- 1Graduate School of Media and Governance, Keio University, Fujisawa, Japan
- 2NTT Communication Science Laboratories, NTT Corporation, Atsugi, Japan
- 3Keio Research Institute at SFC, Keio University, Fujisawa, Japan
- 4Faculty of Health Sciences, Department of Shizuoka Physical Therapy, Tokoha University, Shizuoka, Japan
- 5Faculty of Environment and Information Studies, Keio University, Fujisawa, Japan
Task-specific focal dystonia (TSFD), characterized by the loss of fine motor control and coordination, affects drummers’ lower-limb movements. This study explores lower-limb dystonia’s impact on drumming performance and underlying muscle activity in a professional rock drummer. The drummer executed an eight-beat pattern on a drum kit. The participant reported the occurrence of symptoms when he felt the abnormality such as the loss of control related to involuntary aspects of movement. We measured the peak amplitude of the bass drumhead vibration, synchronization errors as the time elapsed between the metronome onset and the bass drum onset, and amplitude of electromyographic (EMG) recordings centered on metronome beat. Dystonia symptoms primarily manifested in the initial beat, with fewer symptoms on syncopation of the third beat. Analysis revealed decreased bass-drum peak amplitude and earlier synchronization error during the initial beat. EMG measurements of 10 muscles in the affected right lower limb showed significant changes in the Biceps Femoris (BF), Tibialis Anterior (TA), Extensor Digitorum Longus (EDL), and Extensor Digitorum Brevis (EDB) muscles during symptom onset. We observed (1) earlier overactivation of the TA and EDL muscles during the leg lift-up motion or preparatory phase of pedaling, (2) reduced activation of the EDB muscle, and (3) increased activation of the BF muscle during the final pedaling movement when symptoms occurred. These findings suggest that lower-limb dystonia symptoms are characterized by a reduction in amplitude of the bass drumhead vibration and an increase in synchronization error, potentially due to premature overactivation of the ankle dorsiflexor muscles.
1 Introduction
Task-specific focal dystonia (TSFD), characterized by the loss of fine motor control and coordination, significantly affects musicians’ careers (1). The body parts commonly affected include the right hand in pianists, the left hand in violinists (2), the embouchure in wind instrument players (3), the upper limb in drummers and percussionists (4), and the lower limb in drummers (5–7). Reports on TSFD in drummers are limited compared with those on string, keyboard, and wind instrument players. There is a lack of knowledge regarding how dystonia affects drumming performance and the underlying muscle activities in drummers.
A previous study by Lee and Altenmüller (6) reported the drumming performance and underlying muscle activities in a patient performing a uniform accelerando motion with the lower limbs. In this study, the patient could perform this motion with the unaffected left leg up to a maximum speed of approximately 8 Hz, during which alternating muscle contractions were observed between the ankle plantar flexor and dorsiflexor muscles with minimal activation of the thigh muscles. Conversely, with the right leg affected, the patient could not maintain uniform acceleration, and the maximum speed for a stable rhythm was only 4 to 5 Hz. Notably, clear muscle co-contraction was observed in the thigh muscles of the affected right lower limb. Lee and Altenmüller (6) suggested that the control of drumming speed is compromised by lower-limb dystonia, and the co-activation of the thigh muscles is a characteristic of this symptom.
While the study by Lee and Altenmüller (6) provided valuable insights into the characteristics of lower limb dystonia symptoms in drummers, there have been no case reports on drumming performance and the underlying muscle activities when a drummer performs a pattern on a drum kit. Therefore, our study aimed to investigate these aspects in a professional rock drummer experiencing lower limb dystonia symptoms.
2 Methods
2.1 Participant
A 36-year-old male professional rock drummer participated in the study. He reported increased difficulty in his right lower extremity while playing with the drum kit. He began drumming at age 14 and practiced for 1–2 h daily. After enrolling in a music college, he dropped out during his junior year and started his career as a professional drummer at the age of 20, practicing 4 h a day. The first symptoms appeared at the age of 24 during a nationwide tour, manifesting as discomfort in the right foot. He experienced difficulty climbing stairs and driving when the symptoms were severe. To alleviate these issues, he adopted a ‘sensory trick,’ modifying his footwear and adjusting his chair height, which temporarily improved his symptoms. However, the relief was short-lived, and the effectiveness of the sensory trick diminished over time. He was diagnosed at age 29 by a neurologist. His family history was negative for any neurological disorders. Examination revealed a normal gait and no motor or sensory deficits in the lower limbs.
Ethical approval for this study was obtained from the Communication Science Laboratories Research Ethics Committee of Nippon Telegraph and Telephone Corporation (Approval Number: H30-009). The experiments were conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from the participant before the study.
2.2 Experimental setup and task
The experimental setup is illustrated in Figure 1A. The participant was instructed to play an eight-beat drumming pattern on a drumming kit at a tempo of 80 beats per minute (BPM), equivalent to 750 ms between adjacent metronome sounds, as illustrated in Figure 1B. This drumming pattern is a single chunk of four metronome sounds, defined as 1 bar. The task was performed while listening to a pacing metronome-tone sequence via earphones. The dashed vertical lines in Figure 1B denote the beats of the metronome-tone. The pattern involved playing a hi-hat, snare drum, or bass drum. Specifically, for the bass drum, the participant was required to strike two notes per bar, the first on the initial beat and the second on the syncopation of the third beat, represented by the orange and green notes in Figure 1B, respectively.
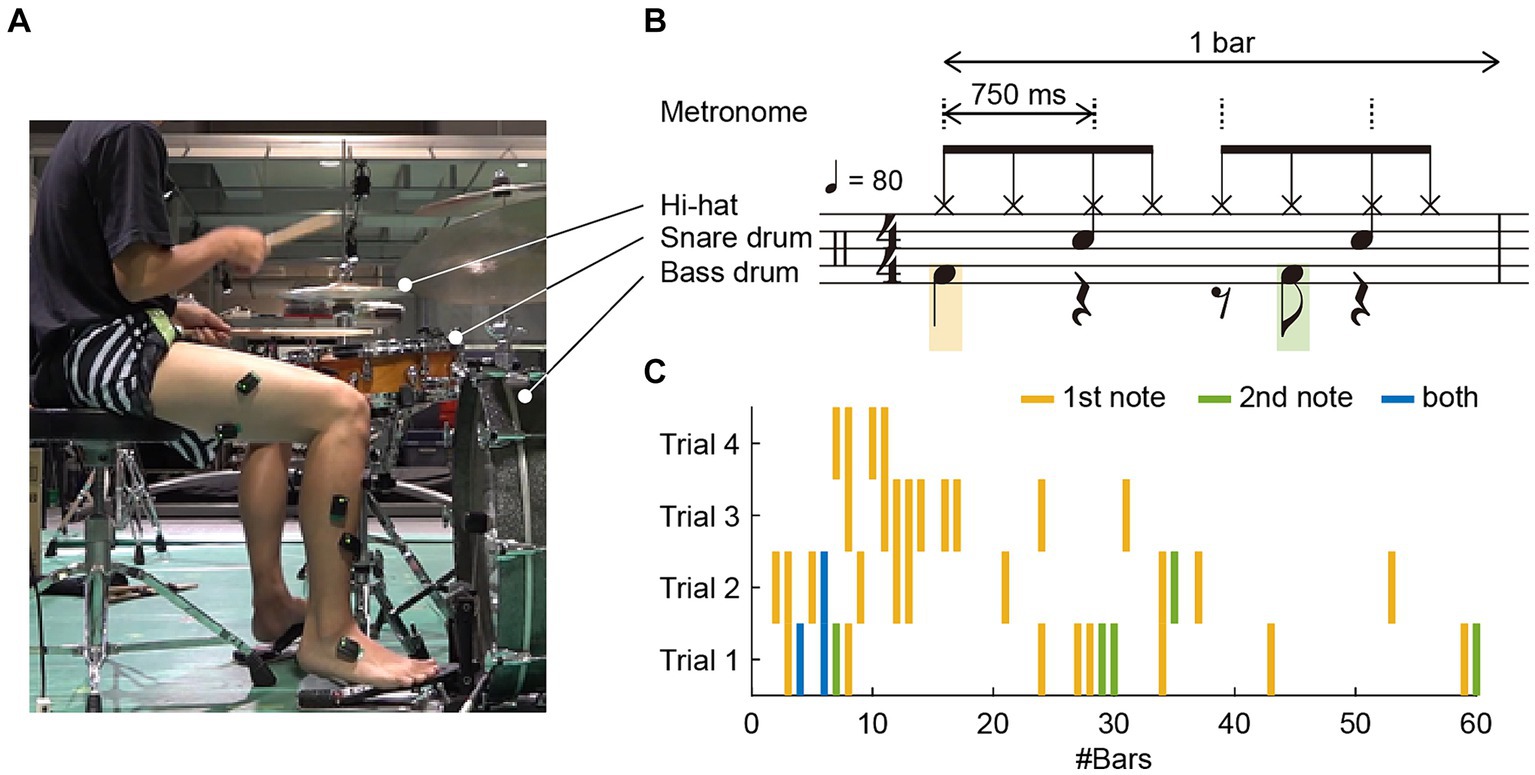
Figure 1. (A) Experimental setup: The participant was instructed to play an eight-beat drumming pattern on a drum kit at a tempo of 80 beats per minute (BPM) and to verbally report any occurrences of dystonia symptoms. (B) The pattern involved playing the high-hat, snare drum, and bass drum. For the bass drum, the participant was required to strike two notes per bar: the first on the initial beat and the second as a syncopation of the third beat, represented by orange and green notes, respectively. A bar consisted of four metronome tones, indicated by dashed vertical lines, denoting the beats of the drumming pattern. (C) Occurrence of dystonia symptoms across four trials: Forty-two instances of symptoms were reported; 31 occurred solely on the first note in a bar, 5 occurred only on the second note, and 3 occurred on both the first and second notes, as indicated by orange, green, and blue vertical lines, respectively.
The participant completed 60 bars in each trial. The start of a trial was signaled by a 1 kHz pure tone, followed by four metronome tones. The experiment comprised four trials. The participant was allowed a rest period of at least 1 min between trials. During the trials, the participant was instructed to promptly and verbally report as an occurrence of the dystonia symptom when he felt an abnormality.
2.3 Data acquisition
A piezoelectric sensor (Model DT-10, YAMAHA) was attached to the head of the bass drum. The value of the piezo sensor indicates the vibration amplitude of the drumhead of the bass drum. The greater the amplitude of the vibration, the greater the sound of the bass drum. Simultaneously, we captured the piezoelectric sensor signal, metronome sound, and trigger signal for the Electromyographic (EMG) sensors using an audio interface (Fireface UCX, RME Audio) with a sampling rate of 48,000 Hz. The input signals were normalized so that the upper limit of the input voltage level was 1 when the audio interface processed the analog-to-digital conversion.
EMG activity was measured using active electrodes (Trigno IM sensor, Delsys Inc.). The EMGs recorded the muscle activities from 10 muscles in the affected right lower limb: (1) Rectus Femoris (RF), (2) Vastus Lateralis (VL), (3) Vastus Medialis (VM), (4) Biceps Femoris (BF), (5) Tibialis Anterior (TA), (6) Extensor Digitorum Longus (EDL), (7) Soleus (SOL), (8) Gastrocnemius (GAS), (9) Peroneus Longus (PL), and (10) Extensor Digitorum Brevis (EDB). Each sensor was carefully placed on shaved skin to avoid innervation zones (8). The EMG signals were amplified, and the sampling rate was set to 1,111 Hz.
2.4 Data analysis
To analyze the bass-drum and metronome sound signals, we identified the onset times as the points at which the envelope signals exceeded 10% of the peak amplitude of each burst. We calculated the synchronization error, defined as the time difference between the onsets of the metronome and bass drum, to assess the performance of the bass drum. In addition, the peak amplitude of the bass-drum sound recorded by the piezoelectric sensor was measured for each beat.
The EMG signals were synchronized with the piezo and metronome signals using the trigger signal. Initially, the EMG signals were rectified, followed by the calculation of the root mean square (RMS) using a 30-millisecond time window. To visualize muscle activation patterns with and without symptoms, we plotted the RMS of the EMG signals for a period of 750 ms before and after the onset of each metronome sound.
To assess the co-activation of agonist and antagonist muscle pairs, we calculated the ratio and sum of antagonist/agonist (AA) muscle contraction (9) for a period of 750 ms before and after the onset of each metronome sound. As pairs of AA muscles, we chose RF-BF, TA-SOL, and TA-GAS. The AA ratio and AA sum between RF and BF muscles indicate the degree of knee-joint extension of the equilibrium-joint angles and the increase in joint stiffness, respectively. The AA ratio and AA sum between TA and SOL muscles and between TA and GAS muscles indicate the degree of ankle dorsiflexion of the equilibrium-joint angles and the increase in joint stiffness, respectively [see also, Hirai et al. (9) for details].
3 Results
3.1 Drumming performance
We identified 42 instances of symptom occurrence (Figure 1C): 31 occurred only on the first note in a bar, five occurred only on the second note, and three occurred on both the first and second notes, indicated by orange, green, and blue vertical lines, respectively, in Figure 1C. This means that 34 instances (81%) occurred on the initial beat, and eight (19%) occurred on the syncopation of the third beat. The drummer reported that these symptoms occurred involuntary.
Owing to the recording failure of the EMG signals, the data from the first trial were excluded from the analysis, and the data of 1 bar from the last three trials were omitted from the analysis due to electrical noise. Thus, a total of 179 bars (60 bars × two trials +59 bars × one trial), were analyzed. During these trials, 25 instances of symptom occurrence were recorded: 23 occurred only on the first note in a bar, one occurred only on the second note, and one occurred on both the first and second notes (as illustrated in Trials 2–4 in Figure 1C). Given that approximately 96% of the symptoms (24 out of 25 instances) occurred in the first note or the first beat, our analysis primarily focused on the performance and EMG signals corresponding to the first beat in each bar. Specifically, of the initial 179 beats per bar in the last three trials, symptoms occurred in 24 beats, whereas no symptoms were observed in the remaining 155 beats. As most symptoms occurred only on the first note of the bass drum, we extracted 750 milliseconds before and after the first beat for further analysis.
The peak amplitude of the bass-drum sound and the synchronization error during bass-drum play are illustrated in Figure 2. The light blue dots and lines represent data without symptoms (n = 154), whereas the orange dots and lines indicate data with symptoms (n = 25). The mean and standard deviation (SD) of the peak amplitude without symptoms were 0.30 ± 0.03 [arbitrary units (a.u.)], whereas with symptoms, they were 0.18 ± 0.09 [a.u.]. The coefficients of variance were 0.10 and 0.52 for without and with symptoms, respectively. The mean amplitudes with symptoms were significantly lower than those without symptoms (Wilcoxon-Mann–Whitney Test: Z = 6.07, p < 0.01, r = 0.45).
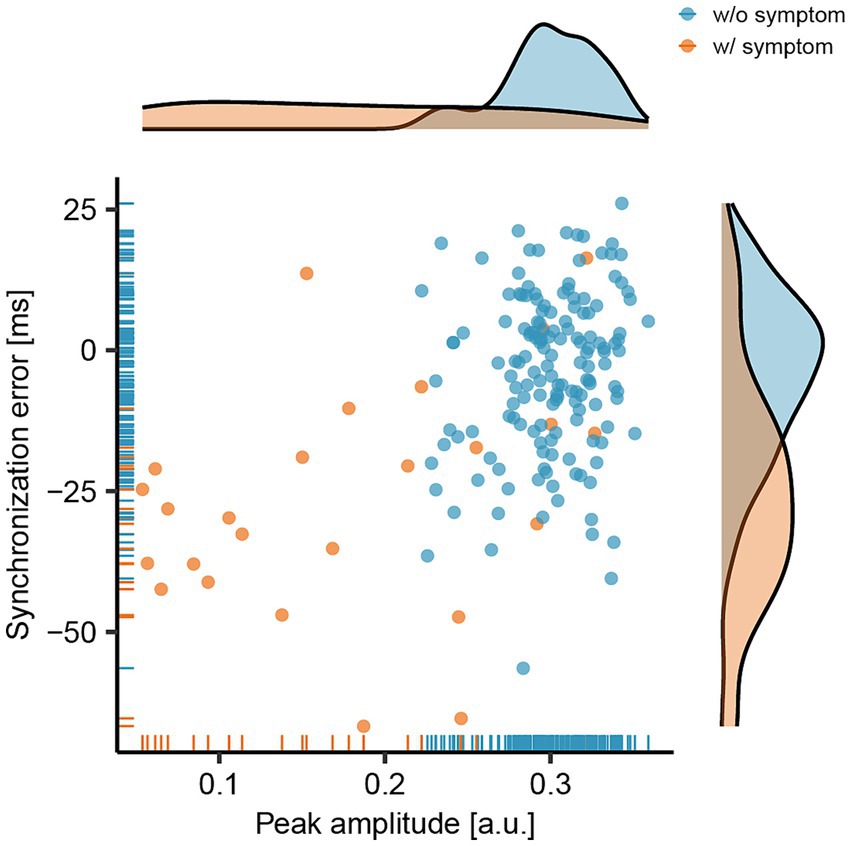
Figure 2. Relationship between the peak amplitude of the bass-drum sound and the synchronization error between the onsets of the metronome and the bass-drum sounds. The light blue dots and lines represent data collected without the presence of symptoms (w/o symptom), while the orange dots and lines indicate data collected with symptoms (w/ symptom).
The mean and SD of synchronization errors without symptoms were − 4.05 ± 14.50 [milliseconds (msec)], and with symptoms, they were − 26.22 ± 20.83 [msec]. The mean synchronization errors with symptoms were significantly earlier than those without symptoms (Wilcoxon-Mann–Whitney Test: Z = 5.00, p < 0.01, r = 0.37). Overall, when symptoms occurred, the peak amplitude of the bass-drum sound decreased, and the synchronization error increased (became earlier).
3.2 Muscle activity
The RMS values of the EMG signals from the RF, VL, VM, BF, TA, EDL, SOL, GAS, PL, and EDB muscles are shown in Figure 3. The data were plotted for 750 milliseconds (msec) before and after the onset of each metronomic sound. The light-blue lines and areas represent the ensemble average and 95% confidence interval across the 155 instances without symptoms, whereas the orange lines and areas correspond to the 24 instances with symptoms. The heat maps display each of the RMS data sorted by the occurrence of symptoms and then by the degree of synchronization errors. Data above the red horizontal lines in the heat maps indicate instances of symptoms, whereas data below these lines represent those without symptoms. Data positioned higher on the heatmap correspond to EMG activity with earlier synchronization errors.

Figure 3. The root mean square (RMS) of the electromyographic (EMG) signals measured from 10 lower-limb muscles: Rectus Femoris (RF), Vastus Lateralis (VL), Vastus Medialis (VM), Biceps Femoris (BF), Tibialis Anterior (TA), Extensor Digitorum Longus (EDL), Soleus (SOL), Gastrocnemius (GAS), Peroneus Longus (PL), and Extensor Digitorum Brevis (EDB). Data were plotted for a period of 750 milliseconds (msec) before and after the onset of each metronome sound. Light blue lines and areas represent the ensemble average and the 95% confidence interval for instances without symptoms (w/o symp.), while orange lines and areas correspond to instances with symptoms (w/ symp.). The heatmaps display each set of RMS data, organized by the occurrence of symptoms and then by the degree of synchronization errors. In the heatmaps, data above the red horizontal lines indicate instances with symptoms, and data below these lines represent those without symptoms. Higher positions on the heatmap correspond to EMG activities with earlier synchronization errors.
There were slight differences in the EMG patterns; however, the overall activities of the RF, VL, VM, SOL, GAS, and PL muscles were comparable between instances with and without symptoms. Conversely, notable differences were observed in the BF, TA, EDL, and EDB during the onset of symptoms. Specifically, TA and EDL activities shifted earlier and increased in magnitude, whereas the BF activity increased without a time shift. In contrast, the EDB activity shifted earlier and decreased when symptoms occurred.
As for the ratio and sum of AA muscle contraction, we found an increased AA ratio and AA sum between BF and RF muscles when the dystonia symptoms occur during the final kick-pedaling movement around the onset of the metronome sound (see the orange lines in the left panel in Figure 4). We also found an earlier shift of AA ratio and increased AA sum between TA and SOL muscles and between TA and GAS muscles when the dystonia symptoms occur during the leg lift-up motion or preparatory motion of pedaling before the onset of the metronome sound (see the orange lines in the middle and right panels in Figure 4).
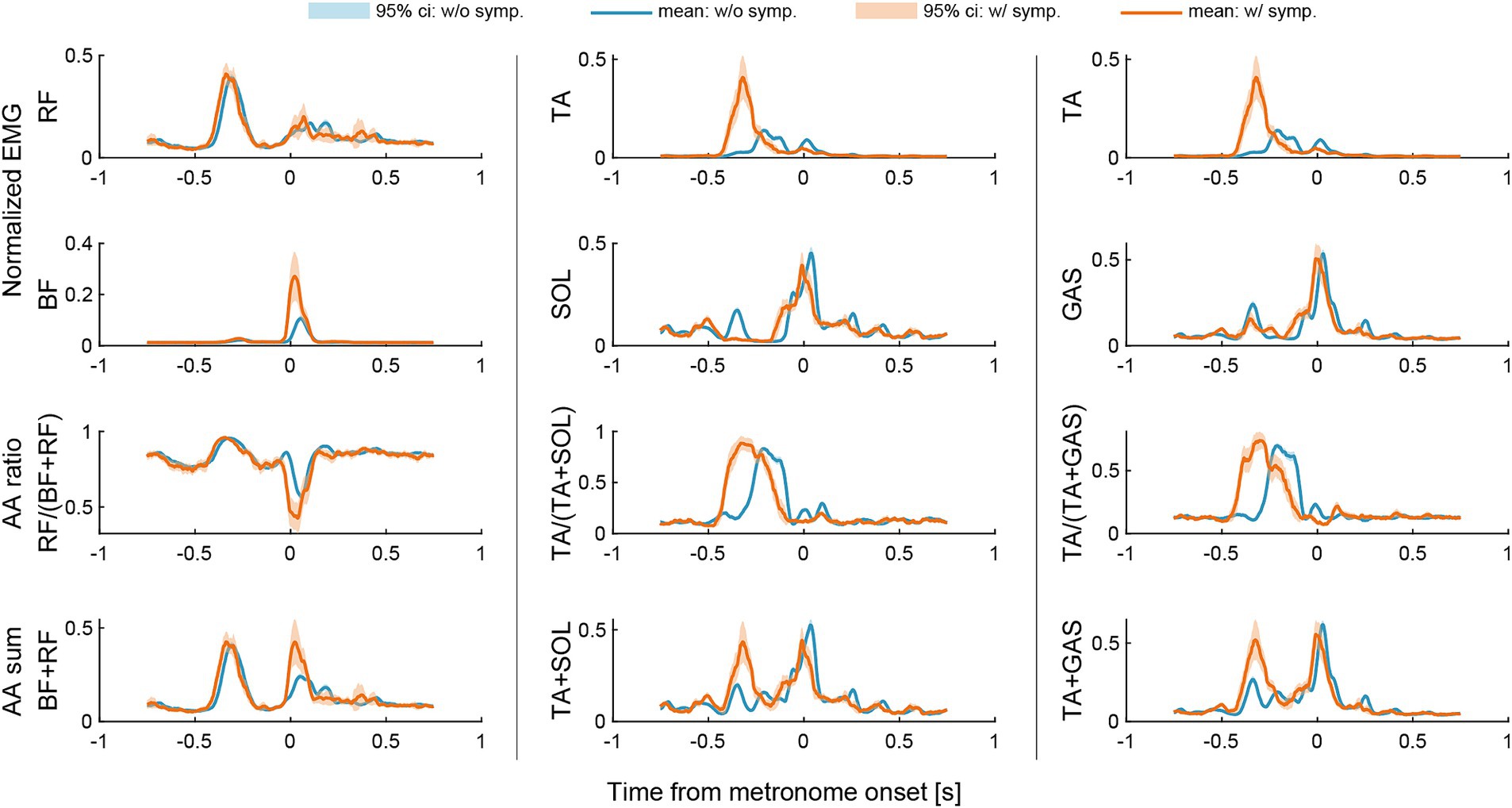
Figure 4. Changes in agonist–antagonist (AA) ratios and AA sums around the metronome onset of the first beat. The AA ratio contributes to the equilibrium position of the joint angle, and the AA sum indicates the stiffness of the joint. Data were plotted for a period of 750 milliseconds (msec) before and after the onset of each metronome sound. Light blue lines and areas represent the ensemble average and the 95% confidence interval for instances without symptoms (w/o symp.), while orange lines and areas correspond to instances with symptoms (w/symp.).
4 Discussion
This study aimed to explore the impact of lower limb dystonia symptoms on drumming performance and the underlying muscle activities in a professional rock drummer. The drummer performed an eight-beat pattern on a drum kit, focusing on kicking the bass drum on the initial beat and syncoping with the third beat. The drummer reported the occurrence of dystonia symptoms, with most symptoms manifesting on the initial beat and a smaller percentage occurring on the syncopation of the third beat. Our analysis of drumming performance indicated that during the initial beat, there was a reduction in the peak amplitude of the bass-drum sound and an increase (becoming earlier) in the synchronization error. The EMG measurements of 10 muscles in the affected right lower limb demonstrated notable differences in the Biceps Femoris (BF), Tibialis Anterior (TA), Extensor Digitorum Longus (EDL), and Extensor Digitorum Brevis (EDB) muscles during symptom occurrence.
4.1 Effect of dystonia symptoms on the downbeat
In our study, the drummer reported lower limb dystonia symptoms primarily on the initial beat, with fewer symptoms appearing upon syncopation of the third beat during an eight-beat drumming pattern. The symptoms were notably more pronounced on the downbeats (i.e., the count “one”) than on the upbeats (i.e., the syncopated beat following the count “three”). The predominance of symptoms during the initial beat may be attributed to factors related to attentional allocation and predictive timing mechanisms. According to the dynamic attending theory, attention is hierarchically allocated over time during the processing of hierarchical rhythmic structures (10–13). In the context of the eight-beat pattern used in our study, a strong attentional focus is likely directed toward the initial beat or the count “one.” Additionally, a neuroimaging study using magnetoencephalography (MEG) by Fujioka et al. (14) revealed that β-band event-related desynchronization is more pronounced at the downbeats compared to the upbeats. Fujioka et al. (14) suggested that the increased β-band activity at the downbeats is indicative of neural processing related to predictive timing or the conversion of timing information into auditory-motor coordination. Based on the findings of Fujioka et al. (14), we propose that the occurrence of dystonia symptoms in drummers may be linked to predictive timing mechanisms or the conversion of timing information into auditory-motor coordination.
4.2 Impact of dystonia symptoms on drumming performance
Our findings indicate a significant reduction in the peak amplitude of the bass-drum play when dystonic symptoms occur. The average peak amplitude with symptoms was measured at 0.18, compared to 0.30 without symptoms. This implied that the relative peak amplitude with symptoms was approximately 60% of that without symptoms, indicating a 40% loss in amplitude. The SD of the peak amplitude without and with symptoms was 0.03 and 0.09, respectively, and the CV of the peak amplitude without and with symptoms was 0.10 and 0.52, respectively, showing larger variability with symptoms. Maintaining a strong bass-drum sound during the initial downbeat is essential for rock music performance. Previous research comparing skilled drummers with unskilled non-drummers found that skilled drummers demonstrated significantly less variability in tapping force, underscoring the importance of stable drumming performance (15, 16). Therefore, it can be inferred that a reduction in peak amplitude may lead to a more variable performance, making it challenging for the drummer to maintain a steady rock beat.
Additionally, this study found that dystonia symptoms resulted in increased synchronization errors in bass-drum playing during the eight-beat pattern at 80 BPM. The average synchronization errors were − 26.22 msec with symptoms and − 4.02 msec without symptoms. A previous study investigating the synchronization error in drum kit playing at 60 and 120 BPMs by professional drummers reported average synchronization errors of −12.91 and − 8.91 msec, respectively, across 15 professional drummers (17). The synchronization error without symptoms in our study (−4.05 msec) was comparable to or even smaller than those reported in the previous study. However, with symptoms, the synchronization error (−26.22 msec) was significantly larger, approximately double or triple the average error observed in professional drummers in the previous study (−12.91 and − 8.91 msecs). Therefore, the extent of synchronization errors with symptoms in our study was considered substantial, potentially impeding professional drummers’ ability to maintain precise timing.
4.3 Muscle activity in the presence of dystonia symptoms
In the absence of dystonia symptoms, sequential muscle activity associated with drum-pedaling movements was observed. Specifically, the rectus femoris (RF) muscle was activated for thigh lifting (hip flexion), whereas the gastrocnemius (GAS), soleus (SOL), and peroneus longus (PL) muscles extended the foot downward (ankle plantar flexion). The tibialis anterior (TA) and extensor digitorum longus (EDL) muscles facilitated upward foot extension (ankle dorsiflexion), while the extensor digitorum brevis (EDB) also contributed to toe extension. The vastus lateralis (VL) was active during knee extension, and the SOL, GAS, PL, vastus medialis (VM), biceps femoris (BF), and EDB were involved in the final striking action of the kick pedal.
In the presence of dystonia symptoms, three significant changes in muscle activity were observed: (1) earlier overactivation of the TA and EDL muscles and disappearance of the first peak of the SOL muscle during the leg lift-up motion or preparatory motion of pedaling; (2) subsequent diminished activation of the EDB muscle; and (3) overactivation of the BF muscle during the final pedaling movement. Premature overactivation of the TA and EDL muscles may cause excessive dorsiflexion of the ankle joint at an unintended earlier phase during symptom occurrence. Excessive dorsiflexion can impede subsequent toe extension, leading to reduced EDB muscle activation. It is possible that when the EDL muscle is overactivated, the EDB muscle is in a shortened position and is less capable of contracting. Consequently, the drummer might activate the BF muscle and extend the hip to compensate for the movement, aiming to achieve the final striking action of the kick pedal. These unintended muscle activities and movements could result in an earlier and insufficient kick of the pedal, contributing to decreased amplitude and increased synchronization errors in drumming performance. These unintentional muscle activities, movements, and altered performance are likely the reasons why the participants could clearly report the occurrence of symptoms.
A previous study by Lee and Altenmüller (6) suggested that lower limb dystonia is characterized by the co-activation of the thigh muscles (BF and RF). When we assessed the co-activation of agonist and antagonist (AA) muscle pairs using the measures of AA ratio and AA sum (9), we found an increased AA ratio and AA sum between BF and RF muscles during the final pedaling movement (Figure 4). The results suggest not only increased co-activation of the thigh muscles but also increased knee-joint extension of the equilibrium-joint angle and increased joint stiffness during the final pedaling movement when the dystonia symptoms occur. This result is consistent with the previous study by Lee and Altenmüller (6) in terms of the increased co-activation of the thigh muscles when the dystonia symptoms occur. The results also suggest that the dystonia symptoms might be characterized not only by increased co-activation of the thigh muscles but also by increased joint stiffness (9).
It is noteworthy that we also found an earlier shift of AA ratio and increased AA sum between TA and SOL muscles and between TA and GAS muscles during the leg lift-up motion or preparatory motion of pedaling (Figure 4). The results suggest increased ankle dorsiflexion of the equilibrium-joint angle and increased joint stiffness during the leg lift-up motion or preparatory motion of pedaling when the dystonia symptoms occur. This finding was not reported in the previous study by Lee and Altenmüller (6). We suggest that this discrepancy could be attributed to the task difference. Namely, while the patient in the study by Lee and Altenmüller (6) performed a uniform accelerando motion task only with the lower limbs, the patient in this study performed a repetitive pattern on a drum kit at a constant tempo with both upper and lower limbs. Compared to the uniform accelerando motion with a limb, the drum pattern used in this study was more repetitive and familiar to the patient. In fact, the eight-beat drumming pattern performed in this study was commonly used by the patient when he was actively performing in his band. This suggests that feedforward predictive motor control was more pronounced in the drum pattern task in this study. The higher load of feedforward and predictive processes might cause the earlier overactivation of the ankle dorsiflexor muscles and increased co-contraction of ankle joint muscles.
Taken together with the results that the symptom was notably more pronounced on the downbeats than on the upbeats, the occurrence of dystonia symptoms in the drummer in this case may be linked to attentional predictive timing mechanisms or the conversion of timing information into feedforward, predictive auditory-motor coordination. Although we observed the co-contraction of thigh muscles (BF and RF), which was consistent with the study by Lee and Altenmüller (6), this may be interpreted as a compensatory movement following the earlier shift of the muscle activities and co-contraction of ankle joint muscles (EDL, TA, GAS, and SOL). Thus, in our study, lower limb dystonia in the drummer might be characterized more by earlier overactivation of the ankle dorsiflexor muscles and an earlier shift of co-contraction in ankle joint muscles.
5 Limitations
The present study has limitations and leaves room for questions to be addressed in future research. First, there was no comparison between the dystonic drummer and the general population of professional drummers. A comparison study will show the relationship among the peak amplitude of the sound, synchronization error, and early involvement of the dorsiflexor muscle of the ankle. At least, a comparison of the healthy and affected sides could have provided more information, as Lee and Altenmüller (6) did. Second, it should also be noted that piezoelectric sensors were not attached to the snare drum and hi-hat. It would be beneficial to evaluate whether the symptom on the right-lower limb affects the performance of other limbs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Communication Science Laboratories Research Ethics Committee of Nippon Telegraph and Telephone Corporation. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KH: Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing, Validation. SS: Data curation, Methodology, Writing – review & editing. MiK: Methodology, Writing – review & editing. SY: Writing – review & editing. SK: Methodology, Writing – review & editing. MaK: Resources, Writing – review & editing. SF: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by a JST COI-NEXT grant (no. JPMJPF2203), a JST PRESTO grant (no. JPMJPR23S9), and a KGRI Pre-startup Grant awarded to SF.
Acknowledgments
We thank T. Narita for her help with recording and editing the videos.
Conflict of interest
KH and MK were employed by the NTT Communication Science Laboratories (Nippon Telegraph and Telephone Corporation, Japan).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schuele, SU, and Lederman, RJ. Occupational disorders in instrumental musicians. Med Probl Perform Art. (2004) 19:123–8. doi: 10.21091/mppa.2004.3021
2. Lim, VK, Altenmüller, E, and Bradshaw, JL. Focal dystonia: current theories. Hum Mov Sci. (2001) 20:875–914. doi: 10.1016/s0167-9457(01)00076-8
3. Steinmetz, A, Stang, A, Kornhuber, M, Röllinghoff, M, Delank, K-S, and Altenmüller, E. From embouchure problems to embouchure dystonia? A survey of self-reported embouchure disorders in 585 professional orchestra brass players. Int Arch Occup Environ Health. (2014) 87:783–92. doi: 10.1007/s00420-013-0923-4
4. Bledsoe, IO, Reich, SG, Frucht, SJ, and Goldman, JG. Twelve drummers drumming… with dystonia. Tremor Other Hyperkinet Mov. (2021) 11:6. doi: 10.5334/tohm.577
5. Rosset-Llobet, J, Fàbregas-Molas, S, and Pascual-Leone, Á. Drummer’s lower limb dystonia. J Neurol. (2012) 259:1236–7. doi: 10.1007/s00415-011-6324-2
6. Lee, A, and Altenmüller, E. Heavy metal curse: a task-specific dystonia in the proximal lower limb of a professional percussionist. Med Probl Perform Art. (2014) 29:174–6. doi: 10.21091/mppa.2014.3035
7. Asahi, T, Taira, T, Ikeda, K, Yamamoto, J, and Sato, S. Full recovery from drummer’s dystonia with foot and arm symptoms after stereotactic ventro-oral thalamotomy: a case report. Acta Neurochir. (2018) 160:835–8. doi: 10.1007/s00701-018-3480-5
8. SENIAM. Recommendations for determination of sensor location. Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles Project, (1999). Available at: (http://seniam.org/).
9. Hirai, H, Miyazaki, F, Naritomi, H, Koba, K, Oku, T, Uno, K, et al. On the origin of muscle synergies: invariant balance in the co-activation of agonist and antagonist muscle pairs. Front Bioeng Biotechnol. (2015) 3:192. doi: 10.3389/fbioe.2015.00192
10. Jones, MR, and Boltz, M. Dynamic attending and responses to time. Psychol Rev. (1989) 96:459–91. doi: 10.1037/0033-295X.96.3.459
11. Large, EW, and Jones, MR. The dynamics of attending: how people track time-varying events. Psychol Rev. (1999) 106:119–59. doi: 10.1037/0033-295X.106.1.119
12. Fitzroy, AB, and Sanders, LD. Musical meter modulates the allocation of attention across time. J Cogn Neurosci. (2015) 27:2339–51. doi: 10.1162/jocn_a_00862
13. Large, EW, Herrera, JA, and Velasco, MJ. Neural networks for beat perception in musical rhythm. Front Syst Neurosci. (2015) 9:159. doi: 10.3389/fnsys.2015.00159
14. Fujioka, T, Ross, B, and Trainor, LJ. Beta-band oscillations represent auditory beat and its metrical hierarchy in perception and imagery. J Neurosci. (2015) 35:15187–98. doi: 10.1523/JNEUROSCI.2397-15.2015
15. Fujii, S, and Oda, S. Tapping speed asymmetry in drummers for single-hand tapping with a stick. Percept Mot Skills. (2006) 103:265–72. doi: 10.2466/pms.103.1.265-272
16. Fujii, S, Kudo, K, Shinya, M, Ohtsuki, T, and Oda, S. Wrist muscle activity during rapid unimanual tapping with a drumstick in drummers and nondrummers. Mot Control. (2009) 13:237–50. doi: 10.1123/mcj.13.3.237
Keywords: task-specific focal dystonia, drummer, electromyography, synchronization error, movement disorder
Citation: Honda K, Sata S, Komine M, Yamaguchi S, Kim S, Kashino M and Fujii S (2024) Drumming performance and underlying muscle activities in a professional rock drummer with lower-limb dystonia: a case study. Front. Neurol. 15:1398476. doi: 10.3389/fneur.2024.1398476
Edited by:
Lucio Marinelli, University of Genoa, ItalyReviewed by:
Cecile Gallea, INSERM U1127 Institut du Cerveau et de la Moelle épinière (ICM), FranceTakanori Oku, Shibaura Institute of Technology, Japan
Copyright © 2024 Honda, Sata, Komine, Yamaguchi, Kim, Kashino and Fujii. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shinya Fujii, ZnVqaWkuc2hpbnlhQGtlaW8uanA=
 Kazuaki Honda
Kazuaki Honda Shizuka Sata
Shizuka Sata Mizuki Komine1
Mizuki Komine1 SungHyek Kim
SungHyek Kim Shinya Fujii
Shinya Fujii