
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 26 June 2024
Sec. Dementia and Neurodegenerative Diseases
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1403567
This article is part of the Research TopicPost-Stroke Cognitive Decline and Dementia: Unraveling Mechanisms, Models, and BiomarkersView all 9 articles
Purpose: The aim was to investigate the associations between cognitive impairment and biopsychosocial factors among older stroke survivors and predictors of poststroke return to daily life.
Materials and methods: This cross-sectional study involved 117 stroke survivors (61% men) with an average age of 77 years (range 65–91). The participants completed two questionnaires (Riksstroke and Short Form 36 questionnaires). The Montreal Cognitive Assessment (MoCA) was used to assess cognitive abilities. The International Classification of Functioning, Disability, and Health (ICF) framework guided the selection of biopsychosocial variables. We used Spearman’s correlation coefficient and multiple logistic regression in the analyses.
Results: The average MoCA score was 21.7 points (range: 4–30, SD 5.6). The need for assistance from relatives and professionals, need for help with dressing and household chores, reliance on others for mobility, and reading and balance problems were correlated with more severe cognitive impairment (r = 0.20–0.33). Cognitive impairment, fatigue, and balance issues predicted an unfavorable return to daily life (odds ratio: 6.2–6.8).
Conclusion: The study indicated that cognitive impairment is associated with difficulties in all ICF domains. Cognitive impairment, fatigue, and balance issues are associated with an unsuccessful return to daily life. Prioritizing these factors and screening for cognitive impairment with objective assessment tools may improve rehabilitation outcomes and enhance overall quality of life poststroke.
Stroke is a leading cause of long-term disability worldwide, impacting the lives of more than 100 million individuals (1, 2). Stroke disrupts cerebral blood flow, causing tissue damage and neurological deficits. The neuroplasticity enables the brain to adapt and reorganize post-stroke, aiding recovery (3) but a common consequence of stroke is poststroke cognitive impairment (PSCI). PSCI is a broad term that includes conditions ranging from mild cognitive impairment to dementia. Common disorders are problems with memory, attention, executive abilities, language, visual processing, and information management (4, 5). Usually, PSCI occurs within 3 months post-stroke, but can also develop over time (3). One year after stroke onset, one in four stroke survivors has PSCI (6, 7), and approximately seven of 10 stroke survivors will develop PSCI over time (8). PSCI often gives negative consequences on problem solving, organized planning and social interaction (9, 10), which has a negative effect on individuals’ overall health and well-being. There is a prioritized and ongoing global effort to understand the complexity of PSCI and develop interventions to prevent cognitive decline (11). PSCI predominantly affects the older population, which is growing globally (11, 12), making it a priority in stroke research (11). An emerging issue in stroke research is investigating how cognitive function is connected to other biopsychosocial factors to develop personalized interventions (4).
Previous research has indicated that PSCI affects several biopsychosocial aspects. It affects the overall recovery process poststroke due to the negative impact on the body’s motor function recovery (13). PSCI makes it more challenging to participate in physical rehabilitation; therefore, persons with PSCI are less physically active (14). Individuals with PSCI are also more prone to psychological deficits such as depression and fatigue (15, 16), which further affects their ability to engage in various leisure and social activities (17). PSCI affects both basic and instrumental activities of daily living, such as dressing, performing personal hygiene and household chores, and cooking (18). It also impairs communication (19), participation in social activities and interactions with others (18, 20). Despite extensive PSCI research, the understanding of how different levels of PSCI affect biopsychosocial factors in older stroke survivors is incomplete. There is also limited research exploring which factors are associated with a successful return to daily life for older persons with PSCI. Previous studies investigating factors associated with return to daily life and leisure activities after stroke have shown that cognitive ability, age, and mobility are important factors (21, 22). However, most related studies have focused on younger stroke survivors (23, 24).
This study adopted a biopsychosocial perspective and used the International Classification of Functioning, Disability and Health (ICF) model (25) as a framework. This approach was chosen to capture the complexity of the interaction between PSCI and daily life. The study aimed to investigate (1) the associations between levels of cognitive impairment and biopsychosocial factors and (2) how these factors are associated with return to daily life. Through comprehensive insights into these biopsychosocial factors, poststroke well-being and recovery can be enhanced, allowing for tailored interventions for affected individuals.
This was a cross-sectional study with data from a stroke population in a medium-sized Swedish municipality. This study was part of the Kumla Stroke Study and received ethical approval from the Swedish Ethical Review Authority (Reference No. 2019-02359).
In the Kumla Stroke Study, a total of 330 people diagnosed with stroke and living in the Kumla municipality were identified as of December 31, 2019 (26). The Swedish Stroke Register (Riksstroke), which is a nationwide quality registry for stroke care (27), medical journals and the local health care center diagnostic register (28), were used to identify the participants. From these 330 persons, we included a subsample who (1) had completed the Montreal Cognitive Assessment (MoCA) (29) and (2) were ≥ 65 years old (Figure 1).
All the data were collected between October 2019 and June 2020. The Riksstroke (27) and Short-Form Health Survey (SF-36) (30) questionnaires were sent by mail and were completed by the participants themselves or with help from a family member. Participants could if they requested also receive support in completing the questionnaires by two physiotherapists, which also conducted the MoCA assessments, either at the local health care center or in the participant’s home. All questionnaires and assessment tools, which were used in the study, were the Swedish versions.
The International Classification of Functioning, Disability and Health (ICF) (25) is a biopsychosocial model from the World Health Organization (WHO). The ICF describes a person’s body functions, body structures, activity, participation, and contextual factors and is used as a framework to describe a person’s overall status of health and well-being. The variables used in this study were selected from the Riksstroke and SF-36 questionnaires to cover a wide range of biopsychosocial factors, which were mapped to ICF codes. ICF mapping rules were used (31) (Table 1).
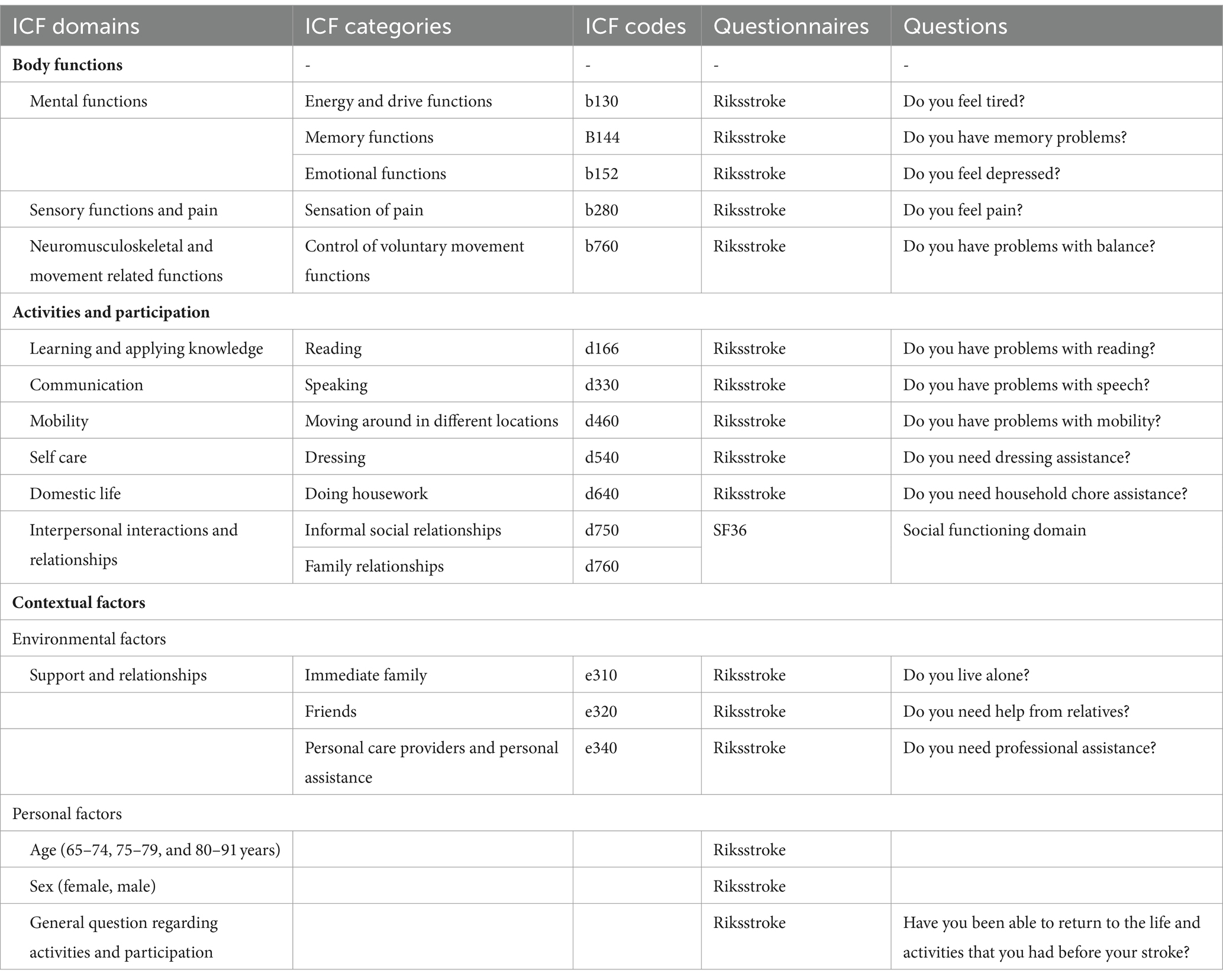
Table 1. Description of ICF domains, categories and codes of the Riksstroke and SF-36 questionnaires.
The Montreal Cognitive Assessment (MoCA) (29) is a screening instrument for cognitive impairment and has been validated for use in stroke survivors. The instrument evaluates attention, concentration, executive functions, memory, language ability, visuoconstructive ability, abstract thinking, numeracy, and orientation. The highest possible score is 30 points. Based on previous studies, the scores were categorized as follows: no cognitive impairment (24–30 points) (32), mild cognitive impairment (18–23 points), and severe cognitive impairment (1–17 points) (33).
The Riksstroke 1-year follow-up questionnaire is a Swedish questionnaire that has been validated for use in stroke survivors (27, 34). It consists of 46 questions regarding stroke survivors’ life situation, daily activities, and need for support and assistance. This study used 15 questions mainly concerning physical, psychological, and activity functions (Table 1). Two questions were converted to trichotomous variables (Do you feel tired? Do you feel depressed? Do you feel pain?) and two questions were converted to dichotomous variables (Do you need household assistance? Have you been able to return to the life and activities that you had before your stroke?).
The Short Form Health Survey 36 (SF-36) is a validated quality of life questionnaire consisting of 36 questions divided into eight domains (30). This study used the Social Function domain (SF) to describe a person’s ability to socialize; the SF domain consists of two questions: (I) During the past 4 weeks, to what extent has your physical health or emotional problems interfered with your usual interactions? and (2) During the last 4 weeks, how much of the time has your physical health or emotional problems interfered with your ability to socialize? (Table 1).
All analyses were conducted by using IBM SPSS statistics version 27 (IBM Corp., Armonk, NY, United States). Descriptive statistics describing the characteristics of the participants are presented as the mean, minimum, maximum, and standard deviation (SD). Spearman’s rank correlation coefficient (Rho) was used to analyze correlations between the independent variables in Table 1 and the dependent variable (cognitive impairment). Cohen’s levels of correlation were used (35).
Logistic regression was used to investigate predictors of return to daily life poststroke. The answer to the question “Have you been able to return to the life and activities that you had before your stroke?” was used as the dependent variable. Univariate logistic regression analysis was used to determine which independent variables should be included in the multiple regression analysis. A p value <0.05 was regarded as statistically significant. A test for multicollinearity with all the independent variables was conducted before the variables were included in the multiple regression analysis, and no substantial collinearity was found (tolerance >0.66 and variance inflation factor < 1.52).
The study sample consisted of 117 persons (61% men), and the average age was 77 years, with a range from 65 to 91 years (SD 6.1). Over half of the participants resided with a spouse, and none were living in care homes; all participants were living in regular housing (Table 2).
The average MoCA score was 21.7 points, with a range from 4 to 30 points (SD 5.6). The results of the MoCA indicated that nearly half of the participants (48.7%) had no cognitive impairment, more than a fourth of the participants (28.2%) had moderate cognitive impairment, and less than a fourth of the participants had severe cognitive impairment (23.1%). In terms of self-reported memory problems, 20% of the individuals without cognitive impairment reported having memory problems. In contrast, among the participants with moderate cognitive impairment, fewer than one-third reported memory problems, and among those with severe cognitive impairment, less than half reported memory issues. More than 80% of the participants experienced fatigue, and more than half-experienced pain and depression. Forty percent of the participants also had problems with balance and socializing. The need for assistance with household chores was the most common assistance need, and most of the assistance was provided by close relatives (Table 2).
Severe cognitive impairment was significantly associated with more difficulties in eight out of 16 biopsychosocial factors: dressing assistance, assistance from close relatives, assistance from professionals, reliance on others for mobility, household chore assistance, reading, balance, and memory problems (Table 2). All eight variables had positive correlations (Rho = 0.02–0.33, weak-moderate); that is, more severe cognitive impairment was associated with more difficulties in these areas. However, the association between cognitive level and balance was not linear; participants who had moderate PSCI had more problems with balance than did those with no PSCI or severe PSCI.
The univariate regression results (Table 3) showed that the biopsychosocial factors household chore assistance, fatigue, balance, cognitive impairment, speech, dressing assistance, reading, and mobility were significantly associated with return to daily life and activities (p ≤ 0.001–0.04).
According to our multiple regression analysis (Table 3), cognitive impairment, balance, and fatigue were identified as significant predictors of nonreturn to daily life for stroke survivors. The odds of returning to daily life and activities were 6.2–6.8 times greater for persons who never or rarely felt tired, had no problems with balance and had no cognitive impairment than for those who had problems in these areas. Less than 20% of the participants who had problems with balance, often or always felt tired or had severe cognitive impairment returned to daily life and activities after stroke.
The overall findings of this study indicate significant associations between cognitive impairment and various biopsychosocial factors across all ICF domains. While the weak to moderate correlations indicate that the relationships between these factors are not notably strong, this study’s findings still emphasize the importance of recognizing cognitive impairment in stroke care but also the need for early detection, considering its impact on stroke survivors’ return to daily life.
One of the aims of this study was to investigate how cognitive impairment was associated with biopsychosocial factors; severe cognitive impairment was most strongly associated with factors within the “Activity and participation” and “Contextual factors” ICF domains. The findings revealed that an increased need for assistance in tasks involving dressing, mobility, and household chores was associated with more severe cognitive impairment. This finding is in line with that of another study highlighting the association between an increased need for support in basic and instrumental activities of daily living and more severe cognitive impairment (36). Severe cognitive impairment was also associated with an increased demand for assistance from both family members and professionals. Earlier findings indicated that people with cognitive impairment required 2–5 times more support than did those without cognitive impairment (37). Our findings imply that cognitive impairment impacts a wide range of daily activities. By combining the ICF model with the MoCA, as demonstrated in this study, we were able to pinpoint the specific biopsychosocial factors most impacted by the severity of cognitive impairment. Understanding individual needs can facilitate the customization of stroke rehabilitation programs and enhance stroke survivors’ daily lives, as well as ease the burden on families and society.
The second aim of this study was to explore the associations between biopsychosocial factors and return to daily life. Return to daily life falls under the ICF—domain of “Activity and participation.” Return to daily life was associated with three factors: cognitive impairment, balance, and fatigue. These factors were all included in the ICF domain of “Body functions.” The first factor associated with an unsuccessful return to daily life was cognitive impairment, which often affects stroke survivors’ executive functions and has a negative impact on problem solving, planning, and social interaction, which are all essential factors for returning to daily life (9, 10). However, a similar study exploring predictors of return to daily activities poststroke found no associations with cognitive impairment (21). However, that study relied on participants’ self-assessments of their cognitive abilities, which may have influenced the results. In our study, using the MoCA, we revealed a significant difference between participants’ self-perceived memory problems and their actual MoCA results. Less than one-third of the participants with moderate cognitive impairment and less than half of the participants with severe cognitive impairment recognized their memory issues. If individuals fail to acknowledge cognitive problems, it becomes crucial for health care providers to step in. Previous research frequently highlights the tendency to overlook cognitive impairment poststroke in favor of physical concerns (4, 38). For instance, a study showed that 70% of stroke survivors discharged with clinical successful recovery according to modified Rankin Scale, and no apparent functional disability, yet demonstrated various cognitive deficits 3 months post-stroke (39). Studies also indicate that health care professionals struggle to detect and prioritize cognitive impairment within brief care periods (4, 5). In essence, recognizing the gap between self-perceptions and objective assessments underscores the urgent need for comprehensive cognitive evaluation in clinical stroke care practice but also the need to do cognitive assessment follow-ups.
Balance challenges were also significantly associated with an unsuccessful return to daily life. Another study revealed that the primary hindrance to resuming physical activity and daily activities was fear of falling due to balance issues (39). Moreover, avoiding situations perceived as posing a fall risk has emerged as the primary cause of the decrease in physical mobility, limited social participation, and reduced quality of life among stroke survivors (40, 41). This underscores the importance of considering balance challenges in a broader context and recognizing the wider psychosocial impact. Using a biopsychosocial approach and combining interventions that provide support regarding both balance and psychological issues may therefore enhance overall recovery for stroke survivors and improve their ability to return to daily life.
In this study, experiencing fatigue emerged as the final factor associated with an unsuccessful return to daily life. A total of 85% of the participants experienced fatigue poststroke, which aligns with previous findings (42). Poststroke fatigue notably affects quality of life and limits individuals’ participation in various daily activities (43). Research has also indicated that stroke survivors with both reduced physical abilities and fatigue have more difficulties returning to previous leisure activities (22). Promoting physical activity, particularly aerobic exercise, is a promising treatment for reducing poststroke fatigue (42). Furthermore, this form of physical activity has also been shown to be effective at preventing or delaying cognitive decline (44, 45); therefore, incorporating this approach in stroke rehabilitation may be beneficial.
We found no association between older age and more severe cognitive impairment or an unfavorable return to daily life. The results of this study contradict those of several other studies that highlight that older age is a strong risk factor for poststroke cognitive impairment (12, 46, 47) and a more challenging return to daily life (21). An explanation for the contradictory result may be that this study focused on stroke survivors aged 65 years and older, which aligns with the retirement age in Sweden. The narrow age range, from 65 to 91 years, might not have been broad enough to impact the results. Alternatively, people with PSCI older than 65 years of age may face similar challenges, regardless of their specific age.
In summary, this study highlights the complex dynamics between cognitive impairment and various biopsychosocial factors impacting daily life poststroke. These findings highlight the importance of screening for cognitive impairment to customize stroke rehabilitation programs and incorporate a broader biopsychosocial perspective in rehabilitation programs.
When interpreting these study results, it is important to consider aspects that might have impacted the study’s outcomes. The lack of homogeneity in stroke severity and years lived with stroke among the participants could have influenced the findings. For example, the participants might have experienced difficulties recalling their return to daily life poststroke, particularly if the stroke had occurred several years ago. The participants had various levels of cognitive impairment, which may affect their ability to answer questions about how the stroke impacted their biopsychosocial and activity performance, especially for those with severe impairment. To ensure the accuracy in the responses, participants could receive support from a family member or a trained research assistant when completing the questionnaires. However, it is important to acknowledge that the participant’s answers are self-reported and should be interpreted considering their cognitive impairment. Furthermore, a cross-sectional study provides only a snapshot of stroke survivors’ lives at a specific point in time. This study may provide a plausible picture of the comprehensive challenges faced by older stroke survivors coping with cognitive impairment today. A notable strength of this study was the use of an objective cognitive assessment tool. This decision ensured a more accurate and reliable evaluation of cognitive impairment, strengthening the robustness of our study’s findings. Additionally, by using the ICF framework, this study comprehensively analyzed a spectrum of biopsychosocial factors, offering a broad perspective on poststroke cognitive impairment. The use of the ICF framework may also facilitate future valuable comparisons with other studies that share a similar analytical framework.
Our findings indicate that cognitive impairment is associated with all ICF domains, and that cognitive impairment, fatigue, and balance predict an unfavorable return to daily life and activities. Notably, we identified a higher prevalence of cognitive impairment than what individuals themselves perceived, highlighting the critical need to prioritize its detection by using objective assessment tools. These findings may provide valuable insights for clinical practice by underscoring the importance of addressing a wide range of biopsychosocial factors associated with cognitive impairment for older stroke survivors understanding these factors can help healthcare professional prioritize interventions and improve rehabilitation outcomes. Furthermore, our research emphasizes the necessity of regular screening for cognitive impairment, allowing for tailored interventions to meet each individual’s specific needs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Swedish Ethical Review Authority. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AB: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. MA: Writing – original draft, Writing – review & editing. GJ: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Swedish Stroke Association (STROKE- Riksförbundet) and Region Örebro County, Sweden.
We extend our sincere gratitude to Peter Appelros, an associate professor, for his expertise and guidance, Sofia Kangedal, a physiotherapist, for her invaluable assistance during the data collection, and all the participants of this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Feigin, VL, Brainin, M, Norrving, B, Martins, S, Sacco, RL, Hacke, W, et al. World stroke organization (WSO): global stroke fact sheet 2022. Int J Stroke. (2022) 17:18–29. doi: 10.1177/17474930211065917
3. Kalaria, RN, Akinyemi, R, and Ihara, M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta. (2016) 1862:915–25. doi: 10.1016/j.bbadis.2016.01.015
4. McDonald, MW, Black, SE, Copland, DA, Corbett, D, Dijkhuizen, RM, Farr, TD, et al. Cognition in stroke rehabilitation and recovery research: consensus-based Core recommendations from the second stroke recovery and rehabilitation roundtable. Neurorehabil Neural Repair. (2019) 33:943–50. doi: 10.1177/1545968319886444
5. Cumming, TB, Marshall, RS, and Lazar, RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke. (2013) 8:38–45. doi: 10.1111/j.1747-4949.2012.00972.x
6. Pendlebury, ST, and Rothwell, PM. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford vascular study. Lancet Neurol. (2019) 18:248–58. doi: 10.1016/S1474-4422(18)30442-3
7. Sexton, E, McLoughlin, A, Williams, DJ, Merriman, NA, Donnelly, N, Rohde, D, et al. Systematic review and meta-analysis of the prevalence of cognitive impairment no dementia in the first year post-stroke. Eur Stroke J. (2019) 4:160–71. doi: 10.1177/2396987318825484
8. Kuźma, E, Lourida, I, Moore, SF, Levine, DA, Ukoumunne, OC, and Llewellyn, DJ. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. (2018) 14:1416–26. doi: 10.1016/j.jalz.2018.06.3061
9. Ho, A, Nicholas, ML, Dagli, C, and Connor, LT. Apathy, cognitive impairment, and social support contribute to participation in cognitively demanding activities Poststroke. Behav Neurol. (2021) 2021:1–8. doi: 10.1155/2021/8810632
10. Chung, CS, Pollock, A, Campbell, T, Durward, BR, and Hagen, S. Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non-progressive acquired brain damage. Cochrane Database Syst Rev. (2013) 2013:Cd008391. doi: 10.1002/14651858.CD008391.pub2
11. Rost, NS, Brodtmann, A, Pase, MP, van Veluw, SJ, Biffi, A, Duering, M, et al. Post-stroke cognitive impairment and dementia. Circ Res. (2022) 130:1252–71. doi: 10.1161/CIRCRESAHA.122.319951
12. Lo Coco, D, Lopez, G, and Corrao, S. Cognitive impairment and stroke in elderly patients. Vasc Health Risk Manag. (2016) 12:105–16. doi: 10.2147/VHRM.S75306
13. Mancuso, M, Iosa, M, Abbruzzese, L, Matano, A, Coccia, M, Baudo, S, et al. The impact of cognitive function deficits and their recovery on functional outcome in subjects affected by ischemic subacute stroke: results from the Italian multicenter longitudinal study CogniReMo. Eur J Phys Rehabil Med. (2023) 59:284–93. doi: 10.23736/S1973-9087.23.07716-X
14. Viktorisson, A, Andersson, EM, Lundström, E, and Sunnerhagen, KS. Levels of physical activity before and after stroke in relation to early cognitive function. Sci Rep. (2021) 11:9078. doi: 10.1038/s41598-021-88606-9
15. van Rijsbergen, MWA, Mark, RE, Kop, WJ, de Kort, PLM, and Sitskoorn, MM. Psychological factors and subjective cognitive complaints after stroke: beyond depression and anxiety. Neuropsychol Rehabil. (2019) 29:1671–84. doi: 10.1080/09602011.2018.1441720
16. Douven, E, Köhler, S, Schievink, SHJ, van Oostenbrugge, RJ, Staals, J, Verhey, FRJ, et al. Temporal associations between fatigue, depression, and apathy after stroke: results of the cognition and affect after stroke, a prospective evaluation of risks study. Cerebrovasc Dis. (2017) 44:330–7. doi: 10.1159/000481577
17. Björkdahl, A . Cognitive Rehabilitation-Theoretical Basis and Practical Application. Lund: Studentlitteratur (2015).
18. Stolwyk, RJ, Mihaljcic, T, Wong, DK, Chapman, JE, and Rogers, JM. Poststroke cognitive impairment negatively impacts activity and participation outcomes: a systematic review and Meta-analysis. Stroke. (2021) 52:748–60. doi: 10.1161/STROKEAHA.120.032215
19. Abualait, TS, Alzahrani, MA, Ibrahim, AI, Bashir, S, and Abuoliat, ZA. Determinants of life satisfaction among stroke survivors 1 year post stroke. Medicine (Baltimore). (2021) 100:e25550. doi: 10.1097/MD.0000000000025550
20. Erler, KS, Sullivan, V, McKinnon, S, and Inzana, R. Social support as a predictor of community participation after stroke. Front Neurol. (2019) 10:1013. doi: 10.3389/fneur.2019.01013
21. Singam, A, Ytterberg, C, Tham, K, and von Koch, L. Participation in complex and social everyday activities six years after stroke: predictors for return to pre-stroke level. PLoS One. (2015) 10:e0144344. doi: 10.1371/journal.pone.0144344
22. Harrison, J, Thetford, C, Reeves, MJ, Brown, C, Joshi, M, and Watkins, C. Returning to leisure activity post-stroke: barriers and facilitators to engagement. Int J Environ Res Public Health. (2022) 19:14587. doi: 10.3390/ijerph192114587
23. Mani, K, Cater, B, and Hudlikar, A. Cognition and return to work after mild/moderate traumatic brain injury: a systematic review. Work. (2017) 58:51–62. doi: 10.3233/WOR-172597
24. Fride, Y, Adamit, T, Maeir, A, Ben Assayag, E, Bornstein, NM, Korczyn, AD, et al. What are the correlates of cognition and participation to return to work after first ever mild stroke? Top Stroke Rehabil. (2015) 22:317–25. doi: 10.1179/1074935714Z.0000000013
25. WHO . International Classification of Functioning, Disability and Health: ICF. Geneva: World Health Organisation (2003).
26. Appelros, P, Matérne, M, Jarl, G, and Arvidsson-Lindvall, M. Comorbidity in stroke-survivors: prevalence and associations with functional outcomes and health. J Stroke Cerebrovasc Dis. (2021) 30:106000. doi: 10.1016/j.jstrokecerebrovasdis.2021.106000
27. RIKS-STROKE TSSR (2021). The Swedish stroke register. Stockholm: RIKS-STROKE TSSR. Available at: https://www.riksstroke.org/sve/ (Accessed January 1, 2023).
28. Appelros, P, Arvidsson-Lindvall, M, and Matérne, M. Stroke prevalence in a medium-sized Swedish municipality. Acta Neurol Scand. (2021) 143:210–6. doi: 10.1111/ane.13357
29. Nasreddine, ZS, Phillips, NA, Bédirian, V, Charbonneau, S, Whitehead, V, Collin, I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
30. Persson, LO, Karlsson, J, Bengtsson, C, Steen, B, and Sullivan, M. The Swedish SF-36 health survey II. Evaluation of clinical validity: results from population studies of elderly and women in Gothenborg. J Clin Epidemiol. (1998) 51:1095–103. doi: 10.1016/S0895-4356(98)00101-2
31. Cieza, A, Geyh, S, Chatterji, S, Kostanjsek, N, Ustün, B, and Stucki, G. ICF linking rules: an update based on lessons learned. J Rehabil Med. (2005) 37:212–8. doi: 10.1080/16501970510040263
32. Ciesielska, N, Sokołowski, R, Mazur, E, Podhorecka, M, Polak-Szabela, A, and Kędziora-Kornatowska, K. Is the Montreal cognitive assessment (MoCA) test better suited than the Mini-mental state examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. (2016) 50:1039–52. doi: 10.12740/PP/45368
33. Trzepacz, PT, Hochstetler, H, Wang, S, Walker, B, and Saykin, AJ. Relationship between the Montreal cognitive assessment and Mini-mental state examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. (2015) 15:107. doi: 10.1186/s12877-015-0103-3
34. Palmcrantz, S, and Sommerfeld, DK. Development and validation of the Swedish national stroke register Riksstroke's questionnaires in patients at 3 and 12 months after stroke: a qualitative study. BMJ Open. (2018) 8:e018702. doi: 10.1136/bmjopen-2017-018702
35. Cohen, J . Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates (1988).
36. Ghaffari, A, Rostami, HR, and Akbarfahimi, M. Predictors of instrumental activities of daily living performance in patients with stroke. Occup Ther Int. (2021) 2021:1–7. doi: 10.1155/2021/6675680
37. SNAC-project . Informal and Formal Care-Older People Living in Ordinary Recidence, Sweden 2001–2015. Stockholm: Socialstyrelsen (2020).
38. Stigen, L, Bjørk, E, and Lund, A. The conflicted practice: municipal occupational therapists' experiences with assessment of clients with cognitive impairments. Scand J Occup Ther. (2019) 26:261–72. doi: 10.1080/11038128.2018.1445778
39. Jokinen, H, Melkas, S, Ylikoski, R, Pohjasvaara, T, Kaste, M, Erkinjuntti, T, et al. Post-stroke cognitive impairment is common even after successful clinical recovery. Eur J Neurol. (2015) 22:1288–94. doi: 10.1111/ene.12743
40. Liu, TW, and Ng, SSM. The reliability and validity of the survey of activities and fear of falling in the elderly for assessing fear and activity avoidance among stroke survivors. PLoS One. (2019) 14:e0214796. doi: 10.1371/journal.pone.0214796
41. Liu, TW, Ng, SS, Kwong, PW, and Ng, GY. Fear avoidance behavior, not walking endurance, predicts the community reintegration of community-dwelling stroke survivors. Arch Phys Med Rehabil. (2015) 96:1684–90. doi: 10.1016/j.apmr.2015.05.005
42. Hinkle, JL, Becker, KJ, Kim, JS, Choi-Kwon, S, Saban, KL, McNair, N, et al. Poststroke fatigue: emerging evidence and approaches to management: a scientific statement for healthcare professionals from the American Heart Association. Stroke. (2017) 48:e159–70. doi: 10.1161/STR.0000000000000132
43. De Vries, EA, Boerboom, W, Van den Berg-Emons, RJG, Van Kooten, F, Visser-Meily, JMA, Ribbers, GM, et al. Fatigue is associated with reduced participation and health-related quality of life five years after Perimesencephalic subarachnoid Haemorrhage: a multicentre cross-sectional study. J Rehabil Med. (2022) 54:jrm00271. doi: 10.2340/jrm.v54.212
44. Hsu, CL, Best, JR, Davis, JC, Nagamatsu, LS, Wang, S, Boyd, LA, et al. Aerobic exercise promotes executive functions and impacts functional neural activity among older adults with vascular cognitive impairment. Br J Sports Med. (2018) 52:184–91. doi: 10.1136/bjsports-2016-096846
45. Liang, YJ, Su, QW, Sheng, ZR, Weng, QY, Niu, YF, Zhou, HD, et al. Effectiveness of physical activity interventions on cognition, neuropsychiatric symptoms, and quality of life of Alzheimer's disease: an update of a systematic review and meta-analysis. Front Aging Neurosci. (2022) 14:830824. doi: 10.3389/fnagi.2022.830824
46. Sachdev, PS, Brodaty, H, Valenzuela, MJ, Lorentz, L, Looi, JC, Berman, K, et al. Clinical determinants of dementia and mild cognitive impairment following ischaemic stroke: the Sydney stroke study. Dement Geriatr Cogn Disord. (2006) 21:275–83. doi: 10.1159/000091434
Keywords: daily life, stroke, international classification of functioning disability and health (ICF), montréal cognitive assessment (MoCA), cognition
Citation: Björck A, Matérne M, Arvidsson Lindvall M and Jarl G (2024) Investigating cognitive impairment, biopsychosocial barriers, and predictors of return to daily life among older stroke survivors. Front. Neurol. 15:1403567. doi: 10.3389/fneur.2024.1403567
Received: 11 April 2024; Accepted: 13 June 2024;
Published: 26 June 2024.
Edited by:
Paco Herson, The Ohio State University, United StatesReviewed by:
Katsuya Sakai, Tokyo Metropolitan University, JapanCopyright © 2024 Björck, Matérne, Arvidsson Lindvall and Jarl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandra Björck, YWxleGFuZHJhLmJqb3Jja0BvcnUuc2U=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.