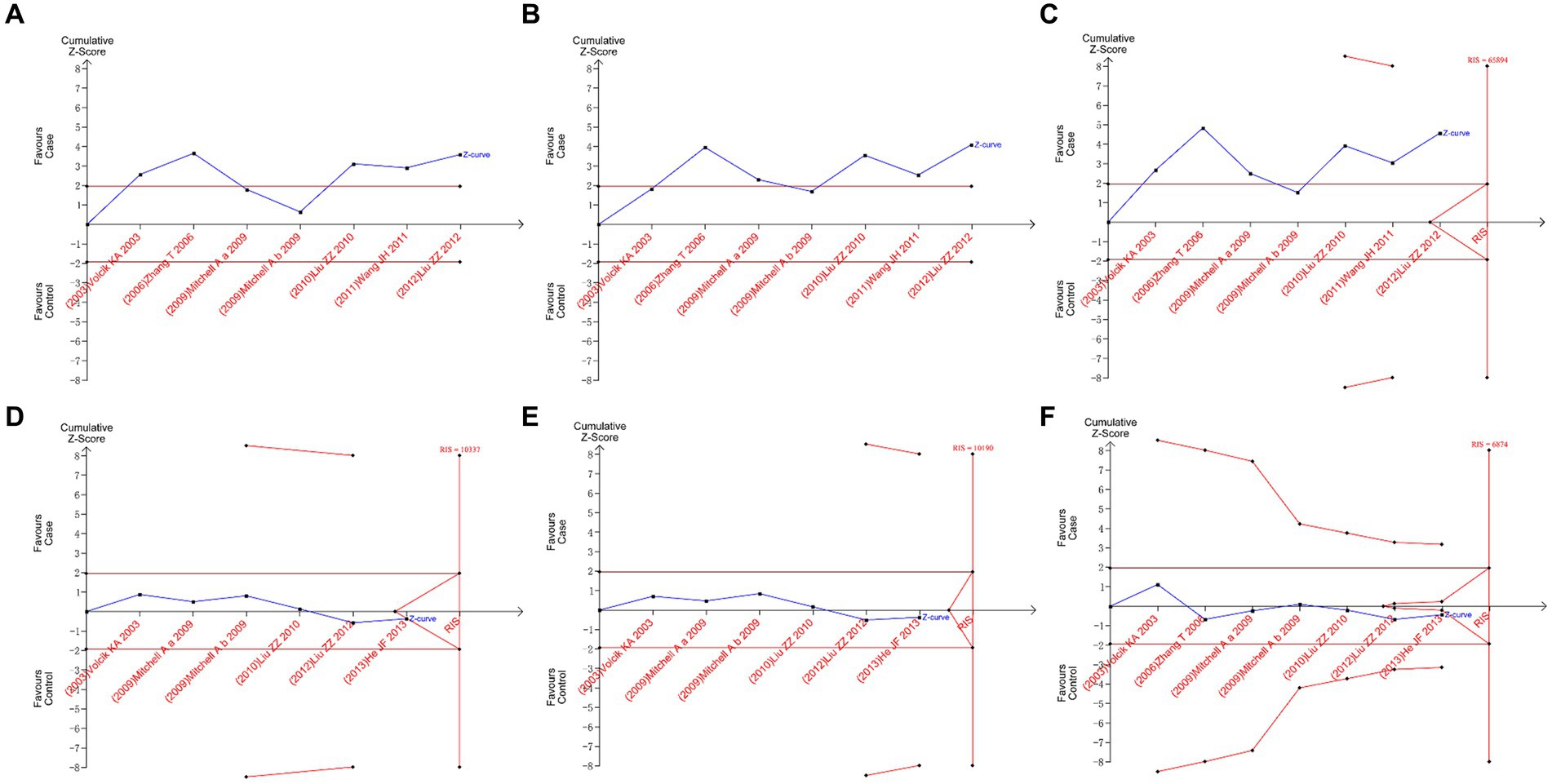
Figure 5. Trial sequential analysis plots. (A) II vs. ID; (B) II vs. DD; (C) I vs. D; (D) AA vs. AV; (E) AA vs. VV; and (F) A vs. V.
Prior research has indicated associations between UCP2 gene polymorphisms, specifically 3’UTR I/D and A55V, and diverse health conditions, with their conflicts prompting our exploration of their potential correlation. The involvement of UCP2 in mitochondrial function influencing energy metabolism was reported to be crucial for neural tube development. The meta-analysis conducted in this study aimed to explore the association between UCP2 gene polymorphisms (3’UTR I/D and A55V) and the susceptibility to NTDs. Our findings would reveal several key insights that warranted discussion.
The overall meta-analysis results for the 3’UTR I/D polymorphism indicated a significant association with NTD risk in the II vs. DD and I vs. D comparisons, whereas the II vs. ID polymorphism showed no statistical significance. These results suggested that individuals with the DD genotype might face a higher risk of NTDs than those with the II genotype, and individuals with the D allele might be at an elevated risk compared to those with the I allele. However, a lack of significance in the II vs. ID comparison was found. Subgroup analysis based on the country of origin revealed divergent outcomes. The II vs. ID meta-analysis demonstrated a significant association with an increased NTD risk in the Chinese population, while no such association was observed in the overall United States populations. These differences could be attributed to various factors, including genetic diversity, environmental influences, and dietary habits. For example, variations in folate metabolism, which is crucial for neural tube closure, might differ between populations. Additionally, differences in sample sizes and study designs could contribute to these discrepancies. Future research should focus on exploring these population-specific factors in greater detail to better understand the genetic and environmental interactions affecting NTD risk. Identifying these differences was crucial for developing targeted prevention strategies and improving public health outcomes. Subgroup analysis based on case group composition did not yield significant changes in heterogeneity, suggesting that genotyping NTD patients or their mothers did not significantly impact the overall meta-analysis results.
The results of the A55V polymorphism meta-analysis indicated no significant association with NTD risk, and this lack of significance persisted in subgroup analyses based on country and case group composition. These findings suggested that the A55V polymorphism might not play a substantial role in NTD susceptibility, at least in the populations considered in the included studies.
Furthermore, the sensitivity analysis demonstrated the stability of our findings, as the exclusion of any single study did not substantially alter the outcomes. Regarding publication bias, it was generally considered that a symmetrical funnel plot from Begg’s test indicated no publication bias. Given the small sample size in this study, we also considered the quantitative Begg’s test, where p > 0.05 indicated no significant publication bias. Similarly, in Egger’s test, all groups had p-values greater than 0.05. These pieces of evidence suggested that the reliability of the conclusions in this meta-analysis was minimally affected by publication bias from a statistical standpoint. This enhanced the credibility of our meta-analysis results, suggesting that the conclusions drawn were reliable.
The impact of UCP2 gene polymorphisms on NTDs prompted exploration into potential mechanisms. While the exact functional implications remained unclear, UCP2, involved in mitochondrial regulation, might affect bioenergetics and redox balance through specific polymorphisms, influencing NTD development (12–14, 23). Polymorphisms might change nutrient availability and interfere with vital pathways in neural tube closure because of the role of UCP2 in energy homeostasis (24–28). Furthermore, the connection to immunological responses and inflammation, possible interactions with environmental factors, and impact on epigenetic modifications also highlighted how UCP2 was involved in the risk of disease (29–31). Recognizing these mechanisms was essential in formulating interventions for the prevention and management of NTDs. Multiple studies have confirmed that obesity was an independent risk factor for NTDs during pregnancy, with a risk increase of 2 to 5 times (10). Additionally, women with diabetes and obesity experience numerous metabolic abnormalities, such as hyperglycemia, which are associated with a higher risk of birth defects (11). Since UCP2 variants might play an important role in energy metabolism, weight regulation, and preventing the accumulation of reactive oxygen species, these conditions were also considered NTD risk factors through obesity and diabetes (10, 11). Therefore, variations in UCP2 were attractive candidates for screening potential risk factors for NTDs.
It was crucial to recognize some limitations. First of all, differences in study designs, population characteristics, and genotyping techniques could have a significant impact on the heterogeneity seen in certain analyses. Although subgroup analysis had addressed some heterogeneity issues, there were still issues in certain analyses that might affect the reliability of the summary results. Furthermore, even though the analysis of publication bias suggested a low risk of bias, it was not completely possible to rule out the possibility of unpublished or undiscovered studies. Finally, a limited number of studies were included in this meta-analysis, which was also indicated by TSA, thus resulting in the limited number of included countries, which restricted the applicability of the study results to other populations and ethnic groups (20). Nevertheless, the study of UCP2 gene polymorphisms in NTDs was still a novel, contentious, and stimulating field of research that could provide foundational knowledge for further research in this developing field.
In conclusion, our meta-analysis sheds significant information on the possible relationship between NTD susceptibility and UCP2 gene polymorphisms. The UCP2 gene 3’UTR I/D polymorphism increased the likelihood of developing NTDs, with D being the risk factor, which was mainly seen in the Chinese population. To confirm and expand on our findings and ultimately provide an improved understanding of the genetic basis of NTDs, more well-designed original studies are expected to be carried out in the future.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
HT: Writing – original draft, Writing – review & editing. ZG: Writing – original draft, Writing – review & editing. SL: Writing – original draft, Writing – review & editing. JW: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (U23A20420), the Beijing Natural Science Foundation (7222016, 7244290), and the Research Foundation of the Capital Institute of Pediatrics (CXYJ-2021-03).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wang, X, Yu, J, and Wang, J. Neural tube defects and folate deficiency: is DNA repair defective? Int J Mol Sci. (2023) 24:2220. doi: 10.3390/ijms24032220
2. Eaves, LA, Choi, G, Hall, E, Sillé, FCM, Fry, RC, Buckley, JP, et al. Prenatal exposure to toxic metals and neural tube defects: a systematic review of the epidemiologic evidence. Environ Health Perspect. (2023) 131:86002. doi: 10.1289/EHP11872
3. Caiaffa, CD, Fonteles, CSR, Yunping, L, and Finnell, RH. Gene-environment interactions underlying the etiology of neural tube defects. Curr Top Dev Biol. (2023) 152:193–220. doi: 10.1016/bs.ctdb.2022.10.007
4. Guan, Z, Wang, J, Guo, J, Wang, F, Wang, X, Li, G, et al. The maternal Itpk1 gene polymorphism is associated with neural tube defects in a high-risk Chinese population. PLoS One. (2014) 9:e86145. doi: 10.1371/journal.pone.0086145
5. Wang, F, Wang, J, Guo, J, Chen, X, Guan, Z, Zhao, H, et al. Pcmt1 gene polymorphisms, maternal folate metabolism, and neural tube defects: a case-control study in a population with relatively low folate intake. Genes Nutr. (2013) 8:581–7. doi: 10.1007/s12263-013-0355-5
6. Tian, XY, Ma, S, Tse, G, Wong, WT, and Huang, Y. Uncoupling protein 2 in cardiovascular health and disease. Front Physiol. (2018) 9:1060. doi: 10.3389/fphys.2018.01060
7. Koziel, A, Sobieraj, I, and Jarmuszkiewicz, W. Increased activity of mitochondrial uncoupling protein 2 improves stress resistance in cultured endothelial cells exposed in vitro to high glucose levels. Am J Physiol Heart Circ Physiol. (2015) 309:H147–56. doi: 10.1152/ajpheart.00759.2014
8. Hsu, Y-H, Niu, T, Song, Y, Tinker, L, Kuller, LH, and Liu, S. Genetic variants in the UCP2-Ucp3 gene cluster and risk of diabetes in the Women's Health Initiative observational study. Diabetes. (2008) 57:1101–7. doi: 10.2337/db07-1269
9. Boyles, AL, Hammock, P, and Speer, MC. Candidate gene analysis in human neural tube defects. Am J Med Genet C Semin Med Genet. (2005) 135C:9–23. doi: 10.1002/ajmg.c.30048
10. Mitchell, A, Pangilinan, F, Van der Meer, J, Molloy, AM, Troendle, J, Conley, M, et al. Uncoupling protein 2 polymorphisms as risk factors for Ntds. Birth Defects Res A Clin Mol Teratol. (2009) 85:156–60. doi: 10.1002/bdra.20520
11. Volcik, KA, Shaw, GM, Zhu, H, Lammer, EJ, and Finnell, RH. Risk factors for neural tube defects: associations between uncoupling protein 2 polymorphisms and Spina bifida. Birth Defects Res A Clin Mol Teratol. (2003) 67:158–61. doi: 10.1002/bdra.10019
12. Li, J, Jiang, R, Cong, X, and Zhao, Y. UCP2 gene polymorphisms in obesity and diabetes, and the role of UCP2 in Cancer. FEBS Lett. (2019) 593:2525–34. doi: 10.1002/1873-3468.13546
13. Robbins, D, and Zhao, Y. New aspects of mitochondrial uncoupling proteins (Ucps) and their roles in tumorigenesis. Int J Mol Sci. (2011) 12:5285–93. doi: 10.3390/ijms12085285
14. Avesani, CM, Kamimura, MA, Utaka, S, Pecoits-Filho, R, Nordfors, L, Stenvinkel, P, et al. Is UCP2 gene polymorphism associated with decreased resting energy expenditure in nondialyzed chronic kidney disease patients? J Ren Nutr. (2008) 18:489–94. doi: 10.1053/j.jrn.2008.08.009
15. He, J, Liu, J, Wang, W, Zhang, Q, Yang, Q, Li, G, et al. A case-control study on the risk factors of neural tube defects in Guizhou Province of China. J Shandong Univ. (2013) 51:50–53. doi: 10.6040/j.issn.1671-7554.2013.07.012
16. Liu, Z, Xie, J, Te, L, and Li, P. Relationship between maternal UCP2 gene polymorphism and neural tube defects of Offsprings. Matern Child Health Care China. (2010) 25:3445–8.
17. Zhang, T. Study on the relationship between UCP2 gene polymorphism and neural tube malformation. [Master]. Taiyuan: Shanxi Medical University (2006).
18. Liu, Z, Xie, J, Te, L, Zhang, T, Zhao, X, Zhao, H, et al. An epidemiologic study of mitochondrial membrane transporter protein gene polymorphism and risk factors for neural tube defects in Shanxi, China. Neural Regen Res. (2012) 7:463–9. doi: 10.3969/j.issn.1673-5374.2011.06.010
19. Wang, J, Liu, C, Zhao, H, Wang, F, Guo, J, Xie, H, et al. Association between a 45-Bp 3'untranslated insertion/deletion polymorphism in exon 8 of UCP2 gene and Neural tube defects in a high-risk area of China. Reprod Sci. (2011) 18:556–62. doi: 10.1177/1933719110393026
20. Tian, H, Yang, C, Song, T, Zhou, K, Wen, L, Tian, Y, et al. Efficacy and safety of Paxlovid (Nirmatrelvir/ritonavir) in the treatment of Covid-19: an updated Meta-analysis and trial sequential analysis. Rev Med Virol. (2023) 33:e2473. doi: 10.1002/rmv.2473
21. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The Prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
22. Rücker, G, Cates, CJ, and Schwarzer, G. Methods for including information from multi-arm trials in pairwise Meta-analysis. Res Synth Methods. (2017) 8:392–403. doi: 10.1002/jrsm.1259
23. Carmichael, SL, Yang, W, Ma, C, Desrosiers, TA, Weber, K, Collins, RT, et al. Oxidative balance scores and neural crest cell-related congenital anomalies. Birth Defects Res. (2023) 115:1151–62. doi: 10.1002/bdr2.2211
24. Rezapour, S, Khosroshahi, SA, Farajnia, H, Mohseni, F, Khoshbaten, M, and Farajnia, S. Association of 45-Bp ins/Del polymorphism of uncoupling protein 2 (UCP2) and susceptibility to nonalcoholic fatty liver and type 2 diabetes mellitus in north-west of Iran. BMC Res Notes. (2021) 14:169. doi: 10.1186/s13104-021-05586-9
25. Sun, H, Zhang, J-T, Xie, X-R, Li, T, Li, X-Y, Wang, N-N, et al. Association of uncoupling protein gene polymorphisms with essential hypertension in a northeastern Han Chinese population. J Hum Hypertens. (2019) 33:524–30. doi: 10.1038/s41371-018-0141-3
26. Mousa, A, Naqash, A, and Lim, S. Macronutrient and micronutrient intake during pregnancy: an overview of recent evidence. Nutrients. (2019) 11:443. doi: 10.3390/nu11020443
27. Suazo, J, Pardo, R, Castillo, S, Martin, LM, Rojas, F, Santos, JL, et al. Family-based association study between Slc2a1, Hk1, and Lepr polymorphisms with myelomeningocele in Chile. Reprod Sci. (2013) 20:1207–14. doi: 10.1177/1933719113477489
28. Avagliano, L, Doi, P, Tosi, D, Scagliotti, V, Gualtieri, A, Gaston-Massuet, C, et al. Cell death and cell proliferation in human Spina bifida. Birth Defects Res A Clin Mol Teratol. (2016) 106:104–13. doi: 10.1002/bdra.23466
29. Labayen, I, Ortega, FB, Sjöström, M, Nilsson, TK, Olsson, LA, and Ruiz, JR. Association of Common Variants of UCP2 gene with low-grade inflammation in Swedish children and adolescents; the European youth heart study. Pediatr Res. (2009) 66:350–4. doi: 10.1203/PDR.0b013e3181b1bd35
30. Su, M, Chen, X, Chen, Y, Wang, C, Li, S, Ying, X, et al. UCP2 and UCP3 variants and gene-environment interaction associated with prediabetes and T2dm in a rural population: a case control study in China. BMC Med Genet. (2018) 19:43. doi: 10.1186/s12881-018-0554-4
Keywords: UCP2 , polymorphism, neural tube defects, meta-analysis, trial sequential analysis
Citation: Tian H, Guan Z, Li S and Wang J (2024) Association between UCP2 gene 3’UTR I/D and A55V polymorphisms and neural tube defects susceptibility: systematic review, meta-analysis, and trial sequential analysis. Front. Neurol. 15:1411184. doi: 10.3389/fneur.2024.1411184
Received: 02 April 2024; Accepted: 26 June 2024;
Published: 16 July 2024.
Edited by:
Anna Maria Lavezzi, University of Milan, ItalyReviewed by:
Ahmed Aljabali, Jordan University of Science and Technology, JordanCopyright © 2024 Tian, Guan, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Wang, Znl3amhAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.