
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol., 05 July 2024
Sec. Neuro-Otology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1403536
This article is part of the Research TopicPaediatric vestibular disorders – a focussed diagnostic approach for best management outcomesView all 7 articles
Vertiginous epilepsy (VE) is a rare and underrecognized epilepsy subtype in the pediatric population. Vertiginous symptoms are the sole or predominant feature, arise from the vestibular cortex, and seizures are usually brief. The incidence is estimated to be between six and 15 percent of pediatric patients presenting with dizziness. Diagnosis is often delayed for many years following the onset of symptoms, as there are no widely accepted diagnostic criteria. Diagnostic work-up should include a detailed history, physical exam, EEG, and brain imaging with MRI. Vestibular testing is helpful if peripheral vestibulopathy is suspected. Vertiginous epilepsy can have many possible causes, but a large majority are idiopathic or suspected to be genetic. Most patients with vertiginous epilepsy achieve seizure freedom with anti-seizure medications.
• VE originates from the known vestibular cortex, specifically parts of the parietal operculum and the posterior insular; symptoms arise from involvement of the multi-sensory vestibular cortical network.
• While vertigo is a hallmark symptom, VE can be differentiated from non-epileptic vestibular disorders (RVC, VMC, and vestibular paroxysmia) based on lack of migraine history/features, brief vestibular symptom duration, and alterations in consciousness.
• Work-up should include a detailed history, EEG, and imaging, as ruling out other causes is crucial for diagnosis.
• VE typically responds well to anti-epileptic medication, with the choice depending on seizure type and individual factors.
In 1977, pediatric neurologists Eviatar and Eviatar reported epilepsy as the most common cause of vertigo in children referred to their clinic (1). Benign Paroxysmal Vertigo of Childhood (BPVC) and Vestibular Migraine of Childhood (VMC) are now widely recognized as the most common causes of pediatric vertigo/dizziness (2–5). On the other hand, vertiginous epilepsy (VE) is an under-recognized cause of dizziness in children. Vertiginous epilepsy (VE) is a specific epilepsy characterized by focal seizures with vestibular symptoms as either the sole or predominant feature. A systematic review by Tarnutzer and colleagues reported children being diagnosed with vertiginous epilepsy 8.7 times more than adults (6). Although isolated vestibular symptoms are rare in those with epilepsy (<0.5%), the current estimates of the incidence of VE among those presenting with dizziness in the pediatric population range between 6.6 to 15% (7–9), likely secondary to the high incidence of epilepsy in pediatric patients (10–12).
Vertiginous epilepsy (VE), also referred to as epileptic vertigo or dizziness (EVD), vestibular epilepsy, or vestibular seizures can be challenging to diagnose, as symptoms of the seizure can manifest as an initial aura of focal or generalized seizures or as an isolated symptom. Hewett and colleagues reported an average of a four-year delay in VE diagnosis following the onset of symptoms (13). Diagnosing VE in the pediatric population is challenging owing to a lack of clear communication skills among children (and their parents) to describe symptoms and difficulty performing vestibular function tests. Such challenges require a unique diagnostic approach. This mini-review provides an up-to-date overview for understanding, diagnosing, and managing VE in the pediatric population.
While focal seizures arising from any brain region may be accompanied by vestibular symptoms if the epileptogenic or symptomatic zone involves the vestibular cortex or its pathways, in VE, the origin of the seizure is presume to be located in the cortex responsible for vestibular semiology (14, 15). Work by Penfield and Jasper (16) as well as Kahane et al. (17) established that vestibular sensations could arise from electrical stimulation of the lateral temporo-parietal cortex (i.e., temporo-peri-Sylvian vestibular cortex). More recently, the vestibular cortex has been described as a distinct network involved in processing multi-sensory vestibular information (15), whereby symptoms may arise from a single region within the vestibular cortex or from the spread of electrical activity to or from nearby regions. While the vestibular cortex has been investigated using primate models (18–24), studies using electrical cortical stimulation and neuroimaging have mapped the human vestibular cortical network to include the parietal operculum and posterior insula primarily (25–28). Additionally, a wide array of vestibular symptoms have been elicited by electrical stimulation of subsites within the temporal and parietal areas, including the superior temporal gyrus, the angular gyrus, the supramarginal gyrus, the hippocampus, the cingulate gyrus, and the precuneus (Figure 1).
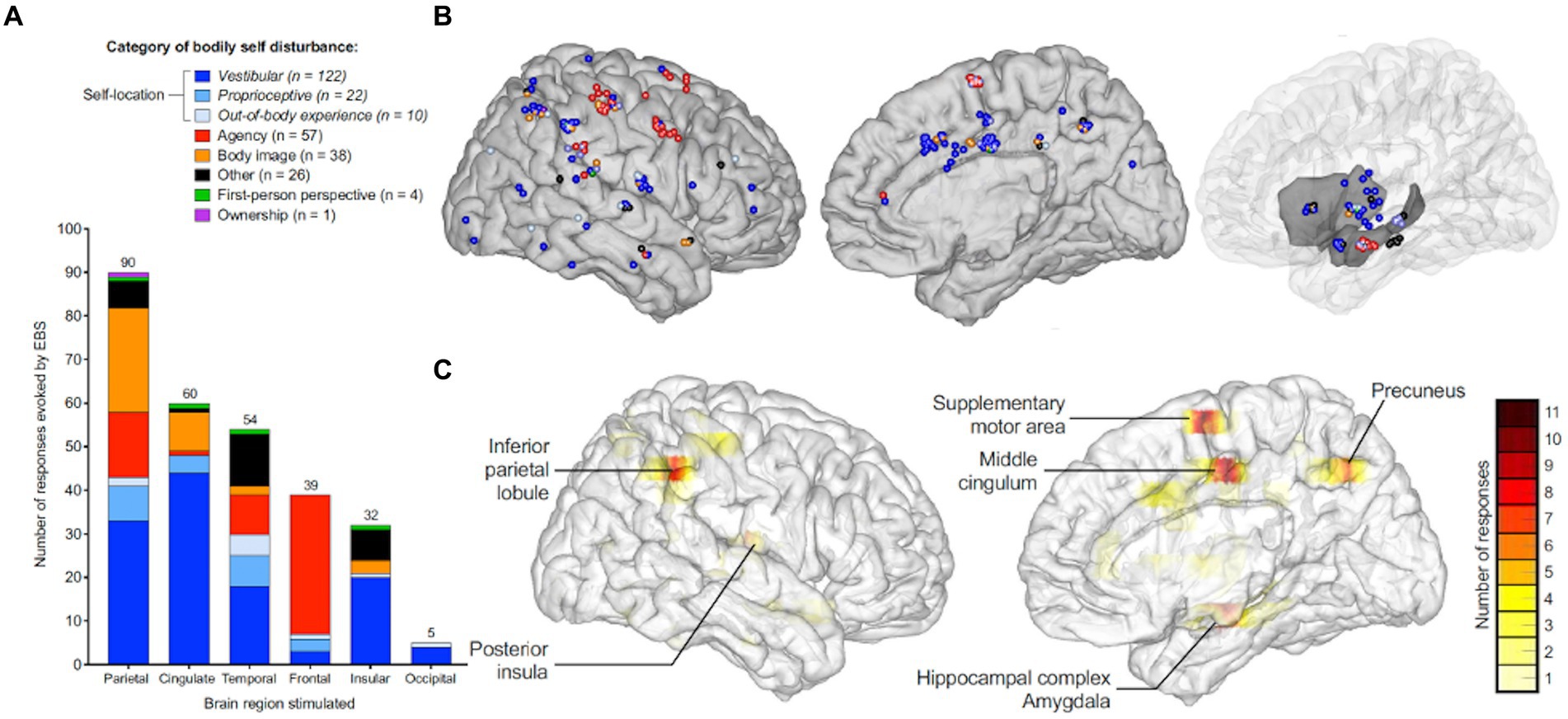
Figure 1. Anatomic and symptom distribution and representation of the areas associated with the multi-sensory vestibular cortical network based on a systematic review of localization studies using intracranial electrical brain stimulation evoking vestibular symptoms. (A) Distribution of reported frequencies in various bodily self disturbances based on stimulated brain region. (B) Color-coded sites within the right cerebral hemisphere indicating the category of bodily self disturbance upon iEBS. (C) Color-coded density maps representing the six main cortical areas eliciting bodily self disturbances on iEBS, based on frequency of response. iEBS= Intra-cranial electrical brain stimulation. Adapted with permission from “Neural bases of the bodily self as revealed by electrical brain stimulation: A systematic review” by Dary et al. (29) in Human Brain Mapping, licensed under CC-BY-NC-ND 4.0 license, published by Wiley Periodicals, LLC.
Before referral to Neurology, children and adolescents with dizziness are often seen in the primary care setting or evaluated by a subspecialist, such as a Pediatric Otolaryngologist or Neurotologist (30–32). As a common chief complaint, “dizziness” is a blanket term for altered spatial orientation that can include vertigo, disequilibrium, imbalance confusion, lightheadedness, or spatial discomfort and should be clarified by the clinician. “Vertigo,” a hallmark symptom of vestibular dysfunction, is defined as experiencing rotatory sensations (self-motion or visual) or other illusions of motion, such as tilting, floating, rocking, rolling, or a sense of falling forward or backward. Vertigo is the sole or primary symptom in VE, lasting seconds to minutes, though the pediatric patient may present with other symptoms (6). Batu et al. (8) reported the following associated symptoms in those diagnosed with VE (15%) among their retrospective analysis of 100 children presenting to their Pediatric Neurology clinic: headache (20%), nausea (26.6%), vomiting (20%), pallor (13.3%), staring (13.3%), and blackout (6.6%). Among these symptoms, staring was the only significant differentiating symptom between VE and other common causes of vertigo in children, such as BPVC, VM, and psychogenic vertigo (8). Due to these findings, it has been suggested that any changes in cognitive function or alterations in consciousness that accompany vertigo in the pediatric population should prompt the clinician to investigate for possible VE (Figure 2) (8, 35). Positional vertigo or hearing loss do not support the diagnosis of VE though auditory hallucinations (e.g., pure tone tinnitus, hearing words/sentences) may occur with temporal lobe activity. Therefore, VE should be considered in a patient with episodic dizziness complaints and a family history of seizures.
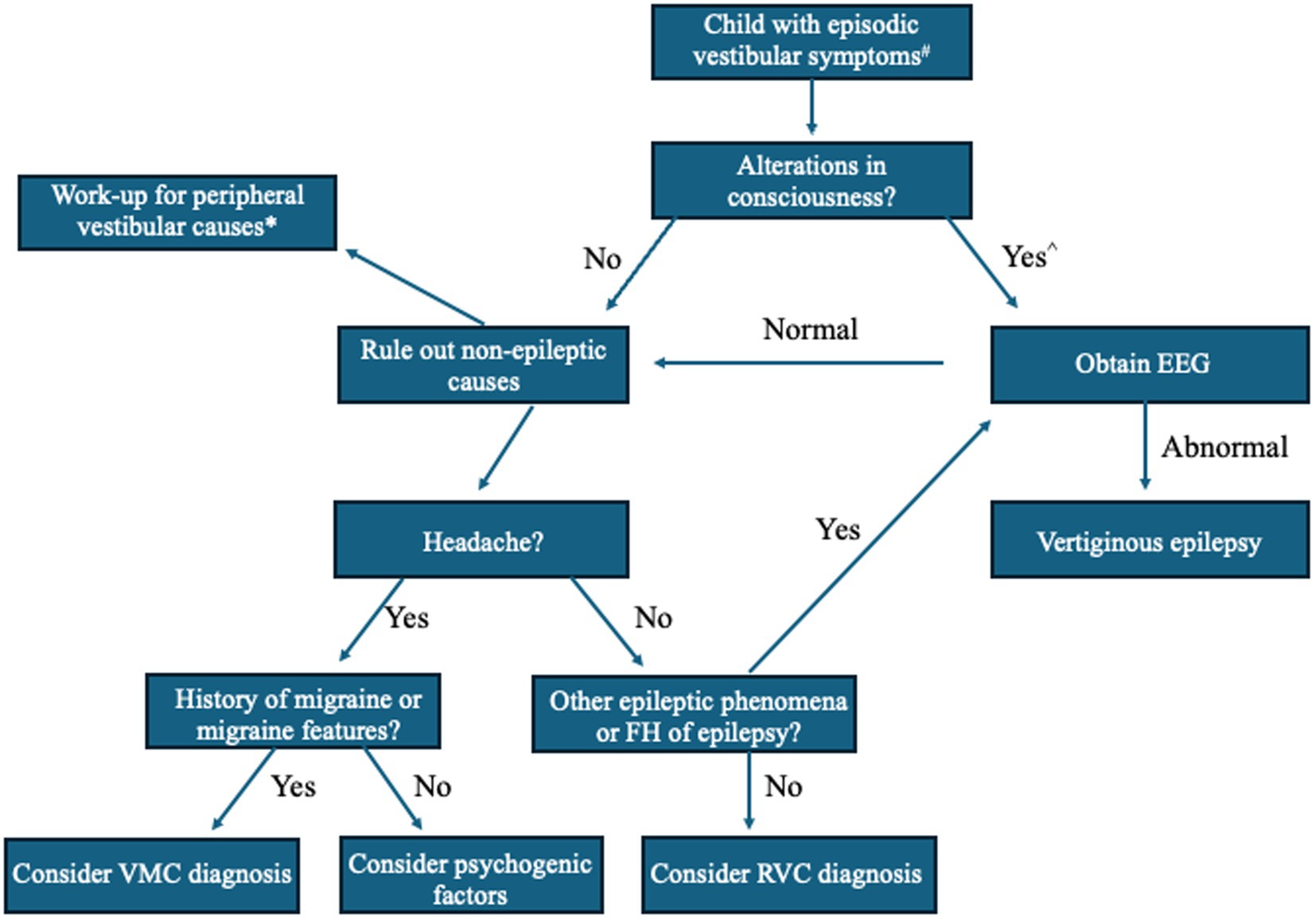
Figure 2. A simplified algorithm for evaluation of vertigo in children suspected of having vertiginous epilepsy (VE). Adapted from Batu et al. European Journal of Pediatric Neurology. 2015 (8), Dasgupta et al. Current Treatment Options in Neurology. 2020 (33), and Peterson and Brodsky, Curr Opin Otolaryngol Head Neck Surg. 2022 (34). # = If focal neurological findings or history of head trauma, obtain brain MRI or head CT. ^ = Perform orthostatic blood pressure measurements, consider ophthalmological exam and electrocardiogram (ECG) or bloodwork (e.g., thyroid function, complete blood cell count, electrolytes). * = Peripheral vestibular disorder work-up should focus on duration and triggers of vestibular episodes, otologic, neurologic, and oculomotor examinations (i.e., rule out middle ear disease, third window phenomena, or nystagmus), and consider vestibular function tests based on age and test tolerance, such as video head-impulse (vHIT), rotatory chair (ROT), and vestibular-evoked myogenic potential (VEMP) testing. EEG, electroencephalography; FH, family history; VMC, vestibular migraine of childhood; RVC, recurrent vertigo of childhood.
There are no established criteria for diagnosing patients with VE. Diagnosis therein relies on a detailed description of episodes, detecting abnormal sleep-deprived electroencephalography (EEG) findings, and ruling out other causes of the origin of epileptic discharge. History-taking should attempt to characterize the vestibular and associated symptoms, including motor activity, with a focus on the episode’s duration, possible triggers, and loss of consciousness (LOC). Physical examination may reveal central findings on oculomotor testing such as abnormal saccades, spontaneous or dynamically-provoked nystagmus that does not fatigue nor suppress with fixation, or abnormal smooth pursuit. An MRI with gadolinium contrast may be ordered to rule out structural etiologies related to symptoms (e.g., infarct, tumor) but is rarely performed (6), likely due to reported low diagnostic yield in the work-up of pediatric vertigo and the challenges of ordering such imaging (e.g., cost, required sedation in younger children) (8, 13). Any patients with LOC should undergo a cardiac work-up, including an electrocardiogram (EKG), an echocardiogram (ECHO), 24 h Holter monitoring, orthostatic vital sign measure, or tilt table testing. Vestibular function testing may rule out peripheral vestibular dysfunction if suspected on clinical history (i.e., episodic positional vertigo lasting seconds to minutes) and/or bedside exam (e.g., positive Romberg). However, abnormal findings on these tests have been reported in children with VE, possibly secondary to the effects of anti-seizure medications (ASM) on the peripheral vestibular system (36). As such, a thorough medication history should screen for off-label use of ASMs in the patient without known epilepsy (e.g., migraine). Otologic examination and audiologic testing are expected to be unremarkable in VE but may be warranted if the patient reports any ear-related symptoms other than vertigo, such as otalgia, otorrhea, or hearing disturbance. Laboratory testing is typically ordered to rule out any metabolic disturbance that may elicit seizures, such as hyponatremia. Genetic testing may be warranted with appropriate counseling if a family history is positive for epilepsy.
One would expect interictal EEG in patients with VE to reveal epileptic discharges within the temporal–parietal-occipital regions, given their participation in the processing of vestibular input (Figure 1) (6, 37, 38). However, Currie and colleagues reported that only 19% of patients with temporal lobe epilepsy presented with vertiginous symptoms alone (39). Indeed, in their retrospective review of 190 pediatric stereoelectroecenphalographies (SEEGs), Taussig and colleagues found that electrical stimulation of the precuneus, the insular region, the anterior temporal operculum, and the retro-insular region/parietal operculum elicited vertiginous symptoms (40). Vestibular symptoms arising from electrical activity within multiple cortical areas underscore current theories supporting the vestibular system’s widespread, multi-sensory cortical representation (28). Additionally, as epileptiform discharge may escape detection during routine scalp EEG (41), especially given the brief duration of vertiginous symptoms, 24 h EEG can be considered to establish the diagnosis before starting ASM.
Epileptic nystagmus (EN), defined as rapid, involuntary repetitive eye movements, is rare as a sole finding in the setting of VE (42). Case reports of EN in the pediatric population report the nystagmus as predominately binocular and horizontal, with the rapid phase of nystagmus contralateral to the epileptic focus noted on EEG. Ictal discharges have been reported in temporal–parietal-occipital regions, likely related to their role in coordinating smooth-pursuit or saccadic eye movements (43, 44).
The term “vestibulogenic epilepsy” refers to seizures evoked by vestibular stimuli and is a separate entity from VE (45, 46). Vestibulogenic seizures are a rare, idiopathic subtype of sensory-evoked epilepsies, also known as reflex epilepsies (47). Proposed pathophysiology involves aberrant afferent peripheral vestibular stimuli or improper central processing of this input. This leads to generalized epileptic activity via the reticular activating system in the brainstem and thalamocortical pathways (45). Kogeorgos et al. (35) found that approximately 23% of all vestibulogenic seizures manifest as general tonic–clonic seizures. While simultaneous abnormalities on VNG caloric testing and ictal EEG would be expected to make the diagnosis of vestibulogenic epilepsy, clinicians would also need to exclude confounding variables (e.g., existing peripheral vestibular disorder, the effect of concurrent ASMs, or other medications).
Given its lack of established diagnostic criteria, VE must be delineated from more common vestibular disorders and vice versa. More than half of children presenting with dizziness also complain of headache (48, 49), so it is no surprise that Recurrent Vertigo of Childhood (RVC) (formerly, Benign Paroxysmal Vertigo of Childhood) and Vestibular Migraine of Childhood (VMC) often lead the diagnostic differential. VE a BPVC can be largely differentiated from VMC by the patient having a current or prior history of migraine and episodes of dizziness accompanied by migraine features (50). The presence of prodromal epileptic phenomena (e.g., visual or auditory aura or hallucinations, impaired consciousness) should alert the clinician toward VE, wherein vestibular symptoms are typically shorter (i.e., a few seconds) compared to RVC and VMC. Additionally, VE may mimic vestibular paroxysms, although the latter may yield horizontal and torsional nystagmus beating toward the affected ear and time-locked to the vestibular episode (51). Response to carbamazepine/oxcarbazepine treatment is also part of the vestibular paroxysmia diagnostic criteria. While an ideal specific test for VE would involve observing SEEG signals during episodes and mapping them to the vestibular cortical network via corticocortical-evoked potential technique (25), this would not be clinically feasible in routine work-up.
There is no consensus regarding first-choice ASMs in the treatment of VE in either the adult or pediatric population, as the evidence is limited to single case reports and small, heterogeneous cohort studies (1, 13, 30, 35, 52, 53). In one systematic review of 1,055 patients with VE [described as “epileptic vertigo or dizziness (EVD)”], the most commonly used ASMs were phenytoin, carbamazepine, and valproate, in descending order (6). Overall, patients with VE respond well to ASMs (14), with drug response rates reported as high as 90% (6). Of note, ASMs have also effectively treated other vestibular disorders of central origin, such as vestibular paroxysmia and vestibular migraine (54, 55).
The 2004 American Academy of Neurology guidelines for the treatment of new-onset focal or generalized epilepsies were updated, but limited recommendations in children to absence seizures (56) Thus, in approaching the treatment for suspected VE, the choice of ASM should be made first by classification of seizure type (focal vs. generalized) and then epilepsy type, which combines EEG data with the seizure semiology (symptoms and observed clinical manifestations). Once the type of epilepsy is determined, the choice of anti-seizure medication should consider the patient’s unique medical history, including active medications, co-morbidities, potential ASM side effects, required laboratory monitoring, and medication formulation.
Many children with epilepsy are unable to swallow pills or tablets, and thus, liquid medication should be utilized. In general, the newer generation ASMs (e.g., lamotrigine, levetiracetam) are better tolerated and have fewer side effects than the first-generation ASMs (e.g., phenobarbital, valproic acid). Clinicians should also be aware that medications such as valproate have been associated with peripheral vestibular function abnormalities on testing, such as electro/video-nystagmography (ENG/VNG) and vestibular-evoked myogenic potential responses (VEMP) (36). ASMs can also cause dizziness, so the side effect profile of the medical regimen should be carefully monitored.
Vertiginous epilepsy (VE) is a specific type of epilepsy characterized by seizures with vestibular symptoms as the most prominent or even sole feature. Despite an increased prevalence in children compared to adults, diagnosis is often delayed and challenging due to the non-specific characterization of vestibular symptoms and the unique spread of electroactivity throughout the multi-sensory vestibular cortical network. Although there are no diagnostic criteria for VE, a thorough evaluation (detailed history, physical examination, MRI, EEG) to rule out structural causes of epilepsy and functional peripheral vestibulopathy is recommended for any pediatric patient presenting with vestibular symptoms accompanied by loss of consciousness. Reassuringly, children with VE generally respond well to ASMs and have a favorable prognosis.
• VE is rare, but diagnostic criteria should be developed to guide clinicians’ work-ups of children from other common causes of pediatric dizziness, including BPVC and vestibular migraine.
• Further research is needed to identify specific biomarkers or clinical characteristics that differentiate VE from other causes of vertigo in children.
• Studies are warranted to investigate the most effective treatment strategies and long-term outcomes for children with VE.
AMW: Conceptualization, Writing – original draft, Writing – review & editing. AT-H: Conceptualization, Writing – original draft, Writing – review & editing. BWK: Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Eviatar, L, and Eviatar, A. Vertigo in children: differential diagnosis and treatment. Pediatrics. (1977) 59:833–8. doi: 10.1542/peds.59.6.833
2. Zhang, J, Zhu, Q, Shen, J, Chen, J, Jin, Y, Zhang, Q, et al. Etiological classification and management of dizziness in children: a systematic review and meta-analysis. Front Neurol. (2023) 14:1125488. doi: 10.3389/fneur.2023.1125488
3. Hung, CY, and Young, YH. Evolution of pediatric vertigo/dizziness during the past two decades. Acta Otolaryngol. (2022) 142:562–7. doi: 10.1080/00016489.2022.2106381
4. Wang, A, Zhou, G, Lipson, S, Kawai, K, Corcoran, M, and Brodsky, JR. Multifactorial characteristics of pediatric dizziness and imbalance. Laryngoscope. (2021) 131:E1308–e1314. doi: 10.1002/lary.29024
5. Davitt, M, Delvecchio, MT, and Aronoff, SC. The differential diagnosis of Vertigo in children: a systematic review of 2726 cases. Pediatr Emerg Care. (2020) 36:368–71. doi: 10.1097/PEC.0000000000001281
6. Tarnutzer, AA, Lee, SH, Robinson, KA, Kaplan, PW, and Newman-Toker, DE. Clinical and electrographic findings in epileptic vertigo and dizziness: a systematic review. Neurology. (2015) 84:1595–604. doi: 10.1212/WNL.0000000000001474
7. Karatoprak, E, Sözen, G, and Yılmaz, K. How often do neurological disorders lead to dizziness in childhood? Turk Arch Pediatr. (2021) 56:249–53. doi: 10.14744/TurkPediatriArs.2020.43410
8. Batu, ED, Anlar, B, Topçu, M, Turanlı, G, and Aysun, S. Vertigo in childhood: a retrospective series of 100 children. Eur J Paediatr Neurol. (2015) 19:226–32. doi: 10.1016/j.ejpn.2014.12.009
9. Kluge, M, Beyenburg, S, Fernández, G, and Elger, CE. Epileptic vertigo: evidence for vestibular representation in human frontal cortex. Neurology. (2000) 55:1906–8. doi: 10.1212/WNL.55.12.1906
10. Symonds, JD, Elliott, KS, Shetty, J, Armstrong, M, Brunklaus, A, Cutcutache, I, et al. Early childhood epilepsies: epidemiology, classification, aetiology, and socio-economic determinants. Brain. (2021) 144:2879–91. doi: 10.1093/brain/awab162
11. Aaberg, KM, Gunnes, N, Bakken, IJ, Lund Søraas, C, Berntsen, A, Magnus, P, et al. Incidence and prevalence of childhood epilepsy: a Nationwide cohort study. Pediatrics. (2017) 139:139. doi: 10.1542/peds.2016-3908
12. Ngugi, AK, Kariuki, SM, Bottomley, C, Kleinschmidt, I, Sander, JW, and Newton, CR. Incidence of epilepsy: a systematic review and meta-analysis. Neurology. (2011) 77:1005–12. doi: 10.1212/WNL.0b013e31822cfc90
13. Hewett, R, Guye, M, Gavaret, M, and Bartolomei, F. Benign temporo-parieto-occipital junction epilepsy with vestibular disturbance: an underrecognized form of epilepsy? Epilepsy Behav. (2011) 21:412–6. doi: 10.1016/j.yebeh.2011.05.017
14. Brandt, T . Vestibular epilepsy Vertigo: its multi-sensory syndromes. New York, NY: Springer New York, (2003):233–239.
15. Hewett, R, and Bartolomei, F. Epilepsy and the cortical vestibular system: tales of dizziness and recent concepts. Front Integr Neurosci. (2013) 7:73. doi: 10.3389/fnint.2013.00073
16. Penfield, W, and Jasper, H. Epilepsy and the functional anatomy of the human brain. AMA Arch Neurol Psychiatry. (1954) 72:663–4. doi: 10.1001/archneurpsyc.1954.02330050133021
17. Kahane, P, Hoffmann, D, Minotti, L, and Berthoz, A. Reappraisal of the human vestibular cortex by cortical electrical stimulation study. Ann Neurol. (2003) 54:615–24. doi: 10.1002/ana.10726
18. Rancz, EA, Moya, J, Drawitsch, F, Brichta, AM, Canals, S, and Margrie, TW. Widespread vestibular activation of the rodent cortex. J Neurosci. (2015) 35:5926–34. doi: 10.1523/JNEUROSCI.1869-14.2015
19. Liu, S, Dickman, JD, and Angelaki, DE. Response dynamics and tilt versus translation discrimination in parietoinsular vestibular cortex. Cereb Cortex. (2011) 21:563–73. doi: 10.1093/cercor/bhq123
20. Chen, A, DeAngelis, GC, and Angelaki, DE. Macaque parieto-insular vestibular cortex: responses to self-motion and optic flow. J Neurosci. (2010) 30:3022–42. doi: 10.1523/JNEUROSCI.4029-09.2010
21. Guldin, WO, and Grüsser, OJ. Is there a vestibular cortex? Trends Neurosci. (1998) 21:254–9. doi: 10.1016/S0166-2236(97)01211-3
22. Buchhalter, JR . Animal models of inherited epilepsy. Epilepsia. (1993) 34:S31–41. doi: 10.1111/j.1528-1167.1993.tb06257.x
23. Guldin, WO, Akbarian, S, and Grüsser, OJ. Cortico-cortical connections and cytoarchitectonics of the primate vestibular cortex: a study in squirrel monkeys (Saimiri sciureus). J Comp Neurol. (1992) 326:375–401. doi: 10.1002/cne.903260306
24. Schwarz, DW, and Fredrickson, JM. Rhesus monkey vestibular cortex: a bimodal primary projection field. Science. (1971) 172:280–1. doi: 10.1126/science.172.3980.280
25. Lopez, C, and Cullen, KE. Electrical stimulation of the peripheral and central vestibular system. Curr Opin Neurol. (2024) 37:40–51. doi: 10.1097/WCO.0000000000001228
26. Lopez, C, Blanke, O, and Mast, FW. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience. (2012) 212:159–79. doi: 10.1016/j.neuroscience.2012.03.028
27. zu Eulenburg, P, Caspers, S, Roski, C, and Eickhoff, SB. Meta-analytical definition and functional connectivity of the human vestibular cortex. NeuroImage. (2012) 60:162–9. doi: 10.1016/j.neuroimage.2011.12.032
28. Lopez, C, and Blanke, O. The thalamocortical vestibular system in animals and humans. Brain Res Rev. (2011) 67:119–46. doi: 10.1016/j.brainresrev.2010.12.002
29. Dary, Z, Lenggenhager, B, Lagarde, S, Medina Villalon, S, Bartolomei, F, and Lopez, C. Neural bases of the bodily self as revealed by electrical brain stimulation: A systematic review. Hum Brain Mapp. (2023) 44:2936-2959. doi: 10.1002/hbm.26253
30. Haripriya, GR, Lepcha, A, Augustine, AM, John, M, Philip, A, and Mammen, MD. Prevalence, clinical profile, and diagnosis of pediatric dizziness in a tertiary care hospital. Int J Pediatr Otorhinolaryngol. (2021) 146:110761. doi: 10.1016/j.ijporl.2021.110761
31. Božanić Urbančič, N, Vozel, D, Urbančič, J, and Battelino, S. Unraveling the etiology of pediatric Vertigo and dizziness: a tertiary pediatric center experience. Medicina (Kaunas). (2021) 57:475. doi: 10.3390/medicina57050475
32. Geser, R, and Straumann, D. Referral and final diagnoses of patients assessed in an academic Vertigo center. Front Neurol. (2012) 3:169. doi: 10.3389/fneur.2012.00169
33. Dasgupta, S, Mandala, M, Salerni, L, Crunkhorn, R, and Ratnayake, S. Dizziness and balance problems in children. Curr Treat Options Neurol. (2020) 22:8. doi: 10.1007/s11940-020-0615-9
34. Peterson, JD, and Brodsky, JR. Evaluation and management of paediatric vertigo. Curr Opin Otolaryngol Head Neck Surg. (2022) 30:431–7. doi: 10.1097/MOO.0000000000000849
35. Kogeorgos, J, Scott, DF, and Swash, M. Epileptic dizziness. Br Med J (Clin Res Ed). (1981) 282:687–9. doi: 10.1136/bmj.282.6265.687
36. Hamed, SA, and Osiely, AM. Vestibular function in children with generalized epilepsy and treated with valproate. Expert Rev Clin Pharmacol. (2022) 15:1479–86. doi: 10.1080/17512433.2022.2130759
37. Surmeli, R, Yalcin, AD, Surmeli, M, and Gunay, G. Vertiginous epilepsy: documentation of clinical and electrophysiological findings of nine patients. Epileptic Disord. (2020) 22:775–81. doi: 10.1684/epd.2020.1232
38. Morano, A, Carnì, M, Casciato, S, Vaudano, AE, Fattouch, J, Fanella, M, et al. Ictal EEG/fMRI study of vertiginous seizures. Epilepsy Behav. (2017) 68:51–6. doi: 10.1016/j.yebeh.2016.12.031
39. Currie, S, Heathfield, KW, Henson, RA, and Scott, DF. Clinical course and prognosis of temporal lobe epilepsy. A survey of 666 patients. Brain. (1971) 94:173–90. doi: 10.1093/brain/94.1.173
40. Taussig, D, Mazzola, L, Petrescu, AM, Aghakhani, N, Bouilleret, V, Dorfmüller, G, et al. Deep retroinsular and parieto-opercular origin of vestibular symptoms: a stereoelectrocenphalography (SEEG) study. Epilepsy Behav. (2023) 149:109509. doi: 10.1016/j.yebeh.2023.109509
41. Baud, MO, Schindler, K, and Rao, VR. Under-sampling in epilepsy: limitations of conventional EEG. Clin Neurophysiol Pract. (2021) 6:41–9. doi: 10.1016/j.cnp.2020.12.002
42. Kaplan, PW, and Tusa, RJ. Neurophysiologic and clinical correlations of epileptic nystagmus. Neurology. (1993) 43:2508–14. doi: 10.1212/WNL.43.12.2508
43. Nicita, F, Papetti, L, Spalice, A, Ursitti, F, Massa, R, Properzi, E, et al. Epileptic nystagmus: description of a pediatric case with EEG correlation and SPECT findings. J Neurol Sci. (2010) 298:127–31. doi: 10.1016/j.jns.2010.08.022
44. Harris, CM, Boyd, S, Chong, K, Harkness, W, and Neville, BG. Epileptic nystagmus in infancy. J Neurol Sci. (1997) 151:111–4. doi: 10.1016/S0022-510X(97)00102-0
45. Behrman, S, and Wyke, BD. Vestibulogenic seizures; a consideration of vertiginous seizures, with particular reference to convulsions produced by stimulation of labyrinthine receptors. Brain. (1958) 81:529–41. doi: 10.1093/brain/81.4.529
46. Barac, B . Vertiginous epileptic attacks and so-called “vestibulogenic seizures”. Epilepsia. (1968) 9:137–44. doi: 10.1111/j.1528-1157.1968.tb05135.x
47. Alpers, BJ . Vertinous epilepsy. Laryngoscope. (1960) 70:631–7. doi: 10.1288/00005537-196005000-00005
48. Cavestro, C, Montrucchio, F, Benci, P, Pompilio, D, Mandrino, S, Cencio, PG, et al. Headache prevalence and related symptoms, family history, and treatment habits in a representative population of children in Alba, Italy. Pediatr Neurol. (2014) 51:348–53. doi: 10.1016/j.pediatrneurol.2014.05.022
49. Langhagen, T, Schroeder, AS, Rettinger, N, Borggraefe, I, and Jahn, K. Migraine-related vertigo and somatoform vertigo frequently occur in children and are often associated. Neuropediatrics. (2013) 44:55–8. doi: 10.1055/s-0032-1333433
50. van de Berg, R, Widdershoven, J, Bisdorff, A, Evers, S, Wiener-Vacher, S, Cushing, SL, et al. Vestibular migraine of childhood and recurrent Vertigo of childhood: diagnostic criteria consensus document of the Committee for the Classification of vestibular disorders of the Bárány society and the international headache society. J Vestib Res. (2021) 31:1–9. doi: 10.3233/VES-200003
51. Strupp, M, Lopez-Escamez, JA, Kim, JS, Straumann, D, Jen, JC, Carey, J, et al. Vestibular paroxysmia: Diagnostic criteria. J Vestib Res. (2017) 26:409–15. doi: 10.3233/VES-160589
52. Tsai, CH, Chen, TS, Lai, MC, and Huang, CW. Unilateral non-lesional temporal lobe epilepsy presenting as isolated ictal vertigo: a case report. J Int Med Res. (2023) 51:3000605231187801. doi: 10.1177/03000605231187801
53. Yang, R, Wu, H, and Gao, Z. Vestibular seizures and spontaneous downbeat nystagmus of ganglioglioma origin: a case report. BMC Urol. (2023) 23:278. doi: 10.1186/s12883-023-03311-6
54. Chen, CC, Lee, TY, Lee, HH, Kuo, YH, Bery, AK, and Chang, TP. Vestibular paroxysmia: long-term clinical outcome after treatment. Front Neurol. (2022) 13:1036214. doi: 10.3389/fneur.2022.1036214
55. Byun, YJ, Levy, DA, Nguyen, SA, Brennan, E, and Rizk, HG. Treatment of vestibular migraine: a systematic review and Meta-analysis. Laryngoscope. (2021) 131:186–94. doi: 10.1002/lary.28546
56. Kanner, AM, Ashman, E, Gloss, D, Harden, C, Bourgeois, B, Bautista, JF, et al. Practice guideline update summary: efficacy and tolerability of the new anti-epileptic drugs I: treatment of new-onset epilepsy: report of the American Epilepsy Society and the guideline development, dissemination, and implementation Subcommittee of the American Academy of neurology. Epilepsy Curr. (2018) 18:260–8. doi: 10.5698/1535-7597.18.4.260
Keywords: vertigo, dizziness, epilepsy, vestibular system, pediatrics, vestibular migraine, benign paroxysmal vertigo of childhood (BPVC)
Citation: Wood AM, Thompson-Harvey A and Kesser BW (2024) Vertiginous epilepsy in the pediatric population. Front. Neurol. 15:1403536. doi: 10.3389/fneur.2024.1403536
Received: 20 March 2024; Accepted: 17 June 2024;
Published: 05 July 2024.
Edited by:
Soumit Dasgupta, Alder Hey Children’s Hospital, United KingdomReviewed by:
Fawad Khan, Ochsner Health System, United StatesCopyright © 2024 Wood, Thompson-Harvey and Kesser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam Thompson-Harvey, VlZOM01TQHV2YWhlYWx0aC5vcmc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.