
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 24 July 2024
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1406157
Objective: This study aimed to assess the impact of multimodal monitoring on predicting the prognosis of patients with spontaneous intracerebral hemorrhage (SICH) and to examine the feasibility of using noninvasive near-infrared spectroscopy (NIRS) for monitoring clinical prognosis.
Methods: Clinical data of 38 patients with SICH who underwent surgery in the Department of Neurosurgery of Shaanxi Provincial People’s Hospital from May 2022 to December 2022 were retrospectively analyzed. The patients were categorized into two groups based on the Glasgow Outcome Scale (GOS) 3 months after operation: poor outcome group (GOSI-III) and good outcome group (GOSIV and V). Multimodal monitoring included invasive intracranial pressure (ICP), brain temperature (BT), internal jugular venous oxygen saturation (SjvO2), and noninvasive NIRS. NIRS monitoring comprised the assessment of brain tissue oxygen saturation (StO2), blood volume index (BVI), and tissue hemoglobin index (THI). The prognostic differences between the two groups were compared. The predictive values were evaluated using the receiver operating characteristic (ROC) curve and the area under the curve (AUC).
Results: ICP, BT, BVI, and THI in the good prognosis group were lower than those in the poor prognosis group. The SjvO2 and StO2 in the group with a good prognosis were higher than those in the group with a poor prognosis.
Conclusion: The levels of ICP, BT, SjvO2, StO2, BVI, and THI reflect the changes in brain function and cerebral blood flow and significantly correlate with the prognosis of patients with SICH. NIRS monitoring has a high clinical utility in assessing the prognosis.
Spontaneous intracerebral hemorrhage (SICH) refers to a hematoma resulting from the rupture of intracranial blood vessels due to nontraumatic factors (1). It is the second leading cause of stroke (2). The causes include primary SICH (hypertension and cerebral amyloid angiopathy) and secondary SICH (vascular malformations, nonatherosclerotic vascular lesions, tumors, coagulation disorders, and exposure to toxicants). The natural mortality rate within 1 month after the onset of SICH is approximately 45%, and approximately 80% of the patients have a poor prognosis (3, 4). Surgical treatment remains the primary treatment modality for SICH.
Although surgery has contributed to a reduction in the mortality rate among patients with SICH, the proportion of patients with poor prognosis in neurological function remains relatively high, which adds to the burden on patients’ families and the national health insurance system (5). There is still a lack of an effective treatment strategy to improve the prognosis of patients with SICH (6). Early and accurate detection of changes in intracranial conditions and timely and effective interventions can dramatically reduce the mortality of patients and substantially improve the prognosis. Near-infrared spectroscopy (NIRS) has been widely used in cardiac surgery to optimize the perfusion of the brain, heart, kidney, liver, and other important organs. However, its use in patients with SICH is limited. Clinicians can optimize oxygen delivery and possibly improve clinical outcomes by measuring brain tissue oxygen saturation (StO2) at the watershed of the anterior and middle cerebral arteries (7). By using multimodal monitoring that integrates intracranial pressure (ICP) with NIRS, this study enables real-time monitoring of intracranial conditions and offers precise treatment guidance for clinicians, thereby overcoming the limitations of empirical treatment approaches and providing an accurate assessment of the prognosis of patients.
The clinical data of 38 patients with SICH who underwent surgery in the Department of Neurosurgery of Shaanxi Provincial People’s Hospital from May 2022 to December 2022 were retrospectively analyzed. The study sample included 17 male participants and 21 female participants (Figure 1). According to the guidelines (8), upon admission, all patients underwent emergency cranial CT examinations. This included dietary restrictions, oxygen inhalation, electrocardiogram (ECG) monitoring, assessment of consciousness, monitoring of basic vital signs, and optimization of relevant blood tests as part of preoperative preparation. The early systolic blood pressure of patients was maintained at <140 mm Hg to prevent the enlargement of hematoma and deterioration of the nervous system to promote functional recovery. The Glasgow Coma Scale (GCS) of the patients was between 4 and 9. The patients were categorized into two groups based on the Glasgow Outcome Scale (GOS) 3 months after discharge: poor outcome group (GOSI-III) and good outcome group (GOSIV-V). This study is in line with the principles of the Helsinki Declaration. An informed consent form for the surgery and placement of an ICP probe was signed by the families of all the patients.
The inclusion criteria for the study included the following: (1) relevant examination upon admission and presence of signs of cerebral hemorrhage based on the clinical symptoms and imaging findings; (2) indication of emergency surgery, including microscopic hematoma evacuation or neuroendoscopic minimally invasive surgery; (3) allowed to monitor ICP and brain temperature (BT); (4) allowed for the placement central venous catheter after surgery; (5) allowed assessing ICP, BT, and internal jugular venous oxygen saturation (SjvO2) and NIRS monitoring at least 3 days after the surgery.
The following patients were excluded from the study: (1) those with a history of head surgery; (2) those with multiple organ failure or other serious underlying diseases affecting the prognosis; (3) patients treated with anticoagulants or antiplatelet drugs for more than three months (due to the associated higher risk of poor prognosis); (4) those with a recurrent stroke within 3 months of follow-up after discharge; and (5) those who died of unknown causes within 24 h after surgery.
All patients underwent emergency surgery within 24 h after diagnosis, and all surgeries were performed by the chief physician or deputy chief physician at Shaanxi Provincial People’s Hospital. The surgical procedures included hematoma clearance and implantation of an ICP probe in the lateral ventricle. Bone flap decompression was performed in patients with severe brain swelling after hematoma clearance. The changes in dynamic ICP were monitored for continuously 3 days after the operation. All patients were transferred to an intensive care unit (ICU) and received standard treatment, including hemostasis, prevention of brain edema, nutritional support, and prevention of complications. Patients in a comatose state underwent tracheotomy to strengthen the management of the respiratory tract. The head CT was reexamined at 1, 3, and 7 days after the operation. ICP and BT were recorded every hour for the first three days post-operation. SjvO2 was monitored every eight hours for three days post-operation. NIRS monitoring was conducted every eight hours, for 20 min at a time, over the course of three days post-operation. The average values of ICP, BT, SjvO2, brain oxygen saturation (StO2), blood volume index (BVI), and tissue hemoglobin index (THI) were calculated.
Some specific treatments were administered as follows:
1. When the postoperative ICP was >22 mm Hg and lasted for >15 min, dehydration drugs (e.g., mannitol, furosemide, albumin) along with analgesic and sedative drugs were administered intravenously.
2. When the postoperative ICP was >30 mm Hg, intermittent and rapid administration of multiple dehydration drugs, cerebrospinal fluid drainage, analgesic and sedative therapy, and hyperventilation therapy were implemented.
3. When the postoperative ICP was >45 mm Hg, the head CT scan was reexamined and reoperation was considered based on the situation.
4. “Postoperative brain temperature management”: The BT was controlled at 37°C. However, in cases where the BT reached >37°C, an ice cap or ice blanket was used.
The ICP probe with BT monitoring was implanted into the ventricle of the patients after the surgery, and the probe was taken out after continuous monitoring for 3 days. The ICP and BT data stored on the monitor were collected, and the average values were calculated in an hour. A central venous catheter was placed in each patient. SjvO2 was measured every 8 h after the operation, and NIRS monitoring of 20 min was performed at the same time. The data of StO2, BVI, and THI stored on the monitor were collected and the average values were calculated (Figure 2).
SPSS software (version 25.0; IBM Corp) was used for statistical analysis. The Student t-test was used to compare the normally distributed data, and the data were expressed in terms of mean ± standard deviation. The Mann–Whitney U test was used to analyze non-normally distributed data between the groups, and the data were expressed using median (M) and interquartile range (IQR). Moreover, the classified variables were expressed by the number of cases and percentage or constituent ratio. The chi-square test and Fisher exact test were used for comparison. A p-value <0.05 was considered statistically significant. The MedCalc software (version 19.0.2) was used to prepare the subject receiver operating characteristic curve (ROC), and the area under the ROC curve (AUC) was calculated to evaluate the significance of each index in predicting the prognosis of patients.
There was no significant difference in age, sex, and GCS score at admission between the good outcome group (n = 20) and the poor outcome group (n = 18). However, the average ICP and BT values in the good outcome group were significantly lower than those in the poor outcome group (ICP: 13.50 ± 5.03 vs. 25.11 ± 5.09; p < 0.001; BT: 36.96 ± 0.69 vs. 38.15 ± 0.78; p < 0.001). The SjvO2 (67.46 ± 4.89 vs. 53.11 ± 11.18, p < 0.001) and StO2 (66.39 ± 3.15 vs. 53.11 ± 17.08, p < 0.004) in the good outcome group were significantly higher than those in the poor outcome group. The values of BVI and THI in the poor outcome group were significantly higher than those in the good outcome group (BVI: 265.66 ± 94.27 vs. 199.05 ± 68.82, p = 0.019; THI: 4.03 ± 1.34 vs. 2.75 ± 0.61, p < 0.001) as shown in Table 1.
Analyze the significance of different monitoring indicators such as CP, BT, SjvO2, and NIRS for poor prognosis of patients with SICH after operation. The results showed that the average postoperative ICP (AUC = 0.928; 95%CI 0.795–0.986; p < 0.001), BT (AUC = 0.871; 95%CI 0.722–0.957; p < 0.001); SjvO2 (AUC = 0.892; 95%CI 0.748–0.969; p < 0.001); StO2 (AUC = 0.828; 95%CI 0.671–0.931; p < 0.001), BVI (AUC = 0.725; 95%CI 0.556–0.857; p < 0.001); and THI (AUC = 0.792; 95%CI 0.629–0.906; p < 0.001). Combined with multiple indexes, it is very important to predict the poor prognosis of patients with SICH as shown in Table 2 and Figure 3.
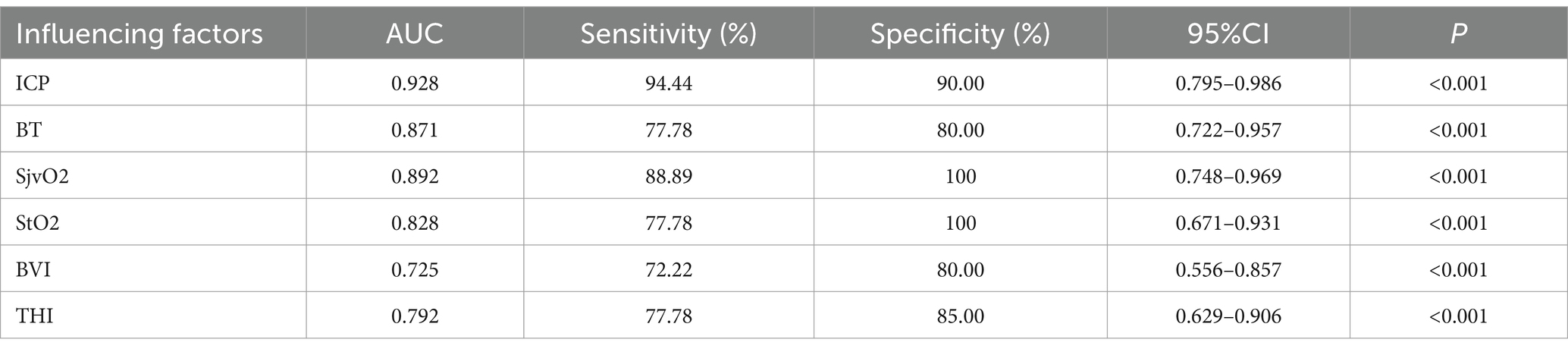
Table 2. The role of different monitor indexes in predicting the poor prognosis of patients with SICH.
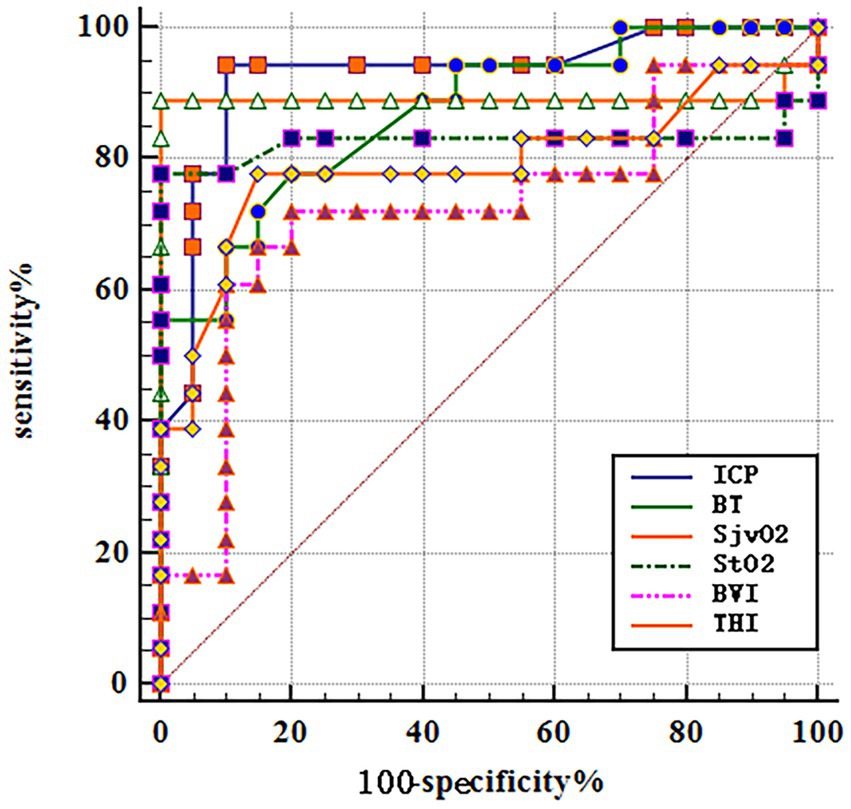
Figure 3. ROC curves for predicting the significance of different monitoring indicators in predicting poor prognosis in SICH patients based on relevant factors for predicting prognosis.
In recent years, a growing body of clinical evidence has demonstrated that multimodal neural monitoring, including parameters such as ICP, BT, and cerebral oxygen metabolism, plays a crucial role in mitigating the risks of secondary brain injury in individual patients and can serve as valuable predictors for assessing the prognosis after SICH (9, 10). Multimodal monitoring is an important reference index for the diagnosis and treatment of clinical neurosurgical diseases, offering valuable insights for the identification and determination of intracranial conditions. Multimodal monitoring can show the changes of various indexes continuously, dynamically, accurately, and intuitively, which has important guiding significance for the treatment of diseases with intracranial hypertension (11). The combined application of a variety of neural monitoring techniques to further integrate brain physiological data can precisely reflect the morbid response of the body, which is important for gaining a deeper understanding of the nature of the disease. Studies have shown that ICP, BT, and SjvO2 hold great importance in predicting the prognosis of TBI, and there are few studies assessing the use of NIRS in monitoring SICH. We aim to use this approach to predict the prognosis of patients after SICH, assessing its potential to improve prognosis and decrease mortality rates.
In recent years, the use of multimodal neural monitoring, including invasive methods such as ICP, BT, and SjvO2, has been suggested to be predictive of patient prognosis following SICH (12, 13). These methods, while highly accurate and specific, pose risks of complications like infection and thrombosis, and are not suitable for long-term monitoring (14). Our study explored the feasibility and advantages of NIRS as an alternative.
NIRS monitoring of StO2, BVI, and THI provides a noninvasive, continuous method to assess cerebral oxygenation and hemodynamics. In our study, NIRS-derived variables correlated well with patient outcomes, although their accuracy and specificity in predicting prognosis were slightly lower than those of invasive methods. Our findings suggest that NIRS can complement or potentially replace invasive methods in certain clinical scenarios. The absence of procedural complications and the capability for long-term monitoring are substantial advantages.
The NIRS technique is a powerful tool to evaluate brain autoregulation, vascular reactivity, and tissue oxygenation in patients. NIRS uses the transmission and absorption of near-infrared light in tissues to measure the relative ratio of oxyhemoglobin to deoxyhemoglobin. NIRS can distinguish complex hemodynamic patterns by simultaneously evaluating oxygenation and perfusion quality (StO2/BVI) parameters and oxygenated and deoxyhemoglobin (THI) distribution (15). StO2 measures cerebral oxygen content in mixed arteries and veins, which itself is affected by cerebral circulation, oxygen content, and oxygen extraction. Therefore, under the assumption that brain metabolism and oxygen transport are relatively constant, a fluctuation in StO2 reflects the local cerebral oxygen metabolism. Many studies have shown that a decrease of 20% or below to 60% in brain StO2 levels is associated with postoperative ischemic brain injury or re bleeding, and this threshold can serve as a reference indicator for clinical monitoring (16), which is consistent with the findings of our study. The average level of StO2 in the group with good prognosis was higher than that in the group with poor prognosis (66.39 ± 3.15 vs. 53.11 ± 17.08). In addition, among the three patients who died in the study, it was found that the significant difference in StO2 between bilateral cerebral hemispheres was associated with mortality. At present, there are no guidelines for measuring blood oxygen saturation in the brain tissues by NIRS. The guidelines issued by the Japanese Society of Cardiovascular Anesthesiologists highlight the efficacy of this noninvasive continuous monitoring technique in monitoring perfusion abnormalities during surgery. Our study showed that brain oxygen saturation monitoring was associated with the prognosis of patients, and the ROC curve showed an AUC of 0.828. It is of certain importance to predict the prognosis of patients with SICH. In the future, more research is required to determine the benefits of NIRS oxygenation monitoring in improving the outcomes related to the nervous system and other factors (17).
THI reflects tissue perfusion, and the increase in THI indicates cerebral vascular congestion (18). In our study, the level of THI in the poor prognosis group was higher than that in the good prognosis group (4.03 ± 1.34 vs. 2.75 ± 0.61). The increase in THI can be explained by the accumulation of red blood cells in the microcirculation of brain tissue, indicating perfusion disorders or rebleeding (19). The brain tissue is hypercongested, leading to reduced oxygen intake, thereby increasing the risk of recurrent cerebral hemorrhage and contributing to a poorer prognosis for the patient. The ROC curve showed an AUC of 0.792, indicating that THI in particular is of great importance in predicting the prognosis of patients. In this study, the BVI of the poor prognosis group was significantly higher than that of the good prognosis group (265.66 ± 94.27 vs. 199.05 ± 68.82; p = 0.019). High BVI in the poor prognosis group may be because of increased brain metabolism, increased cerebral blood flow, which may increase BT, and increased cerebral blood volume, which may increase ICP. Multiple factors interact with each other, resulting in poor neurological prognosis of patients. The ROC curve results showed that the AUC was 0.725, indicating that BVI had a potential utility in predicting the prognosis of SICH patients.
Our results suggest that NIRS monitoring techniques can be used to identify postoperative patients with SICH who exhibit persistent defects in brain tissue oxygenation and perfusion quality. The clinical relevance of StO2, BVI, and THI in different hemodynamic settings should be further evaluated in detail, as they may alert the brain tissue to underperfusion or overperfusion, causing irreversible neurological damage to the patient. Real-time noninvasive optical monitoring of tissue oxygenation levels through NIRS can provide meaningful clinical intervention to improve patient outcomes (20). NIRS could potentially meet ideal neurosurveillance requirements to detect brain tissue at risk of secondary damage and can complement or even replace the current invasive methods (21, 22). NIRS monitoring has several advantages including (1) noninvasive and real-time monitoring of abnormal brain tissue oxygenation status and (2) applicability in various scenarios, such as ICU, during transportation, or surgery (23, 24).
Multimodal monitoring can provide a therapeutic window for continuous prevention, early detection, and timely intervention of hypoxic/ischemic neuronal injury, thereby influencing clinical outcomes. Multimodal monitoring minimizes the limitations of each monitoring mode, and the components complement each other to improve the accuracy of the information obtained.
This study is a single-center study, and the difference in surgical levels in different hospitals might have a certain impact on the prognosis of patients. Moreover, due to various reasons, we could only observe the monitoring indicators 3 days after surgery. In future studies, we should extend the observation time to improve the accuracy of prediction. Furthermore, this is a retrospective study with a small sample size, our study was not suitable for conducting multivariable regression analysis, making it unclear which of the six variables we researched are truly associated with a poor prognosis. Although our sample size is relatively small, it is also limited to single center studies. Due to the limited research on the use of NIRS for monitoring SICH, our research results demonstrate the feasibility of using NIRS for monitoring postoperative SICH patients, which has good research significance and can provide support for future multi center studies.
The goal of postoperative rehabilitation for neurocritical illness is to prevent or mitigate secondary brain injury through prediction, early detection, or monitoring of treatment response. The multimodal monitoring method, combining ICP with NIRS, uses real-time measurement of brain pathophysiology information to comprehensively understand and monitor the brain after injury. This method holds high predictive value, effectively guiding clinical decision-making and treatment methods; the timely intervention facilitated by this approach greatly improves the surgical prognosis of patients with SICH.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
ZS: Writing – review & editing, Writing – original draft, Validation, Data curation. JL: Writing – review & editing. KW: Writing – review & editing. JZ: Writing – review & editing. SL: Writing – review & editing. FX: Writing – review & editing, Resources, Funding acquisition.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by 2023 Natural Science Basic Research Foundation of Shaanxi Province (2023-JC-YB-739).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chu, H, Huang, C, Zhou, Z, Tang, Y, Dong, Q, and Guo, Q. Inflammatory score predicts early hematoma expansion and poor outcomes in patients with intracerebral hemorrhage. Int J Surg. (2023) 109:266–76. doi: 10.1097/JS9.0000000000000191
2. An, SJ, Kim, TJ, and Yoon, B-W. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J Stroke. (2017) 19:3–10. doi: 10.5853/jos.2016.00864
3. Zhang, K, Wei, L, Zhou, X, Yang, B, Meng, J, and Wang, P. Risk factors for poor outcomes of spontaneous supratentorial cerebral hemorrhage after surgery. J Neurol. (2022) 269:3015–25. doi: 10.1007/s00415-021-10888-w
4. Rossi, J, Hermier, M, Eker, OF, Berthezene, Y, and Bani-Sadr, A. Etiologies of spontaneous acute intracerebral hemorrhage: a pictorial review. Clin Imaging. (2023) 95:10–23. doi: 10.1016/j.clinimag.2022.12.007
5. Peng, W-J, Li, Q, Tang, J-h, Reis, C, Araujo, C, Feng, R, et al. The risk factors and prognosis of delayed perihematomal edema in patients with spontaneous intracerebral hemorrhage. CNS Neurosci Ther. (2019) 25:1189–94. doi: 10.1111/cns.13219
6. Che, X-R, Wang, Y-j, and Zheng, H-y. Prognostic value of intracranial pressure monitoring for the management of hypertensive intracerebral hemorrhage following minimally invasive surgery. World J Emerg Med. (2020) 11:169–73. doi: 10.5847/wjem.j.1920-8642.2020.03.007
7. Song, K, Xu, Q, Koenig, HM, Kong, M, Slaughter, MS, Huang, Y, et al. Validation of a novel Neur Os cerebral oximetry monitor against the INVOS monitor during cardiac surgery. J Cardiothorac Vasc Anesth. (2021) 35:2009–18. doi: 10.1053/j.jvca.2020.10.043
8. Lindner, A, Rass, V, Ianosi, B-A, Schiefecker, AJ, Kofler, M, Gaasch, M, et al. Individualized blood pressure targets in the postoperative care of patients with intracerebral hemorrhage. J Neurosurg. (2021) 135:1656–65. doi: 10.3171/2020.9.JNS201024
9. Hutchinson, P, and O'Phelan, KParticipants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. International multidisciplinary consensus conference on multimodality monitoring: cerebral metabolism. Neurocrit Care. (2014) 21 Suppl 2:S148–58. doi: 10.1007/s12028-014-0035-3
10. Cesak, T, Adamkov, J, Habalova, J, Poczos, P, Kanta, M, Bartos, M, et al. The relationship between intracranial pressure and lactate/pyruvate ratio in patients with subarachnoid haemorrhage. Bratislavske lekarske listy. (2018) 119:139–42. doi: 10.4149/BLL_2018_027
11. Hemphill, JC 3rd, Greenberg, SM, Anderson, CS, Becker, K, Bendok, BR, Cushman, M, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2015) 46:2032–60. doi: 10.1161/STR.0000000000000069
12. Senapathi, TGA, Wiryana, M, Sinardja, K, Nada, KW, Sutawan, IBKJ, Ryalino, C, et al. Jugular bulb oxygen saturation correlates with full outline of responsiveness score in severe traumatic brain injury patients. Open Access Emerg Med. (2017) 9:69–72. doi: 10.2147/OAEM.S144722
13. Sun, Z, Liu, J, Dong, S, Duan, X, Xue, F, and Miao, X. Prognostic predictive value of intracranial pressure and cerebral oxygen metabolism monitoring in patients with spontaneous intracerebral hemorrhage. Acta Neurol Belg. (2023) 123:1815–21. doi: 10.1007/s13760-022-02037-5
14. Gomez, A, Griesdale, D, Froese, L, Yang, E, Thelin, EP, Raj, R, et al. Temporal statistical relationship between regional cerebral oxygen saturation (rSO2) and brain tissue oxygen tension (PbtO2) in moderate-to-severe traumatic brain injury: A Canadian high resolution-TBI (CAHR-TBI) cohort study. Bioengineering. (2023) 10:1124. doi: 10.3390/bioengineering10101124
15. Iller, M, Neunhoeffer, F, Heimann, L, Zipfel, J, Schuhmann, MU, Scherer, S, et al. Intraoperative monitoring of cerebrovascular autoregulation in infants and toddlers receiving major elective surgery to determine the individually optimal blood pressure – a pilot study. Front Pediatr. (2023) 11:1110453. doi: 10.3389/fped.2023.1110453
16. Thomas, R, Shin, SS, and Balu, R. Applications of near-infrared spectroscopy in neurocritical care. Neurophotonics. (2023) 10:023522. doi: 10.1117/1.NPh.10.2.023522
17. Ataş, İ, Ersunan, G, Bỉlỉr, Ö, Yavaşỉ, Ö, Altuntaş, M, and Karakullukçu, S. The utility of NIRS in follow-up of patients with acute ischaemic stroke treated with IV thrombolysis and mechanical thrombectomy in the emergency department. J Thromb Thrombolysis. (2023) 12:2920. doi: 10.1007/s11239-023-02920-9
18. Dietrich, M, Antonovici, A, Hölle, T, Nusshag, C, Kapp, A-C, Studier-Fischer, A, et al. Microcirculatory tissue oxygenation correlates with kidney function after transcatheter aortic valve implantation-results from a prospective observational study. Front Cardiovasc Med. (2023) 10:1108256. doi: 10.3389/fcvm.2023.1108256
19. Dietrich, M, Özdemir, B, Gruneberg, D, Petersen, C, Studier-Fischer, A, von der Forst, M, et al. Hyperspectral imaging for the evaluation of microcirculatory tissue oxygenation and perfusion quality in Haemorrhagic shock: a porcine study. Biomedicines. (2021) 9:1829. doi: 10.3390/biomedicines9121829
20. Park, JJ, Kim, C, and Jeon, JP. Monitoring of delayed cerebral ischemia in patients with subarachnoid hemorrhage via near-infrared spectroscopy. J Clin Med. (2020) 9:1595. doi: 10.3390/jcm9051595
21. Mathieu, F, Khellaf, A, Ku, JC, Donnelly, J, Thelin, EP, and Zeiler, FA. Continuous near-infrared spectroscopy monitoring in adult traumatic brain injury: a systematic review. J Neurosurg Anesthesiol. (2020) 32:288–99. doi: 10.1097/ANA.0000000000000620
22. Weigl, W, Milej, D, Janusek, D, Wojtkiewicz, S, Sawosz, P, Kacprzak, M, et al. Application of optical methods in the monitoring of traumatic brain injury: a review. J Cereb Blood Flow Metab. (2016) 36:1825–43. doi: 10.1177/0271678X16667953
23. Variane, GFT, Pietrobom, RFR, Noh, CY, van Meurs, KP, and Chock, VY. Newer indications for neuromonitoring in critically ill neonates. Front Pediatr. (2023) 11:1111347. doi: 10.3389/fped.2023.1111347
Keywords: spontaneous intracerebral hemorrhage, intracranial pressure, cerebral oxygen metabolic indexes, combined prognosis prediction, near-infrared spectroscopy
Citation: Sun Z, Liu J, Wang K, Zhang J, Liu S and Xue F (2024) Feasibility of noninvasive near-infrared spectroscopy monitoring in predicting the prognosis of spontaneous intracerebral hemorrhage. Front. Neurol. 15:1406157. doi: 10.3389/fneur.2024.1406157
Received: 24 March 2024; Accepted: 12 July 2024;
Published: 24 July 2024.
Edited by:
Jason J. Chang, MedStar Washington Hospital Center, United StatesReviewed by:
Pengyu Ren, The Second Affiliated Hospital of Xi’an Jiaotong University, ChinaCopyright © 2024 Sun, Liu, Wang, Zhang, Liu and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Xue, eGZ4MTEyOUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.