
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 05 December 2024
Sec. Movement Disorders
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1397766
Background: Tic disorder, a chronic neurodevelopmental disorder that typically onsets during childhood, is characterized by sudden, involuntary, rapid, and non-rhythmic motor and vocal tics. Individuals with tic disorders often experience physical health issues. The purpose of our retrospective analysis was to elucidate the common comorbid physical diseases and mental disorders and their characteristics of outpatient children with tic disorders in a large public children’s hospital in China over the past 5 years.
Methods: Participants were outpatients with tic disorders who visited Beijing Children’s Hospital, from January 1, 2018 to December 31, 2022. After the inclusion screening, a total of 523,462 patient visits were included in the analysis. Based on the International Classification of Diseases, 10th Revision (ICD-10) diagnostic system, we employed descriptive statistical analysis to examine the frequently co-occurring somatic diseases in patients with tic disorders, as well as the influence of variables such as age and seasonal variation on these comorbidities.
Results: The top five diseases of total outpatient visits were as follows: Respiratory diseases, Mental and behavioral disorders, Diseases of the eye and adnexal, Digestive disorders, Diseases of the skin and subcutaneous tissue. Among the top five comorbid disease system, the most commonly third- level classification of diseases were upper respiratory tract infections, attention deficit and hyperactivity disorder, conjunctivitis, dyspepsia, dermatitis. Respiratory system diseases experienced a peak in April, while the other four types of diseases reached their peak in August. Additionally, each disease system showed the lowest number of patient visits in February. Additional to the mental and behavioral disorders, the other four disease systems would experience a peak in medical visits between the ages of 4 and 6.
Conclusion: Our study highlighted the most common physical diseases and mental disorders in tic disorders, namely the respiratory diseases, specifically upper respiratory tract infections, and mental and behavioral disorders, with ADHD being the most common co-occurring condition.
Tic disorder, a chronic neurodevelopmental disorder that typically onsets during childhood (between the ages of 4–7), is characterized by sudden, involuntary, rapid, and non-rhythmic motor and vocal tics (1–3). The prevalence of common childhood psychiatric disorders, such as tic disorder, attention deficit hyperactivity disorder (ADHD), and autism spectrum disorder (ASD), has been found to be higher in boys than in girls on a global scale (4–6). According to a 2018 epidemiological survey of children aged 6–16 in China, the prevalence of tic disorder was 2.5%, whose incidence significantly declined among adolescents aged 12 and older compared to the group aged 6–11 years (5), consistent with previous research (3, 7–9).
Tic disorder has been found to be highly comorbid with psychiatry disorders such as ADHD and OCD. However, exploration on comorbidity of tic disorder with physical diseases are rare (10). Recent studies have further confirmed the increasingly important role of autoimmune diseases in the onset of tic disorder (11–13). Previous studies have found a correlation between tics and autoimmune encephalitis, with tics being the most common movement disorder in the later stages of encephalitis, and in the aggressive treatment of encephalitis, the patient’s tics improved (14, 15). This may be related to the presence of anti-basal ganglia antibodies (ABGA) in the basal ganglia of patients with autoimmune encephalitis, which contribute to the development of ABGA tics (16). Reviews and studies on multigenerational family clusters suggested a familial aggregation of autoimmune diseases in relation to tic disorders (12, 17). The clinical concept of pediatric acute-onset neuropsychiatric syndrome (PANS) includes an acute onset of obsessive-compulsive behavior or food restriction with at least two severe acute onset neuropsychiatric symptoms (e.g., cognitive, and behavioral symptoms) (18). Inflammation and immune response mediated by Group A Streptococcus (GAS) infection play a predominant role in the development of this disorder (19). Previous studies have also found that patients with tic disorders with PANS have a different clinical presentation and comorbidities than tic patients without PANS, and have identified tic patients with PANS who have reduced levels of M antibodies and elevated CD8+ lymphocytes, findings that may predict the development of autoimmune neuropsychiatric disorders in patient populations (20). Pediatric autoimmune neuropsychiatric disorders (PANDAS) are considered a subset of PANS (18). Previous studies have found a correlation between PANDAS associated with GAS infection and tics, and have noted a clear temporal correlation between Streptococcal infection and the onset or exacerbation of tic symptoms in prepubertal children (21–23). Concurrent treatment of infection, inflammation, and psychiatric and behavioral symptoms in patients with comorbid PANS or PANDS has been found to be helpful in improving patients’ quality of life and social functioning (20).
A 40-year long-term cohort study revealed significant associations between tic disorder and metabolic and cardiovascular risks (24). Another review suggested that the ocular symptoms of benign essential blepharospasm and tic disorder exhibit similar manifestations (25), potentially indicating a common pathophysiological mechanism between ocular tics and blepharospasm (26). The latest research has found that compared to the normal control group, children with tic disorder exhibited lower meibomian gland length and area in their eyes (27). According to Baizabal-Carvallo et al. (28), oral tics were relatively less frequent, but in some cases, they may lead to severe self-injurious damage to oral tissues (29, 30). Budman (31) reported that oral herpesvirus infection potentially exacerbated the psychological and behavioral symptoms of tic disorders. Recent study has indicated an increase in Helicobacter pylori and Bacteroides in the gastrointestinal tract of tic disorder with coprolalia (32). However, current research on comorbid physical diseases in tic disorders is limited, necessitating further investigation. This area of study holds significant clinical value for both the prevention and treatment of tic disorders.
Furthermore, the latest study suggested a possible association between tic disorder and allergic diseases (33–35). Previous studies have also confirmed a positive correlation between tic disorder and asthma, allergic rhinitis, allergic conjunctivitis and eczema with the reason that tic disorders may have the same genetic susceptibility as allergic diseases. Among these, the diagnosis rate of allergic rhinitis was notably higher (36–38). Nevertheless, it is difficult to accurately determine which specific allergic disease is more likely to be accompanied by specific tic disorders (33). A study on serum immunoglobulin E (IgE) levels in children aged 6–9 with tic disorder showed higher IgE levels compared to the healthy control group (39). During the coronavirus disease 2019 (COVID-19) pandemic, it was observed that tic symptoms could worsen during respiratory virus infections (40, 41). Hoekstra (42) found that, compared to the adult Tourette’s disorder group, symptoms in the pediatric group worsen around 4 weeks after contracting a common cold. Therefore, investigating the physical diseases associated with tic disorders is of great significance for the management based on tic disorders and can effectively reduce the fluctuation of tic symptoms.
The purpose of our retrospective analysis is to elucidate the characteristics of children with tic disorder and the comorbidity of physical diseases in tic disorder patients. This analysis would include changes in annual outpatient volumes, comorbidity patterns with which physical diseases, the most common physical diseases, comorbidity rates of psychiatric disorders, age of onset, gender differences, and seasonal variations in outpatient visits. The findings might provide a better basis for preventing fluctuations in tic disorder symptoms and the comorbidity of physical diseases in the future.
The included sample consisted of all children with tic disorders who visited the outpatient department of Beijing Children’s Hospital of Capital Medical University (National Center for Children’s Health, China), from January 1, 2018 to December 31, 2022. Over the past 5 years, patients with tic disorders have sought medical attention at this hospital at least once due to physical illnesses. Patients with tic disorders and common physical illnesses and mental disorders met the diagnostic criteria of the International Statistical Classification of Diseases, 10th revision (ICD-10). We carefully crafted the inclusion and exclusion criteria to accurately delineate the participant cohort for our study.
The ICD-10 diagnostic system is a structured, multilevel classification that organizes diseases and health conditions into a comprehensive hierarchy for global health statistics and information. At the broadest level are the chapters, which categorize conditions into groups influenced by the affected body system or the nature of the disease. Within these chapters, conditions with common characteristics are further grouped into blocks. These blocks are then subdivided into categories, typically defined by the disease’s etiology and the body system it impacts. To achieve greater precision, categories can be divided into subcategories, detailing the specifics of the condition. Moreover, for enhanced detail, extension codes are used to encapsulate particulars such as the cause of an injury or how a disease presents itself. In this study, we have primarily utilized the first three levels of the ICD-10 diagnostic hierarchy, which includes chapters (first-level classification), blocks (second-level classification), and categories (third-level classification), to classify disease diagnoses.
Inclusion criteria:
1. Children aged below 18 years;
2. Diagnoses of physical and mental illnesses falling under the second-level classification of ICD-10, comply with the diagnostic criteria in ICD-10;
3. Children with tic disorders who have experienced at least one type of physical illness in the past 5 years.
Exclusion criteria:
1. Comorbid mental disorders not related to third-level classification;
2. Comorbid physical illnesses not related to third-level classification.
Following the specified Inclusion and Exclusion criteria, our study analyzed a cohort consisting of 41,788 patients (31,808 males and 9,980 females), with a total of 523,462 medical records of patients diagnosed with tic disorders. Our study focuses on the possible co-occurrence of other diseases in different individuals at different times, so we conducted a longitudinal analysis. The same patient with tic disorders who comes to the clinic may have a different comorbidity at each visit. To better capture this information, we included 523,462 patient visits for the final analysis. For more details, please see Figure 1.
We collected data on the number of outpatient visits for tic disorders who concurrently had common physical diseases and mental disorders over a five-year period. Specifically, this included (1) the annual number of outpatient visits per patient, (2) the total number of outpatient visits over the 5 years for each patient; (3) the total number of outpatient visits per year; (4) the total number of outpatient visits over the 5 years for each physical disease; (5) the total number of outpatient visits per month for each physical disease; (6) the total number of male and female patients for each physical disease; (7) the total number of outpatient visits for each age group for each physical disease; (8) the total number of outpatient visits over the 5 years for patients diagnosed with the second-level classification of the ICD-10 in each physical disease; and (9) the total number of outpatient visits over the 5 years for patients diagnosed with the third-level classification of the ICD-10 in each physical disease. For patients in each physical disease where the second-level classification and third-level classification were involved, the data from the top five categories based on the total number of outpatient visits were selected for analysis.
Continuous variables that conformed to normal distribution were described as mean ± standard deviations (SDs). Due to the skewed distribution of the hospitalization duration, its median with interquartile ranges (IQRs) was also used and presented. All categorical variables in the study were presented as counts.
To better show the distribution of the diseases, the top five first-level classification of the diseases in the ICD-10 were described in total, as well as by year, mouth, sex and age, respectively. Then the top five second-level classification and third-level classification of the diseases under the top five first classification in the ICD-10 were also presented. All statistical analyses in this study were conducted with John’s Macintosh Product (JMP) Pro 17.0.
From January 1, 2018 to December 31, 2022, the five-year annual average visitation data indicated that the annual average number of visits in 2018, 2019, and 2021 were quite similar, all of which were higher than those in 2020 and 2022. These visits included a total of 381,501 (72.88%) males and 141,961 (27.12%) females. The mean age of the participants was 6.41 ± 3.17. See Table 1.
The annual average number of visits in 2020 was the lowest over these 5 years (2.0829 ± 5.4782). Please refer to Table 2 for details. Meanwhile, the total outpatient visits also underwent corresponding changes.
In February, relatively lower peaks were found in visits for respiratory diseases (n = 10,906), mental and behavioral disorders (n = 4,398), eye and adnexal disorders (n = 3,211), digestive disorders (n = 2,566), and skin and subcutaneous tissue disorders (n = 1,390). However, in August, a significant increase in visits for mental and behavioral disorders (n = 8,773), eye and adnexal disorders (n = 5,240), digestive disorders (n = 4,046), and skin and subcutaneous tissue disorders (n = 1,995) was noted. Respiratory disorders also experienced a peak in August (n = 16,065). These findings indicated that respiratory disorders exhibited more pronounced fluctuations compared to the other four disorders, which demonstrated a relatively smoother pattern of fluctuation (Figure 2).
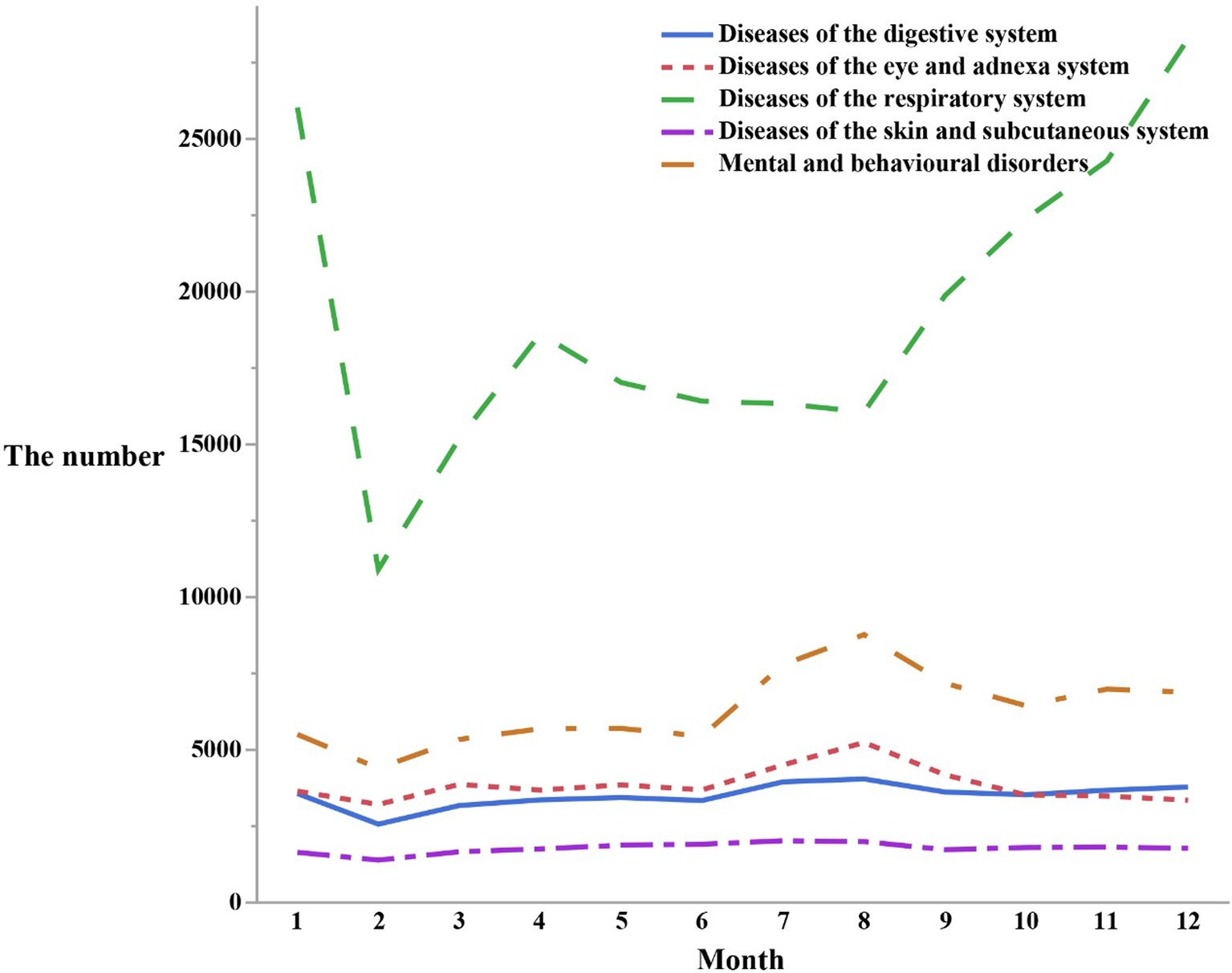
Figure 2. The number of outpatients visits in different months by the top five first classification of the diseases in the ICD-10.
Respiratory disorders reached their peak number of visits around the age of 4, while mental and behavioral disorders exhibited the highest visitation rates at approximately 9 years of age. Eye and appendage disorders showed a peak period of visits at around 5 years old, and digestive disorders tended to have the highest number of visits between the ages of 4 and 6. Similarly, skin and subcutaneous tissue disorders peaked in terms of visits at approximately 6 years old. Prior to reaching the peak period, visits tended to increase as individuals age, and after the peak period, they gradually declined. It has been recognized that a gradual remission would commonly begin after puberty, typically around the age of 12. (For a visual representation of these trends, please refer to Figure 3).

Figure 3. The number of outpatients visits in different ages by the top five first classification of the diseases in the ICD-10.
Diseases ranked in descending order of quantity were as followed: Respiratory diseases (n = 231,432), Mental and behavioral disorders (n = 76,157), Diseases of the eye and adnexal (n = 46,240), Digestive disorders (n = 42,063), Diseases of the skin and subcutaneous tissue (n = 21,385) (Figure 4). Characteristics of changes were also noted: The total number of outpatient visits for these five disease systems was the highest on average in the year 2019. Subsequently, in 2021, another peak in outpatient visits presented, but was lower than that of 2019. In the remaining 3 years, each disease system exhibited its own distinctive patterns of changes in the total number of outpatient visits. Respiratory diseases showed a slight increase in total outpatient visits in the years 2018, 2020, and 2022. The outpatient visits patterns for mental and behavioral disorders, diseases of the eye and adnexal, digestive diseases, and diseases of the skin and subcutaneous tissue all indicated that 2020 had the lowest total number of visits. In 2022, the total number of visits approached the levels of 2020 but remained lower than those in 2018. Interestingly, diseases of the skin and subcutaneous tissue exhibited a much smaller fluctuation in the total number of outpatient visits over the past 5 years compared to the other four disease systems.
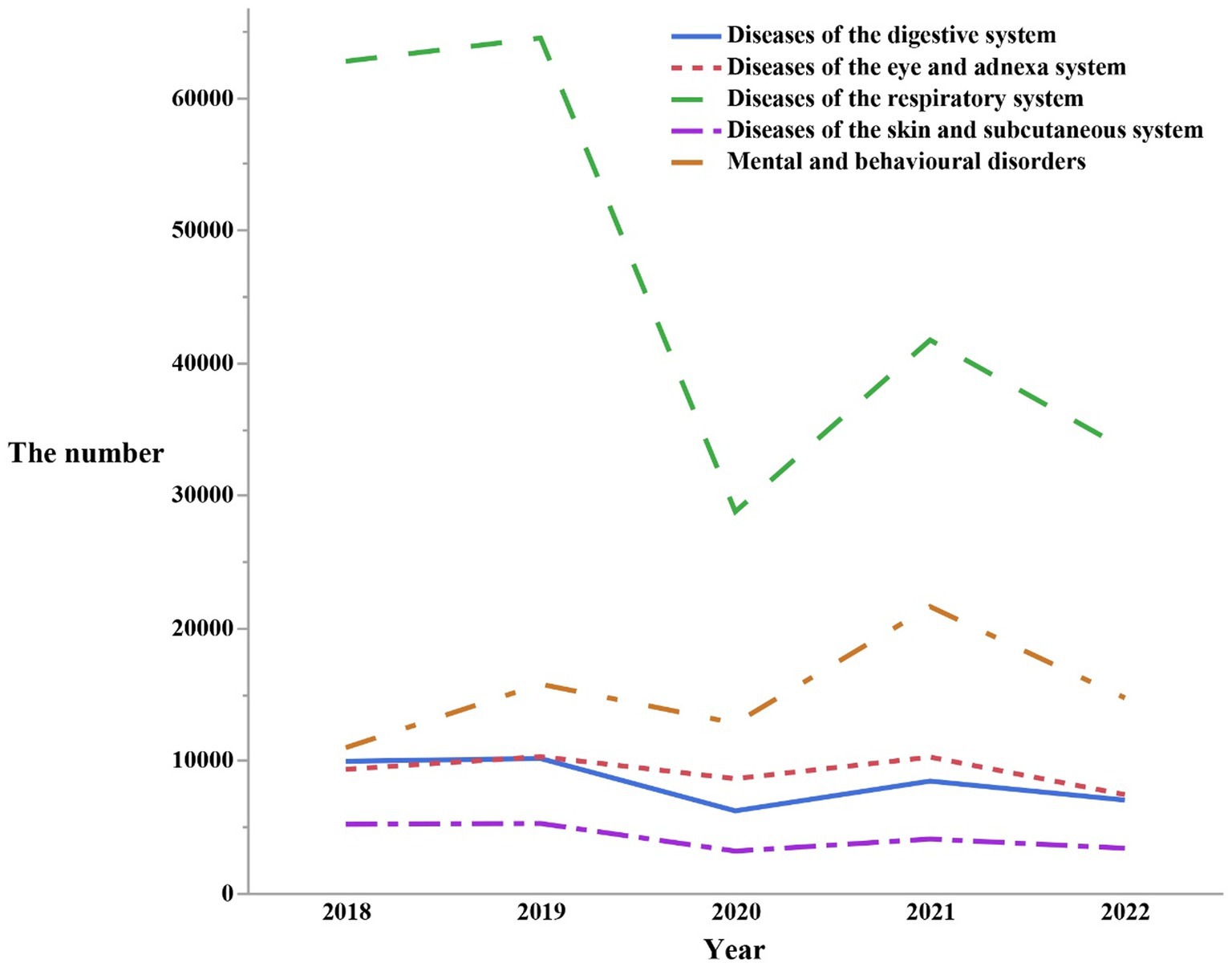
Figure 4. The number of outpatients visits from 2018 to 2022 by the top five first classification of the diseases in the ICD-10.
Among respiratory disorders, mental and behavioral disorders, diseases of the eye and adnexal, digestive disorders, diseases of the skin and subcutaneous tissue, the most common third-level classification of the diseases were, respectively, upper respiratory tract infections, ADHD, conjunctivitis, dyspepsia, and dermatitis (Table 3).

Table 3. The top five tertiary categorized diseases in terms of their respective total number of visits.
The most common second-level classification of diseases for respiratory disorders, mental and behavioral disorders, diseases of the eye and adnexal, digestive disorders, and diseases of the skin and subcutaneous tissue were as followed: respiratory diseases comprised of other upper respiratory diseases (n = 128,174), mental and behavioral disorders commonly observed in children and adolescents (n = 52,578), diseases of the conjunctiva (n = 18,710), esophageal, gastric, and duodenal disorders (n = 15,713), dermatitis and eczema (n = 13,529). For more details, refer to Figure 5.
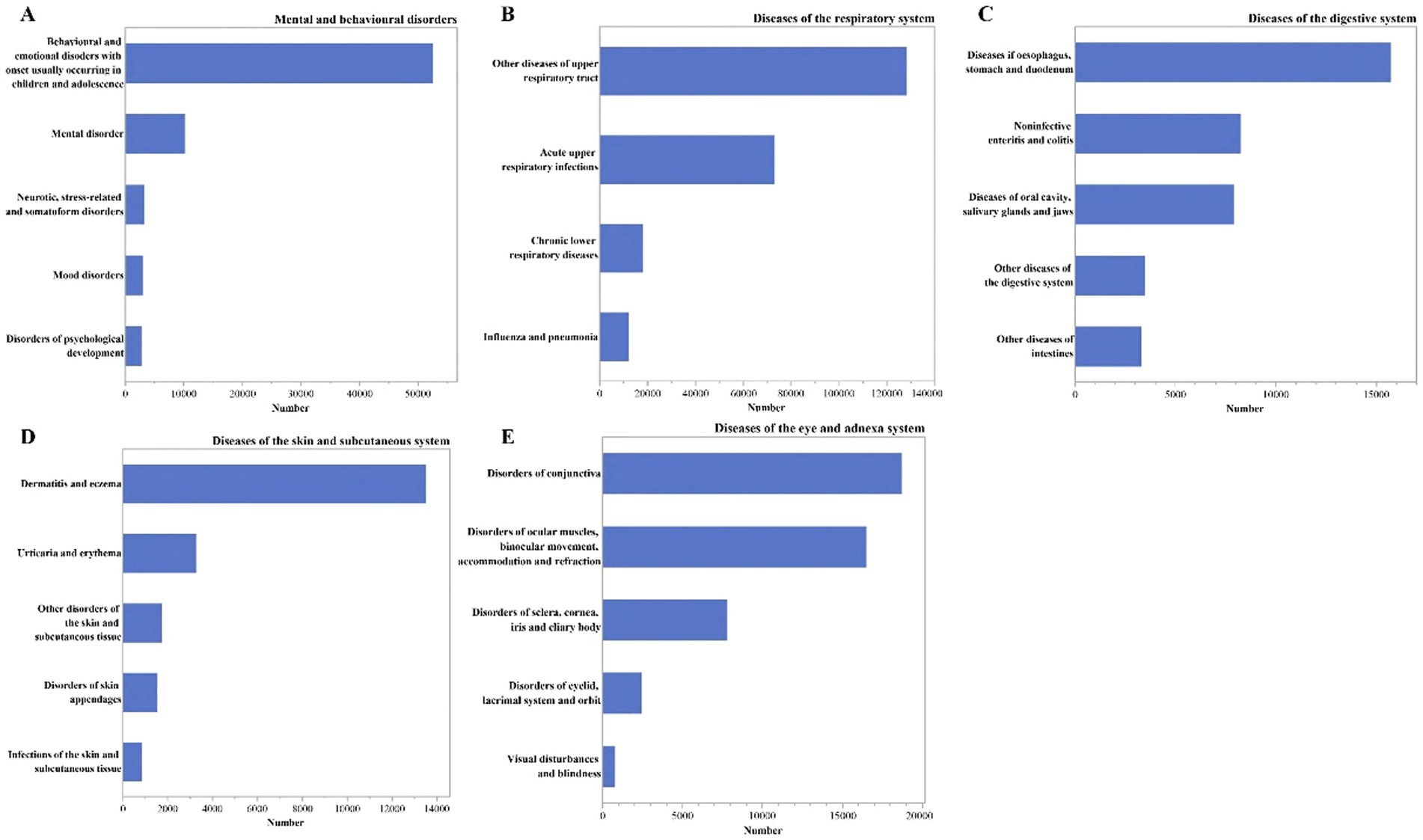
Figure 5. The number of outpatients visits by the secondary classification of the diseases in the ICD-10. (A) Under the mental and behavioral disorders; (B) under the diseases of the respiratory symptom; (C) under the diseases of the digestive symptom; (D) under the diseases of the skin and subcutaneous tissue symptom; (E) under the diseases of the eye and adnexal symptom.
Our retrospective study aimed to investigate the characteristics of coexisting somatic and mental disorders in children with tic disorders attending public children’s hospitals. Most importantly, common physical diseases in tic disorder included respiratory system disorders, eye and adnexal diseases, digestive disorders, and skin and subcutaneous tissue diseases. Among these, the most common comorbid diseases associated with tic disorders were upper respiratory tract infections, conjunctivitis, indigestion, and dermatitis.
Our study found that respiratory disorders were the most commonly comorbid somatic diseases with tic disorders, with other upper respiratory tract diseases being the most common in the second-level classification. Furthermore, among the third-level classification, upper respiratory tract infections were the most common. This suggested a possible association between tic disorders and respiratory tract infections, in accordance with previous studies (43, 44). Previous research has shown that respiratory tract infections triggered by streptococcal infections could lead to autoimmune psychiatric disorders, leading to exacerbation of tic symptoms (45–47). Several studies have already shown that the COVID pandemic has a significant impact on the mental health of young people with TS, exacerbating both tics and emotional and behavioral symptoms (48, 49). An increase in tic symptoms has been reported in some children and adolescents with tic disorders since the onset of the COVID-19 pandemic (49, 50). In addition, another recent study aimed to explore the long-term effects of SARS-CoV-2 infection in children and adolescents with tic disorders reported that patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had more pronounced tics and were more likely to have comorbidities than uninfected patients, suggesting that infection may contribute to the increase in tics and associated comorbidities in patients with tics (51). Some studies have indicated a significant increase in the incidence of movement disorders during the COVID-19 pandemic, such as functional tic disorders (52). Functional tics belong to functional abnormal movement, which phenomenologically shows common features with tic disorders (53). The latest study found an increase in the number of people suffering from functional tics during the COVID-19 pandemic (53, 54). These patient populations are predominantly prevalent in adolescent females and young adult females, mostly suffering from anxiety disorders as well as having been exposed to social media content related to tics, but mostly not benefiting from tic suppression medication (54). A prospective study of functional tics noted that adolescents with functional tics had a better prognosis than adults (55). However, other studies reported no significant association between streptococcal infections and exacerbation of tic symptoms (56).
The most prevalent second-level classification of eye and adnexal disorders associated with tic disorder was conjunctival disease, which was further categorized into tertiary classifications, with conjunctivitis as the most common subtype. Several children attending the tic clinic have documented visits to ophthalmologists, who were also diagnosed as “allergic conjunctivitis.” Previous meta-analyses have indicated a potential link between the development of tic disorder and allergic diseases, with tic disorder showing a positive correlation with allergic asthma, allergic rhinitis, and allergic conjunctivitis (33, 57). A recent study focusing on the relationship between allergic conjunctivitis and tic disorders in 4–10-year-olds in southwestern China found that transient tic disorders exhibited a higher frequency of allergic conjunctivitis, suggesting a potential association between allergic conjunctivitis, dry eye, and transient tic disorders in children (58). Furthermore, a previous study conducted in Taiwan, which involved 99% coverage of the population, revealed that patients with tic disorders had a higher likelihood of experiencing allergic disorders (59).
The most prevalent second-level classification of diseases that often co-occur with tic disorders and involve the digestive system were esophageal, gastric, and duodenal diseases. When further divided into tertiary categories, the most common disorder was dyspepsia. Previous research has indicated that individuals diagnosed with “aerophagy” experience symptoms like abdominal pain, bloating, and vomiting, which were initially treated symptomatically as dyspepsia (60). However, further investigation revealed that frequent swallowing of air due to tic disorders was the underlying cause of their abdominal discomfort (61, 62). It has also been observed that the incidence of tic disorders combined with aerophagy was higher in China compared to that of aerophagy alone (63).
Regarding skin and subcutaneous tissue disorders that frequently co-occur with tic disorders, dermatitis and eczema were the most common second-level classification, which were further divided into third-level classification, with dermatitis being the prevailing co-occurring disorder. Previous studies have found that thimerosal-containing vaccines increased the chances of children developing contact dermatitis and an elevated risk of developing tic disorders (64). A previous case–control study demonstrated a significant correlation between atopic dermatitis and tic disorders (57), suggesting that histaminergic neurotransmission may play a crucial role in the pathogenesis of tic disorders (65, 66).
Our study revealed a relatively high likelihood of comorbidity of psychiatric and behavioral disorders in individuals with tic disorder. Psychiatric and behavioral disorders were the second most common disease system across all tic disorders, which aligns with previous research indicating that a majority of children with tic disorders will have at least one co-occurring psychiatric disorder (9, 10, 67). Our study specifically identified ADHD, childhood mood disorders, depressive states, and childhood autism as common psychiatric disorders that often co-occur with tic disorder. These findings were consistent with previous studies reporting associations between tic disorders and ADHD, dysphoria, anxiety, and ASD (10, 68, 69).
Prior research has shown that the most frequent co-morbid psychiatric disorders in chronic tic disorders were ADHD and OCD (67, 70). Regarding the comorbidity of mental disorders with tic disorders, the most common second-level classification of diseases were behavioral and mental disorders that usually occur in children and adolescents, corresponding to the third-level classification, ADHD ranked as the top co-morbid psychiatric disorder, consistent with previous studies (9, 68). A previous study found an association between emotional dysregulation and severe tic symptoms (71). A recent study also reported that patients with tic disorder were more likely to be depressed than those without the disorder and that there was an association between depression and the severity of tic disorder in children and adolescents (72). This is also in line with previous research findings (73).
Previous studies have found that epilepsy affects autonomic activity, and changes in this area have the potential to influence changes in tic symptoms (74). Stress and anxiety predispose to epilepsy, which is similar to tics (71, 75, 76). Some studies have found that the pathogenesis of tics in patients involves immune mechanisms (77), and there is an association between autoimmune encephalitis associated with streptococcal infection and tic disorders (14, 78). In addition, studies have found that tic disorders may have abnormalities in gait and postural control (78–80). There’s been a case report of severe tics leading to cervical spondylosis (81).
Previous research has indicated that the incidence of tic disorders may be higher during the winter months compared to the spring, with a higher prevalence observed between November and February (2, 82). However, our study found a higher consultation rate during July and August, although this does not necessarily indicate a higher incidence during these months. This observation may be attributed to the specific national situation of our country. July and August marked as summer vacation period for children and adolescents in China, justly, the previous study has shown there is a regular holiday pattern in the months of visits for some pediatric diseases (83). During this time, children are not attending school, and families do not have to worry about disrupting their children’s classes or taking time off from work, making it more convenient for them to bring their children to the hospital for consultations.
In our analysis of the past 5 years, we observed a relatively stable number of visits for tic disorders in the years 2018, 2019, and 2021, with minimal differences. However, there was a significant decrease in the number of visits in 2020 and 2022, particularly in 2020, which was most noticeable. This trend did not align with the prevalence of tic disorders in the past (5, 84, 85). It is important to note that the decrease in the number of visits during these years does not imply that the prevalence of patients with tic disorders decreased. Rather, it may be related to the prevention and control measures implemented during the COVID-19 pandemic in China (86). Factors such as lockdown measures, strict controls, and quarantine protocols may have prevented patients from seeking timely medical attention at hospitals.
This study has three limitations. Firstly, our study was limited to a single center, the National Children’s Medical Center at Beijing Children’s Hospital of Capital Medical University, leading to incomplete sample coverage and potentially limiting the generalizability of our findings. Secondly, the data used in our study were derived solely from outpatient clinics, and thus, the profiles of inpatient hospitalizations were not included, and some patients did not visit the hospital, potentially resulting in incomplete coverage of diseases. Thirdly, some patients with tic disorder were diagnosed with non-psychiatric diseases during visits to non-psychiatric departments. For example, tic disorder symptoms such as blinking may be misdiagnosed as conjunctivitis, or involuntary movements may be erroneously used as diagnostic descriptions. These factors introduced the possibility of errors in disease classification during data collection.
Our study highlighted the most common physical diseases and mental disorders in tic disorders were the respiratory diseases, specifically upper respiratory tract infections, and mental and behavioral disorders, with ADHD being the most common co-occurring condition. The observed patterns in the seasons and years of visits for these comorbidities reflected our specific national circumstances, including the implementation of epidemic prevention and control measures, as well as the concentration of visits during summer and winter vacations.
The datasets presented in this article are not readily available because due to confidentiality agreements with the participants, we are unable to publicly disclose the data. In case of special circumstances, please contact the corresponding author to request access to the data. Requests to access the datasets should be directed to ZG9uZ3l1enVpMUAxNjMuY29t.
The studies involving humans were approved by Ethics Committee of Beijing Children’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
LY: Conceptualization, Data curation, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. HX: Data curation, Funding acquisition, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. ZJ: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. HY: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. YC: Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing. YL: Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (NSFC) under Grant No. 82171538, 82001445, 82301731 and the Natural Science Foundation of Beijing Municipality under Grant No. 7212035, 7232057, Beijing Hospitals Authority Youth Programme Grant No. QML20211203.
We thank all participants of this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Plessen, KJ. Tic disorders and Tourette's syndrome. Eur Child Adolesc Psychiatry. (2013) 22:55–60. doi: 10.1007/s00787-012-0362-x
2. Ueda, K, and Black, KJ. A comprehensive review of tic disorders in children. J Clin Med. (2021) 10:2479. doi: 10.3390/jcm10112479
3. Vermilion, J, and Mink, JW. Tic Disorders. Pediatr Rev. (2023) 44:294–6. doi: 10.1542/pir.2022-005566
4. Kessler, RC, Amminger, GP, Aguilar-Gaxiola, S, Alonso, J, Lee, S, and Ustun, TB. Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry. (2007) 20:359–64. doi: 10.1097/YCO.0b013e32816ebc8c
5. Li, F, Cui, Y, Li, Y, Guo, L, Ke, X, Liu, J, et al. Prevalence of mental disorders in school children and adolescents in China: diagnostic data from detailed clinical assessments of 17,524 individuals. J Child Psychol Psychiatry. (2022) 63:34–46. doi: 10.1111/jcpp.13445
6. Fombonne, E. Epidemiology of pervasive developmental disorders. Pediatr Res. (2009) 65:591–8. doi: 10.1203/PDR.0b013e31819e7203
7. Hassan, N, and Cavanna, AE. The prognosis of Tourette syndrome: implications for clinical practice. Funct Neurol. (2012) 27:23–7.
8. Hallett, M. Tourette syndrome: update. Brain and Development. (2015) 37:651–5. doi: 10.1016/j.braindev.2014.11.005
9. Singer, HS. Tics and Tourette syndrome. Continuum (Minneap Minn). (2019) 25:936–58. doi: 10.1212/CON.0000000000000752
10. Hirschtritt, ME, Lee, PC, Pauls, DL, Dion, Y, Grados, MA, Illmann, C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. (2015) 72:325–33. doi: 10.1001/jamapsychiatry.2014.2650
11. Hsu, CJ, Wong, LC, and Lee, WT. Immunological dysfunction in Tourette syndrome and related disorders. Int J Mol Sci. (2021) 22:853. doi: 10.3390/ijms22020853
12. Orefici, G, Cardona, F, Cox, CJ, and Cunningham, MW. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) In: JJ Ferretti, DL Stevens, and VA Fischetti, editors. Streptococcus pyogenes: basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center (2016).
13. Gilbert, DL. Inflammation in tic disorders and obsessive-compulsive disorder: are PANS and PANDAS a path forward? J Child Neurol. (2019) 34:598–611. doi: 10.1177/0883073819848635
14. Badenoch, J, Searle, T, Watson, I, and Cavanna, AE. Tics in patients with encephalitis. Neurol Sci. (2021) 42:1311–23. doi: 10.1007/s10072-021-05065-w
15. Lai, M, Li, Y, Luo, D, Xu, J, and Li, J. Dopamine-2 receptor antibody encephalitis presenting as pure tongue-biting in a tourette syndrome patient: a case report. BMC Psychiatry. (2022) 22:47. doi: 10.1186/s12888-021-03683-4
17. Perez-Vigil, A, Fernandez de la Cruz, L, Brander, G, Isomura, K, Gromark, C, and Mataix-Cols, D. The link between autoimmune diseases and obsessive-compulsive and tic disorders: a systematic review. Neurosci Biobehav Rev. (2016) 71:542–62. doi: 10.1016/j.neubiorev.2016.09.025
18. Pfeiffer, HCV, Wickstrom, R, Skov, L, Sorensen, CB, Sandvig, I, Gjone, IH, et al. Clinical guidance for diagnosis and management of suspected pediatric acute-onset neuropsychiatric syndrome in the Nordic countries. Acta Paediatr. (2021) 110:3153–60. doi: 10.1111/apa.15875
19. Aman, M, Coelho, JS, Lin, B, Lu, C, Westwell-Roper, C, Best, JR, et al. Prevalence of pediatric acute-onset neuropsychiatric syndrome (PANS) in children and adolescents with eating disorders. J Eat Disord. (2022) 10:194. doi: 10.1186/s40337-022-00707-6
20. Thienemann, M, Murphy, T, Leckman, J, Shaw, R, Williams, K, Kapphahn, C, et al. Clinical management of pediatric acute-onset neuropsychiatric syndrome: part I-psychiatric and behavioral interventions. J Child Adolesc Psychopharmacol. (2017) 27:566–73. doi: 10.1089/cap.2016.0145
21. La Bella, S, Scorrano, G, Rinaldi, M, Di Ludovico, A, Mainieri, F, Attanasi, M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): myth or reality? The state of the art on a controversial disease. Microorganisms. (2023) 11:2549. doi: 10.3390/microorganisms11102549
22. Esposito, S, Bianchini, S, Baggi, E, Fattizzo, M, and Rigante, D. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: an overview. Eur J Clin Microbiol Infect Dis. (2014) 33:2105–9. doi: 10.1007/s10096-014-2185-9
23. Tan, J, Smith, CH, and Goldman, RD. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Can Fam Physician. (2012) 58:957–9.
24. Brander, G, Isomura, K, Chang, Z, Kuja-Halkola, R, Almqvist, C, Larsson, H, et al. Association of Tourette syndrome and chronic tic disorder with metabolic and cardiovascular disorders. JAMA Neurol. (2019) 76:454–61. doi: 10.1001/jamaneurol.2018.4279
25. Kinard, K, Miller, NR, Digre, KB, Katz, BJ, Crum, AV, and Warner, JE. Blepharospasm in children and adolescents. Childs Nerv Syst. (2016) 32:355–8. doi: 10.1007/s00381-015-2938-5
26. Elston, JS, Granje, FC, and Lees, AJ. The relationship between eye-winking tics, frequent eye-blinking and blepharospasm. J Neurol Neurosurg Psychiatry. (1989) 52:477–80. doi: 10.1136/jnnp.52.4.477
27. Qian, S, Dou, R, Wang, Q, Huang, F, Zhao, Y, Zhuo, R, et al. Morphological changes in the meibomian gland in children with tic disorders. Quant Imaging Med Surg. (2023) 13:6374–83. doi: 10.21037/qims-22-390
28. Baizabal-Carvallo, JF, Alonso-Juarez, M, and Jankovic, J. Oromandibular tics associated with Tourette syndrome. J Neurol. (2023) 270:2591–6. doi: 10.1007/s00415-023-11583-8
29. Shimoyama, T, Horie, N, Kato, T, Nasu, D, and Kaneko, T. Tourette's syndrome with rapid deterioration by self-mutilation of the upper lip. J Clin Pediatr Dent. (2003) 27:177–80. doi: 10.17796/jcpd.27.2.u0735u0hh4l3287t
30. Li, YN, Jing, J, Cong, BB, Yu, XX, and Zhang, WY. Diagnosis and treatment of a case of severe oral mucosal traumatic ulcer in a child with Tourette syndrome. Zhonghua Kou Qiang Yi Xue Za Zhi. (2023) 58:1155–8. doi: 10.3760/cma.j.cn112144-20230905-00139
31. Budman, CL, Kerjakovic, M, and Bruun, RD. Viral infection and tic exacerbation. J Am Acad Child Adolesc Psychiatry. (1997) 36:162. doi: 10.1097/00004583-199702000-00004
32. Geng, J, Liu, C, Xu, J, Wang, X, and Li, X. Potential relationship between Tourette syndrome and gut microbiome. J Pediatr. (2023) 99:11–6. doi: 10.1016/j.jped.2022.06.002
33. Huang, J, Li, R, Li, L, Song, Y, and Jin, L. The relationship between allergic diseases and tic disorders: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2022) 132:362–77. doi: 10.1016/j.neubiorev.2021.12.004
34. Liu, X, Wang, X, Zhang, X, and Cao, AH. Allergic diseases influence symptom severity and T lymphocyte subgroups of children with tic disorders. J Investig Med. (2021) 69:1453–7. doi: 10.1136/jim-2021-001788
35. Straughen, JK, Kozyrskyj, AL, and Cassidy-Bushrow, AE. Editorial: allergic diseases and neurodevelopment. Front Pediatr. (2023) 11:1199467. doi: 10.3389/fped.2023.1199467
36. Yuce, M, Guner, SN, Karabekiroglu, K, Baykal, S, Kilic, M, Sancak, R, et al. Association of Tourette syndrome and obsessive-compulsive disorder with allergic diseases in children and adolescents: a preliminary study. Eur Rev Med Pharmacol Sci. (2014) 18:303–10.
37. Tsai, LH, Lin, JW, and Lue, KH. Study protocol to investigate the correlation between Tourette syndrome and allergy in children and adolescents. J Int Med Res. (2020) 48:300060520973921. doi: 10.1177/0300060520973921
38. Ho, CS, Shen, EY, Shyur, SD, and Chiu, NC. Association of allergy with Tourette's syndrome. J Formos Med Assoc. (1999) 98:492–5.
39. Liu, Y, Li, Y, Ma, X, Yu, L, Liang, Y, and Li, C. Comparative analysis of serum total IgE levels and specific IgE levels in children aged 6 to 9 years with tic disorder and normal children. BMC Pediatr. (2023) 23:399. doi: 10.1186/s12887-023-04233-5
40. Martindale, JM, and Mink, JW. The rise of functional tic-like behaviors: what do the COVID-19 pandemic and social media have to do with it? A narrative review. Front Pediatr. (2022) 10:863919. doi: 10.3389/fped.2022.863919
41. Schneider, SA, Hennig, A, and Martino, D. Relationship between COVID-19 and movement disorders: a narrative review. Eur J Neurol. (2022) 29:1243–53. doi: 10.1111/ene.15217
42. Hoekstra, PJ, Manson, WL, Steenhuis, MP, Kallenberg, CG, and Minderaa, RB. Association of common cold with exacerbations in pediatric but not adult patients with tic disorder: a prospective longitudinal study. J Child Adolesc Psychopharmacol. (2005) 15:285–92. doi: 10.1089/cap.2005.15.285
43. Zhang, Y, Xiao, N, Zhang, X, Zhang, Z, and Zhang, J. Identifying factors associated with the recurrence of tic disorders. Brain Sci. (2022) 12:697. doi: 10.3390/brainsci12060697
44. Jiang, J, Chen, M, Huang, H, and Chen, Y. The aetiology of Tourette syndrome and chronic tic disorder in children and adolescents: a comprehensive systematic review of case-control studies. Brain Sci. (2022) 12:1202. doi: 10.3390/brainsci12091202
45. Lin, H, Williams, KA, Katsovich, L, Findley, DB, Grantz, H, Lombroso, PJ, et al. Streptococcal upper respiratory tract infections and psychosocial stress predict future tic and obsessive-compulsive symptom severity in children and adolescents with Tourette syndrome and obsessive-compulsive disorder. Biol Psychiatry. (2010) 67:684–91. doi: 10.1016/j.biopsych.2009.08.020
46. Giulino, L, Gammon, P, Sullivan, K, Franklin, M, Foa, E, Maid, R, et al. Is parental report of upper respiratory infection at the onset of obsessive-compulsive disorder suggestive of pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection? J Child Adolesc Psychopharmacol. (2002) 12:157–64. doi: 10.1089/104454602760219199
47. Singer, HS, Mascaro-Blanco, A, Alvarez, K, Morris-Berry, C, Kawikova, I, Ben-Pazi, H, et al. Neuronal antibody biomarkers for Sydenham's chorea identify a new group of children with chronic recurrent episodic acute exacerbations of tic and obsessive compulsive symptoms following a streptococcal infection. PLoS One. (2015) 10:e0120499. doi: 10.1371/journal.pone.0120499
48. Conte, G, Baglioni, V, Valente, F, Chiarotti, F, and Cardona, F. Adverse mental health impact of the COVID-19 lockdown in individuals with Tourette syndrome in Italy: an online survey. Front Psych. (2020) 11:583744. doi: 10.3389/fpsyt.2020.583744
49. Termine, C, Galli, V, Dui, LG, Berlusconi, V, Taras, R, Vergani, M, et al. Self-reported impact of the COVID-19 pandemic and lockdown on young patients with tic disorders: findings from a case-control study. Neurol Sci. (2022) 43:3497–501. doi: 10.1007/s10072-022-05997-x
50. Heyman, I, Liang, H, and Hedderly, T. COVID-19 related increase in childhood tics and tic-like attacks. Arch Dis Child. (2021) 106:420–1. doi: 10.1136/archdischild-2021-321748
51. Prato, A, Salerno, AM, Saia, F, Maugeri, N, Zanini, A, Scerbo, M, et al. Symptoms compatible with long COVID in an Italian pediatric cohort of Tourette patients with and without SARS-CoV-2 infection: a short-term follow-up assessment. BMC Pediatr. (2023) 23:222. doi: 10.1186/s12887-023-04035-9
52. Han, VX, Kozlowska, K, Kothur, K, Lorentzos, M, Wong, WK, Mohammad, SS, et al. Rapid onset functional tic-like behaviours in children and adolescents during COVID-19: clinical features, assessment and biopsychosocial treatment approach. J Paediatr Child Health. (2022) 58:1181–7. doi: 10.1111/jpc.15932
53. Prato, A, Saia, F, Milana, MC, Scerbo, M, Barone, R, and Rizzo, R. Functional tic-like behaviours during the COVID-19 pandemic: follow-up over 12 months. Front Pediatr. (2022) 10:1003825. doi: 10.3389/fped.2022.1003825
54. Martino, D, Hedderly, T, Murphy, T, Muller-Vahl, KR, Dale, RC, Gilbert, DL, et al. The spectrum of functional tic-like behaviours: data from an international registry. Eur J Neurol. (2023) 30:334–43. doi: 10.1111/ene.15611
55. Howlett, M, Martino, D, Nilles, C, and Pringsheim, T. Prognosis of rapid onset functional tic-like behaviors: prospective follow-up over 6 months. Brain Behav. (2022) 12:e2606. doi: 10.1002/brb3.2606
56. Leckman, JF, King, RA, Gilbert, DL, Coffey, BJ, Singer, HS, Dure, LS, et al. Streptococcal upper respiratory tract infections and exacerbations of tic and obsessive-compulsive symptoms: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. (2011) 50:108–118.e3. doi: 10.1016/j.jaac.2010.10.011
57. Chang, YT, Li, YF, Muo, CH, Chen, SC, Chin, ZN, Kuo, HT, et al. Correlation of Tourette syndrome and allergic disease: nationwide population-based case-control study. J Dev Behav Pediatr. (2011) 32:98–102. doi: 10.1097/DBP.0b013e318208f561
58. Chen, L, Chen, X, Ke, N, Pi, L, and Liu, Q. Association between allergic conjunctivitis and provisional tic disorder in children. Int Ophthalmol. (2020) 40:247–53. doi: 10.1007/s10792-019-01174-w
59. Chen, MH, Su, TP, Chen, YS, Hsu, JW, Huang, KL, Chang, WH, et al. Attention deficit hyperactivity disorder, tic disorder, and allergy: is there a link? A nationwide population-based study. J Child Psychol Psychiatry. (2013) 54:545–51. doi: 10.1111/jcpp.12018
61. Weil, RS, Cavanna, AE, Willoughby, JM, and Robertson, MM. Air swallowing as a tic. J Psychosom Res. (2008) 65:497–500. doi: 10.1016/j.jpsychores.2008.04.001
62. Frye, RE, and Hait, EJ. Air swallowing caused recurrent ileus in Tourette's syndrome. Pediatrics. (2006) 117:e1249–52. doi: 10.1542/peds.2005-2914
63. Zheng, YC, Pan, J, Zhang, ZH, Liu, ZF, Hao, LH, and Qian, R. A single-center retrospective analysis of 46 children with aerophagia. Zhongguo Dang Dai Er Ke Za Zhi. (2020) 22:975–9. doi: 10.7499/j.issn.1008-8830.2003006
64. Dorea, JG. Abating mercury exposure in young children should include thimerosal-free vaccines. Neurochem Res. (2017) 42:2673–85. doi: 10.1007/s11064-017-2277-x
65. Ganos, C. Tics and Tourette's: update on pathophysiology and tic control. Curr Opin Neurol. (2016) 29:513–8. doi: 10.1097/WCO.0000000000000356
66. Udvardi, PT, Nespoli, E, Rizzo, F, Hengerer, B, and Ludolph, AG. Nondopaminergic neurotransmission in the pathophysiology of Tourette syndrome. Int Rev Neurobiol. (2013) 112:95–130. doi: 10.1016/B978-0-12-411546-0.00004-4
67. Simpson, HA, Jung, L, and Murphy, TK. Update on attention-deficit/hyperactivity disorder and tic disorders: a review of the current literature. Curr Psychiatry Rep. (2011) 13:351–6. doi: 10.1007/s11920-011-0223-1
68. Yan, J, Deng, H, Wang, Y, Wang, X, Fan, T, Li, S, et al. The prevalence and comorbidity of tic disorders and obsessive-compulsive disorder in Chinese school students aged 6-16: a national survey. Brain Sci. (2022) 12:650. doi: 10.3390/brainsci12050650
69. Huisman-van Dijk, HM, Matthijssen, S, Stockmann, RTS, Fritz, AV, and Cath, DC. Effects of comorbidity on Tourette's tic severity and quality of life. Acta Neurol Scand. (2019) 140:390–8. doi: 10.1111/ane.13155
70. Cavanna, AE, Servo, S, Monaco, F, and Robertson, MM. The behavioral spectrum of Gilles de la Tourette syndrome. J Neuropsychiatry Clin Neurosci. (2009) 21:13–23. doi: 10.1176/jnp.2009.21.1.13
71. Ruhrman, D, Mikulincer, M, Apter, A, Benaroya-Milshtein, N, and Steinberg, T. Emotion regulation and tic disorders in children. Eur Child Adolesc Psychiatry. (2023) 32:893–902. doi: 10.1007/s00787-021-01912-5
72. Sadeh, DF, Frenk, ML, Simha, T, Horesh, D, Steinberg, T, Geva, N, et al. Moderating role of depression on the association of tic severity with functional impairment in children. Pediatr Neurol. (2023) 144:90–6. doi: 10.1016/j.pediatrneurol.2023.04.013
73. Malek, A, and Golinska, P. Depression in Tourette syndrome. Psychiatr Pol. (2020) 54:69–82. doi: 10.12740/PP/OnlineFirst/94471
74. Nagai, Y. Modulation of autonomic activity in neurological conditions: epilepsy and Tourette syndrome. Front Neurosci. (2015) 9:278. doi: 10.3389/fnins.2015.00278
75. Antebi, D, and Bird, J. The facilitation and evocation of seizures. Br J Psychiatry. (1992) 160:154–64. doi: 10.1192/bjp.160.2.154
77. Martino, D, Zis, P, and Buttiglione, M. The role of immune mechanisms in Tourette syndrome. Brain Res. (2015) 1617:126–43. doi: 10.1016/j.brainres.2014.04.027
78. Leonard, HL, and Swedo, SE. Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). Int J Neuropsychopharmacol. (2001) 4:191–8. doi: 10.1017/S1461145701002371
79. Lemay, M, Termoz, N, Lesperance, P, Chouinard, S, Rouleau, GA, and Richer, F. Postural control anomalies in children with Tourette syndrome. Exp Brain Res. (2007) 179:525–30. doi: 10.1007/s00221-007-0882-7
80. Liu, WY, Lin, PH, Lien, HY, Wang, HS, Wong, AM, and Tang, SF. Spatio-temporal gait characteristics in children with Tourette syndrome: a preliminary study. Res Dev Disabil. (2014) 35:2008–14. doi: 10.1016/j.ridd.2014.04.025
81. Krauss, JK, and Jankovic, J. Severe motor tics causing cervical myelopathy in Tourette's syndrome. Mov Disord. (1996) 11:563–6. doi: 10.1002/mds.870110512
82. Snider, LA, Seligman, LD, Ketchen, BR, Levitt, SJ, Bates, LR, Garvey, MA, et al. Tics and problem behaviors in schoolchildren: prevalence, characterization, and associations. Pediatrics. (2002) 110:331–6. doi: 10.1542/peds.110.2.331
83. Kedia, S, Ginde, AA, Grubenhoff, JA, Kempe, A, Hershey, AD, and Powers, SW. Monthly variation of United States pediatric headache emergency department visits. Cephalalgia. (2014) 34:473–8. doi: 10.1177/0333102413515346
84. Cubo, E. Review of prevalence studies of tic disorders: methodological caveats. Tremor Other Hyperkinet Mov (N Y). (2012) 2:tre-02-61-349-1. doi: 10.7916/D8445K68
85. Knight, T, Steeves, T, Day, L, Lowerison, M, Jette, N, and Pringsheim, T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. (2012) 47:77–90. doi: 10.1016/j.pediatrneurol.2012.05.002
Keywords: tic, Tourette syndrome, comorbid, physical disease, outpatient, retrospective analysis
Citation: Yu L, Xu H, Jiang Z, Yang H, Cui Y and Li Y (2024) Comorbidity of physical illnesses and mental disorders in outpatients with tic disorders: a retrospective study using the outpatient case system. Front. Neurol. 15:1397766. doi: 10.3389/fneur.2024.1397766
Received: 11 March 2024; Accepted: 25 November 2024;
Published: 05 December 2024.
Edited by:
Daniel Martinez-Ramirez, Tecnológico de Monterrey, MexicoCopyright © 2024 Yu, Xu, Jiang, Yang, Cui and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Li, bGl5aW5nQGJjaC5jb20uY24=; Yonghua Cui, Y3VpeW9uZ2h1YUBiY2guY29tLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.