- 1Department of Neurosurgery, The Jikei University School of Medicine, Tokyo, Japan
- 2Department of Neurosurgery, The Jikei University School of Medicine, Katsushika Medical Center, Tokyo, Japan
Introduction: An intermediate catheter (IMC) may pose a risk of intraprocedural rupture (IPR) during coil embolization of ruptured intracranial aneurysms (RIAs), because the pressure on the microcatheter and coil might be more direct. To verify this hypothesis, this study explored whether use of an IMC might correlate with an increased rate of IPR during coil embolization for RIAs.
Methods: We retrospectively reviewed 195 consecutive aneurysms in 192 patients who underwent initial coil embolization for saccular RIAs at our institution between January 2007 and December 2023. Patients were divided into two groups with aneurysms treated either with an IMC (IMC group) or without an IMC (non-IMC group). To investigate whether IMC use increased the rate of IPR, a propensity score-matched analysis was employed to control for age, sex, maximal aneurysm size, neck size, bleb formation, aneurysm location, proximal vessel tortuosity, balloon-assisted coiling, type of microcatheter, and type of framing coil.
Results: Ultimately, 43 (22%) coil embolization used IMC. In univariate analysis, the incidence of IPR was significantly higher in the IMC group compared with the non-IMC group (14.0 vs. 3.3%, p = 0.016). Propensity score matching was successful for pairs of 26 aneurysms in the IMC group and 52 aneurysms in the non-IMC group. The incidence of IPR was still significantly higher in the IMC group than in the non-IMC group (23.1 vs. 3.8%, p = 0.015). No significant differences in the incidences of ischemic complications and IMC-related parent artery dissection were observed between the two groups.
Discussion: When using IMC for coil embolization of RIAs, the surgeons should be more careful and delicate in manipulating the microcatheter and inserting the coils to avoid IPR.
Introduction
Following a report on the International Subarachnoid Aneurysm Trial, endovascular coiling of intracranial aneurysms has become more widespread as a less-invasive treatment associated with a higher independent survival rate compared with neurosurgical clipping (1). However, even with endovascular treatment, a risk of complications is inevitable. Intraprocedural rupture (IPR) is one of the most devastating and serious complications and represents a cause of poor outcome (2–4). Risk factors for IPR during coil embolization have been identified as small size aneurysm, ruptured intracranial aneurysms (RIAs), use of a microballoon, anterior or posterior communicating aneurysm, and irregularly shaped aneurysm (2, 4–28).
In recent years, along with advances and developments in endovascular devices, the intermediate catheter (IMC) has increasingly been employed for the coil embolization of intracranial aneurysms. The IMC might be delivered to more distal arteries, in turn making the microcatheter easier to control and thus facilitating high-density coil packing (29). On the other hand, support with an IMC may pose a risk of IPR during coil embolization of especially RIAs with fragile walls, because the pressure on the microcatheter and coil might be more direct. To verify this hypothesis, we investigated the association between use of an IMC and the incidence of IPR during coil embolization for RIAs.
Materials and methods
Study population
A total of 243 consecutive initial endovascular treatments for ruptured cerebral aneurysms conducted at our institution between January 2007 and December 2023 were retrospectively reviewed. Patients with dissecting aneurysms (n = 38) and fusiform aneurysms (n = 5) were excluded. In addition, patients with extracranial aneurysm (n = 4) and aneurysms treated with parent artery occlusion (n = 1) were also excluded. Ultimately, 195 initial coil embolization of saccular RIAs in 192 patients were included in the present study (Figure 1). Patients were divided into two groups with aneurysms treated either with an IMC (IMC group) or without an IMC (non-IMC group).
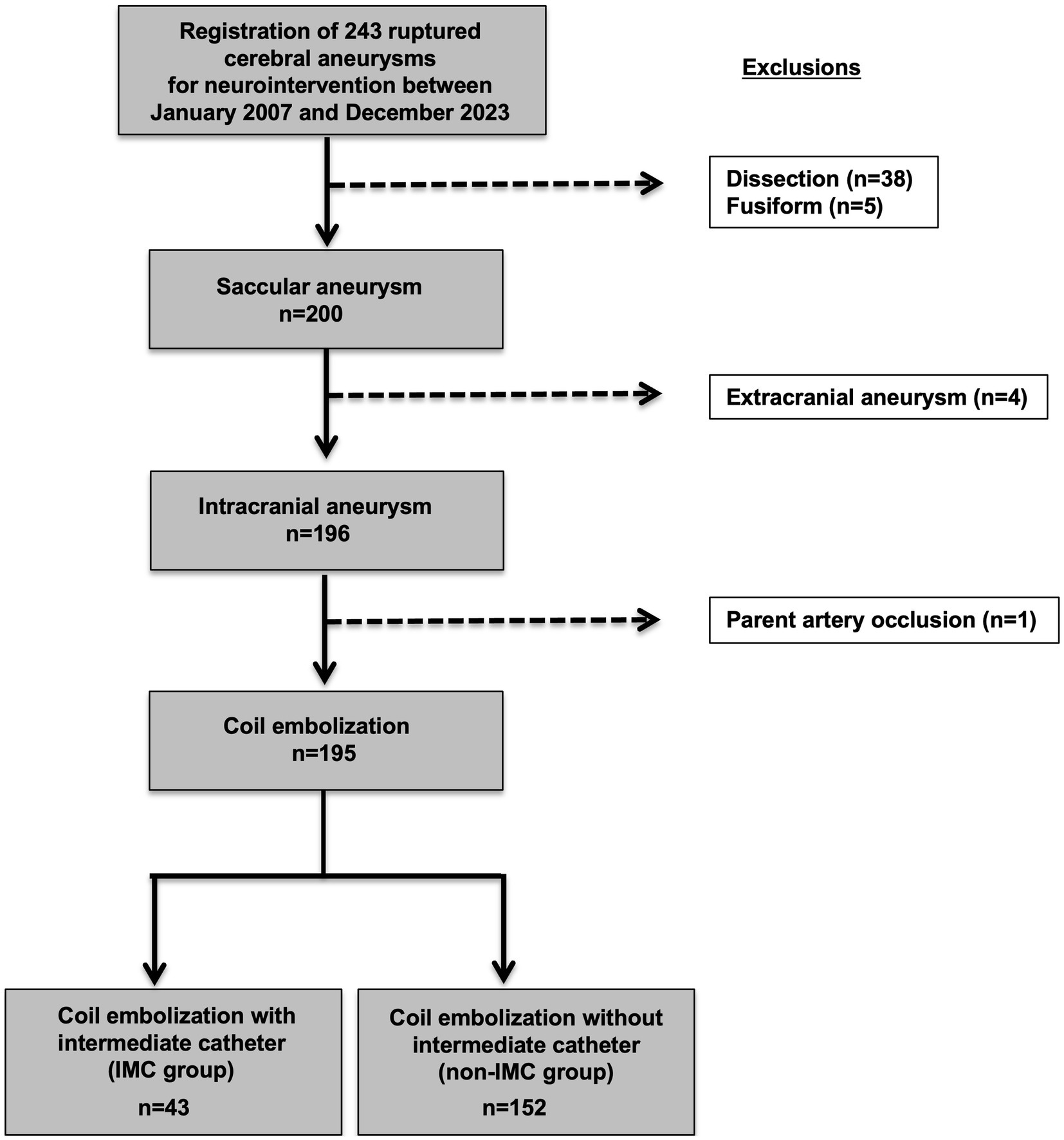
Figure 1. Flowchart for selection of saccular ruptured intracranial aneurysms for initial coil embolization and subsequent classification by use of IMC. A total of 243 consecutive initial endovascular treatments for ruptured cerebral aneurysms conducted at our institution between January 2007 and December 2023 were retrospectively reviewed. Patients with dissecting aneurysms (n = 38) and fusiform aneurysms (n = 5) were excluded. In addition, patients with extracranial aneurysm (n = 4) and aneurysms treated with parent artery occlusion (n = 1) were also excluded. Ultimately, 195 initial coil embolization of saccular ruptured intracranial aneurysms in 192 patients were included in the present study. Of the 195 saccular ruptured intracranial aneurysms, 43 patients were classified to the IMC group and 152 to the non-IMC group. IMC, Intermediate catheter.
Data collection
The medical records and radiological data of these patients were retrospectively reviewed to obtain the following data: age, sex, medical history, family history of cerebral aneurysms, smoking and drinking histories, aneurysm characteristics, proximal vessel tortuosity, endovascular technique, and complications. A proximal vessel tortuosity was defined as two or more bends >90° before reaching the target lesion.
All aneurysms were evaluated for morphology and structure using rotational angiography with three-dimensional image reconstruction (Artis Q Biplane, Siemens Healthcare GmbH, Forchheim, Germany). Based on three-dimensional rotational angiographic images, aneurysm size was calculated using NeuroVision software (Cybernet Systems, Tokyo, Japan), which allows automatic measurement by only placing markers on the aneurysm and parent artery (30).
Definitions of IPR and IMC
In accordance with previous reports (10, 13, 31), IPR was defined as a situation in which a microguidewire, microcatheter, or coil was displaced beyond the boundaries of the aneurysmal sac, with or without evident contrast extravasation on angiography.
An IMC was defined as a catheter inserted coaxially with a guiding catheter for the purpose of improving the maneuverability and stability of the microcatheter. The IMCs used included Tactics (Technorat Corporation, Aichi, Japan), Guidepost (Tokai Medical Products, Aichi, Japan), Cerulean (Medikit Co. Ltd., Tokyo, Japan), Sofia (MicroVention Terumo, Tustin, CA, United States), AXS Vecta (Stryker Neurovascular, Kalamazoo, MI, United States), DAC (Stryker Neurovascular), Navien (Medtronic, Irvine, CA, United States), and Asahi Fubuki (Asahi Intecc, Aichi, Japan).
Endovascular treatment
All coil embolization procedures were accomplished under general anesthesia, in a standardized fashion, and were conducted exclusively by or under the supervision of certified interventional neurosurgeons. Coil embolization was performed without administration of antiplatelet therapy. After the first coil was inserted into the aneurysm, 4,000–5,000 U of heparin was given intravenously as a bolus infusion, followed by 1,000–2,000 U of heparin intermittently to maintain the activated clotting time during the procedure at a level at least twice the baseline value for that patient. Coil embolization was conducted as follows:
The femoral artery was selected as the access site, and for anterior circulation aneurysms, prior to 2010, a 6-8F guiding catheter or sheath without a balloon was placed in the cervical portion of the ICA, which was changed in preparation for IPR to place an 8Fr or 9Fr guiding catheter with a balloon beginning in 2010. For posterior circulation aneurysms, on the other hand, a 5-8F guiding catheter or sheath without a balloon was directed into the V1 (the pre-foraminal segment and ranging from the origin of the VA to the transverse foramen of the sixth cervical vertebra) or V2 (the foraminal segment and ranging from the transverse foramen of the sixth cervical vertebra to the transverse foramen of the second cervical vertebra) segment of the VA. The decision to use IMC was left to the discretion of the surgeon. A 0.0165 or 0.017-in microcatheter was navigated into the aneurysm over a 0.014-in microguidewire. The type of coils used was left to the discretion of the surgeon. In cases of wide-necked aneurysms (neck size >4 mm or dome-to-neck ratio < 2), balloon-assisted, double-catheter, or stent-assisted technique was applied, otherwise primary coiling was employed. In our country, coil embolization using the stent-assisted technique for RIAs has not been covered by national insurance, so it was placed only when no other procedure was available and after obtaining full explanation and consent from the patient or his/her family.
Complications
Complications associated with the procedure were categorized as ischemic, hemorrhagic, or IMC-related parent artery dissection, with IPR assigned as a hemorrhagic complication. Symptomatic complications were defined as an increase of ≥1 in mRS score compared with the preoperative level. This study adhered to the Declaration of Helsinki. Our institutional review board waived the need for informed consent due to the retrospective design.
Statistical analyses
For the comparison of baseline characteristics between IMC and non-IMC groups, the Mann–Whitney U test and Fisher’s exact test were applied to continuous variables and categorical variables, respectively. A 1:2 propensity score matching between IMC and non-IMC groups was performed using the nearest neighbor method, without replacement and with a caliper width of 0.20, to match groups on covariates of age, sex, proximal vessel tortuosity, type of microcatheter, type of framing coil, and previously reported risk factors for IPR, including maximal aneurysm size, neck size, bleb formation, aneurysm location, and balloon-assisted coiling (2, 4–28). All statistical analyses were performed using R and R Commander-based Easy R (EZR) software (Saitama Medical Center, Jichi Medical School, Saitama, Japan) (32). Values of p < 0.05 were considered significant.
Results
Clinical, anatomic, and procedural characteristics
Among the 195 RIAs, 43 (22%) underwent coil embolization using an IMC. Clinical, anatomic, and procedural characteristics are shown in Table 1. No significant differences in age, sex, medical history, family history of cerebral aneurysms, and smoking history were apparent between groups. Patients in the IMC group were significantly more likely to have a history of drinking than those in the non-IMC group (p = 0.026).

Table 1. Clinical, anatomic, and procedural characteristics of saccular ruptured intracranial aneurysms before and after propensity score matching between IMC and non-IMC groups.
Compared with the non-IMC group, the IMC group had significantly smaller aneurysm size (4.1 [IQR: 3.3, 5.5] mm vs. 5.5 [IQR: 4.1, 6.8] mm, p < 0.001) and neck size (2.9 [IQR: 2.0, 3.7] mm vs. 3.4 [IQR: 2.6, 4.7] mm, p = 0.006) and a lower frequency of bleb formation (58.1 vs. 80.3%, p = 0.005). Regarding aneurysm location, use of an IMC was significantly more frequent in the treatment of anterior communicating artery (ACoA) aneurysms (51.2 vs. 30.9%, p = 0.019). The rate of proximal vessel tortuosity, endovascular technique, and type of microcatheter were not significantly different between the two groups. Regarding the type of framing coil, the rate of target coil (Stryker Neurovascular) use was significantly higher in the IMC group compared with the non-IMC group (p < 0.001). Rates of ischemic complications and IMC-related parent artery dissection did not differ significantly between groups. Hemorrhagic complications were all IPR, and the incidence of IPR was significantly higher in the IMC group than in the non-IMC group (14.0 vs. 3.3%, p = 0.016) (Table 1).
Characteristics of IPR in the IMC group
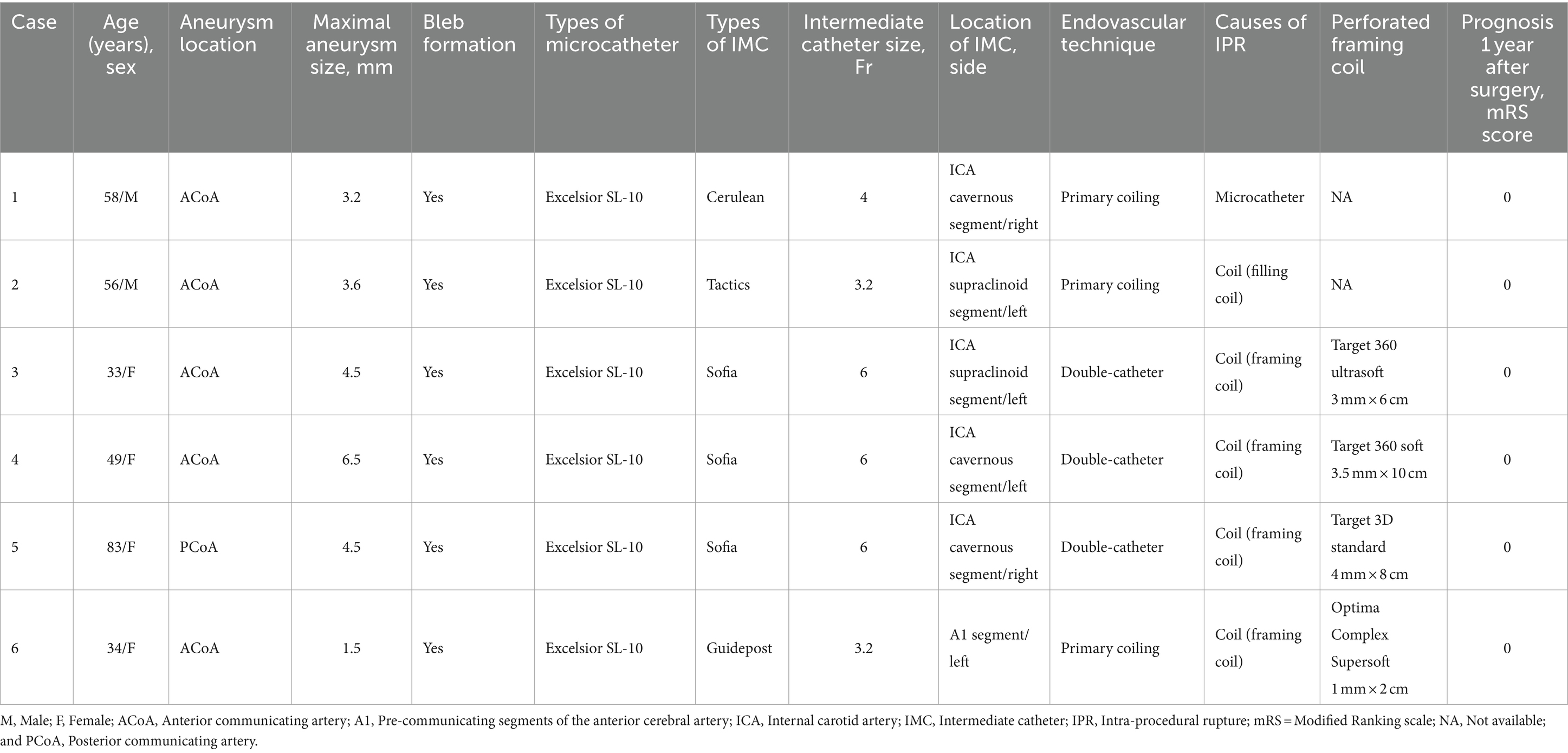
Intraprocedural ruptures with IMC in RIAs are presented in Table 2. Of the six patients who developed IPR, median age was 53 years (IQR, 34–58 years), with a predominance of female patients (n = 4, 66.7%). Aneurysm location was the ACoA in five cases (83.3%) and posterior communicating artery in 1 (16.7%). Median maximal aneurysm size was 4.1 mm (IQR, 3.2–4.5 mm), and bleb formation was observed in all aneurysms. All microcatheters used for endovascular treatment were Excelsior SL-10 (Stryker Neurovascular). The IMC used for endovascular treatment was a 6-Fr Sofia in three patients (50%) and a 4-Fr Cerulean, a 3.2-Fr Guidepost, and a 3.2-Fr Tactics in one patient each (16.7%). The location of the IMC used for coil embolization was the ICA cavernous segment in three patients (50%), the ICA supraclinoid segment in two patients (33.3%), and the pre-communicating segments (A1) of the anterior cerebral artery in one patient (16.7%), with the left side predominating (66.7%), and three IMCs (50%) placed intradurally. The endovascular technique were double-catheter and primary coiling in three patients (50%) each. Causes of IPR were coils in five patients (83.3%) and microcatheter in one patient (16.7%), with IPR occurring most frequently at the time of insertion of the framing coil (66.7%). The types of perforated framing coils included three cases of target coils and one case of optima coil (Balt, Montmorency, France), with a variety of coil stiffness, and coil sizes smaller than the maximum aneurysm diameter were selected for all aneurysms. The mRS score 1 year after surgery was 0 for all patients.
Association between IMC use and IPR after propensity score matching
Propensity score matching was successful for pairs of 26 aneurysms in the IMC group and 52 aneurysms in the non-IMC group (Table 1). After matching for age, sex, maximal aneurysm size, neck size, bleb formation, aneurysm location, proximal vessel tortuosity, balloon-assisted coiling, type of microcatheter, and type of framing coil, the incidence of IPR in the IMC and non-IMC groups was compared. The incidence of IPR was still significantly higher in the IMC group than in the non-IMC group (23.1 vs. 3.8%, p = 0.015) (Table 1). There were no significant differences between the two groups in the incidence of ischemic complications and IMC-related parent artery dissection.
Illustrative case
A 33-year-old woman without previous medical history presented with severe headache and was transported to the emergency department at our institution. CT and DSA of the brain showed SAH due to ruptured ACoA aneurysm with a maximum diameter of 4.5 mm (Figure 2A). Subsequently, the aneurysm was embolized with coil under the double-catheter technique with a 6-Fr Sofia catheter guided as an IMC to the left ICA supraclinoid segment (Figure 2B). During insertion of a framing coil into the aneurysm, the sac of the aneurysm was perforated, and angiography revealed extravasation of contrast medium (Figure 2C). The coil was displaced beyond the boundaries of the aneurysmal sac (Figure 2D). For hemostatic purposes, coils were immediately packed into the aneurysm from the other unperforated catheter. After confirming that the contrast agent had stopped leaking, the perforated coil was inserted in a dumb-bell fashion from outside the aneurysm to inside the aneurysm, sealing the perforated portion (Figures 2E,F). Post-treatment CT scan demonstrated retention of contrast medium in the subarachnoid space.
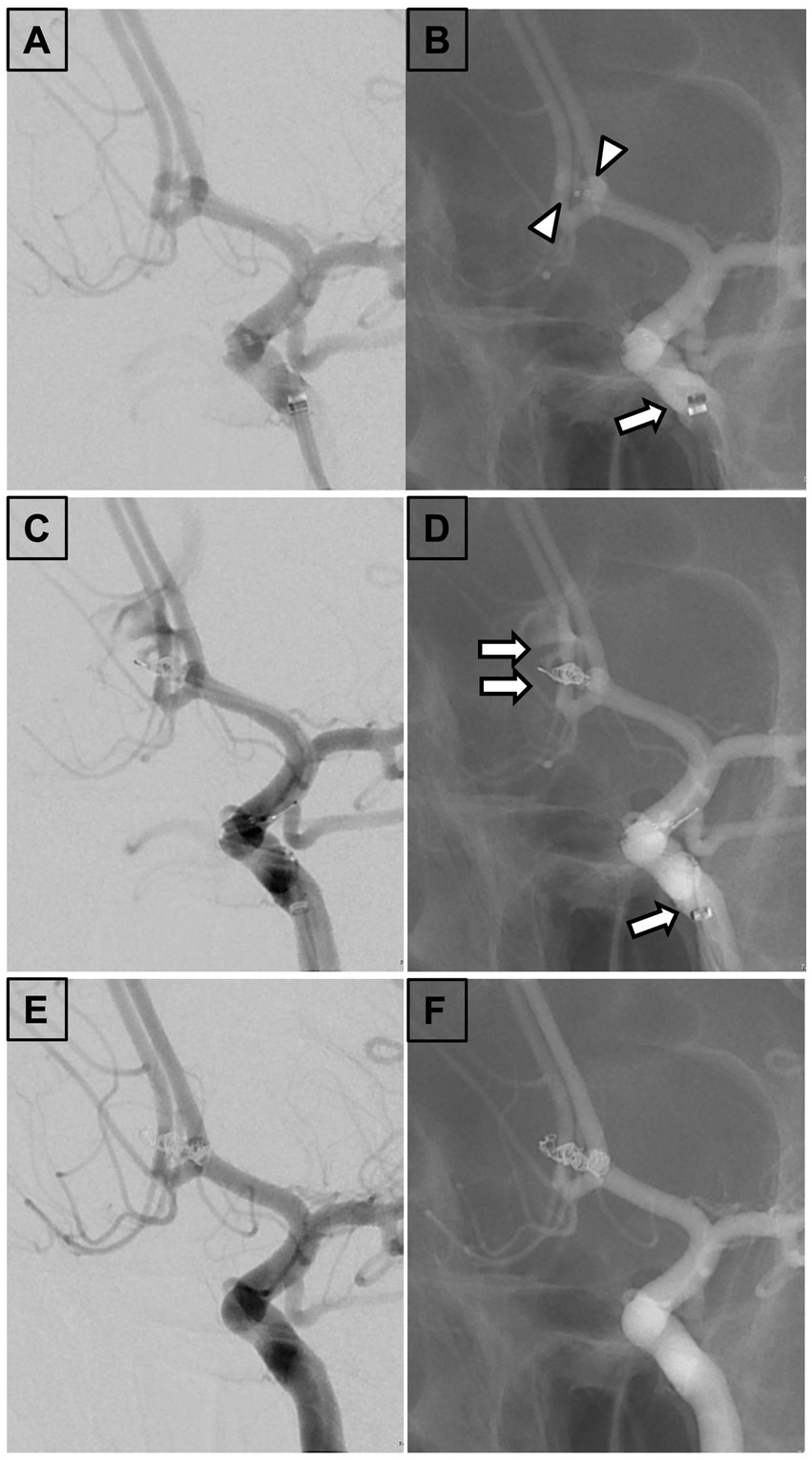
Figure 2. Findings in a 33-year-old woman. (A) Left internal carotid arteriography. A 33-year-old woman presenting with severe headache revealed a ruptured anterior communicating artery aneurysm with a maximum diameter of 4.5 mm. (B) Fluoroscopic view demonstrating aneurysm embolization under a double-catheter technique with a 6-Fr Sophia catheter guided as an IMC to the supraclinoid segment of the left internal carotid artery. (C) Left internal carotid arteriography revealing perforation of the aneurysm sac during insertion of a framing coil into the aneurysm and extravasation of contrast medium. (D) Fluoroscopic view showing coil displacement beyond the boundaries of the aneurysmal sac. (E) Left internal carotid arteriography demonstrating that the coil was immediately filled into the aneurysm from the other unperforated catheter. The perforated coil was inserted in a dumbbell fashion from outside to inside the aneurysm to seal the perforation and stop the bleeding. (F) Fluoroscopic view indicating insertion of the perforated coil in a dumbbell fashion from outside to inside the aneurysm to seal the perforation. IMC, Intermediate catheter; Arrowhead, Tip of the microcatheter; Arrow, Tip of the IMC; and Double arrow, Perforated coil loop.
Discussion
The present study revealed that the use of an IMC was significantly associated with the incidence of IPR in coil embolization of RIAs. RIAs have previously been described as a risk factor for IPR (2, 10, 33). A meta-analysis by Cloft et al. demonstrated that the risk of IPR during coil embolization was significantly higher in RIAs than in unruptured intracranial aneurysms (UIAs) (4.1 vs. 0.5%, p < 0.001) (2). IPR of an UIA requires a new tear in the aneurysm wall, whereas IPR of a RIA can occur either by dislodging a clot that has occluded the original rupture point or by further tearing of the already torn and fragile aneurysm wall. RIAs may have more fragile walls than UIAs and thus require more delicate microcatheter manipulation and coil insertion than UIAs.
IMC and IPR in RIAs
According to a previous literature review, the overall incidence of IPR was 4.47% (393/8791) for RIAs (25). In the present study, the incidence of IPR without IMC in RIAs was 3.3%, which is comparable to the previous meta-analysis. On the other hand, the incidence of IPR with IMC was as high as 14.0%. Causes of IPR have been previously described as microguidewires, coils, and microcatheters (8). The present study revealed coils as the most frequent cause of IPR in coil embolization with IMC for RIAs. Furthermore, the majority of IPRs occurred during first coil insertion in the framing phase. In the present study, the type of framing coil may not affect the incidence of IPR because the rate of IPR in the IMC group was not significantly different from that in the non-IMC group even after adjusting for the two groups in a propensity score-matched analysis. One possible mechanism for the increased risk of rupture with IMC could be that the support by the IMC may have increased the more direct pressure against the microcatheter and coil, which in turn could have increased the more direct pressure against the aneurysm wall. Such phenomena could explain the higher incidence of IPR with IMC use, particularly during framing coil insertion. In RIAs, if an IMC is employed, the coils should be inserted gently and with care, particularly during insertion of the first coil in the framing phase.
In the IMC group as indicated in Table 2, despite IPR, the prognosis 1 year after surgery was favorable with mRS 0 in all patients. The reason for the favorable prognosis may be attributed to the use of balloon guiding catheters for the treatment of RIAs, as our research group previously demonstrated (28). This means that even if the aneurysm ruptured intraoperatively, the balloon dilation enabled rapid hemostasis. Therefore, the rupture point could be treated while stopping the bleeding, which led to a favorable prognosis. As was shown in the results of the present study, the use of IMC in coil embolization for RIAs can increase the risk of IPR. When using IMC, employing balloon guiding catheters may not worsen the patient’s prognosis because even if IPR occurs, rapid hemostasis can be achieved by balloon dilation.
ACoA aneurysms and IMC
In the present study, an IMC was significantly more frequently used for the coil embolization of ACoA aneurysms. This is because aneurysms at this site are distal and along a tortuous path, and navigation with microcatheters is often unstable and technically challenging (34). IMC can contribute to an increased volume embolization ratio by improving the maneuverability and stability of microcatheters (29). However, ACoA aneurysms have previously been alerted as a risk factor for IPR (22, 23). Kawabata et al. performed coil embolization in 1375 patients (1,406 UIAs), and IPR occurred in 20 aneurysms of 20 patients (1.4%). Multivariate analyses revealed that ACoA aneurysms were independently associated with IPR [OR: 7.54 (95% CI: 2.82–19.30), p = 0.0001] (23). In fact, the present study showed that IPR occurred in 5 of 22 (22.7%) ruptured ACoA aneurysms in coil embolization with an IMC. Based on the above, when the use of IMC for coil embolization of ruptured ACoA aneurysms, surgeons should keep in mind that more careful microcatheter manipulation and coil insertion may be necessary to avoid the IPR.
Limitations
The present study has several limitations that should be noted when interpreting the findings. First, the long observation period may have allowed for improvements in endovascular device during that time. Newer coils, microguidewires, and microcatheters may reduce the risk of IPR. However, in the present study, the two groups were compared after propensity score matching with respect to the type of microcatheter and the type of framing coil, both of which are potential confounders of IPR. In addition, more maneuverable IMCs have only recently been developed (35–39). Nevertheless, the incidence of IPR was significantly increased in the coil embolization of RIAs with IMC compared with those without IMC. Therefore, regardless of improvements in endovascular device, IMC can increase the risk of IPR.
Second, this was a retrospective, single-center study, so multi-center and prospective studies should be conducted in the future to verify our findings. Despite these limitations, the present study showed that use of an IMC was significantly associated with IPR in the coil embolization of RIAs.
Conclusion
When using IMC for coil embolization of RIAs, especially in ACoA aneurysms, the surgeons should be more careful and delicate in manipulating the microcatheter and inserting the coils to avoid IPR.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Jikei University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin due to the retrospective nature of the study.
Author contributions
MF: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. TI: Conceptualization, Data curation, Methodology, Supervision, Validation, Writing – review & editing. KA: Data curation, Investigation, Methodology, Supervision, Validation, Writing – review & editing. NK: Data curation, Formal Analysis, Investigation, Project administration, Validation, Visualization, Writing – review & editing. IK: Conceptualization, Data curation, Resources, Supervision, Validation, Visualization, Writing – review & editing. SH: Investigation, Methodology, Project administration, Supervision, Writing – review & editing. GN: Investigation, Methodology, Validation, Visualization, Writing – review & editing. TS: Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. TT: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing. YM: Validation, Writing – review & editing, Conceptualization, Investigation, Methodology, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACoA, Anterior communicating artery; IMC, Intermediate catheter; IPR, Intraprocedural rupture; RIA, Ruptured intracranial aneurysm; UIA, Unruptured intracranial aneurysm.
References
1. Molyneux, AJ, Kerr, RS, Yu, LM, Clarke, M, Sneade, M, Yarnold, JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. (2005) 366:809–17. doi: 10.1016/S0140-6736(05)67214-5
2. Cloft, HJ, and Kallmes, DF. Cerebral aneurysm perforations complicating therapy with Guglielmi detachable coils: a meta-analysis. AJNR Am J Neuroradiol. (2002) 23:1706–9.
3. Elijovich, L, Higashida, RT, Lawton, MT, Duckwiler, G, Giannotta, S, and Johnston, SC. Predictors and outcomes of intraprocedural rupture in patients treated for ruptured intracranial aneurysms: the CARAT study. Stroke. (2008) 39:1501–6. doi: 10.1161/STROKEAHA.107.504670
4. Pierot, L, Spelle, L, and Vitry, F. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke. (2008) 39:2497–504. doi: 10.1161/STROKEAHA.107.512756
5. Cognard, C, Weill, A, Castaings, L, Rey, A, and Moret, J. Intracranial berry aneurysms: angiographic and clinical results after endovascular treatment. Radiology. (1998) 206:499–510. doi: 10.1148/radiology.206.2.9457205
6. McDougall, CG, Halbach, VV, Dowd, CF, Higashida, RT, Larsen, DW, and Hieshima, GB. Causes and management of aneurysmal hemorrhage occurring during embolization with Guglielmi detachable coils. J Neurosurg. (1998) 89:87–92. doi: 10.3171/jns.1998.89.1.0087
7. Kataoka, K, Taneda, M, Asai, T, Kinoshita, A, Ito, M, and Kuroda, R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke. (1999) 30:1396–401. doi: 10.1161/01.STR.30.7.1396
8. Doerfler, A, Wanke, I, Egelhof, T, Dietrich, U, Asgari, S, Stolke, D, et al. Aneurysmal rupture during embolization with Guglielmi detachable coils: causes, management, and outcome. AJNR Am J Neuroradiol. (2001) 22:1825–32.
9. Levy, E, Koebbe, CJ, Horowitz, MB, Jungreis, CA, Pride, GL, Dutton, K, et al. Rupture of intracranial aneurysms during endovascular coiling: management and outcomes. Neurosurgery. (2001) 49:807–13. doi: 10.1227/00006123-200110000-00005
10. Sluzewski, M, Bosch, JA, van Rooij, WJ, Nijssen, PC, and Wijnalda, D. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg. (2001) 94:238–40. doi: 10.3171/jns.2001.94.2.0238
11. Tummala, RP, Chu, RM, Madison, MT, Myers, M, Tubman, D, and Nussbaum, ES. Outcomes after aneurysm rupture during endovascular coil embolization. Neurosurgery. (2001) 49:1059–67. doi: 10.1227/00006123-200111000-00007
12. Murayama, Y, Nien, YL, Duckwiler, G, Gobin, YP, Jahan, R, Frazee, J, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. (2003) 98:959–66. doi: 10.3171/jns.2003.98.5.0959
13. Brisman, JL, Niimi, Y, Song, JK, and Berenstein, A. Aneurysmal rupture during coiling: low incidence and good outcomes at a single large volume center. Neurosurgery. (2005) 57:1103–9. doi: 10.1227/01.NEU.0000185631.20246.1A
14. Layton, KF, Cloft, HJ, Gray, LA, Lewis, DA, and Kallmes, DF. Balloon-assisted coiling of intracranial aneurysms: evaluation of local thrombus formation and symptomatic thromboembolic complications. AJNR Am J Neuroradiol. (2007) 28:1172–5. doi: 10.3174/ajnr.A0490
15. Nguyen, TN, Raymond, J, Guilbert, F, Roy, D, Bérubé, MD, Mahmoud, M, et al. Association of endovascular therapy of very small ruptured aneurysms with higher rates of procedure-related rupture. J Neurosurg. (2008) 108:1088–92. doi: 10.3171/JNS/2008/108/6/1088
16. Schuette, AJ, Hui, FK, Spiotta, AM, Obuchowski, NA, Gupta, R, Moskowitz, SI, et al. Endovascular therapy of very small aneurysms of the anterior communicating artery: five-fold increased incidence of rupture. Neurosurgery. (2011) 68:731–7. doi: 10.1227/NEU.0b013e3182077373
17. Santillan, A, Gobin, YP, Greenberg, ED, Leng, LZ, Riina, HA, Stieg, PE, et al. Intraprocedural aneurysmal rupture during coil embolization of brain aneurysms: role of balloon-assisted coiling. AJNR Am J Neuroradiol. (2012) 33:2017–21. doi: 10.3174/ajnr.A3061
18. Chen, M . A checklist for cerebral aneurysm embolization complications. J Neurointerv Surg. (2013) 5:20–7. doi: 10.1136/neurintsurg-2011-010137
19. Mitchell, PJ, Muthusamy, S, Dowling, R, and Yan, B. Does small aneurysm size predict intraoperative rupture during coiling in ruptured and unruptured aneurysms? J Stroke Cerebrovasc Dis. (2013) 22:1298–303. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.017
20. Kang, DH, Goh, DH, Baik, SK, Park, J, and Kim, YS. Morphological predictors of intraprocedural rupture during coil embolization of ruptured cerebral aneurysms: do small basal outpouchings carry higher risk? J Neurosurg. (2014) 121:605–12. doi: 10.3171/2014.5.JNS132107
21. Thompson, BG, Brown, RD Jr, Amin-Hanjani, S, Broderick, JP, Cockroft, KM, Connolly, ES Jr, et al. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2015) 46:2368–400. doi: 10.1161/STR.0000000000000070
22. Fan, L, Lin, B, Xu, T, Xia, N, Shao, X, Tan, X, et al. Predicting intraprocedural rupture and thrombus formation during coiling of ruptured anterior communicating artery aneurysms. J Neurointerv Surg. (2017) 9:370–5. doi: 10.1136/neurintsurg-2016-012335
23. Kawabata, S, Imamura, H, Adachi, H, Tani, S, Tokunaga, S, Funatsu, T, et al. Risk factors for and outcomes of intraprocedural rupture during endovascular treatment of unruptured intracranial aneurysms. J Neurointerv Surg. (2018) 10:362–6. doi: 10.1136/neurintsurg-2017-013156
24. Kocur, D, Przybyłko, N, Bażowski, P, and Baron, J. Rupture during coiling of intracranial aneurysms: predictors and clinical outcome. Clin Neurol Neurosurg. (2018) 165:81–7. doi: 10.1016/j.clineuro.2018.01.006
25. Park, YK, Yi, HJ, Choi, KS, Lee, YJ, and Chun, HJ. Intraprocedural rupture during endovascular treatment of intracranial aneurysm: clinical results and literature review. World Neurosurg. (2018) 114:e605–15. doi: 10.1016/j.wneu.2018.03.040
26. Jiang, C, Luan, D, Wang, C, Liu, Q, Han, J, and Li, G. Risk and prognostic factors for rupture of intracranial aneurysms during endovascular embolization. World Neurosurg. (2019) 129:e641–9. doi: 10.1016/j.wneu.2019.05.233
27. Li, L, Zhang, X, Feng, Z, Zhao, R, Hong, B, Xu, Y, et al. Risk factors for Intraprocedural rupture in the endovascular treatment of Unruptured intracranial aneurysms: a single-center experience with 1232 procedures. World Neurosurg. (2019) 123:e9–e14. doi: 10.1016/j.wneu.2018.09.164
28. Aoki, K, Murayama, Y, Tanaka, Y, Ishibashi, T, Irie, K, Fuga, M, et al. Risk factors and management of intraprocedural rupture during coil embolization of unruptured intracranial aneurysms: role of balloon guiding catheter. Front Neurol. (2024) 15:1343137. doi: 10.3389/fneur.2024.1343137
29. Fuga, M, Ishibashi, T, Aoki, K, Tachi, R, Irie, K, Kato, N, et al. Intermediate catheter use is associated with complete occlusion and dense packing in coil embolization of unruptured cerebral aneurysms: a propensity score matched study. J Neurointerv Surg. (2024):jnis-2023-021258. doi: 10.1136/jnis-2023-021258 Online ahead of print.
30. Takao, H, Ishibashi, T, Saguchi, T, Arakawa, H, Ebara, M, Irie, K, et al. Validation and initial application of a semiautomatic aneurysm measurement software: a tool for assessing volumetric packing attenuation. AJNR Am J Neuroradiol. (2014) 35:721–6. doi: 10.3174/ajnr.A3777
31. Luo, CB, Mu-Huo Teng, M, Chang, FC, Lin, CJ, Guo, WY, and Chang, CY. Intraprocedure aneurysm rupture in embolization: clinical outcome with imaging correlation. J Chin Med Assoc. (2012) 75:281–5. doi: 10.1016/j.jcma.2012.04.008
32. Kanda, Y . Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. (2013) 48:452–8. doi: 10.1038/bmt.2012.244
33. van Lieshout, JH, Verbaan, D, Donders, R, van den Berg, R, Vandertop, PWP, Klijn, CJM, et al. Periprocedural aneurysm rerupture in relation to timing of endovascular treatment and outcome. J Neurol Neurosurg Psychiatry. (2019) 90:363–5. doi: 10.1136/jnnp-2018-318090
34. Gonzalez, N, Sedrak, M, Martin, N, and Vinuela, F. Impact of anatomic features in the endovascular embolization of 181 anterior communicating artery aneurysms. Stroke. (2008) 39:2776–82. doi: 10.1161/STROKEAHA.107.505222
35. Hauck, EF, Tawk, RG, Karter, NS, Binning, MJ, Khalessi, AA, Natarajan, SK, et al. Use of the outreach distal access catheter as an intracranial platform facilitates coil embolization of select intracranial aneurysms: technical note. J Neurointerv Surg. (2011) 3:172–6. doi: 10.1136/jnis.2010.002535
36. Turk, A, Manzoor, MU, Nyberg, EM, Turner, RD, and Chaudry, I. Initial experience with distal guide catheter placement in the treatment of cerebrovascular disease: clinical safety and efficacy. J Neurointerv Surg. (2013) 5:247–52. doi: 10.1136/neurintsurg-2011-010256
37. Lin, LM, Colby, GP, Huang, J, Tamargo, RJ, and Coon, AL. Ultra-distal large-bore intracranial access using the hyperflexible Navien distal intracranial catheter for the treatment of cerebrovascular pathologies: a technical note. J Neurointerv Surg. (2014) 6:301–7. doi: 10.1136/neurintsurg-2013-010772
38. Chandran, A, Puthuran, M, Eldridge, PR, and Nahser, HC. Distal access using hyperflexible atraumatic distal tip with optimized proximal stability of the benchmark intracranial guide catheter for the treatment of cerebral vascular diseases: a technical note. J Neurointerv Surg. (2016) 8:718–21. doi: 10.1136/neurintsurg-2015-011788
Keywords: distal access catheter, intraoperative aneurysm rupture, intraoperative complication, intraoperative rupture, subarachnoid hemorrhage, balloon guiding catheter, endovascular treatment, hemorrhagic complication
Citation: Fuga M, Ishibashi T, Aoki K, Kato N, Kan I, Hataoka S, Nagayama G, Sano T, Tanaka T and Murayama Y (2024) Intermediate catheter use is associated with intraprocedural rupture during coil embolization of ruptured intracranial aneurysms: a retrospective propensity score-matched study. Front. Neurol. 15:1401378. doi: 10.3389/fneur.2024.1401378
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Redi Rahmani, Barrow Neurological Institute (BNI), United StatesWaldo Rigoberto Guerrero, University of South Florida, United States
Shancai Xu, First Affiliated Hospital of Harbin Medical University, China
Copyright © 2024 Fuga, Ishibashi, Aoki, Kato, Kan, Hataoka, Nagayama, Sano, Tanaka and Murayama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michiyasu Fuga, ZnVnYW1pY2hpeWFzdUBpY2xvdWQuY29t
 Michiyasu Fuga
Michiyasu Fuga Toshihiro Ishibashi
Toshihiro Ishibashi Ken Aoki2
Ken Aoki2 Shunsuke Hataoka
Shunsuke Hataoka Toshihide Tanaka
Toshihide Tanaka