- 1Department of Neurosurgery, The First People's Hospital of Xiaoshan District, Hangzhou, Zhejiang, China
- 2Department of Coloproctology, The First People's Hospital of Xiaoshan District, Hangzhou, Zhejiang, China
Objective: White blood cell (WBC) counts has been identified as a prognostic biomarker which frequently predict adverse outcomes and mortality risk in various conditions. However, evidence for the association between WBC counts and short-term outcomes after intracranial tumor resection remains limited. This study aimed to explore associations between preoperative WBC counts and thirty-day surgical mortality after craniotomy in adult intracranial tumor patients.
Methods: This retrospective cohort study performed secondary analysis of 18,049 intracranial tumor craniotomy patients from the ACS NSQIP database (2012–2015). The major exposure and outcome were preoperative WBC counts and thirty-day surgical mortality, respectively. Cox regression modeling assessed the linear association between them. Non-linear associations between them were evaluated by conducting smooth curve fitting using an additive Cox proportional hazard model in conjunction with segmented linear regression modeling. Subgroup analysis and interaction testing assessed effect modification. Sensitivity analysis evaluated result robustness.
Results: The total thirty-day surgical mortality after craniotomy was 2.49% (450/18,049). The mean of preoperative WBC counts was 9.501 ± 4.402 × 10^9/L. Fully adjusted model shows that elevated preoperative WBC counts was independently associated with increased thirty-day surgical mortality (HR = 1.057, 95%CI: 1.040, 1.076). Further analysis revealed a non-linear association between them: below a WBC threshold of 13.6 × 10^9/L, higher WBC counts elevated thirty-day mortality (HR = 1.117; 95%CI: 1.077, 1.158), while risk plateaued and no significant mortality rise occurred above this level (HR = 1.015, 95%CI: 0.982, 1.050). Steroid usage status has a significant effect modification on the WBC-mortality association (P for interaction = 0.002). The non-linear WBC-mortality association was only present for non-steroid users (HR = 1.158, 95%CI: 1.108, 1.210) but not steroid users (HR = 1.009, 95%CI: 0.966, 1.055). The sensitivity analysis confirmed the result robustness.
Conclusion: Elevated preoperative WBC counts were independently and non-linearly associated with an increased risk of thirty-day surgical mortality in adult non-steroid use patients undergoing craniotomy for intracranial tumors. As a convenient predictor, preoperative WBC data allows improved risk profiling and personalized management in adult intracranial tumor patients.
Introduction
Intracranial tumors refer to abnormal cell growths in the brain or central spinal cord. Intracranial tumors significantly impact global health, responsible for 2% of all cancer deaths and the foremost reason for cancer fatalities among children (1, 2). Intracranial tumor occurs at a rate of 21 per 100,000 population, representing approximately 2% of all human cancer, with rates increasing in recent years (3, 4). Surgical resection by craniotomy is one of primary treatments for intracranial tumors. However, the potential postoperative complications and mortality risks should be considered (5, 6). Studies typically evaluate outcomes and complications during the short-term postoperative period of 30 days, with thirty-day mortality serving as an indicator of surgical risk and safety (7, 8). A study of 8,663 craniotomy patients in China found a 2.3% thirty-day surgical mortality rate for brain tumors (9), consistent with a Norwegian study (7). Meanwhile, a study from American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) reported a 2.6% thirty-day mortality rate after craniotomy in 18,642 patients with primary brain malignancies (10). Current studies have identified several perioperative factors linked to thirty-day mortality after intracranial tumor resection, namely preoperative platelet count, hematocrit, serum sodium, blood urea nitrogen, body mass index, as well as sepsis and septic shock after surgery (10–15).
White blood cells (WBC) or leukocytes, originating from hematopoietic stem cells, are critical elements of the innate and adaptive immune system. These cells are categorized into various subsets, including neutrophils, lymphocytes, monocytes, eosinophils, and basophils, each with distinct functions in immune surveillance and response (16). As the most abundant leukocyte, neutrophils comprise 50–70% of all WBCs and are essential for acute inflammatory responses and pathogen clearance (17). An elevated WBC count, or leukocytosis, can be indicative of an ongoing inflammatory process, infectious disease, or hematological malignancy (18). Many studies have substantiated WBC counts’ prognostic value, highlighting their association with poorer outcomes in cardiovascular disease, cerebrovascular accidents, malignancies, and trauma (19–22). Meanwhile, elevated WBC counts also has been established as a prognostic indicator of surgical mortality and morbidity, associated with increasing mortality across different surgical contexts (23–25). The pathophysiological mechanism may involved is thought to be the WBC-driven inflammatory pathways that contribute to tissue injury and disease progression (26). Additionally, WBC levels also associate with genetic factors and lifestyle habits such as smoking (27). In summary, the WBC count serves as an important biomarker to assess immune function and inflammatory status in vivo, as well as a prognostic marker to predict mortality risk across diverse surgical and medical contexts.
To date, little research has examined associations between preoperative WBC counts and thirty-day surgical mortality in intracranial tumor patients. A study from the ACS NSQIP database showed that patients with sepsis and septic shock postsurgery has significantly higher thirty-day surgical mortality than others, with systemic inflammatory response syndrome (SIRS) as a major risk factor (10). However, the link between preoperative WBC counts, a key biomarker of inflammatory status, and thirty-day surgical mortality in intracranial tumor patients remains to be investigated. Therefore, we aimed to perform a secondary analysis based on the ACS NSQIP database to explore associations between preoperative WBC counts and thirty-day surgical mortality after craniotomy in adult intracranial tumor patients. This study may provide insights into the utility of preoperative WBC counts for prognostication after intracranial tumor resection.
Materials and methods
Study design and data source
This study is a retrospectively designed cohort analysis grounded on the ACS NSQIP database (2012–2015) that originally uploaded and made public by Zhang et al. (10). We performed a secondary analysis of these data (Data source: Sepsis and septic shock after craniotomy: Predicting a significant patient safety and quality outcome measure https://doi.org/10.1371/journal.pone.0235273). The original research was openly published under a Creative Commons Attribution License permitting unrestricted utilization given appropriate attribution. Thus, secondary analysis of these data does not infringe on author copyright.
Study population
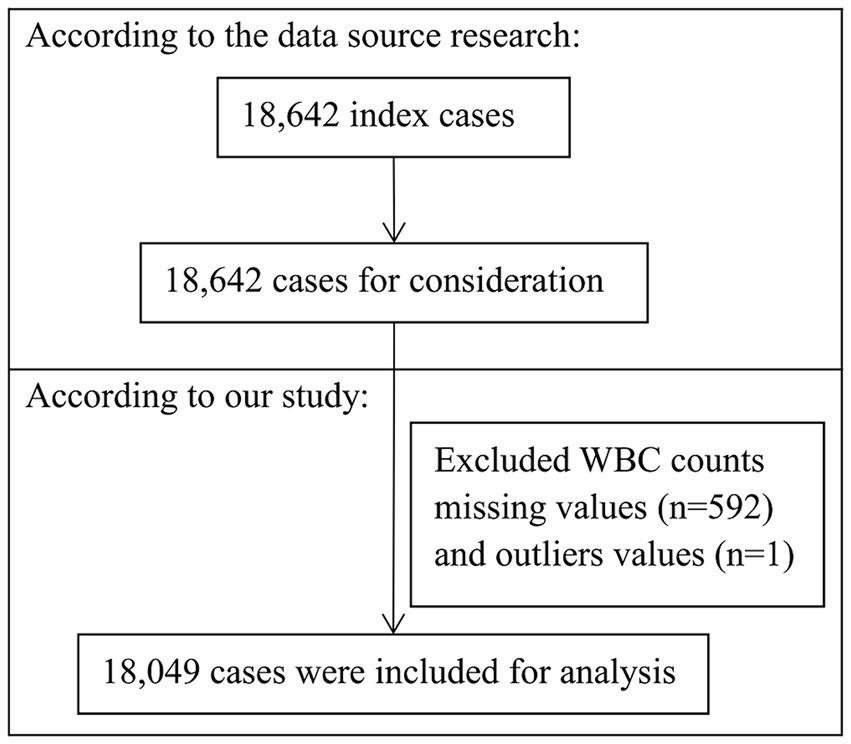
The original study analyzed 18,642 adult intracranial tumor patients undergoing craniotomy across about 400 academic and community hospitals in the United States (2012–2015). All patients were subjected to a thirty-day postoperative follow-up period. After excluding 592 patients missing preoperative WBC data and 1 patient with outliers (WBC = 119.8 × 10^9/L), 18,049 patients were retained for the analytical process (as shown in Figure 1). Informed consent was waived given the retrospective analysis and anonymous data.
Variables
Preoperative WBC count (×10^9/L), the independent variable, was recorded as a continuous variable in the original study. The dependent variable was thirty-day surgical mortality, defined as death occurred within 30 days following craniotomy, which was tracked for 30 days in the ACS NSQIP database (10).
Selection of covariates was informed by existing literature and clinical expertise. Analyzed covariates included: (1) Continuous variables: body mass index [BMI, computed as weight/height squared (kg/m2)], preoperative laboratory parameters [serum sodium (Na), blood urea nitrogen (BUN), creatine (Cr), platelet (PLT) count, hematocrit (HCT), international normalized ratio (INR)], and operation time; (2) categorical variables: sex, race, age ranges, smoking status, surgical site, functional health status, severe chronic obstructive pulmonary disease (COPD), diabetes, hypertension, congestive heart failure (CHF), renal failure, dialysis, disseminated cancer, steroid use for chronic condition, pre-operation systemic infection, emergency case. See original study for more details (10).
Statistical analysis
The percentage of missing data was: BMI 3.97% (n = 716); Na 2.24% (n = 404); BUN 6.22% (n = 1,123); Cr 1.99% (n = 359); HCT 0.07% (n = 12); PLT 0.11% (n = 19); INR 12.88% (n = 2,324); operation time 0.01% (n = 2). The missing values were substituted with their median (non-normal distribution) or mean (normal distribution) values to facilitate analysis.
Participants were stratified into quartiles by WBC counts. Continuous variables with normal distributions were expressed in terms of mean and standard deviation (SD); those non-normally distributed were reported using median values and interquartile ranges. Categorical variables were presented as counts and corresponding percentages. Differences across WBC quartiles were compared by One-way ANOVA (means), Kruskal–Wallis H test (medians), and chi-square test (percentages).
Univariate regression analysis by Cox proportional hazard modeling examined the relationship between baseline characteristics of study subjects and outcomes. Multivariate regression analysis by Cox proportional hazard modeling evaluated the independent impact of preoperative WBC counts on thirty-day surgical mortality risk. A confounding factor is defined as a covariate that, when introduced into the basic model or excluded from the full model, alters the regression coefficient for the exposure variable by >10% or has a regression coefficient with the outcome associated with a p < 0.1 (28).
Following guidelines of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (29), three distinct models were established: Crude model without any adjustments; Model I with minimal adjustments for sex and age; Model II with full adjustments for sex, age, functional health status, COPD, diabetes, hypertension, CHF, renal failure, dialysis, disseminated cancer, steroid use for chronic condition, pre-operation systemic infection, emergency case, Na, BUN, Cr, PLT, HCT, INR and operation time. Hazard ratios (HRs) as effect sizes and 95% confidence intervals (CIs) were reported.
Non-linear associations between preoperative WBC counts and thirty-day surgical mortality were assessed by smooth curve fitting using Additive Cox proportional hazard modeling (30). A segmented regression model with two linear segments was employed to investigate the threshold effect in the exposure-outcome relationship based on the smoothing plot. The WBC threshold at which the exposure-outcome relationship changed was determined by recursive testing (31). The optimal exposure-outcome model was identified using a log-likelihood ratio test.
Subgroup analysis using stratified Cox models with stratification based on following factors: sex, age, diabetes, hypertension, COPD, disseminated cancer, steroid usage, preoperative infection, and emergency case. Interaction terms between subgroup indicators and preoperative WBC counts were used to examine subgroup effect modification via likelihood ratio testing. Results reporting adhered to the STROBE guidelines (29, 32).
The statistical analysis was performed using two statistical software packages, namely R (http://www.R-project.org; The R Foundation; version 4.2.0) and EmpowerStats software (www.empowerstats.net, X&Y Solutions, Inc., Boston, MA, United States). A two-sided p-value <0.05 was considered significant.
Results
Baseline characteristics of participants
The baseline characteristics of the 18,049 cases stratified by preoperative WBC quartiles are depicted in Table 1. The thirty-day surgical mortality was 2.49% (450/18,049). Most parameters significantly differed or trended across quartiles. Patients in higher WBC quartiles showed graded increases in preoperative BUN, HCT, PLT (all p < 0.001) and higher rates of smoking, COPD, diabetes, hypertension, disseminated cancer, steroid use and preoperative infection (all p < 0.001). In contrast, Na levels declined with rising WBC quartiles (p < 0.001). While operation time was shortest for Q4 (p < 0.001).
Furthermore, higher WBC categories contained progressively more high-risk subgroups, including larger proportions of males, non-White ethnicities, elderly patients (age > 60 years), supratentorial tumors, and emergent cases (all p < 0.001). Functional impairment also differed significantly, being more common in higher WBC quartiles (p = 0.016).
The results of univariate regression analysis
The prognostic indicator for thirty-day surgical mortality assessed by univariate analysis are shown in Supplementary Table S1. Several preoperative factors were significantly associated. Each WBC count unit increase conferred a 7.7% rise in surgical mortality (HR = 1.077, 95%CI: 1.062, 1.093), among the strongest associations seen. Higher BUN, Cr, and INR also increased mortality risk (all p < 0.001). By contrast, lower Na, HCT, PLT and operation time portended higher mortality risk (all p < 0.005). In terms of demographics, female sex conferred better prognosis than male (p < 0.001). Mortality progressed higher with older age (p < 0.001). The comorbid conditions including functional impairment, COPD, diabetes, hypertension, CHF, renal issues, metastatic cancer, preoperative infection, steroid usage and emergency cases were also significantly associated with increased mortality risk (all p < 0.01). Whereas BMI, race, smoking status, and surgical site had no significant association with mortality risk (all p > 0.05).
The results of multivariate regression analysis
Association between preoperative WBC counts and thirty-day surgical mortality from multivariate analysis is depicted in Table 2. The unadjusted crude model shows a 7.7% rise in thirty-day mortality risk per unit increase in WBC counts (HR = 1.077, 95%CI: 1.062, 1.093). The Model I (minimally adjusted for age and sex) showed an attenuated but significant 6.9% higher risk per unit rise in WBC (HR = 1.069, 95%CI: 1.054, 1.085). The Model II (fully adjusted for 20 confounders) revealed a 5.7% higher thirty-day mortality risk per unit rise in WBC (HR = 1.057, 95%CI: 1.040, 1.076). The distribution of 95%CI indicate a reliable association between preoperative WBC counts and thirty-day surgical mortality.
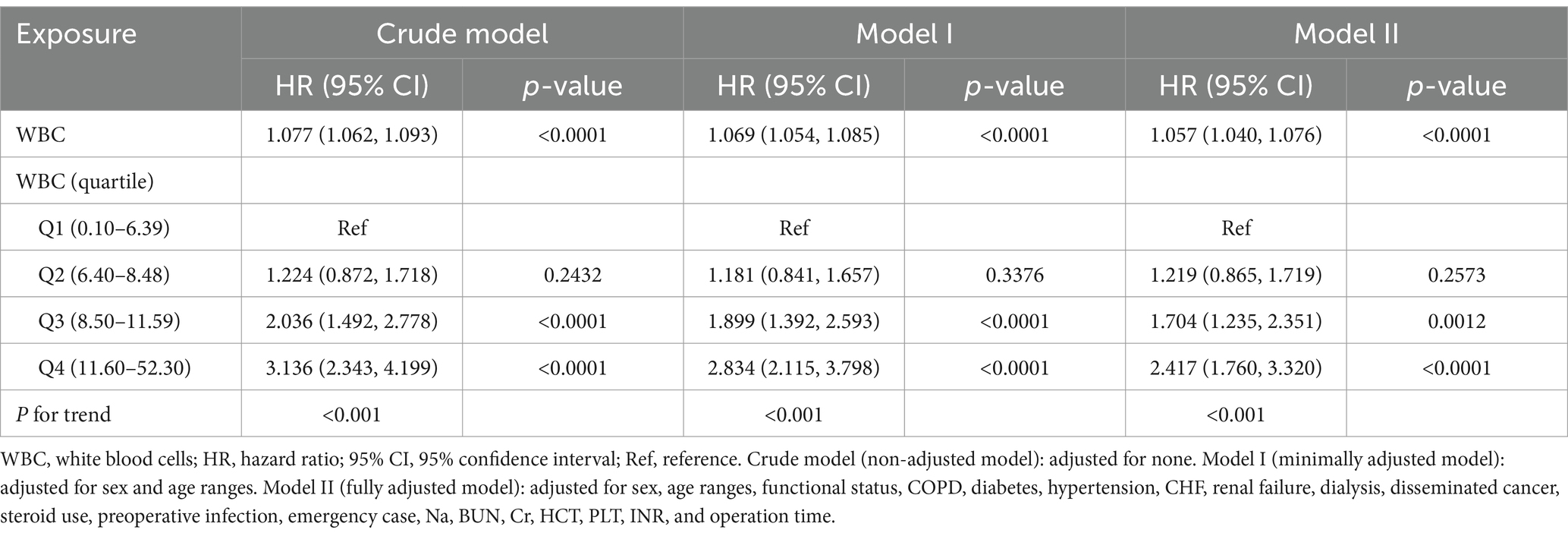
Table 2. The multivariate analysis of the association between preoperative WBC counts and thirty-day surgical mortality.
Non-linear relationship between WBC counts and thirty-day mortality
As depicted in Figure 2, preoperative WBC counts and thirty-day surgical mortality had a non-linear relationship. A segmented regression model with two linear segments identified 13.6 × 10^9/L as the inflection point of WBC counts (depicted in Table 3). Below this threshold, each WBC unit increase conferred a 11.7% rise in surgical mortality (HR = 1.117, 95%CI: 1.077, 1.158). However, above the cutoff, the thirty-day surgical mortality risk plateaued without significant further escalation (HR = 1.015, 95%CI: 0.982, 1.050). Log likelihood ratio testing confirmed superior fit with the non-linear two-segmented model versus standard linear model (p < 0.001).
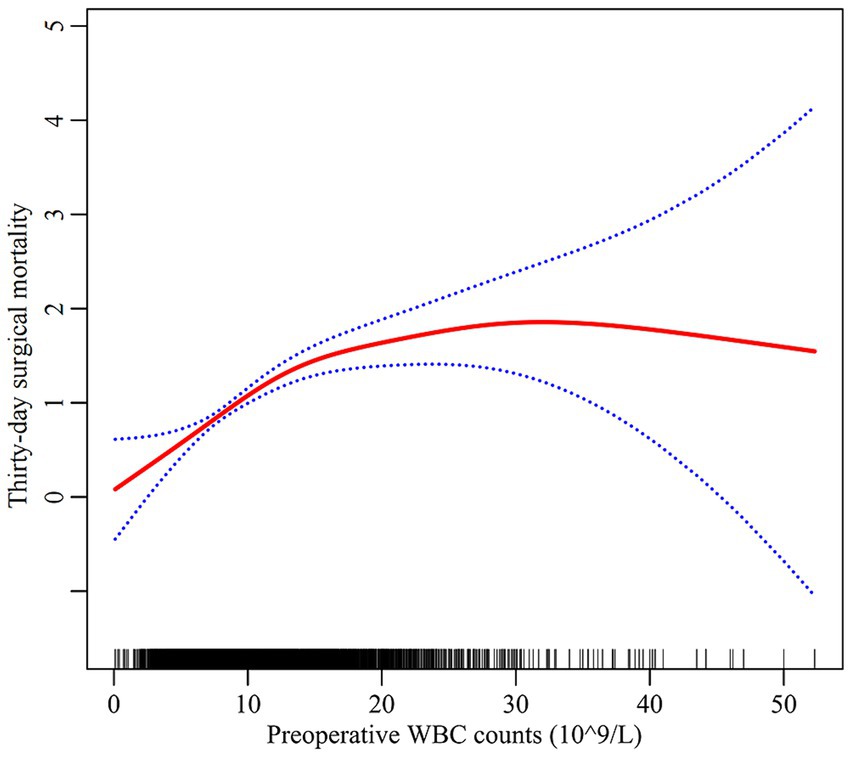
Figure 2. The non-linear relationship between preoperative WBC counts and thirty-day surgical mortality. Red line: thirty-day surgical mortality; Blue line: 95%CI Note: the model adjusted for all covariates in line with the multivariate analysis.
The results of subgroup analysis and interaction testing
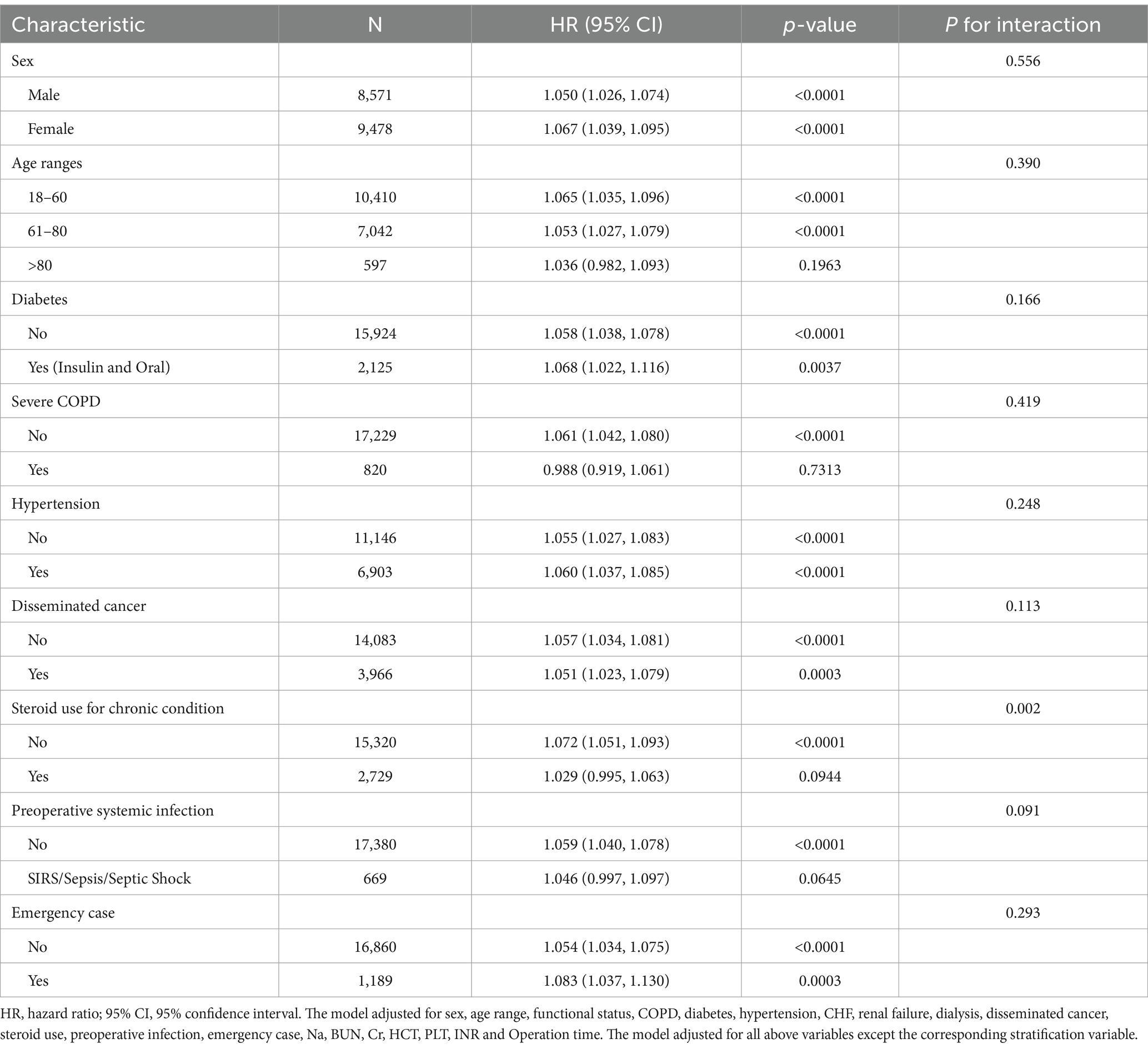
Subgroup analysis was performed to the detect the influence of other stratified factors on the trend of exposure-outcome association (depicted in Table 4). The prognostic impact of preoperative WBC counts was generally consistent across these clinical subgroups except steroid use for chronic condition. A significant interaction was seen with steroid use for chronic condition (P for interaction = 0.002). In non-steroid users, each WBC unit rise conferred a 7.2% higher thirty-day surgical mortality (HR = 1.072, 95%CI: 1.051, 1.093). Whereas no prognostic value of WBC counts was evident in steroid users (HR = 1.029, 95%CI: 0.995, 1.063). No significant effect modifications on the exposure-outcome association were observed in different groups of sex, age, diabetes, hypertension, COPD, disseminated cancer, preoperative infection and emergency case (all P for interaction >0.05).
Steroid usage difference in the non-linear association
We then explored the effect modifications of steroid usage status on the non-linear association between preoperative WBC counts and thirty-day surgical mortality. Steroid usage status was used as a stratification factor to perform smooth curve fitting and segmented linear regression analysis. As depicted in Figure 3, a non-linear exposure-outcome association was present for non-steroid users but not steroid users. As depicted in Table 5, the inflection point of WBC counts was 13.6 × 10^9/L. Below this threshold, each WBC unit increase conferred a 15.8% rise in thirty-day mortality for non-steroid users (HR = 1.158, 95%CI: 1.108, 1.210). Above this threshold, the risk plateaued without significant further escalation (HR = 1.009, 95%CI: 0.966, 1.055). No significant linear or non-linear exposure-outcome association was observed for steroid users. These results suggest that the non-linear WBC-mortality association differed between steroid and non-steroid users.

Figure 3. The effect modification of steroid usage status on the non-linear association between preoperative WBC counts and thirty-day surgical mortality. Note: The model adjusted for all covariates in line with the multivariate analysis except steroid usage.
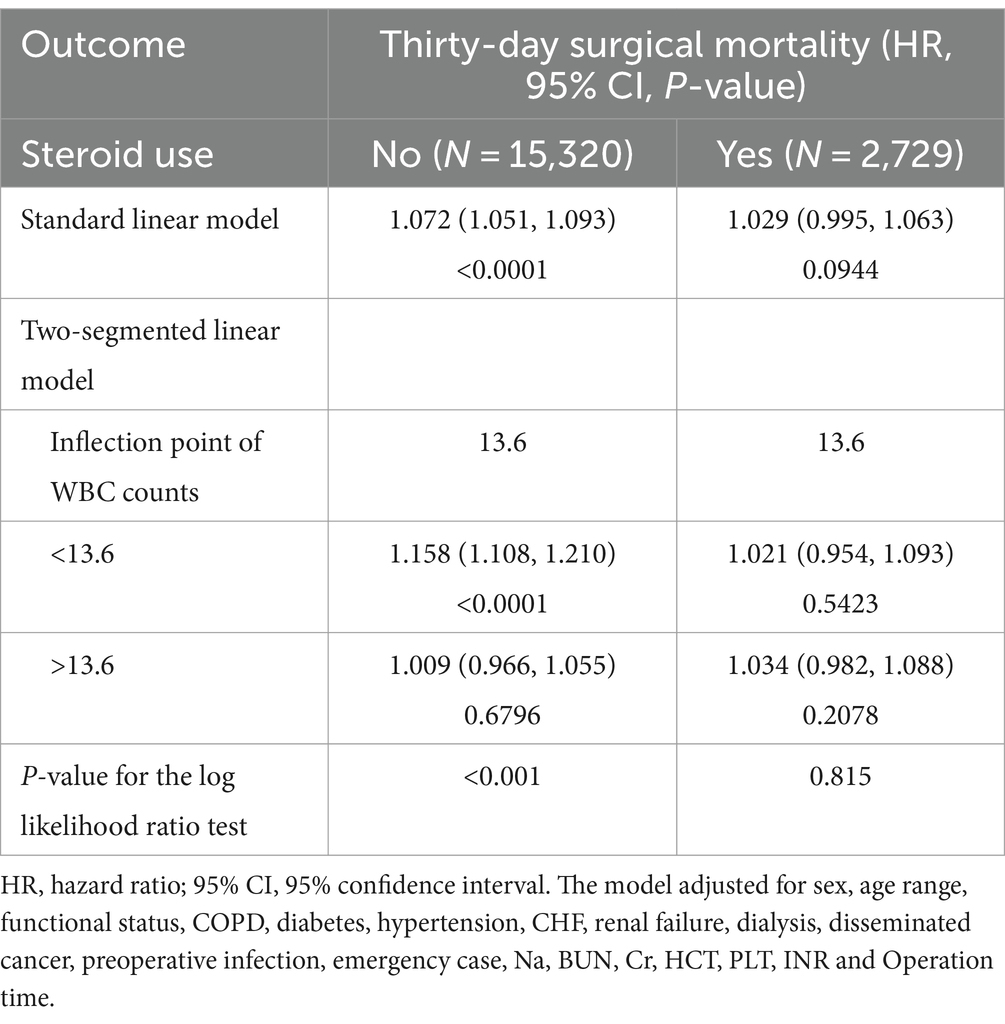
Table 5. The results of the two-segmented linear regression analysis stratified by steroid usage status.
Sensitivity analysis
Our sensitivity analysis evaluated result robustness. As depicted in Table 2, preoperative WBC counts were categorized into quartile. In categorical analysis with the categorical-transformed WBC counts, the upper two quartiles showed higher HRs (Q3 HR = 1.704, 95%CI: 1.235, 2.351; Q4 HR = 2.417, 95%CI: 1.760, 3.320) compared to the lowest quartile (Q1). Significant trends persisted across quartiles (p < 0.001). Furthermore, we reanalyzed the data after excluding 3,009 cases with missing data from 18,049 patients. The thirty-day mortality in the complete cases was 2.71% (407/15,040). In Supplementary Table S2, the multivariate analysis revealed a 5.4% higher thirty-day mortality risk per unit rise in WBC counts (HR = 1.054, 95%CI: 1.035, 1.073). In Supplementary Table S3, the segmented linear regression analysis identified a non-linear exposure-outcome association, showing a 10.7% higher thirty-day mortality risk per unit rise in WBC counts below the threshold (HR = 1.107, 95%CI: 1.064, 1.150) and a plateaued risk above the threshold (HR = 1.017, 95%CI: 0.982, 1.052). These results were consistent with our prior analysis, confirming the robustness of our findings.
Discussion
Analyzing ACS NSQIP data (2012–2015), this large retrospective cohort study of 18,049 cases explored associations between preoperative WBC counts and thirty-day surgical mortality after craniotomy in adult intracranial tumor patients. Our findings indicate that elevated preoperative WBC counts has an independent and non-linear association with increased thirty-day surgical mortality risk, with a significant threshold effect at a WBC count of 13.6 × 10^9/L. Below this threshold, higher WBC counts was associated with elevated thirty-day mortality risk, while the mortality risk plateaued above this level. Furthermore, different steroid usage status has significant effect modification on this association (P for interaction = 0.002). This exposure-outcome association was only present for non-steroid users but not steroid users.
Prior studies have demonstrated a connection between WBC counts and prognosis in diverse diseases, establishing it as a biomarker for mortality risk in conditions such as acute cerebral infarction (20), aortic dissection (33), acute myocardial infarction (34), bladder cancer surgery (35), sepsis (36), and lung cancer (37). Collectively, these findings highlight the WBC count’s role as a predictive indicator for patient outcomes in various clinical contexts. Earlier research has identified preoperative WBC count as an independent prognostic indicator of long-term postoperative outcomes in glioblastoma patients (38). However, the relationship between preoperative WBC counts and short-term outcomes after intracranial tumor resection had not been explored, which our study aimed to investigate. Consistent with previous findings, our study indicates that preoperative WBC counts can independently predicts short-term prognosis in adult intracranial tumor patients without steroid usage undergoing craniotomy, significantly associating elevated WBC levels with an heightened thirty-day surgical mortality risk.
However, the exact mechanisms through which WBC count affects patient outcomes are not yet fully understood. Current research leads us to hypothesize multiple contributing factors: An increased preoperative WBC count could indicate systemic inflammation, as preoperative SIRS has been established as a major risk predictor for postoperative sepsis and septic shock – conditions linked to higher thirty-day surgical mortality (10). The involved pathophysiological mechanisms likely involve WBC-driven inflammatory pathways that exacerbate tissue damage and disease progression (26). Additionally, WBC counts are established as independent predictors of cardiovascular, cerebrovascular, and thrombotic event risks (39–41). Therefore, higher preoperative WBC counts may signal an elevated risk for these events, contributing to increased mortality. Furthermore, research has shown a association between WBC counts and cancer staging (42, 43). A higher preoperative WBC count may imply a more advanced tumor grade and stage, correlated with a worse prognosis. Meanwhile, given that steroids have the ability to cause WBC to demarginate from blood vessel walls into circulation and increase peripheral blood WBC counts, the WBC counts in adult intracranial tumor patients using steroids usually cannot accurately reflect their inflammatory status in vivo. These factors may collectively account for the underlying mechanisms linking WBC counts with short-term postoperative outcomes. Meanwhile, regarding the phenomenon that the association between WBC counts and thirty-day mortality plateaued above 13.6 × 10^9/L, we speculate the following reasons: when the WBC counts exceed a certain threshold, the inflammatory response may have reached its maximum extent, and further elevations in WBC counts may not confer additional harm. Moreover, extremely high WBC counts may indicate advanced-stage tumors with already poor prognosis, thus obscuring any further impact of WBC count on mortality risk. However, given the retrospective nature of this study, we cannot draw definitive conclusions, and further studies are needed to elucidate the underlying mechanisms.
Overall, our findings align with prior evidence across settings showing prognostic value for preoperative WBC counts. Notably, this analysis provides first validation in an American population that preoperative WBC counts independently predicts short-term outcomes of intracranial tumor patients after craniotomy, especially in non-steroid users. This offers a readily available biomarker that aids in the identification of high-risk patients, thereby enhancing preoperative evaluation and preparation to facilitate personalized treatment. Furthermore, our findings lay a foundation for future exploration into the potential application of preoperative WBC counts in prognostic assessments following intracranial tumor surgery.
Our study has the following strengths: Firstly, the study included 18,049 patients, providing a large sample size and solid dataset for analysis. Secondly, there was little missing information on covariates, allowing adjustment for numerous confounders and multi-model effects assessment. Thirdly, sensitivity analysis were conducted to confirm the robustness of results. Fourthly, Smooth curve fitting in conjunction with segmented linear regression modeling and threshold analysis were employed for exploring the non-linear exposure-outcome relationship. Fifthly, subgroup analysis and interaction testing were performed to assess predictive effects across populations. Overall, this study employed a rigorous approach according to the STROBE guidelines, consistent with prior studies and showcasing dependable findings.
Our study has the following limitations: Firstly, as a retrospective cohort study, it can only establish association. Prior studies have revealed that certain other factors, such as platelet counts (11), hematocrit (12), and serum sodium (13), were also associated with postoperative short-term outcomes, suggesting that WBC counts may represent just one of many surrogate parameters for poor prognosis. Given our purely associative results, we cannot establish causality between WBC counts and thirty-day mortality. Secondly, as a secondary analysis based on publicly database, it could not exclude the effect of some unmeasured confounding factors such as genetics, environment and tumor stage. Thirdly, the original database lacked data on WBC subtypes, so the neutrophil to lymphocyte ratio cannot be calculated and used to analyze its impact on outcome indicators. Fourthly, this study was limited to the U.S. population, thereby limiting it’s generalizability to other populations due to differences in baseline characteristics and treatment patterns. Fifthly, the primary outcome measure was thirty-day surgical mortality, the association between WBC counts and long-term survival could not be evaluated. Sixthly, some statistically significant differences in our findings may be caused by the large sample size and multiple testing. For example, many of the significant differences across WBC count quartiles in some factors such as Cr, INR, operation time are actually subtle and not clinically relevant. Seventh, our findings are based on a single registry database, thus should be considered as exploratory. They have not been validated properly in other independent cohorts. As such, further researches in future are warranted to improve the reliability and generalizability of our results, and establish causal mechanisms through robust experimental designs.
Conclusion
For the first time in a large U.S. cohort, this study identified an independent and non-linear association between preoperative WBC counts and thirty-day surgical mortality in adult non-steroid use patients undergoing craniotomy for intracranial tumors. Elevated WBC counts were significantly associated with increased thirty-day mortality risk, while mortality risk stabilized without significant further escalation above a WBC threshold of 13.6 × 10^9/L. This easily accessible predictor can identify intracranial tumor patients facing high postoperative mortality hazards via elevated preoperative WBC levels. Such data may optimize surgical risk profiling and guide WBC management to mitigate thirty-day mortality risk. However, given the retrospective cohort design, causal links and generalizability remain to be established in further studies.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://doi.org/10.1371/journal.pone.0235273.s001.
Ethics statement
The requirement of ethical approval was waived by Ethics Committee of The First People’s Hospital of Xiaoshan District for the studies involving humans because this study represents a secondary analysis utilizing a published public database with retrospective analytical nature. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/Institutional Review Board also waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study is based on a de-identified database, the original personal information was anonymous.
Author contributions
ZG: Data curation, Writing – original draft, Formal analysis, Investigation, Methodology. CH: Data curation, Formal analysis, Writing – original draft, Investigation. SF: Data curation, Writing – original draft, Formal analysis. JG: Writing – original draft, Software. WD: Project administration, Supervision, Writing – review & editing, Resources.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
All authors extend their appreciation to Zhang and co-authors for originally uploading and providing the data available for our analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1394568/full#supplementary-material
References
1. Fouladseresht, H, Ziaee, SM, Erfani, N, and Doroudchi, M. Serum levels of APRIL increase in patients with glioma, meningioma and schwannoma. Asian Pac J Cancer Prev. (2019) 20:751–6. doi: 10.31557/APJCP.2019.20.3.751
2. Hwang, EI, Sayour, EJ, Flores, CT, Grant, G, Wechsler-Reya, R, Hoang-Minh, LB, et al. The current landscape of immunotherapy for pediatric brain tumors. Nat Cancer. (2022) 3:11–24. doi: 10.1038/s43018-021-00319-0
3. Ahammed Muneer, KV, Rajendran, VR, and Paul Joseph, K. Glioma tumor grade identification using artificial intelligent techniques. J Med Syst. (2019) 43:113. doi: 10.1007/s10916-019-1228-2
4. Ostrom, QT, Patil, N, Cioffi, G, Waite, K, Kruchko, C, and Barnholtz-Sloan, JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro-Oncology. (2020) 22:iv1–iv96. doi: 10.1093/neuonc/noaa200
5. Lonjaret, L, Guyonnet, M, Berard, E, Vironneau, M, Peres, F, Sacrista, S, et al. Postoperative complications after craniotomy for brain tumor surgery. Anaesth Crit Care Pain Med. (2017) 36:213–8. doi: 10.1016/j.accpm.2016.06.012
6. Yang, YC, Chen, YS, Liao, WC, Yin, CH, Lin, YS, Chen, MW, et al. Significant perioperative parameters affecting postoperative complications within 30 days following craniotomy for primary malignant brain tumors. Perioper Med. (2023) 12:54. doi: 10.1186/s13741-023-00343-x
7. Lassen, B, Helseth, E, Rønning, P, Scheie, D, Johannesen, TB, Mæhlen, J, et al. Surgical mortality at 30 days and complications leading to recraniotomy in 2630 consecutive craniotomies for intracranial tumors. Neurosurgery. (2011) 68:1259–69. doi: 10.1227/NEU.0b013e31820c0441
8. Senders, JT, Muskens, IS, Cote, DJ, Goldhaber, NH, Dawood, HY, Gormley, WB, et al. Thirty-Day outcomes after craniotomy for primary malignant brain tumors: a National Surgical Quality Improvement Program Analysis. Neurosurgery. (2018) 83:1249–59. doi: 10.1093/neuros/nyy001
9. He, J, He, S, Zhang, Y, Tian, Y, Hao, P, Li, T, et al. Association between intraoperative steroid and postoperative mortality in patients undergoing craniotomy for brain tumor. Front Neurol. (2023) 14:1153392. doi: 10.3389/fneur.2023.1153392
10. Zhang, J, Li, YI, Pieters, TA, Towner, J, Li, KZ, Al-Dhahir, MA, et al. Sepsis and septic shock after craniotomy: predicting a significant patient safety and quality outcome measure. PLoS One. (2020) 15:e0235273. doi: 10.1371/journal.pone.0235273
11. Liu, Y, Hu, H, Li, Z, Yang, J, Zhang, X, Chen, L, et al. Association between preoperative platelet and 30-day postoperative mortality of adult patients undergoing craniotomy for brain tumors: data from the American College of Surgeons National Surgical Quality Improvement Program database. BMC Neurol. (2022) 22:465. doi: 10.1186/s12883-022-03005-5
12. Liu, Y, Li, L, Hu, H, Yang, J, Zhang, X, Chen, L, et al. Association between preoperative hematocrit and postoperative 30-day mortality in adult patients with tumor craniotomy. Front Neurol. (2023) 14:1059401. doi: 10.3389/fneur.2023.1059401
13. Liu, Y, Hu, H, Li, Z, Yang, Y, Chen, F, Li, W, et al. Association between preoperative serum sodium and postoperative 30-day mortality in adult patients with tumor craniotomy. BMC Neurol. (2023) 23:355. doi: 10.1186/s12883-023-03412-2
14. Liu, Y, Hu, H, Li, Z, Han, Y, Chen, F, Zhang, M, et al. Association between pre-operative BUN and post-operative 30-Day mortality in patients undergoing craniotomy for tumors: data from the ACS NSQIP database. Front Neurol. (2022) 13:926320. doi: 10.3389/fneur.2022.926320
15. Liu, Y, Hu, H, Han, Y, Li, L, Li, Z, Zhang, L, et al. Body mass index has a nonlinear association with postoperative 30-Day mortality in patients undergoing craniotomy for tumors in men: an analysis of data from the ACS NSQIP database. Front Endocrinol. (2022) 13:868968. doi: 10.3389/fendo.2022.868968
16. Abbas, AK, Lichtman, AH, and Pillai, S. Cellular and molecular immunology E-book. Philadelphia, PA, USA: Elsevier Health Sciences (2014).
17. Mayadas, TN, Cullere, X, and Lowell, CA. The multifaceted functions of neutrophils. Annu Rev Pathol. (2014) 9:181–218. doi: 10.1146/annurev-pathol-020712-164023
18. Raphael, I, Nalawade, S, Eagar, TN, and Forsthuber, TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. (2015) 74:5–17. doi: 10.1016/j.cyto.2014.09.011
19. Palmerini, T, Mehran, R, Dangas, G, Nikolsky, E, Witzenbichler, B, Guagliumi, G, et al. Impact of leukocyte count on mortality and bleeding in patients with myocardial infarction undergoing primary percutaneous coronary interventions: analysis from the harmonizing outcome with revascularization and stent in acute myocardial infarction trial. Circulation. (2011) 123:2829–37. doi: 10.1161/CIRCULATIONAHA.110.985564
20. Vo, TP, Kristiansen, MH, Hasselbalch, HC, and Wienecke, T. Elevated white blood cell counts in ischemic stroke patients are associated with increased mortality and new vascular events. Front Neurol. (2023) 14:1232557. doi: 10.3389/fneur.2023.1232557
21. Shankar, A, Wang, JJ, Rochtchina, E, Yu, MC, Kefford, R, and Mitchell, P. Association between circulating white blood cell count and cancer mortality: a population-based cohort study. Arch Intern Med. (2006) 166:188–94. doi: 10.1001/archinte.166.2.188
22. Hasjim, BJ, Grigorian, A, Stopenski, S, Swentek, L, Sun, B, Livingston, JK, et al. Moderate to severe leukocytosis with vasopressor use is associated with increased mortality in trauma patients. J Intensive Care Soc. (2022) 23:117–23. doi: 10.1177/1751143720975316
23. Tan, TP, Arekapudi, A, Metha, J, Prasad, A, and Venkatraghavan, L. Neutrophil-lymphocyte ratio as predictor of mortality and morbidity in cardiovascular surgery: a systematic review. ANZ J Surg. (2015) 85:414–9. doi: 10.1111/ans.13036
24. Green, J, Bin Mahmood, SU, Mori, M, Yousef, S, Mangi, AA, and Geirsson, A. Stability across time of the neutrophil-lymphocyte and lymphocyte-neutrophil ratios and associations with outcomes in cardiac surgery patients. J Cardiothorac Surg. (2019) 14:164. doi: 10.1186/s13019-019-0988-6
25. Ali, SO, Welch, JP, and Dring, RJ. Early surgical intervention for fulminant pseudomembranous colitis. Am Surg. (2008) 74:20–6. doi: 10.1177/000313480807400105
26. Apostolakis, S, Vogiatzi, K, Amanatidou, V, and Spandidos, DA. Interleukin 8 and cardiovascular disease. Cardiovasc Res. (2009) 84:353–60. doi: 10.1093/cvr/cvp241
27. Pedersen, KM, Çolak, Y, Ellervik, C, Hasselbalch, HC, Bojesen, SE, and Nordestgaard, BG. Smoking and increased white and red blood cells. Arterioscler Thromb Vasc Biol. (2019) 39:965–77. doi: 10.1161/ATVBAHA.118.312338
28. Vandenbroucke, JP, von Elm, E, Altman, DG, Gøtzsche, PC, Mulrow, CD, Pocock, SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. (2014) 12:1500–24. doi: 10.1016/j.ijsu.2014.07.014
29. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, and Vandenbroucke, JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
30. Perperoglou, A, Sauerbrei, W, Abrahamowicz, M, and Schmid, M. A review of spline function procedures in R. BMC Med Res Methodol. (2019) 19:46. doi: 10.1186/s12874-019-0666-3
31. Lin, L, Chen, CZ, and Yu, XD. The analysis of threshold effect using empower stats software. Zhonghua Liu Xing Bing Xue Za Zhi. (2013) 34:1139–41.
32. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, and Vandenbroucke, JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
33. Fan, X, Huang, B, Lu, H, Zhao, Z, Lu, Z, Yang, Y, et al. Impact of admission white blood cell count on short- and long-term mortality in patients with type a acute aortic dissection: an observational study. Medicine. (2015) 94:e1761. doi: 10.1097/MD.0000000000001761
34. Shiyovich, A, Gilutz, H, and Plakht, Y. White blood cell subtypes are associated with a greater long-term risk of death after acute myocardial infarction. Tex Heart Inst J. (2017) 44:176–88. doi: 10.14503/THIJ-16-5768
35. Gao, M, Yang, Q, Xu, H, Chen, Z, Wang, X, and Guo, H. Preoperative white blood cell-related indicators can predict the prognosis of patients with transurethral resection of bladder Cancer. J Inflamm Res. (2022) 15:4139–47. doi: 10.2147/JIR.S373922
36. Zhao, L, Yang, J, Zhou, C, Wang, Y, and Liu, T. A novel prognostic model for predicting the mortality risk of patients with sepsis-related acute respiratory failure: a cohort study using the MIMIC-IV database. Curr Med Res Opin. (2022) 38:629–36. doi: 10.1080/03007995.2022.2038490
37. Hao, L, Zhang, J, Di, Y, and Tan, Z. Prognostic value of white blood cells detected for the first time after adjuvant chemotherapy in primary operable non-small cell lung Cancer. Technol Cancer Res Treat. (2018) 17:1533033818802813. doi: 10.1177/1533033818802813
38. Pierscianek, D, Ahmadipour, Y, Michel, A, Chihi, M, Oppong, MD, Kebir, S, et al. Preoperative survival prediction in patients with glioblastoma by routine inflammatory laboratory parameters. Anticancer Res. (2020) 40:1161–6. doi: 10.21873/anticanres.14058
39. Rana, JS, Boekholdt, SM, Ridker, PM, Jukema, JW, Luben, R, Bingham, SA, et al. Differential leucocyte count and the risk of future coronary artery disease in healthy men and women: the EPIC-Norfolk prospective population study. J Intern Med. (2007) 262:678–89. doi: 10.1111/j.1365-2796.2007.01864.x
40. Yan, W, Li, M, Lei, Y, Zhang, S, Lv, F, Wang, J, et al. Prognostic impact of white blood cell counts on clinical outcomes in patients with chronic renal insufficiency undergoing percutaneous coronary intervention. Front Cardiovasc Med. (2023) 10:1027107. doi: 10.3389/fcvm.2023.1027107
41. Carobbio, A, Ferrari, A, Masciulli, A, Ghirardi, A, Barosi, G, and Barbui, T. Leukocytosis and thrombosis in essential thrombocythemia and polycythemia vera: a systematic review and meta-analysis. Blood Adv. (2019) 3:1729–37. doi: 10.1182/bloodadvances.2019000211
42. Paik, KY, Lee, IK, Lee, YS, Sung, NY, and Kwon, TS. Clinical implications of systemic inflammatory response markers as independent prognostic factors in colorectal cancer patients. Cancer Res Treat. (2014) 46:65–73. doi: 10.4143/crt.2014.46.1.65
Keywords: white blood cell, thirty-day mortality, non-linear, intracranial tumor, craniotomy, cohort study
Citation: Gao Z, Huang C, Fang S, Guan J and Dong W (2024) Association between preoperative white blood cell counts and thirty-day surgical mortality after craniotomy in adult intracranial tumor patients. Front. Neurol. 15:1394568. doi: 10.3389/fneur.2024.1394568
Edited by:
Christine Marosi, Medical University of Vienna, AustriaReviewed by:
Maximilian J. Mair, Medical University of Vienna, AustriaKarl Roessler, Medical University of Vienna, Austria
Copyright © 2024 Gao, Huang, Fang, Guan and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weifeng Dong, aXdpc2hpZG9AMTI2LmNvbQ==
 Zhichao Gao
Zhichao Gao Cheng Huang
Cheng Huang Shengjie Fang1
Shengjie Fang1